Abstract
Differentiation of the hormone-producing cells of the pituitary represents an informative model of cell fate determination. The generation and maintenance of 2 pituitary lineages, the growth hormone (GH)- producing somatotropes and the prolactin (PRL)- producing lactotropes, are dependent on the pituitary-specific transcription factor, POU1F1. While POU1F1 is expressed in both cell types, and plays a role in activation of both the Gh and Prl genes, expression of Gh and Prl is restricted to somatotropes and lactotropes, respectively. These observations imply the existence of additional factors that contribute to the somatotrope and lactotrope identities and their hormone expressions. Prior transcriptome analysis of primary somatotropes and lactotropes isolated from the mouse pituitary identified enrichment of a transcription factor, Nr4a2, in the lactotropes. Nr4a2 was shown in a cell culture model to bind the Prl promoter at a position adjacent to Pou1f1 and to synergize with Pou1f1 in driving Prl transcription. Here we demonstrate in vivo the role of Nr4a2 as an enhancer of Prl expression by conditional gene inactivation of the Nr4a2 gene in mouse lactotropes. We demonstrate that nuclear orphan receptor transcription factor (NR4A2) binding at the Prl promoter is dependent on actions of POU1F1; while POU1F1 is essential to loading polymerase (Pol) II on the Prl promoter, Nr4a2 plays a role in enhancing Pol II release into the Prl gene body. These studies establish an in vivo role of Nr4a2 in enhancing Prl expression in mouse lactotropes, explore its mechanism of action, and establish a system for further study of the lactotrope lineage in the pituitary.
Keywords: pituitary, somatotrope, lactotrope, RNA-seq, Nr4a2
The divergence of somatotropes and lactotropes of the anterior pituitary represents an ideal system for studying mechanisms of lineage differentiation, establishment, and maintenance. In mice, the pituitary-specific master regulatory transcription factor, POU1F1, is expressed in a subset of developing pituitary cells beginning at e13.5 (1–3) and plays an essential role in establishment of both somatotrope and lactotrope lineages. POU1F1 has a corresponding role in the activation of Gh and Prl genes in these 2 lineages during development and in maintaining their robust expression throughout adult life (4–7). Despite this expression of Pou1f1 in both cell types, the robust expression of the GH and PRL proteins remains primarily restricted to the somatotropes and lactotropes, respectively (1, 8). These observations suggest the presence of cell type-specific transcription factors that work in conjunction with Pou1f1 to support somatotrope and lactotrope lineage differentiation and function.
In prior studies we have identified a nuclear orphan receptor transcription factor, NR4A2, as a lactotrope-enriched factor (9). We demonstrated in cell culture studies that NR4A2 enhances Prl expression by working in concert with POU1F1. Studies in cell culture and in primary lactotropes have further revealed that NR4A2 binds directly to the Prl gene promoter at a site adjacent to the POU1F1 binding site and that the Prl transcription-enhancing activity of NR4A2 is synergistic with, and dependent on, concurrent POU1F1 activity. These observations suggested a model in which NR4A2 works in conjunction with POU1F1 to promote Prl gene transcription (9).
In the present report, we demonstrate an essential role of NR4A2 in stimulating robust expression of the Prl gene in vivo, and we explore its corresponding mechanism of action. These issues were addressed by conditionally inactivating the Nr4a2 locus in the lactotrope lineage, by targeted analyses to determine the impact of NR4A2 on gene expression, and by employing a pituitary cell-based model system to elucidate how NR4A2 exerts its observed transcriptional enhancement of the Prl gene. The observations from these studies comprise an in vivo validation of our earlier cell culture-based studies (9) and provide an expanded mechanistic understanding of NR4A2 actions in the lactotrope lineage.
Materials and Methods
Mouse studies
All mice used in this study were hybrid CD1 × B6SJLF1/J virgin females that were aged 6 to 8 weeks prior to the study to allow for the complete maturation of the pituitary gland. All mouse studies were reviewed and approved by the University of Pennsylvania Laboratory Animal Use and Care Committee. The Nr4a2fl/fl mouse line was provided by Dr Thomas Perlmann (10).
Generation of Prl-Cre transgenic mouse lines
A Prl-Cre transgene construct was generated via bacterial artificial chromosome BAC recombineering as previously described (11). Briefly, a shuttle vector containing the Cre open reading frame (ORF) flanked by 500 bp of sequence homologous to the desired insertion site within the mouse Prl locus (the beginning of exon 2 of the Prl ORF) was generated by conventional subcloning. Bacterial cells were cotransformed with the constructed shuttle vector and a Prl BAC clone (RP23-441I3 BAC; Children’s Hospital Oakland Research Institutes). This Prl BAC contained the mouse Prl locus with more than 50 kb of upstream and downstream sequence. The PRL start codon was maintained in the Cre ORF such that the protein product was the first of approximately 10 amino acids of PRL, followed by the full Cre recombinase protein. Cotransformed cells were grown in lysogeny broth media supplemented with ampicillin to identify co-integrates. After obtaining and validating the shuttle vector/BAC co-integrates by polymerase chain reaction (PCR), a second selection under both ampicillin and chloramphenicol was performed to select for resolution of the co-integrate. Resolved BACs containing the Cre ORF in the proper site within the BAC were confirmed by PCR and pulse field gel analysis and targeted sequencing. Prl-Cre BAC deoxyribonucleic acid (DNA) was linearized by BsiWI (NEB R3553S) digestion, purified by gel extraction, and provided to the Penn Transgenic Mouse Facility for microinjection into mouse embryos. Founder mice carrying the Prl-Cre transgene were genotyped by analysis of tail DNA, and proper function of the Prl-Cre transgene was validated by crossing the Prl-Cre mouse line with a previously described recombination-activated reporter line (results described in text, below) (12).
Cell transfection assays
The Pit-1/Triple and Pit-1/0 cell lines, both previously described (13), were cultured in Dulbecco’s modified eagle medium (DMEM; Life Technologies) plus 10% fetal bovine serum along with 1X antibiotic-antimycotic solution (Invitrogen). A complementary DNA encompassing the full Nr4a2 ORF was amplified from mouse pituitary ribonucleic acid (RNA), and subcloned in cis to an internal ribosome entry sequence (IRES) green fluorescent protein (GFP) cassette (Addgene plasmid #51406). The expression of the bicistronic Nr4a2/IRES/GFP transcription unit was driven from a cytomegalovirus promoter. Pit-1/Triple and Pit-1/0 cells were transfected with 10 μg of plasmid using the TransIT-293 transfection reagent (Mirus Bio). In cotransfection assays, cells were transfected with 5 µg of each plasmid to maintain a consistent ratio of transfected DNA:TransIT-293 reagent, as recommended by the manufacturer. Two days after transfection, GFP+ cells were isolated by FACS (Cell Sorting & Flow Cytometry Core at the University of Pennsylvania). RNA was isolated from the GFP+ cell population as described above, and complementary DNA was generated using SuperScript III reverse transcriptase (Life Technologies). Quantitative real-time (qRT) PCR was performed using the Fast SYBR Green Master Mix kit (Applied Biosystems) on a QuantStudio 7 Flex (Applied Biosystems) platform. Quantitative real time PCR assays were done in biological triplicate. All samples were normalized to Gapdh messenger RNA (mRNA) levels and compared with cells transfected with an empty vector by the ΔΔCt method (14). All transfections were performed using approximately 1.6 million cells per sample, grown to 80% confluence in 10-cm plates. Amplification primer sets are as follows: Gh: 5′-gcccaggctgctttctgc-3′ and 5′-caattccatgtcggttctctgc-3′; Prl: 5′-aggggtcagcccagaaagc-3′ and 5′-tcaccagcggaacagattgg-3′; Pou1f1: 5′-aggtgggagcaaacgaa agg-3′ and 5′-gctccccgaagtgtctctcc-3′; Ghrhr: 5′-tcacttcggctca gcacagg-3′ and 5′-ggcaagccacagggtatgg-3′; Drd2: 5′-tgaacct gtgtgccatcagc-3′ and 5′-gacagtaactcggcgcttgg-3′; Gapdh: 5′-agcttaggttcatcaggtaaactcagg-3′ and 5′-cgttcacaccgaccttcacc-3′; and Nr4a2: 5′-gatcagtgccctcgtcagagc-3′ and 5′-gtcagggttt gcctggaacc-3′.
RNA isolation from pituitary
RNA from total murine pituitary was isolated by standard Trizol LS protocol (Thermo Fisher Scientific) using 10 µg of GlycoBlue coprecipitant (Thermo Fisher Scientific) as a carrier. quantitative real time PCR was performed as described above.
Immunofluorescence microscopy
Pituitaries were harvested at 6 to 8 weeks and dissociated mechanically in enzyme-free cell dissociation buffer (Life Technologies). The dissociated cells were suspended in DMEM + 10% fetal bovine serum culture media, passed through a 40-μm filter, and centrifuged at 1000 g for 5 minutes. The cell pellet was resuspended in phosphate-buffered saline (PBS) plus 0.1% bovine serum albumin (BSA) and layered onto a PBS plus 4% BSA cushion and centrifuged at 100 g for 5 minutes to remove residual cell debris. Immunofluorescent staining was performed as previously described (15). Briefly, the disaggregated cells were placed on poly-L-lysine–coated slides and incubated at room temperature for 10 minutes to allow the cells to adhere to the slide. After attaching to the slides, cells were fixed in 4% formaldehyde for 10 minutes, washed 3 times with PBS for 5 minutes per wash, and permeabilized in a solution of 0.5% triton X-100 and 0.5% saponin for 10 minutes. The cells were washed with PBS before being incubated in a blocking buffer of 4X saline-sodium citrate, 2% BSA, 0.1% Tween 20, and 15% donkey serum for 20 minutes at room temperature. After blocking, slides were incubated with antibodies specific to proteins of interest for 1 hour at room temperature, washed 3 times in PBS containing 0.1% Triton X-100, then incubated for one hour at room temperature with secondary antibodies conjugated to fluorophores for detection. After incubation with secondary antibodies, the slides were washed 3 times, incubated for 1 minute in 1mg/mL 4′,6-diamidino-2-phenylindole dihydrochloride, and cover slips were attached. Slides were imaged with a Leica SP8 confocal microscope platform. The number of lactotropes was quantified via immunofluorescence microscopy assays in a setting in which the genotype of each sample was “blinded” to the observer in order to eliminate issues of bias in cell counting. Images were analyzed using Fiji software (16). Antibodies used to detect GH and PRL were RRID: AB_2629219(17) and RRID: AB_2629220(18), respectively. The antibody used for NR4A2 detection was Abcam ab41917, RRID: AB_776887(19), and the antibody used to detect POU1F1 was RRID: AB_2732812(20).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (21). Chromatin was isolated from approximately 200 000 FACS sorted transfected cells per sample and chromatin from approximately 100 000 cells was used for each ChIP reaction. For the purposes of performing ChIP assays, Pit-1/0 cells were transfected with 20 µg of each plasmid using Lipofectamine 3000 (Thermo Scientific) per the manufacturer’s directions to achieve a high transfection efficiency. Antibodies specific for NR4A2 (Abcam ab41917, RRID: AB_776887(19)) and specific to RNA polymerase II (isoform phosphorylated S5 C-terminal repeat) (Abcam ab5131, RRID: AB_449369) were used in the respective assays. A rabbit antibody specific for POU1F1 was previously generated against the following POU1F1 epitope: PLLAEDPAASEFKQELRRKSKL (Alpha Diagnostics (RRID: AB_2732812(20))) (17). This affinity-purified antibody is specific for POU1F1 as assessed in our laboratory by Western blot analysis (9). An anti-CDK9 antibody (RRID: AB_10881213(22)) was used for pulldowns of CDK9. Normal rabbit immunoglobulin G (Millipore) was used as a control in all ChIP assays. Input and bound DNAs were amplified by quantitative real time PCR using the same QuantStudio 7 Flex platform noted above. A serial dilution of the input chromatin was used to determine the linear range of the amplification, and the enrichment of the bound DNA was calculated as a percentage of the input. ChIP of primary mouse pituitary lactotropes was performed using the same protocol, with cells being collected by FACS prior to isolation of chromatin, as described above. Amplification primer sequences: Gh promoter: 5′-gccttggggtcgaggaaaac-3′ and 5′-gggatttgcgcatgcttacc-3′; Prl upstream control: 5′-gaactggaagctgttgaacttgc-3′ and 5′-ggtcttgggaactgaacttgg-3′; Prl promoter: 5′-tggccaatgtcttcctgaatatg-3′ and 5′-cactggctttataaacctttgaca-3′; Prl downstream control: 5′-catgcattctaccaaaagggtagg-3′ and 5′-tgcatctctatagcacttggtttatcc-3′; and MyoD promoter: 5′-gcgtatggctgccagtctct-3′ and 5′-tgtagtagggcggagcttgg-3′.
Statistical analysis
One-way analysis of variance and t test analyses were performed using GraphPad Prism software, with corrections for multiple comparisons performed using the Prism package.
Results
Generation of a mouse line expressing a lactotrope-restricted Cre recombinase
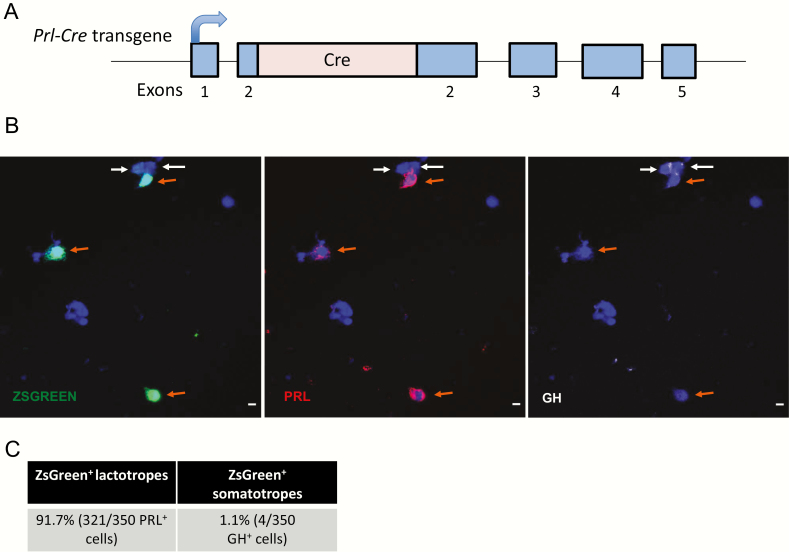
Our prior studies demonstrate that NR4A2 is enriched in the lactotropes of the mouse pituitary and facilitates Prl expression in vitro (9). In the current studies, we explored the functional impact of NR4A2 on the lactotrope specific gene expression in an in vivo setting. A Prl-Cre mouse line was generated for this purpose by inserting the Cre ORF into the second exon of the Prl gene within the context of a 200 kb BAC encompassing the mouse Prl locus (Fig. 1A). The BAC clone used for this insertion contained extensive sequences both 5′ and 3′ of the Prl gene (approximately 85 kb and 100 kb, respectively; see Methods). In the absence of evidence that the mPrl gene is under remote regulatory control (23), the presence of these extensive flanking sequence was assumed to be sufficient to drive lactotrope-specific expression. The recombinant Prl-Cre BAC transgene was microinjected into B6SJLF1/J mouse embryos, and a Prl-Cre mouse line was established. This line was crossed with a previously reported ZsGreen reporter line (Ai6-ZsGreen (12)) to assess activity and specificity of the Cre recombinase expression (12). Pituitaries from mice carrying both the Prl-Cre and ZsGreen transgenes (Prl-Cre/ZsGreen compound transgenics) were disaggregated and immunostained for PRL and ZsGreen (Fig. 1B). The analysis revealed that 91.7% of all PRL+ cells were positive for the ZSGREEN as opposed to only 1.1% of all GH+ cells (somatotropes) (Fig. 1C). These data indicated that the expression of Cre recombinase from the Prl-Cre transgene was robust and lactotrope specific. These findings are in line with a previous report that Prl-driven Cre is robustly expressed in lactotropes, with some measure of expression observed in somatotropes (24).
Figure 1.
The Prl-Cre transgene expresses Cre recombinase selectively in the lactotrope lineage. A. Schematic of the Prl-Cre transgene. A CRE ORF was inserted into the second exon of the Prl gene in an mPrl BAC behind the native Prl translation initiation codon (see Methods). The curved arrow indicates the direction of Prl transgene transcription. The Prl BAC transgene preserves extensive sequences flanking the Prl locus (not shown in diagram; see Results). B. Immunofluorescence microscopy of dispersed pituitary cells from a Prl-Cre/ZsGreen compound transgenic mouse. The ZsGreen fluorescent reporter is preceded by a lox-stop-lox element such that ZsGreen is only expressed in cells that express the Cre recombinase. Lactotrope (PRL+) cells are indicated by orange arrows, and somatotrope (GH+) cells are indicated by white arrows. C. Quantification of somatotropes and lactotropes expressing ZsGreen reporter in a Prl-Cre/ZsGreen compound transgenic mouse (n = 350 cells PRL+ and 350 GH+ cells analyzed). BAC, bacterial artificial chromosome; GH, growth hormone; ORF, open reading frame; PRL, prolactin.
Impact of conditional inactivation of NR4A2 on prolactin expression in the pituitary
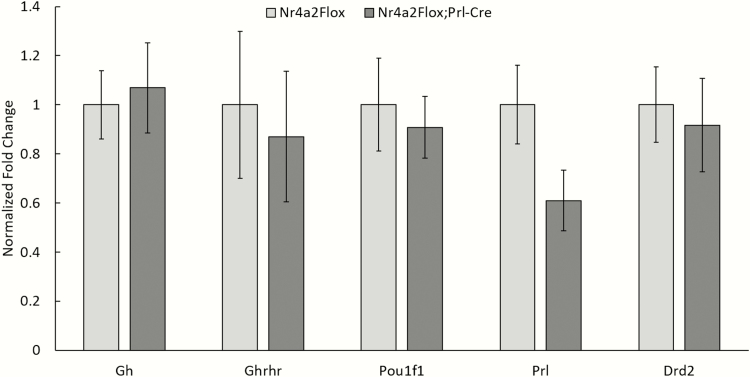
We have previously demonstrated in cell culture studies that NR4A2 binds within the Prl promoter at a site adjacent to POU1F1 and enhances Prl expression in a POU1F1-dependent manner (9). To validate and extend these findings in vivo, we crossed our Prl-Cre line with a line carrying a floxed Nr4a2 allele. The LoxP sites in the floxed Nr4a2 allele flank exon 3(10). Subsequent intercrosses generated Nr4a2fl/fl/Prl-Cre compound transgenics. The impact of the inactivation of Nr4a2 on Prl expression was assessed on the pituitaries of the Nr4a2fl/fl/Prl-Cre mice (n = 2) with a control set comprising Nr4a2fl/fl mice (n = 3) (Fig. 2). This analysis revealed a 40% decrease in Prl mRNA in the pituitaries of Nr4a2fl/fl/Prl-Cre mice as compared with Nr4a2fl/fl littermate controls, while the expression levels of Gh, Pou1f1, Ghrhr, and Drd2 remained unchanged (Fig. 2). These studies led us to conclude that the impact of the Nr4a2 inactivation on lactotrope-specific gene expression appeared to be highly selective to the Prl gene.
Figure 2.
Inactivation of Nr4a2 in lactotropes of the mouse pituitary results in a significant decrease in Prl gene expression. Comparison of Nr4a2fl/fl/Prl-Cre (KO) and Nr4a2fl/fl (control) mice pituitary mRNAs. Targeted RT-qPCR validates selective loss of Prl mRNA in the Nr4a2 knockout mice. mRNA levels are shown for Pou1f1 and for 2 landmark genes for the somatotrope (Gh and Ghrh) and lactotrope (Prl and Drd2) lineages. The only comparison with a significant difference was for Prl (n = 3 mice). KO, knockout; mRNA, messenger ribonucleic acid; RT-qPCR, real-time quantitative polymerase chain reaction.
Deletion of Nr4a2 in lactotropes leads to the presence of ‘prolactin-silent’ lactotropes.
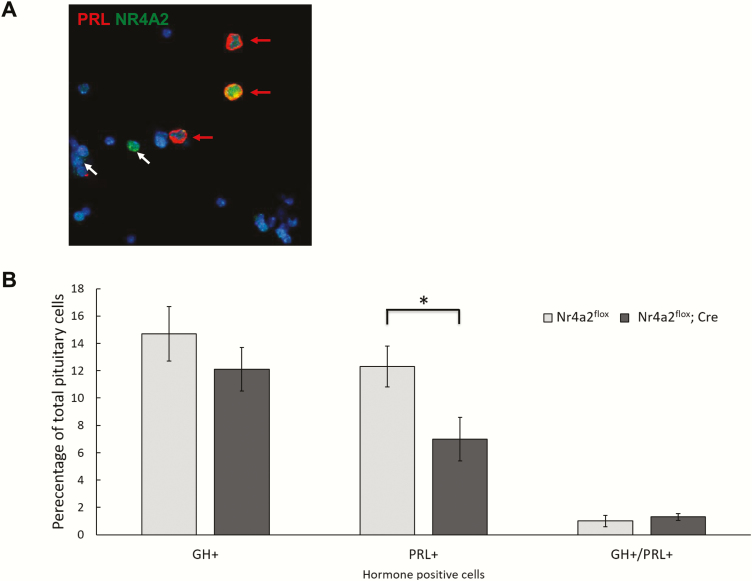
We next assessed the impact of the Nr4a2 inactivation on the representation of the lactotrope population within the pituitary. Immunofluorescent (IF) microscopy for PRL and NR4A2 was carried out on disaggregated cells from the pituitaries of the Nr4a2fl/fl/Prl-Cre mice and their Nr4a2fl/fl littermate controls (Fig. 3A). NR4A2 could be identified in the great majority (90%) of lactotropes in the pituitaries of the control Nr4a2fl/fl mice (n = 371 PRL+ cells) as well as in a subset of nonlactotropes. This percentage of lactotropes expressing the NR4A2 protein in the control pituitary is consistent with that previously observed in a wild-type context (9), confirming that the inserted loxP sites do not adversely affect expression of the Nr4a2 gene. In contrast, only 31% of the PRL+ cells in the pituitaries of the Nr4a2fl/fl/Prl-Cre mice expressed NR4A2 (N = 393 PRL+ cells.). These data confirm successful ablation of Nr4a2 expression in the majority of lactotrope cells in the Nr4a2fl/fl/Prl-Cre mice.
Figure 3.
Targeted inactivation of the Nr4a2 locus in mouse lactotropes results in a decrease in prolactin-positive cells. A. Immunofluorescent detection of PRL and NR4A2 proteins in dispersed pituitary cells. The representative field is from a WT mouse stained for PRL (red) and NR4A2 (green). Lactotropes (red arrows) contain Prl in the cytoplasm and Nr4a2 in the nucleus. A number of nonlactotrope cells in the pituitary also express Nr4a2 (white arrows). B. Quantification of the numbers of GH+, PRL+, and GH+/PRL+ cells in control (Nr4a2fl/fl) and in Nr4a2fl/fl/Prl-Cre conditional knockout mice. N = 500 total pituitary cells. (* indicates P < 0.05 as determined by 1-way analysis of variance test). GH, growth hormone; NR4A2, nuclear orphan receptor transcription factor; PRL, prolactin; WT, wild type.
We next determined the impact of Nr4a2 ablation on the overall representation of lactotropes (PRL+ cells) in the pituitary. We observed that the pituitaries isolated from Nr4a2fl/fl/Prl-Cre mice had approximately half as many PRL+ cells as their Nr4a2flox/flox littermates, while the numbers of GH+ cells and dual GH+/PRL+ cells did not significantly differ between these 2 groups (Fig. 3B). This selective reduction in PRL+ cells paralleled the 40% decrease in Prl mRNA in the pituitaries of Nr4a2flox/flox/ Prl-Cre mice as compared with littermate controls (Fig. 2). We also observed occasional PRL+ cells in the Nr4a2fl/fl/ Prl-Cre mice that lacked an Nr4a2 signal (data not shown), suggesting that Prl expression can be sustained in a subset of lactotropes in the absence of Nr4a2 (also see below). In sum, these IF studies support the overall conclusion that Nr4a2 expression is required in vivo for robust Prl expression in cells within the lactotrope lineage.
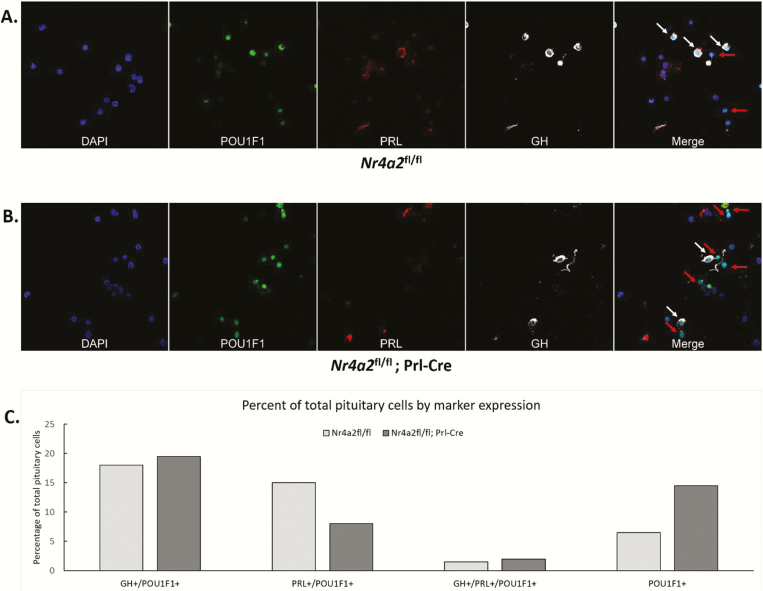
The observed 50% decrease in PRL+ cells in the pituitaries of Nr4a2flox/flox; Prl-Cre mice (Fig. 3B) might reflect loss of lactotrope cells or, alternatively, it may reflect the generation of a population of lactotrope cells that lack Prl expression (PRL-silent lactotropes). To distinguish between these 2 models, we stained the pituitaries of Nr4a2fl/fl/Prl-Cre mice and Nr4a2fl/fl littermates for PRL, GH, and POU1F1 (Fig. 4A). This IF analysis revealed a substantial decrease in the PRL+/POU1F1+ cell population in the Nr4a2fl/fl/Prl-Cre mouse pituitaries and a reciprocal increase in the population of POU1F1+ cells that lacked appreciable levels of PRL or GH (POU1F1+/GH–/PRL– cells; Fig. 4B). These data suggested that inactivation of Nr4a2 generates a population of lactotropes lacking PRL (PRL-silent lactotropes).
Figure 4.
. Targeted inactivation of the Nr4a2 locus in mouse lactotropes results in a decrease in PRL+ cells and a reciprocal increase in cells that are POU1F1+ but lack both PRL and GH. A. Co-immunofluorescent analyses of POUF1, PRL, and GH in pituitary cells from Nr4a2fl/fl (top panel) and Nr4a2fl/fl/Prl-Cre (bottom panel) mice. A single field of disaggregated cells is show for each sample. The cells were stained with a nuclear dye (DAPI) and immunostained for POU1F1, PRL, and GH proteins. The final frame in each of the 2 studies superimposes the POU1F1, PRL, or GH stains. Pou1f1+ cells that coexpress GH or PRL or are negative for both of these hormones were quantified. White arrows indicate POU1F1+ cells that express either GH or PRL, while red arrows indicate POU1F1+ cells that do not express either of the 2 hormones. N = 2 mice used per group. B. Percentage of total POU1F1+ pituitary cells (200 cells counted per genotype, n = 1 for each sample) that express the indicated combinations of GH, PRL, and/or POIU1f1. DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; GH, growth hormone; POU1F1, pituitary-specific master regulatory transcription factor; PRL, prolactin.
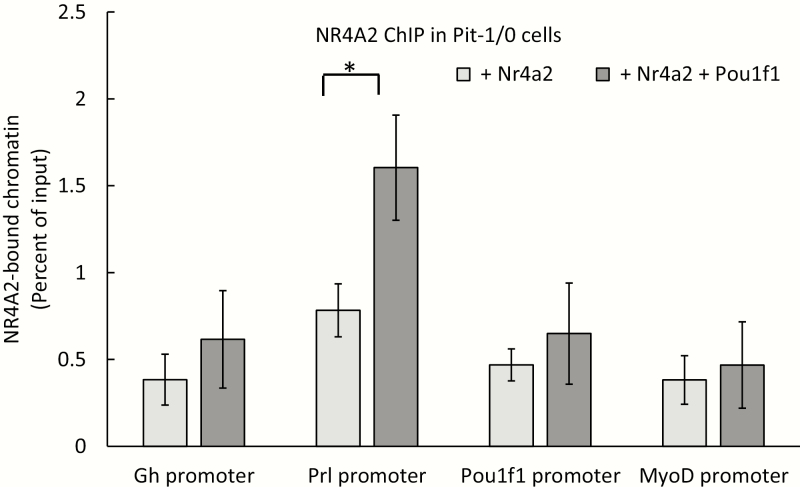
NR4A2 binding at the Prl promoter is POU1F1 dependent
Our prior studies in tissue culture have led us to propose that POU1F1 binding at the Prl promoter generates an activated chromatin environment that is rendered accessible for subsequent NR4A2 binding. To test the model that NR4A2 binding at the Prl promoter is POU1F1 dependent, we transfected an NR4A2-/ POU1F1- pituitary-derived cell line (Pit-1/0 cells (13)) with a plasmid expressing NR4A2 alone or cotransfected with 2 plasmids expressing NR4A2 and POU1F1. NR4A2 binding to the Prl promoter was only observed in cells that coexpressed POU1F1 (Fig. 5). This binding of NR4A2 in the presence of POU1F1 was specific to the Prl promoter when compared with the promoters of the Gh and Pou1f1 genes, which have POU1F1 binding sites but lack consensus NR4A2 binding motifs. These data, in conjunction with the prior observation that NR4A2 enhancement of Prl expression is dependent upon the presence of POU1F1, supports a model in which NR4A2 binding at the Prl promoter is fully dependent on the actions of POU1F1(9).
Figure 5.
NR4A2 occupancy at the Prl promoter is POU1F1 dependent. Pit-1/0 cells were transfected with a plasmid expressing Nr4a2 or with plasmids expressing both Nr4a2 and Pou1f1. All expression vectors contained an IRES/GFP reporter. GFP+ transfected cells were flow sorted, and chromatin was isolated for NR4A2 ChIP analysis. NR4A2 occupancy was assayed at the promoters of Gh, Prl, Pou1f1, and Myod genes (MyoD served as a negative control). * indicates P < 0.05 as determined by 1-way analysis of variance (n = 3 biological replicates). ChIP, chromatin immunoprecipitation; GFP, green fluorescent protein; IRES, internal ribosome entry sequence; NR4A2, nuclear orphan receptor transcription factor; POU1F1, pituitary-specific master regulatory transcription factor.
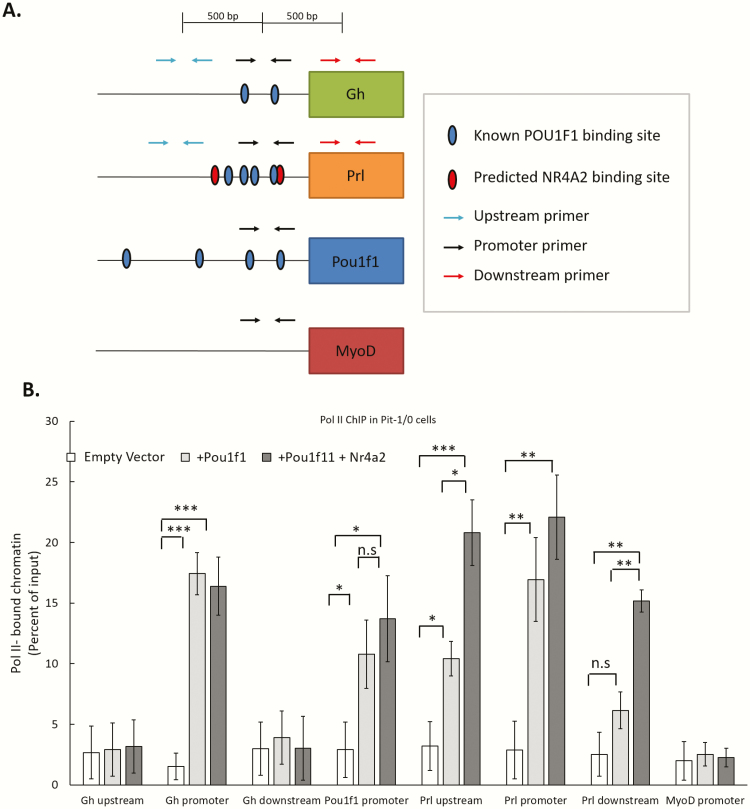
NR4A2 enhances Prl gene transcription by facilitating the release of RNA polymerase II from the Prl promoter into the gene body.
The observation that NR4A2 binding to the Prl promoter and its enhancement of Prl gene expression are both POU1F1 dependent led us to ask if NR4A2 augments POU1F1-dependent recruitment of Pol II to the Prl promoter. To test this model, we transfected Pit-1/0 cells with plasmids that express POU1F1 and NR4A2 (see Methods) and assayed Pol II occupancy at the Prl locus as well as at POU1F1 binding sites at the Gh and Pou1f1 loci (Fig. 6). Pol II ChIP revealed that Pol II recruitment to the Prl promoter was dependent on POU1F1 and that this recruitment of Pol II was not significantly enhanced by NR4A2. NR4A2 did, however, enhance Pol II density within the body of the Prl gene (Fig. 6, “Prl downstream”). These data suggested a model in which NR4A2 enhances Prl gene expression by facilitating the release of Pol II from the Prl promoter into the gene body rather than by enhancing the assembly of the Pol II initiation complex.
Figure 6.
NR4A2 enhances the release of Pol II from the Prl promoter into the gene body. A. Schematic of primer locations used in subsequent assays. B. Pit-1/0 cells were transfected with a Pou1f1 expression plasmid or with a combination of expression plasmids encoding both Pou1f1 and Nr4a2 proteins. Transfection with an empty GFP vector served as a negative control. ChIP was performed on flow-sorted GFP+ cells with a Poll II antibody (Ser 5-P isoform), and occupancy was quantified by qPCR. The Gh, Pou1f1, and Prl promoters were assayed for Pol II occupancy along with regions 500 bp upstream and downstream of the respective promoters. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001 as determined by 1-way analysis of variance. n.s. indicates no significance. n = 3 biological replicates. ChIP, chromatin immunoprecipitation; GFP, POL II, polymerase II.
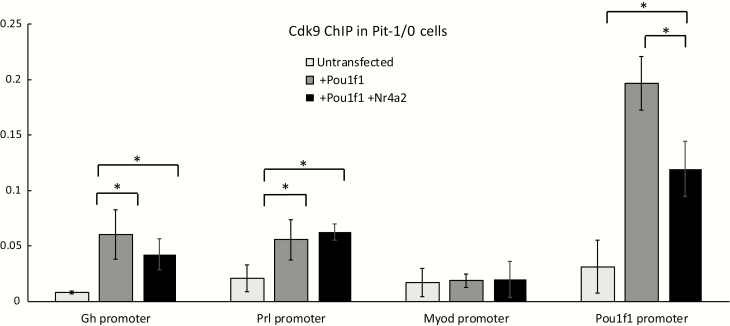
The observation that NR4A2 may act on the Prl gene by enhancing release of Pol II from the Prl promoter suggested that it might function by recruitment of Pol II release factors. The release factor most clearly linked to Pol II release from eukaryotic promoters is positive transcription elongation factor b (P-TEFb) (25). P-TEFb is composed of 2 major subunits; Cdk9 and cyclin T1(26,27). To determine if NR4A2 enhances recruitment of P-TEFb to the Prl promoter, we transfected Pit-1/0 cells with plasmids expressing POU1F1 and NR4A2 and performed ChIP for the Cdk9 component of P-TEFb. Expression of POU1F1 was observed to stimulate significant increases in Cdk9 occupancy at both the Gh and Prl promoters, as well as a robust recruitment of Cdk9 to the autoregulated Pou1f1 promoter (5) (Fig. 7). Remarkably, the POU1F1-dependent recruitment of Cdk9 to the Prl promoter was not impacted by the coexpression of NR4A2, and the POU1F1-dependent recruitment Cdk9 recruitment at the Pou1f1 was actually repressed by NR4A2 coexpression. These studies lead us to conclude that NR4A2 stimulates Pol II release from the Prl promoter via a P-TEFb independent pathway.
Figure 7.
NR4A2 fails to enhance POU1F1-dependent Cdk9 recruitment at the Prl promoter. A. Schematic of relative positions of “upstream” (blue arrows), “promoter” (black arrows), and “downstream” (red arrows) primers, with known POU1F1 and predicted NR4A2 binding sites in blue and red ovals, respectively. Known POU1F1 sites are adapted from (21). B. Pit-1/0 cells were transfected with plasmids expressing Pou1f1 alone or with a combination of expression plasmids for both Pou1f1 and Nr4a2 (as in Fig. 6). ChIP was performed using an antibody against the Cdk9 subunit of the release factor P-TEFb. Cdk9 occupancy was assessed at the Gh and Prl promoters, at the autoregulated Pou1f1 promoter, and the promoter for the muscle-specific Myod gene. * indicates P < 0.05 as determined by 1-way analysis of variance. n = 3 biological replicates. NR4A2, nuclear orphan receptor transcription factor; POU1F1, pituitary-specific master regulatory transcription factor.
Discussion
Here we demonstrate in vivo a positive role of the orphan receptor transcription factor NR4A2 in the enhancement of Prl gene transcription in pituitary lactotropes, and we explore its corresponding mechanism of action. Lactotrope-specific inactivation of NR4A2 expression resulted in a substantial loss of Prl mRNA in the pituitary and a corresponding decrease in PRL+ cells. The data further suggested that lactotropes could be maintained as POU1F1+ cells in the absence of PRL expression, that is, “PRL-silent” lactotropes. Finally, our studies suggest that NR4A2 enhances Prl gene expression by working in conjunction with POU1F1 and that the mechanism of the enhanced Prl gene transcription by NR4A2 reflects a stimulation of Pol II release from the Prl promoter into the gene body.
In order to specifically inactivate the Nr4a2 loci in lactotropes, we generated a mouse line that expressed Cre recombinase under control of the Prl gene regulatory elements (Fig. 1). The expression of this Prl-Cre transgene was shown to be highly effective and lactotrope specific as demonstrated by its activation of a Cre-dependent reporter in 91.7% of PRL+ cells and in only 1.1% of Gh+ cells (Fig. 1C). We proceeded to generate compound transgenic mice that carry this Prl-Cre transgene as well as the floxed Nr4a2 alleles. Analysis of pituitaries isolated from these Nr4a2flox/flox; Prl-Cre mice compared with Nr4a2flox/flox littermate controls demonstrated significant loss of Nr4a2 expression, a 40% decrease in Prl mRNA levels (Fig. 2), and a 45% decrease in the number of PRL+ cells (Fig. 3). These data directly support a positive role for Nr4a2 in Prl gene expression in the pituitary lactotropes.
We noted in the course of these studies that the inactivation of Nr4a2 gene expression by Prl-Cre appeared to be only partial as 31% of lactotropes in Nr4a2flox/flox; Prl-Cre mice continued to express NR4A2. This partial activity, consistent with that reported for an independently generated Prl-Cre transgenic line (24), could reflect incomplete efficiency of Cre recombinase to trigger recombination at the floxed Nr4a2 locus and/or to the existence of a subpopulation of lactotropes within normal pituitary that does not effectively support the expression of the Prl-Cre transgene. Our recently reported single-cell RNA sequencing analysis revealed a marked heterogeneity within lactotrope cell populations, and it is possible that various subgroups may support Prl-Cre expression to different extents (28). One additional model that needs to be considered is that the representation of a small population of lactotropes in the Nr4a2flox/flox; Prl-Cre mouse pituitary that had escaped Nr4a2 inactivation in some manner, either because it was Cre– or it was Cre+ but failed to have an inactivated the Nr4a2 locus, might undergo selective expansion over time to maintain PRL homeostasis. This might occur via a feed-forward mechanism activated by the reduction of circulating PRL in these Nr4a2flox/flox; Prl-Cre mice. Such an expansion of a population of Prl-expressing cells might be further stimulated by one or more physiologic signals. Such a model could be tested by following the representation of a Prl+ cell population in Nr4a2flox/flox; Prl-Cre mice as these mice age or as they respond to a major lactogenic stress such as postpartum lactation.
In order to further characterize the consequences Prl gene inactivation in the lactotropes of the Nr4a2flox/flox; Prl-Cre mice, we performed immunofluorescence microscopy by costaining for POU1F1, Gh and Prl, (Fig. 4). This analysis revealed that the Cre-mediated inactivation of NR4A2 loci in lactotropes results in not only a significant decrease in PRL+/POU1F1+ cells but also a reciprocal increase in POU1F1+ cells that lacked PRL. The possibility that these cells might represent lactotropes that had converted to somatotropes was ruled out by demonstrating that they were negative for GH as well. These data suggested that the silencing of the Prl gene expression by Nr4a2 ablation may generate a population of PRL-silent lactotrope cells, meaning. that ablation of NR4A2 expression causes a loss of prolactin expression in lactotrope cells rather than an outright loss of the cells in the lactotrope lineage.
Having previously demonstrated that POU1F1 binds the Prl promoter independent of NR4A2 occupancy (9), we sought to explore the mechanism by which the NR4A2 protein exerts its enhancement of Prl gene expression. Consistent with our previous data in cell culture (9), we determined that NR4A2 occupancy at the Prl promoter requires the presence of POU1F1 (Fig. 5). The data revealed that Pou1f1 is sufficient to recruit Pol II at the Prl promoter, and this activity is not enhanced by NR4A2 (Fig. 6). Surprisingly, we further observed that Pol II occupancy downstream in the Prl gene body was markedly enhanced by coexpression of NR4A2 with POU1F1. Pol II occupancy at the region approximately 500 bp upstream of the Prl promoter is also enhanced by expression of POU1F1 and further enhanced by the coexpression of POU1F1 and NR4A2. This increase in Pol II occupancy at regions both upstream and downstream of the Prl promoter may be indicative of bidirectional transcription, which is a previously described phenomenon at many active promoters (29–31). As was expected, given the lack of NR4A2 in somatotropes and the lack of binding sites at the Gh promoter, the expression of NR4A2 in the Pit-1/0 cell line failed to impact on Pol II occupancy at the promoter or within the body of the Gh gene. These data led us to conclude that NR4A2 enhances Prl transcription, at least in part, by facilitating Pol II release from the Prl promoter into the Prl gene body.
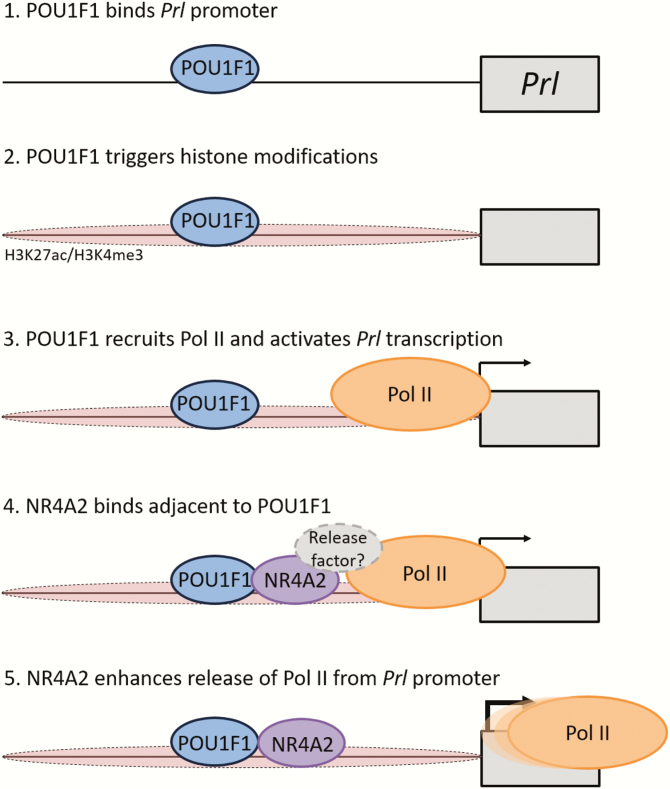
The basis for the NR4A2-enhanced release of Pol II from the Prl promoter was investigated by asking if it reflected an enhanced recruitment of known release cofactors. These studies failed to support an involvement of the prominent Pol II release factor, P-TEFb in this process (Fig. 7). Thus, alternative models involving the interaction of NR4A2 with as yet unidentified release factor(s), or NR4A2-mediated ejection of release inhibitory factor(s), remain to be further explored. The data further suggests that NR4A2 may play an unanticipated negative role in P-TEFb recruitment. This was indicated by the significant negative impact of NR4A2 on POU1F1-dependent Cdk9 occupancy at the autoregulated Pou1f1 promoter (Fig. 7). In sum, these data led us to hypothesize that a model in which POU1F1 occupancy at the Prl promoter sets the stage for NR4A2 binding and that NR4A2 then acts to increase the rate of transcription of Prl by triggering the release of Pol II from the Prl promoter (outlined in Fig. 8).
Figure 8.
. Model: Proposed role of NR4A2 in enhancement of Prl transcriptional activity. The Prl gene is represented as a single box along with its promoter region (line). The activation process is shown as 5 hypothetical steps. 1. POU1F1 binds the Prl promoter. This binding is an initiating event and is independent of NR4A2(9). 2. POU1F1 triggers activating histone modifications at the Prl promoter (9). 3. POU1F1 recruits Pol II to the Prl promoter. This event activates transcription initiation and leads to low level Prl transcription. 4. NR4A2 binds the Prl promoter in the presence of POU1F1. The bound Nr4a2 enhances the recruitment of one or more Pol II release factor(s). 5. The efficient release of Pol II into the Prl gene body enhances transcription of the Prl gene. NR4A2, nuclear orphan receptor transcription factor; POL II, polymerase II; POU1F1, pituitary-specific master regulatory transcription factor.
The observation that a novel, cell type-enriched transcription factor binds to the promoter of the Prl gene and acts in conjunction with POU1F1 to enhance gene expression offers a potential explanation for the observation that Pou1f1 is expressed in both somatotropes and lactotropes, yet selectively drives the production of one hormone protein per cell type. While POU1F1 binding at the Gh and Prl promoters may be ubiquitous in somatotropes and lactotropes, and prime the respective promoters via targeted histone acetylation, the presence or absence of additional lineage-enriched factors such as NR4A2 may be the crucial determinant for which hormone gene is productively activated by POU1F1 in a cell.
Defining a cell type by its expression of a hormone alone may be problematic. For example, if a lactotrope ceases to express PRL, should it still be classified as a lactotrope? Only with a more complete understanding of the set of genes that makes a somatotrope a somatotrope can one deal with classifying cells in which the hormones, currently the only landmarks used to determine cell identity, are perturbed. Further study of the somatotrope, lactotrope, and somatolactotrope cell identities will be needed to resolve this dilemma. The work presented here in characterizing a novel transcription factor acting on lactotropes provides a critical resource that will be essential in reaching that goal.
Taken together, the present data reconfigure and expand upon the previous standard model of pituitary cell identity. POU1F1 acts in conjunction with other transcription factors that are enriched in either cell type. These transcription factors may exert either enhancing or repressive effects on the expression of the Prl gene, and NR4A2 is likely to represent only one example of the factors involved in this process.
Acknowledgments
We acknowledge the assistance of the following Core facilities at the Perelman School of Medicine in this study: Dr Andrea Stout of the Microscopy Core in the Department of Cell and Development Biology, Dr Jean Richa of the Transgenic and Chimeric Mouse facility in the Department of Genetics, and Thomas Williams and members of the Flow Cytometry & Cell Sorting Facility Department of Pathology. We also acknowledge Dr Thomas Perlmann (Karolinska Institute) for his generous gift of the Nr4a2Flox transgenic mouse line.
Financial Support: This work was supported by National Institutes of Health (NIH) Grant DK107453 to S.A.L. and Y.H.
Glossary
Abbreviations
- BAC
bacterial artificial chromosome
- BSA
bovine serum albumin
- ChIP
chromatin immunoprecipitation
- DMEM
Dulbecco’s modified eagle medium
- DNA
deoxyribonucleic acid
- GH
growth hormone
- IF
immunofluorescent
- mRNA
messenger RNA
- NR4A2
nuclear orphan receptor transcription factor
- ORF
open reading frame
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- Pol
polymerase
- POU1F1
pituitary-specific master regulatory transcription factor
- PRL
prolactin
- qRT
quantitative real-time
- RNA
ribonucleic acid
Additional Information
Disclosure Summary: The authors have no conflicts of interest to disclose.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87(3):933-963. [DOI] [PubMed] [Google Scholar]
- 2. Zhu X, Wang J, Ju BG, Rosenfeld MG. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19(6):605-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersen B, Rosenfeld MG. Pit-1 determines cell types during development of the anterior pituitary gland. A model for transcriptional regulation of cell phenotypes in mammalian organogenesis. J Biol. Chem. 1994;269(47):29335-29338. [PubMed] [Google Scholar]
- 4. Mangalam HJ, Albert VR, Ingraham HA, et al. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989;3(7):946-958. [DOI] [PubMed] [Google Scholar]
- 5. Cohen LE, Wondisford FE, Radovick S. Role of Pit-1 in the gene expression of growth hormone, prolactin, and thyrotropin. Endocrinol Metab Clin North Am. 1996;25(3):523-540. [DOI] [PubMed] [Google Scholar]
- 6. Tatsumi K, Miyai K, Notomi T, et al. Cretinism with combined hormone deficiency caused by a mutation in the PIT1 gene. Nat Genet. 1992;1(1):56-58. [DOI] [PubMed] [Google Scholar]
- 7. Nelson C, Albert VR, Elsholtz HP, Lu LI, Rosenfeld MG. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988;239(4846):1400-1405. [DOI] [PubMed] [Google Scholar]
- 8. Nakane PK. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem. Cytochem. 1970;18(1):9-20. [DOI] [PubMed] [Google Scholar]
- 9. Peel MT, Ho Y, Liebhaber SA. Transcriptome analyses of female somatotropes and lactotropes reveal novel regulators of cell identity in the pituitary. Endocrinology. 2018;159(12):3965-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadkhodaei B, Alvarsson A, Schintu N, et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci U S A. 2013;110(6):2360-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong S, Yang XW, Li C, Heintz N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 2002;12(12):1992-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sizova D, Ho Y, Cooke NE, Liebhaber SA. Research resource: T-antigen transformation of pituitary cells captures three novel cell lines in the Pit-1 lineage. Mol Endocrinol. 2010;24(11):2232-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101-1108. [DOI] [PubMed] [Google Scholar]
- 15. Ho Y, Liebhaber SA, Cooke NE. The role of the hGH locus control region in somatotrope restriction of hGH-N gene expression. Mol Endocrinol. 2011;25(5):877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. RRID: AB_2629219. In. [Google Scholar]
- 18. RRID: AB_2629220. In. [Google Scholar]
- 19. RRID: AB_776887. In. [Google Scholar]
- 20. RRID: AB_2732812. In. [Google Scholar]
- 21. Shewchuk BM, Ho Y, Liebhaber SA, Cooke NE. A single base difference between Pit-1 binding sites at the hGH promoter and locus control region specifies distinct Pit-1 conformations and functions. Mol Cell Biol. 2006;26(17):6535-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RRID: AB_10881213. In. [Google Scholar]
- 23. Crenshaw EB 3rd, Kalla K, Simmons DM, Swanson LW, Rosenfeld MG. Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev. 1989;3(7):959-972. [DOI] [PubMed] [Google Scholar]
- 24. Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J. Endocrinol. 2010;205(1):49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paparidis NF, Durvale MC, Canduri F. The emerging picture of CDK9/P-TEFb: more than 20 years of advances since PITALRE. Mol Biosyst. 2017;13(2):246-276. [DOI] [PubMed] [Google Scholar]
- 26. Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16(3):167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen FX, Smith ER, Shilatifard A. Born to run: control of transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol. 2018;19(7):464-478. [DOI] [PubMed] [Google Scholar]
- 28. Ho Y, Hu P, Peel MT, et al. Single-cell transcriptomic analysis of adult mouse pituitary reveals sexual dimorphism and physiologic demand-induced cellular plasticity. Protein Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Z, Wei W, Gagneur J, et al. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457(7232):1033-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young RS, Kumar Y, Bickmore WA, Taylor MS. Bidirectional transcription initiation marks accessible chromatin and is not specific to enhancers. Genome Biol. 2017;18(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei W, Pelechano V, Järvelin AI, Steinmetz LM. Functional consequences of bidirectional promoters. Trends Genet. 2011;27(7):267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]