Abstract
Full-thickness acetabular articular cartilage defects (FAACD) are found on most hips with femoroacetabular impingement (FAI) with a wave sign in the acetabulum. When not repaired it can produce pain and catching sensation. Multiple arthroscopic techniques for repairing this chondral lesion exist, but only few show the quality of the repair on a second look. The purpose of this study is to evaluate the quality of the repaired cartilage during revision hip arthroscopy (RHA) allowing a second look in patients treated of FAACD. A total of 13 hips with FAACD repaired in the past underwent RHA for ongoing pain. Signs of persistent chondral defects or the ability to elevate the articular cartilage from subchondral bone were evaluated by zones. Those with persistent defects were re-repaired. All patients had FAACD lesions in zones I, II and III diagnosed in the index hip arthroscopy. The most common finding at the RHA was the presence of bone growth or residual impingement. Before FAACD repair, 11 (85%) hips had the wave sign, while 2 (15%) hips had it in RHA. Five (38%) hips had residual delamination in the second look, these patients had residual FAI, were ≥58 years or waited >6 months to be revised. The wave sign was not observed in 85% of the revised hips, indicating the technique was successful in most cases and was not the principal cause of their ongoing pain. This technique achieved the stated goal of stabilizing the articular cartilage seen in the wave sign.
INTRODUCTION
The articular cartilage is a highly hydrated, avascular, aneural and alymphatic tissue that provides a smooth gliding of the joints during skeletal motion [1]. The cartilage has limited capacity to repair or regenerate and when it is damaged surgical repair is needed [2, 3].
Delamination of the acetabular articular cartilage is a full-thickness detachment of the cartilage from the underlying subchondral bone with an intact chondrolabral junction surface [4–6]. The senior author (T.G.S.) has described this lesion as a ‘full-thickness acetabular articular cartilage defect’ (FAACD) [5].The FAACD produces bulging effect of the affected articular cartilage when is pressed to the adjacent labrum resulting in a ‘wave’ or ‘carpet’ sign (Fig. 1) [5–7]. This kind of lesion is commonly associated with femoroacetabular impingement (FAI) and anterosuperior labral tears [6, 7]. The common symptoms are pain and a catching sensation and if those are not repaired the cartilage may break off and become a loose body into the joint, leaving behind a substantial defect, leading to an early progression of hip osteoarthritis [4, 7–9].
Fig. 1.
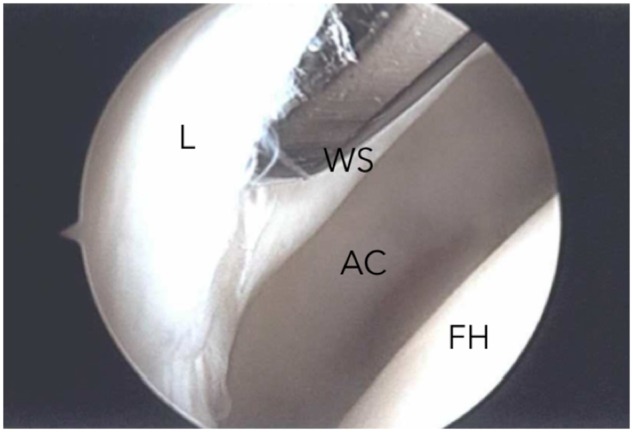
Right hip arthroscopy showing debonding of the articular cartilage with the wave sign (WS) at the acetabulum. L, Labrum; AC, acetabulum cartilage; FH, femoral head.
Histological studies have shown that between 40% and 90% of chondrocytes in delaminated flaps are still viable with the result of continued deposition of hyaline cartilage and fibrocartilage that indicates repair [10, 11]. These findings, along with positive follow-up results after refixation of the chondral defects, are encouraging when considering repair over excising the cartilage.
Previously techniques had been described to repair FAACD including chondroplasty, debridement, microfracture, use of fibrin adhesive, autologous chondrocyte implantation or bridging sutures, some of them adapted from studies of the knee [5, 7, 8, 12–14]. Some of these techniques had shown positive follow-up results and a few have reported the findings on revision arthroscopies.
A cartilage repair technique has been introduced by the senior author (T.G.S.) with the aim of fixing the FAACD. At the time of detecting the lesion, an elevation of the cartilage defect is performed; microfractures are done in the subchondral bone under the cartilage lesion and the adjacent labrum is fixed with bone anchors. This will stimulate the bone marrow to release mesenchymal stem cells to the articular lesion to heal the detachment of the cartilage supported by anchor fixation [7, 13].
The purpose of this study was to evaluate the quality of the repair during the revision hip arthroscopy (RHA) in patients with FAACD treated with our proposed technique.
METHODS
After approval by our institutional review board, a single-center retrospective review of all patients who underwent RHA for persistent symptoms after FAACD was repaired at the index hip arthroscopy between January 2013 and January 2017 was performed. Inclusion criteria were patients 18 years of age or older and persistent symptoms related to the hip or onset of further symptoms with at least 6 months of follow-up with no improvement. Patients with an index hip arthroscopy by a different surgeon or those who had incomplete data were excluded from this study. The characteristic and details of the primary diagnosis were collected from the patient’s chart. Operative reports were investigated and details such as chondral lesion zone, pattern and concomitant procedures were recorded. All patients were evaluated with history, examination and radiological findings including x-rays with standardized anteroposterior view, false profile, Dunn view and magnetic resonance imaging before RHA. At the RHA signs of persistent defects such as a wave sign or the ability to elevate the articular cartilage from the substrate acetabulum with a blunt elevator were evaluated by zones and allowed a second look of the index chondral repair [15]. Those with persistent defects were re-repaired by removing more acetabular rim and repeat the microfracture with a larger pick into the cancellous bone before refixation of the labrum.
Patient position
After anesthesia, patients were placed in lateral decubitus position. Axillary rolls, as well as extra padding for downside bony prominences and hip positioners, stabilize the pelvis. The operated leg was then set up in the hip distractor, taking care to adequately pad the foot and provide a large perineal post. The hip was then prepared and draped in standard fashion. A standard fluoroscope is positioned in an anteroposterior view (Fig. 2).
Fig. 2.
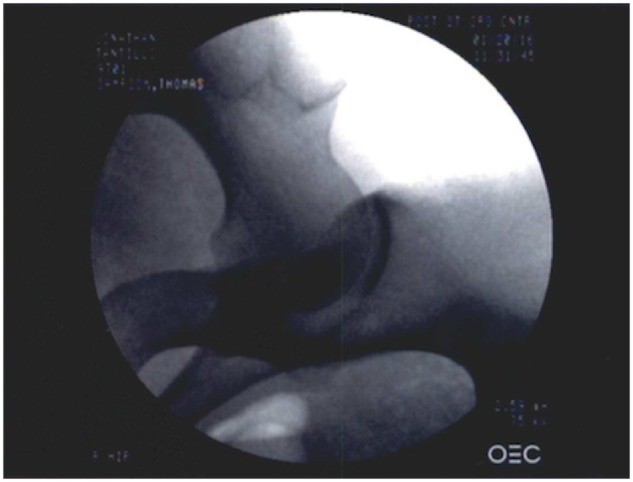
Right hip fluroscopy in an anteroposterior view for an adequate portal placement and resection of the FAI.
Portal placement
Hip arthroscopy was initiated by entering the peripheral compartment first (Fig. 3). A spinal needle and nitinol wire were used to place the anterolateral portal first followed by the mid-anterior portal, both aiming toward the superior lateral articulation of the head of the femur and acetabulum with fluoroscopic visualization. After the insertions of a 70° arthroscope and working instruments, the pump is activated, and the space above the capsule is distended to provide a view of the anterior fat pad and the reflected head of the rectus femoris. The capsulotomy is made along its anterolateral portion adjacent and through the iliofemoral ligament from the base of the neck to the acetabular rim with a 50° radiofrequency, further dissecting it laterally and anteriorly, taking care to preserve the labrum. When adequately opened, traction is applied and the arthroscope is advanced into central compartment for inspection with a probe.
Fig. 3.
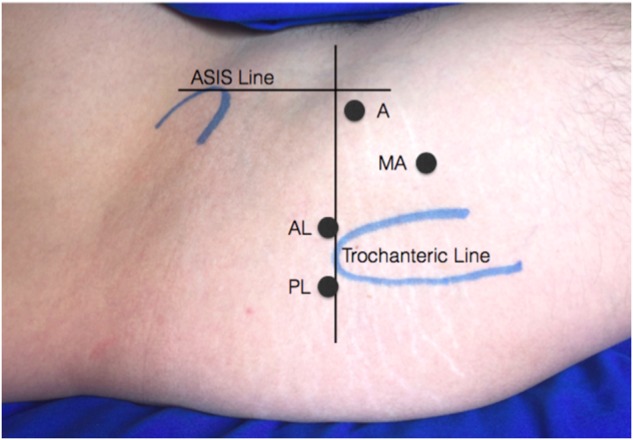
Right hip portals marking in lateral decubitus positioning. A, anterior; AL, anterolateral; PL, posterolateral; MA, Mid-anterior; ASIS, anterior superior iliac spine.
Repair technique
First examination of the central compartment of the hip is made to identify pathologies associated with CAM impingement, including articular cartilage (delamination, fibrillation, flap or fissure) and labral damage (tear or detachment). Then examination of the peripheral compartment is done by releasing the traction and flexing the hip to 45° to inspect the head–neck junction to identify the CAM impingement area. Once the CAM is defined it can be resected with a 5.5-mm-round burr to restore the head–neck junction offset. The traction is then applied and if no major cartilage damage was found and the FAACD lesion was confirmed with a blunt elevator, the pincer trimming was proceeded. Trimming of the rim with the 5.5-mm-round burr was done until the bone overhang was resected. In this step, multiple fluoroscopic view is taken to secure an adequate resection. The labrum was detached with a blunt instrument from the rim in the zones where the FAACD was identified. Care must be taken to not delaminate when inserting the blunt elevator. A rasp or curete was used to prepare the subchondral bone for bone marrow stimulation. The microfractures were performed with a 60° pick at a depth of 4 mm to promote bone bleeding and cartilage healing. PEEK knotless suture anchors (Speedlocks, Smith & Nephew Endoscopy, Andover, MA) were placed by drilling holes between the involved zones (Fig. 4). High-strength suture was placed through the labrum in a mattress configuration. The step was repeated if more anchors were needed. The hip was reduced through direct scope viewing to assess the range of motion and evaluation of labral sealing.
Fig. 4.
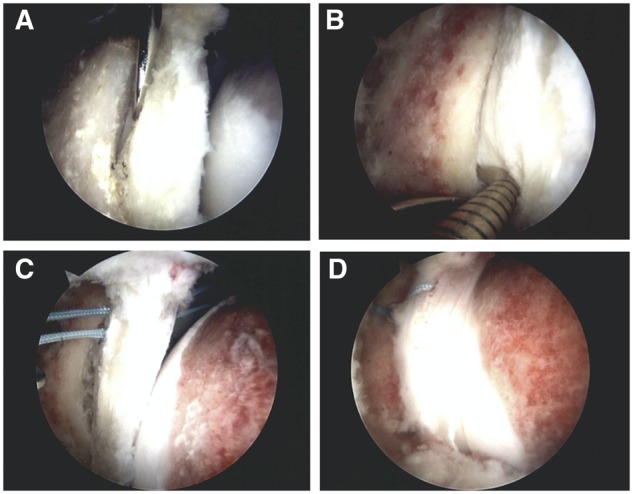
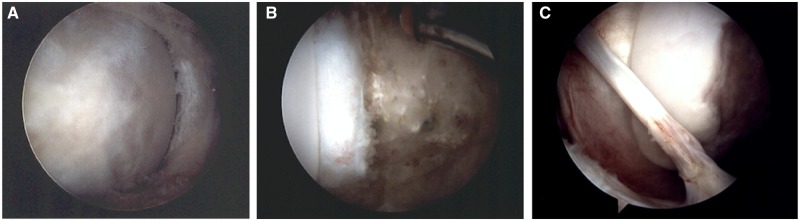
Right hip arthroscopy showing the sequence of the repair technique initiating with (A) elevation of the cartilage defect, (B) microfracture beneath through the substrate bone and (C and D) repaired using the adjacent labrum with bone anchors.
The capsule was plicated with high-strength suture using a suture passer adjacent to the orbicularis zone, tightening it. Fluroscopy was used during the procedure and at the end to confirm the amount of resected bone. Skin was sutured with nylon and a standard bulky dressing was applied during the first 24 h to absorb any leakage or blood. The dressing was removed the day after surgery and the patient was allowed to shower.
Rehabilitation protocol
Partial weight bearing was allowed with crutches increasing to full weight bearing as patient tolerate. Rehabilitation starts as soon as pain was tolerated with a stationary bicycle, followed by an elliptical trainer, with range of motion exercises and a specific rehabilitation protocol. The author typically has patients doing self-rehabilitation for the first 3 weeks and then assesses their needs for range of motion, stability and strength to determine if formal therapy is required.
RESULTS
Demographic results
A total of 47 patients were identified and analysed. Of this cohort, only 10 of them met the inclusion criteria with 3 patients involving both hip (total of 13 hips). The pre-operative diagnosis was FAI in all cases (10 patients, 13 hips) (Table I). The average age of the group was 36.3 years (SD ±15.54). Of the 10 patients, six were male, two of them had bilateral FAI with a mean age of 31.75 years (SD ±13.33). Four patients were female and one had bilateral FAI with the mean age of 43.6 years (SD ± 7.47). Seven hips were left while 6 were right for a total of 13 hips. The mean time from the index hip arthroscopy to revision hip arthroscopy was 26.3 months (SD ±13.85) (range 6–47 months).
Table I.
Findings of FAACD on revision hip arthroscopy
| Age | Delamination | Wave sign |
Defect found | Time to revision | ||||
|---|---|---|---|---|---|---|---|---|
| No. hips | Years | Zone | Index HA | Revision HA | Zone | Months | Indication for revision | Re-repaired |
| 1 | 19 | I–II–III | Yes | No | No | 13 | Adhesion, residual FAI | No |
| 2 | 19 | I–II–III | Yes | No | No | 18 | Adhesion, residual FAI | No |
| 3 | 39 | I–II–III | Yes | No | Delamination I–II | 20 | Ongoing pain, residual FAI | Yes |
| 4 | 58 | I–II–III | Yes | Yes | Delamination I–II–III–IV | 37 | Ongoing pain, residual FAI | Yes |
| 5 | 21 | I–II–III | No | No | No | 47 | Adhesion, residual FAI | No |
| 6 | 60 | I–II–III | Yes | Yes | Delamination I–II–III | 35 | Ongoing pain, residual FAI | Yes |
| 7 | 60 | I–II–III | Yes | No | Delamination I–II–III | 34 | Ongoing pain, residual FAI | Yes |
| 8 | 41 | I–II–III | Yes | No | No | 23 | Adhesion, residual FAI | No |
| 9 | 33 | I–II–III | Yes | No | No | 28 | Adhesion, residual FAI | No |
| 10 | 33 | I–II–III | Yes | No | No | 20 | Adhesion, residual FAI | No |
| 11 | 42 | I–II–III | Yes | No | No | 51 | Ongoing pain after trauma | No |
| 12 | 29 | I–II–III | Yes | No | No | 10 | Adhesion, residual FAI | No |
| 13 | 18 | I–II–III | No | No | Delamination II–III | 6 | Ongoing pain, residual FAI | Yes |
HA, hip arthroscopy; FAI, femoroacetabular impingement.
Findings in the index surgery
All hips had delamination on the anterior and anterosuperior part of the acetabulum that corresponded to zones I, II and III (Fig. 5). The wave sign was observed only in 11 hips (85%) but cartilage was able to detached easily from the adjacent subchondral bone in all operated hips indicating a hidden delamination. The FAACD was repaired in all cases.
Fig. 5.
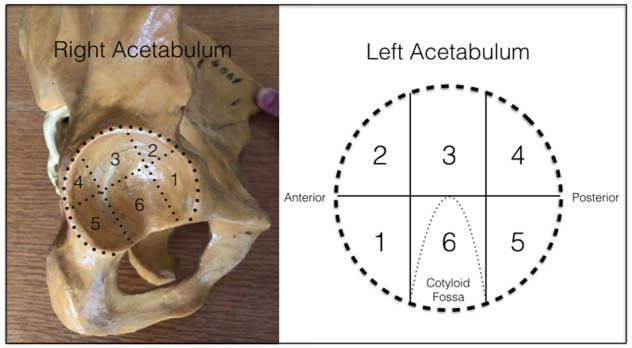
Topographic classification that divide the acetabulum in six zones using the cotyloid fossa as a reference. In this classification, zone 2 (anterolateral) and zone 3 (lateral) are areas where the acetabular cartilage damage occurs more frequently corresponding to the zone of the mechanical impact in patients with FAI or mechanical overload.
Findings in the revision surgery
During RHA, the wave sign was found in 2 (15%) hips. The repaired FAACD was stable on 8 (62%) hips when the chondrolabral junction was tested (Fig. 6). Five (38%) hips persisted with some delamination on the repaired area (Fig. 7). From those hips with persistent delamination, two had a smaller area, two the same size as in the index procedure and in one case the delamination was bigger than the initial defect. All five patients had residual FAI, three patients were 58 years of age or older when the index hip arthroscopy was performed and four of them waited >6 months for the hips to be revised. The treatment in two of the hips was only microfracture because the cartilage defect was smaller. The three remaining cases had to be re-repaired with the same technique. Either recurrent bone growth, residual conflict from impingement or adhesion was the most common cause and finding at RHA (Fig. 8).
Fig. 6.
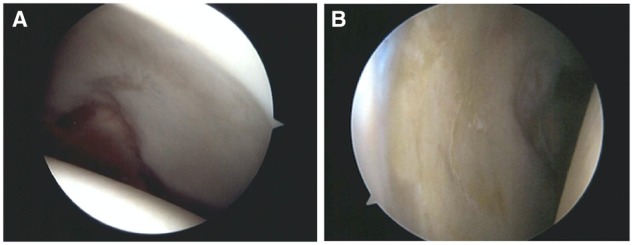
(A) Right hip on an 18-year old and (B) left hip on a 44-year old on a revision hip arthroscopy showing no sign of wave sign or delamination.
Fig. 7.
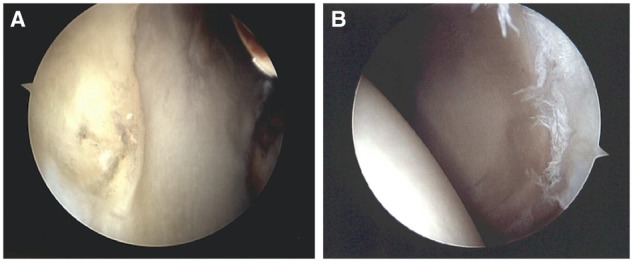
(A) Acetabulum showing a wave sign and (B) cartilage delamination on repaired FAACD with persistent pain.
Fig. 8.
(A) Recurrent bone growth on femoral head–neck junction, (B) acetabulum rim and (C) adhesion were the most common findings at the revision hip arthroscopy.
DISCUSSION
The FAACD lesion can be stabilized with different techniques. In a study of 19 patients with chondral delamination injuries of acetabular cartilage with wave sign, Tzaveas and Villar [8] managed chondral delamination lesions of the hip arthroscopically with fibrin adhesive. Nineteen patients underwent hip arthroscopy for labral tears with FAI. The overall cartilage structure was intact in all patients. The authors performed microfracture of the underlying subchondral bone and then injected fibrin adhesive under the flap, pressing down until the adhesive had set. Five patients underwent revision hip arthroscopy for multiple reasons, and the repaired chondral lesion was found to be stable in all patients. At 1-year follow-up, the mean of Modified Harris Hip Scores (MHHS) improved from 53.3 to 80.3, and the mean pain score improved from 15.7 to 28.9. Later Stafford and Bunn with the same technique showed the clinical results of 43 patients with FAI. They completed the MHHS pre- and post-operatively [7]. Average MHHS pain scores improved from 21.8 pre-operatively to 35.8 at a mean of 28 months post-operatively (P < 0.0001). MHHS functional score also improved post-operatively from 40.0 to 43.6 (P = 0.0006). No statistically significant change in scores between 1 and 3 years post-procedure was found.
Sekiya et al. [9] reported on a case of chondral delamination in a 17-year-old male athlete with FAI, an anterosuperior labral tear, and an adjacent area of delaminated acetabular articular cartilage that measured 1 cm2. This area was found to be unstable but looked healthy enough for salvage. Microfracture was performed under the flap, and the flap was sutured with absorbable polydioxanone monofilament. At 2-year follow-up, the patient reported feeling 95% normal. He scored 96 on the mHSS, 93 on the Hip Outcome Score Activities of Daily Living subscale and 81 on the Hip Outcome Score Sports subscale.
Kaya et al. [12] elaborated a technique using stitches to press and stabilize the delaminated cartilage to the subchondral bone. This novel technique reports to be a safe and simplified method for arthroscopic repair of the acetabular carpet delamination. It achieves a rigid stability of the delaminated area. Early clinical results have shown excellent pain relief and no complications. The disadvantage the study mention is the necessity of inserting the suture anchors in the medial acetabulum, which may induce iatrogenic damage to the acetabular cartilage. To minimize the risk of cartilage damage, soft anchors are used.
Articular cartilage repair is appropriate for FAACD with a wave sign. They are small lesions of 2–4 cm2 with an intact cartilage surface that can respond well with the proper management [14, 16]. Microfracture is used on cartilage lesions <4 cm2 and has been demonstrated at second-look hip arthroscopy to be an effective treatment in acetabular chondral defects [13, 17]. Philipon et al. [13] investigated the percent of fill and repair grade of microfracture lesion. The average percent fill of the acetabular chondral lesion was 91% at an average time of 1.61 years until second look. Only one patient had a poor outcome 25% of fill but was likely associated with the advanced stage of degenerative joint disease at the time of index arthroscopy. Karthikeyan et al. [17] reported in a series of 20 patients the macroscopic and microscopic appearances of repaired tissue after microfracture. At the second-look arthroscopy, the area of microfracture had a mean fill rate of 96% with macroscopically good quality repair tissue. Microscopic findings were primarily fibrocartilage with some staining of type II collagen in the region closest to the bone. Microfracture beneath the intact cartilage on the subchondral bone in our study stabilized 85% of the wave sign on the second-look arthroscopy. This is an encouraging finding that it may be due to the vascularity and proximity of multipotent mesenchymal stem cells and growth factors from the rich bone marrow of the pelvis to reach the chondral defect [18].
CAM impingement has been well-known to delaminate the articular cartilage of the acetabulum [19–21]. During flexion, the CAM slides into the anterosuperior acetabulum and induces compression and shear forces between the chondrolabral junction [19]. The labrum is stretched and pushed outwards and the cartilage is compressed and pushed centrally. In our study, 38% of the hips presented some delamination on the second look. This may be explained by the residual FAI that was the most common indication for RHA and this persistent morphological abnormality did not let a proper healing of the cartilage tissue [22, 23]. Ross et al. [24] compared computed tomography of symptomatic hips after arthroscopy with those who had undergone a successful arthroscopy and found 90% of the symptomatic group had residual osseous deformity and reduced motion compared with the asymptomatic group due to bony contact. This may also explain that patients who waited >6 months to be revised had poor cartilage healing of the repaired area.
Articular cartilage undergoes substantial changes in matrix structure, molecular composition, metabolic activity and mechanical functions during aging [25]. These age-related changes can result in impaired efficacy of cartilage repair with marrow stimulation treatments [25–27]. Our case series showed three hips of 58 years of age or older, with no healing or a greater defect of the articular cartilage. Cartilage cellular senescence has been notice to play a role in the failure of the cartilage repair on these group of patients [25]. A case report by Martinez et al. [28] described a 58-year-old woman with FAI that underwent hip arthroscopy and was found to have a degenerative labral tear, an anterosuperior chondral defect on the acetabulum and CAM impingement. The labrum was repaired with anchors, femoroplasty of CAM area was done and microfracture of the chondral defect. Two months later the patient began with increased pain on the groin area, treated with conservative management that failed. She underwent surgery 4 months later and was found to have the acetabulum chondral defect with no fill of fibrous tissue and the femoral head had cartilage separation of the subchondral bone. However, multiple studies report good to excellent outcomes scores with hip microfracture [29–31]. It appears to be an effective and safe method for the treatment of chondral defects in short- to midterm follow-up, but a limitation is that most studies of microfracture on the hip are made in patients with a mean age of 38.8 years reported in a systematic review by MacDonald [31]. More cartilage treatment studies on patients of 50 years or older may be beneficial for future management.
The strengths of this study are as follows: (i) the second look of the repaired FAACD, let us analyse directly how the cartilage responded with the treatment; (ii) it is a safe technique that can be done by any hip arthroscopy surgeon; and (iii) the hip arthroscopy was performed by 1 surgeon. The following limitations are also noticed on this study: (i) it is a retrospective review of the data of these case series; (ii) there was no control group of patients with no treatment of the FAACD lesion; (iii) the absence of clinical and functional scores did not allow to correlate the findings of the cartilage repair in the second-look arthroscopy; and (iv) it is a small sample.
CONCLUSION
In this study, 38% of the patients who had the FAACD lesion with residual hip pain demonstrated delamination in the same zones. These patients had residual FAI, were 58 years of age or older or waited >6 months to be revised. Two of the hips had a smaller area of delamination that only required revision microfracture. The wave sign was not observed in 85% of the revised hips, indicating the technique worked in most, and was not the principal cause of their ongoing pain. It also was the first evidence that repairing the FAACD lesion using this proposed technique achieved the stated goal of stabilizing the articular cartilage seen in the wave sign. Hips on patients on their fifties or older should be studied to identify if the cartilage at this age is a good candidate to be repaired and should be carefully selected. It is a safe and reproducible technique that makes an anatomic repair that will prevent progress to a torn cartilage and present early signs of osteoarthritis in the future.
ACKNOWLEDGMENTS
The author would like to thank the senior author for his wisdom and goodwill during his mentor. Also would like to thank for the assistance of every author who make this article possible.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. McCarthy JC, Noble Philip C, Villar RN.. Chapter 2: Anatomy: cartilage In: Ulici V, Chen AF, Tuan RS (eds). Hip Joint Restoration, 1st edn. New York: Springer, 2017, 15–22. [Google Scholar]
- 2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy 2000; 16: 578–87. [DOI] [PubMed] [Google Scholar]
- 3. Beck M, Leunig M, Parvizi J. et al. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res 2004; 418: 67–73. [PubMed] [Google Scholar]
- 4. Beck M, Kalhor M, Leunig M. et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87: 1012–8. [DOI] [PubMed] [Google Scholar]
- 5. Sampson TG. Arthroscopic treatment for chondral lesions of the hip. Clin Sports Med 2011; 30: 331–48. [DOI] [PubMed] [Google Scholar]
- 6. Jannelli E, Parafioriti A, Acerbi Alberto Ivone A. et al. Acetabular delamination: epidemiology, histological features, and treatment. Cartilage 2018; 10: 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stafford GH, Bunn JR, Villar RN.. Arthroscopic repair of delaminated acetabular articular cartilage using fibrin adhesive: results at one to three years. Hip Int 2011; 21: 744–50. [DOI] [PubMed] [Google Scholar]
- 8. Tzaveas AP, Villar RN.. Arthroscopic repair o f acetabular chondral delamination with fibrin adhesive. Hip Int 2010; 20: 115–9. [DOI] [PubMed] [Google Scholar]
- 9. Sekiya JK, Martin RL, Lesniak BP. Arthroscopic repair of delaminated acetabular articular cartilage in femoroacetabular impingement. Orthopedics 2009; 32: 692–6. [DOI] [PubMed] [Google Scholar]
- 10. Hembree WC, Ward BD, Furman BD. et al. Viability and apoptosis of human chondrocytes in osteochondral fragment following joint trauma. J Bone Joint Surg Br 2007; 89: 1388–95. [DOI] [PubMed] [Google Scholar]
- 11. Ball ST, Jadin K, Allen RT. et al. Chondrocyte viability after intra-articular calcaneal fractures in humans. Foot Ankle Int 2007; 28: 665–8. [DOI] [PubMed] [Google Scholar]
- 12. Kaya M, Hirose T, Yamashita T. Bridging suture repair for acetabular chondral carpet delamination. Arthrosc Tech 2015; 4: e345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Philippon MJ, Schenker ML, Briggs KK. et al. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy 2008; 24: 46–50. [DOI] [PubMed] [Google Scholar]
- 14. Mardones R, Larrain C.. Cartilage restoration technique of the hip. J Hip Preserv Surg 2016; 3: 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ilizaliturri VM Jr, Byrd JW, Sampson TG. et al. A geographic zone method to describe intra-articular pathology in hip arthroscopy: cadaveric study and preliminary report. Arthroscopy 2008; 24: 534–9. [DOI] [PubMed] [Google Scholar]
- 16. Dallich A, Rath E, Atzmon R. et al. Chondral lesions in the hip: a review of relevant anatomy, imaging and treatment modalities. J Hip Preserv Surg 2019; 6: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karthikeyan S, Roberts S, Griffin D.. Microfracture for acetabular chondral defects in patients with femoroacetabular impingement results at second-look arthroscopic surgery. Am J Sports Med 2012; 40: 2725–30. [DOI] [PubMed] [Google Scholar]
- 18. Frisbie D, Oxford J, Southwood L. et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res 2003; 407: 215–27. [DOI] [PubMed] [Google Scholar]
- 19. Beck M, Kalhor M, Leunig M. et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip . J Bone Joint Surg Br 2005; 87; 1012–8 [DOI] [PubMed] [Google Scholar]
- 20. Pfirrmann C, Mengiardi B, Dora C. et al. Cam and pincer femoroacetabular impingement: characteristic MR arthrographic findings in 50 patients. Radiology 2006; 240: 778–85. [DOI] [PubMed] [Google Scholar]
- 21. Anderson L, Peters C, Park B. et al. Acetabular cartilage delamination in femoroacetabular impingement risk factors and magnetic resonance imaging diagnosis. J Bone Joint Surg Am 2009; 91: 305–13. [DOI] [PubMed] [Google Scholar]
- 22. Shin J, de SA D, Burnham J. et al. Refractory pain following hip arthroscopy: evaluation and management. J Hip Preserv Surg 2018; 5: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sardana V, Philippon M, D de Sa. et al. Revision hip arthroscopy indications and outcomes: a systematic review. Arthroscopy 2015; 31: 2047–55. [DOI] [PubMed] [Google Scholar]
- 24. Ross J, Larson C, Adeoyo O. et al. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study . Clin Orthopaed Rel Res 2014; 473: 1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toh WS, Brittberg M, Farr J. et al. Cellular senescence in aging and osteoarthritis: implications for cartilage repair. Acta Orthopaedica 2016; 87: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinwachs M, Guggi T, Kreuz PC.. Marrow stimulation techniques. Injury 2008; 39: 26–31. [DOI] [PubMed] [Google Scholar]
- 27. Miller B, Briggs K, Downie B. et al. Clinical outcomes following the microfracture procedure for chondral defects of the knee: a longitudinal data analysis. Cartilage 2010; 1: 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mas Martinez J, Sanz Reig J, Morales Santias M. et al. Chondrolysis after hip arthroscopy. Arthroscopy. 2014; 31: 167–72. [DOI] [PubMed] [Google Scholar]
- 29. Domb BG, Gupta A, Dunne KF. et al. Microfracture of the hip: a two-year follow-up with a matched-pair control group. Orthopaed J Sports Med 2014; 2: 2325967114S00031. [Google Scholar]
- 30. Haviv Barak Singh P, Takla A, O'Donnell J.. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br 2010; 92: 629–33. [DOI] [PubMed] [Google Scholar]
- 31. Macdonald AE, A Bedi, Horner NS. et al. Indications and outcomes for microfracture as an adjunct to hip arthroscopy for treatment of chondral defects in patients with femoroacetabular impingement: a systematic review. Arthroscopy 2016; 32: 190–200. [DOI] [PubMed] [Google Scholar]