Abstract
Background:
Although in 2013 the American College of Obstetricians and Gynecologists recommended early screening for gestational diabetes in obese women, no studies demonstrate an improvement in perinatal outcomes with this strategy.
Objective:
We sought to determine if early screening for gestational diabetes improves perinatal outcomes in obese women.
Study Design:
Randomized controlled trial comparing early gestational diabetes screening (14–20 weeks) to routine screening (24–28 weeks) in obese women (BMI≥30 kg/m2) at two tertiary care centers in the US. Screening was performed using a 50-g, 1-hr glucose challenge test followed by a 100-g, 3-hr glucose tolerance test if initial screen ≥135 mg/dL. Gestational diabetes was diagnosed using Carpenter-Coustan criteria. Women not diagnosed at 14–20 wks were rescreened at 24–28 wks. Exclusion criteria were pre-existing diabetes, major medical illness, bariatric surgery, and prior cesarean. The primary outcome was a composite of macrosomia (>4000g), primary cesarean, hypertensive disease of pregnancy, shoulder dystocia, neonatal hyperbilirubinemia, and neonatal hypoglycemia (assessed within 48 hours of birth).
Results:
A total of 962 women were randomized, and outcomes were available for 922. Of these 922, 459 (49.8%) to early screen and 463 (50.2%) to routine screen. Baseline characteristics were balanced between groups. In the early screening group, 69 (15.0%, 95% CI 11.9–18.6%) were diagnosed with gestational diabetes: 29 (6.3%, 95% CI 4.3–8.9%) <20 wks and 40 (8.7%, 95% CI 6.3–11.7%) >24 wks. Of those randomized to routine screening, 56 (12.1%, 95% CI 9.3–15.4%) had gestational diabetes. Early screening did not reduce the incidence of the primary outcome (56.9% in early screen vs 50.8% in routine screen, p=0.07, RR 1.12, 95% CI 0.99–1.26).
Conclusion:
Early screening for gestational diabetes in obese women did not reduce the composite perinatal outcome.
Keywords: early screening, gestational diabetes, obesity, randomized trial, cesarean, macrosomia, preeclampsia, shoulder dystocia
Condensation:
Compared to routine screening (24–28 weeks), early screening (14–20 weeks) for gestational diabetes in obese women did not reduce adverse perinatal outcomes.
Introduction
In the US, 36.8% of reproductive age women are obese (BMI≥30 kg/m2).1 Obesity substantially increases the risk of gestational diabetes (odds ratio 2–5),2 which is associated with macrosomia, cesarean delivery, preeclampsia, shoulder dystocia, and neonatal hypoglycemia.3 Gestational diabetes treatment has been shown to improve pregnancy outcomes,4, 5 but obese women with gestational diabetes continue to have worse outcomes compared to normal weight women with gestational diabetes.6
In 2005 and again in 2013, The American College of Obstetricians and Gynecologists (ACOG) suggested screening obese women for pre-existing diabetes or early gestational diabetes in the first trimester or upon presentation, much earlier than the 24–28 weeks screen recommended for low-risk women.7, 8 Early screening and diagnosis of gestational diabetes allows earlier treatment, by as many as 14 weeks, decreasing fetal exposure to hyperglycemia during critical periods of fetal growth and development. As treatment of gestational diabetes diagnosed at 24–28 weeks may lead to decreased cesarean, macrosomia, shoulder dystocia, and preeclampsia, earlier treatment may further decrease these risks.4, 9 However, this recommendation, which is based solely on expert opinion, is not widely adopted and the majority of obese women do not undergo GDM screening until 24–28 weeks gestation,10 possibly due to lack of level I evidence demonstrating benefit and lack of guidance in how screening should be accomplished. In contrast to ACOG, the US Preventive Services Task Force determined there was insufficient evidence to recommend early screening.11 The lack of level I evidence and the contradictory recommendations led the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to identify the risks and benefits of early screening for GDM as an important research gap and called for level I evidence on this topic.12
Therefore, we designed this pragmatic randomized, controlled trial to test the hypothesis that early screening (at 14–20 weeks) would improve the selected perinatal outcomes compared to routine screening (24–28 weeks) for gestational diabetes in obese women.
Materials and Methods
Trial Design
Patients were randomly assigned to early screening for gestational diabetes at 14–20 weeks gestation or routine screening for gestational diabetes at 24–28 weeks gestation. We used broad inclusion criteria and routine clinical procedures. Outcomes were analyzed according to the intention-to-treat principle. The full trial protocol is available with the full text of this article in supplementary materials. Institutional review board approval was obtained from both participating sites and all participants gave written informed consent. Prior to trial start, the trial protocol was registered at clinicaltrials.gov (NCT01864564).
Patient Selection
All participants provided written informed consent. Pregnant women receiving prenatal care prior to 20 weeks gestation at University of Alabama at Birmingham with a BMI≥30 kg/m2 at enrollment were eligible. Enrollment began at the University of Alabama at Birmingham in June, 2013; Ochsner Medical Center began enrolling in December 1, 2015. We excluded women with a prior cesarean delivery, pre-existing diagnosis of diabetes mellitus, a history of bariatric surgery, major medical illness (eg cardiac disease, sickle cell disease), known fetal anomalies, or chronic steroid use. Enrollment concluded January 31, 2018. Primary cesarean was a component of the primary outcome; women with a prior cesarean delivery could not experience a key component of the primary outcome and were therefore excluded. Known pre-existing diabetes and a history of bariatric surgery are both contraindications to glucola screening; therefore these women were excluded. Women with major medical illness, known fetal anomalies, and chronic steroid use were excluded because they have increased risks of the primary outcomes for reasons unrelated to gestational diabetes.
Treatment Allocation
Enrolled patients underwent randomization, in a 1:1 ratio in blocks of equal size, with the use of a computer-generated sequence produced by the study statistician using SAS for Windows software, version 9.4 (SAS Institute Inc, Cary, NC). Study personnel were notified of randomization assignment online.
Randomization was stratified by BMI≥40 kg/m2 and study site. Patients were assigned to receive either early screening (at 14–20 weeks) or routine screening (at 24–28 weeks) for gestational diabetes.
Gestational Diabetes Screening
All screening (regardless of gestational age or group assignment) was performed using the two-step method. Women underwent a non-fasting, 1-hour, 50-g glucose challenge test. If results were ≥135 mg/dL, including a 1-hour result ≥200 mg/dL, patients were asked to undergo a fasting, 3-hour, 100-g glucose tolerance test within 7–10 days. Carpenter-Coustan criteria were used to diagnose gestational diabetes (fasting ≥95 mg/dL, 1-hour ≥ 180 mg/dL, 2-hour ≥ 155 mg/dL, 3-hour ≥ 140mg/dL).13, 14
If women randomized to early screening were negative for gestational diabetes prior to 20 weeks, they repeated the same gestational diabetes screening at 24–28 weeks, starting with a non-fasting, 1-hour, 50-g glucose challenge test and using the same thresholds for positive screen and diagnosis.
Trial Procedures
Women were screened and randomized at a prenatal visit prior to 20 weeks gestation. Clinicians and patients were informed of their randomization group (open label). All patients, regardless of randomization group, underwent a blood draw between 14–20 weeks for hemoglobin A1c. The hemoglobin A1c was performed in order to screen patients for pre-existing diabetes and to provide a baseline measure of glycemic status at enrollment. Providers were notified of hemoglobin A1c values >6.2% due to potential association of GDM with thresholds below 6.5%.15 Patients were treated for diabetes if hemoglobin A1c values at 14–20 weeks were ≥6.5%,16 for values between 6.2–6.5, providers performed gestational diabetes screening (one-hour testing followed by three-hour testing if abnormal) regardless of randomization arm and treated for diabetes if diagnosed with gestational diabetes on three-hour testing.
After a diagnosis of gestational diabetes, patients were managed according to institutional guidelines on gestational diabetes management. Within one week of diagnosis, patients received diabetic education and instructions on self-blood glucose monitoring. Goals of fasting blood sugar was <95 mg/dL and two-hour postprandial was <120 mg/dL. Patients were started on medication (glyburide, metformin, or insulin at the discretion of the provider) and adjusted if more than half of blood sugars were above goal. During the time of this study, ACOG had indicated that insulin and oral medications were “equivalent in efficacy” and either could be considered as first-line agents;17 thus, providers were given discretion as to the first-line agent based on patient preference, insurance, and glycemic control in the previous week. Allowing practitioners and patients to determine the most appropriate first-line medication mimics real-world practice. Patients receiving medication to manage gestational diabetes underwent antenatal testing weekly after 32 weeks. Ultrasounds were performed for fluid and growth serially in most patients due to the presence of obesity. Women were not treated for an elevated one-hour glucose test regardless of timing as this is not currently the standard of care.
Perinatal outcomes were abstracted from the medical records at delivery and through the 6-week postpartum visit. If patients did not deliver at the participating institution, medical records were obtained from the delivering hospital/physician. We collected demographic information, obstetrical and medical history, and details of gestational diabetes treatment. Data were supplemented by direct interview with the patients.
Trial Oversight
The study was approved by the institutional review board at the University of Alabama at Birmingham and at Ochsner Medical Center. Study progress and safety events were monitored by an independent data and safety monitoring board (DSMB).
Trial Outcomes
The primary outcome was defined as an adverse perinatal composite outcome that included any one of the following: macrosomia, primary cesarean delivery, pregnancy-induced hypertension (defined as gestational hypertension, preeclampsia or eclampsia), shoulder dystocia, neonatal hypoglycemia, or neonatal hyperbilirubinemia. These outcomes were selected both as adverse outcomes associated with gestational diabetes and because they may be reduced by treatment of gestational diabetes.4, 5 If a patient had any single component, she was considered to have the adverse perinatal composite outcome. Individual components of the composite outcome were considered as secondary outcomes. Additionally, gestational age at delivery, severity of pregnancy induced hypertension (gestational hypertension, preeclampsia without severe features, and preeclampsia with severe features, and eclampsia), and use of antidiabetic medications (oral agents and/or insulin) were considered as individual secondary outcomes (prespecified). Large for gestational age, as defined as ≥90th percentile by Duryea et al,18 was also considered as a secondary outcome (not specified a priori).
Macrosomia was defined as an absolute birth weight of greater than 4000 g given the increased risk with labor abnormalities and newborn complications above this birth weight.19 Primary cesarean delivery for any indication was included in the primary outcome; since women with a previous cesarean were excluded, all cesarean deliveries in this cohort were primary cesarean deliveries. Pregnancy induced hypertension was defined as either gestational hypertension or preeclampsia, with or without severe features, according to ACOG guidelines.20 Gestational hypertension was defined as new onset hypertension (systolic ≥140 mm Hg or diastolic ≥90 mm Hg) without proteinuria. Preeclampsia was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg with either proteinuria or serum laboratory abnormalities (platelets <100,000, AT >80 IU/mL, creatinine >1.2 mg/dL). Proteinuria was defined as protein excretion exceeding 300 mg in 24-hours, a protein:creatinine ratio of ≥0.3, or 1+ proteinuria or greater on urine dipstick. Preeclampsia was further classified as with or without severe features. Severe features were considered present if systolic blood pressure ≥160 mm Hg, diastolic blood pressure ≥110 mm Hg, seizures (eclampsia), persistent headache, pulmonary edema, or any serum laboratory abnormalities. Eclampsia was also considered in the diagnosis of pregnancy induced hypertension, however, there were no cases of eclampsia in the study population. The PI reviewed all diagnoses of gestational hypertension, preeclampsia and preeclampsia with severe features for classification, blinded to randomization group.
A shoulder dystocia was considered to have occurred if documented by the delivering physician and requiring at least one maneuver to resolve. Neonatal hypoglycemia was defined as blood sugar <35 mg/dL within the first 48 hours of life. Neonatal hyperbilirubinemia was defined as >95th percentile for gestational age and hour of life or as requiring phototherapy for treatment.21, 22
Sample Size and Interim Analysis
As the benefits of early screening and diagnosis of gestational diabetes are expected primarily in women diagnosed with gestational diabetes, the sample size was calculated to ensure a sufficient number of women with gestational diabetes to detect an effect in this group. A sample size of 58 women with gestational diabetes per group was necessary to have 80% power with two-sided alpha of 0.05, to detect a 50% relative reduction in the incidence of the primary outcome, from an estimated baseline rate of 50% (based on prior institutional data). A 50% reduction in the incidence of the primary outcome was based on a prior study that demonstrated a relative risk of 0.37–0.79 associated with treatment at 24–28 weeks for the selected outcomes of interest.9 Based on an incidence of gestational diabetes of 10% in obese women, approximately 580 women per screening group (1160 total) were needed. The total sample size allowed for >90% power to detect an 8% absolute change in the primary outcome for the entire population, assuming a baseline incidence of 20% in pregnancies complicated by obesity.
A planned, blinded interim analysis was conducted after the first 600 randomized subjects were delivered. An O’Brien-Fleming alpha spending function determined critical stopping boundaries of 0.005 at the interim analysis. Due to the extended recruitment time (five years), a sample size review was also performed. The DSMB recommended continuation of the trial and approved a sample size reduction to 950 patients total, considering the higher baseline incidence of GDM in our population (13.8% at the interim analysis). Based on our original estimates of a 50% incidence of the primary adverse outcome in women with GDM, this adjusted sample size maintained >80% power to detect a 50% reduction. This sample size also maintained >90% power to detect an 8% absolute change in the primary outcome for the entire population.
Statistical Analysis
Final analyses were by intention-to-treat. Patient characteristics and outcomes were compared by randomization group. Categorical measures were presented as numbers and percentages, and evaluated using the chi-square test of association. Relative risks (95% confidence intervals) were calculated for outcomes. Continuous variables were presented as means (SD) or median (1st – 3rd quartile) and compared using the Student t-test and Wilcoxon rank-sum test as appropriate. Pre-specified subgroup analysis was conducted among those diagnosed with gestational diabetes. The level of statistical significance was set at 0.048 based on the O’Brien-Fleming alpha spending function. All statistical analyses were performed by the UAB Center for Women’s Reproductive Health Biostatistics and Data Management Core with the use of SAS for Windows software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Trial Participants
From June 1, 2013 to January 31, 2018, 3,922 women were screened for eligibility (Figure 1). Of these, 2,946 were excluded because they did not meet the inclusion criteria or they declined to participate. Of the remaining 976 women consented and randomized to the study, 14 were excluded prior to intervention for not meeting inclusion criteria (one twin pregnancy, seven missed abortions diagnosed prior to 14 weeks, one previously randomized, one without intention to deliver at UAB, one already diagnosed with gestational diabetes, and three with prior cesarean deliveries). Of the 962 women randomized and not subsequently excluded, 40 were lost to follow up, leaving 922 (95.8%) women in the intent to treat analysis (459 early screen, 463 routine screen). In the early screen group, 387 (84.3%) women received the early screen. The two most common reasons patients in the early screen group did not receive the assigned intervention was that the provider did not order (27/72) or the patient refused (11/72). Only two (2/72) early screens were not performed due to patient inability to tolerate the glucose challenge test. In the routine screening group, 443 (95.9%) received the routine screen. Patients were similar regarding age, race, BMI at randomization, medical comorbidities, gestational age at randomization, and baseline hemoglobin A1c (Table 1).
Figure 1:
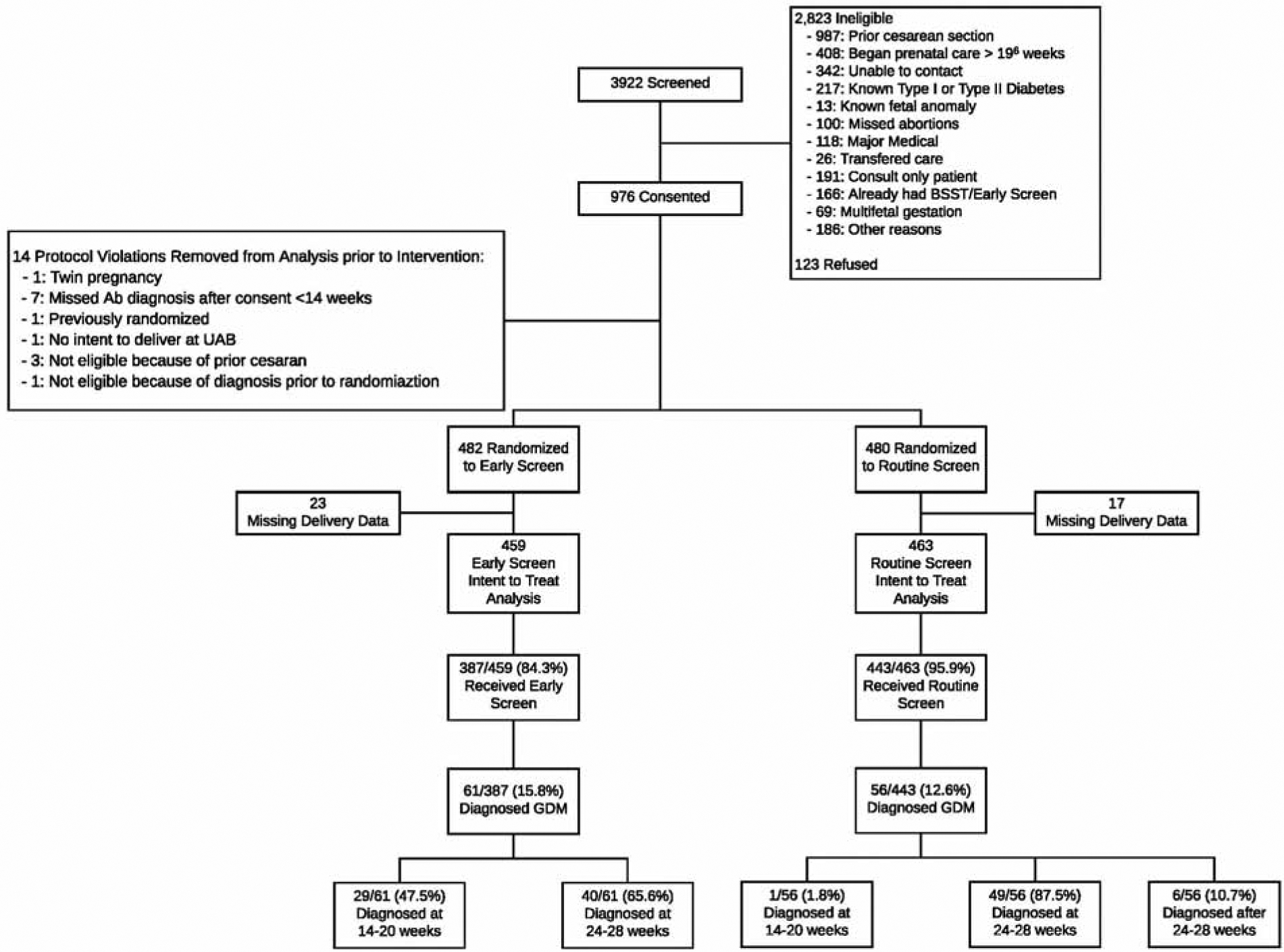
CONSORT Diagram
Table 1:
Maternal baseline characteristics
| Variable | Early Screen (n=459) | Routine Screen (n=463) |
|---|---|---|
| Age (years) | 27.2 (5.9) | 26.8 (5.9) |
| Race/Ethnicity | ||
| White, non-Hispanic | 52 (11.3%) | 35 (7.6%) |
| Black, non-Hispanic | 280 (61.0%) | 299 (64.6%) |
| Native American | 2 (0.4%) | 3 (0.7%) |
| Asian | 1 (0.2%) | 2 (0.4%) |
| Hispanic | 122 (26.6%) | 123 (26.6%) |
| Other | 2 (0.4%) | 1 (0.2%) |
| BMI at Randomization (kg/m2) | 37.2 (6.6) | 37.0 (6.5) |
| Medicaid/No Insurance | 434 (95.8%) | 441 (96.5%) |
| Married | 98 (21.4%) | 96 (20.8%) |
| High School Education or Greater | 309 (70.9%) | 305 (71.1%) |
| Parous | 329 (71.7%) | 338 (73.0%) |
| Any Smoking (%) | 83 (18.1%) | 98 (21.2%) |
| Any Alcohol Use (%) | 76 (16.6%) | 61 (13.1%) |
| Any Drug Use (%) | 41 (8.9%) | 49 (10.6%) |
| Hypertension | 61 (13.3%) | 50 (10.8%) |
| Asthma | 61 (13.3%) | 53 (11.5%) |
| Depression | 55 (12.0%) | 50 (10.8%) |
| Hemoglobin A1c at 14–20 weeks (%) | 5.3 (5.0–5.6) | 5.3 (5.0–5.6) |
| Gestational age at Randomization (weeks) | 13.8 (3.8) | 13.6 (3.7) |
Data presented as n(%) or mean (standard deviation) as appropriate
Gestational Diabetes
In the early screening group, 69/459 (15.0%, 95% CI 11.9–18.6%) of the early screening group ultimately received a diagnosis of gestational diabetes: 29 (6.3%, 95% CI 4.3–8.9%) prior to 20 weeks and 40 (8.7%, 95% CI 6.3–11.7%) at the 24–28 week screen.
Of the 463 women analyzed in the routine screening group, one patient (0.2%) was diagnosed and treated for gestational diabetes prior to 20 weeks due to an A1c ≥6.5% at intake. Fifty-five women were diagnosed after 24 weeks with routine screening or an A1c≥6.5% (n=1). In total, 56 (12.1%) women in the routine screening group were diagnosed with gestational diabetes.
The overall incidence of gestational diabetes in the study cohort was 13.6% (95% CI 11.4–15.8%). In the early screen group, the average gestational age at GDM diagnosis was 24.3 ± 5.2 weeks, compared to 27.1 ± 1.7 weeks in the routine screen group.
Primary and Key Secondary Outcomes
The composite primary outcome occurred in a total of 261 (56.9%) patients in the early screening group and 235 (50.8%) patients in the routine screening group (relative risk 1.12, 95% confidence interval [CI] 0.99–1.26, p=0.06) (Table 2).
Table 2.
Primary and Key Secondary Outcomes
| Outcome | Early Screen (n = 459) | Routine Screen (n = 463) | P | Relative Risk (95% CI) |
|---|---|---|---|---|
| Primary Composite Outcome* | 261 (56.9%) | 235 (50.8%) | 0.06 | 1.12 (0.99–1.26) |
| Secondary Outcomes | ||||
| Macrosomia | 25 (5.5%) | 21 (4.6%) | 0.51 | 1.21 (0.69–2.12) |
| Primary Cesarean | 79 (17.2%) | 93 (20.1%) | 0.26 | 0.86 (0.65–1.12) |
| Gestational Hypertension | 74 (16.2%) | 58 (12.6%) | 0.12 | 1.29 (0.94–1.77) |
| Preeclampsia | 62 (13.6%) | 44 (9.5%) | 0.06 | 1.42 (0.99–2.05) |
| Without Severe Features | 32 (7.0%) | 26 (5.6%) | 0.39 | 1.24 (0.75–2.05) |
| With Severe Features | 30 (6.6%) | 18 (3.9%) | 0.07 | 1.68 (0.95–2.98) |
| Hyperbilirubinemia | 90 (19.6%) | 72 (15.6%) | 0.11 | 1.26 (0.95–1.66) |
| Shoulder Dystocia | 30 (6.6%) | 32 (6.9%) | 0.83 | 0.95 (0.59–1.54) |
| Neonatal Hypoglycemia | 22 (4.8%) | 19 (4.1%) | 0.61 | 1.17 (0.64–2.13) |
| Gestational Age at Delivery | 38.2 (4.4) | 38.5 (3.4) | 0.34 | - |
| Any Diabetic Medication | 31 (6.8%) | 20 (4.3%) | 0.11 | 1.56 (0.90–2.70) |
| Insulin Medication | 11 (2.4%) | 3 (0.7%) | 0.03 | 3.70 (1.04–13.17) |
| Large for Gestational Age | 27 (5.9%) | 26 (5.6%) | 0.86 | 1.05 (0.62–1.77) |
Individual components of the composite outcome were not significantly different between groups. Gestational age at delivery, rates of induction, and large for gestational age were similar between randomization groups. Women in the early screening group were more likely to be placed on insulin than the routine screening group (2.4% versus 0.7%, p=0.03).
Among those diagnosed with gestational diabetes, the primary outcome occurred in 51 (73.9%) women with gestational diabetes in the early screening group compared to 37 (66.1%) women with gestational diabetes in the routine screening group. The average gestational age at delivery in women with gestational diabetes in women who were screened early was significantly earlier than those diagnosed at the routine time (36.7 ± 4.5 weeks versus 38.7 ± 1.7, p<0.01).
Comment
Principal Findings of the Study
In this randomized controlled trial, we found that screening obese women for gestational diabetes between 14–20 weeks gestation was not associated with a decrease in a composite adverse perinatal outcome of primary cesarean, macrosomia, pregnancy-induced hypertension, shoulder dystocia, hyperbilirubinemia, or neonatal hypoglycemia. When comparing only women diagnosed with gestational diabetes, the results were similar.
Results in Context of What is Known
To our knowledge (based on a search of Pubmed, Clinicaltrials.gov, and Embase), this is the first completed US-based trial to compare early versus routine screening for gestational diabetes in a randomized fashion, although other trials are ongoing in the US and elsewhere (NCT02377531, ACTRN12616000924459). However, prior retrospective studies suggest that early screening in a high-risk population may not be beneficial. Hong et al reported that women who were screened early required oral antidiabetic agents or insulin more frequently than those without an early screen, but had similar rates of cesarean delivery, preeclampsia, and macrosomia.23 Although this study only included women with an indication for early screening (obesity, prior pregnancy affected by gestational diabetes or macrosomia) and examined women by the timing of when the first glucose challenge test (screening) was performed rather than the timing of the first abnormal glucose tolerance test (diagnosis), the early screen group remained significantly higher risk with more hypertension, higher body mass index, and older ages. Similarly, Feghali et al reported that those with a diagnosis of gestational diabetes prior to 24 weeks had an increased risk of preterm birth, macrosomia, and NICU admission than those diagnosed after 24 weeks, although most of these differences disappeared after adjusting by propensity score analysis.24 As such, both retrospective studies were likely limited by confounding by indication: women screened early were higher risk than women screened after 24 weeks, leading to more adverse outcomes in the early screening group. Importantly, in this randomized trial, randomization groups were similar at baseline with respect to body mass index, hypertension, and hemoglobin A1c, suggesting similar baseline risks for gestational diabetes.
Two intervention trials of early treatment for GDM in an already screened population also suggest that early treatment is not beneficial. Osmundson et al examined the benefits of intervention prior to 14 weeks gestation in women with a hemoglobin A1c in the pre-diabetes range (5.7–6.4%). In this study of 95 women, 50 women were randomized to early gestational diabetes treatment (self-blood glucose monitoring and treatment for elevated blood sugars) and 45 were randomized to standard of care. There was no difference in the primary outcome of gestational diabetes diagnosis by 24–28 weeks nor any of the secondary outcomes of primary cesarean delivery, infant birth weight, or umbilical cord blood C-peptide.25 Similarly, Roeder et al randomized women with a hemoglobin A1c in the pre-diabetes range (5.7–6.4%) or fasting plasma glucose ≥92 mg/dL prior to 15 weeks.26 Women were randomly assigned to early pregnancy treatment (n=82) compared to third trimester treatment (n=75) that included nutritional counseling, glucose monitoring, and medications as needed. No difference was detected in the primary and secondary outcomes of cord blood C-peptide>90th percentile, fat mass, weight for length percentile at birth, macrosomia, or maternal gestational weight gain. Early treatment of hyperglycemia did not prevent a gestational diabetes diagnosis on a blinded two-hour glucose tolerance test at 24–28 weeks.
Clinical and Research Implications
In this trial, the initial screen was performed at 14–20 weeks gestation in the intervention group, rather than at the initial visit as recommended by ACOG. We selected 14–20 weeks for several reasons. First, in this high-risk population, the initial visit occurs as late as 20 weeks gestation. In fact, the study inclusion criteria was expanded from a maximum gestational age of 18 weeks up to 20 weeks due to the number of women being excluded for presentation at 18–20 weeks. Additionally, performance of the 1-hour test at 14–20 weeks enabled patients to 1) consider enrollment in the study prior to consent, 2) plan a convenient time to present for the 1-hour to coincide with another clinic visit. As the initial prenatal visit is typically a lengthy visit, scheduling the 1-hour at another visit, frequently to coincide with an ultrasound or another laboratory draw, was much more convenient for patients.
Although more women in the early screening group were diagnosed with gestational diabetes, it is worth noting that 58% of women diagnosed with gestational diabetes in the early screening group were not diagnosed until their repeat screen at 24–28 weeks. Consequently, the majority of women with gestational diabetes who were screened early did not receive a diagnosis and treatment until 24–28 weeks, which may have reduced the difference between groups. It is possible that different screening and diagnostic thresholds are necessary earlier in pregnancy. Further studies are needed that assess early screening at the first visit with potentially lower thresholds for screening and diagnosis.
Limitations
We acknowledge several limitations of this trial. First, the lack of blinding of patients and providers may have introduced bias and may explain the increased use of insulin and the early timing of delivery associated with early screening, as early diagnosis of gestational diabetes is frequently conflated with pregestational diabetes. However, lack of blinding is the pragmatic approach and mimics a real world practice where patients and providers are aware of the timing of diagnosis of gestational diabetes for treatment. Another feature of this pragmatic trial is that while maternal-fetal medicine specialists managed patients according to the institutional guidelines, management of glycemic control was not monitored by the study team and deviations from institutional guidelines based on patient and provider preferences may have occurred. The study population consisted largely of black or Hispanic women without private insurance, potentially limiting the generalizability of these results. However, this is a high-risk population and therefore most likely to benefit from early screening and diagnosis. Additionally, 83.3% of women in the early screening group actually received early screening. As we performed an intent to treat analysis, this may have reduced the observed efficacy of early screening and reduced the detectable effect size. However, in this study the direction of effect actually favored routine screening, rather than suggesting a benefit of early screening in an underpowered study. Finally, we were powered to detect a relatively large difference between groups (8% absolute change in the primary outcome for the entire population), although smaller effects which may be clinically meaningful and differences in rare outcomes would not have been detected.
Conclusions
In conclusion, early screening for gestational diabetes at 14–20 weeks in obese women did not improve the perinatal outcomes examined. Since this trial was started, ACOG expanded the recommendations for early screening for pre-existing diabetes or early gestational diabetes to overweight women with additional risk factors, corresponding to the ADA recommendations for early screening and diagnosis for pre-existing diabetes in pregnancy.16, 27 Further studies of early screening of gestational diabetes should focus on this population in a geographically, racially, and ethnically diverse cohort.
Supplementary Material
Table 3.
Outcomes among women diagnosed with gestational diabetes, by screening group
| Outcome | Early Screen (n=69) | Routine Screen (n=56) | P | Relative Risk (95% CI) |
|---|---|---|---|---|
| Primary Composite Outcome* | 51 (73.9%) | 37 (66.1%) | 0.34 | 1.12 (0.89–1.41) |
| Secondary Outcomes | ||||
| Macrosomia | 4 (5.9%) | 5 (8.9%) | 0.73 | 0.66 (0.19–2.34) |
| Primary Cesarean | 16 (23.2%) | 13 (23.2%) | >0.99 | 1.0 (0.53–1.90) |
| Gestational Hypertension | 14 (20.3%) | 8 (14.3%) | 0.38 | 1.42 (0.64–3.14) |
| Preeclampsia | 15 (21.7%) | 9 (16.1%) | 0.42 | 1.35 (0.64–2.86) |
| Without Severe Features | 9 (13.0%) | 7 (12.5%) | 0.93 | 1.04 (0.41–2.63) |
| With Severe Features | 6 (8.7%) | 2 (3.6%) | 0.30 | 2.43 (0.51–11.60) |
| Hyperbilirubinemia | 18 (26.1%) | 13 (23.2%) | 0.71 | 1.12 (0.60–2.09) |
| Shoulder Dystocia | 4 (5.8%) | 5 (8.9%) | 0.51 | 0.65 (0.18–2.30) |
| Neonatal Hypoglycemia | 7 (10.1%) | 8 (14.3%) | 0.48 | 0.71 (0.27–1.84) |
| Gestational Age at Delivery | 36.7 (4.5) | 38.7 (1.7) | 0.001 | .. |
| Any Diabetic Medication | 30 (43.5%) | 18 (32.1%) | 0.20 | 1.35 (0.85–2.16) |
| Insulin Medication | 11 (15.9%) | 3 (5.4%) | 0.06 | 2.98 (0.87–10.15) |
| Large for Gestational Age | 6 (8.7%) | 7 (12.5%) | 0.49 | 0.70 (0.25–1.95) |
AJOG at a Glance:
Why was the study conducted? The American College of Obstetricians and Gynecologists recommends early screening for preexisting diabetes or early gestational diabetes in a high-risk population, based on expert opinion. The National Institute of Diabetes and Digestive and Kidney Disease Consensus workshop on research gaps in gestational diabetes called for a randomized controlled trial examining the risks and benefits of early screening. The study was performed to compare perinatal outcomes in obese women undergoing early screening (14–20 weeks) for gestational diabetes compared to routine screening (24–28 weeks).
What are the key findings? Early screening did not reduce the incidence of the primary composite outcome (macrosomia (>4000g), primary cesarean, hypertensive disease of pregnancy, shoulder dystocia, neonatal hyperbilirubinemia, and neonatal hypoglycemia): 56.9% in early screen vs 50.8% in routine screen, p=0.07, RR 1.12, 95% CI 0.99–1.26).
What does this study add to what is already known? This randomized controlled trial did not demonstrate benefit of early screening for gestational diabetes in obese women. To our knowledge, this is the first US-based trial to compare early versus routine screening for gestational diabetes in a randomized fashion, although other trials are ongoing (NCT02377531, ACTRN12616000924459).
Acknowledgments
This trial was supported by K12HD001258-13, PI WW Andrews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as an oral presentation, The Society for Maternal Fetal Medicine, The Pregnancy Meeting, February 14–16, 2019, Las Vegas, Nevada.
Registered on clinicaltrials.gov, NCT01864564, on 5/29/13. First participant enrolled 6/18/13.
The authors report no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. National Center for Health Statistics, 2016.
- 2.Dodd JM, Grivell RM, Nguyen AM, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. The Australian & New Zealand journal of obstetrics & gynaecology 2011;51:136–40. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstetrics and gynecology 2001;98:525–38. [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. The New England journal of medicine 2005;352:2477–86. [DOI] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. The New England journal of medicine 2009;361:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman AS, Rebarber A, FOX NS, et al. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J Matern Fetal Neonatal Med 2011;24:723–7. [DOI] [PubMed] [Google Scholar]
- 7.ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstetrics and gynecology 2005;106:671–5. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstetrics and gynecology 2013;122:406–16. [DOI] [PubMed] [Google Scholar]
- 9.Landon MB, Rice MM, Varner MW, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes care 2015;38:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mission JF, Catov J, Deihl TE, Feghali M, Scifres C. Early Pregnancy Diabetes Screening and Diagnosis: Prevalence, Rates of Abnormal Test Results, and Associated Factors. Obstetrics and gynecology 2017;130:1136–42. [DOI] [PubMed] [Google Scholar]
- 11.US Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine 2008;148:759–65. [DOI] [PubMed] [Google Scholar]
- 12.Wexler DJ, Powe CE, Barbour LA, et al. Research Gaps in Gestational Diabetes Mellitus: Executive Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Obstetrics and gynecology 2018;132:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American journal of obstetrics and gynecology 1982;144:768–73. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan JB, Mahan CM. CRITERIA FOR THE ORAL GLUCOSE TOLERANCE TEST IN PREGNANCY. Diabetes 1964;13:278–85. [PubMed] [Google Scholar]
- 15.Stiewig M, Jackson DN, Howard DL. Does serum hemoglobin A1C during early pregnancy predict performance on the 1-hour glucose challenge test? J Matern Fetal Neonatal Med 2019:1–3. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Professional Practice Committee. Professional Practice Committee: Standards of Medical Care in Diabetes—2019. Diabetes care 2019;42:S13–S27. [DOI] [PubMed] [Google Scholar]
- 17.Gynecologists; ACoOa. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstetrics and gynecology 2013;122:406–16. [DOI] [PubMed] [Google Scholar]
- 18.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstetrics and gynecology 2014;124:16–22. [DOI] [PubMed] [Google Scholar]
- 19.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. American journal of obstetrics and gynecology 2003;188:1372–8. [DOI] [PubMed] [Google Scholar]
- 20.American College OF Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstetrics and gynecology 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics Subcommittee on H. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316. [DOI] [PubMed] [Google Scholar]
- 22.Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol 2012;32:660–4. [DOI] [PubMed] [Google Scholar]
- 23.Hong WY, Biggio JR, Tita A, Harper LM. Impact of Early Screening for Gestational Diabetes on Perinatal Outcomes in High-Risk Women. American journal of perinatology 2016;33:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feghali MN, Abebe KZ, Comer DM, Caritis S, Catov JM, Scifres CM. Pregnancy outcomes in women with an early diagnosis of gestational diabetes mellitus. Diabetes research and clinical practice 2018;138:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osmundson SS, Norton ME, El-Sayed YY, Carter S, Faig JC, Kitzmiller JL. Early Screening and Treatment of Women with Prediabetes: A Randomized Controlled Trial. American journal of perinatology 2016;33:172–9. [DOI] [PubMed] [Google Scholar]
- 26.Roeder HA, Moore TR, Wolfson MT, Gamst AC, Ramos GA. Treating hyperglycemia in early pregnancy: a randomized controlled trial. American Journal of Obstetrics & Gynecology MFM 2019;1:33–41. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics and gynecology 2018;131:e49–e64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


