Abstract
Background and Objectives:
The number of patients diagnosed with head and neck squamous cell carcinoma (HNSCC) at an advanced age has increased. The aim of this study is to evaluate the age at which disease-specific survival (DSS) significantly decreases in HNSCC.
Methods:
We performed a retrospective study of 5,469 patients with HNSCC treated at our center (1985–2016). An external validation with 2,082 oral squamous cell carcinomas from a collaborative institution from another continent was performed.
Results:
We observed an orderly decrease in overall survival as age at diagnosis increased. There were no differences in DSS based on age for patients <80 years-old (P=0.623), while older patients had a significant decrease in DSS. These results were validated in the independent dataset. In a multivariable analysis performed in the test set, compared to patients <80 years-old, patients between 80–85 had a 1.50 times higher risk of disease-specific death (95% CI: 1.19–1.89, P=0.001), and patients >85 had a 2.19 times higher risk (95% CI: 1.68–2.87, P<0.001).
Conclusions:
DSS started to significantly decrease in HNSCC at 80 years-old. These findings, validated in an independent cohort, indicate that chronological age on its own should not withhold curative treatment in the majority of HNSCC patients.
Keywords: Elderly, Head and Neck Neoplasms, Oral Cavity Neoplasms, Cancer-specific Survival
Synopsis for Table of Contents.
We evaluated the age at which disease-specific survival started to significantly decrease in head and neck cancer based on a retrospective cohort study of over 5,000 patients with head and neck cancer. There were no differences in disease-specific survival in patients < 80 years-old, conversely, patients > 80 years-old had a significant decrease in disease-specific survival. Our results were validated in a large independent cohort of over 2,000 patients with oral cavity cancer from another continent.
INTRODUCTION
Over the past several decades life expectancy has improved, leading to an increase in the elderly population. Epidemiological studies have shown that life expectancy of the population of the European Union in 1960 was 69.2 years (66.6 years for males and 72.0 for females), reaching 80.6 years (78.0 years for males and 83.3 for females) in 2016. Similar results were found in the United States (US). [1] The number of patients diagnosed with head and neck squamous cell carcinoma (HNSCC) at an advanced age is also increasing.
Several authors have analyzed the differences in clinical and tumor characteristics, type of treatment and outcomes in patients with HNSCC at an advanced age compared to younger patients. [2–10] There is, however, no consensus in the age used to define the elderly population between the different studies, which ranges between 60 and 80 years. [4,8]
Elderly patients usually have a higher number of comorbidities and are more fragile than younger patients, which can limit therapeutic options and decrease survival. All authors agree that elderly patients with HNSCC have a lower overall survival (OS) than younger patients. [2,9,10] In contrast, there are discrepancies in the reported influence of age on disease-specific survival (DSS), with some authors reporting no significant relationship, while others report decreased DSS in elderly patients. [8,9,11]
The aim of this study is to evaluate at what age DSS significantly decreases in patients with HNSCC.
MATERIALS AND METHODS
The present study was performed retrospectively using our departmental database that prospectively collects data related to epidemiological and oncological characteristics, treatment, and follow-up of patients with malignant HNSCC treated at our center since 1985. [12]
All patients with squamous cell carcinomas located in the oral cavity, naso-oro-hypopharynx, larynx, and cervical metastases of unknown primary treated during the period between 1985 and 2016 were included in the study. During this period, a total of 5,526 patients received treatment at our center. Patients with a follow-up of less than 1 year were excluded (n = 57). The final cohort included 5,469 patients which were considered the test set.
For patients with multiple HNSCC primaries, the first diagnosed tumor was considered the index tumor, and all successive tumors were considered second primaries. Second primaries were defined according to the criteria established by Warren and Gates: every second neoplasm must have a histological confirmation, a metastatic origin must be ruled out, and there should not be submucosal continuity with a previous tumor. [13]
Age at time of diagnosis of the HNSCC index tumor was used to group patients in intervals of 10 years if they were younger than 70 years-old, and in 5-year intervals if they were older.
Patientś sex, history of tobacco and alcohol use, location of the primary tumor, performance status measured using the Karnofsky index, local, regional and distant extension of disease, overall staging, and type of treatment were analyzed according to age at time of diagnosis of the index HNSCC tumor. All patients were evaluated prior to treatment by our tumor board, which staged all tumors according to the current version of the Union for International Cancer Control (UICC) TNM classification at the time, and proposed treatment according to our institutional therapeutic guidelines.
For patients with synchronous tumors, location and staging of the most advanced tumor was considered. Given the interaction between tobacco and alcohol use, a combined variable was created in which toxic use was grouped (no use; moderate use: less than 20 cigarettes/day and/or less than 80g of alcohol/day; severe use: more than 20 cigarettes/day and/or more than 80g of alcohol/day). The intent of initial treatment was classified as curative or palliative. Information regarding the type of treatment for palliative patients was categorized as: chemotherapy vs supportive care. Curative treatment was categorized as surgical (including adjuvant treatment with radiotherapy or chemoradiotherapy), or radiotherapy (including patients treated with chemoradiotherapy or bioradiotherapy). For patients with a cervical metastasis of unknown primary, information regarding treatment of the neck was included.
OS and DSS for the index HNSCC tumor were analyzed according to age at time of diagnosis. Cause of death was classified as mortality associated with the HNSCC index tumor, mortality due to second malignancies, and non-tumor-related mortality.
A multivariable analysis was performed considering DSS as dependent variable, and sex, history of tobacco and alcohol use, performance status using the Karnofsky index, location of the primary tumor, overall tumor stage and age as independent variables.
Lastly, we carried out an external validation with an independent cohort of oral cavity squamous cell carcinomas (OSCC) from a US collaborative institution, which is a population from a different continent, given the fact that our test set population is European. A total of 2,082 OSCC diagnosed and treated with primary surgery consecutively from 1985 to 2015 were used as the validation set. [14] Our test set included patients with index tumors from all head and neck sites, which are typically treated not only with surgery but also chemo/radiotherapy, and patients treated with palliative intent. In selecting a validation set, we chose a cohort of OSCC treated with primary surgery with curative intent.
Comparisons between categorical variables were made with the Chi-square test, and between categorical and continuous variables with the ANOVA test. Survival curves were estimated using the Kaplan-Meier actuarial method, and the comparison between curves was analyzed using the log-rank test. For the multivariable analysis, the Cox proportional hazards model was used. A P value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS (v25.0, IBM Corporation; Somers, NY).
The study was approved by Institutional Review Boards at both participating institutions and was carried out according to the principles indicated in the Declaration of Helsinki. Due to the retrospective nature of the study no formal consent was required.
RESULTS
Patientś characteristics of the test set (n = 5,469):
The characteristics of the patients from the test set are shown in Table 1. Mean age at diagnosis was 61.9 years, with a standard deviation (SD) of 11.5 years and a range of 10.6–98.7 years. Mean age for patients diagnosed during the period 1985–1994 (n=1,973) was 59.9 years (SD 11.1 years), for those diagnosed during the period 1995–2004 (n=1,803) it was 61.8 years (SD 11.4 years), and for patients diagnosed during the 2005–2016 period (n=1,693) it was 64.2 years (SD 11.8 years). An increase in the patients’ mean age was observed throughout the study period (P<0.001).
Table 1.
Patient’s characteristics of the test set (n = 5,469)
| N | % | ||
|---|---|---|---|
| Age, years | <50 | 817 | 14.9 |
| 50–60 | 1,598 | 29.2 | |
| 60–70 | 1,712 | 31.3 | |
| 70–75 | 580 | 10.6 | |
| 75–80 | 419 | 7.7 | |
| 80–85 | 218 | 4.0 | |
| >85 | 125 | 2.3 | |
| Sex | Male | 4,898 | 89.6 |
| Female | 571 | 10.4 | |
| Tobacco and alcohol use | No | 483 | 8.8 |
| Moderate | 873 | 16.0 | |
| Severe | 4,113 | 75.2 | |
| Karnofsky index | 90% | 4,446 | 81.3 |
| 80% | 771 | 14.1 | |
| ≤70% | 252 | 4.6 | |
| Location of the primary tumor | Nasopharynx | 239 | 4.4 |
| Oral cavity | 767 | 14.0 | |
| Oropharynx | 1,110 | 20.3 | |
| Hypopharynx | 529 | 17.9 | |
| Supraglottis | 979 | 17.9 | |
| Glottis | 1,720 | 31.4 | |
| Unknown primary | 125 | 2.3 | |
| T classification | T1-T2 | 3,147 | 57.5 |
| T3-T4 | 2,322 | 42.5 | |
| N classification | N0 | 3,409 | 62.3 |
| N+ | 2,060 | 37.7 | |
| M classification | M0 | 5,402 | 98.8 |
| M1 | 67 | 1.2 | |
| Treatment | Palliative | 343 | 6.3 |
| -Chemotherapy | 202 | 3.7 | |
| -Supportive care | 141 | 2.6 | |
| Surgery | 1,952 | 35.7 | |
| -Surgery alone | 1,011 | 18.5 | |
| -Surgery and radiotherapy | 829 | 15.2 | |
| -Surgery and chemoradiotherapy | 112 | 2.0 | |
| Radiotherapy | 3,174 | 58.0 | |
| -Radiotherapy | 2,599 | 47.5 | |
| -Chemoradiotherapy | 504 | 9.2 | |
| -Bioradiotherapy | 71 | 1.3 | |
| Induction Chemotherapy | No | 3,903 | 71.4 |
| Yes | 1,566 | 28.6 | |
Table 2 shows the distribution of patientś characteristics according to age at time of diagnosis of the index HNSCC tumor. As age increased, we found a significant increase in the proportion of female patients, a lower percentage of patients with history of tobacco and alcohol use, a decrease in the percentage of patients with tumors located in the pharynx, and an increase in the proportion of tumors located in the oral cavity. The percentage of tumors with advanced stages at time of diagnosis decreased progressively as patientś age increased, breaking this trend for patients older than 85 years-old. Regarding the treatment performed, we observed a significant increase in the percentage of patients treated palliatively as the patientś age increased. Moreover, from 75 years-old onward, there was a decrease in the number of patients receiving adjuvant radiotherapy/chemoradiotherapy, and a reduction in the use of chemotherapy either as induction treatment or as concomitant with radiotherapy, with an increase in the proportion of patients who were considered candidates for treatment with bioradiotherapy.
Table 2.
Distribution of patients’ characteristics according to age in the test set (n = 5,469)
| Age, years | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <50 | 50–60 | 60–70 | 70–75 | 75–80 | 80–85 | >85 | |||
| Sex | Male | 722 (88.4%) | 1,444 (90.4%) | 1,578 (92.2%) | 522 (90.0%) | 366 (87.4%) | 179 (69.6%) | 87 (69.6%) | <0.001 |
| Female | 95 (11.6%) | 154 (9.6%) | 134 (7.8%) | 58 (10.0%) | 53 (12.6%) | 39 (17.9%) | 38 (30.4%) | ||
| Tobacco and alcohol use | No | 67 (8.2%) | 90 (5.6%) | 111 (6.5%) | 57 (9.8%) | 58 (13.8%) | 48 (22.0%) | 52 (41.6%) | <0.001 |
| Moderate | 94 (11.5%) | 159 (9.9%) | 282 (16.5%) | 128 (22.1%) | 107 (25.5%) | 68 (31.2%) | 35 (28.0%) | ||
| Severe | 656 (80.3%) | 1,349 (84.4%) | 1,319 (77.0%) | 395 (68.1%) | 254 (60.6%) | 102 (46.8%) | 38 (30.4%) | ||
| Karnofsky index | 90% | 707 (86.5%) | 1,331 (83.3%) | 1,414 (82.6%) | 469 (80.9%) | 302 (72.1%) | 145 (66.5%) | 78 (62.4%) | <0.001 |
| 80% | 95 (11.6%) | 203 (12.7%) | 231 (13.5%) | 81 (14.0%) | 83 (19.8%) | 55 (25.2%) | 23 (18.5%) | ||
| ≤70% | 15 (1.8%) | 64 (4.0%) | 67 (3.9%) | 30 (5.2%) | 34 (8.1%) | 18 (8.3%) | 24 (19.2%) | ||
| Location of the primary tumor | Nasopharynx | 88 (10.8%) | 68 (4.3%) | 49 (2.9%) | 18 (3.1%) | 9 (2.1%) | 6 (2.8%) | 1 (0.8%) | <0.001 |
| Oral cavity | 105 (12.9%) | 209 (13.1%) | 203 (11.9%) | 85 (14.7%) | 74 (17.7%) | 47 (21.6%) | 44 (35.2%) | ||
| Oropharynx | 196 (24.0%) | 382 (23.9%) | 329 (19.2%) | 100 (17.2%) | 54 (12.9%) | 32 (14.6%) | 17 (13.6%) | ||
| Hypopharynx | 94 (11.5%) | 181 (11.3%) | 155 (9.1%) | 43 (7.4%) | 33 (7.9%) | 16 (7.3%) | 7 (5.6%) | ||
| Larynx | 321 (39.3%) | 722 (45.2%) | 937 (54.7%) | 321 (55.3%) | 235 (56.1%) | 110 (50.5%) | 53 (42.4%) | ||
| Unknown primary | 13 (1.6%) | 36 (2.3%) | 39 (2.3%) | 13 (2.2%) | 14 (3.3%) | 7 (3.2%) | 3 (2.4%) | ||
| Overall stage | I-II | 297 (36.4%) | 599 (37.5%) | 785 (45.9%) | 284 (49.0%) | 212 (50.6%) | 110 (50.5%) | 47 (37.6%) | <0.001 |
| III-IV | 520 (63.6%) | 999 (62.5%) | 927 (54.1%) | 296 (51.0%) | 207 (49.4%) | 108 (49.5%) | 78 (62.4%) | ||
| Treatment | Palliative | 31 (3.8%) | 83 (5.1%) | 106 (6.2%) | 49 (8.4%) | 33 (7.9%) | 18 (8.3%) | 24 (19.2%) | <0.001 |
| - Supportive care | 6 (0.7%) | 24 (1.5%) | 36 (2.1%) | 17 (2.9%) | 17 (4.1%) | 17 (7.8%) | 24 (19.2%) | ||
| - Chemotherapy | 25 (3.1%) | 58 (3.6%) | 70 (4.1%) | 32 (5.5%) | 16 (3.8%) | 1 (0.5%) | 0 (0%) | ||
| Surgery | 275 (33.7%) | 585 (36.6%) | 614 (35.9%) | 182 (31.4%) | 172 (41.1%) | 82 (37.6%) | 42 (33.6%) | ||
| - Surgery alone | 119 (14.6%) | 265 (16.6%) | 314 (18.3%) | 120 (20.7%) | 110 (26.3%) | 49 (22.5%) | 34 (27.2%) | ||
| - Surgery and radiotherapy | 140 (17.1%) | 268 (16.8%) | 269 (15.7%) | 55 (9.5%) | 56 (13.4%) | 33 (15.1%) | 8 (6.4%) | ||
| - Surgery and chemoradiotherapy | 16 (2.0%) | 52 (3.3%) | 31 (1.8%) | 7 (1.2%) | 6 (1.4%) | 0 (0%) | 0 (0%) | ||
| Radiotherapy | 511 (62.5%) | 931 (58.3%) | 992 (57.9%) | 349 (60.2%) | 214 (51.1%) | 118 (54.1%) | 59 (47.2%) | ||
| - Radiotherapy | 418 (51.2%) | 728 (45.6%) | 812 (47.4%) | 292 (50.3%) | 188 (44.9%) | 104 (7.7%) | 57 (45.6%) | ||
| - Chemoradiotherapy | 91 (11.1%) | 190 (11.9%) | 156 (9.1%) | 46 (7.9%) | 15 (3.6%) | 6 (2.8%) | 0 (0%) | ||
| - Bioradiotherapy | 2 (0.2%) | 13 (0.8%) | 24 (1.4%) | 11 (1.9%) | 11 (2.6%) | 8 (3.7%) | 2 (1.6%) | ||
| Induction chemotherapy | No | 395 (48.3%) | 833 (52.1%) | 1,019 (59.5%) | 397 (68.4%) | 350 (83.5%) | 206 (90.5%) | 123 (98.4%) | <0.001 |
| Yes | 422 (51.7%) | 765 (47.9%) | 693 (40.5%) | 183 (31.6%) | 69 (16.5%) | 12 (5.5%) | 2 (1.6%) | ||
Survival analyses according to age stratification in the test set (n = 5,469):
OS curves at 20 years and DSS curves at 5 years according to patients’ age at time of diagnosis of the index tumor are shown in Figure 1. An orderly decrease in OS was observed as the age group increased (P<0.001). Conversely, there were significant differences in DSS only at the expense of patients with an age equal to or greater than 80 years-old (P<0.001). Analyzing only patients under 80 years-old, there were no differences in DSS based on age at diagnosis of the index tumor (P=0.623). Twenty-year OS, 5-year DSS, and the corresponding hazard ratios obtained with the Cox proportional hazards model for the test set are shown in Supplementary Table 1.
Figure 1.
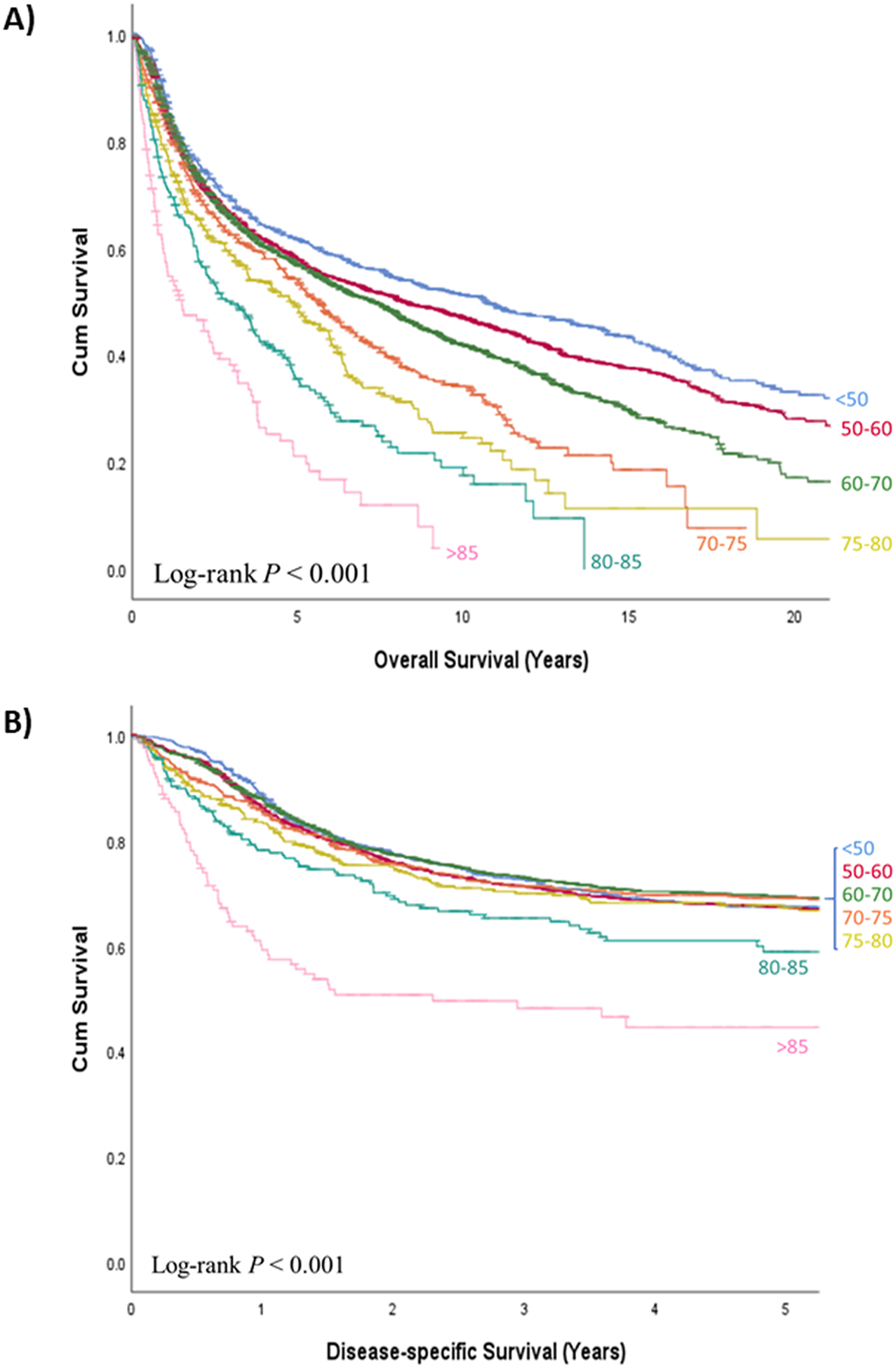
a) Overall survival and b) disease-specific survival curves according to age (in years) at time of diagnosis of the head and neck index tumor in the test set (n = 5,469)
Supplementary Figure 1 shows the OS and DSS curves according to age at time of diagnosis of the index HNSCC tumor excluding patients who were treated with palliative intent. Similar to the analyses in the entire cohort, an orderly decrease in OS as age increased, and significant differences in DSS at the expense of patients older than 80 years-old (P<0.001) were observed. There were no significant differences in DSS in patients under 80 years-old treated with curative intent (P=0.171).
Table 3 shows the 5-year DSS according to the age group for the entire test set cohort and depending on the type of treatment performed (in this case excluding patients treated palliatively). There were significant differences in DSS based on age groups for patients treated surgically (P=0.05), and for patients treated with radiotherapy (P<0.001). For patients treated with surgery (including cases treated with adjuvant radiotherapy or chemoradiotherapy), differences in survival appeared at the expense of patients older than 80 years-old, who had worse survival. There were no differences in DSS for patients treated with surgery if they were younger than 80 years-old (P=0.993). Regarding patients treated with radiotherapy (including patients treated with chemoradiotherapy or bioradiotherapy), differences in survival appeared at the expense of patients older than 85 years-old, who had worse survival. There were no significant differences in DSS for patients treated with radiotherapy under the age of 85 years-old (P=0.077).
Table 3.
Disease-specific survival according to age at time of diagnosis of the head and neck index tumor in the test set (n = 5,469)
| Age | 5-year disease-specific survival (95% CI) | ||
|---|---|---|---|
| All patientsa
(n = 5,469) |
Curative intent | ||
| Surgery (n = 1,952) |
Radiotherapy (n = 3,174) |
||
| <50 years | 67.4 % (64.1–70.7%) | 75.2% (69.9–80.5%) | 67.4% (63.1–71.7%) |
| 50–60 years | 67.3% (64.9–69.7%) | 74.6% (70.9–78.3%) | 68.7% (65.6–71.8%) |
| 60–70 years | 69.4% (67.0–71.8%) | 74.9% (71.2–78.6%) | 73.5% (70.6–76.4%) |
| 70–75 years | 69.1% (65.2–73.0%) | 75.6% (68.9–82.3%) | 75.9% (71.2–80.6%) |
| 75–80 years | 67.2% (62.3–72.1%) | 73.4% (66.0–80.8%) | 72.5% (65.8–79.2%) |
| 80–85 years | 58.9% (51.5–66.3%) | 61.5% (48.0–75.0%) | 65.7% (56.1–75.3%) |
| >85 years | 44.6% (34.6–54.6%) | 64.1% (48.0–80.2%) | 49.6% (34.1–65.1%) |
Including patients treated with palliative intention
Table 4 shows the cause of death based on age at diagnosis of the HNSCC index tumor for the 3,214 patients who died during the study period. We observed a trend of higher proportion of deaths associated to the index tumor in the extreme ages (<50 years-old and > 85 years-old), and as the age increased there was a progressive decrease in the percentage of deaths associated with second primaries and a trend towards an increase in the proportion of deaths associated with non-tumor-related causes.
Table 4.
Cause of death for patients who died during the study follow-up period based on patients’ age at time of diagnosis of the head and neck index tumor in the test set (n = 3,214)
| Age | CAUSE OF DEATH N (%) |
|||
|---|---|---|---|---|
| Index tumor | Second primary | Non-tumor-related | Total | |
| <50 years | 264 (60.4%) | 136 (31.1%) | 37 (8.5%) | 437 (100%) |
| 50–60 years | 515 (56.7%) | 298 (32.8%) | 96 (10.6%) | 909 (100%) |
| 60–70 years | 506 (50.9%) | 317 (31.6%) | 171 (17.2%) | 994 (100%) |
| 70–75 years | 173 (47.7%) | 98 (27.0%) | 92 (25.3%) | 363 (100%) |
| 75–80 years | 125 (47.9%) | 55 (21.1%) | 81 (31.0%) | 261 (100%) |
| 80–85 years | 78 (50.3%) | 21 (13.5%) | 56 (36.1%) | 155 (100%) |
| >85 years | 63 (66.3%) | 6 (6.3%) | 26 (27.4%) | 95 (100%) |
| Total | 1,724 (53.6%) | 931 (29.0%) | 559 (17.4%) | 3,214 (100%) |
According to the results of a multivariable analysis (Table 5), the variables that were significantly correlated with DSS were patientś performance status measured by the Karnofsky index, primary location of the tumor, tumor stage, and patient’s age at time of diagnosis. Compared to patients under 80 years-old, patients that were 80–85 years-old had a 1.50 times higher risk of disease-specific death (95% CI: 1.19–1.89, P=0.001), and patients over 85 years-old had a 2.19 times higher risk (95% CI: 1.68–2.87, P<0.001).
Table 5.
Multivariable analysis considering disease-specific survival as dependent variable in the test set (n = 5,469)
| HR (95% CI) | P | ||
|---|---|---|---|
| Sex | Male | 1 | |
| Female | 0.93 (0.78–1.11) | 0.477 | |
| Tobacco and alcohol use | No | 1 | |
| Moderate | 1.07 (0.85–1.34) | 0.534 | |
| Severe | 1.15 (0.94–1.41) | 0.155 | |
| Karnofsky index | 90% | 1 | |
| 80% | 1.70 (1.50–1.91) | <0.001 | |
| ≤70% | 3.36 (2.84–3.97) | <0.001 | |
| Location of the primary tumor | Nasopharynx | 1 | |
| Oral cavity | 1.24 (0.98–1.57) | 0.071 | |
| Oropharynx | 1.41 (1.13–1.76) | 0.002 | |
| Hypopharynx | 1.32 (1.04–1.69) | 0.021 | |
| Larynx | 0.54 (0.43–0.69) | <0.001 | |
| Unknown primary | 1.46 (1.07–1.99) | 0.015 | |
| Overall stage | I-II | 1 | |
| III-IV | 3.57 (3.11–4.09) | <0.001 | |
| Age | <80 years | 1 | |
| 80–85 years | 1.50 (1.19–1.89) | 0.001 | |
| >85 years | 2.19 (1.68–2.87) | <0.001 | |
Patientś characteristics and survival analyses according to age stratification in the validation set (n = 2,082):
Patientś characteristics of the validation set are shown in Supplementary Table 2. Only 192/2,082 (9%) of the cohort were older than 80 years-old, 1,174/2,082 (56%) were men and history of tobacco and alcohol use was reported in 1,378/2,082 (66%) and 1,486/2,082 (71%), respectively. Comorbidities described as a Washington University Head and Neck Comorbidity Index (WUHNCI) score of 1 or above were present in 563/2,082 (27%) of the cohort. [15] In terms of pathological stage using the American Joint Committee on Cancer (AJCC) 8th edition TNM classification, 849/2,082 (41%) had advanced stage tumors (III-IV). [16] All patients were treated primarily with surgery, 608/2,082 (29%) received adjuvant radiotherapy and 126/2,082 (6%) received adjuvant chemoradiotherapy.
OS and DSS curves are shown in Figure 2. An ordered decrease in OS was observed as the age category increased. Conversely, only patients in the last 2 age categories (80–85 years-old and > 85 years-old) had a poorer DSS compared to younger patients, reaching statistically significant differences in the group of patients between 80 and 85 years-old (Pairwise log-rank, P=0.046). Twenty-year OS, 5-year DSS, and the corresponding hazard ratios obtained with the Cox proportional hazards model for the validation set are shown in Supplementary Table 3.
Figure 2.
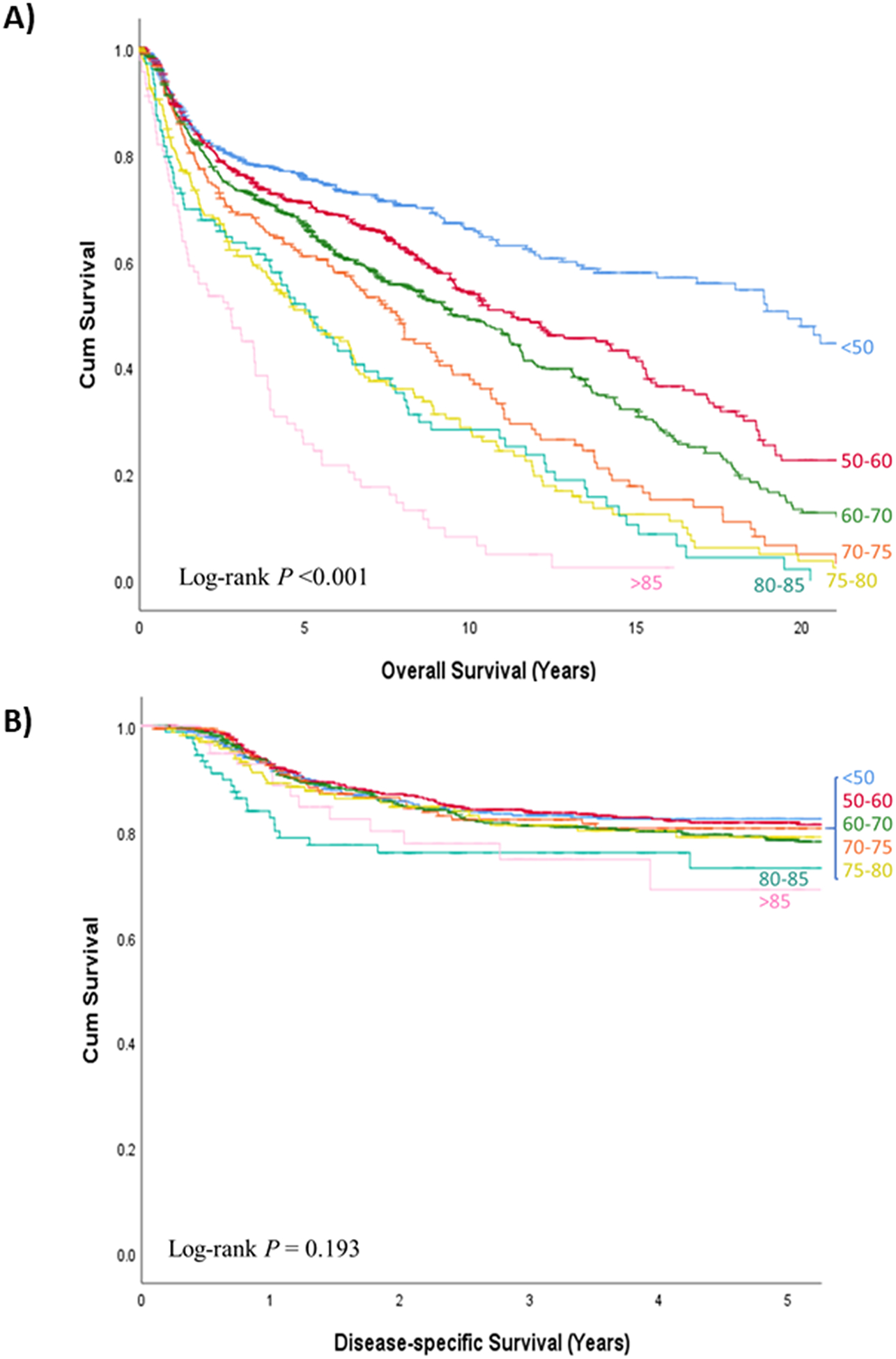
a) Overall survival and b) disease-specific survival curves according to age (in years) at time of diagnosis of the index tumor in the validation set (n = 2,082)
DISCUSSION
According to our results, although patientś characteristics and type of treatment were modified according to patients’ age at time of diagnosis of the HNSCC index tumor, there were no differences in DSS until 80 years-old. From that age onward, patients had a significant decrease in DSS with increasing age.
The first thing to take into consideration is that throughout the study period we were able to observe a progressive increase in the mean age at diagnosis of the HNSCC index tumor, which rose from 59.9 years in the group diagnosed during the first decade (1985–1994) to 64.2 years for those diagnosed during the last period (2005–2016). Several authors have described an increase in the mean age of patients with HNSCC over the past decades. [17–19]
Based on the existence of differences in pharmacokinetics and treatment tolerance, most clinical trials in oncology consider the age of 70 as the reference to define a patient as elderly. In studies conducted on patients with HNSCC, patients with ages above 60, 65, 70, 75 or 80 years-old have been considered as elderly patients, and others have defined different categories within patients over 65 years-old. [2–4,7–11,20–24] The rationale to establish a certain cutoff is different across the studies: definition of advanced age established by the national health system, retirement age, or age limit for the inclusion of patients in clinical trials.
There is a general agreement that there is a decrease in OS as patients’ age increases, especially associated with comorbidities and intercurrent pathology, which increases progressively with age. [2,8–10] On the contrary, there is no consensus on the influence of age on DSS, with some authors who report lower DSS in elderly patients, while other authors suggest that age does not significantly influence disease control. [8,9,11] We believe that this discrepancy is a consequence of the variability in the type of population analyzed in each of these studies, physician bias in the treatment of elderly patients as well as the age cutoff used to define elderly patients.
It is natural that as patientś age increases there is a tendency to modify the treatment protocols, with an increase in the number of patients who refuse treatment, an increase in the number of cases that are not considered eligible for treatment with curative intent, and a decrease in the percentage of patients treated with extensive surgeries, combined treatments, or treatments that include chemotherapy. [2–7] As a consequence, the proportion of patients who do not receive standard treatment is higher in the elderly population. [25] According to our results, the proportion of patients who were not considered candidates for curative treatment increased progressively as the age of the patients increased, reaching nearly 20% (24/125) in the group of patients over 85 years-old. In addition, we observed a decrease in the percentage of patients in whom the treatment included some type of chemotherapy, either as induction treatment or concomitant with radiotherapy.
Several authors have analyzed the outcomes in elderly patients with HNSCC treated with surgery or chemoradiotherapy without finding significant differences in disease control based on patientś age. [11,21,26,27] The results of these studies should be interpreted with caution, taking into account the selection bias created when including only the elderly patients who were considered candidates for aggressive treatment. In addition to the chronological age, the patient’s biological age is an element to consider when deciding treatment options. While the term elder refers to chronological age, biological age depends on the fragility and the decrease in resistance, which is related to both chronological age and comorbidities. Although chronological age is an important consideration, biological age should be the dominant factor in selecting the best treatment for patients with HNSCC.
One criterion that can be considered to define the elderly population in oncology is to identify the age beyond which a deterioration in DSS occurs. We observed a decrease in disease control starting at the age of 80 years-old in our patients. Although there were appreciable differences in patientś characteristics and treatment protocols according to age in patients under 80 years-old at time of diagnosis, these differences did not translate into the final control of the disease (DSS). However, over the age of 80 years-old, we observed a significant decrease in DSS, which was more profound in patients who were older.
When analyzing the competing causes of death, we were able to identify differences according to age (Table 4). The proportion of deaths due to second primaries progressively decreased as a result of the patientś shorter time exposure as their age at diagnosis of the index HNSCC tumor increased. On the other hand, we observed a progressive increase in mortality associated with non-tumor-related causes as age increased, which was related to the increase in morbidity and comorbidities. Moreover, studies conducted in patients treated surgically or with combinations of radiotherapy and chemotherapy have found an increase in toxicity and treatment-related complications in elderly patients. [2,10]
The results of the multivariable analysis suggested an independent prognostic relationship between age at diagnosis and DSS. However, this analysis must be interpreted with caution as our model does not control for physician bias in the treatment of elderly patients. It is possible that the decrease in DSS is largely a consequence of the modifications in treatment protocols applied to patients with advanced age at time of diagnosis i.e. surgeons, radiation and medical oncologists are likely to be less aggressive in their treatment when patients are older than 80 years of age.
We performed a sub-analysis in the group of patients that were treated with curative intent and we observed similar results (Supplementary Figure 1). Moreover, we selected as the validation set a cohort of patients with OSCC treated with primary surgery with curative intent in a different health care system, reducing the possible bias introduced by palliative patients and the possible bias of modifications in treatment protocols that we would encounter with other tumor sites where non-surgical treatments are considered first-line options.
In the external validation, the OS and DSS curves from the validation set (Figure 2) mimicked the OS and DSS curves from the test set (Figure 1), even though the differences in DSS for the oldest patients (> 85 years-old) did not reach statistical significance in the validation set. Furthermore, the external validation has been carried out in an independent cohort with geographical and clinicopathological differences, which suggests that our results might be extendable outside our population.
A salient limitation of our study is that, although we observed a worsening of the performance status measured according to the Karnofsky index with increasing age at diagnosis, we did not have more detailed information regarding the burden of comorbidities associated with patients at that time point. Another caveat of this study is that we could not assess retrospectively how many patients treated with curative intent from the older groups had less than standard of care treatment (reduced surgery extent, lesser adjuvant chemo or radiotherapy, etc.), and this could be a potential confounder.
Although limited by the aforementioned biases, our study provides data related to a large cohort of patients followed prospectively over a prolonged period of time, and adds an external validation in an independent large cohort from another continent, which ensures the robustness of the results achieved. Therefore, we believe that age at diagnosis should not be a treatment selection criterion per se and should not be a deterrent to appropriate treatment if the patient is able to tolerate it.
CONCLUSIONS
There were no significant differences in DSS according to age at diagnosis for patients with HNSCC if the age at diagnosis was less than 80 years-old. From that age onward, we observed a progressive decrease in DSS as the patient’s age at diagnosis increased. Our results were validated in a large independent cohort of OSCC with geographical and clinicopathological differences.
Supplementary Material
Funding:
This study was supported by grants from Instituto de Salud Carlos III (FIS PI19/01661), Fondo Europeo de Desarrollo Regional (FEDER) - A Way to Build Europe, Fundación Alfonso Martín Escudero and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest pertinent to this work.
Data Availability Statement
Research data are not shared.
REFERENCES
- 1.https://data.worldbank.org. Last accessed 1/13/2020.
- 2.Sarini J, Fournier C, Lefebvre JL, et al. : Head and neck squamous cell carcinoma in elderly patients: a long-term retrospective review of 273 cases. Arch Otolaryngol Head Neck Surg 2001;127:1089–1092. [DOI] [PubMed] [Google Scholar]
- 3.van der Schroeff MP, Derks W, Hordijk GJ, de Leeuw RJ: The effect of age on survival and quality of life in elderly head and neck cancer patients: a long-term prospective study. Eur Arch Otorhinolaryngol 2007;264:415–422. [DOI] [PubMed] [Google Scholar]
- 4.Italiano A, Ortholan C, Dassonville O, et al. : Head and neck squamous cell carcinoma in patients aged > or = 80 years: patterns of care and survival. Cancer 2008;113:3160–3168. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg D, Mackley H, Koch W, et al. : Age and stage as determinants of treatment for oral cavity and oropharyngeal cancers in the elderly. Oral Oncol 2014;50:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, Kim SD, Shim YJ, et al. : Is there any age cutoff to treat elderly patients with head and neck cancer? Comparing with septuagenarians and octogenarians. J Korean Med Sci 2016;31:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juarez JE, Choi J, St John M, et al. : Patterns of care for elderly patients with locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2017;98:767–774. [DOI] [PubMed] [Google Scholar]
- 8.Yang CC, Su YC, Lin YW, et al. : Differential impact of age on survival in head and neck cancer according to classic Cox regression and decision tree analysis. Clin Otolaryngol 2019;44:244–253. [DOI] [PubMed] [Google Scholar]
- 9.Sommers LW, Steenbakkers RJHM, HP Bijl, et al. : Survival patterns in elderly head and neck squamous cell carcinoma patients treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2017;98:793–801. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SH, Roh JL, Choi SH, et al. : Incidence and risk factors for morbidity and mortality in elderly head and neck cancer patients undergoing major oncological surgery. J Cancer Res Clin Oncol 2016;142:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teymoortash A, Bohne F, Kissing L, et al. : Oncological and surgical outcome of total laryngectomy in combination with neck dissection in the elderly. Eur Arch Otorhinolaryngol 2016;273:1825–1833. [DOI] [PubMed] [Google Scholar]
- 12.Leon X, Orus C, Quer M: Design, maintenance, and exploitation of an oncologic database for patients with malignant tumors of the head and neck. Acta Otorrinolaringol Esp 2002;53:185–190. [DOI] [PubMed] [Google Scholar]
- 13.Warren S, Gates O: Multiple malignant tumors: a survey of literature and statistical study. Am J Cancer 1932;51:1358–1414. [Google Scholar]
- 14.Zanoni DK, Montero PH, Migliacci JC, et al. : Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol 2019;90:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL: Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg 2002;128:1172–1179. [DOI] [PubMed] [Google Scholar]
- 16.Amin MB, Edge S, Greene F, et al. (eds). AJCC Cancer Staging Manual. 8th ed New York: Springer, 2017. [Google Scholar]
- 17.Chen SW, Zhang Q, Guo ZM, et al. : Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag Res 2018;10:4523–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windon MJ, D’Souza G, Rettig EM, et al. : Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer 2018;124:2993–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaseviciene L, Gurevicius R, Obelenis V, et al. : Trends in laryngeal cancer incidence in Lithuania: a future perspective. Int J Occup Med Environ Health 2004;17:473–477. [PubMed] [Google Scholar]
- 20.Bonomo P, Desideri I, Loi M, et al. : Elderly patients affected by head and neck squamous cell carcinoma unfit for standard curative treatment: Is de-intensified, hypofractionated radiotherapy a feasible strategy? Oral Oncol 2017;74:142–147. [DOI] [PubMed] [Google Scholar]
- 21.Müller von der Grün J, Martin D, Stöver T, et al. : Chemoradiotherapy as definitive treatment for elderly patients with head and neck cancer. Biomed Res Int 2018;2018:3508795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woody NM, Ward MC, Koyfman SA, et al. : Adjuvant chemoradiation after surgical resection in elderly patients with high-risk squamous cell carcinoma of the head and neck: A National Cancer Database analysis. Int J Radiat Oncol Biol Phys 2017;98:784–792. [DOI] [PubMed] [Google Scholar]
- 23.L’Esperance HE, Kallogjeri D, Yousaf S, et al. : Prediction of mortality and morbidity in head and neck cancer patients 80 years of age and older undergoing surgery. Laryngoscope 2018;128:871–877. [DOI] [PubMed] [Google Scholar]
- 24.Porceddu SV, Haddad RI: Management of elderly patients with locoregionally confined head and neck cancer. Lancet Oncol 2017;18:e274–e283. [DOI] [PubMed] [Google Scholar]
- 25.Derks W, de Leeuw RJ, Hordijk GJ: Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head NeckSurg 2005;13:92–96. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Zhang B, Huang Z, et al. : Study of surgical treatment for elderly patients with head and neck cancer. Int J Oral Maxillofac Surg 2018;47:824–829. [DOI] [PubMed] [Google Scholar]
- 27.Amini A, Jones BL, McDermott JD, et al. : Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer 2016;122:1533–1543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


