Abstract
Establishment of enduring sex differences in brain and behavior occurs during pre- or perinatal development, depending on species. For over 50 years the focus has been on gonadal steroid production by male fetuses and the impact on developing brain. An increasing awareness of the importance of sex chromosome complement has broadened the focus but identifying specific roles in development has yet to be achieved. Recent emphasis on transcriptomics has revealed myriad and unexpected differences in gene expression in specific regions of male and female brains which may produce sex differences, serve a compensatory role or provide latent sex differences revealed only in response to challenge. More surprising, however, has been the consistent observation of a central role for inflammatory signaling molecules and immune cells in masculinization of brain and behavior. The signal transduction pathways and specific immune cells vary by brain region, as does the neuroanatomical substrate subject to differentiation, reflecting substantial complexity emerging from what may be a common origin, the maternal immune system. A working hypothesis integrating these various ideas is proposed.
Keywords: Androgens, estrogens, amygdala, preoptic area, prostaglandins, microglia, mast cells
Introduction
Sexual differentiation of the brain is a developmental process occurring during a restricted critical window which is operationally defined by the onset of androgen production by the fetal testis of males. The closing of the critical window is when the blockade or removal of androgens in males is without effect or similarly, treating females with testosterone is ineffective at inducing masculinization. In the first scenario the process of masculinization has proceeded to the point of no return and the same is true for feminization in the second scenario. Defining the critical period as beginning with the onset of hormone secretion and ending with loss of sensitivity to hormones frames our view of the entire process as being driven by hormones. This myopic view prevents consideration of other plausible biological variables that may be equally, if not more important, for establishing and maintaining sex differences in physiology and behavior. This review will highlight the importance of two such variables, sex chromosome complement and the immune system.
Sex determination begins with chromosome complement.
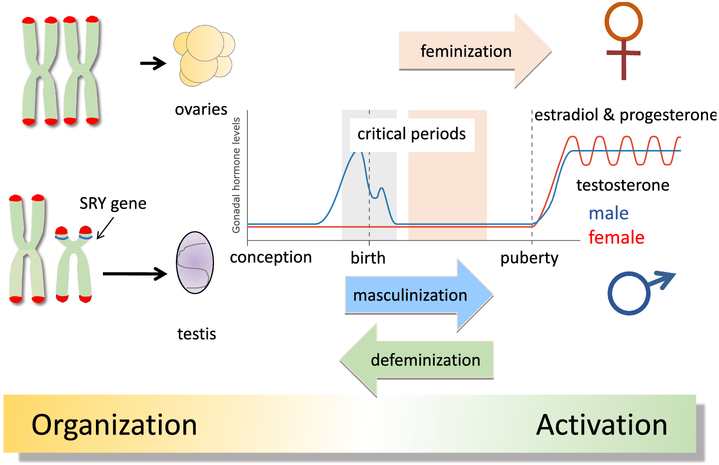
The biological construct of maleness and femaleness begins with the establishment of sex chromosome complement in the fertilized zygote and ends with reproductive competence of the mature adult (Figure 1). In mammals, a single gene on the Y chromosome, Sry (Sex-determining region of the Y), codes for the testis-determining factor protein (Tdf), which initiates a cascade of gene expression within days of fertilization that will lead to the creation of a testis from the bipotential gonadal anlage (Goodfellow and Lovell-Badge, 1993). If there is no Y chromosome, i.e. XX, or if the Sry gene is missing or mutated, the gonadal anlage will proceed down a developmental pathway that culminates in the formation of an ovary. As the embryo develops the formation of the reproductive tract and external genitalia are sculpted to match the demands created by gonadal phenotype. One of the most profound consequences of the formation of a testis versus an ovary is not the production of many gametes versus only a few or the cyclical versus static production of steroid hormones and creation of a sex specific hormonal milieu. The most important difference is that one gonad, the testis, is engineered to release gametes outside the body and therefore must be transferred to a viable mate, while the other gonad, the ovary, is restricted to releasing gametes inside the body cavity which then with some assistance hopefully find their way to the inside of the uterus. In order for those gametes to merge and become zygotes, in other words be fertilized, there must be a mechanism by which external gametes also gain access to the interior of the uterus. Both scenarios require a series of coordinated behaviors by both sexes that begin with mate seeking, mate recognition and selection followed by copulation.
Figure 1: Classic View of Sexual Differentiation of the Brain.
Sexual differentiation begins with chromosome complement and is driven by the Sry gene of the Y chromosome directing the formation of a testis from the bipotential gonad. Testosterone production by the resultant testis will commence during late gestation and into the early postnatal period of the rodent and masculinize select regions of the male brain. A separate process of defeminizaton specific to sex behavior removes the capacity for female-like receptive behavior (i.e. lordosis) in males. The female ovary will develop in the absence of signals from the Sry-induced gene expression cascade and will remain quiescent until puberty when cyclical production of estrogens and progestins begins. There is evidence for a sensitive period for feminization that occurs slightly later in development. The masculinizing actions of testosterone and its aromatized by product, estradiol, are considered organizational and are essential for the activational effects of hormones at puberty on various reproductive parameters.
Sexual differentiation of behavior begins with the brain
A central question in the early days of behavioral neuroendocrinology (not called that at the time), was whether the behaviors associated with male versus female copulation were a function of the peripheral apparatus, i.e. a penis versus a vagina, or controlled internally by the brain. Hard as it is to believe now, the prevailing and favored theory was that the body was the determinant of behavior. Thus, if an individual found themselves with a penis, regardless of any other variables, they would attempt to mount and copulate with other individuals possessing a vagina, and vice versa. Moreover, while hormones were clearly required for adult copulatory behavior to be displayed, the actions of the hormones were considered independent of any organizational effect during development. This notion was empirically tested by Phoenix and his trainees and essentially rejected in what is now considered a land mark paper published in Hormones and Behavior in 1959 (Phoenix et al., 1959). The experiment was simple, they treated pregnant Guinea pigs with testosterone and then assessed the copulatory behavior of the offspring as adults. The critical component was the use of different doses of testosterone, a high dose that fully masculinized the genitalia of the females in utero so that they were externally indistinguishable from male littermates, and a lower dose that did not noticeably impact the genitalia. Offspring of both treatment groups were compared to those from untreated dams. The animals were tested in two important ways. First was to determine the impact of prenatal androgen exposure on the display of female sexual receptivity (lordosis). They found that females exposed to either high or low dose testosterone did not exhibit lordosis in response to mounting by a male, thus the behavior change was independent of the peripheral genitalia. Secondly, they assessed the propensity of the neonatally exposed animals to engage in male copulatory behavior, finding that females exposed to high dose testosterone developmentally where highly responsive to adult testosterone and vigorously mounted other females. Interestingly they did not test the low dose exposed females, presumably because they were so inculcated with the prevailing view that without a penis no animal would attempt to mate like a male. Nonetheless, they concluded that the actions of the prenatal hormones were organizational and enduring. They went on to assert the notion that the tissues which had been organized developmentally to modify behavior was probably neural, but they were “not prepared to suggest whether the site of action is generalized or localized”. They further speculated that any effects of androgens on the neural substrate would likely reflect subtle changes in function as opposed to structure. The intervening 60 years have proved that wrong and filled in the gaps by convincingly demonstrating that hormones have highly localized and varied impact on the developing brain, playing major roles in determining cell birth, death, differentiation and connectivity. The impact of steroids expands beyond neurons, also profoundly influencing astrocytes, oligodendrocytes and cells of non-neuronal origin such as the immune microglia and mast cells. The impact of steroids is so powerful, complex and widespread one could argue the field has been held prisoner by the all encompassing effort to understand this class of signaling molecules. One could also say the field has been myopic in its view, attributing every and all sex difference in brain or behavior to the action of steroid hormones. Indeed, the power of hormones was revered as so great that the circulating levels observed in adult females across the estrus cycle was considered so remarkable in its power to control brain and behavior that females could not be used as reliable subjects in experimental neuroscience. We will never know for certain, but this may have contributed to the fact that within a few short years the majority of neuroscience research narrowed its focus to only male subjects (Beery and Zucker, 2010). The study of females was only for purposes of understanding reproduction.
Now we are in a new era. In response to a growing awareness of the negative consequences of not considering sex as a biological variable during preclinical research, the NIH created a new mandate that requires all funded studies include subjects of both sexes and that data be disaggregated and analyzed for an impact of sex. If an NIH funded investigator insists on using subjects of only one sex in preclinical research it requires extraordinary justification, i.e. studies of ovarian cancer can only be done in females for instance. Attention to the importance of sex as a biological variable has extended to Canadian and European funding agencies as well. Some consider this to be over reach and possibly damaging, particularly in the neurosciences, but others have embraced the new mandate and are finding unexpected and surprising differences in the characteristics of various biological processes. Observations of this sort often lead naturally to questions of “how” does said biological sex difference come about? There are three generic answers to this question. Sex differences can occur because; 1) the steroid hormone milieu is markedly different in reproductively mature animals, 2) developmental programming by steroids dictates adult responses or 3) every cell in the body, including the brain, is either XX or XY within a given individual and genes on sex chromosomes could have direct effects. All three or any combination of two of these is also possible.
Sex differences in the brain involve more than just hormones: Sex chromosome complement
Because of the hegemony of hormones, the notion that genes on the sex chromosomes might contribute to male and female differences in brain and behavior was largely ignored until the early 2000’s when Art Arnold made use of a novel mouse strain in which gonadal sex and genetic sex could be dissociated (De Vries et al., 2002). Known as the 4-core-genotype, this mouse strain is engineered so that the Sry gene is removed from the Y chromosome and translocated to an autosome. This allows for animals that are XX-Sry+ and XY-Sry−. In other words, it creates XX animals with testis and XY animals with ovaries. By comparing endpoints in these mice to those that are XX with ovaries and XY with testis, the relative contribution of chromosomal sex versus gonadal sex can be assessed. Many behaviors have been examined and generally those directly associated with reproduction, i.e. mating and parenting, are entirely controlled by the gonads (Arnold et al., 2004). But many other behaviors and some physiological endpoints are at least “influenced” by chromosomal sex. One of the most notable is body weight, which is markedly impacted by chromosomal sex but only if the gonads are removed (Chen et al., 2013). In another particularly remarkable study involving an animal model of multiple sclerosis (MS), the chromosomal sex of the immune system and the nervous system were separated so that individuals were XX in the periphery and XY in the brain. This revealed that the higher vulnerability of females to MS was a function of the peripheral immune system, whereas the more severe disease pathology observed in males is the result of XY in the nervous system (Du et al., 2014). This reinforces the view that the relationship between gonadal sex and chromosomal sex can be both contextual and tissue dependent.
What is missing in our understanding of potential impact of chromosome complement on sex differences is whether there is any effect during development. An in vitro examination of dopamine neuron survival in cells derived from embryos of the 4-core-genotypes model demonstrates a clear effect of sex chromosome complement (Carruth et al., 2002), and is consistent with inferential findings in vivo (Dewing et al., 2003), so there is potential for direct effects but whether they constitute sexual differentiation. per se, is unclear. The sexual differentiation of the brain is classically viewed as occurring during a critical period when testicularly produced androgens in the male fetus gain access to the CNS and initiate a variety of molecular and cellular processes that are the manifestation of masculinization. The critical period begins with the production of steroids late in gestation in rodents and about mid-gestation in primates, including humans, and ends within the first few days of postnatal life or prior to birth, respectively (reviewed in (McCarthy et al., 2017a). In females there is a parallel sensitive period, which is distinct from a critical period in that it involves sensitivity to an exogenous substance. In our rodent models, a newborn female can be injected with testosterone or its aromatized byproduct estradiol, for up to 6-10 days postnatally and still be masculinized for some endpoints, i.e. remain “sensitive”. This has provided an excellent tool for revealing the mechanisms of brain masculinization as it is relatively easy to manipulate the hormonal milieu and other physiological parameters in a newborn pup compared to a developing fetus, which is further confounded by the hormonal environment of pregnancy. However, in newborn males masculinization cannot be blocked or reversed easily because the process was initiated in utero and like a train that has left the station, it is difficult to impede its forward progression. In both cases, natural masculinization in males or induced masculinization in females, the brain is remarkably immature at the time that it is being “programmed” or “organized”, especially in light of the fact that most of the physiology and behavior that will be impacted does not appear until the animals are reproductively mature. The pulsatile versus cyclic release of luteinizing hormone from the pituitary is a hallmark of puberty, and sexual behavior is not expressed until well after that, nor is territorial aggression, courtship, nest building and maternal aggression. And so there is tremendous dynamism occurring from the time of sexual differentiation and sex differences in brain and behavior, but what is constant across this period is sex chromosome complement. This makes it difficult to identify effects that might be restricted to a critical or sensitive period.
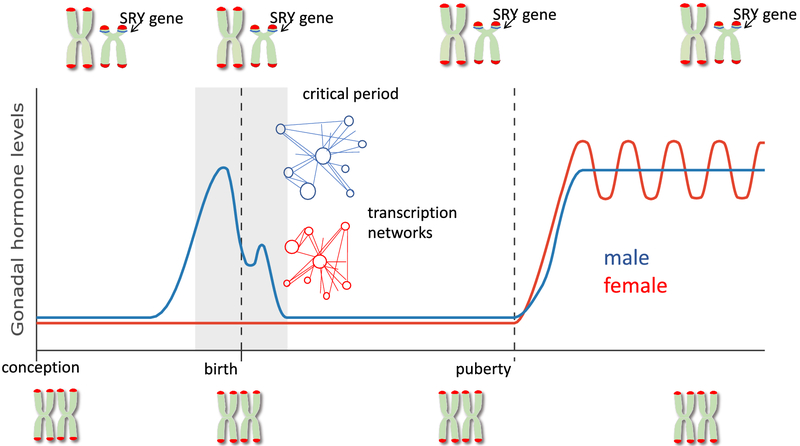
Further complicating our ability to attribute developmental effects to sex chromosome complement is the increasing awareness that autosomes are impacted by whether they share the nucleus with an XX or XY set. There are several ways this can occur. First is that when a cell has an XX compliment, one of the X chromosomes is subject to inactivation. This inactivation is achieved by epigenetic repression via DNA methylation and histone modification, an energetically expensive process that could deplete the cell of precious enzymes and substrates that may be needed for other epigenetic regulatory processes, thereby creating a heterochromatic sink. A second route for sex chromosome regulation of autosomes is via microRNAs, which are particularly abundant on the X chromosome (Pinheiro et al., 2011). MicroRNAs provide regulatory control of transcription and a single mRNA might impact multiple genes, thereby modulating coordinated gene networks. Third, there are some genes that escape X-inactivation, resulting in a double dose in females and thereby creating the potential for a sex difference in gene expression (reviewed in (Arnold et al., 2016). In addition to being rich in microRNAs, the X-chromosome is also notably rich in genes relevant to brain development and neural functioning, as well as immune system genes (Bianchi et al., 2012). Lastly, while the Y chromosome is small, it is not completely devoid of genes and these too could have direct effects on the developing brain. All of these are in the realm of possibility and none of them exclude another, but to-date there has been no empirical evidence that sex chromosome complement directly impacts sexual differentiation of the brain (Figure 2).
Figure 2: Incorporation of sex chromosomes and transcriptomics in sexual differentiation of the brain.
In recent years it has become increasingly clear that sex chromosome complement in mammals (XX vs XY) is an important contributor to sex differences in brain and behavior. However because of the continuous presence of this modifier since the time of conception, it is difficult to attribute particular aspects of sex chromosome complement to the process of sexual differentiation. Future studies addressing convergence of sex chromosome effects and steroid hormones will be valuable in this regard. A second emerging concept is sex differences in the transcriptome, and in particular the potential for differences in networks as opposed to isolated gene expression.
Sex differences in the brain involve more than just hormones: transcription regulatory networks (TRNs)
Steroid receptors are nuclear transcription factors and this logically led to the assumption that androgen receptors (AR), estrogen receptors (ER) or both would induce a set of genes that drive the brain masculinization process. This reasonable and plausible scenario leads to a series of testable predictions; 1) there will be sex differences in gene expression, some genes more highly expressed in males and some higher in females, 2) treatment of females with testosterone will recapitulate the pattern seen in males, 3) the androgen-induced genes will be directly relevant to brain development, and 4) identifying those genes will solve the mystery of brain sexual differentiation. An analysis of the transcriptome of the neonatal preoptic area, arguably the most sexually dimorphic region of the brain, found equal numbers of genes expressed at higher levels in males versus females (Nugent et al., 2015), in direct contradiction to prediction #1. Treating females with testosterone (or estradiol) does increase the level of some mRNA and protein from some genes normally higher in males (Quadros et al., 2002), but just as frequently does not. More importantly, the genes that are regulated do not seem to have direct relationship to neural development, thus neither prediction #2 or #3 are strongly supported. One of the more surprising findings to emerge in recent years are the profound and pervasive sex differences in the transcriptome, both developmentally and in adulthood, in animals and humans (Nugent et al., 2015; Werling et al., 2016; Labonte et al., 2017). The differences involve large number of genes, belying the notion that a few select genes are responsible for the masculinization of the brain.
So where do sex differences in the transcriptome come from and what does it mean? Independent of, or, in tandem with sex chromosome complement, there is a strong potential for sex differences in transcription regulatory networks (TRNs). Transcription factors (TF) interact directly with DNA or via co-activators and co-repressors in a multi-component transcriptional complex. They modulate the activity at gene promoters and interact with distal regulatory elements known as enhancers. The binding of individual TF’s can be weak and imprecise and so cooperativity between them and associated proteins provides the robust regulation needed to precisely regulate the roughly 20,000 genes in the mammalian genome. TRNs determine cell fate during development and adult maintenance of homeostasis. How networks are established and maintained is still being explored experimentally. Network stability is influenced by both intrinsic (i.e. cell cycle progression) and extrinsic (i.e. metabolic state) factors (reviewed in (Wilkinson et al., 2017). Steroid receptors are classical TFs that recognize and interact with palindromic sequences embedded in promoters but also associate with a variety of co-factors, some specific to steroid receptors and others more general, such as c-fos (Barrett, 1998; Beato and Klug, 2000). Steroids can also influence TRNs by modulating the activity of DNA methylating enzymes (Nugent et al., 2015), and histone modifying enzymes (Matsuda et al., 2011). Evidence that TRNs differ as a function of sex is found in differential gene expression, alternative splice variants and promoter usage, genome wide methylation and histone modifications associated with promoter activation in rats, mice and humans (Trabzuni et al., 2013; Ghahramani et al., 2014; Nugent et al., 2015; Shen et al., 2015; Werling et al., 2016; Labonte et al., 2017). Of particular interest would be to know if TRNs are established developmentally during the critical period and then maintained across the life span (Figure 2).
Sex differences in the brain involve more than just hormones: Immune and inflammatory mediators
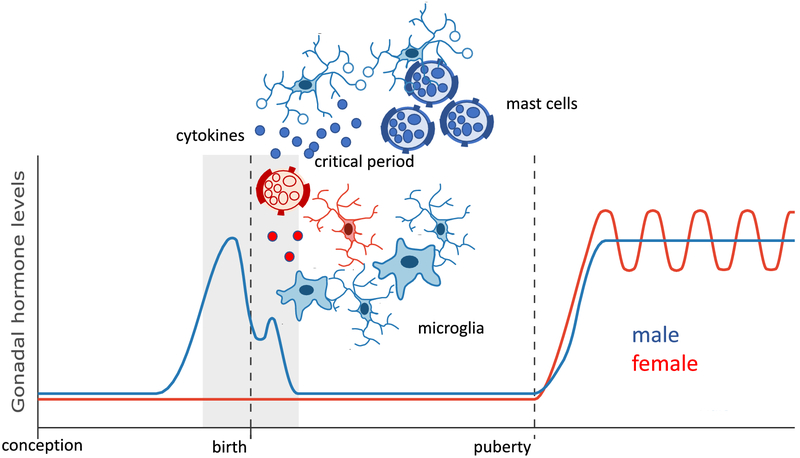
The cellular and molecular mechanisms mediating the masculinization of specific brain regions has been elucidated in considerable detail for some regions and a surprising pattern has emerged. It was predicted that factors impacting cell number, dendritic growth and synapse density would most likely be growth factors, neurotransmitters and other modulators of neuronal activity such as ion changes. Instead, an over preponderance of signaling pathways and cell types involved in immune and inflammatory responses was discovered to be central to masculinization of multiple endpoints in multiple brain regions (Figure 3).
Figure 3: New view of sexual differentiation of the brain involving immune modulators.
Converging evidence from studies exploring the cellular and molecular mechanisms establishing sex differences in brain and behavior implicates the innate immune cells of the brain, microglia, as well as mast cells which are found both in the periphery and centrally. Males consistently have either more of these immune cells in specific brain regions or the cells are in a more activated state and producing inflammatory signaling molecules are engaging in high levels of phagocytosis during the critical period for sexual differentiation, thereby modulating divers endpoints such as synaptogenesis and cell survival. Cytokines and associated proteins are also expressed at higher levels in males and in one brain region result in the death of neurons that would otherwise survive. Thus there both a complexity and a consistency in the involvement of the immune system in masculinization of brain and behavior in rodents.
Sex differences in synaptic patterning are directed by prostaglandins and histamine
The preoptic area was noted above as touting some of the most robust sex differences in the brain which come in the form of synaptic patterning and differential cell death. This brain region is also of interest due to its centrality to the control of both mating and parenting behavior (Numan, 1994; Hull and Dominguez, 2007). The neurons in this region are replete with estrogen and androgen receptors but the key players initiating the differentiation of the synaptic pattern (i.e. more dense dendritic spine synapses in males), are two types of immune cells, microglia and mast cells (Lenz et al., 2013; Lenz et al., 2018). Microglia are immune cells unique to the brain and are modified macrophages that take up permanent residence there early in embryonic development (Ginhoux et al., 2013). Mast cells are also immune cells but originate in the bone marrow and are distributed throughout the body, particularly at surfaces such as skin, nasal epithelium, mouth eyes etc. They are considered first responders to any tissue disturbances and are packed with large secretory granules ready to dump histamine, serotonin and other signaling molecules (Silver and Curley, 2013). They are also capable of making prostaglandins and cytokines (Theoharides et al., 2012). In the brain mast cells are usually found in the meninges but during the critical period for sexual differentiation there is a population deep in the neuropil of the preoptic area, and there are significantly more in males than females. At the close of the sensitive period the numbers drop precipitously in both sexes (Lenz et al., 2018). During the time that mast cells are residing within the neuropil, they are also constitutively degranulating in males and releasing histamine. Nearby microglia respond to the released histamine with increased prostaglandin production, in particular PGE2, which is usually associated with fever (Lazarus et al., 2007), but also has signaling capacity through G-protein coupled receptors linked to adenyl cyclase, cAMP production and activation of protein kinase A (PKA) (Sugimoto and Narumiya, 2007). In the preoptic area this activated PKA phosphorylates a subunit of the AMPA glutamate receptor and this in turn promotes the trafficking of the receptor to the membrane and, via mechanisms not entirely understood, promotes the formation and stabilization of excitatory dendritic spine synapses (Lenz et al., 2011). The increase in dendritic spines endures into adulthood and positively correlates with the expression of male sexual behavior (Wright et al., 2008). The preoptic area is essential for male sexual behavior and if it is damaged or lesioned males lose interest in copulating. The increase in excitatory input created by generating a higher density of glutamatergic synapses promotes mating by enhancing the neural responsiveness to salient cues from receptive females. The critical point is that two immune cells, microglia and mast cells, are essential to the establishment of this node in the neural circuit controlling male copulation. In support of this conclusion, depleting microglia selectively during the critical period completely abolishes the capacity for mating in males when they mature, but has no impact on females (VanRyzin et al., 2016).
Sex differences in cell death are directed by immune signaling
In addition to a sex difference in synaptic patterning in the preoptic area are volumetric differences in which a subnucleus is larger in one sex. The most famous such nucleus is actually named for that fact, the sexually dimorphic nucleus of the preoptic area, or more simply the SDN (Gorski et al., 1980). The topic of intense study, the SDN is arguably the most robust volumetric sex difference in the brain and its discovery in the 1970’s heralded a new era of connecting brain morphology to physiology and behavior. It also provided the first opportunity to identify the specific cellular mechanisms by which steroids mediate sexual differentiation. Ironically, attempts at both have largely failed. The precise function of the SDN remains unclear although it is strongly implicated in partner preference (Roselli and Balthazart, 2011). As to mechanism, we know that the SDN starts with essentially the same number of neurons in males and females but they die in females due to lack local of estradiol, aromatized from peripheral androgens in males (Jacobson and Gorski, 1981; Davis et al., 1996a). But HOW estradiol is providing survival support to the SDN neurons in males has been a mystery, and many obvious answers such as regulation of cell death and survival pathways have been largely negative (Forger, 2009). The possibility that immune cells such as microglia and mast cells may be key mediators of SDN volume is an active area of investigation.
A second nucleus within the preoptic area also differs in volume but in the opposite direction, being larger in females than males (Davis et al., 1996b). The anteroventral periventricular nucleus, or more simply the AVPV, is a key node for the control of LH release from the pituitary by controlling the activity of GnRH neurons (Gu and Simerly, 1997; Clarkson and Herbison, 2006; Polston and Simerly, 2006). The pattern of LH release is sexually dimorphic, being continuously pulsatile in males but cyclical in females and characterized by a surge that is essential for inducing ovulation. It requires markedly distinct control of GnRH neuronal activity to achieve these diverse ends and this control is established during the critical period for sexual differentiation. If a neonatal female is treated with a large dose of testosterone or its aromatized end product, estradiol, she will be sterile as an adult (Gorski and Barraclough, 1963). The steroid-induced sterility is not because of any fault with the ovary or the pituitary but rather the loss of the synchronized firing of the GnRH neurons required for a surge of LH from the pituitary to induce ovulation. The follicules of the ovary develop perfectly well, they simply cannot release the ova, nor do they form the corpus luteum required to maintain pregnancy. The firing rate of GnRH neurons is not controlled by direct action of estrogens because they do not express estrogen receptors (Shivers et al., 1983). Instead it is the afferent input to the GnRH neurons which is critical, and an essential node for the synchronization required for the LH surge is the AVPV. The AVPV is a region of the preoptic area and contains a variety of different cell types. Dopamine neurons residing here are more numerous in females than males (Simerly et al., 1997), but they are only a small percentage of the population (which is not to suggest they are unimportant). Inhibitory GABAergic neurons in this region all express estrogen receptors, and some have a dual identity as glutamatergic as well, but regardless there are more of them in females and they project monosynaptically onto GnRH neurons (Ottem et al., 2004). The AVPV is just one part of a complex intercalating network of collections of cells that control GnRH activity but in a very simplistic sense the more neurons in the AVPV the greater the potential to induce the surge of LH necessary for ovulation. The number of neurons in the AVPV is established during the critical period for sexual differentiation, just as with the SDN, but in this case estradiol appears to induce cell death, thereby reducing the number of surviving cells in males (Forger, 2006).
That estradiol is acting as a cell killer instead of a cell survival factor is surprising, but what is even more intriguing is that the process involves signaling pathways normally associated with inflammation (Krishnan et al., 2009). Tumor necrosis factor-alpha, or Tnfα, is a cytokine involved in the acute phase of an inflammatory response, and it acts through two related receptors, Tnfr1 and Tnfr2. Tnfr1 is directly associated with cell death, but it is Tnfr2 that is critical to cell survival in the AVPV of females. The impact of Tnfα is indirect via phosphorylation of NFκB, which increases the cell survival factor Bcl2. In males, estrogens induce yet another protein, Traip, which inhibits NFκB and allows for increased expression of the Bax mediated cell death pathway and thereby reducing the overall cell population of the AVPV selectively in males (reviewed in (Petersen et al., 2012).
Sex differences in cell death are directed by immune cells
The amygdala is a complex collection of subnuclei involved in a variety of social and emotional behaviors ranging from juvenile social play, to mating to fear memories. Play behavior in rats is expressed predominantly during the period between weaning and puberty, and is often seen more frequently in males but depends upon context (Bredewold et al., 2014; Argue and McCarthy, 2015). The medial amygdala has long been identified as a brain region critical to this sex difference (Meaney and McEwen, 1986), but how that is the case was unknown until microglia were considered as potential sculptures of the neural circuit of play. In developing males, the microglia of the amygdala are highly phagocytic and they selectively target newly born astrocytes (VanRyzin et al., 2019). The ability of microglia to engulf and kill new born cells in a directed manner is a recently discovered phenomenon referred to as “phagoptosis”, and about which we still have much to learn (Brown and Neher, 2014). In the developing amygdala, newly born astrocytes express proteins on their surface that are interpreted by microglia as “eat me” signals, as opposed to “don’t eat me” signals found on other cells. In males, the number of astrocytes expressing eat me signals is higher, and they are therefore more frequently consumed. The end result is a reduced population of astrocytes in the male medial amygdala compared to females, and this then correlates with greater neuronal activation during play bouts, which occur some 3-4 weeks later (VanRyzin et al., 2019). Thus, once again, the immune system is the key determinant of neural circuit construction that will impact sex differences in physiology and behavior as the animal matures.
A new view of sexual differentiation of the brain
Based on the above observations, we propose the novel working hypothesis that neuroinflammatory mediators and immune cells are primary drivers of the masculinization of specific brain regions and behaviors, while steroids are either enablers that act to augment and enhance the inflammatory signaling, or perhaps are actually acting in an anti-inflammatory capacity in order to prevent runaway inflammation. But this raises the essential question of why do males have more of these signaling molecules and immune cells than females? There are multiple potential sources, beginning with a direct effect of testosterone or estradiol on the transcription of inflammation associated genes. This does seem to be the case for the genes coding for the COX-1 and COX-2 enzymes (Amateau and McCarthy, 2004), but there are no other clear demonstrations of direct steroid-hormone induced immune related gene expression. On a more global level immune-related genes appear to be epigenetically repressed in females as opposed to males, suggesting higher overall immune related transcription in regions of male brain (McCarthy et al., 2017b). This is puzzling given that androgens are generally anti-inflammatory, not pro-inflammatory. A second source is the sex chromosome complement, with the X chromosome being enriched in immune associated genes (Bianchi et al., 2012). As discussed above, there are many genes that escape X-inactivation, resulting in a double dose in females, which would be counter to the notion of higher immune activation in males. Lastly there is the potential that the source is external to the individual and instead derives from the maternal immune system. During pregnancy the maternal immune system must be repressed in order for successful growth of the fetus. However, there is increasing evidence that repression against male fetuses is less strong than that against female fetuses (reviewed in (McCarthy, 2019). Flipping that around, the maternal immune system is more apt to recognize a male fetus as foreign and mount a reaction against it. This could result in increased levels of diffusible inflammatory signals, such as prostaglandins or cytokines, in the male fetuses, or could evoke a reactive immune response from the male fetus itself. Lastly, there is the potential for maternal immune cells gaining access to the male fetus and even invading the brain. Demonstrating any or all of these will be technically challenging for many reasons. One of the biggest is separating what is maternal in origin from what is fetal in origin. Equally daunting is the accurate identification and quantification of immune cells in the nervous system as these cells are both small and rare. Overcoming these hurdles will provide essential information for determining the veracity of this new view of sexual differentiation of the brain.
Acknowledgments
Funding: This work was supported by RO1MH52716 and R01DA039062 to MMM.
Bibliography
- Amateau SK, McCarthy MM (2004) Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650. [DOI] [PubMed] [Google Scholar]
- Argue KJ, McCarthy MM (2015) Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol Sex Differ 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y (2004) Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology 145:1057–1062. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, Williams-Burris SM (2016) The importance of having two X chromosomes. Philosophical transactions of the Royal Society of London Series B, Biological sciences 371:20150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett TJaS Thomas C. (1998) Steroid Receptors at the Nexus of Transcriptional Regulation. Journal of Cellular Biochemistry Supplements 30:198–193. [DOI] [PubMed] [Google Scholar]
- Beato M, Klug J (2000) Steroid hormone receptors: an update. Hum Reprod Update 6:225–236. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I (2010) Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews 35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi I, Lleo A, Gershwin ME, Invernizzi P (2012) The X chromosome and immune associated genes. Journal of autoimmunity 38:J187–192. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH (2014) Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in behavioral neuroscience 8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ (2014) Microglial phagocytosis of live neurons. Nature reviews Neuroscience 15:209–216. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP (2002) Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5:933–934. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Itoh Y, Reue K, Arnold AP (2013) X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 154:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE (2006) Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA (1996a) The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res 734:10–18. [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA (1996b) Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology 63:142–148. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E (2003) Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res 118:82–90. [DOI] [PubMed] [Google Scholar]
- Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR (2014) XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America 111:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG (2006) Cell death and sexual differentiation of the nervous system. Neuroscience 138:929–938. [DOI] [PubMed] [Google Scholar]
- Forger NG (2009) Control of cell number in the sexually dimorphic brain and spinal cord. Journal of neuroendocrinology 21:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E (2014) The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Frontiers in cellular neuroscience 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow PN, Lovell-Badge R (1993) SRY and sex determination in mammals. Annual review of genetics 27:71–92. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Barraclough CA (1963) Effects of low dosages of androgen on the differentiation of hypothalamic regulatory control of ovulation in the rat. Endorcrinology 73:210–216. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM (1980) Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol 193:529–539. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB (1997) Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. The Journal of comparative neurology 384:142–164. [PubMed] [Google Scholar]
- Hull EM, Dominguez JM (2007) Sexual behavior in male rodents. Hormones and behavior 52:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Gorski RA (1981) Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. The Journal of comparative neurology 196:519–529. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL (2009) Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proceedings of the National Academy of Sciences of the United States of America 106:16692–16697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B et al. (2017) Sex-specific transcriptional signatures in human depression. Nature medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB (2007) EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 10:1131–1133. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Wright CL, Martin RC, McCarthy MM (2011) Prostaglandin E regulates AMPA receptor phosphorylation and promotes membrane insertion in preoptic area neurons and glia during sexual differentiation. PloS one 6:e18500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM (2013) Microglia are essential to masculinization of brain and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Pickett LA, Wright CL, Davis KT, Joshi A, McCarthy MM (2018) Mast Cells in the Developing Brain Determine Adult Sexual Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:8044–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M (2011) Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152:2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2019) Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, De Vries GJ, Forger NG (2017a) Sexual differentiation of the brain: A Fresh look at mode, mechanisms and meaning In: Hormones, Brain and Behavior. (Pfaff DaJ M, ed), pp 3–32. San Diego: Elsevier. [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM (2017b) Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nature reviews Neuroscience 18:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, McEwen BS (1986) Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res 398:324–328. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M (1994) Maternal Behavior In: Physiology of Reproduction (Knobil E, Neill JD, eds), pp 108–302. New York: Raven Press. [Google Scholar]
- Ottem EN, Godwon JG, Krishnan S, Petersen SL (2004) Dual-phenotype GABA/glutamate neurons in adult preoptic area: Sexual dimorphism and function. J Neurosci 24:8097–8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Krishnan S, Aggison LK, Intlekofer KA, Moura PJ (2012) Sexual differentiation of the gonadotropin surge release mechanism: a new role for the canonical NfkappaB signaling pathway. Frontiers in neuroendocrinology 33:36–44. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC (1959) Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382. [DOI] [PubMed] [Google Scholar]
- Pinheiro I, Dejager L, Libert C (2011) X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays : news and reviews in molecular, cellular and developmental biology 33:791–802. [DOI] [PubMed] [Google Scholar]
- Polston EK, Simerly RB (2006) Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. The Journal of comparative neurology 495:122–132. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Goldstein AY, De Vries GJ, Wagner CK (2002) Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. Journal of neuroendocrinology 14:761–767. [DOI] [PubMed] [Google Scholar]
- Roselli C, Balthazart J (2011) Sexual differentiation of sexual behavior and its orientation. Frontiers in neuroendocrinology 32:109. [DOI] [PubMed] [Google Scholar]
- Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, de Vries GJ, Akbarian S, Forger NG (2015) Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Experimental neurology 268:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW (1983) Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304:345–347. [DOI] [PubMed] [Google Scholar]
- Silver R, Curley JP (2013) Mast cells on the mind: new insights and opportunities. Trends in neurosciences 36:513–521. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS (1997) Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proceedings of the National Academy of Sciences of the United States of America 94:14077–14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S (2007) Prostaglandin E receptors. The Journal of biological chemistry 282:11613–11617. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D (2012) Mast cells and inflammation. Biochimica et biophysica acta 1822:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME, Hardy J, Ryten M, North American Brain Expression C (2013) Widespread sex differences in gene expression and splicing in the adult human brain. Nature communications 4:2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM (2016) Temporary Depletion of Microglia during the Early Postnatal Period Induces Lasting Sex-Dependent and Sex-Independent Effects on Behavior in Rats. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, McCarthy MM (2019) Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron. [DOI] [PubMed] [Google Scholar]
- Werling DM, Parikshak NN, Geschwind DH (2016) Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nature communications 7:10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AC, Nakauchi H, Gottgens B (2017) Mammalian Transcription Factor Networks: Recent Advances in Interrogating Biological Complexity. Cell Syst 5:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM (2008) Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol 68. [DOI] [PMC free article] [PubMed] [Google Scholar]