Abstract
BACKGROUND:
Spontaneous preterm birth (SPTB) is a leading cause of neonatal morbidity and mortality. Early identification of at-risk women by reliable screening tests could reduce the SPTB rate, but conventional methods such as obstetrical history and maternal cervical length screening identify only a minority of SPTB cases. Cervicovaginal fluid (CVF) might prove to be a useful, readily available biological fluid for identifying SPTB biomarkers.
OBJECTIVE:
To identify CVF biomarkers of early SPTB in a high-risk cohort of pregnant women with a history of SPTB using targeted and shotgun proteomic analyses.
STUDY DESIGN:
A nested case control study (cases = SPTB <34 weeks in the current pregnancy; controls = spontaneous labor and delivery at 39–41 weeks) was performed using CVF samples collected at three study visits (100/7–186/7 weeks, 190/7–236/7 weeks, and 280/7–316/7 weeks). All participants had a history of at least one prior SPTB. Targeted proteomic analysis was performed using a stable-isotope labeled proteome (SILAP) derived from endocervical and vaginal mucosal cells. This served as a standard to quantitate candidate protein levels in individual CVF samples from the second and third study visits using liquid chromatography-multiple reaction monitoring mass spectrometry. The ratio of endogenous peptide area/SILAP-derived peptide area was used to measure levels of 42 peptides in 22 proteins. In order to maximize biomarker discovery in the CVF samples, shotgun proteomic analysis also was performed utilizing liquid chromatography and ion trap mass spectrometry. A validation study was performed in second-trimester CVF samples from an independent study group (12 SPTB cases, 19 term delivery controls) using enzyme-linked immunosorbent assay (ELISA) for five proteins expressed at higher levels in SPTB cases compared to controls in targeted or shotgun proteomic analyses.
RESULTS:
For targeted proteomics, CVF samples from 33 cases and 32 controls at 190/7–236/7 weeks, and 16 cases and 14 controls at 280/7–316/7 weeks from the same pregnancies, were analyzed. When samples were compared between cases and controls, the relative abundance of five proteins was greater (P=0.02–0.05) in cases at both visits, while the relative abundance of one protein was lower (P=0.03) in cases at both visits. For shotgun proteomics analyses, CVF samples were pooled for nine SPTB cases and nine term delivery controls at each study visit. Shotgun proteomics yielded 28 proteins that were detected at levels >2× higher and one protein that was detected at a level <0.5× lower in SPTB cases compared to controls at all three study visits. Validation ELISA for five proteins that were detected at higher levels in CVF samples from SPTB cases compared to term delivery controls in proteomics analyses did not demonstrate statistically significant differences between SPTB cases and controls.
CONCLUSIONS:
Potential biomarkers of SPTB were identified by targeted and shotgun proteomics analyses in CVF samples from high-risk, asymptomatic women. Many of the proteins detected at higher levels in CVF samples from SPTB cases are extracellular matrix proteins and/or regulate cell membrane physiology. These proteins have substantial biological interest, but validation ELISA for five of these proteins did not yield clinically useful biomarkers for SPTB.
Keywords: fibronectin-1, extracellular matrix protein 1 isoform 1 precursor, laminin alpha 3 subunit isoform, laminin A/C isoform 2, calsyntenin 1 isoform 2
CONDENSATION:
Proteomic analyses using cervicovaginal fluid samples identified biologically relevant proteins that could not be validated by ELISA as useful biomarkers for predicting spontaneous preterm birth.
INTRODUCTION
Preterm birth, defined as birth before 37 weeks of gestation, is a leading cause of infant morbidity and mortality in non-anomalous infants. In the US, approximately 9.6 percent of all births are preterm,1 and two-thirds of preterm births are spontaneous following idiopathic preterm labor or preterm premature rupture of membranes (PPROM).2 Preterm birth also is a leading cause of long-term neurological disabilities in children. Unfortunately, traditional research approaches have yielded little progress in understanding the underlying cause(s) of spontaneous preterm birth, and current clinical strategies have failed to identify most women who will deliver preterm.
The overarching goal of the Genomic and Proteomic Network for Preterm Birth Research (GPN-PBR) is to identify possible biomarkers that could predict the susceptibility to spontaneous preterm birth as well as to shed light on the molecular mechanisms involved in its etiologies. Novel biomarkers and a better understanding of the mechanisms involved in spontaneous preterm birth may facilitate the introduction of more effective prevention and treatment strategies. The GPN-PBR investigators hypothesized that maternal proteomic profiles could prospectively identify women at risk for spontaneous preterm birth. To test this hypothesis, a longitudinal cohort study of 500 women with a history of spontaneous preterm birth <37 weeks enrolled at <18 weeks of pregnancy was conducted at the three GPN-PBR clinical sites. The previous preterm delivery criterion was selected to enrich the cohort for preterm delivery cases in the current pregnancy. Maternal blood and cervicovaginal fluid were collected at three study visits (enrollment visit between 100/7–186/7 weeks gestation, second study visit between 190/7–236/7 weeks, and third study visit between 280/7–316/7 weeks). Results for maternal serum proteomics in this high-risk cohort were published previously;3 here the results for maternal cervicovaginal fluid proteomics are presented.
Cervicovaginal fluid (CVF) is a complex mixture of secretions from the vagina, endocervix, endometrial decidua, and amniochorion, and therefore may serve as an important diagnostic site for monitoring maternal and fetal health in pregnant women.4 Collection of CVF is minimally invasive and relatively safe, as compared to collection of amniotic fluid by amniocentesis or tissue excisions from the placenta or uterus, and therefore is expected to be more readily available and potentially useful as a source of biomarkers in pregnant women.
Recent advances in the application of various proteomics-based platforms have facilitated the process of discovery of novel protein biomarkers of spontaneous preterm birth.3,5–8 Shotgun proteomics using various separation techniques such as multidimensional liquid chromatography (LC) coupled with high-resolution tandem mass spectrometry (MS/MS) has dramatically improved the discovery of novel protein biomarkers of spontaneous preterm birth.5,9,10 Targeted proteomics utilizing stable isotope labeling by amino acids in cell culture (SILAC) allows for the generation of labeled proteomes that can be used as internal standards to facilitate accurate identification and quantification of low-abundance proteins in biological samples.8 Finally, LC-multiple reaction monitoring/mass spectrometry (LC-MRM/MS) provides speed, sensitivity, selectivity, and the ability to quantitate multiple peptides simultaneously.3,11,12 In the current study, both targeted and shotgun proteomics analyses were performed to: 1) utilize the quantification capabilities of SILAC to generate a stable isotope labeled proteome (SILAP) standard and LC-MRM/MS methodology; and 2) utilize the enhanced search capabilities of LC-MS/MS based shotgun proteomics to identify candidate protein biomarkers of spontaneous preterm birth in CVF samples.
MATERIALS AND METHODS
Longitudinal cohort
The GPN-PBR longitudinal cohort of 500 women with a history of spontaneous preterm birth was projected to have 30 spontaneous preterm births <34 weeks (six percent of the cohort) and 60 spontaneous preterm births between 34–37 weeks (12 percent of the cohort; overall spontaneous preterm birth rate 18 percent) that could be matched with term deliveries from the same cohort between 39–41 weeks for nested case-control analyses.3 Women were eligible for enrollment at the three GPN-PBR clinical sites (University of Alabama at Birmingham, University of Texas Medical Branch at Galveston, and University of Utah) from 2007 to 2010 if they had a history of at least one spontaneous preterm delivery of a singleton pregnancy at a gestational age between 200/7 and 366/7 weeks in a previous pregnancy and a current singleton pregnancy. Study participants were recruited and enrolled during routine prenatal visits before 19 weeks of pregnancy, and gestational dating was based upon the first day of the patient’s last menstrual period and ultrasound examination performed before enrollment.3 Maternal blood, urine, and CVF samples were collected at three study visits (enrollment visit between 100/7–186/7 weeks gestation, second study visit between 190/7–236/7 weeks, and third study visit between 280/7–316/7 weeks) and at admission for delivery.
Exclusion criteria were maternal uterine anomalies, cervical cerclage, multi-fetal gestation, fetal aneuploidy or lethal fetal anomalies, polyhydramnios (amniotic fluid index ≥25 cm or deepest vertical pocket ≥12 cm), planned or probable delivery at a non-network site, no availability for prospective specimen/data collection, and serious maternal medical conditions.3
Maternal CVF samples were collected at all three study visits and at admission for delivery. Briefly, speculum examinations were performed by study coordinators and a Dacron swab was inserted into the posterior fornix of the vagina and rotated for 10 seconds. The swab was swirled in a 2-mL tube containing 1 mL phosphate-buffered saline and discarded. The tube was frozen and stored in liquid nitrogen immediately after collection. Protein expression was studied in these samples to identify potential biomarkers that predict spontaneous preterm birth.
Outcomes data were recorded in a shared database by trained research coordinators at the three study sites. Maternal CVF samples were shipped on dry ice to the network’s analytical core at the University of Pennsylvania, where all proteomic assays were performed by experienced laboratory personnel who were blinded to demographics and outcomes data for each subject. Proteomics results and clinical outcomes were analyzed at the network’s data coordinating and analysis center at Yale University. The GPN-PBR scientific protocol was approved by the investigational review boards at all 5 institutions.
Targeted proteomics techniques
In a previous study from the same laboratory, SILAC methodology was employed to identify 15 biologically relevant candidate proteins that were expressed differentially in pooled CVF samples collected at 240/7–276/7 weeks gestation from five asymptomatic women who delivered preterm at 280/7–316/7 weeks following spontaneous preterm labor compared to five term delivery controls.8 For the current study, seven more proteins were added to the 15 protein panel based on the network’s previous serum biomarker study for spontaneous preterm birth.3 These additional seven proteins were detected in the SILAC proteome.3,8 Two physiologically relevant transformed epithelial cell lines were utilized to create the SILAC proteome – human endocervical (End1, American Type Culture Collection) cells and human vaginal mucosa (VK2, ATCC) cells.3,8 Two peptides were selected for 20 of the proteins comprising the proteome, while one peptide was analyzed for two of the proteins comprising the proteome (total of 42 peptides in 22 proteins, Table 1).
Table 1.
Stable isotope labeled proteins used for targeted proteomic analyses.
| Protein | Peptide Sequence |
|---|---|
| Cadherin 1; type 1 preprotein | DTANWLEINPDTGAISTR |
| NTGVISVVTTGLDR | |
| Calreticulin precursor | FYALSASFEPFSNK |
| EQFLDGDGWTSR | |
| SerpinB7 | YVEVFFPQFK |
| Glia derived nexin isoform c precursor | VLGITDMFDSSK |
| TIDSWMSIMVPK | |
| Galectin-3 binding protein | STHTLDLSR |
| ELSEALGQIFDSQR | |
| Peptidylprolyl isomerase 3 | VSFELFADK |
| FEDENFILK | |
| Heat shock protein b1 | VSLDVNHFAPDELTVK |
| LATQSNEITIPVTFESR | |
| Thrombospondin-1 precursor | TIVTTLQDSIR |
| SITLFVQEDR | |
| Fibronectin-1 isoform 1 preprotein | FLATTPNSLLVSWQPPR |
| NTFAEVTGLSPGVTYYFK | |
| Stratifin | YEDMAAFMK |
| YLAEVATGDDK | |
| Extracellular matrix protein 1 isoform 1 precursor | LVWEEAMSR |
| NVALVSGDTENAK | |
| Fatty acid binding protein 5 (psoriasis associated) | MGAMAKPDCIITCDGK |
| TTQFSCTLGEK | |
| Calsyntenin 1 isoform 2 | AASEFESSEGVFLFPELR |
| IPDGVVSVSPK | |
| Plasminogen activator inhibitor-1 | FIINDWVK |
| FSLETEVDLR | |
| Secretory leukocyte peptidase inhibitor precursor | CLDPVDTPNPTR |
| Cathepsin L2 preprotein | LYGANEEGWR |
| FDQNLDTK | |
| Laminin alpha 3 subunit isoform-1 | LNDTVGVTK |
| VWQDACSPLPK | |
| Cystatin C precursor | ALDFAVGEYNK |
| LVGGPMDASVEEEGVR | |
| Tissue inhibitor of metalloproteinase 1 precursor | GFQALGDAADIR |
| SEEFLIAGK | |
| Urokinase plasminogen activator preprotein | VSHFLPWIR |
| LNSNTQGEMK | |
| Desmoplakin isoform 1 | AITGFDDPFSGK |
| VLLQEEGTR | |
| Lamin A/C isoform 2 | LAVYIDR |
| EGDLIAAQAR |
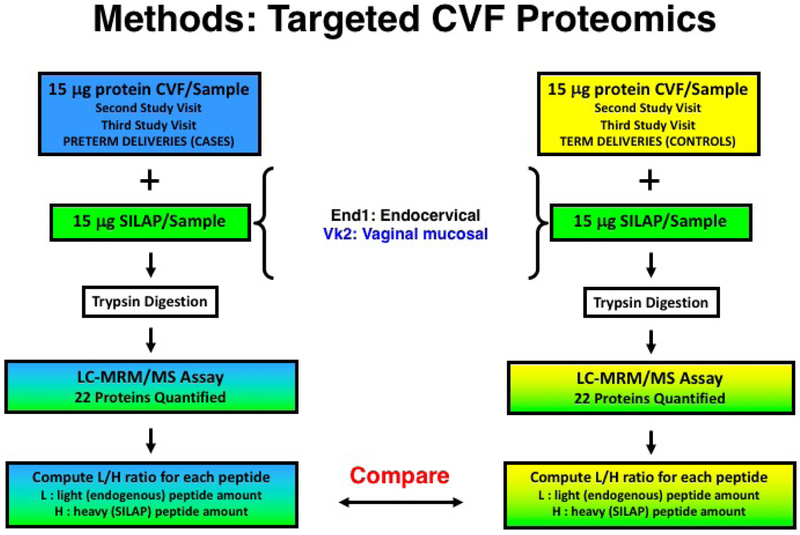
For targeted proteomic analyses, protein levels in individual CVF samples from the second and third study visits (190/7–236/7 weeks and 280/7–316/7 weeks, respectively) were compared between preterm delivery cases and term delivery controls. Three transitions for each peptide were quantified by LC-MRM/MS (Figure 1) using a TSQ-Vantage mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA).
Figure 1.
Methods for targeted cervicovaginal fluid (CVF) proteomics assays, based on stable isotope labeling of proteins secreted by endocervical (End1) and vaginal mucosal (Vk2) cells. Levels of 22 peptides were compared in individual CVF samples from spontaneous preterm birth cases and term delivery controls. SILAP, stable isotope labeled proteome; LC, liquid chromatography; MRM, multiple reaction monitoring; MS, mass spectrometry; MS/MS, tandem mass spectrometry.
Shotgun proteomics techniques
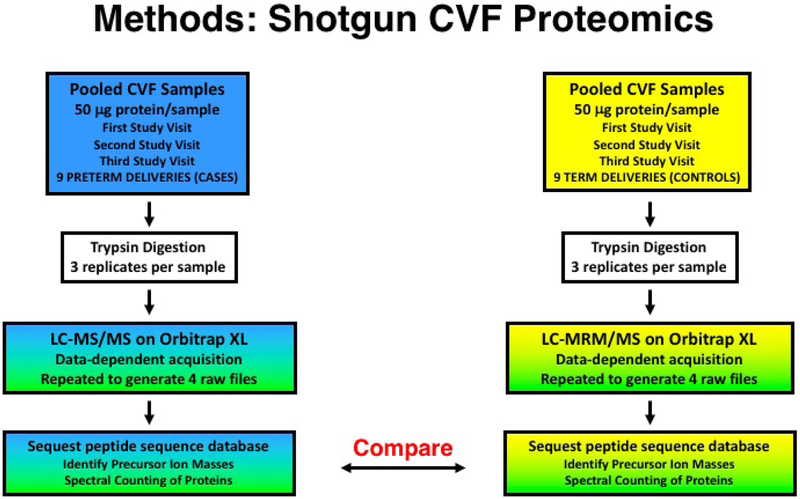
In order to maximize biomarker discovery in the CVF samples, shotgun proteomic analysis was performed utilizing LC and ion trap MS. Briefly, CVF samples (50 μg protein/sample) from nine women in each study group (preterm birth cases, term delivery controls) were pooled for each of the three study visits, yielding a total of six pooled CVF samples (Figure 2). For each analysis, 50 mg protein/pooled CVF sample underwent trypsin digestion and LC-MS/MS utilizing an LTQ Orbitrap XL ion-trap mass spectrometer (Thermo Fisher Scientific). Analyses were performed in triplicate for each pooled CVF sample. The MS/MS spectra were searched against an indexed human peptide database (Proteome Discoverer, Thermo Fisher Scientific) using the Sequest search algorithm (Thermo Fisher Scientific). Strict trypsin cleavage rules with maximum of two missed cleavage sites, fragment mass tolerance 0.8 Da, precursor mass tolerance 10 ppm, and variable modifications of methionine oxidation, carboxyamidomethlyation on cysteine, [13C6 15N2]-lysine, and [13C6 15N1]-leucine were applied in the search criteria. Exclusion lists were created by repeating LC-MS/MS three times to generate four raw files for each pooled CVF sample. Assignment of peptide sequences was performed using the Sequest database search algorithm. Sequest accounts for the distribution of scores over an entire data set to calculate the probability of a correct assignment for every peptide. Sequest calculates false-positive error rates at specific probability score cutoff values for each data set. A minimum Sequest probability score of ≥0.5 was used to remove low probability peptides. At this cutoff, the estimated false-positive error rate was 10.8 percent.
Figure 2.
Methods for shotgun cervicovaginal fluid (CVF) proteomics assays utilizing liquid chromatography and tandem mass spectroscopy (LC-MS/MS). Peptide expression was compared in 6 pooled CVF (50mg/subject) samples: 1) 9 spontaneous preterm birth cases, 1st study visit; 2) 9 spontaneous preterm birth cases, 2nd study visit; 3) 9 spontaneous preterm birth cases, 3rd study visit; 4) 9 term delivery controls, 1st study visit; 5) 9 term delivery controls, 2nd study visit; and 6) 9 term delivery controls, 3rd study visit.
Validation study
Cervicovaginal fluid samples were collected during the second trimester from a separate cohort of women at the same time period (2007–2010) at one of the GPN-PBR clinical sites (University of Alabama at Birmingham). Samples were collected from asymptomatic women during the second trimester (160/7 to 206/7 weeks gestation). The validation cohort consisted of 31 women, 12 of whom underwent spontaneous preterm delivery at <370/7 weeks and 19 of whom delivered after 370/7 weeks. Levels of five proteins in CVF samples were measured by enzyme-linked immunosorbent assay (ELISA) and compared between women who underwent spontaneous preterm delivery at <370/7 weeks and term delivery after >370/7 weeks gestation.
Statistics
Clinical data and protein expression levels were compared between spontaneous preterm deliveries and term deliveries using standard statistical tests for nested case-control studies – Fisher exact tests for categorical data and Student’s t-tests for continuous data. The primary analysis for targeted proteomics was the comparison of maternal CVF protein levels at the second and third study visits (190/7–236/7 weeks and 280/7–316/7 weeks, respectively) between spontaneous preterm delivery cases and term delivery controls. The L/H ratio (light endogenous peptide area/heavy SILAP peptide area) was determined for each peptide in individual CVF samples, and L/H ratios were averaged for the 20 proteins for which two peptides were analyzed. The L/H ratios were compared between spontaneous preterm birth cases and term delivery controls using the Student’s t-test (α<0.05). The primary analysis for shotgun proteomics was the comparison of spectral counts in pooled CVF samples between cases and controls. Spectral count ratios with cut-offs of >2, >3, >5, and >10-fold differences between cases and controls were reported. For validation studies, protein levels were compared in CVF samples from women who delivered preterm compared to term delivery controls. Analyses were not adjusted for multiple comparisons. Normally distributed sample sets were compared by Student’s t-tests, while nonparametric samples sets were compared by Mann Whitney Rank Sum tests.
RESULTS
Among the 500 women enrolled in the high-risk cohort, 33 women had a subsequent spontaneous preterm birth <34 weeks gestation (33/500 = 6.6 percent incidence; range, 236/7–335/7 weeks). The 33 spontaneous preterm birth cases were matched to the next study participant (according to subject number) by age (± 4 years), race, clinical site, and who delivered at 39–41 weeks’ gestation without complications. Cervicovaginal fluid samples from second study visits (190/7–236/7 weeks) were available for all 33 spontaneous preterm birth cases and 32 controls, while CVF samples from the third study visit (280/7–316/7 weeks) were available for only 16 spontaneous preterm birth cases, largely because many of these women delivered before the third study visit. Third study visit CVF samples were available for 14 of the 16 matched term delivery controls. Demographic characteristics and obstetrical outcomes for the 33 spontaneous preterm birth cases <34 weeks’ gestation and 32 term delivery controls whose CVF samples were analyzed are listed in Table 2.
Table 2.
Demographic characteristics and obstetrical outcomes for spontaneous preterm birth cases and term delivery controls.
| Variable | Cases (n=33) | Controls (n=32) | P value1 |
|---|---|---|---|
| Maternal age, mean (SD) | 26.7 (5.6) | 26.7 (4.9) | 0.71 |
| Maternal race (%) | 1.00 | ||
| Black or African American | 12 (36.4) | 12 (37.5) | |
| Caucasian | 18 (54.5) | 19 (59.4) | |
| Asian | 1 (3.0) | 0 (0) | |
| Other | 2 (6.1) | 1 (3.1) | |
| Parity (%) | 0.37 | ||
| 1 | 9 (27.3) | 10 (31.3) | |
| 2 | 7 (21.2) | 13 (40.6) | |
| 3 | 6 (18.2) | 3 (9.4) | |
| 4 | 8 (24.2) | 4 (12.5) | |
| 5 or 6 | 3 (9.1) | 2 (6.3) | |
| Previous preterm delivery (%) | 0.79 | ||
| 1 | 22 (66.7) | 24 (75.0) | |
| 2 | 7 (21.2) | 6 (18.8) | |
| >2 | 4 (12.1) | 2 (6.2) | |
| Previous term delivery (%) | 0.24 | ||
| 0 | 17 (51.5) | 19 (59.4) | |
| 1 | 9 (27.3) | 11 (34.4) | |
| >1 | 7 (21.2) | 2 (6.3) | |
| Spontaneous/elective abortion (%) | 0.44 | ||
| 0 | 22 (66.7) | 22 (68.8) | |
| 1 | 7 (21.2) | 9 (28.1) | |
| >1 | 4 (12.1) | 1 (3.1) | |
| Stillbirth (%) | 0.49 | ||
| 0 | 33 (100) | 31 (96.9) | |
| 1 | 0 (0) | 1 (3.1) | |
| Maternal BMI (lb/in2), mean (SD) | 25.6 (4.8) | 27.0 (7.3) | 0.37 |
| Maternal education (%) | 0.93 | ||
| K-5 Elementary | 2 (6.1) | 1 (3.1) | |
| 6–8 Middle School | 4 (12.1) | 5 (15.6) | |
| 9–12 High School or GED | 17 (51.5) | 15 (46.9) | |
| 13–16 College | 10 (30.3) | 11 (34.4) | |
| Gestational age at 2nd study visit (wk), mean (SD) | 21.0 (1.6) | 21.5 (1.5) | 0.18 |
| Gestational age at 3rd study visit (wk), mean (SD) | 29.5 (1.6) | 30.0 (1.5) | 0.18 |
| Gestational age at delivery (wk), mean (SD) | 30.0 (3.5) | 39.7 (0.6) | <0.0001 |
| Birthweight (gm), mean (SD) | 1607 (629) | 3381 (453) | <0.0001 |
Fisher exact tests were used for categorical variables, and t tests were used for continuous variables
For targeted proteomics assays, L/H ratios were calculated in each maternal CVF sample for 22 proteins (42 peptides, Table 1) by targeted LC-MRM/MS proteomics techniques. The method for calculating the L/H ratio for one peptide (secretory leukocyte peptidase inhibitor precursor) in a CVF sample from one participant who delivered at term (control) is presented in Figure 3. The relative abundance of four proteins (based on two peptides) was significantly greater in CVF samples from cases at the second and third study visits (Figure 4). Meanwhile, the relative abundance of one protein (based on one peptide) was significantly greater, and the relative abundance of one protein (based on one peptide) was significantly lower, in CVF samples from cases at the second and third study visits (see summary of targeted proteomics results in Table 3).
Figure 3.
Method for calculating semi-quantitative protein expression levels in cervicovaginal fluid (CVF) samples utilizing targeted proteomics techniques. Illustrated here is the L/H ratio (light endogenous peptide area/heavy stable isotope labeled proteome [SILAP] peptide area) of secretory leukocyte peptidase inhibitor precursor (one peptide) in one CVF sample collected at the second study visit (190/7–236/7 weeks) for a participant who delivered at term (control). The total intensity (Y-axis) of three transitions of the light endogenous peptide was divided by the total intensity of three transitions of the heavy labeled (SILAP) peptide, yielding an L/H ratio of 6.33.
Figure 4.
Proteins with significantly greater levels of two peptides in cervicovaginal fluid (CVF) samples from spontaneous preterm birth cases compared to term delivery controls. Samples from the 2nd study visit (33 cases, 32 controls) and 3rd study visit (16 cases, 14 controls) were analyzed together. Measured on the Y-axis is the L/H ratio (endogenous peptide area/internal standard peptide area). PTB, spontaneous preterm birth.
Table 3.
Proteins expressed at significantly different levels in CVF samples from spontaneous preterm birth cases and term delivery controls.
| Protein | Peptides | Fold Change | +/− | P |
|---|---|---|---|---|
| Second Study Visit (190/7–236/7 wk) | ||||
| Laminin alpha 3 subunit isoform | 2 | 2.6 | + | 0.05 |
| Cystatin C precursor | 2 | 2.3 | + | 0.002 |
| Laminin A/C isoform 2 | 2 | 1.8 | + | 0.05 |
| Calsyntenin 1 isoform 2 | 1 | 1.7 | + | 0.007 |
| Third Study Visit (280/7–316/7 wk) | ||||
| Extracellular matrix protein 1 isoform 1 precursor | 2 | 4.7 | + | 0.02 |
| Tissue inhibitor of metalloproteinase 1 precursor | 2 | 1.7 | − | 0.05 |
| HSP-beta 1 | 1 | 1.9 | − | 0.04 |
| Calsyntenin 1 isoform 2 | 1 | 2.3 | − | 0.05 |
| Laminin alpha 3 subunit isoform | 1 | 2.2 | − | 0.04 |
| Urokinase plasminogen activator preprotein | 1 | 2.1 | − | 0.05 |
| Both Visits | ||||
| Fibronectin-1 | 2 | 1.7 | + | 0.05 |
| Extracellular matrix protein 1 isoform 1 precursor | 2 | 2.3 | + | 0.02 |
| Laminin alpha 3 subunit isoform | 2 | 2.5 | + | 0.03 |
| Laminin A/C isoform 2 | 2 | 1.7 | + | 0.04 |
| Desmoplakin isoform 1 | 1 | 1.5 | − | 0.03 |
| Calsyntenin 1 isoform 2 | 1 | 1.4 | + | 0.02 |
Peptides: number of peptides for each protein demonstrating different L/H (light endogenous peptide area/heavy stable isotope labeled proteome [SILAP] peptide area); +/−: increase/decrease;
P values: determined by two-sample t tests.
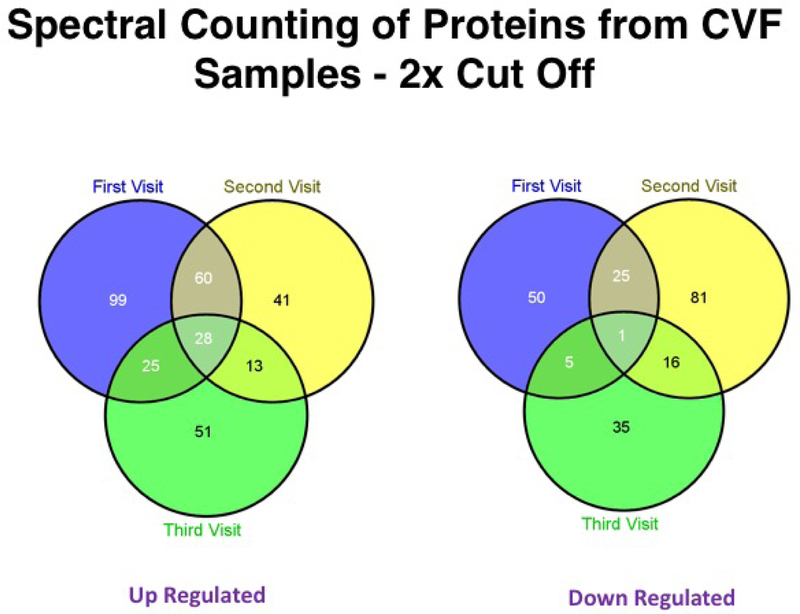
Shotgun proteomics was utilized to compare protein expression in pooled CVF samples between 9 spontaneous preterm birth cases and 9 term delivery controls. Spectral count ratios were >2 (i.e., two-fold greater) in CVF samples from cases compared to controls for 212 proteins at the first study visit, 142 proteins at the second study visit, and 117 proteins at the third study visit (Table 4). Twenty-eight proteins were expressed at >2× greater levels at all three study visits (Table 5), while 1 protein was expressed at <0.5× lower levels at all three study visits (Figure 5).
Table 4.
Spectral counting of proteins from cervicovaginal fluid samples (shotgun proteomics techniques).
| Spectral Count Ratio | First Study Visit (Number of proteins) | Second Study Visit (Number of proteins) | Third Study Visit (Number of proteins) | |||
|---|---|---|---|---|---|---|
| Increased | Decreased | Increased | Decreased | Increased | Decreased | |
| >10 | 0 | 2 | 0 | 2 | 0 | 1 |
| >5 | 17 | 5 | 7 | 16 | 4 | 2 |
| >3 | 65 | 19 | 28 | 43 | 21 | 12 |
| >2 | 212 | 81 | 142 | 123 | 117 | 57 |
Table 5.
Individual molecular functions of the 28 proteins detected by shotgun proteomics at greater levels in spontaneous preterm births for all three study visits.
| # | Protein Name | Protein ID (Accession Number | Molecular function |
|---|---|---|---|
| 1 | Destrin isoform A | gi|5802966|ref|NP_006861.1| | Actin depolymerization |
| 2 | Titin isoform N2-A | gi|291045225|ref|NP_596869.4| | Actin filament binding |
| 3 | Beta-catenin | gi|148227672|ref|NP_001091680.1| | Activator |
| 4 | Protein-glutamine gamma-glutamyltransferase K | gi|4507475|ref|NP_000350.1| | Acyltransferase |
| 5 | Protein S100-A8 | gi|21614544|ref|NP_002955.2| | Anti-microbial |
| 6 | ATP synthase subunit alpha, mitochondrial precursor | gi|50345984|ref|NP_001001937.1| | ATP binding |
| 7 | ATP synthase subunit beta, mitochondrial precursor | gi|32189394|ref|NP_001677.2| | ATP binding |
| 8 | Plastin-3 isoform 1 | gi|209862851|ref|NP_001129497.1| | Calcium binding |
| 9 | Protein S100-A6 | gi|7657532|ref|NP_055439.1| | Calcium-dependent protein binding |
| 10 | Heat shock 60 kDa protein, mitochondrial | gi|41399285|ref|NP_955472.1| | Chaperone |
| 11 | Heat shock-related 70 kDa protein 2 | gi|13676857|ref|NP_068814.2| | Chaperone |
| 12 | Sacsin | gi|163659918|ref|NP_055178.3| | Chaperone binding |
| 13 | Proteasome 26S non-ATPase subunit 2 | gi|25777602|ref|NP_002799.3| | Enzyme regulator |
| 14 | Leukotriene A4 hydrolase | gi|4505029|ref|NP_000886.1| | Epoxide hydrolase |
| 15 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 2 isoform 2 precursor | gi|209413738|ref|NP_001129243.1| | Glycotransferase |
| 16 | Cytosolic purine 5’-nucleotidase | gi|197276664|ref|NP_001127845.1| | Hydrolase |
| 17 | Exportin-2 | gi|29029559|ref|NP_001307.2| | Importin-alpha export receptor activity |
| 18 | Nicotinate phosphoribosyltransferase | gi|194394158|ref|NP_660202.3| | Nicotinate phosphoribosyltransferase |
| 19 | Thymidine phosphorylase isoform 1 proprotein | gi|166158925|ref|NP_001107228.1| | Nucleotide phosphorylase |
| 20 | Heme oxygenase 1 | gi|4504437|ref|NP_002124.1| | Oxidoreductase |
| 21 | Cytosolic phospholipase A2 delta | gi|116174754|ref|NP_828848.3| | Phospholipase |
| 22 | Protein disulfide-isomerase A3 precursor | gi|21361657|ref|NP_005304.3| | Protein disulfide isomerase |
| 23 | Cellular retinoic acid binding protein 2 | gi|4503029|ref|NP_001869.1| | Retinoid binding |
| 24 | Eukaryotic translation initiation factor 5A-1 isoform A | gi|219555707|ref|NP_001137232.1| | Ribosome binding |
| 25 | Sulfotransferase family cytosolic 2B member 1 isoform B | gi|31563386|ref|NP_814444.1| | Steroid sulfotransferase |
| 26 | Talin 1 | gi|223029410|ref|NP_006280.3| | Structural constituent of cytoskeleton |
| 27 | Plectin isoform 1E | gi|41322908|ref|NP_958781.1| | Structural constituent of muscle |
| 28 | 14-3-3 protein beta/alpha | gi|21328448|ref|NP_647539.1| | Transcription corepressor activity |
Figure 5.
Spectral counting of proteins in cervicovaginal fluid samples using shotgun proteomics. Twenty-eight proteins were detected at >2× greater levels in cervicovaginal fluid (CVF) samples from spontaneous preterm birth cases compared to term delivery controls for all 3 study visits, while 1 protein was detected at <0.5× lower levels in CVF samples from spontaneous preterm birth cases compared to term delivery controls for all 3 study visits.
Validation studies were performed in an independent study cohort for five proteins of interest that were expressed at significantly greater levels in CVF samples from preterm birth cases compared to term delivery controls: 1–4) fibronectin-1, extracellular matrix protein 1 isoform 1 precursor, laminin alpha 3 subunit isoform, and calsyntenin 1 isoform 2, which were detected by targeted proteomics from the second and third study visits; and 5) heme oxygenase 1, which was detected by shotgun proteomics at all three study visits. Enzyme-linked immunosorbent assays were performed for each protein according to the manufacturer’s instructions: 1) Human Fibronectin Quantikine ELISA Kit, R&D Systems, Minneapolis, MN; 2) Human ECM-1 ELISA Kit for Cell Culture Supernatants, Plasma, and Serum samples, Ray Biotech, Norcross, GA; 3) Laminin Human ELISA Kit, Abcam, Cambridge, MA; 4) Human Calsyntenin-1 Elisa Kit, BlueGene Biotech, Shanghai, China; and 5) Human Total HO-1/HMOX1 DuoSet IC ELISA, R&D Systems. Levels of laminin and fibronection were 6–12× greater in spontaneous preterm birth cases, but wide standard deviations and relatively small sample sizes yielded differences that were not statistically significant (P=0.08 for laminin, P=0.06 for fibronectin) in CVF samples from women who delivered preterm compared to term delivery controls (Figure 6).
Figure 6.
Enzyme-linked immunosorbent assays comparing protein levels in cervicovaginal fluid samples from spontaneous preterm birth cases (PTB) and term delivery controls (TERM) in the validation cohort. A) fibronectin-1, B) extracellular matrix protein 1 isoform 1 precursor (ECM), C) laminin alpha 3 subunit isoform, D) calsyntenin 1 isoform 2, and E) heme oxygenase 1 (HMOX). Normally distributed sample sets (B, D, E) were compared by Student’s t-tests; nonparametric samples sets (A, C) were compared by Mann Whitney Rank Sum tests. None of the protein levels were significantly different (P<0.05) between spontaneous preterm birth cases and term delivery controls.
COMMENT
Principal findings
Targeted proteomics analyses yielded a total of 10 proteins that were expressed at different levels in CVF samples (second and third study visits) from spontaneous preterm birth cases compared to term delivery controls. Shotgun proteomics analyses generated a list of 28 proteins that were expressed at >2× greater levels at all three study visits in preterm birth cases compared to term delivery controls. Five proteins were selected for validation using ELISA techniques and CVF samples from an independent cohort of women, but none of the proteins were detected at significantly different levels in spontaneous preterm birth cases compared to term delivery controls.
Results
Previous proteomic analyses to identify potential biomarkers in CVF associated with spontaneous preterm birth utilized primarily pooled CVF samples from smaller groups of patients than the current study.6,8,10,13–15 In a preliminary study performed by GPN-PBR investigators, three proteins were detected by SILAP methods at significantly higher levels in pooled CVF samples collected at 24–28 weeks gestation from five women who later delivered preterm compared to five women who delivered at term. One of these proteins, desmoplakin-1, was detected at significantly lower levels in the women who delivered preterm in the current study and in a previous study performed at Oregon Health and Science University using pooled CVF samples from six women with symptomatic preterm labor compared to asymptomatic controls.10 Desmoplakin is involved in intercellular junctions and cell-cell communication, and desmoplakin has been localized in human fetal membranes.16 In both GPN-PBR studies, extracellular matrix protein 1 isoform 1 precursor was detected at 2.3–3.7-fold higher levels in the women who delivered preterm, but the difference was not significant in the preliminary study due to wide standard deviations.8 The functional significance of extracellular matrix protein 1 in human fetal membranes has not been determined. Investigators from the University of Melbourne performed two-dimensional gel electrophoresis and MS using pooled CVF samples from small groups of patients (N=4–10 samples per group) to identify candidate proteins associated with preterm premature rupture of membranes, preterm labor, and spontaneous preterm delivery.13–15 Validation studies utilizing ELISA techniques demonstrated that albumin and vitamin-D binding protein levels yielded better predictive values than fetal fibronectin for predicting spontaneous preterm birth in women with preterm contractions.14 The major strengths of the current study compared to previous studies are: 1) larger numbers, 2) rigorous targeted (SILAP) techniques, and 3) analysis of individual CVF samples.
Clinical implications
Although differences in expression levels of five proteins were not validated by ELISA techniques in an independent cohort, the proteomics studies generated a list of 38 candidate proteins with relevant molecular functions that could inform future biomarker or therapeutic studies. Importantly, the proteomics assays were performed using samples from a highly selective cohort of recurrent spontaneous preterm birth. In addition, cases were restricted to spontaneous preterm births <34 weeks while controls delivered at 39–41 weeks, so late preterm births at 34–37 weeks, which constitute approximately half of all spontaneous preterm births, were not considered in the proteomics analyses. Hence, results may differ in a more generalized population that has different etiologies and risk factors for preterm birth.
Research implications
Both proteomics techniques (targeted and shotgun) employed multiple parameters that were implemented to reduce the number of false positive results reported. Targeted proteomics focused exclusively on proteins in the SILAP secretome, while shotgun proteomics utilized three replicates per sample, strict trypsin cleavage rules, and repeated LC-MRM/MS to generate four raw data files. Rigorous proteomics assays based on labeled molecules that include additional proofreading steps have the best combination of sensitivity and specificity, and yield biomarkers with the greatest clinical potential.17 Unfortunately, validation was negative for five candidate proteins, similar to most biomarker studies that have not been translated into clinical tests.17 Failure to validate the proteomics results by ELISA may be attributed to several factors: 1) relatively small sample size; 2) earlier sampling of CVF in the validation study (160/7 to 206/7 weeks) compared to the second study visit (190/7 to 236/7 weeks); 3) different gestational age cutoff for spontaneous preterm births; and 4) inability of ELISA to measure protein levels with the same sensitivity as targeted and shotgun proteomics techniques.
Strengths and limitations
Proteomic analyses are likely to offer guidance on the diagnosis of disease and potential therapeutic targets, but limitations in proteomic analyses, including reproducibility, sensitivity, sample requirements, and limited multiplexing capacity, must be overcome in order to fill a major void in systems biology research and precision medicine.18 Proteomic analyses have not been applied widely in perinatal medicine.3,19,20 A major strength of the current study is that both targeted and shotgun proteomics techniques were utilized to identify potential biomarkers of spontaneous preterm birth in CVF samples, because limitations are inherent with any single proteomics approach.18 Targeted proteomics utilizing SILAP permits semi-quantitive analysis of low-abundance proteins that are secreted by biologically relevant cells (in this study, cervical and vaginal epithelial cells). Shotgun proteomics permits enhanced search capabilities, but rigorous techniques including strict trypsin cleavage rules, replication of LC-MS/MS to generate four raw files for each pooled CVF sample, and a minimum Sequest probability score of ≥0.5, were employed to minimize reporting of low probability peptides. Hence, the study design incorporated a balance between casting a wide net to identify multiple proteins in CVF samples and employing rigorous techniques to reduce the likelihood of false positive results.
Conclusions
Based on the current results, future studies should explore the biology of identified proteins in relation to cervical remodeling, fetal membrane degradation, and spontaneous preterm birth. Validation of these potential biomarkers in other cohorts should be conducted. Source of samples, racial and ethnic populations of samples, and timing of sample collection should be considered. Challenges remain with proteomics methodologies, but additional studies utilizing rigorous techniques are vital for spontaneous preterm birth biomarker discovery.
AJOG AT A GLANCE.
- Why was the study conducted?
- The study was conducted to identify biomarkers of spontaneous preterm birth in cervicovaginal fluid samples using targeted and shotgun proteomic analyses.
- What are the key findings?
- Potential biomarkers of spontaneous preterm birth were identified by targeted and shotgun proteomics analyses in cervicovaginal fluid samples from high-risk, asymptomatic women.
- Many of the proteins detected at higher levels in cervicovaginal fluid samples from spontaneous preterm birth cases are extracellular matrix proteins and/or regulate cell membrane physiology, including fibronectin-1, extracellular matrix protein 1 isoform 1 precursor, laminin alpha 3 subunit isoform, laminin A/C isoform 2, and calsyntenin 1 isoform 2.
- What does this study add to what is already known?
- Based on the current results, future studies should explore the biology of identified proteins in relation to cervical remodeling, fetal membrane degradation, and spontaneous preterm birth.
- Validation of these potential biomarkers in other cohorts should be conducted.
ACKNOWLEDGMENTS
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Advancing Translational Sciences provided grant support for the NICHD Genomic and Proteomic Network for Preterm Birth (GPN) through cooperative agreements. NICHD staff provided input into the study design, conduct, analysis, and manuscript drafting; NCRR and NCATS cooperative agreements provided infrastructure support to the GPN. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data collected at participating sites of the GPN were transmitted to Yale University, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the GPN, Drs. Heping Zhang (DCC Principal Investigator) and Hao Huang (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
Steering Committee Chair: Yoel Sadovsky, MD, Magee Womens Research Institute, University of Pittsburgh, Pittsburgh, PA.
Eunice Kennedy Shriver National Institute of Child Health & Human Development – John V. Ilekis, PhD; Uma M. Reddy, MD; Stephanie Wilson Archer, MA.
University of Alabama at Birmingham Health System (U01 HD50094, UL1 RR25777) -- Joseph R. Biggio, Jr., MD; William W. Andrews, MD PhD; Rachel L. Copper, MSN CRNP; Pamela B. Files, MSN CRNP; Stacy L. Harris, BSN RN.
University of Pennsylvania (U01 HD5088) – Samuel Parry, MD; Don A. Baldwin, PhD; Rita Leite, MD; Ian A Blair, PhD.
University of Texas Medical Branch at Galveston (U01 HD50078) – Radek K. Bukowski, MD PhD; George R. Saade, MD; Margaret L. Zimmerle, BSN; Janet L. Brandon, RN MSN; Sonia Jordan, RN BSN; Angela Jones, RN BSN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, McKay-Dee Hospital, and Utah Valley Regional Medical Center (U01 HD50080) – Michael W. Varner, MD; M. Sean Esplin, MD; Kelly Vorwaller, RN BSN; Sharon Quinn, RN; Valerie S. Morby, RN CCRP; Kathleen N. Jolley, RN BSN; Julie A. Postma, RN BSN CCRP.
Yale University School of Public Health, Collaborative Center for Statistics in Science (U01 HD50062) -- Heping Zhang, PhD; Kei-Hoi Cheung, PhD; Donna Losi DelBasso; Buqu Hu, MS; Hao Huang, MD MPH; Lina Jin, PhD; Analisa L. Lin, MPH; Charles C. Lu, MS; Lauren Perley, MA; Laura Jeanne Simone, BA; Chi Song, PhD; Feifei Xiao, PhD; Yaji Xu, PhD.
Alpert Medical School of Brown University, Women & Infants Hospital of Rhode Island – Dwight J. Rouse, MD MSPH; Donna Allard, RNC.
Columbia University Hospital, Drexel University, Christiana Care Health Systems, and St. Peter’s University Hospital – Ronald Wapner, MD; Michelle Divito, RN MSN; Sabine Bousleiman, RN MSN MsPH; Vilmarie Carmona, MA; Rosely Alcon, RN BSN; Katty Saravia, MA; Luiza Kalemi, MA; Mary Talucci, RN MSN; Lauren Plante, MD MPH; Zandra Reid, RN BSN; Cheryl Tocci, RN BSN; Marge Sherwood; Matthew Hoffman, MD; Stephanie Lynch, RN; Angela Bayless, RN; Jenny Benson, RN; Jennifer Mann, RN; Tina Grossman, RN; Stephanie Lort, RN; Ashley Vanneman; Elisha Lockhart; Carrie Kitto; Edwin Guzman, MD; Marian Lake, RN; Shoan Davis; Michele Falk; Clara Perez, RN.
Northwestern University – Alan M Peaceman MD, Lara Stein RN, Katura Arego, Mercedes Ramos-Brinson B.S., Gail Mallett RN BSN.
University of North Carolina – John M. Thorp, Jr, MD MPH; Karen Dorman, RN MS; Seth Brody, MD MPH.
University of Texas Health Science Center at Houston and Lyndon Baines Johnson General Hospital/Harris County Hospital District – Sean C. Blackwell, MD; Maria Hutchinson, MPH.
GPN Advisory Board – Anthony Gregg (chair), MD, University of South Carolina School of Medicine; Reverend Phillip Cato, PhD; Traci Clemons, PhD, The EMMES Corporation; Alessandro Ghidini, MD, Inova Alexandria Hospital; Emmet Hirsch, MD, Feinberg School of Medicine, Northwestern University; Jeff Murray, MD, University of Iowa; Emanuel Petricoin, PhD, George Mason University; Caroline Signore, MD MPH, Eunice Kennedy Shriver National Institute of Child Health and Human Development; Charles F. Sing, PhD, University of Michigan; Xiaobin Wang, MD, Children Memorial Hospital.
In addition, the NICHD Maternal-Fetal Medicine Units Network and the Stillbirth Collaborative Research Network provided specimen samples for the validation analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
REFERENCES
- 1.Murphy SL, Mathews TJ, Martin JA, Minkovitz CS, Strobino DM. Annual Summary of Vital Statistics: 2013–2014. Pediatrics 2017;139. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry S, Zhang H, Biggio J, et al. Maternal serum serpin B7 is associated with early spontaneous preterm birth. Am J Obstet Gynecol 2014;211:678 e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasari S, Pereira L, Reddy AP, et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res 2007;6:1258–68. [DOI] [PubMed] [Google Scholar]
- 5.Esplin MS, Merrell K, Goldenberg R, et al. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol 2011;204:391 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo JO, Reddy AP, Wilmarth PA, et al. Proteomic analysis of cervical vaginal fluid proteins among women in recurrent preterm labor. J Matern Fetal Neonatal Med 2014;27:1183–8. [DOI] [PubMed] [Google Scholar]
- 7.Saade GR, Boggess KA, Sullivan SA, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol 2016;214:633 e1–e24. [DOI] [PubMed] [Google Scholar]
- 8.Shah SJ, Yu KH, Sangar V, Parry SI, Blair IA. Identification and quantification of preterm birth biomarkers in human cervicovaginal fluid by liquid chromatography/tandem mass spectrometry. J Proteome Res 2009;8:2407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt RH, Lee MW, Pirshahid SA, Backlund PS, Wood S, Coorssen JR. An initial proteomic analysis of human preterm labor: placental membranes. J Proteome Res 2006;5:3161–72. [DOI] [PubMed] [Google Scholar]
- 10.Pereira L, Reddy AP, Jacob T, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res 2007;6:1269–76. [DOI] [PubMed] [Google Scholar]
- 11.Han B, Higgs RE. Proteomics: from hypothesis to quantitative assay on a single platform. Guidelines for developing MRM assays using ion trap mass spectrometers. Brief Funct Genomic Proteomic 2008;7:340–54. [DOI] [PubMed] [Google Scholar]
- 12.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics 2007;6:2212–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liong S, Di Quinzio MK, Fleming G, Permezel M, Rice GE, Georgiou HM. Prediction of spontaneous preterm labour in at-risk pregnant women. Reproduction 2013;146:335–45. [DOI] [PubMed] [Google Scholar]
- 14.Liong S, Di Quinzio MK, Fleming G, Permezel M, Rice GE, Georgiou HM. New biomarkers for the prediction of spontaneous preterm labour in symptomatic pregnant women: a comparison with fetal fibronectin. BJOG 2015;122:370–9. [DOI] [PubMed] [Google Scholar]
- 15.Liong S, Di Quinzio MK, Heng YJ, et al. Proteomic analysis of human cervicovaginal fluid collected before preterm premature rupture of the fetal membranes. Reproduction 2013;145:137–47. [DOI] [PubMed] [Google Scholar]
- 16.Ockleford C, Malak T, Hubbard A, et al. Confocal and conventional immunofluorescence and ultrastructural localisation of intracellular strength-giving components of human amniochorion. J Anat 1993;183 (Pt 3):483–505. [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson R Sensitivity and specificity: twin goals of proteomics assays. Can they be combined? Expert Rev Proteomics 2013;10:135–49. [DOI] [PubMed] [Google Scholar]
- 18.Cayer DM, Nazor KL, Schork NJ. Mission critical: the need for proteomics in the era of next-generation sequencing and precision medicine. Hum Mol Genet 2016;25:R182–R9. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Nunez J, Valdes-Yong M. Utility of proteomics in obstetric disorders: a review. Int J Womens Health 2015;7:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch AM, Wagner BD, Deterding RR, et al. The relationship of circulating proteins in early pregnancy with preterm birth. Am J Obstet Gynecol 2016;214:517 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]