Abstract
Background
Studies investigating the effects of pain-relieving medication use on conceiving a pregnancy have shown conflicting results. Furthermore, no previous study has examined medication use around ovulation or implantation, and the associations with the probability of conception, fecundability.
Objective
To explore the association between fecundability and analgesic use in three different menstrual cycle windows – pre-ovulation, peri-ovulation and implantation – as well as across the entire menstrual cycle.
Study Design
We analyzed data from a prospective cohort study of women between 30 to 44 years of age who were trying to conceive naturally from 2008–2015. Using daily diaries, medication usage was classified as acetaminophen, aspirin, or non-aspirin nonsteroidal anti-inflammatory drug (NSAID), during four time periods of interest - pre-ovulatory, peri-ovulatory and implantation – as well as the overall non-menstrual bleeding days of the cycle. Menstrual cycles during the prospective attempt to become pregnant were enumerated using daily diary menstrual bleeding information. Conception was defined as a positive home pregnancy test. Discrete time fecundability models were used to estimate the fecundability ratio (FR) and 95% confidence interval (CI) in each of the four time windows of interest and for each pain reliever (aspirin use, non-aspirin NSAID use, acetaminophen) compared with no medication use, after adjustment for several covariates including age, race, education, body mass index, alcohol and caffeine use, frequency of intercourse, and a history of migraines or uterine fibroids.
Results
Medication use was infrequent in the 858 women and 2366 cycles in this analysis. Use of non-aspirin NSAIDs or acetaminophen were not associated with fecundability in any of the time windows of interest. Although the sample size was small, aspirin use during the implantation window was associated with increased fecundability (adjusted FR(CI): 2.05 (1.23, 3.41). This association remained when limiting the analysis to cycles with minimal missing data or when adjusting for gravidity. None of the other medications were associated with fecundability.
Conclusion
Aspirin use around implantation was associated with increased fecundability. These results expand previous literature to suggest that: 1) implantation may be an important target for the effects of aspirin on conception and 2) aspirin may be beneficial regardless of pregnancy loss history. These observations should be tested with a clinical trial.
Keywords: pain medication, pain reliever, ovulation, implantation, fertility, time to pregnancy, conception, NSAID, acetaminophen, aspirin
Condensation
Aspirin use during the implantation window was associated with increased probability of conception. Use of other NSAIDs or acetaminophen was not associated with probability of conception.
Introduction
The three most commonly used drugs in the US, based on weekly prevalence of consumption, are over-the-counter pain relieving medications, also known as analgesics.1 In a study of over 10,000 participants, 60% of pregnant women reported using over-the-counter pain-relieving medications three months before conception.2 The most common analgesic taken in the 3 months before pregnancy was acetaminophen (48%), followed by ibuprofen (21%), a nonsteroidal anti-inflammatory drug (NSAID).2 NSAIDs are a class of pain-relieving medications that includes ibuprofen, naproxen, and aspirin. NSAIDs act as analgesics by inhibiting the enzymes that synthesize prostaglandins (PG).3 Pre-ovulatory increases in prostaglandin levels are essential for reproduction as they enable ovulation4 and implantation.5 These connections between reproductive function, prostaglandins, and NSAIDs provide biologic plausibility for an effect on fecundability.
On the other hand, acetaminophen, the most commonly used non-NSAID analgesic2, does not have the anti-inflammatory properties of NSAIDs,6 and does not appear to inhibit any COX pathways. Thus, acetaminophen would not be expected to show an association with female fecundability. This has been observed in two studies.7,8 In one study,8 women who used the NSAID naproxen while trying to conceive had lower fecundability than women who did not use any analgesic medications, while other NSAIDs such as aspirin and ibuprofen, were not associated with fecundability. This conflicts somewhat with the findings of a randomized trial of aspirin which found that preconception-initiated low dose aspirin increased fecundability by 28%.9 In this latter study, the association was attributed to the beneficial effect of aspirin on vasodilation and increased blood flow to the uterus. The difference between the two studies may be due to their underlying populations: the latter study only enrolled women with a history of pregnancy loss while the former study enrolled women regardless of their pregnancy history.
Given the conflicting results from the prior two human studies, the large percentage of reproductive age women who take over-the-counter pain medications, and the biological plausibility of the effects of analgesic medications, further research is needed to investigate NSAIDs and other pain-relieving medications and their associations with fecundability. Furthermore, no previous study has examined the timing of medication use during a given menstrual cycle, for example around ovulation or implantation, and the corresponding associations with fecundability. Analgesic medications may have specific effects on these reproductive events. Thus, the primary objective of this study was to explore the association between fecundability and analgesic use in three different menstrual cycle windows – pre-ovulation, peri-ovulation and implantation – as well as the non-bleeding days of the entire menstrual cycle.
Materials and Methods
Study population
The Time to Conceive (TTC) study was a prospective cohort study conducted in the Triangle area of North Carolina from 2008–2015 of women with no history of infertility, between 30 to 44 years of age, who were trying to conceive for three months or less at study recruitment.10 Women reported the amount of time they had been “having regular intercourse without doing anything to prevent pregnancy”. Women intending to become pregnant were recruited via introductory letters and emails as well as radio and web advertising. Participants completed a self-administered baseline questionnaire that elicited information on behavioral habits, sociodemographic background, and reproductive and contraceptive history. Women were excluded from the study if they reported a history of infertility, polycystic ovarian syndrome, or endometriosis, or if they had a partner with infertility, or if they were currently breastfeeding. They were also asked to schedule a study visit on days 2–4 of their next menses. During the study visit the women provided a blood sample and written informed consent. They were also given pregnancy tests and ovulation tests, and instructions to test for pregnancy every third day starting on day 28 of the menstrual cycle.
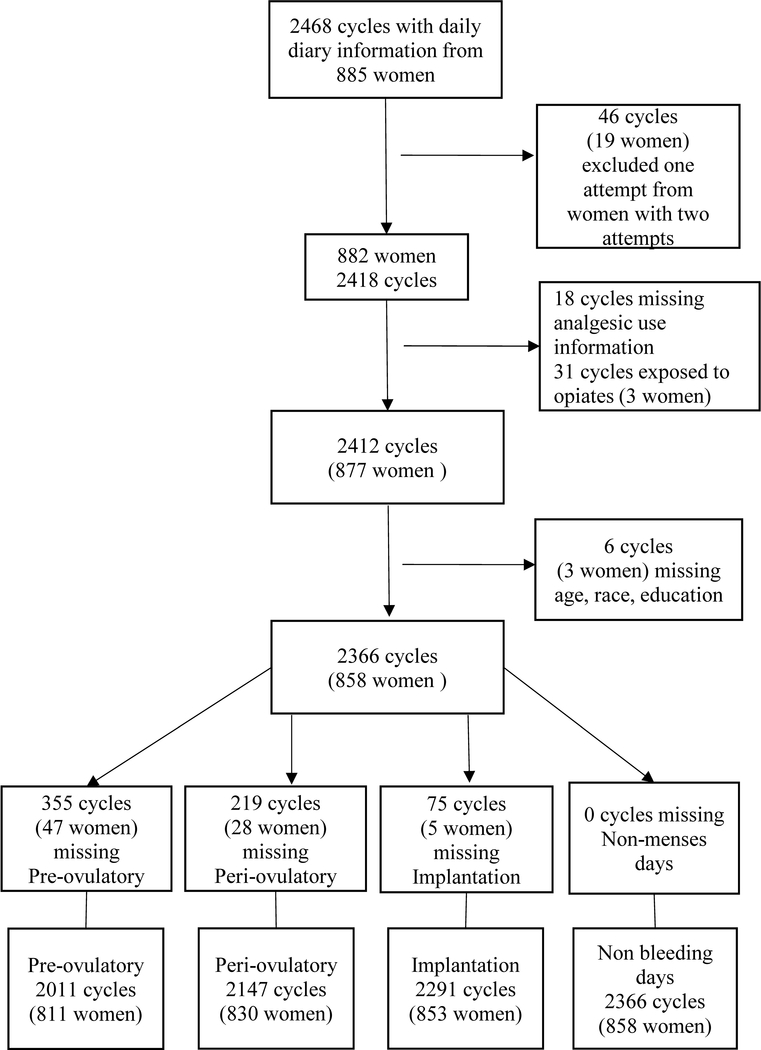
For up to four months, participants kept daily diaries in which they recorded their menstrual bleeding, the results of their ovulation and pregnancy tests as well as their medication usage (described below). They were withdrawn from the study if they began fertility treatment or stopped trying to conceive. There was a total of 2468 cycles from 885 women recorded in the daily diaries (Figure 1).
Figure 1.
Flowchart of women and cycles in the analytical sample.
Nineteen women participated in Time to Conceive more than once, and therefore had two attempts recorded in the study. Because this number was too small to support a clustered analysis, we randomly selected one of the women’s attempts to be included in the analysis (excluding 19 observations and 46 cycles, Figure 1).
Study activities were approved by the Institutional Review Board at the University of North Carolina (#08–0423).
Ovulation definition
Ovulation was defined using the participants’ daily diary recording of ovulation test results, cervical mucus monitoring, or basal body temperature. Ovulation test kits were distributed to the participants starting in 2013 and, prior to this year, if participants voluntarily purchased kits they were able to record their test results, however, the kits were not frequently used, and ovulation information was often missing for these earlier cycles. If cycles were missing ovulation information, ovulation was assigned as day 15 of the cycle, the mode for the entire study sample when measured by ovulation predictor kit.
Time windows of exposure
Using the assigned day of ovulation, the “pre-ovulation” window included days −5 to −1 where 0 is the day of ovulation. This corresponds to the fertile window of the menstrual cycle11 excluding day of ovulation. The day of ovulation was excluded from this window because ovulation can sometimes cause pain, which may lead to analgesic use. However, since ovulation itself may be an important time window to consider, the “peri-ovulatory” window was defined to include days 0 to +4. The “implantation” window included days 6 through 12, corresponding to the previously reported window of implantation.12 Finally, to characterize the entire menstrual cycle, the “non-menstrual” time window included all the non-menstrual bleeding days of the menstrual cycle. Menstrual bleeding days were excluded due to their likely correlation with pain and pain medication use.
Assessment of medication usage
In the daily diary, women reported any vitamins, supplements and/or medications that they had taken that day (time of day was not recorded) by entering the drug name or active ingredient in a search box. The search box retrieved results from the Cerner Multum drug database.13 Participants could then select the correct medication from the retrieved results. Two authors (PP and AMZJ) reviewed all the reported medications from each participant and identified the active ingredients in each. Pain relieving medications were classified as acetaminophen, aspirin, non-aspirin NSAIDs (ibuprofen and naproxen), and opioids. Because some medications contain more than one active ingredient, these are overlapping categories. Medication use was assessed as any or none in the four previously described time windows of interest. Opioid use was rare in this cohort; it could not be examined as an independent exposure. Thus, 31 cycles and 3 women who reported opioid use were excluded from further analyses.
The three drug exposures of interest (acetaminophen, aspirin, and non-aspirin NSAIDs) were analyzed in each of the four time windows of interest (pre-ovulatory, peri-ovulatory, implantation and the non-menstrual bleeding days of the cycle) to make 12 dichotomous exposure variables (use versus non-use in each time window). We could not calculate the exact dosage taken per day because the diary records did not include the number of pills taken at a time or the number of times the medication was taken per day. Additionally, 19 cycles were missing analgesic use information and were also excluded. This left 882 women and 2418 cycles eligible for analysis.
Assessment of pregnancy and attempt time
Pregnancy was defined as a positive home pregnancy test and women were instructed to inform study staff of a positive test. Women were offered a free early ultrasound to encourage notification of pregnancy results. The cycle of attempt at enrollment was determined using answers from the self-administered baseline questionnaire. Time since discontinuation of contraception was divided by the reported usual cycle length to quantify the cycle of attempt at enrollment. While women self-reported the amount of time they had been trying to conceive on the phone screen, this quantity was not always equivalent to our assessment of the cycle of attempt at enrollment because, 1) months and cycles are not the same, 2) the women’s reports may differ from our exact quantification based on these reported dates, and 3) women may call to join the study, but then not fully enroll for another 1–2 cycles. Women who had tried to conceive for more than 6 menstrual cycles at study entry according to the baseline questionnaire were excluded. Menses information from daily diaries was used to enumerate subsequent menstrual cycles from enrollment until either a positive pregnancy test or four months had elapsed whichever came first.
Covariates
Variables examined as potential confounders included age at the beginning of each menstrual cycle and several self-reported variables: race (Caucasian, African-American, other), gravidity (yes/no), body mass index, education, a self-reported history of migraines (yes/no), and a self-reported history of uterine fibroids (yes/no). Prospective monthly reports of average daily caffeine intake (drinks per day) and average alcohol intake (drinks per month) were matched with menstrual cycles to characterize behavior during each menstrual cycle. If a monthly report was missing an adjacent report was used, with preference for a previous report. If no monthly reports were available, the values reported on the baseline questionnaire were used. The estimated day of ovulation was used to define the fertile window as previously described (see “Ovulation definition”). We used this information in conjunction with sexual intercourse information from the daily diary to calculate the frequency of sexual intercourse during the fertile window (the 6-day window up to and including ovulation).
Statistical analysis
We examined the unadjusted associations between pain medication use in each time window of the first menstrual cycle of observation and characteristics of the women using a frequency table (Table 1). Discrete-time, time-to-event models were used to estimate the associations between medication variables and covariates with fecundability. Log-binomial regression with conception as the outcome, and the attempt cycle number as a predictor, was used to estimate fecundability ratios (FR) and 95% confidence intervals (CI).
Table 1.
Distribution of baseline characteristics stratified by analgesic use in the first menstrual cycle of observation among women in the Time to Conceive cohort, 2008–2016 (N=858 a).
| Medication use in the first observed cycle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Acetaminophen | Aspirin | NSAID | None | ||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| 150 | 17 | 10 | 1 | 33 | 4 | 684 | 78 | |||
| Age (years) | ||||||||||
| 29 – 30 | 176 | (21) | 28 | (19) | 2 | (20) | 5 | (15) | 141 | (21) |
| 31 – 32 | 262 | (31) | 46 | (31) | 3 | (30) | 13 | (39) | 200 | (30) |
| 33 – 35 | 233 | (27) | 43 | (29) | 1 | (10) | 7 | (21) | 182 | (27) |
| 36 – 40 | 160 | (19) | 25 | (17) | 1 | (10) | 8 | (24) | 126 | (19) |
| >40 | 27 | (3) | 5 | (3) | 3 | (30) | 0 | 0 | 19 | (3) |
| Race | ||||||||||
| African-American | 79 | (9) | 7 | (5) | 1 | (10) | 0 | 0 | 71 | (11) |
| Caucasian | 664 | (77) | 132 | (90) | 7 | (70) | 29 | (88) | 496 | (74) |
| Other | 115 | (13) | 8 | (5) | 2 | (20) | 4 | (12) | 101 | (15) |
| Education | ||||||||||
| Some college or less | 62 | (7) | 8 | (5) | 0 | 0 | 3 | (9) | 51 | (8) |
| College graduate | 164 | (19) | 24 | (16) | 2 | (20) | 6 | (18) | 132 | (20) |
| Some graduate school or Master’s degree | 413 | (48) | 81 | (55) | 4 | (40) | 17 | (52) | 311 | (47) |
| Doctoral degree (MD, PhD) | 219 | (26) | 34 | (23) | 4 | (40) | 7 | (21) | 174 | (26) |
| Body mass index (kg/m3) | ||||||||||
| <20 | 103 | (12) | 15 | (10) | 1 | (10) | 3 | (9) | 84 | (13) |
| 20 – 25 | 454 | (53) | 84 | (57) | 5 | (50) | 17 | (52) | 348 | (52) |
| >25 – 30 | 174 | (20) | 26 | (18) | 3 | (30) | 10 | (30) | 135 | (20) |
| >30 | 126 | (15) | 21 | (14) | 1 | (10) | 3 | (9) | 101 | (15) |
| Nulligravid | ||||||||||
| No | 424 | (49) | 62 | (42) | 7 | (70) | 16 | (48) | 339 | (51) |
| Yes | 434 | (51) | 85 | (58) | 3 | (30) | 17 | (52) | 329 | (49) |
| Frequency of intercourse in the fertile window (days) | ||||||||||
| 0 | 314 | (37) | 28 | (19) | 2 | (20) | 7 | (21) | 277 | (41) |
| 1 | 96 | (11) | 27 | (18) | 0 | 0 | 7 | (21) | 62 | (9) |
| 2 | 172 | (20) | 36 | (24) | 2 | (20) | 7 | (21) | 127 | (19) |
| 3 | 143 | (17) | 28 | (19) | 3 | (30) | 6 | (18) | 106 | (16) |
| 4 | 70 | (8) | 14 | (10) | 1 | (10) | 5 | (15) | 50 | (7) |
| 5 or 6 | 63 | (7) | 14 | (10) | 2 | (20) | 1 | (3) | 46 | (7) |
| Alcoholic drinks per month | ||||||||||
| 0 – 1 | 308 | (36) | 41 | (28) | 4 | (40) | 10 | (30) | 253 | (38) |
| 2 – 8 | 274 | (32) | 54 | (37) | 2 | (20) | 9 | (27) | 209 | (31) |
| 9 – 60 | 273 | (32) | 50 | (34) | 4 | (40) | 13 | (39) | 206 | (31) |
| Caffeinated drinks per day | ||||||||||
| 0 | 141 | (16) | 15 | (10) | 2 | (20) | 4 | (12) | 120 | (18) |
| 1 | 409 | (48) | 66 | (45) | 4 | (40) | 13 | (39) | 326 | (49) |
| 2 | 235 | (27) | 47 | (32) | 2 | (20) | 12 | (36) | 174 | (26) |
| >2 | 72 | (8) | 19 | (13) | 2 | (20) | 3 | (9) | 48 | (7) |
| History of migraines | ||||||||||
| Yes | 220 | (26) | 54 | (37) | 3 | (30) | 9 | (27) | 154 | (23) |
| No | 638 | (74) | 93 | (63) | 7 | (70) | 24 | (73) | 514 | (77) |
| History of fibroids | ||||||||||
| Yes | 31 | (4) | 4 | (3) | 1 | (10) | 1 | (3) | 25 | (4) |
| No | 827 | (96) | 143 | (97) | 9 | (90) | 32 | (97) | 643 | (96) |
Variables that don’t sum to 858 are due to missing values.
NSAID: Nonsteroidal anti-inflammatory drug
We independently evaluated the associations between several parameterizations of the continuous variables: age, body mass index, caffeine and alcohol with fecundability after adjustment for age, race, and education. We used the Akaike information criterion (AIC) to choose the best-fitting parameterization of each variable. This led to using linear, continuous variables for age and alcohol while BMI was categorized as obese or non-obese and caffeine was divided into four categories.
We examined the associations between fecundability and use of each pain reliever (aspirin use, non-aspirin NSAID, and acetaminophen) in each of the four time windows (pre-ovulatory, peri-ovulatory, implantation and non-menstrual days) using separate multivariable models for each time window, all three medication variables were mutually adjusted for within a given time window. We present models with two different adjustment sets. One adjustment set (“minimal adjustment) included age, race, and education, and the second adjustment set (“full adjustment”) included age, race, education, obesity, alcohol, caffeine, frequency of intercourse in the fertile window, a history of migraines, and history of uterine fibroids. Observations missing covariate information were excluded. To address confounding by indication, the full adjustment set includes self-reported conditions that might cause pain (history of migraines and history of uterine fibroids). Additionally, we adjusted for frequency of intercourse in the fertile window which may also be the result of the underlying pathology that causes pain. Frequency of intercourse may be either a confounder or a causal mediator and thus we present models with and without this adjustment.
Both the minimal and full adjustment sets included the cycle number of attempt, which accounts for left censoring and delayed entry into the risk-sets. The FR is a measure of the per-cycle probability of conception when comparing exposed (the drug of interest in the window of interest) with unexposed (no medication use in that window). An FR less than 1.0 indicates reduced fecundability in the exposed group while an FR greater than 1.0 indicates increased fecundability.
Sensitivity analyses
First, there may be carryover of an effect of medication use in one window on the outcome of another time window, and correlation between the use of a given medication across time windows. If an association was found with a given medication and time window, we further examined the association after mutually adjusting for that same medication’s use in the other time windows. Second, because the medication use variables are calculated across several days within a time window, they can be influenced by days women did not complete the daily diary. To examine the influence of these missing days, we performed a sensitivity analysis limited to cycles in which at least 4 days of each exposure window were recorded. For the non-menstrual window, we limited the analysis to cycles in which at least 13 days were recorded in the daily diary. Third, we examined the influence of adjusting for gravidity and in a separate analysis excluded women who had previously been pregnant, for comparison with previous research9. Fourth, we calculated a Bonferroni adjusted confidence interval to account for multiple comparisons. Fifth, we adjusted for partner characteristics. Sixth, we randomly excluded one attempt from the 19 women who provided more than one attempt during the study period. We confirmed with within-cluster resampling14 that this exclusion did not alter the findings of our fully adjusted model. And finally, we used multiple imputation to determine the sensitivity of our results to missing ovulation data or other missing variables (frequency of intercourse, medication use variables, and other variables)15. For this imputation we allowed ovulation to be missing instead of assigning day 15.
Results
Of the 858 women in our analyses, 596 eventually conceived, 69 were lost to follow-up, 110 completed 12 cycles of follow-up with no conception, 83 women withdrew either to take fertility medications, because they stopped trying to conceive, or they moved. Of the 858 women, 478 conceived during their daily diary participation and 327 of these conceived in their first three cycles of attempt.
Baseline characteristics
Characteristics of the 858 women included in this analysis, stratified by pain medication use in the first menstrual cycle of observation, are shown in Table 1. Pain medication use was infrequent in the first menstrual cycle of observation, with 78% unexposed to any of the medications of interest (acetaminophen, aspirin, non-aspirin NSAID). Acetaminophen was taken most often (17%) followed by non-aspirin NSAIDs (3.6%) and aspirin (1.2%). A majority of participants were Caucasian and highly educated, with approximately three quarters of participants in each medication category having some graduate education or higher. One-third of the women were overweight or obese, and aspirin and NSAID users were slightly less frequently obese. Half of the participants were nulligravid, and aspirin users were more likely to have previously been pregnant. Caffeine and alcohol consumption were slightly higher in women who used medications. Medication use was more common for women with a history of migraines.
Multivariate Analysis
While the number of cycles was small, aspirin use in the implantation window was associated with increased fecundability (FR (95% CI): 2.05 (1.23, 3.40) (Table 2). Aspirin in the other time windows was not associated with fecundability. Of the 47 cycles contributed by the 26 women who reported using aspirin in at least one time window, only 15 cycles had aspirin use in all three time windows. Other NSAIDs were not associated with fecundability in any of the time windows of interest. Excluding the 12 cycles to current smokers did not alter these results (FR(CI): 1.95 (1.17, 3.25)).
Table 2.
Associations between analgesic medication use at three specific time points of the menstrual cycle and fecundability in the Time to Conceive cohort, 2008–2016
| Time windows | N of cyclesa | N of conceptions | Medications | Minimally adjusted FR b (95% CI) | Fully adjusted FRc (95% CI) |
|---|---|---|---|---|---|
| Pre-ovulatory | 146 | 35 | Acetaminophen | 1.08 (0.77, 1.51) | 1.02 (0.73, 1.44) |
| 26 | 6 | Aspirin | 1.15 (0.57, 2.32) | 1.05 (0.53, 2.09) | |
| 145 | 34 | NSAID | 1.04 (0.74, 1.46) | 1.03 (0.73, 1.46) | |
| 1773 | 370 | None | 1 | 1 | |
| Peri-ovulatory | 148 | 35 | Acetaminophen | 1.05 (0.74, 1.49) | 0.97 (0.68, 1.37) |
| 30 | 7 | Aspirin | 1.18 (0.61, 2.27) | 1.20 (0.62, 2.29) | |
| 151 | 36 | NSAID | 1.08 (0.77, 1.52) | 1.15 (0.82, 1.62) | |
| 1904 | 376 | None | 1 | 1 | |
| Implantation | 180 | 49 | Acetaminophen | 1.25 (0.92, 1.69) | 1.16 (0.85, 1.59) |
| 25 | 9 | Aspirin | 1.97 (1.19, 3.27) | 2.05 (1.23, 3.41) | |
| 146 | 37 | NSAID | 1.09 (0.77, 1.54) | 1.11 (0.79, 1.57) | |
| 2032 | 377 | None | 1 | 1 | |
| Non- Menstrual Days | 405 | 96 | Acetaminophen | 1.17 (0.93, 1.48) | 1.14 (0.90, 1.44) |
| 58 | 14 | Aspirin | 1.14 (0.72, 1.82) | 1.11 (0.70, 1.75) | |
| 341 | 74 | NSAID | 0.93 (0.72, 1.21) | 0.96 (0.74, 1.25) | |
| 1813 | 348 | None | 1 | 1 |
Categories are not mutually exclusive and do not sum to the total number of cycles
Minimally adjusted model contains all medication variables, age, race and education
Adjusted model is the minimal model plus frequency of intercourse in the fertile window, body mass index, caffeine intake, alcohol intake, and a history of migraines or uterine fibroids.
To further investigate whether the association between aspirin and fecundability was specific to implantation, we used generalized estimating equations to estimate the association between pre-ovulatory aspirin use and ovulation timing based only on ovulation test kit information. We did not find associations with either long (>17 days, N=128) or short (<12 days, N=69) follicular phases, (Risk Ratio (RR) (CI): 1.4 (0.68, 2.8) and 0.58 (0.08, 3.9), respectively). There was some suggestion of increased risk of a long follicular phase with pre-ovulatory acetaminophen use (RR(CI): 1.5 (1.0, 2.1).
Sensitivity Analyses
Simultaneous adjustment for aspirin use in the pre- or peri-ovulatory time windows also did not change our interpretation of aspirin in the implantation window (FR (CI): 2.91 (1.55, 5.44)). When limited to cycles with minimal missing data, aspirin use in the implantation window was still strongly associated with fecundability, FR (CI): 1.89 (1.10, 3.26) (Table A1). Adjustment for gravidity did not alter our interpretations (FR (CI): 1.73 (1.01, 2.97)) (Table A1). A previous study suggested an association between aspirin use and increased fecundability among women with a history of pregnancy loss9. To explore whether our observed association was driven by women who had been previously pregnant, we excluded women who had previously been pregnant. This exclusion did not alter the observed association (FR (CI): 2.63 (1.04, 6.63)), suggesting that aspirin may benefit women who have not previously conceived.
Adjusting the confidence interval with a Bonferroni correction for multiple comparisons did not alter our interpretation (FR: 2.05, 99.6% CI: 0.97, 4.32). Adjustment for partner age, education, obesity, and current smoking did not alter the implantation aspirin results: FR(CI): 1.95 (1.17, 3.25).
The results of our analysis based on within-cluster resampling (to account for the 19 women with two pregnancy attempts in our study) were consistent with the results presented in Table 2, (FR (CI): 2.04 (1.23, 3.39). (Table A2). Further, the results of the multiple imputation analysis also support the results in Table 2 (Table A2).
Comment
Principal Findings
In this prospective cohort study of women aged 30–44 years, use of non-aspirin NSAIDs and acetaminophen were not appreciably associated with fecundability in any of the windows of interest – pre-ovulatory, peri-ovulatory, implantation or the non-menstrual days of the cycle. Although medication use was rare, aspirin use in in the implantation window was associated with higher fecundability. This association was robust to sensitivity analyses.
Results – what is known?
The NSAID indomethacin, available only by prescription, has been associated with delayed ovulation and is used to prevent ovulation during natural in vitro fertilization (IVF) cycles16. Ibuprofen on the other hand, which was the most commonly reported non-aspirin NSAID in our study, did not cause delayed ovulation or altered luteal progesterone in a small (N=11), randomized, placebo-controlled trial.17 It is possible then, that different NSAIDs have specific mechanisms of action, and therefore differing associations with ovulation and fertility.
The Effects of Aspirin on Gestation and Reproduction (EAGeR) trial explored the association between preconception aspirin use and fecundability in women between 18–40 years old with history of 1 or 2 prior pregnancy losses.9 Women with one early pregnancy loss in the previous year who were randomized to low dose aspirin (LDA) treatment had an increased rate of positive pregnancy tests, and a shorter time to pregnancy, compared to women receiving placebo (FR 1.28, 95% CI: 1.02, 1.62). The EAGeR trial’s study population was younger than ours and they had all been previously pregnant, compared to the mix of gravidity in the participants in our study. Despite this, the EAGeR trial’s findings are consistent with our results.
Our aspirin results and those of the EAGeR trial contrast with the findings of McInerney et al. who did not find an association between aspirin use and fecundability (FR: 1.00, 95% CI: 0.80–1.25),).8 However, when limited to women who only took aspirin, there was some evidence of increased fecundability FR: 1.27 (95% CI: 0.82–1.97). Thus, the use of more than one medication, each with different effects on the likelihood of conception, may influence the overall observed associations.
McInerney et al. reported a significant decrease in fecundability among women who used the NSAID naproxen (FR 0.71 95% CI: 0.57–0.89).8 However, ibuprofen was not associated with fecundability (FR 1.00, 95% CI: 0.89–1.11).8 Naproxen use was rare in our study population; thus, we were unable to examine it as a single exposure. In our study, NSAIDs other than aspirin were not associated with fecundability. Ibuprofen was the most commonly reported non-aspirin NSAID in our population, which would agree with the results from McInerney et al. The differing effects on fecundability of each NSAID (aspirin, naproxen, ibuprofen) could be because various NSAID medications inhibit prostaglandin synthesizing enzymes to differing degrees18. Moreover, aspirin modifies the metabolism of prostaglandin precursors such that alternative metabolites are produced18. The biological significance for fecundability of these alternative metabolites is unknown.
Clinical implications
Women are often advised to avoid NSAIDs in the peri-ovulatory period when trying to conceive. Our results suggest that this avoidance may be unnecessary, particularly for ibuprofen, but further research of specific NSAIDs is warranted.
The use of aspirin in the implantation window was associated with increased fecundability. It has been hypothesized that one of the reasons for higher fecundability in the implantation window is due to aspirin’s vasodilatory and anti-inflammatory properties. These properties are thought to improve implantation rates in women undergoing IVF19, although IVF studies are conflicting regarding aspirin and fecundability20–23. Low dose aspirin was also shown to improve fertility in women with polycystic ovary syndrome24.
Our results expand the previous finding to suggest that 1) the implantation window may be an important time window for the effects of aspirin on conception and 2) aspirin may also be beneficial to women who are nulligravid, and/or have no history of pregnancy loss.
Research implications
Our results suggest no association of non-aspirin NSAIDs with fecundability but given the low prevalence of use in our study, further research is warranted of specific NSAIDs and their mechanisms of action. Future studies also should investigate the implantation window as a target for aspirin’s effects on conception and implantation.
Strengths and Limitations
Medication use was rare in this population leading to small numbers of women and cycles in certain strata. The number of pills or the number of times per day that a woman took a medication was not recorded. We did not have any information on the reason for taking analgesic medications. To address confounding by indication, we adjusted for self-reported conditions that might cause pain (history of migraines and history of uterine fibroids). Additionally, we adjusted for frequency of intercourse in the fertile window which may also be the result of the underlying pathology that causes pain. These adjustments did not alter our results. Our assessment of ovulation may have been misclassified. We would not expect any errors in our ovulation assessment to be related to medication use or time to pregnancy, and thus are non-differential. Non-differential misclassification may have reduced our ability to detect associations. The sensitivity analysis that included multiple imputation of all missing variables did not change our conclusions.
There are also strengths of this study. While medication use was rare, the sample size was large, including over 800 women and more than 2000 cycles. This is the first study to look at analgesic use in specific time windows of interest (pre-ovulatory, peri-ovulatory and implantation) with fecundability. Furthermore, having data from daily diaries decreases the likelihood of recall bias or exposure misclassification. Having the authors review and categorize all reported medications, masked to outcome and based on identified active ingredients also helped with proper classification of exposure.
Conclusions
The use of aspirin in the implantation window was associated with increased fecundability. Future clinical trials should investigate the implantation window as a target for aspirin’s effects on conception and implantation.
AJOG at a Glance.
A. Why was the study conducted?
Analgesic medications may influence ovulation or implantation and medication use around these events may influence the probability of conception.
B. What are the key findings?
Aspirin use around the time of implantation was associated with increased fecundability. Use of other NSAIDs or acetaminophen was not associated with probability of conception.
C. What does this study add to what is already known?
These results suggest that: 1) implantation may be an important target for the effects of aspirin on conception and 2) aspirin may also be beneficial to women who do not have a history of pregnancy loss.
Acknowledgements
We appreciate comments on an earlier draft of this manuscript provided by Drs. Kristen Moore and Donna Baird. We thank Dr. Clarice R. Weinberg for providing statistical advice. This research was supported the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award number R01HD067683 and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Financial support
This research was supported the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award number R01HD067683 and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Z01ES103333–01. Ovulation predictor kits were generously donated to A.M.J. by Swiss Precision Diagnostics. The funding sources had no role in the design, collection, analysis, or interpretation of these data.
This study was conducted in Raleigh, Durham, Chapel Hill, North Carolina.
Appendix
Table A1.
Sensitivity analyses of the fully-adjusted associations between analgesic use and fecundability in the Time to Conceive cohort, 2008–2016.
| Time windows | Medicationsa | FRb (95% CI) | FRc (95% CI) |
|---|---|---|---|
| Pre-ovulatory | Acetaminophen | 1.03 (0.73, 1.44) | 1.09 (0.78, 1.53) |
| Aspirin | 1.04 (0.52, 2.06) | 1.02 (0.51, 2.01) | |
| NSAID | 1.03 (0.73, 1.44) | 0.96 (0.68, 1.37) | |
| Peri-ovulatory | Acetaminophen | 0.94 (0.66, 1.34) | 1.01 (0.71, 1.44) |
| Aspirin | 1.20 (0.62, 2.29) | 1.14 (0.60, 2.16) | |
| NSAID | 1.14 (0.81, 1.62) | 1.09 (0.77, 1.54) | |
| Implantation | Acetaminophen | 1.15 (0.84, 1.59) | 1.27 (0.92, 1.74) |
| Aspirin | 1.89 (1.10, 3.26) | 1.73 (1.01, 2.97) | |
| NSAID | 1.13 (0.79, 1.64) | 1.07 (0.75, 1.51) | |
| Non-Bleeding Days | Acetaminophen | 1.14 (0.90, 1.44) | 1.19 (0.94, 1.51) |
| Aspirin | 1.10 (0.70, 1.75) | 1.14 (0.72, 1.80) | |
| NSAID | 0.97 (0.74, 1.25) | 0.93 (0.71, 1.21) |
The reference group for each medication are cycles in which none of that medication was reported for that particular window.
Sensitivity analysis of the full model, limiting each exposure variable to cycles in which at least four days of the corresponding time window was observed in the daily diary.
Sensitivity analysis of the full model, adjusting for gravidity.
Table A2.
Analysis of the associations between analgesic use and fecundability in the Time to Conceive cohort (2008–2016), after within-cluster resampling or multiple imputation.
| Time windows | Medicationsa | FRb (95% CI) | FRc (95% CI) |
|---|---|---|---|
| Pre-ovulatory | Acetaminophen | 1.02 (0.73, 1.43) | 1.00 (0.68, 1.47) |
| Aspirin | 1.04 (0.52, 2.08) | 1.60 (0.94, 2.73) | |
| NSAID | 1.05 (0.74, 1.49) | 1.11 (0.73, 1.70) | |
| Peri-ovulatory | Acetaminophen | 0.97 (0.68, 1.37) | 1.07 (0.73, 1.56) |
| Aspirin | 1.19 (0.62, 2.28) | 1.26 (0.65, 2.46) | |
| NSAID | 1.17 (0.83, 1.65) | 1.01 (0.64, 1.57) | |
| Implantation | Acetaminophen | 1.15 (0.84, 1.58) | 1.09 (0.72, 1.64) |
| Aspirin | 2.04 (1.23, 3.39) | 2.21 (1.33, 3.69) | |
| NSAID | 1.16 (0.81, 1.64) | 1.04 (0.68, 1.59) | |
| Non-Bleeding Days | Acetaminophen | 1.13 (0.89, 1.4 | 1.10 (0.77, 1.57) |
| Aspirin | 1.10 (0.69, 1.74) | 1.17 (0.73, 1.87) | |
| NSAID | 0.99 (0.76, 1.29) | 1.05 (0.74, 1.50) |
The reference group for each medication are cycles in which none of that medication was reported for that particular window.
Analysis based on 5 datasets formed from within-cluster resampling to account for 19 women with two attempts in the study, fully adjusted model, complete case analysis.
Analysis based on 20 multiply imputed datasets, N=2390.
Footnotes
Disclosure statement: A.M.J received vitamin D supplements from Theralogix, LLC for a clinical trial unrelated to the present work. M.B.B is, or has been, a consultant to Forest Laboratories, GlaxoSmithKline, and Eli Lilly, all on pharmaceutical products unrelated to the present paper. The other authors report no conflict of interest.
References
- 1.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002;287:337–44. [DOI] [PubMed] [Google Scholar]
- 2.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771–7. [DOI] [PubMed] [Google Scholar]
- 3.Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs 1996;52 Suppl 5:13–23. [DOI] [PubMed] [Google Scholar]
- 4.Ando M, Kol S, Irahara M, Sirois J, Adashi EY. Non-steroidal anti-inflammatory drugs (NSAIDs) block the late, prostanoid-dependent/ceramide-independent component of ovarian IL-1 action: implications for the ovulatory process. Mol Cell Endocrinol 1999;157:21–30. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal SS, Alvin Jose M. Anti-implantation activity of H2 receptor blockers and meloxicam, a COX-inhibitor, in albino Wistar rats. Eur J Contracept Reprod Health Care 2009;14:444–50. [DOI] [PubMed] [Google Scholar]
- 6.Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin Infect Dis 2000;31 Suppl 5:S202–10. [DOI] [PubMed] [Google Scholar]
- 7.Smarr MM, Grantz KL, Sundaram R, et al. Urinary paracetamol and time-to-pregnancy. Hum Reprod 2016;31:2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McInerney KA, Hatch EE, Wesselink AK, Rothman KJ, Mikkelsen EM, Wise LA. Preconception use of pain-relievers and time-to-pregnancy: a prospective cohort study. Hum Reprod 2017;32:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schisterman EF, Mumford SL, Schliep KC, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab 2015;100:1785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA 2017;318:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995;333:1517–21. [DOI] [PubMed] [Google Scholar]
- 12.Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. The association of maternal factors with delayed implantation and the initial rise of urinary human chorionic gonadotrophin. Hum Reprod 2011;26:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qato DM, Schumm LP, Johnson M, Mihai A, Lindau ST. Medication data collection and coding in a home-based survey of older adults. J Gerontol B Psychol Sci Soc Sci 2009;64 Suppl 1:i86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman EB, Sen PK, Weinberg CR. Within-cluster resampling. Biometrika 2001;88:1121–34. [Google Scholar]
- 15.Harel O, Mitchell EM, Perkins NJ, et al. Multiple Imputation for Incomplete Data in Epidemiologic Studies. Am J Epidemiol 2018;187:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadoch IJ, Al-Khaduri M, Phillips SJ, et al. Spontaneous ovulation rate before oocyte retrieval in modified natural cycle IVF with and without indomethacin. Reprod Biomed Online 2008;16:245–9. [DOI] [PubMed] [Google Scholar]
- 17.Uhler ML, Hsu JW, Fisher SG, Zinaman MJ. The effect of nonsteroidal anti-inflammatory drugs on ovulation: a prospective, randomized clinical trial. Fertil Steril 2001;76:957–61. [DOI] [PubMed] [Google Scholar]
- 18.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1993;268:6610–4. [PubMed] [Google Scholar]
- 19.Dentali F, Ageno W, Rezoagli E, et al. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost 2012;10:2075–85. [DOI] [PubMed] [Google Scholar]
- 20.Dirckx K, Cabri P, Merien A, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? A randomized double-blind placebo controlled trial. Hum Reprod 2009;24:856–60. [DOI] [PubMed] [Google Scholar]
- 21.Lambers MJ, Hoozemans DA, Schats R, Homburg R, Lambalk CB, Hompes PG. Low-dose aspirin in non-tubal IVF patients with previous failed conception: a prospective randomized double-blind placebo-controlled trial. Fertil Steril 2009;92:923–9. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril 1999;71:825–9. [DOI] [PubMed] [Google Scholar]
- 23.Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database Syst Rev 2016;11:CD004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Du B, Jiang X, et al. Effects of combining lowdose aspirin with a Chinese patent medicine on follicular blood flow and pregnancy outcome. Mol Med Rep 2014;10:2372–6. [DOI] [PubMed] [Google Scholar]