Abstract
Streptococcus pneumoniae is a major cause of pneumonia wherein infection of respiratory mucosa drives a robust influx of neutrophils. We have previously shown that S. pneumoniae infection of the respiratory epithelium induces the production of the 12-lipoxygenase- (12-LOX-) dependent lipid inflammatory mediator hepoxilin A3 (HXA3), which promotes recruitment of neutrophils into the airways, tissue damage and lethal septicemia. Pneumolysin (PLY), a member of the cholesterol-dependent cytolysin (CDC) family, is a major S. pneumoniae virulence factor that generates ~25 nm diameter pores in eukaryotic membranes and promotes acute inflammation, tissue damage, and bacteremia. We show that a PLY-deficient S. pneumoniae mutant was impaired in triggering human neutrophil transepithelial migration in vitro. Ectopic production of PLY endowed the nonpathogenic Bacillus subtilis with the ability to trigger neutrophil recruitment across human cultured monolayers. Purified PLY, several other CDC family members, and the α-toxin of Clostridium septicum, which generates pores with cross-sectional areas nearly 300-times smaller than CDCs, reproduced this robust neutrophil transmigration. PLY non-pore forming point mutants that are trapped at various stages of pore assembly did not recruit neutrophils. PLY triggered neutrophil recruitment in a 12-LOX-dependent manner in vitro. Instillation of wild type PLY, but not inactive derivatives, into the lungs of mice induced robust 12-LOX-dependent neutrophil migration into the airways, although residual inflammation induced by PLY in 12-LOX deficient mice indicates that 12-LOX-independent pathways also contribute to PLY-triggered pulmonary inflammation. These data indicate that PLY is an important factor in promoting HXA3-dependent neutrophil recruitment across pulmonary epithelium in a pore-dependent fashion.
Keywords: PLY, neutrophil, transepithelial migration, S. pneumoniae, pore formation
Introduction
The Gram-positive bacterium Streptococcus pneumoniae is the leading cause of community-acquired pneumonia and also causes several other infections including otitis media, bacteremia, and meningitis. Asymptomatic colonization by S. pneumoniae has been estimated to be as high as 95% in children and 40% in adults, and is considered to be an important prerequisite for invasive disease(1, 2). In the United States alone there are approximately 900,000 cases of pneumococcal pneumonia annually, with a mortality rate of 5-7%, making the disease both a significant health and financial burden(3, 4). According to the World Health Organization, S. pneumoniae pneumonia accounts for ~500,000 deaths in children under 5 years old in developing countries(5).
A hallmark of a S. pneumoniae lung infection is a robust proinflammatory host response characterized by a massive influx of neutrophils (polymorphonuclear leukocytes, or PMNs)2 into the alveoli. PMNs, which confront the invading S. pneumoniae with a number of antibacterial effector mechanisms, are beneficial for the host during early stages of the infection(6). Indeed, murine infection studies have found that decreased neutrophil recruitment leads to higher bacterial loads in the lungs by 12 to 24 hours after pulmonary challenge with S. pneumoniae(7, 8). However, a strong PMN response to control bacterial outgrowth during the first phase of infection can also lead to epithelial barrier disruption, pulmonary edema and significant lung damage(9, 10), and mice containing high numbers of pulmonary PMNs several days post-infection suffer bacteremia and succumb to infection(11, 12). These findings suggest that for a favorable host outcome, the timing and degree of PMN recruitment must be carefully orchestrated.
S. pneumoniae encodes pneumolysin (PLY)3, a 53kDa member of a large family of cholesterol-dependent cytolysins (CDCs)4 that form ~25 nm diameter pores in eukaryotic membranes(13). CDCs have been identified in over 40 bacterial species, and include intermedilysin (ILY)5 of Streptococcus intermedius, streptolysin O (SLO)6 of S. pyogenes, and perfringolysin O (PFO)7 of Clostridium perfringens(14). Pore formation by CDCs is a multistep process involving membrane binding, oligomerization, and membrane insertion, and several PLY toxoids that are defective at discrete steps have been characterized(15-17). CDCs damage diverse host cell types and contribute to disease in many infection models(18). Indeed, after pulmonary challenge in mice, S. pneumoniae deficient for PLY exhibit decreased tissue damage and inflammation, lower bacterial burden, and less bacteremia(12, 19, 20). In addition to directly damaging host cells, PLY has a major influence on the host immune response. Relative to infection by a PLY-deficient strain, WT S. pneumoniae infection results in an earlier and greater influx of PMNs and in greater numbers, resulting in more severe lung damage(19-21). PLY also activates complement, an activity that has been shown to contribute to cellular influx during pulmonary infection (22, 23).
PLY triggers an early step in movement of PMNs into airways, i.e. transmigration across the endothelial cell barrier(24). For example, a PLY-deficient S. pneumoniae mutant exhibits a two- to four-fold defect for inducing PMN migration across cultured endothelial monolayers, and purified PLY is capable of promoting PMN movement(24). Although the specific host signaling molecules underlying this process have not been identified, purified PLY activates phospholipase A in endothelial cells, with concomitant release of arachidonic acid (AA)8, suggesting that eicosanoid signaling molecules may be involved(25).
The final step of PMN entry into airways during S. pneumoniae infection is transmigration across the lung epithelium, a step that has been associated with disruption of the mucosal barrier function and spread of S. pneumoniae into the bloodstream(11). Interestingly, given that PLY activates phospholipase in cultured endothelial cells(25), we previously showed that the final step in PMN movement into the airways is promoted by epithelial production of 12-lipoxygenase (12-LOX)9, which is required for the synthesis of the potent eicosanoid chemoattractant hepoxilin A3 (HXA3)10(26). HXA3 has been implicated in both intestinal and pulmonary inflammation induced during bacterial infection(26-29). Disruption of 12-LOX activity by chemical inhibition or genetic ablation dramatically reduces pulmonary inflammation, bacteremia and host morbidity in a murine infection model(11).
In this study, we identify S. pneumoniae PLY as a bacterial factor necessary and sufficient to induce 12-LOX-dependent PMN migration across epithelial monolayers. We found that the pore-forming activity of PLY is central to induction of inflammation, and purified PLY triggered recruitment of PMNs into the murine airway in a manner dependent on both its pore-forming activity and host 12-LOX activity.
Materials and Methods
Bacterial strains
Mid-exponential growth phase aliquots of S. pneumoniae TIGR4, D39, and 23F strains (serotype 4), were grown in Todd-Hewitt broth (BD Biosciences) supplemented with 0.5% yeast extract in 5% CO2 and Oxyrase (Oxyrase, Mansfield, OH), were frozen in growth media with 20% (v/v) glycerol. Bacterial titers in aliquots were confirmed by plating serial dilutions on Tryptic Soy Agar plates supplemented with 5% sheep blood agar (Northeast Laboratory Services, Winslow, ME). Bacillus subtilis strains were grown overnight at 37°C in Luria broth. The TIGR4 pneumolysin mutant (Δply) was a gift from Andy Camilli, and D39 and 23F wildtype, Δply mutants, and revertant strains were a gift from Jeff Weiser. Strains D39 and 23F wildtype and Δply mutants have been previously described.(30-34) The D39 revertant strain was generated by transformation of the D39Δply mutant with D39 chromosomal DNA and screening for hemolytic transformants. Insertion of wild type ply at the original locus was confirmed by sequencing.
Bacterial growth conditions
S. pneumoniae strains TIGR4 (serotype 4), D39 (serotype 2), E134 (serotype 23F) were grown in Todd-Hewitt broth (BD Biosciences) supplemented with 0.5% yeast extract and used in late log phase. For mouse experiments and H292 membrane repair experiments, bacteria were stored at −80°C in media with 25% (v/v) glycerol, thawed, and diluted in PBS to appropriate concentrations as required. Bacterial titers in thawed stocks were confirmed by plating serial dilutions on blood agar. B. subtilis strain 168 was grown overnight at 37°C in Luria broth.
Growth and maintenance of epithelial cells
Human pulmonary mucoepidermoid carcinoma-derived NCI-H292 (H292)11 cells were grown on the underside of collagen-coated Transwell filters (0.33-cm2, Corning Life Sciences) in RPMI 1640 medium (ATCC) with 2 mM L-glutamine, 10% FBS, and 100 U penicillin/streptomycin.
Animals
C57BL/6J female mice and Alox12/15 knockout (Alox15 −/−) female mice (B6.129S2ALOX15 tm1Fun /J) were obtained from Jackson Laboratories. Mice were housed in a specific pathogen-free facility at Tufts University and all procedures were performed in accordance with Institutional Animal Care and Use Committee approved protocols.
Toxin Purification
The expression and purification of recombinant toxins and toxin derivatives from Escherichia coli were carried out as previously described(35) with the following modifications. Growth of E. coli XL-1 Blue containing pRT20 or derivatives thereof was initiated by inoculating 1 L of sterile TB broth(36) with a 1:100 inoculum of an overnight culture grown at room temperature with 100 μg/mL ampicillin. The 1 L culture was incubated at 37 °C with shaking at 200 rpm. At OD600 0.5-0.6, expression of toxin was induced by the addition of isopropyl β-D-thiogalactopyranoside (IPTG, Fisher Scientific) to a final concentration of 1 mM. Ampicillin was also added to a final concentration of 100 μg/mL. The induced culture was grown overnight at room temperature and pelleted by centrifugation. Cell pellets were resuspended in a total of 30 mL of PBS. Halt Protease Inhibitor Cocktail (Thermo Fisher Scientic) was added to prevent proteolytic degradation of the toxin. Cells were lysed by two passages through a microfluidizer, and cell debris was removed by centrifugation at 10000g for 30 min at 4 °C. The supernatant containing the polyhistidine-tagged toxin was loaded onto a Ni-NTA agarose column (Qiagen). The column was then washed with a 20–120 mM gradient of imidazole to remove additional contaminating proteins. Bound toxin was then eluted (2 mL/min) with 10mL of PBS containing 500mM imidazole. SDS-PAGE was performed on the collected fractions to confirm toxin purity. 10% (v/v) glycerol and 5μM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) was added to toxin-containing fractions, which were then flash-frozen and stored at −80 °C until use.
Toxin Activity Assay
Toxin aliquots were thawed on ice on and then spun at 14,000rpm in a microcentrifuge at 4 °C to remove protein precipitate for 10 min. Toxin concentration was determined using Bradford Reagent (BioRad) per the manufacturer’s instructions. H292 cells were placed in 100ul volumes in a non-adherent 96 well plate. Toxin was incubated at varying concentrations, in triplicate, with the H292 cells for 15 minutes at 37 °C. Addition of 5% Triton-X 100 and HBSS+ were used as positive and negative controls, respectively. The plate was then spun at 1200rpm for 5 minutes and cells were resuspended in 1mg/ml of propdium iodide (PI). Cells were filtered through 100μM filters into 100ul of PBS with 10% serum, placed on ice, and kept in the dark until analysis by flow cytometry. Cells were run through a FACSCalibur flow cytometer (BD Biosciences) and a minimum of 5 x 104 events were analyzed per replicate. Collected data were analyzed using FlowJo software (Tree Star, Inc.) to determine the toxin concentration that resulted in 50% of the H292 cells becoming PI+. This concentration was defined as “1 Suspension Unit” of toxin activity. Transwell monolayers were then treated with varying suspension units to determine the number of units that resulted in 50% of the polarized H292 cells on the transwell becoming PI+. This concentration was defined as “1 Transwell Unit” of toxin activity, which we refer to as “1 Unit” of toxin activity. For some experiments, ionomycin activity was determined identically to the method described above except ionomycin, not toxin, was incubated at varying concentrations, in triplicate, with the H292 cells for 15 minutes at 37 °C.
Preparation and assessment of polarized H292 monolayers.
Polarized H292 monolayers were prepared as previously described(37). The transepithelial resistance of lung epithelial monolayers are typically very low ((38); unpublished data). Hence, to assess the generation of intact, confluent H292 monolayers, for a sampling of polarized monolayers, we measured the ability of HRP added to the basolateral compartment to be detected in the apical chamber after 20 minutes, as previously described (39, 40). In addition, some samples were analyzed by fluorescent microscopy to confirm confluent and intact monolayers. To prepare samples for fluorescent microscopy, monolayers were permeabilized with 0.1% Triton-X 100 in PBS plus 3% BSA and fixed in fixed in 4% paraformaldehyde. Monolayers were then stained with DAPI12 and phalloidin (data not shown) and visualized on excised filters. Finally, defects in Transwell filter integrity after collagen coating were detect by buffer loss, and changes in pH of cell media were used to detect potential defects in cell growth. Approximately 3% of H292 monolayers were excluded from our experiments.
PMN transepithelial migration assay
The apical surface of inverted Transwells was infected with 1 x 107 bacteria for 2.5 hours, treated with the indicated units of toxin for 1 hour, treated with the indicated units of ionomycin for 1 hour, or incubated with HBSS+Ca/Mg at 37°C. After toxin treatment, transwells were washed and placed in 24-well plates containing HBSS+Ca/Mg. 1 x 106 PMNs, isolated from whole blood obtained from healthy human volunteers as previously described (28, 41), were added to the basolateral chamber and after 2.5 hours, PMNs in the apical chamber were quantified by MPO assay, as described(41). Briefly, monolayers and their underlying Transwell filters were removed from individual wells of a 24-well plates, leaving the 24-well plate containing the apical buffer and migrated neutrophils for each sample and control well. 50μl of 10% Triton X-100 was added to each well and gently rocked for 20 min at 4°C. 50μl of citrate buffer was added to each sample and the 24-well plate was gently rocked for 20 min at 4°C. ABTS solution was freshly prepared and 50μl of hydrogen peroxide was added to the ABTS solution. 100μl from each well was transferred to a 96-well plate and 100μl of ABTS solution was added to each sample in the 96-well plate. The 96-well plate was incubated in the dark at room temperature for 5-10 min until it was read on a microplate reader for absorbance at a wavelength of 405nm. Formylated Met-Leu-Phe (“fMLP”)13 and HBSS+Ca/Mg were used as positive and negative controls, respectively.
To determine if epithelial cells, in the absence of PMNs, were capable of generating chemoattractants, after treatment with PLY, Transwell filters were incubated in 24-well plates containing HBSS+Ca/Mg for an additional 2.5 hours at 37°C to collect potential chemoattractants released by epithelial monolayers into the apical supernatant. Treated Transwell filters were removed and naïve Transwell filters were placed in the apical supernatant, with the remainder of the transmigration assay proceeding as described above to detect chemoattractant activity. For select Transwell filters, we used fluorescence microscopy to visually assess the effect of toxin treatment on the health of the H292 monolayers. To prepare samples for fluorescent microscopy, monolayers were permeabilized with 0.1% Triton-X 100 in PBS plus 3% BSA and fixed in fixed in 4% paraformaldehyde. Monolayers were then stained with DAPI and PI and visualized on excised filters. The number of PI+ and DAPI+ cells were counted manually in a blinded manner and then the percentage of PI+ cells was calculated.
Treatment of monolayers with inhibitors
Cinnamyl-3,4-dihydroxy- α-cyanocinnamate (Ci-Di-Cy)14 and baicalein (bcn)15 were obtained from Enzo Life Sciences, Plymouth Meeting, PA. Cells were pretreated with Ci-Di-Cy (25μM)(42) or bcn (0.5μM)(11, 27) for 3-5 hours. To minimize potential direct effects of the inhibitors, drugs were removed prior to addition of toxin to the transwells.
H292 Membrane Repair Assay
2x105 H292 cells were placed in 200ul volumes/well in a non-adherent 96 well plate and incubated at 37 °C with CO2. 1mg/mL of propidium iodide was added was to each well and the plate was protected from light. H292 cells were infected with WT TIGR4 or the TIGR4 ply-deficient derivative and the plate was briefly spun at 1000rpm for 3 min. The plate was incubated at 37 °C with CO2 and protected from light for 1 hour. The plate was then spun at 1200rpm for 5 minutes and cells were resuspended in FACS buffer before RedDot™2 was added to designated wells, transferred to a round bottom 96-well plate and spun at 1200rpm for 5 minutes. Cells were filtered through 100μM filters into a final volume of 250ul of FACS Buffer. Cells were placed on ice, and kept in the dark until analysis by flow cytometry. Cells were run through a FACSCalibur flow cytometer (BD Biosciences) and a minimum of 2 x 104 events were analyzed per replicate. Collected data were analyzed using FlowJo software (Tree Star, Inc.).
Leached PLY activity assay
The apical surface of H292 cell monolayers on Transwell filters was treated with the indicated units of toxin or incubated with HBSS+Ca/Mg at 37°C for 1 hour. After toxin treatment, monolayers were washed and placed in 24-well plates containing HBSS+Ca/Mg for 2.5 hours at 37°C. Basolateral and apical supernatants were collected and added to a 96 well plate. 7.8x103 PMNs were added to wells containing basolateral or apical supernatant and incubated for 1.5 hours at 37°C. Addition of 0.05 Units of PLY (as defined by the Toxin Activity Assay) and HBSS+ were used as positive and negative controls, respectively. The plate was then spun at 1200rpm for 5 minutes and cells were resuspended in 1mg/ml of propidium iodide (PI)16. Cells were filtered through 100μM filters into 100ul of PBS with 10% serum, placed on ice, and kept in the dark until analysis by flow cytometry. Cells were run through a FACSCalibur flow cytometer (BD Biosciences) and a minimum of 1 x 104 events were analyzed per replicate. Collected data were analyzed using FlowJo software (Tree Star, Inc.) to measure the percentage of PI+ cells.
Murine infection studies
Mice were intratracheally instilled with PLY or the indicated PLY toxoids (in 50 μl PBS). Control mice received PBS alone. Mice were sacrificed at 48hrs and H&E-stained lung sections were prepared. Sections were examined by light microscopy (at original magnification x20). Bronchoalveolar lavage fluid (BALF)17 was collected and cells in the BALF were quantitated by flow cytometry as described below.
Flow Cytometry
For flow cytometry studies, anti-Ly-6G-PE (clone 1A8), anti-CD11c-FITC (clone N418), anti-F4-80-PE-Cy7 (clone BM8) were obtained from BD Biosciences. Mice were euthanized at 48hrs and BALF was collected. Cells present in the BALF were stained on ice for 30 min with appropriate MAbs and then washed. Cells were then run through a FACSCalibur flow cytometer (BD Biosciences) and the fluorescence intensities of the stained cells were determined. Data were analyzed using FlowJo software (Tree Star, Inc.) to determine the numbers of PMNs (Ly6G+) in the BALF.
Presentation of data and statistical analyses
Due to intrinsic donor-to-donor variability of human PMNs and their transmigration, efficiency of transmigration was compared within individual experiments but not between experiments. The conclusions drawn were those found to be reproducible and statistically significant across independent experiments. Statistical analysis was carried out using the program GraphPad Prism (GraphPad Software, San Diego, CA). P values <0.05 were considered significant in all cases. For all graphs, the mean values +/− SEM are shown.
Results
PLY is required for maximal S. pneumoniae-induced transepithelial PMN migration.
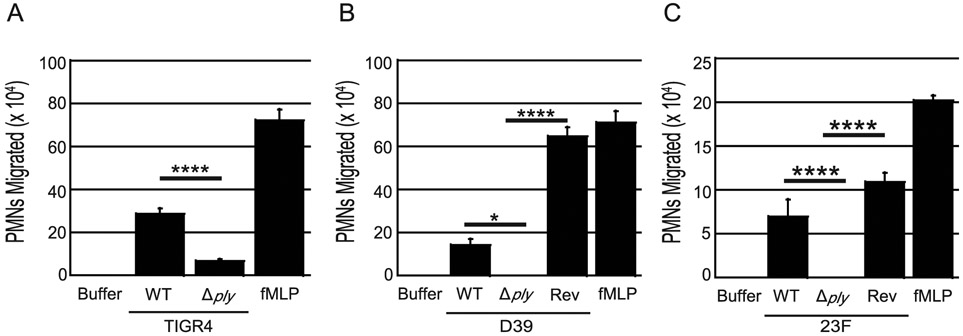
We previously showed that apical infection of polarized H292 lung epithelial cell monolayers cultured on Transwell filters with S. pneumoniae triggers the basolateral-to-apical transmigration of human PMNs, as assessed by myeloperoxidase (MPO)18 activity (see Materials and Methods)(43-45). We confirmed that S. pneumoniae strain TIGR4, a capsular serotype 4 clinical isolate, triggers 35-fold higher levels of transmigration compared to the buffer control, although somewhat less than the transmigration triggered by imposed gradients of the potent chemoattractant N-Formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) (Fig. 1A). An isogenic TIGR4 ply-deficient derivative recruited 4-fold less PMNs (p < 0.0001), indicating that PLY contributes to S. pneumoniae-induced transmigration across respiratory epithelial monolayers (Fig. 1A). To determine if PLY-induced transmigration was a common property of S. pneumoniae, we apically infected polarized H292 monolayers with WT or ply-deficient derivatives of the capsular serotype 2 S. pneumoniae strain D39, and the serotype 23F S. pneumoniae strain E134(32, 46). The wild type versions of these strains elicited robust PMN transmigration, at least 8-fold greater than buffer control (Fig. 1B-C). The degree of PMN migration varied with each experiment, a finding common to human PMN migration studies and thought to be related to donor variation. Consistent with our findings with strain TIGR4, the ply-deficient derivatives of both D39 and 23F recruited fewer PMNs relative to the WT parental strain (p < 0.05, p < 0.0001; Fig. 1B-C). Reversion of these ply mutants by insertion of a wild type ply at the original locus(47) resulted in restoration of PMN migration to at least wild type levels. These findings indicate that PLY is required for maximal PMN transepithelial migration upon apical infection by S. pneumoniae.
Fig. 1. Pneumolysin is required for PMN transepithelial migration during S. pneumoniae infection.
Apical sides of H292 monolayers on transwells were infected with 1X107 CFU per transwell of wild type, ply-deficient, or complemented S. pneumoniae strains for 2.5 hours. After infection, 1x106 human PMNs were added to the basolateral side post-infection. PMN transepithelial migration was quantified by MPO activity. fMLP and buffer alone were used as positive and negative controls, respectively. For each panel, shown is a representative of two to four independent experiments where each condition was tested in triplicate per experiment. Asterisk(s) indicate migration is significantly (* = p<0.05, **** = p<0.0001) greater than the corresponding S. pneumoniae Δply mutant, as determined by one-way ANOVA, Post-hoc: Tukey.
PLY is sufficient to recruit transepithelial PMN migration.
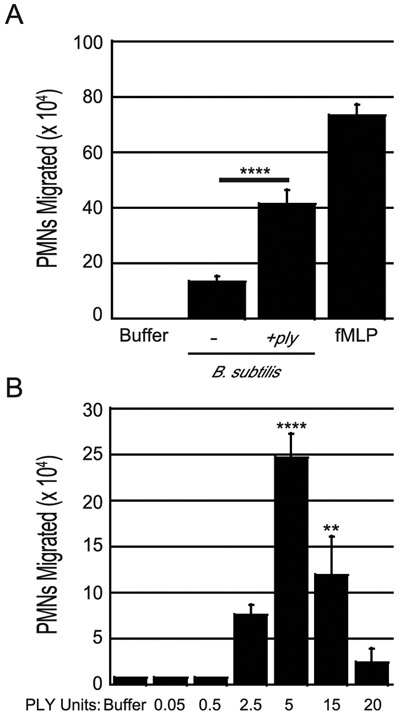
To determine if PLY requires one or more S. pneumoniae-specific factors to promote PMN transepithelial migration in vitro, we infected polarized H292 monolayers with either strain AC4052 of the nonpathogenic soil bacterium B. subtilis, or strain AC4050, a derivative that ectopically expresses surface-localized PLY(48, 49). B. subtilis strain AC4052 elicited low levels of PMN migration, consistent with the notion that this bacterium encodes some molecules (e.g. peptidoglycan(50)) that are capable of triggering inflammation. In contrast, the B. subtilis strain expressing PLY exhibited three-fold more PMN migration relative to strain AC4050 (p < 0.0001), indicating that PLY plays a role in eliciting the host immune response independent of S. pneumoniae-specific factors (Fig. 2A).
Fig. 2. Pneumolysin is sufficient to induce PMN transepithelial migration in a dose dependent manner.
(A) Apical sides of H292 monolayers on transwells were infected with 1x107 wild type B. subtilis or B. subtilis expressing ply. After infection, transepithelial migration of 1x106 human PMNs added to the basolateral side was quantified by MPO assay. fMLP and buffer alone were used as positive and negative controls, respectively. Asterisks indicate migration is significantly (**** = p<0.0001) greater than migration induced by the parental B. subtilis strain, as determined by one-way ANOVA, Post-hoc: Tukey. Shown is a representative of two independent experiments where each condition was tested in triplicate per experiment. (B) Apical sides of H292 monolayers were treated with indicated units of recombinant PLY (PLY). Transepithelial migration of 1x106 PMNs added to the basolateral side post-infection was quantified. fMLP and buffer alone were used as positive and negative controls, respectively. Shown is one representative experiment of four independent experiments where each condition was tested in triplicate per experiment. Asterisks indicate migration is significantly (** = p<0.005, **** = p<0.0001) greater than migration induced by buffer alone, as determined by one-way ANOVA, Post-hoc: Dunnett.
To determine if bacterial factors other than PLY are required to elicit PMN transmigration in vitro, we measured the PMN response to purified PLY. We found that the pore-forming activity of an N-terminally His-tagged PLY varied even when stored as aliquots at −80°C (See Materials and Methods). Thus, to expose monolayers to standardized levels of PLY activity, we defined the concentration of PLY as one unit that resulted in permeabilization (indicated by propidium iodide staining) of 50% of polarized H292 cells (See Materials and Methods). We found that as few as 2.5 units of PLY, which triggered permeabilization of 62% of cells (Sup. Fig 1A-D), were sufficient to induce significant PMN migration when applied to H292 cell polarized monolayers (Fig. 2B). Five units induced maximal PMN transmigration, whereas no transmigration was observed at 20 units (or more; Fig. 2B), which resulted in propidium iodide staining of 100% H292 cells (Sup. Fig. 1A-D). Treatment of human embryonic kidney cells and neuroblastoma SH-SY5Y cells with the equivalent of 20 units of PLY results in irreversible cell lysis(51), providing an explanation for the lack of PMN transmigration at high doses observed in our study.
The lack of PMN transmigration in response to levels of PLY that induced pore formation (i.e. PI+ staining) in 100% of cells raised the question as to whether any PI+ H292 cells might be viable and actively produce chemoattractant. In fact, limited pore formation by PLY or the related cholesterol-dependent cytolysin SLO triggers rapid membrane repair that prevents cell death(52, 53). At a PLY dose that induces permeabilization of 70% of human embryonic kidney cells and neuroblastoma SH-SY5Y cells, all but ~5% are capable of repairing their cytoplasmic membrane (51). To test whether limited PLY-mediated pore formation was also reversible, we mock-infected H292s or infected them with either wild type or PLY-deficient S. pneumoniae TIGR4 in the presence of propidium iodide for 1 hour. After washing to remove bacteria and propidium iodide, H292s were incubated with RedDot™2 (RD2), which would stain host cells only if the plasma membrane remained compromised (54). Using flow cytometry, we found that 40% of H292 cells were permeabilized (“PI+”) by wild type infection, a percentage that was at least 14-fold higher (p < 0.01) than the percentage of permeabilized cells after mock infection or infection by the ply-deficient strain (Sup. Fig. 1E). Importantly, using RD2 staining, we found that 69% of cells had intact (PI+RD2−) membranes, compared to 31% with permeabilized (PI+RD2+) membranes (Sup. Fig. 1F, “RD2−” or “RD2+”, respectively). Thus, the majority of H292s permeabilized in a PLY-dependent manner retain sufficient viability to undergo rapid membrane repair.
In our transmigration assays, although monolayers were washed after apical treatment with PLY prior to the addition of PMNs to the basolateral chamber, PLY might diffuse into the basolateral chamber and directly alter PMN function. Contrary to this hypothesis, by propidium iodide staining of PMNs, no pore-forming activity was detected in buffer collected from the basolateral chamber 2.5 h after apical treatment of H292 cell monolayers with 2.5 or 5 units of PLY (Sup. Fig. 2A; see Methods). PLY that binds to the H929 cell monolayers and then is released into the apical medium might also interact with PMNs, potentially contributing to transmigration by directly eliciting PMN chemoattractant secretion. However, we attempted to capture this putative ‘leached’ PLY by apically treating H292 cell monolayers with 2.5 or 5 units of PLY, washing the monolayers, and adding fresh apical buffer. After 2.5 hours of incubation, this buffer also was found to contain no PLY when assayed for the ability to generate pores in PMNs (Sup. Fig. 2B). We conclude that the PLY-induced transmigration in our Transwell system is due to its interaction with H292 cells rather than PMNs.
To rigorously test if PLY could elicit chemoattractant from epithelial cells in the complete absence of PMNs, we collected supernatant from PLY-treated H292 cells. This supernatant was found to be capable of eliciting neutrophil migration across naïve monolayers, and in a 12-LOX-dependent manner (Sup. Fig. 3), demonstrating that PLY-epithelial interaction in the absence of neutrophils is sufficient to drive initial basolateral-to-apical neutrophil migration.
Diverse pore-forming agonists induce PMN migration.
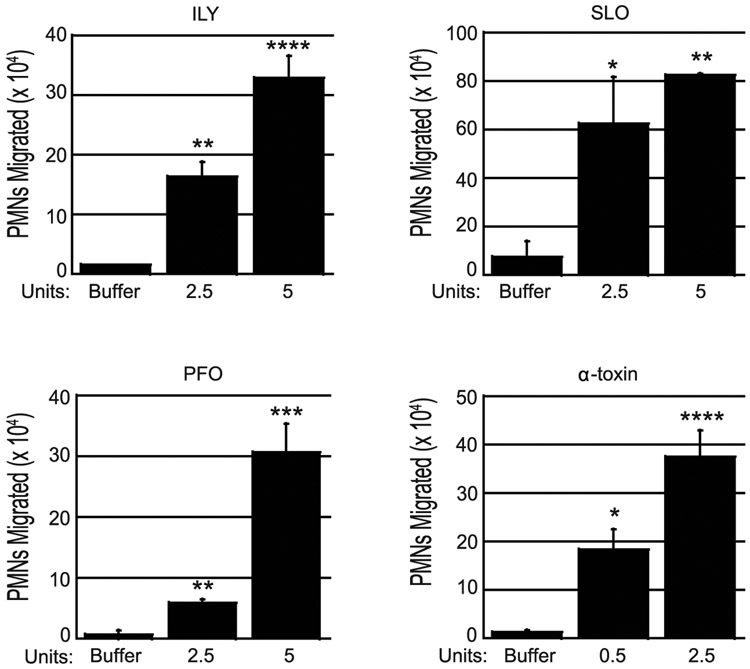
The CDCs show substantial structural and functional conservation, so we investigated whether other CDCs could also induce PMN migration. We treated polarized H292 cell monolayers with 2.5 units (2.5U) or 5 units (5U) of intermedilysin (ILY), streptolysin O (SLO), and perfringolysin O (PFO), with activity units determined as described above (see also Materials and Methods). ILY, SLO, and PFO triggered a dose-dependent increase in PMN migration that at 5U reached at least 6-fold greater than buffer controls, respectively (p < 0.05); Fig. 3). Similar to our other PMN migration experiments, each toxin induced significant PMN migration in a dose-dependent manner, although we observed donor-to-donor variability in maximal levels of PMN migration.
Fig. 3. Diverse pore-forming agonists induce PMN transmigration.
The apical face of H292 monolayers were treated with the indicated units of intermedilysin (ILY) of S. intermedius, streptolysin O (SLO) of S. pyogenes, perfringolysin O (PFO) of C. perfringens, or alpha toxin of C. septicum. Transepithelial migration of 1 x 106 human PMNs added to the basolateral side was quantified by MPO assay. Buffer alone was used as negative control. Each panel depicts one representative of between two and four independent experiments where each condition was tested in triplicate per experiment. Asterisks indicate migration is significantly (* = p<0.05, ** = p<0.005, *** = p<0.0005, **** = p<0.0001) greater than migration induced by buffer alone, as determined by one-way ANOVA. Post-hoc: Dunnett.
To assess whether a non-CDC toxin that generates pores of a different nature could also elicit PMN migration, we tested α-toxin of C. septicum, which generates pores 1.3-1.6 nm in diameter, with cross-sectional areas some 300 times smaller than those generated by CDCs(55, 56). We found that 0.5 and 2.5 units of C. septicum α-toxin induced PMN migration at 14-fold and 30-fold higher levels than the buffer-treated control (p < 0.05) (Fig 3). These results indicate that the ability to induce PMN trafficking is a property of diverse pore-forming agonists.
PLY requires pore-forming activity to induce PMN migration.
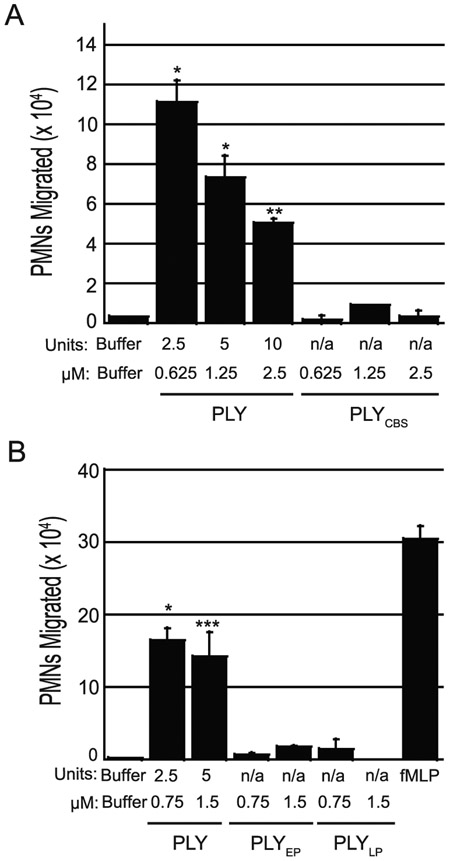
Pore formation by PLY is the result of a multi-step process(57). First, CDC monomers bind to cholesterol in the host cell membrane. Thirty to 50 monomers then oligomerize to form a large ‘ring’, termed an “early prepore complex”, on the cell surface(35, 58, 59). The oligomer undergoes a conformational change to generate a “late prepore complex” which is stable to dissociation by SDS and heat. Finally, the late prepore complex refolds two α-helical bundles from each monomer in the prepore complex into membrane-spanning β-hairpins to form the large 25 nm β-barrel pore(60).
To determine which step of pore formation is associated with triggering PMN migration, we tested whether PLY mutants defective at different stages retain the ability to induce PMN migration. The PLY mutant PLYCBS19, containing a single amino acid substitution in the cholesterol binding sequence in domain 4, is deficient in membrane association and pore formation(61). Treatment of epithelial monolayers with as much as 2.5 μM of PLYCBS, a concentration that corresponds to 10 units of wild type PLY, did not induce PMN migration significantly over background levels (Fig. 4A) whereas as little as 2.5 units of wild type PLY induced considerable PMN migration. PLYEP20, which is locked at the early prepore stage due to single amino acid substitutions in domains 2 and 3, and PLYLP21, which is locked at the late prepore stage due to a double substitution mutation in domain 3, demonstrated only 3% and 2% of WT PLY pore-forming activity, respectively. Neither toxoid triggered PMN migration across epithelial monolayers at concentrations of 0.75 or 1.5 μM, whereas equivalent molar concentrations of wild type PLY, corresponding to 2.5 and 5 units, respectively, were sufficient to induce robust PMN migration (Fig. 4B). These results indicate that PLY-mediated pore formation is necessary for PLY induced PMN migration.
Fig. 4. PLY requires pore-forming activity to induce PMN migration.
(A) Apical sides of H292 monolayers were treated for one hour with equimolar concentrations of functional PLY or a PLY cholesterol binding site mutant (PLYCBS). (B) Apical sides of H292 monolayers were treated for 1 hour with equimolar concentrations of PLY, a PLY mutant locked in an early prepore conformation (PLYEP), or a PLY mutant locked in a late prepore conformation (PLYLP). 1x106 human PMNs were added to the basolateral side and PMN transepithelial migration was quantified by MPO assay. Buffer alone and fMLP were used as negative and positive controls respectively. Shown is one representative experiment of two independent experiments where each condition was tested in triplicate per experiment. Asterisks indicate migration induced by wild type PLY is significantly greater (* = p<0.05, ** = p<0.005, **** = p<0.0001) than equimolar concentrations of toxoid as determined by (A) Welch’s t-test or (B) one-way ANOVA, Post-hoc: Tukey.
12-LOX inhibitors impair PLY induced PMN migration.
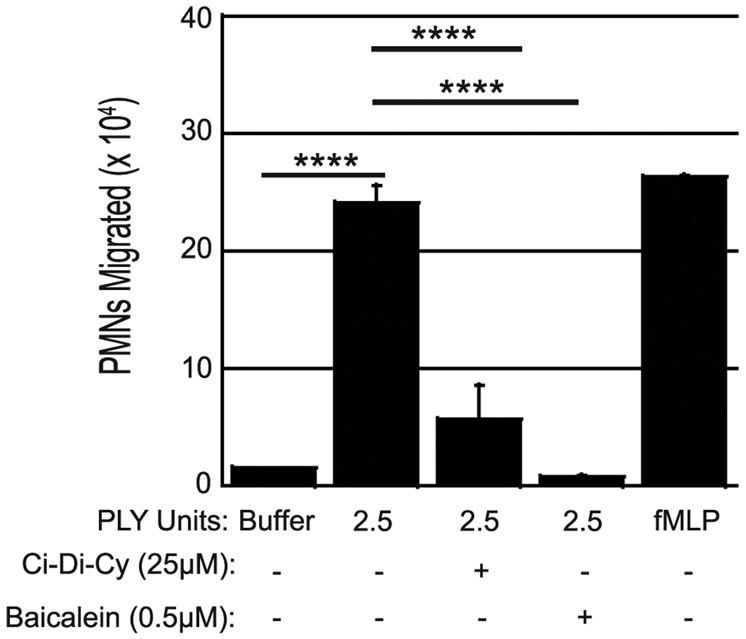
S. pneumoniae-induced PMN epithelial transmigration is dependent on 12-LOX, an enzyme that converts AA into 12(S)-HPETE and is required for the production of HXA3(11, 26, 62, 63). As noted above, purified PLY has been previously shown to activate phospholipase A in endothelial cells, with concomitant release of arachidonic acid(25). To determine whether neutrophil migration in response to PLY is dependent on the 12-LOX pathway, we tested the ability of 12-LOX pharmacologic inhibitors Ci-Di-Cy or baicalein to inhibit neutrophil transmigration in response to apically applied PLY. H292 cells treated with either 25μM Ci-Di-Cy or 0.5μM baicalein (see Methods) exhibited significantly less PMN migration (Fig. 5). We also investigated whether neutrophil migration in response to other pore forming toxins (e.g. ILY, PFO, SLO, and α-toxin) is dependent on the 12-LOX pathway using the same pharmacologic inhibitors, but our results were inconsistent (data not shown), so definitive identification of the signaling pathway(s) activated by these other pore forming toxins will require further study. Nevertheless, the observed neutrophil migration in response to PLY is dependent on the 12-LOX pathway, supporting the hypothesis that PLY contributes to the neutrophil transmigration induced by S. pneumoniae.
Fig. 5. 12-LOX is required for PLY induced PMN transmigration.
H292 monolayers were incubated for 3 hours with buffer alone, the 12-LOX inhibitor Ci-Di-Cy, or the 12-LOX inhibitor Baicalein. Following this incubation, apical sides of H292 monolayers were treated for one hour with 2.5 units of PLY. 1x106 human PMNs were added to the basolateral side and PMN transepithelial migration was quantified by MPO assay. Buffer alone and fMLP were used as negative and positive controls respectively. Shown is a representative of six independent experiments where each condition was tested in triplicate per experiment. Treatment with inhibitors reached statistical significance in five out of six independent experiments. Asterisks indicate that PMN migration is significantly greater (**** = p<0.0001) than migration induced by the negative control of buffer without PLY, 2.5 units PLY with Ci-Di-Cy, and 2.5 units PLY with Baicalein as determined by one-way ANOVA, Post-hoc: Tukey.
PLY-mediated pore-forming activity triggers 12-LOX-dependent pulmonary inflammation in mice.
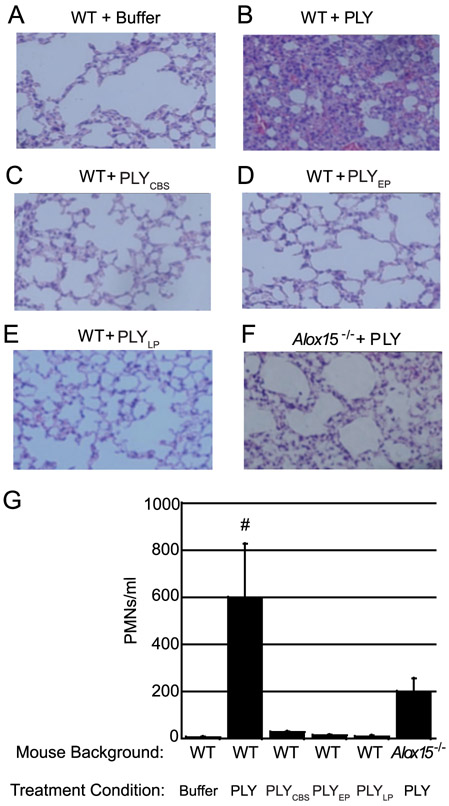
PLY is sufficient to induce pulmonary inflammation upon intratracheal instillation(64-66). To determine if PLY pore-forming activity is required for this phenotype, we instilled intratracheally into mice wild type PLY, or PLY mutants that cannot form pores or are severely deficient in pore formation. As expected, when evaluated at 48 h post-instillation, buffer alone induced no pathology and bronchial alveolar lavage fluid (BALF) contained less that 13 PMNs/ml (Fig. 6A), whereas wild type PLY triggered considerable infiltration of PMNs into the airways and more than 900 PMNs/ml in the BALF (Fig. 6B,G, p < 0.05). PLYCBS, PLYEP or PLYLP mutant toxoids did not trigger pulmonary inflammation when evaluated by histopathology and there was no significant difference in PMN infiltration between these treatment conditions and buffer alone. In fact, the number of PMNs in BALF of these mice was at least 28-fold lower than the number in BALF from mice instilled with wild type PLY (Fig. 6C-E,G).
Fig. 6. PLY requires pore formation and the 12-LOX pathway to induce robust PMN migration in vivo.
Female WT mice were challenged intratracheally (i.t.) with PLY, PLYCBS, PLYEP, or PLYLP. Female Alox15−/− mice were challenged i.t. with PLY. Control mice received buffer alone. (A-F) Mice were sacrificed and H&E-stained lung sections were prepared. Sections were examined by light microscopy (at original magnification x 20). Histology images shown are representative data from one of three independent experiments (using three to five mice per condition). (G) BALF samples were collected and the number of PMNs was measured by flow cytometry. The data presented is the average value for each treatment condition across three independent experiments (using three to five mice per condition). # indicates that PMN number in the BALF was significantly greater in WT mice challenged with PLY than in all other groups (# = p<0.05) except Alox15−/− mice challenged with PLY (p = 0.1012) as determined by one-way ANOVA, Post-hoc: Tukey. Alox15−/− mice challenged with PLY were not significantly different than WT mice that received buffer alone (p = 0.7135) as determined by one-way ANOVA, Post-hoc: Tukey.
As in vitro inhibition of 12-LOX activity diminishes PLY-mediated PMN transmigration across polarized H292 cell monolayers, we asked whether pulmonary inflammation also displays a similar dependence on 12-LOX. To address this question we instilled purified PLY into the lungs of Alox15−/− mice, which are deficient for 12/15-LOX(26, 67, 68). Histopathological analysis revealed that PLY-mediated inflammation, like inflammation triggered by infection with S. pneumoniae(11), was dramatically diminished in 12-LOX-deficient mice, and the number of PMNs in BALF was 3-fold lower than in wild type mice (Fig. 6F-G). These findings support our model that PLY-mediated pore-forming activity triggers activation of the HXA3 synthesis pathway, resulting in robust pulmonary inflammation, a hallmark of pneumococcal pneumonia.
Discussion
PMN recruitment to the lung in response to a microbial intrusion is a multi-stage process that is dictated by a combination of haptotactic and chemotactic gradients. In vitro modeling of the initial step, exit from the vasculature, indicates that wild type S. pneumoniae elicits significantly more PMN recruitment than a PLY-deficient strain,(24) and that purified PLY alone is sufficient to induce PMN migration across endothelial monolayers(24). After traversing the basement membrane and lung interstitium, the final step in migration of PMNs to the site of infection is movement across the lung epithelium and into the alveoli(69). We previously demonstrated that S. pneumoniae infection of pulmonary epithelial monolayers leads to PMN transmigration in vitro, and that blocking this step not only diminished pulmonary inflammation, but abrogated high level bacteremia and lethal outcome(11). This earlier work also revealed that the S. pneumoniae polysaccharide capsule promoted PMN recruitment, but because capsule-deficient strains still elicited PMN migration, we posited that other bacterial products must also contribute to this process. Here we found that ply-deficient S. pneumoniae strains of multiple serotypes were significantly impaired in eliciting PMN migration across polarized respiratory epithelial cell monolayers. Similarly, ectopic expression of PLY by B. subtilis boosted the ability of this non-pathogen to elicit PMN movement. (Note that B. subtilis alone was also able to induce low levels of PMN migration, suggesting that bacterial components that are also produced by B. subtilis, such as peptidoglycan or lipoproteins, may trigger PMN trafficking with less efficiency than PLY, consistent with our observation that high inoculums of ply-deficient S. pneumoniae induce PMN migration; data not shown). In addition, we found that purified PLY was sufficient to induce transepithelial PMN migration. This activity of purified PLY is consistent with previous findings that instillation of PLY into mouse lungs causes acute lung injury, including hyperpermeability, pulmonary edema and PMN infiltration,(64, 65, 70) that mice infected with ply-deficient S. pneumoniae exhibit reduced PMN recruitment(20), and that in vitro infection by ply-deficient S. pneumoniae induced a diminished number of specific transcriptional immune responses in epithelial cells compared to WT TIGR4 infection (71). Thus, PLY plays a central role in eliciting an acute inflammatory response, promoting movement of PMNs across both endothelial and epithelial barriers in response to S. pneumoniae infection.
Purified PLY activates phospholipase A in endothelial cells, with concomitant release of arachidonic acid(25). We previously showed that S. pneumoniae infection of cultured pulmonary epithelial cells activates cPLA2α22 and the synthesis pathway for the arachidonic acid metabolite HXA3, a potent PMN chemoattractant(11, 72). Hence, we investigated whether the synthetic pathway for HXA3 is required for robust PMN migration in response to purified PLY. Two structurally unrelated pharmacological inhibitors that block HXA3 synthesis, used at concentrations thought to specifically inhibit 12-LOX(11, 27, 42), each significantly decreased PLY-induced PMN migration in vitro. In addition, when we instilled purified PLY into a mouse genetically ablated for HXA3 production, the numbers of PMNs present in the airways were markedly reduced relative to WT mice. These findings support a model in which PLY, by activating phospholipase activity, releases arachidonic acid from the plasma membrane and stimulates the production of HXA3 to promote PMN influx.
PLY might activate phospholipase activity through its best characterized activity, the formation of ~25 nm pores in eukaryotic membranes(13, 73). In fact, the pore-forming activity of PLY is required to activate phospholipase A in cultured endothelial cells(25). Here we found that PLY derivatives deficient in any of the several steps leading to pore formation, such as cholesterol binding or conformational changes leading to pore insertion into the lipid bilayer, were unable to elicit significant neutrophil migration in vitro or in the lung after intratracheal instillation. Three related cholesterol-dependent cytolysins, ILY, SLO and PFO, as well as C. septicum α-toxin, which generates pores with cross-sectional areas nearly 300-times smaller than the cholesterol-dependent cytolysins, all induced PMN transmigration in vitro. Given that cPLA2α is calcium-regulated(74, 75), an attractive hypothesis is that PLY-induced influx of ions, which includes Ca2+, into host cells (76-80) leads to the activation of cPLA2α or other phospholipases and the production of chemoattractant lipids such as HXA3. Indeed, we found that treatment of lung epithelial monolayers with the calcium ionophore ionomycin also induced significant PMN migration (Sup. Fig. 4). Furthermore, HXA3 itself induces Ca2+ flux, first rapidly from intracellular stores, then slowly from the extracellular environment (81), potentially amplifying Ca2+-dependent signaling such as that mediated by cPLA2α.
PLY has been reported to recognize TLR4(82), activation of which has been found to induce cPLA2 activity, and TLR4-deficient mice exhibit lower levels of PMNs in the BALF following PLY instillation (82-85). In addition, PLY has been demonstrated to activate complement(22), raising the possibility that TLR4 binding or complement activation plays a central role in inducing PMN chemotaxis. However, the set of PLY toxoids described in this manuscript harbor single or double amino acid substitutions that are (collectively) distributed throughout all four PLY domains(86, 87): PLYCBS (L460D) is altered in domain 4, whereas PLYLP (G25C-E159C) and PLYEP (K288C-V303C) are altered in domains 2 and/or 3(86, 87). Given the diversity of the location of the lesions, defects in their interaction with TLR4 or complement that are common to all three mutants would likely reflect global misfolding. Contrary to this possibility, each of the mutants retains the native structure of WT PLY (83, 84, Tweten, unpublished data). For example, PLYLP and PLYEP retain cholesterol binding activity, indicating that domain 4, the cholesterol binding domain, is functional, and both these toxoids retain full pore-forming activity when reduced (83, 84, Tweten, unpublished data), indicating that the structure of domains 1-3 has not been perturbed. For PLYCBS (L460D), which does not bind cholesterol(61), the sidechain affected does not significantly interact with other nearby amino acids. Rather, the major interactions of L460 are exclusively via its backbone amide and carbonyl, which are not altered by the mutation. Note also that although the putative complement activation site near N385 (90) is in domain 4, N385 lies at the extreme end of the domain away L460 (86, 87). These considerations rule out the possibility that the lack of chemoattractant-inducing activity of the toxoids is due to an inability to bind TLR4 or activate complement. Instead, these findings show that the inability to form pores by mutants inhibited at 3 different stages of the pore-forming mechanism (i.e., binding, early prepore oligomer assembly and late prepore oligomer assembly) prevents chemoattractant secretion by epithelial cells. Therefore, PLY-mediated pore formation is necessary to stimulate the observed chemoattractant secretion and subsequent influx of neutrophils, an assertion consistent with our analysis of other pore-forming toxins (see above).
Although PLY-mediated pore formation is required to trigger PMN transmigration, the production and secretion of chemoattractant is not simply a reflection of cell lysis, but instead is an active process. Exposure of H292 cells to 20 units or more of PLY, which triggers pore formation in 100% of H292 cells, did not induce PMN migration. In contrast, 2.5 units of PLY, which resulted in membrane permeabilization (indicated by propidium iodide staining) of 62% of H292 cells, triggered significant chemoattractant activity. Several studies have revealed that host cell repair mechanisms are quickly initiated to prevent cell death after CDC-dependent membrane permeabilization (51-53), and here we found that the majority of H292 cells that became permeabilized upon exposure to PLY-producing S. pneumoniae strain TIGR4 were capable of membrane repair, as indicated by exclusion of a subsequently added (different) membrane impermeant dye. Finally, we found that chemical inhibitors of 12-LOX reduced PMN transmigration, indicating that an active metabolic pathway capable of producing HXA3 is required for production and/or release of chemoattractant activity.
Intratracheal instillation of PLY into mice lacking 12/15 LOX, which is required for production of HXA3, resulted in a three-fold fewer BALF PMNs compared to i.t. PLY instillation into wild type mice (Fig. 6). This decrease is similar to the six-fold reduction of BALF PMNs after intratracheal challenge of 12/15 LOX-deficient mice with S. pneumoniae (11). Notably the residual level of inflammation observed in 12/15 LOX-deficient mice upon challenge with either PLY or S. pneumoniae was 35- to 20-fold higher than basal levels (Fig. 6; (11)), suggesting that HXA3-independent pathways that contribute to PMN trafficking to the lung. For example, treatment of either epithelial cells or peripheral blood monocytes with purified PLY induces TNFα and IL-1β production, both of which play important roles in PMN recruitment during S. pneumoniae infection(91-93). Pulmonary instillation of PLY resulted in elevated IL-6 and MIP-2, and PMN recruitment was diminished in IL-6-deficient mice or mice treated with anti-MIP-2 rabbit antiserum(94). PLY triggers IL-1β, IL-8 and LTB4 production by PMNs, which could amplify PMN migration to the lung after initial infiltration(95-97). These potential pathways of HXA3-independent PMN migration are consistent with our finding that migration triggered by purified ILY, SLO, or C. septicum α-toxin was not consistently inhibited by12-LOX pharmacological inhibitors (data not shown).
In summary, this work identifies the pore-forming activity of PLY as an important contributor to PMN infiltration into the lung by virtue of triggering a 12-LOX-dependent inflammatory pathway. Given the importance of inflammatory damage during pneumococcal lung infection, a comprehensive understanding of the microbial factors and host signaling pathways that trigger exaggerated PMN pulmonary influx may provide targets for therapeutic intervention.
Supplementary Material
Key Points.
Pneumolysin (PLY) induces PMN migration across pulmonary epithelial cells
PLY-dependent PMN migration requires pore-formation and epithelial 12-LOX activity
Diverse pore-forming toxins induce PMN migration
Acknowledgements.
We thank Andrew Camilli for strains and Urmila Powale for technical assistance.
This work was supported by NIH Award Number 5R37AI037657-22 to RKT, NIGMS Award Number K12GM074869 Training in Education and Critical Research Skills (TEACRS) to WA, and the American Lung Association Senior Research Training Fellowship RT 194942 N to RB.
Footnotes
polymorphonuclear leukocytes
pneumolysin
cholesterol dependent cytolysins
intermedilysin
streptolysin O
perfringolysin O
Arachidonic acid
12-lipoxygenase
Hepoxilin A3
Human pulmonary mucoepidermoid carcinoma-derived NCI-H292
4′,6-diamidino-2-phenylindole
Formylated Met-Leu-Phe
Cinnamyl-3,4-dihydroxy- α-cyanocinnamate
baicalein
Propidium iodide
bronchoalveolar lavage fluid
myeloperoxidase
PLY cholesterol binding site toxoid
PLY early prepore toxoid
PLY late prepore toxoid
Cytosolic phospolipase A2α
References
- 1.Lloyd-Evans N, O’Dempsey TJ, Baldeh I, Secka O, Demba E, Todd JE, Mcardle TF, Banya WS, and Greenwood BM. 1996. Nasopharyngeal carriage of pneumococci in Gambian children and in their families. Pediatr. Infect. Dis. J 15: 866–71. [DOI] [PubMed] [Google Scholar]
- 2.Trzciński K, Bogaert D, Wyllie A, Chu MLJN, van der Ende A, Bruin JP, van den Dobbelsteen G, Veenhoven RH, and Sanders EAM. 2013. Superiority of Trans-Oral over Trans-Nasal Sampling in Detecting Streptococcus pneumoniae Colonization in Adults. PLoS One 8: e60520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckenhaupt P, Bryant P, Kroger A, Weaver D, Wolicki J, W. P 2012. Centers for Disease Control and Prevention Epidemiology and Prevention of Vaccibe-Preventable Diseases Epidemiol. Prev. Vaccine-Preventable Dis 12th Editi: 512. [Google Scholar]
- 4.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, and Finkelstein JA. 2011. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 29: 3398–3412. [DOI] [PubMed] [Google Scholar]
- 5.2013. WHO ∣ Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. WHO. [Google Scholar]
- 6.Dockrell DH, Whyte MKB, and Mitchell TJ. 2012. Pneumococcal Pneumonia: Mechanisms of Infection and Resolution. Chest 142: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadioglu A, De Filippo K, Bangert M, Fernandes VE, Richards L, Jones K, Andrew PW, and Hogg N. 2011. The Integrins Mac-1 and 4 1 Perform Crucial Roles in Neutrophil and T Cell Recruitment to Lungs during Streptococcus pneumoniae Infection. J. Immunol 186: 5907–5915. [DOI] [PubMed] [Google Scholar]
- 8.Bou Ghanem EN, Clark S, Roggensack SE, McIver SR, Alcaide P, Haydon PG, and Leong JM. 2015. Extracellular Adenosine Protects against Streptococcus pneumoniae Lung Infection by Regulating Pulmonary Neutrophil Recruitment. PLoS Pathog. 11: e1005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lax S, Wilson MR, Takata M, and Thickett DR. 2014. Using a non-invasive assessment of lung injury in a murine model of acute lung injury. BMJ Open Respir. Res 1: e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.José RJ, Williams AE, Mercer PF, Sulikowski MG, Brown JS, and Chambers RC. 2015. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J. Immunol 194: 6024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhowmick R, Tin Maung NH, Hurley BP, Ghanem EB, Gronert K, McCormick BA, and Leong JM. 2013. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J. Immunol 191: 5115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, and Janoff EN. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Invest 95: 142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell TJ, and Dalziel CE. 2014. The Biology of Pneumolysin. In Sub-cellular biochemistry vol. 80 145–160. [DOI] [PubMed] [Google Scholar]
- 14.Heuck AP, Moe PC, and Johnson BB. 2010. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell. Biochem 51: 551–77. [DOI] [PubMed] [Google Scholar]
- 15.Hotze EM, and Tweten RK. 2012. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim. Biophys. Acta - Biomembr 1818: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall JE, Faraj BHA, Gingras AR, Lonnen R, Sheikh MA, El-Mezgueldi M, Moody PCE, Andrew PW, and Wallis R. 2015. The Crystal Structure of Pneumolysin at 2.0 Å Resolution Reveals the Molecular Packing of the Pre-pore Complex. Sci. Rep 5: 13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence SL, Feil SC, Morton CJ, Farrand AJ, Mulhern TD, Gorman MA, Wade KR, Tweten RK, and Parker MW. 2015. Crystal structure of Streptococcus pneumoniae pneumolysin provides key insights into early steps of pore formation. Sci. Rep 5: 14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy SKB, and O’Riordan MXD. 2013. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins (Basel). 5: 618–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, and Mitchell TJ. 1995. The Role of Pneumolysin and Autolysin in the Pathology of Pneumonia and Septicemia in Mice Infected with a Type 2 Pneumococcus. J. Infect. Dis 172: 119–123. [DOI] [PubMed] [Google Scholar]
- 20.Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, and Andrew PW. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun 68: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadioglu A, Taylor S, Iannelli F, Pozzi G, Mitchell TJ, and Andrew PW. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun 70: 2886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton JC, Rowan-Kelly B, and Ferrante A. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun 43: 1085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jounblat R, Kadioglu A, Mitchell TJ, and Andrew PW. 2003. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun 71: 1813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreland JG, and Bailey G. 2006. Neutrophil transendothelial migration in vitro to Streptococcus pneumoniae is pneumolysin dependent. AJP Lung Cell. Mol. Physiol 290: L833–L840. [DOI] [PubMed] [Google Scholar]
- 25.Rubins JB, Mitchell TJ, Andrew PW, and Niewoehner DE. 1994. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect. Immun 62: 3829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, and McCormick BA. 2004. From The Cover: Identification of hepoxilin A3 in inflammatory events: A required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci 101: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, and McCormick BA. 2008. Distinct Isoforms of Phospholipase A2 Mediate the Ability of Salmonella enterica Serotype Typhimurium and Shigella flexneri To Induce the Transepithelial Migration of Neutrophils. Infect. Immun 76: 3614–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley BP, Siccardi D, Mrsny RJ, and McCormick BA. 2004. Polymorphonuclear Cell Transmigration Induced by Pseudomonas aeruginosa Requires the Eicosanoid Hepoxilin A3. J. Immunol 173: 5712–5720. [DOI] [PubMed] [Google Scholar]
- 29.Pazos MA, Pirzai W, Yonker LM, Morisseau C, Gronert K, and Hurley BP. 2015. Distinct Cellular Sources of Hepoxilin A 3 and Leukotriene B 4 Are Used To Coordinate Bacterial-Induced Neutrophil Transepithelial Migration. J. Immunol 194: 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCool TL, Cate TR, Moy G, and Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med 195: 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthias KA, Roche AM, Standish AJ, Shchepetov M, and Weiser JN. 2008. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J. Immunol 180: 6246–54. [DOI] [PubMed] [Google Scholar]
- 32.Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, and Weiser JN. 2006. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem 281: 12994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, and Weiser JN. 2007. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell. Microbiol 9: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura S, Davis KM, and Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Invest 121: 3657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepard LA, Heuck AP, Hamman BD, Rossjohn J, Parker MW, Ryan KR, Johnson AE, and Tweten RK. 1998. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry 37: 14563–74. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E, and Maniatis T 1989. Molecular Cloning; A Laboratory Manual,. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 37.Kusek ME, Pazos MA, Pirzai W, and Hurley BP. 2014. In vitro coculture assay to assess pathogen induced neutrophil trans-epithelial migration. J. Vis. Exp e50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason RJ, and Williams MC. 1980. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim. Biophys. Acta 617: 36–50. [DOI] [PubMed] [Google Scholar]
- 39.Hurley BP, Siccardi D, Mrsny RJ, and McCormick BA. 2004. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J. Immunol 173: 5712–20. [DOI] [PubMed] [Google Scholar]
- 40.Chen H-R, and Yeh T-M. 2017. In vitro Assays for Measuring Endothelial Permeability by Transwells and Electrical Impedance Systems. BIO-PROTOCOL 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, and Madara JL. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol 131: 1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamang DL, Pirzai W, Priebe GP, Traficante DC, Pier GB, Falck JR, Morisseau C, Hammock BD, McCormick BA, Gronert K, and Hurley BP. 2012. Hepoxilin A3 Facilitates Neutrophilic Breach of Lipoxygenase-Expressing Airway Epithelial Barriers. J. Immunol 189: 4960–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, and McCormick BA. 2004. From The Cover: Identification of hepoxilin A3 in inflammatory events: A required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci 101: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCormick BA, Parkos CA, Colgan SP, Carnes DK, and Madara JL. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol 160: 455–66. [PubMed] [Google Scholar]
- 45.Pazos MA, Pirzai W, Yonker LM, Morisseau C, Gronert K, and Hurley BP. 2015. Distinct cellular sources of hepoxilin A3 and leukotriene B4 are used to coordinate bacterial-induced neutrophil transepithelial migration. J. Immunol 194: 1304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCool TL, Cate TR, Moy G, and Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med 195: 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis KM, Nakamura S, and Weiser JN. 2011. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J. Clin. Invest 121: 3666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price KE, Greene NG, and Camilli A. 2012. Export Requirements of Pneumolysin in Streptococcus pneumoniae. J. Bacteriol 194: 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoch JA 1993. Regulation of the Phosphorelay and the Initiation of Sporulation in Bacillus Subtilis. Annu. Rev. Microbiol 47: 441–465. [DOI] [PubMed] [Google Scholar]
- 50.Stewart-Tull DES 1980. The Immunological Activities of Bacterial Peptidoglycans. Annu. Rev. Microbiol 34: 311–340. [DOI] [PubMed] [Google Scholar]
- 51.Wolfmeier H, Radecke J, Schoenauer R, Koeffel R, Babiychuk VS, Drücker P, Hathaway LJ, Mitchell TJ, Zuber B, Draeger A, and Babiychuk EB. 2016. Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack. Biochim. Biophys. Acta - Gen. Subj 1860: 2498–2509. [DOI] [PubMed] [Google Scholar]
- 52.Wolfmeier H, Schoenauer R, Atanassoff AP, Neill DR, Kadioglu A, Draeger A, and Babiychuk EB. 2015. Ca2 +-dependent repair of pneumolysin pores: A new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim. Biophys. Acta - Mol. Cell Res 1853: 2045–2054. [DOI] [PubMed] [Google Scholar]
- 53.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, and Andrews NW. 2008. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol 180: 905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skindersoe ME, and Kjaerulff S. 2014. Comparison of three thiol probes for determination of apoptosis-related changes in cellular redox status. Cytom. Part A 85: 179–187. [DOI] [PubMed] [Google Scholar]
- 55.Ballard J, Bryant A, Stevens D, and Tweten RK. 1992. Purification and characterization of the lethal toxin (alpha-toxin) of Clostridium septicum. Infect. Immun 60: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imagawa T, Dohi Y, and Higashi Y. 1994. Cloning, nucleotide sequence and expression of a hemolysin gene of Clostridium septicum. FEMS Microbiol. Lett 117: 287–92. [DOI] [PubMed] [Google Scholar]
- 57.Tweten RK, Hotze EM, and Wade KR. 2015. The Unique Molecular Choreography of Giant Pore Formation by the Cholesterol-Dependent Cytolysins of Gram-Positive Bacteria. Annu. Rev. Microbiol 69: 323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shatursky O, Heuck AP, Shepard LA, Rossjohn J, Parker MW, Johnson AE, and Tweten RK. 1999. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99: 293–9. [DOI] [PubMed] [Google Scholar]
- 59.Dang TX, Hotze EM, Rouiller I, Tweten RK, and Wilson-Kubalek EM. 2005. Prepore to pore transition of a cholesterol-dependent cytolysin visualized by electron microscopy. J. Struct. Biol 150: 100–8. [DOI] [PubMed] [Google Scholar]
- 60.van Pee K, Neuhaus A, D’Imprima E, Mills DJ, Kühlbrandt W, and Yildiz Ö. 2017. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, and Tweten RK. 2010. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl. Acad. Sci. U. S. A 107: 4341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pace-Asciak C 1984. Arachidonic Acid Epoxides. J. Biol. Chem 259: 8332–8337. [PubMed] [Google Scholar]
- 63.Arora JK, Lysz TW, and Zelenka PS. 1996. A role for 12(S)-HETE in the response of human lens epithelial cells to epidermal growth factor and insulin. Invest. Ophthalmol. Vis. Sci 37: 1411–8. [PubMed] [Google Scholar]
- 64.Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, and Lohmeyer J. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J. Immunol 173: 1307–12. [DOI] [PubMed] [Google Scholar]
- 65.Shigematsu M, Koga T, Ishimori A, Saeki K, Ishii Y, Taketomi Y, Ohba M, Jo-Watanabe A, Okuno T, Harada N, Harayama T, Shindou H, Li J-D, Murakami M, Hoka S, and Yokomizo T. 2016. Leukotriene B4 receptor type 2 protects against pneumolysin-dependent acute lung injury. Sci. Rep 6: 34560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, Oseghale A, Verin AD, Chakraborty T, Matthay MA, Zemskov EA, White R, Block NL, and Schally AV. 2012. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci 109: 2084–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Funk CD, Chen X-S, Johnson EN, and Zhao L. 2002. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 68–69: 303–312. [DOI] [PubMed] [Google Scholar]
- 68.Pace-Asciak CR 2009. The hepoxilins and some analogues: a review of their biology. Br. J. Pharmacol 158: 972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reglero-Real N, García-Weber D, Millán J, Millá, and n. J 2016. Cellular Barriers after Extravasation: Leukocyte Interactions with Polarized Epithelia in the Inflamed Tissue. Mediators Inflamm. 2016: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, and Schütte H. 2006. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit. Care Med 34: 1947–1954. [DOI] [PubMed] [Google Scholar]
- 71.Weight CM, Venturini C, Pojar S, Jochems SP, Reiné J, Nikolaou E, Solórzano C, Noursadeghi M, Brown JS, Ferreira DM, and Heyderman RS. 2019. Microinvasion by Streptococcus pneumoniae induces epithelial innate immunity during colonisation at the human mucosal surface. Nat. Commun 10: 3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhowmick R, Clark S, Bonventre JV, Leong JM, and McCormick BA. 2017. Cytosolic phospholipase A 2 α promotes pulmonary inflammation and systemic disease during Streptococcus pneumoniae infection. Infect. Immun IAI.00280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Pee K, Neuhaus A, D’Imprima E, Mills DJ, Kühlbrandt W, and Yildiz Ö. 2017. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. Elife 6: e23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schievella AR, Regier MK, Smith WL, and Lin LL. 1995. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J. Biol. Chem 270: 30749–54. [DOI] [PubMed] [Google Scholar]
- 75.Gijón MA, and Leslie CC. 1999. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol 65: 330–6. [DOI] [PubMed] [Google Scholar]
- 76.Korchev YE, Bashford CL, Pederzolli C, Pasternak CA, Morgan PJ, Andrew PW, and Mitchell TJ. 1998. A conserved tryptophan in pneumolysin is a determinant of the characteristics of channels formed by pneumolysin in cells and planar lipid bilayers. Biochem. J 329 (Pt 3): 571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Rachkidy RG, Davies NW, and Andrew PW. 2008. Pneumolysin generates multiple conductance pores in the membrane of nucleated cells. Biochem. Biophys. Res. Commun 368: 786–792. [DOI] [PubMed] [Google Scholar]
- 78.Korchev YE, Bashford CL, and Pasternak C. 1992. Differential sensitivity of pneumolysin-induced channels to gating by divalent cations. J. Membr. Biol 127: 195–203. [DOI] [PubMed] [Google Scholar]
- 79.Alhamdi Y, Neill DR, Abrams ST, Malak HA, Yahya R, Barrett-Jolley R, Wang G, Kadioglu A, and Toh C-H. 2015. Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection. PLOS Pathog. 11: e1004836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iliev AI, Djannatian JR, Nau R, Mitchell TJ, and Wouters FS. 2007. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc. Natl. Acad. Sci. U. S. A 104: 2897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reynaud D, Demin PM, Sutherland M, Nigam S, and Pace-Asciak CR. 1999. Hepoxilin signaling in intact human neutrophils: biphasic elevation of intracellular calcium by unesterified hepoxilin A3. FEBS Lett. 446: 236–8. [DOI] [PubMed] [Google Scholar]
- 82.Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, and Malley R. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun 73: 6479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qi H-Y, and Shelhamer JH. 2005. Toll-like Receptor 4 Signaling Regulates Cytosolic Phospholipase A 2 Activation and Lipid Generation in Lipopolysaccharide-stimulated Macrophages. J. Biol. Chem 280: 38969–38975. [DOI] [PubMed] [Google Scholar]
- 84.Dessing MC, Hirst RA, de Vos AF, van der Poll T, and Andrew P. 2009. Role of Toll-Like Receptors 2 and 4 in Pulmonary Inflammation and Injury Induced by Pneumolysin in Mice. PLoS One 4: e7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, and Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A 100: 1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall JE, Faraj BHA, Gingras AR, Lonnen R, Sheikh MA, El-Mezgueldi M, Moody PCE, Andrew PW, and Wallis R. 2015. The Crystal Structure of Pneumolysin at 2.0 Å Resolution Reveals the Molecular Packing of the Pre-pore Complex. Sci. Rep 5: 13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawrence SL, Feil SC, Morton CJ, Farrand AJ, Mulhern TD, Gorman MA, Wade KR, Tweten RK, and Parker MW. 2015. Crystal structure of Streptococcus pneumoniae pneumolysin provides key insights into early steps of pore formation. Sci. Rep 5: 14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hotze EM, Wilson-Kubalek EM, Rossjohn J, Parker MW, Johnson AE, and Tweten RK. 2001. Arresting pore formation of a cholesterol-dependent cytolysin by disulfide trapping synchronizes the insertion of the transmembrane beta-sheet from a prepore intermediate. J. Biol. Chem 276: 8261–8. [DOI] [PubMed] [Google Scholar]
- 89.Ramachandran R, Tweten RK, and Johnson AE. 2004. Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment. Nat. Struct. Mol. Biol 11: 697–705. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell TJ, Andrew PW, Saunders FK, Smith AN, and Boulnois GJ. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol 5: 1883–8. [DOI] [PubMed] [Google Scholar]
- 91.Yoo I-H, Shin H-S, Kim Y-J, Kim H-B, Jin S, and Ha U-H. 2010. Role of pneumococcal pneumolysin in the induction of an inflammatory response in human epithelial cells. FEMS Immunol. Med. Microbiol 60: 28–35. [DOI] [PubMed] [Google Scholar]
- 92.Houldsworth S, Andrew PW, and Mitchell TJ. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun 62: 1501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones MR, Simms BT, Lupa MM, Kogan MS, and Mizgerd JP. 2005. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol 175: 7530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rijneveld AW, van den Dobbelsteen GP, Florquin S, Standiford TJ, Speelman P, van Alphen L, and van der Poll T. 2002. Roles of Interleukin-6 and Macrophage Inflammatory Protein–2 in Pneumolysin-Induced Lung Inflammation in Mice. J. Infect. Dis 185: 123–126. [DOI] [PubMed] [Google Scholar]
- 95.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, and Pearlman E. 2015. Neutrophil IL-1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol 194: 1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cockeran R, Durandt C, Feldman C, Mitchell TJ, and Anderson R. 2002. Pneumolysin Activates the Synthesis and Release of Interleukin-8 by Human Neutrophils In Vitro. J. Infect. Dis 186: 562–565. [DOI] [PubMed] [Google Scholar]
- 97.Cockeran R, Steel HC, Mitchell TJ, Feldman C, and Anderson R. 2001. Pneumolysin potentiates production of prostaglandin E(2) and leukotriene B(4) by human neutrophils. Infect. Immun 69: 3494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.