Abstract
Most vasovagal syncope patients lose consciousness due to hypotension before severe bradycardia /asystole occurs. Patients that benefit from dual-chamber pacing are typically older with highly symptomatic, late-onset syncope with short/no prodrome and documented severe cardioinhibition. Tilt-testing is of value in patients with recurrent syncope to identify important hypotensive susceptibility as a result of reduction in stroke volume. A negative tilt test in patients with implantable/insertable loop recorder documented spontaneous asystole is associated with lower syncope recurrence rates after pacing. Pacing may be more effective if triggered by sensor detection of a parameter changing earlier in the reflex than bradycardia when stroke volume may still be preserved. In this regard, detection of right ventricular impedance offers promise. In conclusion, guidelines remain conservative in recommending pacing in vasovagal syncope to a very limited subset of symptomatic older patients with clearly documented cardioinhibition in particular regard to the timing of loss of consciousness. Understanding the physiology of VVS and its different forms will permit application of well-timed and appropriate pacing that may yield further benefit for highly symptomatic patients.
Keywords: vasovagal syncope, reflex syncope, pacemaker, pacing, head-up tilt test, asystole, physiology, ILR
Introduction
Asystole occurs in vasovagal syncope (VVS), known as severe cardioinhibition,1 but it usually follows hypotension and may even occur after loss of consciousness.2 It is tempting for the electrophysiologist, having seen asystole on tilt or implantable loop recorder (ILR) in association with syncope to offer permanent pacing to combat bradycardia/asystole. This approach fails to address the physiology of the vasovagal reflex, in particular the relationship between timing of asystole and the moment of loss of consciousness and, further, the details of how pacing is triggered. The mechanism by which pacing is effective, when it ameliorates symptoms, has also received little attention.
Certain aspects of this clinical conundrum are already established:
During tilt-testing asystole occurs late in majority of patients when blood pressure (BP) is already very low, implying that pacing may have little effect.1,2
When bradycardia/asystole present during tilt-testing, pacing can prolong vasovagal presyncope by attenuating extreme hypotension, thereby offering patients time to protect themselves.3
Physiological studies, performed in supine/sitting position, have shown that high rate pacing (>80 bpm) is inefficient in increasing CO.4
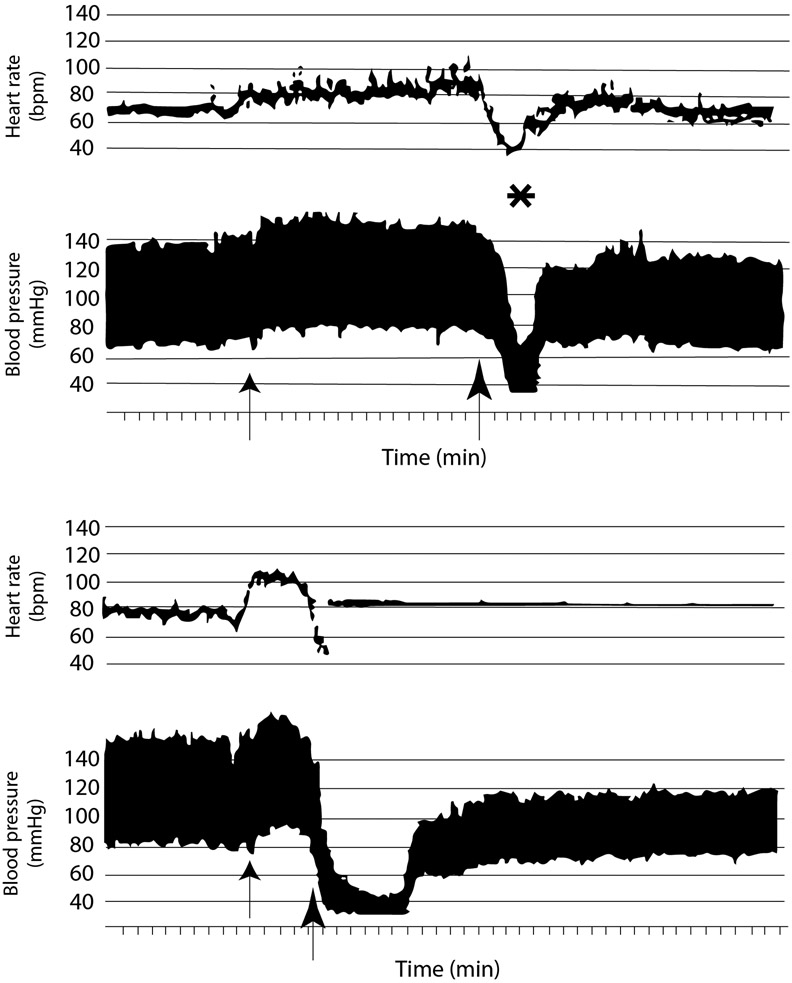
Pacing early, before/coincident with HR fall, seems more effective, but underlying hemodynamics need further study (Figure 1).5,6
Figure 1.
Upper panel shows HR/BP during tilt. First arrow denotes tilt-up and second denotes start of bradycardia. Star denotes loss of consciousness. Lower panel also shows heart rate/blood pressure in second tilt of same patient. First arrow denotes tilt-up; second arrow denotes onset of symptoms. Fall in heart rate was steep. Pacing was triggered at heart rate 45bpm and delivered at 90ppm. With pacing, blood pressure was low (<50 mmHg systolic), but patient remained conscious while upright for ~5 min. Petersen et al. (1994).5 Reproduced with permission.
Finally, tilt-testing results may not replicate spontaneous VVS events,7 but may identify those with hypotensive susceptibility by downward gravitational blood pooling and splanchnic venodilation causing hypotension preceding loss of consciousness/asystole.2
Although there are physiological arguments that pacing cannot work, there are recent positive pacing clinical trials in vasovagal syncope that show clear benefit in a subgroup of patients. This review discusses these trials, the different pacemaker modes and what we have learnt from them in selection of patients who benefit from pacing and to what extent.
Recent clinical trials of pacing in vasovagal syncope: ISSUE-3 (Randomized controlled trial - RCT and Registry), SUP-2 (Prospective Registry) and SPAIN (RCT)
The results of ISSUE-3, SUP-2, SPAIN and ISSUE-3 Registry8-12 (Table 1), four recent studies that show some different aspects of pacing in clinically defined VVS which prompt a reconsideration of the role of pacing in amelioration of VVS, will be discussed. As in previous studies on pacing in reflex syncope, small numbers of patients-included by a large number of centers (~1 inclusion center/year) confirm that such patients are rare.
Table 1:
Recent studies of pacing in vasovagal syncope
| Author | Year | Intervention | Inclusion criteria | Cardioinhibitory criteria |
N | Inclusions/ centre/yr |
Syncope, % | P |
|---|---|---|---|---|---|---|---|---|
| Brignole et al.8 | 2012 | DDD/RDR vs. ODO | ≥40y old, ≥3 syncopes with a clinically presumed neurally-mediated mechanism in previous 2y, severe clinical presentation to warrant specific treatment | HUTT+ ILR ≥3s asystole or non-syncopal ≥6s asystole | 77 | 0.76 | 21 vs. 48.7 | 0.039** |
| Brignole et al.9,10 | 2016 | DDD/RDR vs. ILR | ≥40y old, ≥2 syncopes last y or ≥3 syncopes last 2y, absence of prodromes | HUTT+ with (observational) ≥3s asystole or CI CSM or ILR >3s asystole + syncope or >6s asymptomatic | 201 | 2 | 18 vs. 30.3 | 0.01 |
| Baron-Esquivias et al.11 | 2017 | CLS vs. DDI 30ppm | ≥40y old, ≥5 VVS lifetime, ≥2 last y | HUTT+ with HR <40 bpm for ≥10s or > 3s asystole | 46 | 0.79 | 8.7 vs. 46 | 0.017† |
p : p-value of relative risk reduction
p-value of log rank of time to first recurrence
p-value for number of patients with ≥50% reduction of recurrences
bpm,: beats per minute; CI CSM: cardioinhibitory carotid sinus massage; CLS: DDD pacemaker with closed loop system; DDD: DDD mode; HR: heart rate; HUTT(+): (positive) head-up tilt test; ILR: implantable loop recorder; NS: non-significant; PM: pacemaker; RDR: Rate-drop-response; ODO: sensing only mode; HUTT: Head-up tilt test; ILR: implantable loop recorder; CICSM: Cardioinhibitory carotid sinus massage; CLS: Closed loop system; DDI: see pacemaker modes13; HR: heart rate; SBR: sudden-brady-response;; VVS: vasovagal syncope; y: year(s)
In 2012, ISSUE-3,8 a multicenter, prospective, randomized, double-blind controlled trial compared dual chamber pacing (DDD) + Rate-drop-response (RDR) with sensing-only mode (ODO)13 in reflex syncope patients, aged ≥40 years. Carotid sinus/cardiac syncope were excluded. Patients were of mean age 63±12 years, had frequent syncope (median last 2 years, 4-5 episodes) often without prodrome (~50%). The first syncope had occurred at mean age 45 years. Tilt was not obligatory but was performed in 87%; tilt data were not used for inclusion. All patients received ILRs (n=511) and when asystole (sinus arrest or atrioventricular block (AVB) ≥3 s during syncope (n=72), or ≥6 s asymptomatic/presyncope) was documented (n=17), patients received pacemakers. Seventy-seven of 89 patients were assigned to DDD12 with RDR or to ODO.13 After 24 months follow-up, syncope recurrence was 25% in paced vs. 49% in unpaced patients (p<0.04); the authors concluded that pacing was effective but less than perfect treatment for clinically defined reflex syncope when asystole was documented by ILR.
An ISSUE-3 Registry sub-study compared ILR and tilt data.12 Twenty-one percent of 136 patients had asystole during tilt, but 60% showed asystole on ILR. With tilt asystole, there was 86% chance of asystole on ILR. Surprisingly, when pre-implant tilt-testing was negative and ILR recorded spontaneous asystole, pacing was effective; syncope recurrence in pre-implant tilt-positive was 31% vs. 8% in tilt-negative.12 The 8% recurrence rate in tilt-negative patients is comparable with 3% syncope recurrence in paced AVB.14,15 Similar observations have been made in tilt-negative carotid sinus syndrome (CSS) patients.16
Attempting an explanation, a recent viewpoint concluded that tilt-testing reveals a hypotensive susceptibility, co-existing with cardioinhibition in vasovagal and other reflex syncope, playing an important part in syncope recurrence.17 The hypotensive susceptibility can be attributed to decreased stroke volume /preload as a result of gravitational pooling and splanchnic venodilation18 in combination leading to brain hypoperfusion preventing pacing efficacy.
The Syncope Unit Project 2 (SUP-2) study followed in 2015/6.9,10 This multicenter, prospective, observational study in older patients assessed an algorithm of diagnosis/therapy based on European Society of Cardiology Guidelines.1 Patients with severely impaired quality of life, unpredictable (short/absent prodromes) and recurrent clinically diagnosed reflex syncope were included. Patients were aged ≥40years (mean, 73.3±11 years), the age at first syncope was 65±19 years. Patients first underwent carotid sinus massage (CSM - method of symptoms)16 If positive for cardioinhibition patients were paced without further tests. Negative CSM patients proceeded to tilt-testing where, if positive with cardioinhibition (=VASIS IIB19), they were paced. However, if they were tilt-negative or demonstrated no asystole (=VASIS I,IIA,III),19 they received an ILR. If ILR showed asystole, as in ISSUE-3, patients were also paced.
In total 137 patients received pacing: CSS was the most frequent indication (n=78). Asystolic tilt-tests occurred in 38 patients; ILR asystole in 21. The 3-year syncope recurrence rate in patients with asystole on tilt or ILR was 23% and 24% respectively. One-hundred-and-forty-two patients who received ILR but no pacemaker, had a 3-year syncope-recurrence rate of 43%. The SUP-2 study 9,10 reproduced findings of ISSUE-3 sub-study12 that patients with negative-tilt (20/137) have more benefit from pacing (5% recurrence) than those with a positive tilt-test (61/137 or 23%).
In a recent meta-analysis of four ILR studies including 1046 patients affected by certain or likely reflex syncope, asystole was captured by ILR in 201 patients (19%).20 Among those asystolic, 105/201 (52%) were classified as slowing sinus rhythm followed by sinus arrest without escape rhythm (1a), 23/201(11%) as sinus bradycardia + AVB (1b) and 41/201(20%) as AVB without slowing sinus rhythm (1c).21 In 121 patients receiving a pacemaker, syncope recurred in 18 patients. At three years, syncope recurrence was estimated to occur in 2% of tilt-negative (upper confidence interval 6%) and in 33% of tilt-positive patients (confidence interval 13-53%). The 2% recurrence rate in tilt-negative patients in this meta-analysis is similar to that observed in paced patients with intrinsic AVB.14,15 Undiagnosed intrinsic atrioventricular conduction abnormality might have played a role.22 However, it is also possible that the AVB was vagally induced.22 The authors describe three types of AVB, intrinsic (ventricular conduction abnormality), extrinsic vagally-induced and extrinsic idiopathic (possibly due to hypoadenosinemia).22 The latter two forms cover patients in both ISSUE-2 & 3.8,12,23 In the ISSUE studies, there were no clinical differences between those with AVB and others with more typical VVS onset including initial sinus slowing21 Adenosine levels were not measured in ISSUE-studies.8,13,23 Further work is required for better understanding of these phenomena. This should include Modelflow analysis in patients with VVS analyzing both cardioinhibition and hypotensive susceptibility. The ISSUE-38 and SUP-2 studies9,10 have shown that clinical diagnosis of VVS based on history in combination with careful interpretation of tilt and ILR-data allow selection of patients who will benefit from pacing and to what extent. More recently, VVS pacing studies have focused on DDD-Closed loop system (CLS) pacemakers hypothesizing that early pacing is more effective.5,6 In 2017, the SPAIN study,11 a randomized, prospective, double-blind, cross-over, multicenter trial, compared DDD-CLS (lower-rate (LR) 45ppm, CLS rate limit 110ppm, dynamic setting “high”) with DDI pacing (LR 30ppm, DDI-30 -essentially ineffective-called 'sham'),13 crossing-over after 12 months or after syncope in 46 patients (younger than in ISSUE-38) with cardioinhibitory-VVS. Patients were aged ≥40 years (mean, 56.3±10.63 years) with ≥5 life-time VVS, ≥2 VVS in last year and positive-tilt with asystole ≥3s or HR<40 bpm for ≥10s. SPAIN11 did not select patients on relative absence of prodrome or ILR documentation of asystole in contrast to ISSUE-3.8,12 Only four (8.7%) patients had syncope recurrence during DDD-CLS, compared with 21 (46%) in DDI-30. Pacing with DDD-CLS (8.7%) approached the effectiveness of pacing in AVB (3%).14,15 BIOSync-study has almost completed recruitment of 128 tilt-positive (VASIS-2B) patients to a randomized, double-blind, controlled trial of CLS-on vs. CLS-off.24 In a recent tertiary center consecutive series of paced patients predominantly with reflex syncope, both HUT and ILR predicted similar recurrence rates (~15%).25 In contrast to this report, the trials discussed above8-12 did not document if recurrences were associated with treated hypertension, atrial fibrillation or renal failure, factors that should be better monitored in future trials. In this respect, one small trial, STOP-VD,26 has given evidence that reduction in hypotensive medication for hypertension reduces syncope recurrence.
Timing and mode of pacing relevant to vasovagal syncope
When considering pacing in VVS, it is necessary to consider timing of onset and duration of pacing. A Dutch group led by van Dijk has shown that during tilt in a relatively young group of patients one third of patients lose consciousness before intense bradycardia/asystole2. In order to avoid ineffective pacing, it will be necessary to define accurately the timing of syncope by head-flop using video or precise marking of the event in the recorded data by the supervising clinician,2 so as to relate the two events (asystole and loss of consciousness) in selection of those who will benefit from pacing in real life. Little information exists with respect to the duration of pacing necessary. It appears that several minutes are necessary with CLS pacing to prevent syncope or extend duration of pre-syncope, ultimately pacing is inhibited by a rising sinus rate in RDR or by falling right ventricular (RV) impedance as in CLS (see also below).
RDR mode of pacing delivery attempted to detect rate-falls that occur in VVS distinct from natural falls on relaxing/sleeping and from precipitous falls at AVB onset.26 Unfortunately, in practice, there is much overlap between these different rate-falls implying that RDR is often falsely triggered. RDR also delivers a programmable higher pacing rate (90-120 ppm) at onset assuming that higher HR in presyncope is more physiological. However, Kurbaan et al. addressed optimal rate selection in this context and concluded that rates >90 bpm gave no greater benefit.4 Some patients have shown benefit from RDR pacing in VVS3 and a minority of them have reported that they experience a prodrome included a feeling of palpitation which coincides with relief of symptoms.
The HR falls late in VVS when preload (central blood volume) and BP are already low18 prompting search for alternative sensed parameters than HR to achieve earlier pacing introduction when stroke volume may still be preserved. RV volume derived from its impedance (detected between two electrodes in RV or one RV with other on pulse-generator can) is presumed to be a tenable alternative because impedance rises much before HR fall during VVS. Impedance rise parallels reduction in preload implying that pacing will occur earlier when there may remain enough blood in the ventricles to pump This facility is available in the CLS device of Biotronik, Berlin, Germany.
Palmisano et al27 compared DDD pacing mode with DDD-CLS pacing during tilt-testing in 30 patients with cardioinhibitory-VVS. DDD was programmed LR 60ppm, and DDD-CLS at LR 60 ppm and sensor-determined rate up to 130ppm. The study was a randomized-crossover design. Patients were 62.2±13.5 years. In DDD, 17 patients (56.7%) had syncope during tilt but not in DDD-CLS, six (20%) had syncope in both modes, three (10%) had syncope in DDD-CLS but not in DDD, and four (13.3%) had no syncope. Thus, CLS pacing was far more effective than DDD pacing supporting the concept of earlier pacing being more effective. Pacing intervention in CLS started at around eight minutes prior to (pre)syncope which implies that a substantial period of pacing during presyncope may be required. Two other acute studies are relevant to these observations.6,28 The first28 showed that short AV-delay VDD pacing failed to prevent syncope in VVS patients when applied from the beginning of tilt denying the possibility of prophylactic pacing and the second6 affirmed in ten severe VVS patients, undergoing four tilts in random order in one day with temporary dual chamber pacing, that early manually triggered pacing, based on physical signs, such as pallor/yawning, performed better than RDR pacing. Both atrioventricular sequential pacing at tilt onset and no pacing were 100% ineffective in prevention of VVS in these severely affected patients. The available data are sparse but are in favour of interventional pacing in an evolving VVS but with no role for prophylactic pacing. Early onset of pacing may possibly also ameliorate the situation where the patient loses consciousness before the onset of asystole but this aspect has not yet been explored.
Should a placebo effect be considered?
Blinding was applied in ISSUE-38/SPAIN11 studies but its effectiveness was not reported. RDR-pacing in ISSUE 38 may have triggered higher-rate pacing, and similarly in DDD-CLS11, causing palpitations, which may have influenced pacing success in two ways: bias results by insufficient blinding, but also offer early warning for patients without prodromes, providing time to sit, lie or employ physical counter-maneuvers. While it is important that blinding is effective,29 especially in VVS with a likely placebo effect,30 it will be difficult completely to dismiss placebo playing a role.
Dilemmas
Currently, two dilemmas exist. First, how can a cardioinhibitory tilt-test be an inclusion criterion for pacing VVS when a positive tilt-test predicts less effectiveness of pacing? Positive-tilt patients, however, still show benefit over no pacing, possibly related to degree of hypotensive susceptibility.12,17 Explanations of the better results of DDD-CLS pacemakers in studies on presumed reflex syncope include 1) induction of early onset pacing by restriction of the fall in venous return as discussed above; 2) a placebo effect; 3) afferent brain or cardiac ganglia signals elicited by pacing the right ventricle at an early point in development of VVS. The latter concept is new but since cardioneuroablation has been shown to influence VVS favourably,31,32 it is believed possible that the mere delivery of electrical stimuli to the right ventricle could have cardiovascular autonomic effects in addition to creation of ventricular dyssynchrony33. These might be sufficient to reverse or modify the evolution of the reflex.34 Additional support for this concept is given by the studies of Palmisano,27 Petersen et al5 and Sutton6 mentioned above.
The second dilemma is closely related and concerns the cause of the observed sudden AVB in patients who are clinically presenting VVS? The clinical contention based on the patient's history is that it is all extrinsic, either vagal or idiopathic (possibly hypoadenosinemia35). Sceptics believe this is undiagnosed intrinsic AVB which is unsupported by other evidence of ventricular conduction tissue disease, such as bundle branch block at assessment or in follow-up. In such situations, where clinical aspects are so important, an expert committee for critical follow up, may have value, as was undertaken in the FAST study.36 Electrophysiological studies might potentially offer a better understanding of this issue but it is doubtful whether they will ever be performed given such good effects of pacing in this patient category. Neither of these dilemmas can be resolved without further work.
In conclusion
Recent clinical trials in VVS have demonstrated benefit of pacing in carefully selected older patients with severe symptoms and clear evidence of intense cardioinhibition. The degree of benefit varies according to hypotensive susceptibility in the reflex as demonstrated by tilt-testing, which predicts pacing outcomes. Also, the timing of asystole in relation to loss of consciousness will help to select patients that will benefit from pacing. From a physiological viewpoint, more research is needed to explain the benefit of pacing and from where it arises. Studies reporting influence of decreased CBV/preload on HR/CO relationship during pacing are needed to select optimal pacing rate/timing.
Future trials need to be blinded and include asking patients at study exit to identify their therapy. Critical follow-up of paced VVS-patients is required. Finally, precise determination of which patients to pace is not yet possible but is becoming more clear with increasing clinical experience and physiological understanding.
Acknowledgments
Funding
JS reports National Institutes of Health grants; NHLBI RO1-HL112736 and NHLBI RO1-HL134674. No other funding.
ABBREVIATIONS:
- AVB
atrioventricular block
- BP
blood pressure
- CBV
central blood volume
- CLS
closed-loop stimulation
- CO
cardiac output
- CSM
carotid sinus massage
- HUT
head-up tilt
- HR
heart rate
- ISSUE
International Study of Syncope of uncertain origin
- LR
Lower-rate
- MAP
mean arterial blood pressure
- ILR
Implantable/insertable loop recorder
- RDR
rate drop response
- RV
right ventricular
- SBP
systolic blood pressure
- SUP
Syncope Unit Project
- SV
stroke volume
- SVR
systemic vascular resistance
- VASIS
Vasovagal syncope International Study
- VVS
vasovagal syncope
Footnotes
Conflicts of interest
AF consultant to Medtronic Inc; patent royalties from ThermoFisher Scientific (biomarkers in syncope), RS consultant to Medtronic Inc., member of Speakers' Bureau of Abbott Laboratories and shareholder in Boston Scientific Inc., Edwards Lifesciences Corp, and holds RDR patent in name only.
References
- 1.Brignole M, Moya A, Lange D et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018. June 1;39(21): 1883–1948. [DOI] [PubMed] [Google Scholar]
- 2.Saal DP, Thijs RD, van Zwet EW, Bootsma M, Brignole M, Benditt DG, van Dijk JG. Temporal relationship of asystole to onset of transient loss of consciousness in tilt-induced reflex syncope. JACC: Clin Electrophysiol 2017;3:1592–1598. [DOI] [PubMed] [Google Scholar]
- 3.Benditt DG, Sutton R, Gammage MD, Markowitz T, Gorski J, Nygaard GA, Fetter J. Clinical experience with Thera DR rate-drop response pacing algorithm in carotid sinus syndrome and vasovagal syncope. The International Rate-Drop Investigators Group. Pacing Clin Electrophysiol 1997; 20: 832–839. [DOI] [PubMed] [Google Scholar]
- 4.Kurbaan AS, Franzen AC, Stack Z, Heaven D, Mathur G, Sutton R. Determining the optimal pacing intervention rate for vasovagal syncope. J Interv Card Electrophysiol 2000;4:585–589. [DOI] [PubMed] [Google Scholar]
- 5.Petersen MEV, Chamberlain-Webber R, Fitzpatrick AP, Ingram A, Williams T, Sutton R. Permanent pacing for cardioinhibitory malignant vasovagal syndrome. Heart 1994;71:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton R, Petersen MEV. Invasive tilt testing: the search for a new sensor to permit earlier pacing therapy in vasovagal syncope In Cardiac Arrhythmias 1995 Ed. Raviele A Springer-Verlag Italia; Milano Italy: 1996. pp132–133. [Google Scholar]
- 7.Moya A, Brignole M, Menozzi C, Garcia-Civera R, Tognarini S, Mont L, Botto G, Giada F, Cornacchia D. Mechanism of syncope in patients with isolated syncope and in patients with tilt positive syncope. Circulation 2001; 104: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 8.Brignole M, Menozzi C, Moya A, et al. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012;125:2566–2571. [DOI] [PubMed] [Google Scholar]
- 9.Brignole M, Ammirati F, Arabia F, Quartieri F, Tomaino M, Ungar A, Lunati M, Russo V, Del Rosso A, Gaggioli G. Assessment of a standardized algorithm for cardiac pacing in older patients affected by severe unpredictable reflex syncopes. Eur Heart J 2015; 36: 1529–1535. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Arabia F, Ammirati F, Tomaino M, Quartieri F, Rafanelli M, Del Rosso A, Vecchi MR, Russo V, Gaggioli G, on behalf of the Syncope Unit Project 2 (SUP 2) investigators. Standardized algorithm for cardiac pacing in older patients affected by severe unpredictable reflex syncope: 3-year insights from the Syncope Unit Project 2 (SUP 2) study. Europace 2016;17:DOI: 10.1093/europace/euv343. [DOI] [PubMed] [Google Scholar]
- 11.Baron-Esquivias G, Morillo CA, Moya-Mitjans A, Martinez-Alday J, Ruiz-Granell R, Lacunza-Ruiz J, Garcia-Civera R, Gutierrez-Carretero E, Romero-Garrido R. Dual-chamber pacing with closed loop stimulation in recurrent reflex vasovagal syncope: The SPAIN Study. J Am Coll Cardiol 2017;70:1720–172. [DOI] [PubMed] [Google Scholar]
- 12.Brignole M, Donateo P, Tomaino M, et al. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014;7:10–16. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein AD, Camm AJ, Fisher JD, Fletcher RD, Mead RH, Nathan AW, Parsonnet V, Rickards AF, Smyth NPD, Sutton R, Tarjan PP. NASPE Policy Statement: The NASPE/BPEG Defibrillation code. Pacing Clin Electrophysiol 1993; 16: 1776–1780. Also published in J Interv Cardiol 1993; 6: 235-239. [DOI] [PubMed] [Google Scholar]
- 14.Langenfeld H, Grimm W, Maish B, Kochsiek K. Course of symptoms and spontaneous ECG inpacemaker patients: a 5-year follow-up study. Pacing Clin Electrophysiol 1988; 11: 2198–2206. [DOI] [PubMed] [Google Scholar]
- 15.Aste M, Oddone D, Donateo P, Solano A, Maggi R, Croci F, Solari D, Brignole M. Syncope in patients paced for atrioventricular block. Europace 2016;18:1735–1739. [DOI] [PubMed] [Google Scholar]
- 16.Solari D, Maggi R, Oddone D, Solano A, Croci F, Donateo P, Brignole M. Clinical context and outcome of carotid sinus syndrome diagnosed by means of the 'method of symptoms'. Europace 2014;16:928–934. [DOI] [PubMed] [Google Scholar]
- 17.Sutton R, Brignole M. Twenty-eight years of research permit reinterpretation of tilt-testing: hypotensive susceptibility rather than diagnosis Eur Heart J 2014; 35: 2211–2212. [DOI] [PubMed] [Google Scholar]
- 18.Jardine DL, Wieling W, Brignole M, Lenders JWM, Sutton R, Stewart J. The pathophysiology of the vasovagal response. Heart Rhythm 2018;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N, Bartoli P, Sutton R. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000;2:66–76. [DOI] [PubMed] [Google Scholar]
- 20.Brignole M, Deharo JC, Menozzi C, Moya A, Sutton R, Tomaino M, Ungar A. The benefit of pacemaker therapy in patients with neurally mediated syncope and documented asystole: a meta-analysis of implantable loop recorder studies. Europace; 2018; 20:1362–1366. [DOI] [PubMed] [Google Scholar]
- 21.Brignole M, Moya A, Menozzi C, Garcia-Civera R, Sutton R. Proposed electrocardiographic classification of spontaneous syncope documented by an implanted loop recorder. Europace 2005; 7: 14–18. [DOI] [PubMed] [Google Scholar]
- 22.Aste M, Brignole M. Syncope and paroxysmal atrioventricular block. J Arrhythmia 2017; 33: 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Moya A, Wieling W, Andresen D, Benditt DG, Vardas P for the International Study on Syncope of Uncertain Etiology [ISSUE 2] Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 24.Brignole M, Tomaino M, Aerts A, et al. Benefit of dual-chamber pacing with Closed Loop Stimulation in tilt-induced cardio-inhibitory reflex syncope (BIOSync trial): study protocol for a randomized controlled trial. Trials 2017;18:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasa E, Ricci F, Holm H, Persson T, Melander O, Sutton R, Hamrefors V and Fedorowski A. Pacing therapy in the management of unexplained syncope: a tertiary care centre prospective study. Open Heart. 2019;6:e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton R, Petersen MEV. First steps toward a pacing algorithm for vasovagal syncope. Pacing Clin Electrophysiol 1997;20:827–828. [DOI] [PubMed] [Google Scholar]
- 27.Palmisano P, Dell'Era G, Russo V, et al. Effects of closed-loop stimulation vs. DDD pacing on haemodynamic variations and occurrence of syncope induced by head-up tilt test in older patients with refractory cardioinhibitory vasovagal syncope: the Tilt test-Induced REsponse in Closed-loop Stimulation multicentre, prospective, single blind, randomized study. Europace 2018;20:859–866. [DOI] [PubMed] [Google Scholar]
- 28.Petersen MEV, Price D, Williams T, Jensen N, Riff K, Sutton R. Short AV interval VDD pacing does not prevent tilt-induced vasovagal syncope in patients with cardio-inhibitory vasovagal syndrome. Pacing Clin Electrophysiol 1994; 17: 882–891. [DOI] [PubMed] [Google Scholar]
- 29.Hrobjartsson A, Forfang E, Haahr MT, Als-Nielsen B, Brorson S. Blinded trials taken to the test: an analysis of randomized clinical trials that report tests for the success of blinding. Int J Epidemiol 2007;36:654–663. [DOI] [PubMed] [Google Scholar]
- 30.Sud S, Massel D, Klein GJ, Leong-Sit P, Yee R, Skanes AC, Gula LJ, Krahn AD. The expectation effect and cardiac pacing for refractory vasovagal syncope. Am J Med 2007:120:54–62. [DOI] [PubMed] [Google Scholar]
- 31.Pachon JC, Pachon EI, Pachon JC, Lobo TJ, Pachon MZ, Vargas RN et al. “Cardioneuroablation” – new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF ablation. Europace 2005; 7: 1–13. [DOI] [PubMed] [Google Scholar]
- 32.Sun W, Zheng L, Qiao Y, Shi R, Hou B, Wu L, Guo J, Zhang S, Yao Y. Catheter ablation as a treatment for vasovagal syncope: Long-Term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. 2016;5:e003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton R Reply to ‘Letter to the Editor’ regarding the article ‘Should we treat severe vasovagal syncope with a pacemaker?’ J Int Med 2017; 10.1111/jce.13920. [DOI] [PubMed]
- 34.Salavatian S, Yamaguchi N, Hoang J, Lin N, Patel S, Ardell JL, Armour JA, Vaseghi M. Premature ventricular contractions activate vagal afferents and alter autonomic tone: implications for premature contraction-induced cardiomyopathy, Am J Physiol Heart Circ 2019; 317: H607–H617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton R, Deharo JC, Brignole M, Hamdan MH. Emerging concepts in diagnosis and treatment of syncope by pacing. Trends Cardiovasc Med 2018; 10.1016/j.tcm.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 36.van Dijk N, Boer KR, Colman N, et al. High diagnostic yield and accuracy of history, physical examination and ECG in patients with transient loss of consciousness in FAST: the Fainting Assessment Study. J Cardiovasc Electrophysiol 2008; 19: 48–55. [DOI] [PubMed] [Google Scholar]