Abstract
Background:
Most prostate cancer cases are diagnosed with low-grade, localized disease and may not require definitive treatment. In 2012 the US Preventive Services Task Force (USPSTF) recommended against prostate cancer screening to address over-detection and over-treatment.
Objective:
To determine the effect of guideline changes on prostate-specific antigen (PSA) screening and initial diagnostic stage for prostate cancer.
Design:
A difference-in-differences analysis to compare changes in PSA testing (exposure), relative to cholesterol testing (control) following the 2012 USPSTF change.
Setting:
Data were derived from a tertiary academic medical center’s electronic health records (EHRs), a national commercial insurance database (Optum), and the Surveillance, Epidemiology, and End Results Program (SEER).
Participants:
Men aged 35+ before (2008–2011) and after (2013–2016) the guideline changes.
Intervention:
2012 USPSTF guideline change.
Measurements:
PSA screening rates and diagnostic stage for prostate cancer.
Results:
In both the academic center and insurance database, PSA testing significantly decreased for all men compared to the control. The greatest decrease at the academic center was among men aged 55–75 and 35–55 in the commercial database. The proportion of early stage prostate cancer diagnoses (<T2) decreased across age groups at the academic center and in SEER.
Conclusions and Relevance
This study highlights the impact of the USPSTF’s 2012 PSA screening recommendation on primary care testing patterns and an associated effect on early stage diagnoses for prostate cancer patients. In primary care, PSA testing decreased significantly and fewer prostate cancers were diagnosed at an early stage, suggesting provider guideline adherence. Long-term follow-up is needed to understand the effect of decreased screening on prostate cancer survival.
Introduction
Prostate cancer is the most common cancer in US men; 2018 is projected to see 164,700 new cases, accounting for 19% of all new cancers in men, and 29,500 deaths.1 However, there remains a lack of consensus as to best practices for screening and for treatment, partly due to the difficulty in distinguishing aggressive from indolent cancers.2 The majority of prostate cancers are asymptomatic, are detected by primary-care directed screening, are slow growing and will not become clinically evident during the lifetime of a patient. Autopsy studies detect prostate cancer in 30% of men by age 55 and 60% of men by age 80.3 Widespread implementation of prostate-specific antigen (PSA) screening has led to a significant increase in the diagnosis and treatment of prostate cancer, including many inconsequential tumors,4 with minimal or no effect on mortality rates.5–9 Meanwhile, treatment of these cancers can lead to treatment-related side effects such as urinary incontinence or sexual dysfunction.10,11
The Prostate Lung, Colon and Ovarian (PLCO) trial demonstrated that systematic PSA testing resulted in higher prostate cancer diagnosis rates - particularly of early-stage disease - but without improvements in mortality.7 In addition, the Prostate Intervention Versus Observation Trial (PIVOT), conducted in the Veterans Health Administration, showed no survival advantage for surgery compared to no treatment for localized prostate cancer.12 Based on the results of these trials, the US Preventative Services Task Force (USPSTF) published new guidelines in 2012 that recommended against PSA screening in all men (D rating),13 expanding a 2008 guideline that recommended against screening in the over-75 age group.14 However, these recommendations were highly controversial since the death rate from prostate cancer had dropped 50% since the initiation of PSA testing in the US, and randomized trials of PSA screening and surgical treatment of localized prostate cancer conducted in Europe showed significant survival benefits for both screening and treatment.5,15,16 The controversy continued to grow based on criticisms of PLCO due to contamination of the control arm, and criticisms of PIVOT for selection of patients with co-morbidities and indolent disease.17,18 Finally, since 2012, the increased use of active surveillance for management of indolent disease in both the U.S. and Europe changed the risk/benefit ratio for prostate cancer screening by decoupling screening from treatment side effects.19 Because of this, in 2018 the USPSTF rolled back the 2012 recommendations and advises men aged 55–69 to discuss the risks and benefits of screening with their health care provider (C rating).20
Better understanding the effects of guideline changes, particularly in controversial topics such as cancer screening, may help inform future policy. Studies of Medicare beneficiaries have shown that the 2008 guidelines changes were associated with: a 2% decline in PSA screening for men over the age of 75, and decline in treatment by 42% at the population level but only by 8% among diagnosed men, suggesting that declines in screening and diagnosis were driving the decline rather than changes in the patterns of treatment.21 Studies examining men of all ages have found conflicting results, observing significant declines22,23 or no change24 in PSA screening rates in the wake of the 2012 changes, and declines in testing have been suggested to underly increases in late-stage disease burden from 2010 to 2014.23 However, these studies did not control for secular trends like the Affordable Care Act that might influence screening and diagnosis and only encompassed a year or two of data following the guideline changes.
Given the controversy over this guideline change, clinician adherence and impacts on prostate cancer diagnosis are poorly understood. Using multiple datasets from 2008–2016, the purpose of this study was 2-fold: (1) determine whether PSA testing rates changed in primary care following the 2012 USPSTF guideline changes; and (2) to determine if early-stage, low-risk prostate cancer diagnoses decreased following the downgrade in PSA screening recommendations. This finding can highlight the impact of even controversial guideline changes on clinical practice.
Methods
Study Design
We used a quasi-experimental, difference-in-differences design25 to compare PSA vs cholesterol testing rates among men aged 35+ before (2008–2011) vs after (2013–2016) the 2012 USPSTF prostate cancer screening guideline changes. We focused on primary care providers because they are tasked with disease screening, while subspecialists likely use PSA for disease monitoring after therapy. Cholesterol testing, like PSA, addresses conditions that are asymptomatic at onset, targets similar risk populations, is administered as a blood test, is widely accessible across care settings and is mainly used by primary care physicians. The difference-in-differences allows the control to serve as the counterfactual, thereby accounting for secular trends such as increased access to care following the Affordable Care Act. We adjusted for potential time-varying confounders that could bias estimates and tested for parallelism in pre-policy trends between the study and control populations before the guideline changes, adhering to published best practices to asses validity of the control as a suitable counterfactual.25,26 We further compared rates of prostate and colorectal cancers diagnosed at early stage before (2008–2011) and after (2013–2016) USPSTF guideline changes. Colorectal cancer like prostate cancer has a similar patient population and a clinically silent period, yet screening guidelines were stable over the study period.
Data Sources
Primary data were derived from the Electronic Health Records (EHR) of a tertiary academic medical center containing encounter-level data from 2008 to 2016, including demographics, lab orders, insurance payer, clinical features, and provider specialty. The clinical data warehouse is described elsewhere.27
Optum Labs was founded in early 2013 by Mayo Clinic and Optum, a commercial data, infrastructure services, and care organization that is part of UnitedHealth Group. Optum Labs now has eleven collaborators and a database of deidentified information on more than 150 million people that is compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Records include inpatient, outpatient, pharmacy, and laboratory claims. Socioeconomic status (SES) was established using net worth as coded by the Optum database. We used a 1% sample of the population from 2008–2016.28
Surveillance, Epidemiology, and End Results Program (SEER) is a national cancer registry covering about a third of the US population. We used 2008–2015 data for both prostate and colorectal diagnoses, including demographics and diagnostic stage. SEER data was available up to 2015 and lacked comorbidity scores.29 Due to data structure, insurance was categorized as insured, any Medicaid, or other/unknown/uninsured.
Study Participants
The screening population consisted of undiagnosed men aged 35+ seen by a primary care provider. Primary care was defined in EHR data by provider specialty (family medicine, family practice, geriatric medicine, or nurse practitioner-family), while the Optum database already included a variable for provider type that identified records from primary care providers. Charlson comorbidity scores were assigned at the start of each year, and ages were calculated between birth and encounter dates. Race included White, Asian, Black, Hispanic, and other/unknown. Insurance payer was categorized to Medicare, Medicaid, private, and other/unknown. Annual testing rates were assessed independently: patients could be counted as receiving screening or not only once per annual eligibility period. Diagnosed patients were excluded following their diagnosis date.
Diagnostic stage was assessed in all first-time cancer diagnoses by calendar year. We defined low-grade based on AJCC (American Joint Committee on Cancer) prognostic stage groups.30 Early stage was defined as localized (summary stage ≤ 2) at initial diagnosis. Cancers with unrecorded initial stage were excluded.
Statistical Analysis
Linear regression difference-in-differences models compared changes in PSA screening relative to cholesterol testing following the 2012 USPSTF recommendation. The models account for secular changes, which include factors like expanded access to care following the Affordable Care Act, by assuming the control is a counterfactual for the exposure group had the policy not existed; we adhered to published best practices in assessing this assumption by testing for parallelism in the pre-intervention period (Supplement eMethods and eTable 1).25,26 Linear probability models were a function of separate binary indicator variables for exposure status, post-policy status (2013–2016), and their product yielding their interaction (Supplement eMethods). The difference-in-differences estimate is represented by the interaction term, which describes the differential change between exposure and control following policy implementation. Charlson comorbidity, age, race, and insurance or socioeconomic status (net worth) were included in the models. The pre-policy and post-policy periods were defined as 1 January 2008 to 31 December 2011 and 1 January 2013 to 31 December 2016 (2015 for SEER). The implementation year, 2012, was excluded as a ‘washout’ period.13 Screening trends compared PSA (exposure) and cholesterol (control) testing. Diagnostic stage separately examined prostate and colorectal cancers by Chi squared test. We stratified analysis by age group. Statistical significance was defined by 2-sided p value < 0.05. All analyses were performed with the open source statistical program R, version 3.4.1 via RStudio version 1.0.153.
Results
In the academic center’s EHR, we identified 18,559 pre-policy and 78,281 post-policy patients; 296 (1.6%) pre-policy and 874 (1.1%) post-policy were excluded due to prostate cancer diagnosis prior to tabulated annual PSA screening. Pre-policy, 3,252 received any PSA tests (3,456 tests ordered) and 5,686 received any cholesterol tests (6,410 tests ordered); post-policy, 8,306 patients received any PSA tests (8,914 tests ordered total) and 24,491 received any cholesterol tests (28,161 tests ordered total). Compared to the pre-policy patients, the post-policy patients (Table 1) were slightly older, with a greater proportion of men 55–74, slightly fewer on Medicare, and consisted of a similar proportion of Whites, slightly more Black and Hispanic, and fewer Asian patients.
Table 1.
Characteristics of Patients Eligible for Screening, By Policy Period, 2008–2016.
| Tertiary Academic Center | Optum 1% Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Pre-policy, % (95% CI) | Post-policy, % (95% CI) | Unadjusted Difference, % (95% CI) | Pre-policy, % (95% CI) | Post-policy, % (95% CI) | Unadjusted Difference, % (95% CI) | ||||||
| N | 18,559 | 78,281 | 93,334 | 110,067 | ||||||||
| Mean age, years (95% CI) | 56.2 | (56.0 to 56.4) | 56.9 | (56.8 to 57.0) | 0.7 | (0.5 to 1.0) | 54.9 | (54.8 to 55.0) | 58.4 | (58.4 to 58.5) | 3.5 | (3.4 to 3.7) |
| Charlson comorbidity – mean (95% CI), | 1.1 | (1.1 to 1.2) | 1.0 | (1.0 to 1.1) | −0.1 | (−0.1 to 0.0) | ||||||
| Eligible patients by age – % (95% CI) | ||||||||||||
| 35 – 54 years old | 51.6 | (50.9 to 52.3) | 46.6 | (46.3 to 47.0) | −4.9 | (−5.7 to −4.1) | 53.1 | (52.8 to 53.4) | 42.1 | (41.8 to 42.4) | −11 | (−10.6 to −11.4) |
| 55 – 74 years old | 35.3 | (34.6 to 36.0) | 41.8 | (41.5 to 42.2) | 6.5 | (5.7 to 7.3) | 37.7 | (37.3 to 38.0) | 43.8 | (43.5 to 44.1) | 6.1 | (5.7 to 6.6) |
| 75+ years old | 13.1 | (12.6 to 13.6) | 11.5 | (11.3 to 11.7) | −1.6 | (−2.1 to −1.0) | 9.2 | (9.0 to 9.4) | 14.1 | (13.9 to 14.3) | 4.8 | (4.6 to 5.1) |
| Race – % (95% CI) | ||||||||||||
| White | 54.1 | (53.3 to 54.8) | 53.4 | (53.0 to 53.7) | −0.7 | (−1.5 to 0.1)b | 68.8 | (68.5 to 69.1) | 60.3 | (60.1 to 60.6) | −8.5 | (−8.9 to −8.0) |
| Asian | 16.4 | (15.8 to 16.9) | 15.5 | (15.2 to 15.8) | −0.9 | (−1.4 to −0.3)a | 3.0 | (2.9 to 3.2) | 3.3 | (3.2 to 3.4) | 0.3 | (0.1 to 0.4) |
| Black | 3.3 | (3.1 to 3.6) | 5.6 | (5.5 to 5.8) | 2.3 | (2.0 to 2.6) | 8.2 | (8.0 to 8.4) | 7 | (6.8 to 7.1) | −1.2 | (−1.5 to −1.0) |
| Hispanic | 7.8 | (7.4 to 8.2) | 9 | (8.8 to 9.2) | 1.2 | (0.8 to 1.7) | 7.6 | (7.4 to 7.8) | 8.2 | (8.0 to 8.4) | 0.6 | (0.4 to 0.8) |
| Other or unknown | 18.4 | (17.9 to 19.0) | 16.5 | (16.2 to 16.7) | −2.0 | (−2.6 to −1.3) | 12.4 | (12.2 to 12.6) | 21.2 | (20.9 to 21.4) | 8.8 | (8.5 to 9.1) |
| Insurance – % (95% CI) | ||||||||||||
| Medicare | 32.0 | (31.3 to 32.7) | 25.0 | (24.7 to 25.3) | −7.0 | (−7.7 to −6.2) | N/A | |||||
| Medicaid | 3.1 | (2.9 to 3.4) | 2.0 | (1.9 to 2.1) | −1.1 | (−1.3 to −0.8) | ||||||
| Private | 54.6 | (53.9 to 55.3) | 53.5 | (53.1 to 53.8) | −1.2 | (−1.9 to −0.4)a | ||||||
| Other or Unknown | 10.3 | (9.8 to 10.7) | 19.5 | (19.2 to 19.7) | 9.2 | (8.7 to 9.7) | ||||||
Notes: All comparisons are between the pre-policy (2008–2011) and post-policy (2013–2016) periods in primary care. Time intervals were defined as calendar years and evaluated independently for patient-level eligibility. CI, confidence interval. All p-values <0.001, except:
= <0.01;
= 0.094.
In the Optum 1% sample, we identified 93,334 pre-policy and 110,067 post-policy patients by year. Post-policy patients (Table 1) were slightly older, with larger proportions of men aged 55–74, fewer aged 35–54, with fewer Whites compared to other groups and a larger proportion with unknown socioeconomic status (net worth). The number of patients in the academic center’s population increased over the course of the study due to an expanded primary care initiative, which is controlled for along with other background temporal trends through the difference-in-differences model via the cholesterol control.
Annual primary care testing rates for PSA (exposure) and cholesterol (control), including composite rates and rates stratified by age group, are shown unadjusted in Figure 1 for the tertiary academic center and Optum. PSA testing declined in both the academic medical center and in Optum, with the greatest decreases in PSA testing observed in men 55–74 and 75+, respectively. Modeled estimates accounting for background temporal trends (Table 2) show significant decreases in PSA testing both overall and by age group (all p < 0.001). PSA testing declined across all age groups by 8.0% [95% CI: −8.9% to −7.1%] in the academic center and by 3.6% [95% CI: −4.1% to −3.2%] in the Optum population. The academic center had largest changes in men 55–74 (−13.0%) and smaller declines in men 35–54 (−4.8%) and 75+ (−8.5%). Optum had its largest decrease in men 75+ (−8.2%) with smaller drops in men 55–74 (−2.8%) and 35–54 (−4.1%).
Figure 1.
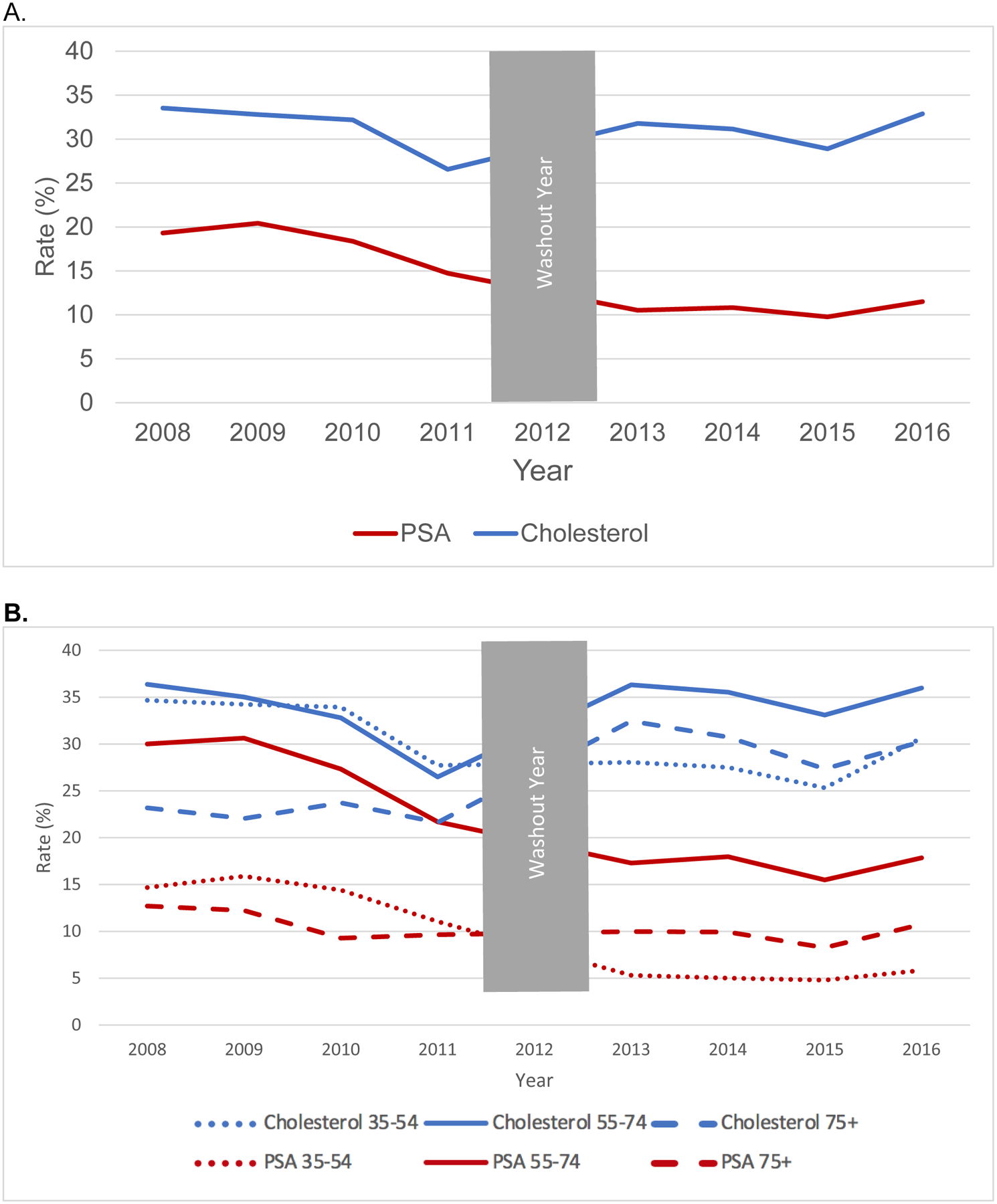
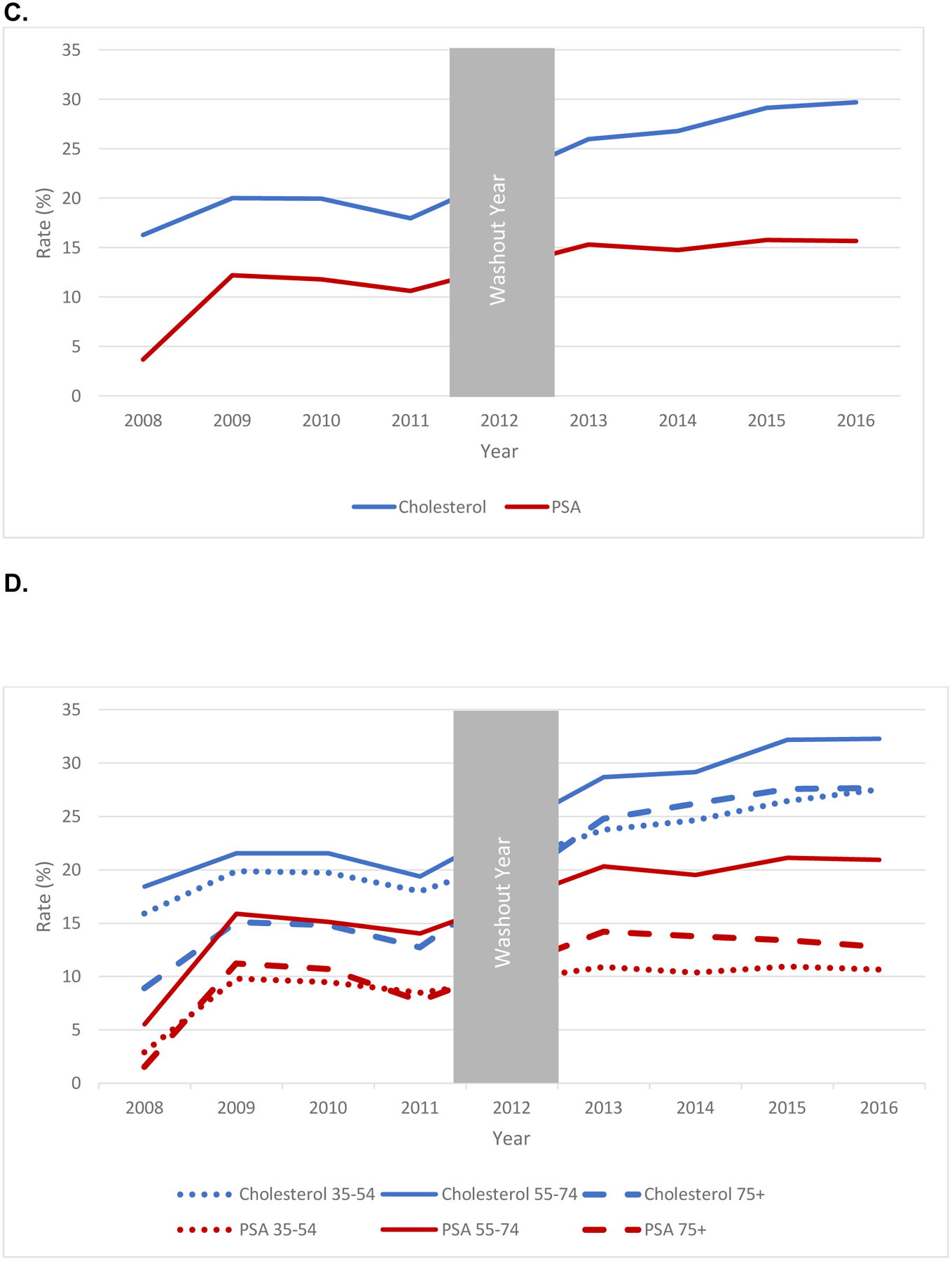
Unadjusted Trends in PSA (Exposure) and Cholesterol (Control) Testing in Primary Care Settings for Men, (A) Academic Center, All Ages; (B) Academic Center, By Age Group, (C) Optum, All Ages; (B) Optum, By Age Group, 2008–2016.
Table 2.
Changes in PSA (Exposure) and Cholesterol (Control) Testing, By Policy Period, 2008–2016
| Eligible No. | PSA Screening (Exposure), % | Cholesterol Screening (Control), % | Adjusted Difference-in-Differences Estimate (95% CI), Percentage Points | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group | Pre-policy (Control; Exposed) | Post-policy (Control; Exposed) | Pre-policy | Post-policy | Unadjusted Difference (95% CI), Percentage Points | Pre-policy | Post-policy | Unadjusted Difference (95% CI), Percentage Points | |
| Tertiary Academic Medical Center | |||||||||
| All Men | 18,559 | 78,281 | 17.8 | 10.7 | −7.1 | 30.6 | 31.3 | 0.7 | −8 |
| 18,303 | 77,407 | (−7.7 to −6.5) | (0.0 to 1.4) | (−8.9 to −7.1) | |||||
| 35–54 | 9,574 | 36,517 | 13.6 | 5.3 | −8.3 | 32 | 28.1 | −3.9 | −4.8 |
| 9,566 | 36,484 | (−9.0 to −7.6) | (−4.9 to −2.9) | (−6.0 to −3.7) | |||||
| 55–74 | 6,556 | 32,756 | 26.5 | 17.1 | −9.4 | 31.6 | 35.2 | 3.6 | −13 |
| 6,389 | 32,256 | (−10.6 to −8.2) | (2.4 to 4.8) | (−14.7 to −11.4) | |||||
| 75+ | 2,429 | 9,008 | 10.8 | 9.8 | −1 | 22.6 | 29.9 | 7.3 | −8.5 |
| 2,348 | 8,667 | (−2.4 to 0.4) | (5.4 to 9.2) | (−11.0 to −6.1) | |||||
| Optum 1% Sample | |||||||||
| All Men | 93,334 | 110,067 | 9.6 | 15.4 | 5.8 | 18.6 | 28 | 9.4 | −3.6 |
| (5.5 to 6.1) | (9.0 to 9.8) | (−4.1 to −3.2) | |||||||
| 35–54 | 49,571 | 46,359 | 7.6 | 10.7 | 3.1 | 18.3 | 25.6 | 7.3 | −4.1 |
| (2.7 to 3.5) | (6.8 to 7.8) | (−4.8 to −3.5) | |||||||
| 55–74 | 35,148 | 48,215 | 12.9 | 20.5 | 7.6 | 20.2 | 30.7 | 10.5 | −2.8 |
| (7.1 to 8.1) | (9.9 to 11.1) | (−3.6 to −2.0) | |||||||
| 75+ | 8,615 | 15,493 | 8.2 | 13.5 | 5.3 | 13.1 | 26.7 | 13.6 | −8.2 |
| (4.5 to 6.1) | (12.6 to 14.6) | (−9.6 to −6.9) | |||||||
Notes: All comparisons are between the pre-policy (2008–2011) and post-policy (2013–2016) periods in primary care. Time intervals were defined as calendar years and evaluated independently for patient-level eligibility. Adjusted difference-in-differences estimates are corrected for age, Charlson comorbidity, race, and insurance provider type for the academic center, while the Optum sample is corrected for age, race, and socioeconomic status (net worth). Charlson comorbidity and age were derived on an annual basis. CI, confidence interval. All p-values <0.001.
In the EHRs, we identified 2,572 prostate and 413 colorectal pre-policy diagnoses after dropping 288 (10.1%) and 204 (33.1%) without stage, respectively. There were 1,397 prostate and 521 colorectal post-policy diagnoses after dropping 593 (29.8%) and 176 (25.3%) without stage. Compared to pre-policy, post-policy (Supplement eTable 2) prostate cancer patients had similar age, slightly higher comorbidity scores, larger White and Asian proportions, and larger Medicaid and private insurance proportions. Colorectal cancer patients had similar age, comorbidity, and insurance but smaller proportions of White and Asian men. Prostate cancer patients were generally older than colorectal cancer patients and had a larger proportion of Asians.
In SEER, there were 75,641 prostate and 20,250 colorectal pre-policy diagnoses after dropping 1,945 (2.5%) and 821 (3.9%) without stage, respectively; there were 44,904 prostate and 15,077 colorectal post-policy diagnoses after dropping 1,477 (3.2%) and 681 (4.3%) without stage. Post-policy (Supplement eTable 2), age slightly increased for prostate cancer patients and decreased for colorectal cancer patients; both had fewer Whites and a larger Medicaid proportion. Prostate cancer patients were slightly older comparison to men with colorectal cancer.
Decreases in the unadjusted proportion of cancers with early stage (Table 3) were seen in both the academic medical center and SEER. The academic center had nearly uniform decreases across age groups with an overall decline from 79.0% to 63.4% (−15.6%). In comparison, colorectal diagnoses did not display significant changes except for an increase from 46.3% to 58.6% (+12.3%, p < 0.01) in men 55–74. SEER showed smaller decreases in early stage prostate cancer, decreasing overall from 82.6% to 77.7% (−4.9%). The largest decline was in men 75+ (−10.1%), followed by men 55–74 (−4.1%) and men 35–54 (−2.6%). SEER colorectal diagnoses also decreased, but to a lesser degree, from 44.0% to 41.7% overall (−2.3%) with men 75+ showing the largest decrease (−4.0%), followed by men 55–74 (−2.2%), while men 35–54 had no significant change. All results were significant at p < 0.001 unless noted otherwise.
Table 3.
Changes in Proportion of Early Stage Diagnoses for Prostate Cancer (Exposure) Compared to Colorectal Cancer (Controls) at an Academic Medical Center and in the SEER Registry, By Age Group, 2008–2016.
| Prostate Cancer | Colorectal Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-policy | Post-policy | Pre-policy | Post-policy | |||||||
| Age Group | Total Diagnosed | No. Early Stage (%) | Total Diagnosed | No. Early Stage (%) | Difference, (95% CI) | Total Diagnosed | No. Early Stage (%) | Total Diagnosed | No. Early Stage (%) | Difference, (95% CI) |
| Academic Medical Center | ||||||||||
| All Men | 2,572 | 2,032 (79.0) | 1,397 | 885 (63.4) | −15.6 | 413 | 198 (47.9) | 521 | 279 (53.6) | 5.6a |
| (−18.6, −12.7) | (−0.8, 12.1) | |||||||||
| 35–54 | 332 | 267 (80.4) | 143 | 93 (65.0) | −15.4 | 122 | 48 (39.3) | 168 | 73 (43.5) | 4.1a |
| (−24.3, −6.5) | (−7.4, 15.6) | |||||||||
| 55–74 | 1,895 | 1,488 (78.5) | 1,095 | 691 (63.1) | −15.4 | 203 | 94 (46.3) | 266 | 156 (58.6) | 12.3 |
| (−18.8, −12.0) | (3.3, 21.4) | |||||||||
| 75+ | 345 | 277 (80.3) | 159 | 101 (63.5) | −16.8 | 88 | 56 (63.6) | 87 | 50 (57.5) | −6.2a |
| (−25.3, −8.2) | (−20.6, 8.3) | |||||||||
| SEER | ||||||||||
| All Men | 75,641 | 62,469 (82.6) | 44,904 | 34,875 (77.7) | −4.9 | 20,250 | 8,915 (44.0) | 15,077 | 6,286 (41.7) | −2.3 |
| (−5.4, −4.4) | (−3.4, −1.3) | |||||||||
| 35–54 | 8,587 | 6,901 (80.4) | 4,476 | 3,481 (77.8) | −2.6 | 4,841 | 2,062 (42.6) | 3,802 | 1,576 (41.5) | −1.1a |
| (−4.1, −1.1) | (−3.2, 1.0) | |||||||||
| 55–74 | 53,875 | 44,525 (82.6) | 33,256 | 26,110 (78.5) | −4.1 | 10,437 | 4,661 (44.7) | 8,067 | 3,423 (42.4) | −2.2 |
| (−4.7, −3.6) | (−3.7, −0.8) | |||||||||
| 75+ | 13,179 | 11,043 (83.8) | 7,172 | 5,284 (73.7) | −10.1 | 4,972 | 2,192 (44.1) | 3,208 | 1,287 (40.1) | −4 |
| (−11.3, −8.9) | (−6.2, −1.8) | |||||||||
Notes: All comparisons are between the pre-policy (2008–2011) and post-policy (2013–2016) periods. Time intervals were defined as calendar years and evaluated independently for patient-level eligibility. Differences are unadjusted and evaluated by Chi square test. CI, confidence interval.
p-value - <0.0001 unless otherwise specified;
p-value >0.05
Discussion
In this large, retrospective, observational study, we found PSA testing rates in the primary care setting declined relative to cholesterol screening across age groups in both an academic medical center and a large commercial claims database following the controversial 2012 USPSTF recommendation against PSA screening in men of all ages. The academic center saw largest declines in men 55–74, the population that many clinicians view as the target prostate cancer screening population, and while the USPSTF has never endorsed PSA screening, in their updated 2018 recommendations they upgraded from a grade “D” rating to a “C” for men 55–69, softening an explicit recommendation against screening to leaving the decision to patients and their doctor after discussing the risks and benefits.20 In the commercial database, the greatest decline was seen in men 75+, reflecting continued improvement in adherence to 2008 PSA USPSTF guideline recommendations. Coinciding with these declines in PSA testing, there was a decrease in the proportion of prostate cancer patients diagnosed with early stage disease, as would be expected based on the results of randomized PSA screening trials showing fewer diagnoses in the non-screened control arm.6,7,30 It is notable that the decline in diagnoses was confined to early stage cancers, potentially decreasing the number of men identified when their disease is curable, but also diminishing rates of overdiagnosis (and subsequent overtreatment).16
These findings demonstrate that the guideline changes had durable effects on practice patterns through 2016, in line with previous work that demonstrated declines of 3–10% in PSA screening across age groups with data through 2013.31 A recent survey study demonstrated men aged 55–59, 60–74, and over 75 had similar decreases in screening following the 2012 guidelines,32 while another found significant declines of 5–10% in men under 75.33 These studies only included data through 2013, one year after the recommendation changes, therefore, our findings demonstrate that the guideline changes had durable effects on practice patterns through 2016. An analysis of Optum’s privately insured patients found a 38.4% decline in PSA testing that was restricted to men 75+ while no significant changes were seen in younger men. Again, this study was limited to data through 2013 and calculate raw rates of PSA testing that did not account for secular trends.34 By including data through 2016 and accounting for secular trends through the difference-in-differences method, we found declines in PSA testing across ages, demonstrating the declines were generalized and not restricted to an academic practice. It is of interest that the academic practice showed greater declines in PSA testing, suggesting a closer adherence to the guidelines. Whether this is true generally, or due to other factors such as regional difference in practice patterns, should be tested by analyzing EHR extracted data in academic and non-academic settings.
We observed reductions in unadjusted proportions of prostate cancers diagnosed at early stage, particularly compared to colorectal cancers, showing that the number and proportion of low-risk prostate cancer diagnoses decreased after the guideline changes, consistent with ongoing attempts to reduce overtreatment.11,19,35,36 Data from SEER has shown that prostate cancer incidence as a whole has declined substantially in recent years,31 including early-stage cancers, although the drop in early stage cancers has become attenuated over time after 2013.37 Our study included more years following the guideline changes and suggests continued but modest declines in the proportion of early stage cancers. Future work will need to evaluate whether the upgrading of the recommendations to a C rating in 201820 will affect PSA testing rates.
Our findings suggest, at least for the controversial recommendations for prostate cancer screening, that guideline changes impact physician behavior. In addition, the decrease in diagnosis of early stage prostate cancer shows that the guidelines affect important disease characteristics in patients. Fewer low stage cancers will decrease the number of men overtreated for prostate cancer and thereby diminish the burden of treatment side effects such as incontinence and erectile dysfunction in the male population.10,11,38,39 However, some randomized trials of screening and treatment have shown mortality reductions in screened populations and in men treated, as opposed to those observed, with early stage prostate cancer.5,15 Since prostate cancer has a long natural history, changes in mortality rates in the population might not be seen for several years following guideline changes.
Our study has limitations that should be mentioned. First, we used a nonrandomized design and thus could not prove that the 2012 USPTF recommendations caused any of the observed changes in PSA testing; however, studying changes over time, using multiple datasets, and controlling for patient demographics reduces the chance that the results were confounded by variations in unobserved patient characteristics. In our analysis, we assumed that cholesterol testing patterns serve as a counterfactual that reflects secular changes in practice patterns since it is widely practiced, administered via blood test, applied in men across a spectrum of ages, and guidelines regarding its use did not change during the study period. We followed published best practices in assessing this assumption by testing for parallelism in the pre-policy period.25,26 If this assumption was inaccurate, the results of our analysis could be biased.
Conclusions
We observed declines in primary care PSA testing rates following the USPSTF’s 2012 grade D recommendation relative to cholesterol testing patterns. Coincident with this drop, we observed a decrease in the proportion of prostate cancers diagnosed at early stage. Our study shows that primary care physicians respond to guidelines changes by changing their practice patterns. Guideline adherence was not absolute, evidenced by the continued use of PSA testing, and likely reflects controversies surrounding PSA testing. As our healthcare system moves to a more efficient, patient-centered focus and guidelines and quality metrics become widespread, our findings show that providers rapidly respond to guideline recommendations. However, technological advances in screening technologies are likely to beget clinical scenarios that parallel the controversies surrounding PSA testing. It is important to consider how to best meet needs for disease screening in adults and the efficient use of health services. Further research is needed to understand the effects of the guideline change on cancer survival.
Supplementary Material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Ghani KR, Miller DC. Variation in Prostate Cancer Care. JAMA. 2015;313(20):2066–2067. doi: 10.1001/jama.2015.0607 [DOI] [PubMed] [Google Scholar]
- 3.Bell KJL, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer. 2015;137(7):1749–1757. doi: 10.1002/ijc.29538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roach M, Thomas K. Overview of randomized controlled treatment trials for clinically localized prostate cancer: implications for active surveillance and the United States preventative task force report on screening? J Natl Cancer Inst Monographs. 2012;2012(45):221–229. doi: 10.1093/jncimonographs/lgs039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Leeuwen PJ, Kranse R, Hakulinen T, et al. Impacts of a population-based prostate cancer screening programme on excess total mortality rates in men with prostate cancer: a randomized controlled trial. J Med Screen. 2013;20(1):33–38. doi: 10.1258/jms.2013.012026 [DOI] [PubMed] [Google Scholar]
- 7.Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;123(4):592–599. doi: 10.1002/cncr.30474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin RM, Donovan JL, Turner EL, et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018;319(9):883–895. doi: 10.1001/jama.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354–360. [DOI] [PubMed] [Google Scholar]
- 11.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. [DOI] [PubMed] [Google Scholar]
- 12.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyer VA , U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459 [DOI] [PubMed] [Google Scholar]
- 14.Lin K, Lipsitz R, Miller T, Janakiraman S, U.S. Preventive Services Task Force. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(3):192–199. [DOI] [PubMed] [Google Scholar]
- 15.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–942. doi: 10.1056/NEJMoa1311593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks JD. Managing localized prostate cancer in the era of prostate-specific antigen screening. Cancer. 2013;119(22):3906–3909. doi: 10.1002/cncr.28301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoag JE, Mittal S, Hu JC. Reevaluating PSA Testing Rates in the PLCO Trial. N Engl J Med. 2016;374(18):1795–1796. doi: 10.1056/NEJMc1515131 [DOI] [PubMed] [Google Scholar]
- 18.Barbosa PV, Thomas I-C, Srinivas S, et al. Overall Survival in Patients with Localized Prostate Cancer in the US Veterans Health Administration: Is PIVOT Generalizable? Eur Urol. 2016;70(2):227–230. doi: 10.1016/j.eururo.2016.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015;193(1):95–102. doi: 10.1016/j.juro.2014.07.111 [DOI] [PubMed] [Google Scholar]
- 20.Grossman DC, Curry SJ, Owens DK, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 21.Borza T, Kaufman SR, Shahinian VB, et al. Sharp Decline In Prostate Cancer Treatment Among Men In The General Population, But Not Among Diagnosed Men. Health Aff (Millwood). 2017;36(1):108–115. doi: 10.1377/hlthaff.2016.0739 [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Fedewa SA, Ma J, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314(19):2054–2061. doi: 10.1001/jama.2015.14905 [DOI] [PubMed] [Google Scholar]
- 23.Negoita S, Feuer EJ, Mariotto A, et al. Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124(13):2801–2814. doi: 10.1002/cncr.31549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchinson R, Akhtar A, Haridas J, Bhat D, Roehrborn C, Lotan Y. Testing and referral patterns in the years surrounding the US Preventive Services Task Force recommendation against prostate-specific antigen screening. Cancer. 2016;122(24):3785–3793. doi: 10.1002/cncr.30330 [DOI] [PubMed] [Google Scholar]
- 25.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. doi: 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 26.Ryan AM, Burgess JF, Dimick JB. Why We Should Not Be Indifferent to Specification Choices for Difference-in-Differences. Health Serv Res. 2015;50(4):1211–1235. doi: 10.1111/1475-6773.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seneviratne MG, Seto T, Blayney DW, Brooks JD, Hernandez-Boussard T. Architecture and Implementation of a Clinical Research Data Warehouse for Prostate Cancer. EGEMS (Wash DC). 2018;6(1):13. doi: 10.5334/egems.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Bleicher PD, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038 [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2015), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017. submission. [Google Scholar]
- 30.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. 8th ed Springer International Publishing; 2017; //www.springer.com/us/book/9783319406176. Accessed August 13, 2018. [Google Scholar]
- 31.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14(1):26–37. doi: 10.1038/nrurol.2016.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drazer MW, Huo D, Eggener SE. National Prostate Cancer Screening Rates After the 2012 US Preventive Services Task Force Recommendation Discouraging Prostate-Specific Antigen-Based Screening. J Clin Oncol. 2015;33(22):2416–2423. doi: 10.1200/JCO.2015.61.6532 [DOI] [PubMed] [Google Scholar]
- 33.Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-Specific Antigen Screening After 2012 US Preventive Services Task Force Recommendations. JAMA. 2015;314(19):2077–2079. doi: 10.1001/jama.2015.7273 [DOI] [PubMed] [Google Scholar]
- 34.Kim SP, Karnes RJ, Gross CP, et al. Contemporary National Trends of Prostate Cancer Screening Among Privately Insured Men in the United States. Urology. 2016;97:111–117. doi: 10.1016/j.urology.2016.06.067 [DOI] [PubMed] [Google Scholar]
- 35.Gandaglia G, Briganti A, Fossati N, et al. The Problem Is Not What to Do with Indolent and Harmless Prostate Cancer-The Problem Is How to Avoid Finding These Cancers. Eur Urol. 2016;70(4):547–548. doi: 10.1016/j.eururo.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 36.Polascik TJ, Passoni NM, Villers A, Choyke PL. Modernizing the diagnostic and decision-making pathway for prostate cancer. Clin Cancer Res. 2014;20(24):6254–6257. doi: 10.1158/1078-0432.CCR-14-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemal A, Ma J, Siegel R, Fedewa S, Brawley O, Ward EM. Prostate Cancer Incidence Rates 2 Years After the US Preventive Services Task Force Recommendations Against Screening. JAMA Oncol. 2016;2(12):1657–1660. doi: 10.1001/jamaoncol.2016.2667 [DOI] [PubMed] [Google Scholar]
- 38.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582–1592. [DOI] [PubMed] [Google Scholar]
- 39.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


