Abstract
Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is an oncofetal protein expressed in various cancers including leukemia. In this study, we assessed the role of IGF2BP1 in orchestrating leukemia stem cell properties. Tumor-initiating potential, sensitivity to chemotherapeutic agents and expression of cancer stem cell markers were assessed in a panel of myeloid, B-, and T-cell leukemia cell lines using gain- and loss-of-function systems, cross-linking immunoprecipitation (CLIP), and photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) techniques. Here we report that genetic or chemical inhibition of IGF2BP1 decreases leukemia cells’ tumorigenicity, promotes myeloid differentiation, increases leukemia cell death, and sensitizes leukemia cells to chemotherapeutic drugs. IGF2BP1 affects proliferation and tumorigenic potential of leukemia cells through critical regulators of self-renewal HOXB4 and MYB and through regulation of expression of the aldehyde dehydrogenase, ALDH1A1. Our data indicate that IGF2BP1 maintains leukemia stem cell properties by regulating multiple pathways of stemness through transcriptional and metabolic factors.
Keywords: IGF2BP1, cancer stem cells, leukemia, HOXB4, MYB, ALDH
Introduction
Leukemia, a wide spectrum of diseases with altered proliferation and differentiation capacity of myeloid or lymphoid blood progenitors, is the most frequent type of cancer in children and one of the most common in adults1. Despite the significant progress in treatments and improved survival outcomes, cancer resistance to chemotherapeutics is the major cause of disease relapse. The 5-year recurrence estimate for acute myeloid and lymphoblastic leukemia (AML, ALL) is 20–30% in children and 70% in adults2, 3. The incidence of molecular recurrence in treatment-free patients with chronic myeloid leukemia (CML) is 30% at 3 years4.
The relapse of leukemia and other cancers is associated with tumor heterogeneity and the presence of groups of cells that possess stem cell properties, such as self-renewal, differentiation block, resistance to apoptosis, drug efflux and detoxification that make them resistant to therapies. The ability of human leukemia-initiating or leukemia stem cells (LICs/LSCs) to regenerate the disease is assessed by leukemia engraftment into immunodeficient mice and expression of hallmark stem cell markers such as ALDH1A, ABC transporters, CD34, DNMT3A and others5–7.
Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), also known as CRD-BP, IMP1, ZBP1 or VICKZ1, is an oncofetal RNA-binding protein that plays a major role in RNA transport, translation, and stability8. IGF2BP1 belongs to the IGF2BP family that consists of three members: IGF2BP1, IGF2BP2, and IGF2BP3, with 56% overall sequence homology. Among the three paralogs, IGF2BP1 and IGF2BP3 display greater resemblance in structure and expression patterns. They function mostly during embryogenesis, display low or no expression in adult tissues, and are often reactivated in cancer.
While the tumorigenic and metastatic properties of IGF2BPs have been intensively investigated in solid tumors, less is known about their role in leukemia9. The overexpression of IGF2BP1 and 3 was linked to certain types of B-cell acute lymphoblastic leukemia (B-ALL) with ETV-RUNX1 and MLL rearrangements, respectively10. In addition, IGF2BP1 was shown to mediate tumorigenic functions of LIN28B in AML cells11.
Given the physiological role of IGF2BP1 in stem cell maintenance and development8, we sought to investigate the impact of IGF2BP1 expression on LSC properties. To this end, we assessed the role of IGF2BP1 in leukemia cells with respect to their capacity to engraft, differentiate, and respond to differentiating or cytotoxic agents.
We found that IGF2BP1 regulates the LSC phenotype affecting leukemia engraftment upon xenotransplantation, differentiation capacity in response to all-trans retinoic acid (ATRA), and induction of cell death by various drugs. We identified a number of novel IGF2BP1 targets with known functions in regulating hematopoietic stem cells (HSC) self-renewal and showed that HOXB4, MYB, and ALDH1A1 mediate the IGF2BP1-dependent LSC phenotype. The results of this study delineate novel mechanisms of IGF2BP1-mediated regulation of leukemogenisis.
Methods
For the detailed description of materials and methods, please refer to the supplemental information.
Cell lines selection and characterization of leukemia stem cell phenotype
The thirteen human leukemia cell lines with different histological and genetic backgrounds randomly chosen for this study are listed in Supplementary Table 1. SKNO1, TANOUE, REH, and MOLT16 were purchased from Leibniz Institute DSMZ, Germany. Other cell lines were obtained from ATCC (Manassas, VA). For leukemia stem cell phenotype characterization, cell lines were assessed for leukemia initiating capacity in NSG mice, colony forming cell (CFC) potential, and expression of stem cell markers. The antibodies used for flow cytometry and western blotting are listed in Supplementary Table 2.
Gain- and loss-of-function systems
The lentivirus constructs for constitutive and doxycycline-inducible expression of short-hairpin (sh) RNAs are listed in Supplementary Table 3. The constitutive expression of shIGF2BP1 (short-hairpin sequence 1 (SH1)), shIGF2BP3 and shControl were obtained from Sigma (St. Louis, MO). The doxycycline-inducible shIGF2BP1 (sequences 2 and 3 (SH2 and SH3)) and scrambled shControl were obtained from GE Dharmacon (Lafayette, CO). Plasmids and lentiviral vectors for doxycycline-inducible and constitutive protein overexpression are listed in Supplementary Table 4.
Chemical compounds
For chemical compounds used in this study, please refer to Supplementary Table 5.
Gene expression analysis
Quantitative PCR (qPCR) reactions were assembled with at least two technical replicates, and at least three biological replicates were performed for each experiment. qPCR data are presented as a mean value of biological replicates (n) and the error bars indicating standard error of mean (± SEM). The primers are listed in Supplementary Table 6. RNA sequencing analysis of 697 (EU3) ALDH+ subpopulations with and without IGF2BP1 knockdown was performed from four biological replicates (n=4). Each biological replicate represents de novo selection of ALDH+ cells and independent doxycycline treatments.
In vivo experiments
Non-obese diabetic/severe combined immunodeficient gamma (NSG) mice were obtained from Jackson Laboratory. For the engraftment experiments, 1×103 −1×106 cells were injected into tail veins of non-irradiated 6–10 week-old female mice in 100 μL of DPBS per mouse. No blinding or randomization was applied to mice experiments. Routinely, each in vivo experiment was performed with three technical replicates (three mice per group) and independently repeated two to three times for each cell line. The biological replicates were conducted with the de novo transduced, puromycin or GFP selected cells, and with the efficient IGF2BP1 knockdown verified by western blot.
Data deposition
Gene expression profiling data by high throughput sequencing of 697 (EU3) ALDH+ cells with IGF2BP1 knockdown were deposited to NCBI Gene Expression Omnibus (GEO) and can be accessed through GEO series number GSE138704. The PAR CLIP data of IGF2BPs RNA targets in K562 CML can be accessed through GEO series number GSE138063. The hyperlinks to submissions are provided in the supplemental methods.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) and R programming language version 3.4.4 (R Foundation, Vienna, Austria). A log-rank (Mantel-Cox) test was used to determine p values in Kaplan-Meier survival curves comparison. For two-group analysis, two-sample Student’s or Welch’s t-tests were used. All tests were two-sided, and values with *P< 0.05, **P<0.01, ***P<0.001 were considered statistically significant.
Results
The role of IGF2BP1 in leukemia cell proliferation and tumorigenesis.
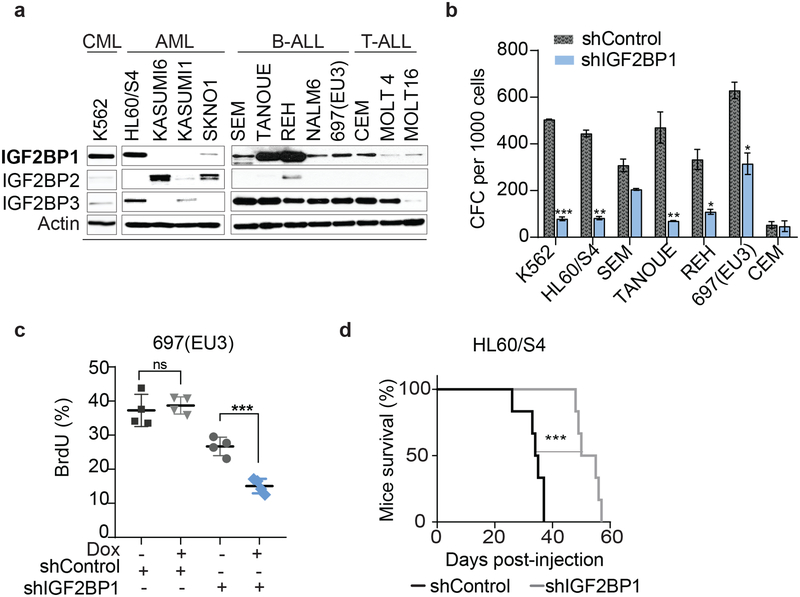
The role of IGF2BP1 expression in LSC properties was examined in multiple leukemia cell lines of diverse histological and genetic backgrounds (Supplementary Table 1), using in vitro and in vivo assays. The expression of IGF2BP1 and its two paralogs was analyzed together with each cell lines’ tumorigenicity, colony-forming cell (CFC) potential, the presence of CD34+CD38− subpopulation, and expression of aldehyde dehydrogenase (ALDH) (Fig. 1a, Supplementary Fig. 1a–e, Supplementary Table 2). Among the 13 tested cell lines, the least colony-forming and non-tumor-initiating cells had low levels of IGF2BP1 and IGF2BP3 (KASUMI1 and MOLT16) or expressed mostly IGF2BP2 protein (SKNO1 and KASUMI6) (Fig. 1a, Supplementary Fig. 1a, 1b). Although activation of CD34 expression was seen upon xenotransplantation (Supplementary Fig. 1c), only KASUMI1 cells expressed a well-defined CD34+CD38− subpopulation in culture (Supplementary Fig. 1d), but the cells were slow-growing and did not engraft. ALDH+ subpopulations were detected by flow cytometry in three of the cell lines: K562, 697(EU3), and MOLT4 (Supplementary Fig. 1e). Based on the cumulative screening results, the leukemia cell lines were stratified for LSC-relevant phenotypes (Supplementary Fig. 1f). Based on endogenous levels of IGF2BP1, we established stable and inducible IGF2BP1 loss- and gain-of-function systems where high IGF2BP1 expressers were used for knockdown and low IGF2BP1 expressers for overexpression (Supplementary Fig. 2a, 4a, Supplementary Tables 3, 4). Three cell lines, K562, 697(EU3) and HL60/S4, scored highest for LSCs properties and had high levels of IGF2BP1. IGF2BP1 knockdown reduced colony-forming capacity in most cell lines with high levels of IGF2BP1 expression (Fig. 1b, Supplementary Fig. 2b, 2c). Similarly, in leukemia cells with higher endogenous levels of IGF2BP1 paralog, IGF2BP3, inhibition of IGF2BP3 reduced colony formation (Supplementary Fig. 2d). Decreased cell numbers in CFCs were associated with reduced staining of the proliferation marker Ki67 (Supplementary Fig. 2e). BrdU staining of 697(EU3) showed statistically significant reduction of cells replicating their DNA (Fig. 1c, Supplementary Fig. 2f). Lower levels of IGF2BP1 expression are associated with better survival in patients with AML (Supplementary Fig. 2g). IGF2BP1 downregulation also resulted in the reduced tumorigenicity of various subtypes of leukemia as measured by survival after leukemia cell injection into healthy NSG mice (Fig. 1d, Supplementary Fig. 2h). These data suggest that IGF2BP1, expressed at varied levels in leukemia cells of different histological subtypes, is important for maintaining leukemogenesis.
Figure 1. IGF2BP1 loss-of-function reduces leukemia cell growth and tumorigenesis.
a, Western blot analysis of IGF2BP1, 2, and 3 endogenous expression in leukemia cells (30 μg of total protein per sample); b, Colony-forming cell (CFC) assay for shIGF2BP1(SH1) and shControl expressing cells, (t-test, n=3, ± SEM); c, Quantitative analysis of BrdU+ cells in S-phase of a cell cycle, 697(EU3) with doxycycline-induced shIGF2BP1(SH2) (t-test, ***P=0.0005, n=4) and shControl (t-test, P=0.60, n=4, non-significant (ns)); d, Kaplan-Meier survival analysis of mice transplanted with HL60/S4 cells with and without IGF2BP1 knockdown (Mantel-Cox test, ***P=0.0008, n=6).
Inhibition of IGF2BP1 sensitizes leukemia cells to chemotherapeutics.
The acute myeloblastic leukemia cell line HL60/S4 is a good model to study the differentiation of myeloid leukemia cell upon retinoid acid treatment targeting the nuclear retinoic acid receptors (RARs)12, 13. Downregulation of IGF2BP1 stimulated spontaneous differentiation of HL60/S4, producing ~20% CD11b+ cells in DMSO treatments, and further potentiated ATRA-induced differentiation (Supplementary Fig. 3a, and Supplementary Table 5). IGF2BP1 knockdown sensitized various leukemia cell lines to treatments with doxorubicin, cytarabine, and cyclophosphamide measured by the staining with cell-impermeant DNA-binding dye 7-Amino-Actinomycin D (7-AAD) (Supplementary Fig. 3b). Treatment with the recently identified small molecule inhibitor of IGF2BP1 BTYNB14 was effective in inducing cytotoxicity in AML, B-, and T-cell leukemia cells, and significantly increased the effect of doxorubicin in these assays (Supplementary Fig. 3c–e). Conversely, the ectopic expression of IGF2BP1 in MOLT16, characterized by very low or undetectable endogenous expression of IGF2BP proteins, reduced cellular death and increased resistance to doxorubicin compared to control (Supplementary Fig. 3f). The elevated levels of IGF2BP1 expression in leukemia cells were also associated with a modest increase in amount of Ki67+ cells (Supplementary Fig. 4b). IGF2BP1-dependent changes in leukemia cell proliferation, tumorigenicity, and sensitivity to drugs are likely not associated with changes in expression of fusion proteins since no statistically significant differences were detected in the mRNA levels of AML1/ETO, MLL/AF4, and TCF/PBX1 measured by qPCR in both loss- and gain-of function systems (Supplementary Fig. 4c, Supplementary Table 6).
IGF2BP1 supports leukemia stem cell phenotype.
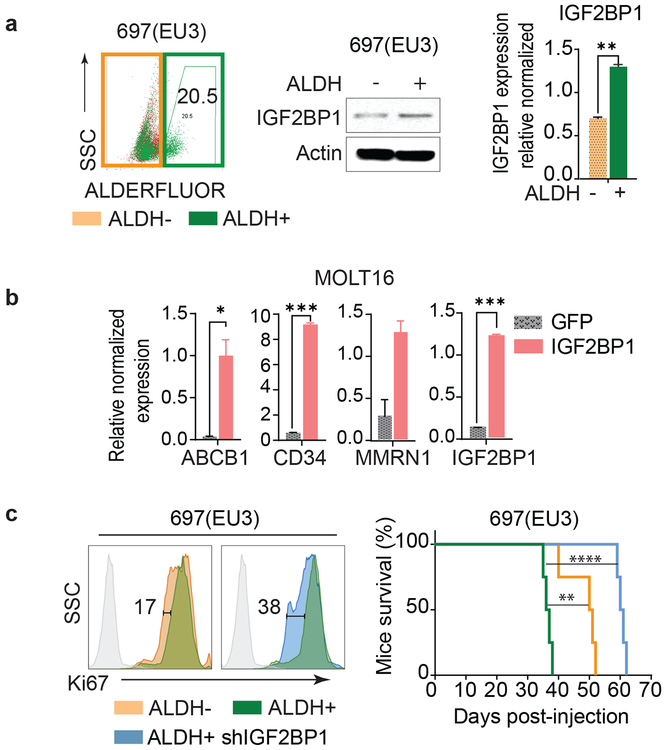
To investigate the role of IGF2BP1 in LSC gene expression, we separated ALDH-enriched (ALDH+) and depleted (ALDH−) subpopulations of 697(EU3) cells that scored highly for LSC properties, including ALDH expression. As expected ALDH+ 697(EU3) cells expressed higher levels of ALDH1A1 and 2, ABC transporters, and some LSC markers associated with acute leukemia7 (Supplementary Fig. 5a). They also displayed higher tumorigenic potential than ALDH− cells (Supplementary Fig. 5b). Importantly, the ALDH+ population expressed higher mRNA and protein levels of IGF2BP1 compared to ALDH− cells (Fig. 2a). Ectopic expression of IGF2BP1 in MOLT16 cells with low endogenous IGF2BPs expression, resulted in elevated levels of ABCB1 transporter, CD34 and MMRN1 mRNAs (Fig. 2b). IGF2BP1 knockdown in ALDH+ cells led to a two-fold reduction of their proliferative potential, making them equally or less tumorigenic than 697(EU3) ALDH− cells (Fig. 2c, Supplementary Fig. 5c). The anti-proliferative effect of IGF2BP1 downregulation in ALDH− cells was half as effective when compared to ALDH+ cells (Supplementary Fig. 5d) indicating that IGF2BP1’s regulatory potential is higher in the ALDH-enriched cells. No difference in cell proliferation nor tumorigenicity was observed in cells expressing shControl (Supplementary Fig. 5e). Together these data suggest that IGF2BP1 is an important regulator of the leukemia stem cell phenotype.
Figure 2. IGF2BP1 expression supports leukemia cancer stem cell properties.
a, Schematic representation of ALDH− (depleted) and ALDH+ (enriched, 20.5%) sorted subpopulations of 697(EU3) (left); endogenous levels of IGF2BP1 mRNA and protein in 697(EU3) ALDH− and ALDH+ cells (t-test, **P<0.01, n=3, ± SEM); b, Expression of selected leukemia stem cell markers in MOLT16 with overexpression of IGF2BP1 and GFP control (t-test, *P<0.05, ***P<0.001, n=3, ± SEM); c, Representative flow cytometric analysis of Ki67 staining of 697(EU3) ALDH− and ALDH+ subpopulations, and ALDH+ cells with IGF2BP1 knockdown (SH2), and Kaplan-Meier survival analysis of mice injected with 1×103 of those cells (Mantel-Cox test, **P=0.0067, ****P<0.0001, n=4).
Identification of IGF2BP1 targets responsible for IGF2BP1-mediated phenotypes in LSCs.
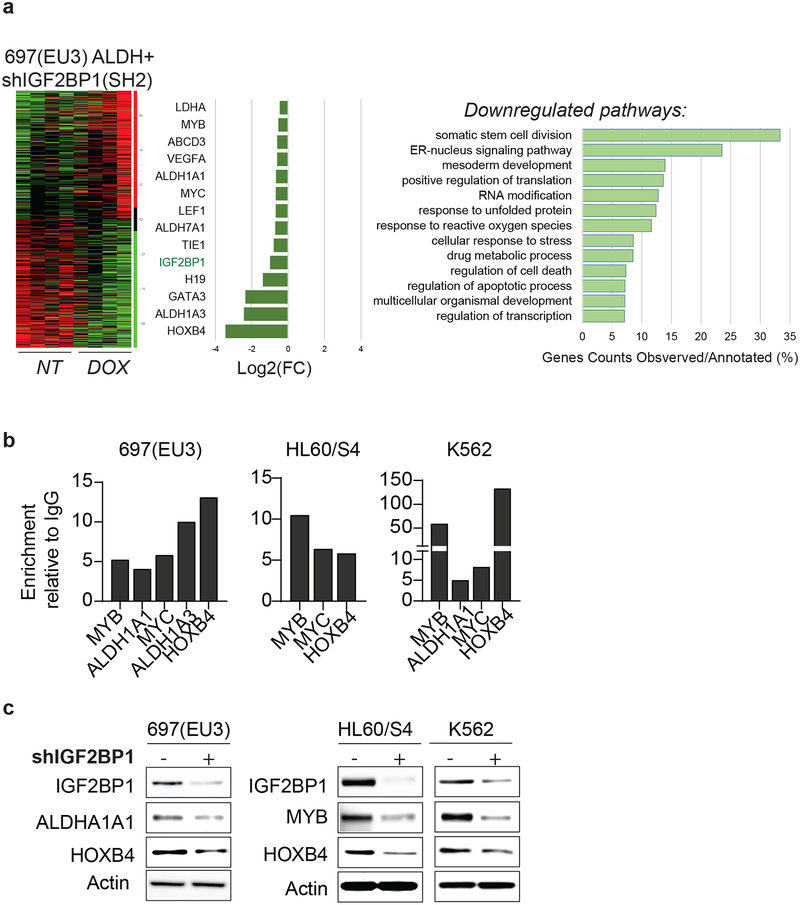
Gene expression profiling of IGF2BP1 knockdown in ALDH-enriched cell populations revealed downregulation of 1,234 genes (*p<0.05, FDR<0.3). The Gene Ontology enrichment analysis for inhibited mRNAs (GO confidence interval 98%, FDR<0.05) indicates negative regulation of somatic stem cell division, cellular metabolic processes, and translation (Fig. 3a). Multiple genes involved in drug metabolic processes, including ALDH1A1, ALDH1A3, ALDH2, and LDHA, were downregulated in IGF2BP1 knockdown cells. Downregulated stem cell factors include the key regulators of hematopoietic stem cell division HOXB4 and MYB and previously characterized IGF2BP1 mRNA targets c-MYC, H19, and LEF115–17 (Fig. 3a). The cross-linking and immunoprecipitation analysis (CLIP) in 697(EU3) and other cells with the highest LSC properties, resulted in 10- to 120-fold enrichment of the selected mRNAs, including HOXB4, ALDH1A1, and MYB, compared to control immunoprecipitation with IgG (Fig. 3b, IgG is set at zero). The endogenous protein levels of ALDH1A1, HOXB4, and MYB were reduced upon shIGF2BP1 knockdown in these cells (Fig. 3c).
Figure 3. IGF2BP1-dependent changes in gene expression profile of ALDH-enriched 697(EU3) leukemia cells.
a, The analysis of differentially expressed genes in ALDH+ subpopulation of 697(EU3) leukemia cells with shIGF2BP1(SH2) knockdown: a heatmap, a list of selected downregulated genes, and Gene Ontology (GO) enrichment analysis for downregulated genes upon IGF2BP1 knockdown; b, qPCR analysis of selected gene targets in the cross-linked immunoprecipitations with anti-IGF2BP1 antibodies relative to anti-IgG, (IgG set at zero). c, Western blot analysis of endogenous ALDH1A1, HOXB4, and MYB expression in 697(EU3), HL60/S4, and K562 cells with and without IGF2BP1 knockdown.
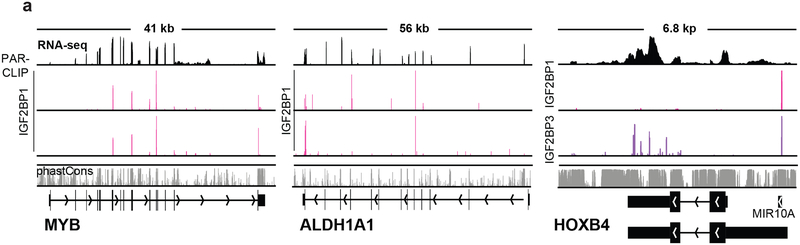
To determine IGF2BP1 direct binding mRNA targets, we performed photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) analysis in K562 CML. The GO analysis of biological processes indicated enrichment with regulators of cell division, protein modification and ubiquitination, cellular metabolic processes, and regulation of cellular transport (Supplementary Fig. 6a). KEGG pathway analysis demonstrated enrichment with regulators of proteolysis, DNA replication and repair, and programmed cell death (Supplementary Fig. 6a). In line with the RNA sequencing and CLIP analysis, PAR-CLIP identified MYB and ALDH1A1 among the mRNAs bound by IGF2BP1 (Fig. 4a). HOXB4 mRNA, significantly enriched in CLIP, displayed a stronger binding with IGF2BP3 rather than IGF2BP1 in PAR-CLIP, probably due to technical limitations of the method. However, a number of other homeobox (HOX)-encoding mRNAs also bound to IGF2BP1, such as HOXB2 and HOXB9 (Supplementary Fig. 6b). CLIP analysis confirmed the enrichment with HOXB9 mRNA compared to CEBPA mRNA, which did not indicate binding either with IGF2BP1 or IGF2BP3 in PAR-CLIP (Supplementary Fig. 6c, d).
Figure 4. PAR-CLIP analysis of K562 cells.
a, The representative photographs of the screenshots of two independent PAR-CLIP experiments illustrating the amplification (vertical red bars) in IGF2BP1-mRNA-binding sites of MYB, ALDH1A1, HOXB4, binding HOXB4-IGF2BP3 (lower panel, blue bars);
Forced expression of stem cell genes ALDH1A1, HOXB4 and MYB rescues IGF2BP1 loss-of-function phenotype.
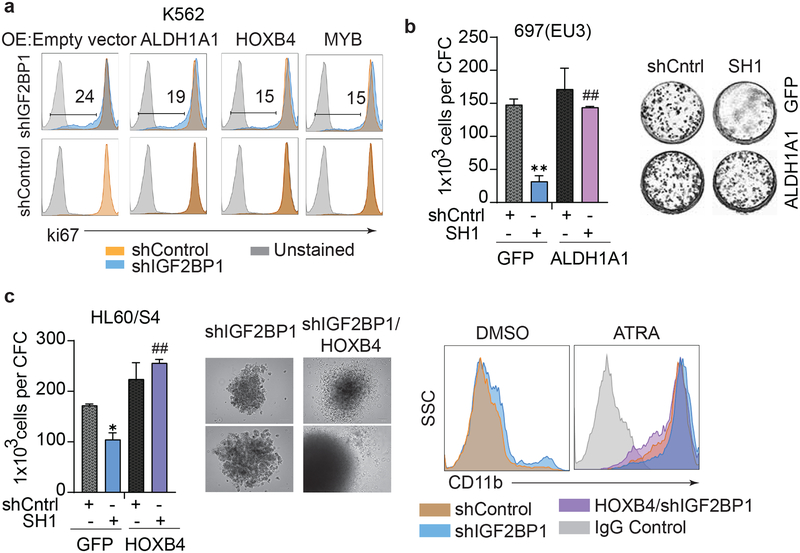
The ectopic expression of ALDH1A1, HOXB4 and MYB in K562 cells with shIGF2BP1 knockdown reversed the anti-proliferative effect of IGF2BP1 inhibition with varied efficiency (Fig. 5a, Supplementary Fig. 7a). The Ki67 staining intensity notably increased upon HOXB4 and MYB overexpression compared with a control vector overexpression (Fig. 5a, upper panel). Forced expression of ALDH1A1, HOXB4 and MYB in K562 cells expressing scrambled shRNAs (shControl) did not have any effect on K562 cell proliferation (Fig.5a, lower panel). Forced expression of either ALDH1A1 or HOXB4 completely rescued the colony forming capacity of 697(EU3) and HL60/S4 cells (Fig. 5b, c; Supplementary Fig. 7b, 7c). The overexpression of HOXB4 in the HL60/S4 cell line with IGF2BP1 knockdown, restored the morphology of the colony forming units and enhanced the HL60/S4 resistance to ATRA-mediated differentiation (Fig. 5c). The overexpression of either HOXB4 or MYB partially rescued colony forming capacity of K562 cells expressing shIGF2BP1 and significantly improved survival of K562 cells (Supplementary Fig. 7d–f).
Figure 5. Ectopic expression of stem cell genes rescues IGF2BP1 loss-of-function phenotype in leukemia.
a, Ki67 staining of K562 cells co-expressing shIGF2BP1(SH1) or shControl together with the indicated constructs (over expression (OE): empty control vector, ALDH1A1, HOXB4, MYB). b, Co-expression of shIGF2BP1(SH1) or shControl with ALDH1A1 in 697(EU3) cells: quantitative assessment and phase contrast photographs of CFC assay of 697(EU3) cells expressing shIGF2BP1(SH1) and shControl in combination with GFP (t-test for shCntrl-GFP and SH1-GFP: **P<0.01, n=3, ± SEM) or ALDH1A1 (t-test for SH1-GFP and SH1-ALDH1A1: ##P<0.01, n=3, ± SEM), day 14. c, Co-expression of shIGF2BP1(SH1) or shControl with HOXB4 in HL60/S4 cells: CFC assay of HL60/S4 cells expressing shIGF2BP1(SH1) and shControl in combination with GFP (t-test for shCntrl-GFP and SH1-GFP: *P<0.05, n=3, ± SEM) or HOXB4 (t-test for SH1-GFP and SH1-HOXB4: ##P<0.01, n=3, ± SEM), day 14; phase contrast photograph of CFC colonies expressing only shIGF2BP1 (left), and HOXB4-coexpressing (right), day 14; flow cytometric analysis of CD11b expression in HL60/S4 cells transduced with indicated constructs and treated with DMSO or all-trans retinoic acid (ATRA, 1μM, 4 days).
Discussion
IGF2BP1 expression is elevated in a variety of human cancers, including melanoma, breast, colon, lung, ovarian, and hepatocellular tumors, and its high expression is associated with metastasis and poor prognosis18–22.
Whereas the oncogenic role of IGF2BPs has been reported for a number of solid tumors, the role of IGF2BP1 and its paralogs in normal and aberrant hematopoiesis is not well understood. Several reports indicate association of IGF2BPs with leukemia driven by specific chromosomal rearrangements: IGF2BP1 is associated with ETV6/RUNX1C traslocation23–25, while IGF2BP3 is commonly overexpressed in B-ALL with MLL rearrangements10. It has been shown that overexpression of IGF2BP3 in normal murine progenitors directs hematopoiesis towards the myeloid lineage10, and orchestrates a fetal-like B cell development program in collaboration with LIN28B26. Another study describes the shift of definitive erythropoiesis to a fetal-like state upon IGF2BP1 expression27. Therefore, IGF2BPs may have a distinct role in stem and progenitor lineage specification and the development of myeloid, B-, or T-cell leukemia. In addition, there is controversy concerning the role of IGF2BP1 in blood cells as an oncogene or tumor, suppressor with one report indicating that downregulation of IGF2BP1 increases proliferation of K56228.
Our study showed that IGF2BP1 expression is associated with LSC properties that can be acquired regardless of the histological and genetic subtype of advanced leukemia. The hallmarks of human LSCs are their engraftment potential upon xenotransplantation and a colony forming capacity in vitro. Among 13 tested cell lines, the least colony-forming and non-tumor-initiating leukemia cells had low levels of IGF2BP1 and IGF2BP3 or express mostly IGF2BP2 protein. IGF2BP1 enrichment was detected in fast engrafting ALDH+ population of leukemia cells. IGF2BP1 knockdown significantly delayed leukemia development in mice and reduced leukemia cell proliferation by more than 30%. RNA sequencing and PAR-CLIP analysis identified a hematopoiesis-specific factor of self-renewal, HOXB4, and the proto-oncogene, MYB, that can both rescue the anti-proliferative phenotype upon IGF2BP1 withdrawal.
Homeobox B4 (HOXB4) protein plays an important role in the self-renewal of normal HSCs and, unlike other HOX proteins, does not possess strong leukemogenic capacity29. This associates HOXB4 expression with a favorable prognosis in de novo acute leukemia30. However, a stem cell therapy with HOXB4-expressing vectors led to a high incidence of leukemia in large animals31. The overexpression of HOXB4 in CD34+ cells of dogs and monkeys had contributed to leukemia development in those animals, while the knockdown of HOXB4 induced leukemia cells differentiation31. A recent study on the K562/ADM chemo resistant line provides evidence that downregulation of HOXB4 increases sensitivity to drugs and decreases drug efflux32.
Our PAR-CLIP analysis revealed IGF2BP1 binding sites within mRNAs of multiple HOXB genes. A recent study in synthetic modeling of T-cell leukemia showed that HOXB genes are critical for leukemia initiation and maintenance, providing growth advantage in both pre-leukemia cells and established clones33.
The MYB proto-oncogene encodes a nuclear transcription factor with an essential role in the proliferation, lineage commitment, and differentiation of hematopoietic progenitor cells34. Genomic duplications and high levels of MYB expression, often in cooperation with NOTCH1 mutations, are associated with T-cell acute lymphoblastic leukemia pathogenesis, increased leukemia cell proliferation, viability, and differentiation block35, 36. MYB is responsible for maintaining an aberrant self-renewal program that is essential for the initiation and progression of MLL-rearranged AML37, 38. Our data demonstrate the importance of IGF2BP1-mediated post-transcriptional regulation of MYB in leukemia cell proliferation.
In addition to the self-renewal factors, HOXB4 and MYB, we found that IGF2BP1 post-transcriptionally regulates the expression of the ubiquitous, hallmark stem cell marker ALDH1A1. The aldehyde dehydrogenase (ALDH) superfamily consists of 19 NAD(P)-dependent enzymes that can oxidize a structurally diverse group of endogenous and exogenous aldehyde substrates39. High levels of ALDH1A1 activity protect normal and leukemia hematopoietic stem cells from the toxicity of the alkylating agents40. In our study, downregulation of IGF2BP1 in ALDH-expressing 697(EU3) and K562 cells sensitized them to chemotherapeutic-induced cell death. In addition to cellular detoxification functions, ALDH expression is associated with cell proliferation and cancer aggressiveness41–44. ALDH1A1 overexpression in IGF2BP1-depleted 697(EU3) cells fully restored their clonogenic potential indicating an important role for ALDH1A1 in mediating the effects of IGF2BP1 in leukemia cell proliferation and survival.
IGF2BP1 expression in LSCs is in line with the recent report that the stem cell factor LIN28B, which is often upregulated in advanced forms of blood cancer, promotes leukemia aggressiveness at least partially via interaction with IGF2BP111. It is also plausible that IGF2BP1 functions in LSCs as a reader of m6A-methylated RNA. Members of the IGF2BP family were recently shown to recognize the consensus GG(m6A)C sequence, promoting the stability and storage of their target mRNAs in an m6A-dependent manner in HepG2 cells and human embryonic stem cells (hESCs)45.
Our study provides further evidence that IGF2BP1 maintains tumorigenicity and drug resistance of leukemia cells by enhancing critical transcriptional and metabolic regulators. For the first time, we have found that ALDH1A1, HOXB4, and MYB are directly regulated by IGF2BP1 at the post-transcriptional level.
Given the fact that IGF2BP1 is often upregulated in various types of malignancies and not expressed in most normal tissues, IGF2BP1 could become an important target for anti-cancer therapy. Developing clinically relevant IGF2BP1 inhibitors will help sensitize leukemia cells to programmed cell death and induce differentiation by targeting stem cell pathways that are common for various types of advanced cancers.
Supplementary Material
Key points:
IGF2BP1 promotes leukemia stem cell phenotype by regulating HOXB4, MYB and ALDH1A1.
Inhibition of IGF2BP1 reduces leukemia tumorigenicity, and enhances leukemia cell death and differentiation.
Acknowledgements
This study was supported by the NIH grant R01 AR063361 (V.S.S.), NIH Intramural Research Program of the NIAID (S.A.M.) and NIAMS (M.H.). The authors thankful to Dr. Chunhua Song and Dr. Joel Yisraeli for the gift of reagents. We also thankful to Yuka Imamura and Penn State Cancer Institute Genomics Sciences, Joe Bednarczyk and Flow Cytometry Core for help with data acquisition and analysis. We thank Gustavo Gutierrez-Cruz and Stefania Dell’Orso (NIAMS) for sequencing the PAR-CLIP libraries, and NIAID Office of Cyber Infrastructure and Computational Biology for high-performance computing.
Footnotes
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019. April 15; 144(8): 1941–1953. [DOI] [PubMed] [Google Scholar]
- 2.de Rooij JD, Zwaan CM, van den Heuvel-Eibrink M. Pediatric AML: From Biology to Clinical Management. J Clin Med 2015. January 9; 4(1): 127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017. January 26; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Boluda JC, Pereira A, Pastor-Galan I, Alvarez-Larran A, Savchuk A, Puerta JM, et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J 2018. December 2; 8(10): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997. July; 3(7): 730–737. [DOI] [PubMed] [Google Scholar]
- 6.Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017. July 6; 547(7661): 104–108. [DOI] [PubMed] [Google Scholar]
- 7.Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016. December 15; 540(7633): 433–437. [DOI] [PubMed] [Google Scholar]
- 8.Degrauwe N, Suva ML, Janiszewska M, Riggi N, Stamenkovic I. IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev 2016. November 15; 30(22): 2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Zhang H, Guo X, Zhu Z, Cai H, Kong X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J Hematol Oncol 2018. June 28; 11(1): 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanichamy JK, Tran TM, Howard JM, Contreras JR, Fernando TR, Sterne-Weiler T, et al. RNA-binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J Clin Invest 2016. April 1; 126(4): 1495–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Bi C, Ching YQ, Chooi JY, Lu X, Quah JY, et al. Inhibition of LIN28B impairs leukemia cell growth and metabolism in acute myeloid leukemia. J Hematol Oncol 2017. July 11; 10(1): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark Welch DB, Jauch A, Langowski J, Olins AL, Olins DE. Transcriptomes reflect the phenotypes of undifferentiated, granulocyte and macrophage forms of HL-60/S4 cells. Nucleus 2017. March 4; 8(2): 222–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olins AL, Buendia B, Herrmann H, Lichter P, Olins DE. Retinoic acid induction of nuclear envelope-limited chromatin sheets in HL-60. Exp Cell Res 1998. November 25; 245(1): 91–104. [DOI] [PubMed] [Google Scholar]
- 14.Mahapatra L, Andruska N, Mao C, Le J, Shapiro DJ. A Novel IMP1 Inhibitor, BTYNB, Targets c-Myc and Inhibits Melanoma and Ovarian Cancer Cell Proliferation. Transl Oncol 2017. October; 10(5): 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leeds P, Kren BT, Boylan JM, Betz NA, Steer CJ, Gruppuso PA, et al. Developmental regulation of CRD-BP, an RNA-binding protein that stabilizes c-myc mRNA in vitro. Oncogene 1997. March 20; 14(11): 1279–1286. [DOI] [PubMed] [Google Scholar]
- 16.Runge S, Nielsen FC, Nielsen J, Lykke-Andersen J, Wewer UM, Christiansen J. H19 RNA binds four molecules of insulin-like growth factor II mRNA-binding protein. J Biol Chem 2000. September 22; 275(38): 29562–29569. [DOI] [PubMed] [Google Scholar]
- 17.Zirkel A, Lederer M, Stohr N, Pazaitis N, Huttelmaier S. IGF2BP1 promotes mesenchymal cell properties and migration of tumor-derived cells by enhancing the expression of LEF1 and SNAI2 (SLUG). Nucleic Acids Res 2013. July; 41(13): 6618–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoshal A, Rodrigues LC, Gowda CP, Elcheva IA, Liu Z, Abraham T, et al. Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis. Oncogene 2019. April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld YB, Krumbein M, Yeffet A, Schiffmann N, Mishalian I, Pikarsky E, et al. VICKZ1 enhances tumor progression and metastasis in lung adenocarcinomas in mice. Oncogene 2019. January 30. [DOI] [PubMed] [Google Scholar]
- 20.Dimitriadis E, Trangas T, Milatos S, Foukas PG, Gioulbasanis I, Courtis N, et al. Expression of oncofetal RNA-binding protein CRD-BP/IMP1 predicts clinical outcome in colon cancer. Int J Cancer 2007. August 1; 121(3): 486–494. [DOI] [PubMed] [Google Scholar]
- 21.Kobel M, Weidensdorfer D, Reinke C, Lederer M, Schmitt WD, Zeng K, et al. Expression of the RNA-binding protein IMP1 correlates with poor prognosis in ovarian carcinoma. Oncogene 2007. November 29; 26(54): 7584–7589. [DOI] [PubMed] [Google Scholar]
- 22.Bell JL, Turlapati R, Liu T, Schulte JH, Huttelmaier S. IGF2BP1 harbors prognostic significance by gene gain and diverse expression in neuroblastoma. J Clin Oncol 2015. April 10; 33(11): 1285–1293. [DOI] [PubMed] [Google Scholar]
- 23.Stoskus M, Eidukaite A, Griskevicius L. Defining the significance of IGF2BP1 overexpression in t(12;21)(p13;q22)-positive leukemia REH cells. Leuk Res 2016. August; 47: 16–21. [DOI] [PubMed] [Google Scholar]
- 24.Stoskus M, Gineikiene E, Valceckiene V, Valatkaite B, Pileckyte R, Griskevicius L. Identification of characteristic IGF2BP expression patterns in distinct B-ALL entities. Blood Cells Mol Dis 2011. April 15; 46(4): 321–326. [DOI] [PubMed] [Google Scholar]
- 25.Stoskus M, Vaitkeviciene G, Eidukaite A, Griskevicius L. ETV6/RUNX1 transcript is a target of RNA-binding protein IGF2BP1 in t(12;21)(p13;q22)-positive acute lymphoblastic leukemia. Blood Cells Mol Dis 2016. March; 57: 30–34. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Chim B, Su Y, Khil P, Wong M, Wang X, et al. Enhancement of LIN28B-induced hematopoietic reprogramming by IGF2BP3. Genes Dev 2019. August 1; 33(15–16): 1048–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vasconcellos JF, Tumburu L, Byrnes C, Lee YT, Xu PC, Li M, et al. IGF2BP1 overexpression causes fetal-like hemoglobin expression patterns in cultured human adult erythroblasts. Proc Natl Acad Sci U S A 2017. July 11; 114(28): E5664–E5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao B, Patel M, Hu Y, Charles S, Herrick DJ, Brewer G. Targeted knockdown of the RNA-binding protein CRD-BP promotes cell proliferation via an insulin-like growth factor II-dependent pathway in human K562 leukemia cells. J Biol Chem 2004. November 19; 279(47): 48716–48724. [DOI] [PubMed] [Google Scholar]
- 29.Abramovich C, Pineault N, Ohta H, Humphries RK. Hox genes: from leukemia to hematopoietic stem cell expansion. Ann N Y Acad Sci 2005. June; 1044: 109–116. [DOI] [PubMed] [Google Scholar]
- 30.Umeda S, Yamamoto K, Murayama T, Hidaka M, Kurata M, Ohshima T, et al. Prognostic significance of HOXB4 in de novo acute myeloid leukemia. Hematology 2012. May; 17(3): 125–131. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XB, Beard BC, Trobridge GD, Wood BL, Sale GE, Sud R, et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J Clin Invest 2008. April; 118(4): 1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Jia XH, Chen JR, Yi YJ, Wang JY, Li YJ, et al. HOXB4 knockdown reverses multidrug resistance of human myelogenous leukemia K562/ADM cells by downregulating P-gp, MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J Oncol 2016. December; 49(6): 2529–2537. [DOI] [PubMed] [Google Scholar]
- 33.Kusakabe M, Sun AC, Tyshchenko K, Wong R, Nanda A, Shanna C, et al. Synthetic modeling reveals HOXB genes are critical for the initiation and maintenance of human leukemia. Nat Commun 2019. July 2; 10(1): 2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelicci PG, Lanfrancone L, Brathwaite MD, Wolman SR, Dalla-Favera R. Amplification of the c-myb oncogene in a case of human acute myelogenous leukemia. Science 1984. June 8; 224(4653): 1117–1121. [DOI] [PubMed] [Google Scholar]
- 35.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, Graux C, Cauwelier B, Lambert F, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet 2007. May; 39(5): 593–595. [DOI] [PubMed] [Google Scholar]
- 36.Clappier E, Cuccuini W, Kalota A, Crinquette A, Cayuela JM, Dik WA, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood 2007. August 15; 110(4): 1251–1261. [DOI] [PubMed] [Google Scholar]
- 37.Somervaille TC, Matheny CJ, Spencer GJ, Iwasaki M, Rinn JL, Witten DM, et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell 2009. February 6; 4(2): 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev 2011. August 1; 25(15): 1628–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics 2011. May; 5(4): 283–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasparetto M, Smith CA. ALDHs in normal and malignant hematopoietic cells: Potential new avenues for treatment of AML and other blood cancers. Chem Biol Interact 2017. October 1; 276: 46–51. [DOI] [PubMed] [Google Scholar]
- 41.Flahaut M, Jauquier N, Chevalier N, Nardou K, Balmas Bourloud K, Joseph JM, et al. Aldehyde dehydrogenase activity plays a Key role in the aggressive phenotype of neuroblastoma. BMC Cancer 2016. October 10; 16(1): 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, et al. Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma. PLoS One 2012; 7(8): e43664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med 2012. February 15; 52(4): 735–746. [DOI] [PubMed] [Google Scholar]
- 44.Vassalli G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int 2019; 2019: 3904645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 2018. March; 20(3): 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.