Abstract
Background:
Frequent and severe vasomotor symptoms during menopause are linked with adverse health outcomes. Understanding modifiable lifestyle factors for the risk of vasomotor menopausal symptoms is important to guide preventive strategies.
Objective:
We investigated the associations between body mass index and smoking, and their joint effects with the risk of vasomotor symptoms, and whether the associations differed by menopausal stage.
Study Design:
The International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events pooled data on 21,460 midlife women from eight studies (median age 50 years, interquartile range 49–51 years) for the cross-sectional analysis. Four studies provided data for the prospective analysis (n=11,986). Multinomial logistic regression models with four categories of frequency/severity for the outcome of vasomotor symptoms were used to estimate relative risk ratios (RRR) and 95% confidence intervals (CI) adjusted for within-study correlation and covariates.
Results:
At baseline, nearly 60% of the women experienced vasomotor symptoms. Half of them were overweight (30%) or obese (21%), and 17% were current smokers. Cross-sectional analyses showed that a higher body mass index and smoking more cigarettes with longer duration and earlier initiation were all associated with more frequent or severe vasomotor symptoms. Never smokers who were obese had a 1.5-fold (RRR, 1.52; 95% CI, 1.35–1.73) higher risk of often/severe vasomotor symptoms, compared with never smokers who were of normal-weight. Smoking strengthened the association as the risk of often/severe vasomotor symptoms was much greater among smokers who were obese (RRR, 3.02; 95% CI, 2.41–3.78). However, smokers who quit before 40 years of age were at similar levels of risk as never smokers. Prospective analyses showed a similar pattern, but the association attenuated markedly after adjustment for baseline vasomotor symptoms. Furthermore, we found that the association between body mass index and vasomotor symptoms differed by menopausal status. Higher body mass index was associated with increased risk of vasomotor symptoms in pre- and perimenopause but with reduced risk in postmenopause.
Conclusion:
High body mass index (≥25 kg/m2) and cigarette smoking substantially increased women’s risk for experiencing frequent or severe vasomotor symptoms in a dose-response manner, and smoking intensified the effect of obesity. However, the effect of body mass index on the risk vasomotor symptoms was opposite among postmenopausal women. Maintaining a normal weight before the menopausal transition and quitting smoking before age 40 years may mitigate the excess risk of VMS in midlife.
Keywords: hot flushes, night sweats, overweight, obesity, smoking, vasomotor symptoms
CONDENSATION
Obesity and cigarette smoking substantially increased women’s risk of frequent or severe vasomotor symptoms in a dose-response manner, and smoking intensified the effect of obesity.
INTRODUCTION
Vasomotor menopausal symptoms (VMS), including hot flushes and night sweats, are considered the cardinal symptoms of menopause1 and are one of the main reasons for menopause-related health service use.2,3 It is estimated that up to 80% of women will report VMS at some time during the menopausal transition,4–6 though the percentage of women experiencing symptoms varies from as low as 20% among some Asian populations4,5 to 60%–80% in some North American4 and European6 sub-groups. VMS also vary by intensity or severity, with some women reporting only mild transient symptoms and others reporting intense heat spreading over the body and profuse sweating that can disrupt sleep.3 Early-onset VMS has been linked with endothelial dysfunction7 and is considered a biomarker for the development of cardiovascular disease (CVD) in later life.8
Although menopause-related hormonal changes are primarily associated with VMS,9,10 evidence from population-based studies suggests that certain lifestyle and socio-demographic factors are also associated with frequency and severity of VMS.11–13 For instance, epidemiologic data have revealed that current smokers have a significantly higher odds of VMS compared to non-smokers,4 and this has been attributed to the anti-estrogenic effects of tobacco smoking.12 Another notable lifestyle factor associated with a higher risk of VMS is overweight and obesity, where increased subcutaneous adipose tissue is likely to provide an insulating layer that blunts abdominal heat transfer,14 which during the menopausal transition, reduces the body’s ability to respond to changes in core temperature. In addition, smoking and body weight are also interrelated. Given the increased risk of VMS conferred by both smoking and overweight/obesity, a better understanding of their joint associations would provide important information for women at midlife as weight gain is common during the menopausal transition. Also, it is possible that the relative contribution of body fat to the risk of VMS in the early and late stage of menopause may differ.15
Determining the modifiable health behaviours, as well as identifying those individuals at an increased risk of developing symptoms across racial/ethnic groups, is essential for developing preventative strategies to reduce both the individual and societal burden associated with VMS. Therefore, this study investigated the cross-sectional and prospective associations between body mass index (BMI) and smoking and their joint effects with the risk of VMS in a pooled sample from the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease (InterLACE) consortium. We further examined whether the effects of BMI and smoking on the risk of VMS differ by menopausal status.
MATERIALS AND METHODS
Study participants
InterLACE is an individual-level pooled study of 20 observational studies from ten countries. Full details on the study aims, data harmonisation, and characteristics across the studies were published previously.16,17 Each participating study has been undertaken with ethical approval from the Institutional Review Board or Human Research Ethics Committee at each research institution, and all participants provided consent for that study. For this analysis, eight studies which had collected information on BMI, smoking status, and degree of VMS (either reporting in frequency or severity) were included: Australian Longitudinal Study on Women’s Health (ALSWH),18 MRC National Survey of Health and Development (NSHD),19 National Child Development Study (NCDS),20 Study of Women’s Health Across the Nation (SWAN),21 Whitehall II Study (WHITEHALL),22 Seattle Midlife Women’s Health Study (SMWHS),23 Healthy Ageing of Women Study (HOW),24 and Japanese Midlife Women’s Health Study (JMWHS).24
For the longitudinal studies, data for women around the age of 50 years were used as an analytic baseline to make the distribution of menopausal status and VMS more comparable across studies. For instance, Survey 2 (1998) was selected as analytic baseline for ALSWL as the median age was 50 years; Visit 4 (2000–2002) was selected for SWAN and Survey 3 (1991–1994) for WHITEHALL (Table 1). At this baseline, 21,460 women who had reported their BMI, smoking status and frequency or severity of VMS and provided complete information on the covariates (listed below) were included for the cross-sectional analyses. Four studies (ALSWH, NSHD, SWAN, and WHITEHALL) had longitudinal data to examine the association with the risk of subsequent VMS at three-year follow-up. We excluded 3,791 women who did not return to the study or had incomplete follow-up data on VMS, menopausal status, or hormone therapy, leaving 11,986 women for prospective analyses. The excluded women were more likely to be current smokers, obese, less educated, or to report VMS at baseline, compared with the included women (data not shown).
Table 1.
Characteristics of eight studies in the InterLACE consortium
| Study | Country | N | Age at baseline Median (IQR) |
Survey (year) selected for analytic baselinea |
Survey (year) selected for three-year follow up |
|---|---|---|---|---|---|
| Australian Longitudinal Study on Women’s Health (ALSWH) | Australia | 10,323 | 50 (48, 51) | Survey 2 (1998) | Survey 3 (2001) |
| National Survey of Health and Development (NSHD) | UK | 1,068 | 50a | Survey 1996 (1996) | Survey 1999 (1999) |
| National Child Development Study (NCDS) | UK | 3,983 | 50a | Survey 8 (2008) | N/A |
| Study of Women’s Health Across the Nation (SWAN) | USA | 2,345 | 50 (48, 52) | Visit 4 (2000–2002) | Visit 7 (2003–2005) |
| Whitehall II Study (WHITEHALL) | UK | 2,041 | 50 (45, 55) | Survey 3 (1991–1994) | Survey 4 (1995–1996) |
| Seattle Midlife Women’s Health Study (SMWHS) | USA | 189 | 50 (46, 53) | Survey 2000 (2000) | N/A |
| Healthy Ageing of Women Study (HOW) | Australia | 768 | 54 (52, 57) | Survey 1 (2001) | N/A |
| Japanese Midlife Women’s Health Study (JMWHS) | Japan | 743 | N/Ab | Survey 1 (2002) | N/A |
| Overall | 21,460 | 50 (49, 51) |
N/A, not applicable; IQR, interquartile range.
For the longitudinal studies, data for women around the age of 50 years were used as analytic baseline to make the data more comparable across studies. Women who participated in the NSHD (1946 British birth cohort) and NCDS (1958 British birth cohort) were at age 50 years in the 1996 and 2008 survey, respectively.
JMWHS provided age by category only (≤55 and >55 years), and 48% of women were aged more than 55 (age range from 45 to 60 years).
Main outcome and exposure variables
Hot flushes and night sweats were collected at analytic baseline using self-reported menopausal symptom checklists recalling the symptoms over a specific period. VMS were defined as either hot flushes or night sweats. In ALSWH, women were asked how frequently they have experienced VMS in the last 12 months, while SWAN asked frequency in the past 2 weeks. The frequency responses were categorised as never, rarely, sometimes, and often. In NSHD and NCDS, women were asked how severely they have been bothered by VMS in the last 12 months, and the severity responses were categorised as never, mild, moderate, and severe. In the other four studies, women also reported their severity of VMS but in a recent period (in the last 24 hours or at the moment). For the pooled analysis, the degree of VMS was harmonised as never, rarely, sometimes, and often (if reporting frequency) or never, mild, moderate, and severe (if reporting severity). Subsequent VMS was defined based on frequency/severity of VMS reported at three-year follow-up.
Height and weight were self-reported or measured at analytic baseline. BMI was computed as weight divided by the square of height and categorised as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2), according to the WHO classification.25 Because only 357 women (1.7%) were classified as underweight, they were combined into the normal weight group (BMI <25 kg/m2). For the Asian population (Japanese and other Asian), we performed a sensitivity analysis by using a lower BMI cut-off of 23 and 27.5 kg/m2 for overweight and obesity.25 Smoking status was self-reported and categorised as never smoker, former smoker and current smoker. For the current smokers, data on number of cigarettes smoked per day, duration of smoking, and pack-years were collected in ALSWH, SWAN and WHITEHALL (n=14,709), while these details were not available for the former smokers at analytic baseline. The average number of cigarettes smoked per day was categorised as 1–9, 10–19, and ≥20 cigarettes/day. Smoking duration was defined by the time between age at initiation and age at baseline and categorised as <20, 20–29, and ≥30 years. Pack-years (number of cigarettes smoked per day divided by 20 and multiplied by the duration of smoking) was categorised as <10, 10–19, 20–29, 30–39, and ≥40 pack-years. Age at smoking initiation was collected for both former and current smokers and categorised as ≤15, 16–19, and ≥20 years of age. In ALSWH, data on age at quitting smoking (categorised as <30, 30–39, and ≥40 years of age) and years since quitting smoking (categorised as 1–5, 6–14, 15–19, ≥20 years) were collected for former smokers. To test the joint effects of body weight and smoking status, a new variable with nine levels was created. It was made up of the combinations of BMI (underweight/normal, overweight, and obese) and smoking status (never, former, and current).
Confounding factors
Participants reported on a range of demographic and reproductive factors at baseline, including birth year, race/ethnicity/region, education level, menopausal status, and use of menopausal hormone therapy (MHT). Responses for birth year were categorised as <1940, 1940–1949, and 1950–1959. Race/ethnicity/region was defined based on self-identified race/ethnicity, country of birth, the language spoken at home, or the country where the study was conducted (residency). Seven racial/ethnic groups with regional status were defined here: Caucasian-Australian, Caucasian-European, Caucasian-American, Japanese, other Asian (Chinese, South/Southeast Asian), African American/Black/Caribbean, and Other (Hispanic, Middle Eastern, Aboriginal, and mixed). For education level, responses were categorised as completing ≤10 years (corresponding to less than high school or O-level in the UK), 11–12 years (high school or A-level in the UK), and >12 years (at least post high school education). Menopausal status was collapsed and categorised into five groups based on menstrual bleeding patterns and gynaecological surgery: 1) unknown due to surgery (hysterectomy and/or oophorectomy, including bilateral oophorectomy (surgical menopause) due to insufficient information to define surgical menopause for all studies), 2) unknown due to hormone use (unless natural menopause specified), 3) premenopause (regular menstrual cycles in the last 3 months and 12 months), 4) perimenopause (menses in the past 3 months and changes/irregularity in menstrual patterns in the past 12 months; or no menses in the previous 3 months but menses in the preceding 11 months), and 5) natural postmenopause (amenorrhea for at least 12 months). Women who were taking MHT (e.g. estrogen) were classified as current hormone users.
Statistical analyses
Multinomial logistic regression models with four categories of outcome for VMS (never, rarely/mild, sometimes/moderate, and often/severe) were used to examine the associations between BMI, smoking status, and their joint effects with the risk of VMS at baseline (cross-sectional analysis) and three-year follow-up (prospective analysis). A generalised logit model was used to estimate relative risk ratios (RRR) and 95% confidence intervals (CIs) for each VMS category using no symptom as the reference category. In the cross-sectional analysis, the associations were obtained separately for the studies of VMS frequency and VMS severity, followed by the overall estimates that incorporated study design (study cluster) into the analyses. The models were first adjusted for menopausal status, use of MHT at baseline (Model 1), and additionally adjusted for race/ethnicity/region, education level, and included both BMI and smoking status in the same model (Model 2). Furthermore, we included an interaction term between the two exposures in the model and analysed their joint associations. As Asian women are less likely to be overweight or obese and less likely to have frequent or severe VMS, we performed a sensitivity analysis by excluding Asian women (996 Japanese and 488 other Asian).
The dose-response relationships between the different aspects of smoking and risk of VMS were examined using data from ALSWH, SWAN, and WHITEHALL (n=14,709). The number of cigarettes, duration, and pack-years of smoking were analyzed for current smokers, while age at having initiated smoking was analyzed for both former and current smokers. Age at quitting and years since quitting smoking for former smokers could only be analysed using data from ALSWH. Never smoker was used as the reference group for all smoking measures. All models were adjusted for the confounding variables mentioned above including BMI.
For the prospective analysis, four studies provided data (n=11,986). BMI and smoking status at baseline and subsequent VMS at three-year follow-up were examined in the model fully adjusted for menopausal status and use of MHT at three-year follow-up and baseline covariates mentioned in Model 2, and additionally adjusted for frequency/severity of VMS at baseline.
We further investigated whether menopausal status modified the association between BMI, smoking and VMS. The interaction term between BMI and menopausal status and between smoking status and menopausal status was included in the models. If there is a statistical interaction, the association was further stratified by concurrent menopausal status at baseline (cross-sectional analyses) and at three-year follow-up (prospective analyses). The SURVEYLOGISTIC procedure in SAS 9.4, which incorporated the study cluster into the analyses, was used for the multinomial logistic regression.
RESULTS
Baseline characteristics
A total of 21,460 women with a median age of 50 years (interquartile range: 49–51 years) from eight studies were included at baseline (Table 1). HOW and JMWHS recruited women at slightly older ages around 55 years. In the overall sample, almost half were premenopausal or perimenopausal (19% and 27% respectively), 19% had a natural menopause, 20% had had a hysterectomy or oophorectomy, and 14% were classified as unknown menopausal status due to hormone use before menopause (Table 2). Nearly 20% of the women were currently taking MHT, regardless of menopausal status. Across studies, half of the women were either overweight (30%) or obese (21%); 28% were former smokers, and 17% were current smokers. Overall, up to 55% of the women experienced hot flushes (rarely/mild to often/severe), and 45% reported night sweats.
Table 2.
Analytic baseline characteristics of study sample
| Study | Overall | ALSWH | NSHD | NCDS | SWAN | WHITEHALL | SMWHS | HOW | JMWHS |
|---|---|---|---|---|---|---|---|---|---|
| n | 21,460 | 10,323 | 1,068 | 3,983 | 2,345 | 2,041 | 189 | 768 | 743 |
| Birth year | |||||||||
| <1940 | 3.8 | N/A | N/A | N/A | N/A | 39.5 | 0.5 | N/A | N/A |
| 1940–1949 | 54.9 | 74.3 | 100 | N/A | 41.3 | 48.5 | 46.6 | 85.4 | 47.5c |
| 1950–1959 | 41.3 | 25.7 | N/A | 100 | 58.7 | 12.0 | 52.9 | 14.6 | 52.5c |
| Race/ethnicity/region | |||||||||
| Caucasian- Australian | 40.8 | 78.8 | N/A | N/A | N/A | N/A | N/A | 82.3 | N/A |
| Caucasian- European | 40.1 | 16.9 | 100 | 98.2 | N/A | 87.7 | N/A | 12.5 | N/A |
| Caucasian- American | 6.3 | 0.7 | N/A | N/A | 48.0 | N/A | 85.2 | N/A | N/A |
| Japanese | 4.6 | 0.1 | N/A | N/A | 10.5 | N/A | N/A | N/A | 100 |
| Other Asian | 2.3 | 2.1 | N/A | 0.6 | 9.5 | N/A | 7.9 | 1.0 | N/A |
| African American/Black/Caribbean | 3.0 | N/A | N/A | 0.4 | 25.9 | N/A | 5.8 | N/A | N/A |
| Other | 2.9 | 1.5 | N/A | 0.8 | 6.1 | 12.3 | 1.1 | 4.2 | N/A |
| Education level | |||||||||
| ≤10 years | 46.0 | 48.1 | 67.8 | 62.2 | 5.6 | 54.2 | 0 | 51.7 | 9.4 |
| 11–12 years | 17.4 | 17.1 | 25.8 | 10.3 | 15.8 | 16.2 | 13.8 | 15.6 | 59.4 |
| >12 years | 36.6 | 34.9 | 6.4 | 27.5 | 78.6 | 29.6 | 86.2 | 32.7 | 31.2 |
| Menopausal status | |||||||||
| Unknown due to surgery | 19.8 | 25.6 | 17.9 | 16.9 | 4.5 | 15.9 | 3.2 | 28.4 | 11.0 |
| Unknown due to hormone use | 14.2 | 16.1 | 21.6 | 13.1 | 11.3 | 12.0 | 25.9 | 7.6 | 2.3 |
| Premenopause | 19.4 | 23.0 | 19.8 | 18.8 | 6.7 | 22.1 | 26.5 | 3.4 | 19.8 |
| Perimenopause | 27.4 | 24.2 | 24.5 | 30.1 | 56.2 | 18.3 | 30.7 | 11.6 | 11.3 |
| Natural postmenopause | 19.1 | 11.0 | 16.2 | 21.0 | 21.2 | 31.8 | 13.8 | 49.1 | 55.6 |
| Current use of menopausal hormone therapy | |||||||||
| No | 80.9 | 76.6 | 79.7 | 90.4 | 80.6 | 84.9 | 78.3 | 65.1 | 96.8 |
| Yes | 19.1 | 23.4 | 20.3 | 9.6 | 19.4 | 15.1 | 21.7 | 34.9 | 3.2 |
| Body mass index | |||||||||
| Normal weight (<25 kg/m2)a | 48.5 | 48.2 | 63.2 | 44.5 | 36.5 | 52.7 | 50.8 | 42.8 | 85.6 |
| Overweight (25–29.9 kg/m2) | 30.4 | 31.6 | 24.3 | 33.0 | 27.6 | 32.2 | 25.4 | 32.4 | 13.2 |
| Obese (≥30 kg/m2) | 21.0 | 20.2 | 12.5 | 22.5 | 35.9 | 15.1 | 23.8 | 24.7 | 1.2 |
| Smoking status | |||||||||
| Never smoker | 54.9 | 56.2 | 34.2 | 48.8 | 59.4 | 52.2 | 50.8 | 62.9 | 86.7 |
| Former smoker | 27.6 | 26.7 | 40.5 | 29.3 | 26.5 | 30.9 | 39.2 | 27.6 | 4.0 |
| Current smoker | 17.4 | 17.1 | 25.3 | 21.9 | 14.1 | 16.9 | 10.1 | 9.5 | 9.3 |
| Frequency/severity of hot flushes | |||||||||
| Never | 47.2 | 44.8 | 47.8 | 35.5 | 56.0 | 63.4 | 67.7 | 56.1 | 54.9 |
| Rarely/mild | 17.1 | 15.7 | 21.3 | 8.6 | 26.4 | 17.8 | 16.9 | 28.8 | 33.0 |
| Sometimes/moderate | 22.3 | 24.9 | 20.3 | 36.5 | 7.0 | 10.6 | 9.0 | 11.1 | 7.8 |
| Often/severe | 13.5 | 14.6 | 10.5 | 19.4 | 10.6 | 8.2 | 6.3 | 4.0 | 4.3 |
| Frequency/severity of night sweats | |||||||||
| Never | 57.2 | 54.9 | 57.6 | 48.3 | 63.4 | 68.7 | 77.8 | 62.1 | 75.2 |
| Rarely/mild | 15.0 | 14.3 | 18.9 | 6.9 | 24.6 | 15.3 | 13.8 | 25.7 | 20.7 |
| Sometimes/moderate | 17.8 | 19.7 | 15.2 | 31.2 | 4.9 | 8.8 | 2.6 | 8.7 | 3.0 |
| Often/severe | 9.9 | 11.1 | 8.3 | 13.7 | 7.1 | 7.2 | 5.8 | 3.5 | 1.1 |
| Frequency/severity of vasomotor symptomsb | |||||||||
| Never | 41.9 | 40.3 | 42.2 | 30.1 | 47.5 | 59.1 | 63.5 | 49.7 | 49.5 |
| Rarely/mild | 18.4 | 16.5 | 22.4 | 8.4 | 31.6 | 17.7 | 18.5 | 32.7 | 37.4 |
| Sometimes/moderate | 24.2 | 26.9 | 22.4 | 39.1 | 8.3 | 12.3 | 9.0 | 12.2 | 8.6 |
| Often/severe | 15.4 | 16.2 | 13.0 | 22.4 | 12.6 | 10.8 | 9.0 | 5.3 | 4.4 |
Data are presented as percentage (%).
ALSWH, Australian Longitudinal Study on Women’s Health; NSHD, National Survey of Health and Development; NCDS, National Child Development Study; SWAN, Study of Women’s Health Across the Nation; WHITEHALL, Whitehall II Study; SMWHS, Seattle Midlife Women’s Health Study; HOW, Healthy Ageing of Women Study; JMWHS, Japanese Midlife Women’s Health Study.
Only 357 (1.7%) women were underweight (BMI<18.5 kg/m2) and thus they were categorised into the normal weight group.
Vasomotor symptoms were defined as having either hot flushes or night sweats.
JMWHS provided age by category only (≤55 and >55 years). Thus, birth year was categorised based on age categories.
Cross-sectional associations
Table 3 shows results separately for studies of VMS frequency, VMS severity, and the overall sample. Overall, the pattern of results was similar regardless of whether VMS were assessed as frequency or severity. BMI and smoking status were associated with the risk of VMS, even when both were included in the same model (Model 2). We found that women who were overweight and obese and current smokers were more likely to report some degree of VMS (rarely/mild to often/severe). For instance, in the overall sample, compared with the normal weight group, a dose-response relationship was observed between overweight and the frequency/severity of VMS, with adjusted RRR (95% CI) of 1.24 (1.18–1.30), 1.30 (1.17–1.46), and 1.53 (1.42–1.65) for rarely/mild, sometimes/moderate and often/severe VMS, respectively. Similar trends were seen for the obese group, with adjusted RRR (95% CI) of 1.15 (1.08–1.24), 1.32 (1.20–1.44), and 1.59 (1.41–1.78), respectively. When we applied a lower cut-off point of overweight (BMI ≥23 kg/m2) and obesity (BMI ≥27.5 kg/m2) for the Asian population, the estimated effects remained unchanged. Compared with never smoking, current smoking was also associated with frequency/severity of VMS, with adjusted RRR (95% CI) of 1.21 (1.08–1.35), 1.39 (1.24–1.56), and 1.83 (1.45–2.30), respectively. Former smokers were only at a slightly increased risk of having often/severe VMS (RRR, 1.17; 95% CI, 0.99–1.38). By examining the RRRs in this table, it appeared that current smoking conveyed greater risk for VMS than being overweight or obese.
Table 3.
Adjusted cross-sectional associations of body mass index and smoking status with the risk of vasomotor symptoms at baseline (n=21,460)
| VMS (hot flushes and night sweats) (%) |
Model 1 RRR (95% CI) |
Model 2 RRR (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Never | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | |
| Frequency of VMS (ALSWH, SWAN; n=12668) | |||||||||||
| Body mass index | |||||||||||
| Normal (<25 kg/m2) | 5830 | 46.0 | 18.4 | 22.4 | 13.3 | – | – | – | – | – | – |
| Overweight (25–29.9 kg/m2) | 3906 | 37.9 | 19.7 | 25.4 | 17.0 | 1.31 (1.26–1.36) | 1.37 (1.16–1.61) | 1.54 (1.40–1.68) | 1.26 (1.21–1.31) | 1.38 (1.21–1.56) | 1.51 (1.34–1.71) |
| Obese (≥30 kg/m2) | 2932 | 38.0 | 20.7 | 23.1 | 18.2 | 1.32 (1.08–1.61) | 1.17 (0.91–1.49) | 1.50 (1.49–1.50) | 1.17 (1.15–1.18) | 1.30 (1.27–1.32) | 1.51 (1.50–1.51) |
| Smoking status | |||||||||||
| Never smoker | 7193 | 44.1 | 19.7 | 22.3 | 13.9 | – | – | – | – | – | – |
| Former smoker | 3381 | 41.1 | 18.9 | 24.4 | 15.6 | 1.02 (1.02–1.02) | 1.16 (1.03–1.30) | 1.17 (0.98–1.39) | 1.01 (1.00–1.02) | 1.15 (1.03–1.28) | 1.12 (0.95–1.32) |
| Current skomer | 2094 | 33.9 | 18.8 | 26.2 | 21.1 | 1.20 (1.17–1.23) | 1.41 (0.99–2.01) | 1.72 (1.33–2.24) | 1.17 (1.12–1.22) | 1.33 (1.11–1.61) | 1.58 (1.46–1.70) |
| Severity of VMS (NSHD, NCDS, WHITEHALL, SMWHS, HOW, JMWH; n=8792) | |||||||||||
| Body mass index | |||||||||||
| Normal (<25 kg/m2) | 4583 | 46.1 | 18.1 | 23.6 | 12.3 | – | – | – | – | – | – |
| Overweight (25–29.9 kg/m2) | 2625 | 39.2 | 16.8 | 26.2 | 17.8 | 1.10 (0.90–1.34) | 1.31 (1.06–1.62) | 1.70 (1.30–2.23) | 1.20 (1.08–1.33) | 1.20 (1.16–1.24) | 1.56 (1.31–1.85) |
| Obese (≥30 kg/m2) | 1584 | 36.9 | 14.4 | 28.9 | 19.8 | 0.98 (0.70–1.36) | 1.48 (1.09–2.02) | 1.92 (1.30–2.82) | 1.07 (0.87–1.33) | 1.37 (1.07–1.74) | 1.79 (1.38–2.33) |
| Smoking status | |||||||||||
| Never smoker | 4598 | 46.4 | 18.6 | 22.9 | 12.1 | – | – | – | – | – | – |
| Former smoker | 2547 | 41.2 | 15.3 | 27.8 | 15.7 | 0.97 (0.79–1.20) | 1.43 (1.07–1.91) | 1.53 (1.08–2.16) | 1.12 (0.99–1.26) | 1.19 (0.95–1.48) | 1.26 (0.99–1.59) |
| Current skomer | 1647 | 32.9 | 15.4 | 28.2 | 23.6 | 1.17 (0.81–1.68) | 1.80 (1.54–2.10) | 2.70 (1.91–3.82) | 1.33 (1.01–1.76) | 1.43 (1.33–1.52) | 2.11 (1.69–2.64) |
| Overall sample (n=21460) | |||||||||||
| Body mass index | |||||||||||
| Normal (<25 kg/m2) | 10413 | 46.0 | 18.3 | 22.9 | 12.8 | – | – | – | – | – | – |
| Overweight (25–29.9 kg/m2) | 6531 | 38.4 | 18.6 | 25.7 | 17.3 | 1.23 (1.11–1.36) | 1.34 (1.17–1.55) | 1.61 (1.42–1.84) | 1.24 (1.18–1.30) | 1.30 (1.17–1.46) | 1.53 (1.42–1.65) |
| Obese (≥30 kg/m2) | 4516 | 37.6 | 18.5 | 25.1 | 18.8 | 1.22 (1.00–1.48) | 1.28 (1.05–1.56) | 1.67 (1.37–2.05) | 1.15 (1.08–1.24) | 1.32 (1.20–1.44) | 1.59 (1.41–1.78) |
| Smoking status | |||||||||||
| Never smoker | 11791 | 45.0 | 19.3 | 22.5 | 13.2 | – | – | – | – | – | – |
| Former smoker | 5928 | 41.2 | 17.3 | 25.8 | 15.6 | 0.99 (0.91–1.07) | 1.26 (1.01–1.57) | 1.30 (0.99–1.71) | 1.03 (0.97–1.09) | 1.16 (1.05–1.27) | 1.17 (0.99–1.38) |
| Current smoker | 3741 | 33.5 | 17.3 | 27.1 | 22.2 | 1.17 (1.03–1.33) | 1.55 (1.20–2.00) | 2.07 (1.45–2.96) | 1.21 (1.08–1.35) | 1.39 (1.24–1.56) | 1.83 (1.45–2.30) |
| Joint effect | |||||||||||
| Normal weight & never smoker | 5824 | 49.2 | 19.0 | 21.1 | 10.8 | – | – | – | – | – | – |
| Normal weight & former smoker | 2675 | 45.2 | 17.9 | 24.9 | 12.1 | 1.04 (0.89–1.21) | 1.29 (1.03–1.63) | 1.24 (0.96–1.61) | 1.12 (1.02–1.23) | 1.15 (1.06–1.24) | 1.07 (0.96–1.18) |
| Normal weight & current smoker | 1914 | 37.3 | 16.7 | 25.8 | 20.3 | 1.13 (0.96–1.33) | 1.54 (1.20–1.99) | 2.28 (1.47–3.53) | 1.18 (0.97–1.44) | 1.31 (1.21–1.42) | 1.86 (1.37–2.52) |
| Overweight & never smoker | 3583 | 41.3 | 19.7 | 23.9 | 15.2 | 1.25 (1.11–1.40) | 1.35 (1.13–1.60) | 1.68 (1.37–2.06) | 1.28 (1.18–1.38) | 1.26 (1.08–1.46) | 1.50 (1.35–1.66) |
| Overweight & former smoker | 1849 | 37.5 | 16.6 | 28.0 | 17.9 | 1.17 (0.97–1.41) | 1.77 (1.41–2.22) | 2.23 (1.64–3.02) | 1.23 (1.09–1.40) | 1.56 (1.38–1.76) | 1.87 (1.59–2.19) |
| Overweight & current smoker | 1099 | 30.5 | 18.4 | 27.8 | 23.3 | 1.53 (1.15–2.03) | 2.02 (1.71–2.39) | 3.17 (2.38–4.23) | 1.59 (1.37–1.84) | 1.73 (1.56–1.93) | 2.54 (2.22–2.89) |
| Obese & never smoker | 2384 | 40.1 | 19.5 | 24.0 | 16.4 | 1.25 (1.03–1.50) | 1.33 (1.07–1.66) | 1.76 (1.37–2.26) | 1.21 (1.08–1.35) | 1.30 (1.18–1.42) | 1.52 (1.35–1.73) |
| Obese & former smoker | 1404 | 38.3 | 17.4 | 24.9 | 19.4 | 1.15 (0.86–1.54) | 1.43 (1.17–1.76) | 2.13 (1.38–3.28) | 1.13 (0.94–1.38) | 1.38 (1.23–1.55) | 1.85 (1.33–2.57) |
| Obese & current smoker | 728 | 27.9 | 17.3 | 29.4 | 25.4 | 1.55 (1.20–2.02) | 2.29 (1.73–3.03) | 3.72 (2.56–5.40) | 1.50 (1.28–1.75) | 2.14 (1.79–2.56) | 3.02 (2.41–3.78) |
Data are presented as percentage (%) or relative risk ratio (RRR) and their 95% confidence intervals (95% CI) using multinomial logistic regression with a generalised logit link. SURVEYLOGISTIC procedure in SAS was used to incorporate the study cluster into the analyses.
Model 1 included menopausal status and use of menopausal hormone therapy at baseline.
Model 2 additionally included race/ethnicity/region, education, and included both BMI and smoking status in the same model. The model for joint effect only additionally included race/ethnicity/region and education.
ALSWH, Australian Longitudinal Study on Women’s Health; NSHD, National Survey of Health and Development; NCDS, National Child Development Study; SWAN, Study of Women’s Health Across the Nation; WHITEHALL, Whitehall II Study; SMWHS, Seattle Midlife Women’s Health Study; HOW, Healthy Ageing of Women Study; JMWHS, Japanese Midlife Women’s Health Study.
Joint effects of BMI and smoking
Table 3 also shows the joint effect of BMI and smoking. A significant interaction was observed between BMI and smoking status for the risk of VMS (P <.001). Never-smokers who were obese had a 1.5-fold increased risk of often/severe VMS (RRR, 1.52; 95% CI, 1.35–1.73) compared to never-smokers who were of normal-weight. Smoking enhanced the association as the risk of often/severe VMS among smokers who were obese was much higher (RRR, 3.02; 95% CI, 2.41–3.78), and the joint effect was not additive (i.e., greater than the sum of individual effects). We also observed a higher risk of often/severe VMS among smokers who were overweight but to a lesser extent (RRR, 2.54; 95% CI, 2.22–2.89). Quitting smoking appeared to mitigate excess risk as the risk of often/severe VMS among obese former-smokers (RRR, 1.85; 95% CI, 1.33–2.57) and overweight former-smokers (RRR, 1.87; 95% CI, 1.59–2.19) was much lower. Further exclusion of Asian women (n=1,484) did not change the observed associations (data not shown).
Dose-response relationship between smoking and VMS
Among current smokers, dose-response relationships were observed in all measures of smoking characteristics, i.e., higher number of cigarettes smoked, longer duration of smoking, higher number of pack-years, and earlier age at initiating smoking were associated with more frequent/severe VMS (Table 4). For instance, compared with never smokers, current smokers with ≥40 pack-years were at more than two-fold increased risk of often/severe VMS (RRR, 2.21; 95% CI, 2.06–2.37). Smoking initiation at ≤15 years was associated with increased risk of often/severe VMS in both current and former smokers, while current smokers had a much higher risk (RRR, 2.19; 95% CI, 1.88–2.54) than former smokers (RRR, 1.29; 95% CI, 1.15–1.46). Women who quit after the age of 40 years and those who had recently quit smoking within five years, had a similar risk of VMS to those of current smokers. However, smokers who quit before 40 years of age or had quit for more than five years had similar levels of risk as never smokers.
Table 4.
Adjusted cross-sectional dose-response relationships between smoking and the risk of vasomotor symptoms at baseline (n=14,709; data from ALSWH, SWAN and WHITEHALL)
| VMS (hot flushes and night sweats) (%) |
Model 1 RRR (95% CI) |
Model 2 RRR (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Never | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | |
| Smoking status (n=14,709) | |||||||||||
| Never smoker | 8259 | 46.3 | 19.4 | 20.9 | 13.4 | – | – | – | – | – | – |
| Former smoker | 4011 | 44.0 | 18.7 | 22.4 | 14.9 | 1.00 (0.94–1.06) | 1.11 (0.98–1.25) | 1.13 (0.98–1.30) | 1.00 (0.99–1.02) | 1.13 (1.06–1.20) | 1.12 (0.98–1.28) |
| Current smoker | 2439 | 36.6 | 18.9 | 24.6 | 19.9 | 1.19 (1.16–1.21) | 1.38 (1.08–1.77) | 1.66 (1.39–1.98) | 1.18 (1.15–1.21) | 1.35 (1.17–1.55) | 1.58 (1.51–1.65) |
| Intensity of smoking (n=14,442) | |||||||||||
| Never smoker | 8259 | 46.3 | 19.4 | 20.9 | 13.4 | – | – | – | – | – | – |
| Former smoker | 4011 | 44.0 | 18.7 | 22.4 | 14.9 | 1.00 (0.94–1.05) | 1.11 (0.98–1.25) | 1.13 (0.98–1.30) | 1.01 (0.99–1.02) | 1.13 (1.06–1.21) | 1.12 (0.98–1.28) |
| Current smoker 1–9 cigarettes/day | 362 | 43.7 | 22.4 | 18.8 | 15.2 | 1.15 (0.96–1.38) | 0.92 (0.69–1.23) | 1.11 (0.76–1.63) | 1.08 (1.07–1.08) | 1.13 (0.77–1.67) | 1.17 (0.94–1.45) |
| Current smoker 10–19 cigarettes/day | 675 | 39.6 | 18.1 | 23.7 | 18.7 | 1.04 (0.76–1.43) | 1.20 (0.88–1.65) | 1.41 (1.00–1.97) | 1.01 (0.77–1.33) | 1.19 (0.93–1.52) | 1.33 (1.04–1.70) |
| Current smoker ≥20 cigarettes/day | 1135 | 32.2 | 18.0 | 27.7 | 22.2 | 1.27 (1.05–1.52) | 1.71 (1.31–2.23) | 2.02 (1.65–2.47) | 1.29 (1.11–1.49) | 1.58 (1.47–1.70) | 1.87 (1.75–1.99) |
| Duration of smoking (n=14,684) | |||||||||||
| Never smoker | 8259 | 46.3 | 19.4 | 20.9 | 13.4 | – | – | – | – | – | – |
| Former smoker | 4011 | 44.0 | 18.7 | 22.4 | 14.9 | 1.00 (0.94–1.05) | 1.11 (0.98–1.25) | 1.13 (0.98–1.30) | 1.01 (0.99–1.02) | 1.13 (1.06–1.21) | 1.12 (0.98–1.28) |
| Current smoker duration <20 years | 103 | 42.7 | 17.5 | 18.5 | 21.4 | 0.93 (0.64–1.35) | 0.90 (0.47–1.71) | 1.57 (1.35–1.82) | 0.93 (0.64–1.34) | 0.84 (0.47–1.48) | 1.45 (1.17–1.79) |
| Current smoker duration 20–29 years | 566 | 44.7 | 17.0 | 23.0 | 15.4 | 0.91 (0.64–1.32) | 1.15 (1.00–1.31) | 1.22 (1.01–1.46) | 0.92 (0.66–1.30) | 1.17 (1.11–1.23) | 1.22 (0.99–1.49) |
| Current smoker duration ≥30 years | 1745 | 33.5 | 19.5 | 25.6 | 21.4 | 1.32 (1.11–1.56) | 1.52 (1.10–2.11) | 1.85 (1.40–2.44) | 1.30 (1.12–1.50) | 1.46 (1.19–1.80) | 1.74 (1.49–2.03) |
| Cumulative dose of smoking (n=14,431) | |||||||||||
| Never smoker | 8259 | 46.3 | 19.4 | 20.9 | 13.4 | – | – | – | – | – | – |
| Former smoker | 4011 | 44.0 | 18.7 | 22.4 | 14.9 | 1.00 (0.94–1.05) | 1.11 (0.98–1.25) | 1.13 (0.98–1.30) | 1.01 (1.00–1.02) | 1.13 (1.06–1.21) | 1.12 (0.98–1.28) |
| Current smoker <10 pack-years | 285 | 44.2 | 21.1 | 16.8 | 17.9 | 1.10 (0.85–1.42) | 0.83 (0.59–1.17) | 1.35 (1.01–1.80) | 1.03 (0.82–1.30) | 1.01 (0.67–1.53) | 1.44 (1.20–1.72) |
| Current smoker 10–19 pack-years | 431 | 40.1 | 20.0 | 22.3 | 17.6 | 1.11 (0.84–1.46) | 1.12 (0.84–1.48) | 1.31 (1.14–1.51) | 1.05 (0.89–1.23) | 1.13 (0.90–1.44) | 1.23 (1.15–1.32) |
| Current smoker 20–29 pack-years | 436 | 40.1 | 16.5 | 26.8 | 16.5 | 0.96 (0.76–1.22) | 1.40 (0.99–1.97) | 1.30 (0.98–1.72) | 0.97 (0.76–1.22) | 1.39 (1.10–1.76) | 1.26 (1.00–1.60) |
| Current smoker 30–39 pack-years | 493 | 32.9 | 19.9 | 26.6 | 20.7 | 1.35 (1.18–1.54) | 1.59 (1.25–2.01) | 1.77 (1.18–2.68) | 1.35 (1.15–1.59) | 1.54 (1.40–1.69) | 1.69 (1.19–2.42) |
| Current smoker ≥40 pack-years | 516 | 28.5 | 17.3 | 28.7 | 25.6 | 1.36 (1.27–1.47) | 1.95 (1.22–3.12) | 2.55 (2.05–3.16) | 1.39 (1.28–1.51) | 1.68 (1.35–2.09) | 2.21 (2.06–2.37) |
| Age initiated smoking (n=14,543) | |||||||||||
| Never smoker | 8259 | 46.3 | 19.4 | 20.9 | 13.4 | – | – | – | – | – | – |
| Former smoker initiated at ≥20 years | 854 | 44.7 | 19.2 | 20.7 | 15.3 | 1.00 (0.78–1.28) | 1.00 (0.87–1.16) | 1.12 (0.91–1.37) | 1.04 (0.86–1.26) | 1.06 (0.98–1.15) | 1.17 (1.07–1.28) |
| Former smoker initiated at 16–19 years | 2149 | 45.5 | 19.3 | 21.3 | 14.0 | 1.00 (0.93–1.08) | 1.03 (0.91–1.16) | 1.04 (0.87–1.25) | 1.01 (0.98–1.05) | 1.05 (0.96–1.15) | 1.06 (0.86–1.30) |
| Former smoker initiated at ≤15 years | 882 | 39.3 | 17.2 | 26.4 | 17.0 | 1.01 (0.81–1.26) | 1.42 (1.17–1.72) | 1.38 (1.19–1.60) | 0.98 (0.80–1.20) | 1.41 (1.21–1.64) | 1.29 (1.15–1.46) |
| Current smoker initiated at ≥20 years | 605 | 40.0 | 17.9 | 22.3 | 19.8 | 1.00 (0.80–1.26) | 1.13 (0.92–1.39) | 1.48 (1.35–1.61) | 1.00 (0.78–1.28) | 1.11 (1.00–1.22) | 1.43 (1.35–1.50) |
| Current smoker initiated at 16–19 years | 1124 | 37.5 | 19.8 | 25.8 | 16.9 | 1.22 (1.12–1.32) | 1.42 (1.16–1.73) | 1.40 (1.21–1.62) | 1.23 (1.11–1.36) | 1.38 (1.27–1.50) | 1.37 (1.32–1.42) |
| Current smoker initiated at ≤15 years | 670 | 31.6 | 17.8 | 25.4 | 25.2 | 1.29 (1.17–1.42) | 1.63 (1.09–2.45) | 2.41 (1.81–3.20) | 1.25 (1.22–1.28) | 1.57 (1.14–2.17) | 2.19 (1.88–2.54) |
| Age at quitting smoking (n=10,034)a | |||||||||||
| Never smoker | 5800 | 42.5 | 16.7 | 26.0 | 14.8 | – | – | – | – | – | – |
| Current smoker | 1764 | 33.5 | 16.5 | 28.6 | 21.5 | 1.19 (1.02–1.40) | 1.26 (1.10–1.45) | 1.58 (1.35–1.85) | 1.19 (1.01–1.39) | 1.26 (1.10–1.45) | 1.54 (1.31–1.81) |
| Former smoker quit at <30 years | 807 | 46.1 | 15.0 | 25.5 | 13.4 | 0.85 (0.69–1.06) | 0.95 (0.79–1.15) | 0.91 (0.71–1.15) | 0.85 (0.68–1.06) | 0.96 (0.80–1.16) | 0.90 (0.71–1.15) |
| Former smoker quit at 30–39 years | 834 | 40.3 | 17.6 | 28.4 | 13.7 | 1.11 (0.90–1.37) | 1.14 (0.95–1.37) | 0.97 (0.77–1.22) | 1.10 (0.89–1.35) | 1.13 (0.94–1.36) | 0.94 (0.74–1.18) |
| Former smoker quit at ≥40 years | 829 | 32.7 | 16.2 | 31.1 | 20.0 | 1.18 (0.94–1.47) | 1.40 (1.16–1.68) | 1.50 (1.21–1.86) | 1.14 (0.91–1.43) | 1.34 (1.11–1.62) | 1.37 (1.10–1.71) |
| Years since quitting smoking (n=10,031)a | |||||||||||
| Never smoker | 5800 | 42.5 | 16.7 | 26.0 | 14.8 | – | – | – | – | – | – |
| Current smoker | 1764 | 33.5 | 16.5 | 28.6 | 21.5 | 1.19 (1.02–1.40) | 1.26 (1.10–1.45) | 1.58 (1.35–1.85) | 1.18 (1.01–1.39) | 1.26 (1.10–1.45) | 1.54 (1.31–1.81) |
| Former smoker quit 1–5 years | 445 | 31.2 | 14.8 | 33.7 | 20.2 | 1.11 (0.82–1.51) | 1.54 (1.21–1.97) | 1.52 (1.14–2.03) | 1.06 (0.78–1.44) | 1.47 (1.15–1.88) | 1.37 (1.03–1.83) |
| Former smoker quit 6–14 years | 739 | 37.5 | 18.1 | 28.2 | 16.2 | 1.22 (0.98–1.53) | 1.20 (0.99–1.46) | 1.21 (0.96–1.53) | 1.20 (0.96–1.50) | 1.17 (0.96–1.43) | 1.14 (0.90–1.45) |
| Former smoker quit 15–19 years | 450 | 42.2 | 15.8 | 29.3 | 12.7 | 0.96 (0.73–1.28) | 1.17 (0.92–1.48) | 0.91 (0.66–1.25) | 0.96 (0.72–1.28) | 1.18 (0.93–1.50) | 0.89 (0.65–1.23) |
| Former smoker quit ≥20 years | 833 | 44.8 | 15.6 | 25.2 | 14.4 | 0.90 (0.72–1.11) | 0.93 (0.77–1.12) | 0.94 (0.75–1.19) | 0.89 (0.72–1.10) | 0.94 (0.78–1.13) | 0.93 (0.74–1.17) |
Data are presented as percentage (%) or relative risk ratio (RRR) and their 95% confidence intervals (95% CI) using multinomial logistic regression with a generalised logit link. SURVEYLOGISTIC procedure in SAS was used to incorporate the study cluster into the analyses.
Model 1 included menopausal status and use of menopausal hormone therapy at baseline.
Model 2 additionally included race/ethnicity/region, education, and BMI at baseline.
ALSWH, Australian Longitudinal Study on Women’s Health; SWAN, Study of Women’s Health Across the Nation; WHITEHALL, Whitehall II Study.
The analysis was only based on data from the ALSWH study.
Prospective associations
At the three-year follow-up, 23% of the women reported no VMS at baseline and follow-up, 47% experienced some degree of VMS (rarely/mild to often/severe) at both times, 11% reported VMS at baseline but no VMS at follow-up, and 20% reported VMS only at follow-up (n = 11,986, data not shown). Like the results from the cross-sectional analysis, overweight/obesity and smoking at baseline were associated with subsequent risk of VMS at three-year follow-up, and smoking strengthened the effect of BMI, but to a much lesser extent (Table 5). Also, former smokers had a lower risk of often/severe VMS at three-year follow-up than current smokers. Similar results were observed for studies of VMS frequency and VMS severity (data not shown). However, these associations attenuated markedly after adjusting for baseline VMS.
Table 5.
Adjusted prospective associations of body mass index and smoking status at baseline with the risk of subsequent vasomotor symptoms at three-year follow-up (n=11,986; data from ALSWH, NSHD, SWAN and WHITEHALL)
| VMS (hot flushes and night sweats) (%) |
Fully adjusted model RRR (95% CI) |
Fully adjusted model + baseline VMS RRR (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Never | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | |
| Body mass index | |||||||||||
| Normal (<25 kg/m2) | 5859 | 35.7 | 19.7 | 25.3 | 19.3 | – | – | – | – | – | – |
| Overweight (25–29.9 kg/m2) | 3638 | 31.0 | 21.6 | 25.8 | 21.7 | 1.21 (1.12–1.31) | 1.11 (1.01–1.23) | 1.17 (0.99–1.38) | 1.13 (1.09–1.18) | 0.99 (0.94–1.05) | 1.01 (0.89–1.14) |
| Obese (≥30 kg/m2) | 2489 | 31.3 | 21.6 | 24.3 | 22.8 | 1.08 (1.00–1.17) | 1.12 (1.03–1.21) | 1.12 (0.92–1.37) | 1.00 (0.94–1.05) | 0.97 (0.91–1.03) | 0.93 (0.78–1.11) |
| Smoking status | |||||||||||
| Never smoker | 6629 | 34.6 | 20.3 | 25.0 | 20.1 | – | – | – | – | – | – |
| former smoker | 3406 | 33.0 | 22.0 | 25.0 | 19.9 | 1.18 (1.11–1.26) | 1.13 (1.11–1.16) | 1.13 (1.09–1.17) | 1.18 (1.11–1.25) | 1.11 (1.07–1.15) | 1.09 (1.03–1.15) |
| Current skomer | 1951 | 29.7 | 19.7 | 26.2 | 24.5 | 1.16 (1.07–1.26) | 1.22 (1.02–1.45) | 1.39 (1.22–1.59) | 1.07 (1.00–1.15) | 1.08 (0.92–1.27) | 1.17 (1.02–1.33) |
| Joint effect | |||||||||||
| Normal weight & never smoker | 3251 | 37.1 | 19.5 | 24.9 | 18.6 | – | – | – | – | – | – |
| Normal weight & former smoker | 1592 | 36.0 | 20.5 | 25.1 | 18.4 | 1.14 (1.06–1.23) | 1.12 (1.06–1.19) | 1.12 (1.02–1.24) | 1.12 (1.02–1.24) | 1.09 (1.01–1.16) | 1.09 (0.96–1.24) |
| Normal weight & current smoker | 1016 | 31.0 | 19.3 | 26.9 | 22.8 | 1.23 (1.10–1.37) | 1.24 (0.91–1.70) | 1.39 (1.09–1.76) | 1.15 (1.04–1.27) | 1.12 (0.85–1.48) | 1.19 (0.95–1.50) |
| Overweight & never smoker | 2029 | 32.1 | 20.8 | 26.0 | 21.0 | 1.18 (1.07–1.31) | 1.13 (1.05–1.21) | 1.17 (1.00–1.36) | 1.10 (1.01–1.21) | 1.00 (0.93–1.08) | 1.01 (0.89–1.14) |
| Overweight & former smoker | 1053 | 30.0 | 24.0 | 25.6 | 20.4 | 1.54 (1.32–1.79) | 1.27 (1.11–1.45) | 1.31 (1.03–1.67) | 1.45 (1.26–1.67) | 1.12 (1.02–1.24) | 1.11 (0.88–1.40) |
| Overweight & current smoker | 556 | 28.4 | 20.0 | 25.4 | 26.3 | 1.30 (1.09–1.56) | 1.26 (1.17–1.34) | 1.60 (1.41–1.82) | 1.10 (0.92–1.31) | 0.98 (0.86–1.13) | 1.15 (1.04–1.27) |
| Obese & never smoker | 1349 | 32.3 | 21.6 | 23.9 | 22.2 | 1.12 (0.92–1.35) | 1.09 (1.02–1.17) | 1.11 (0.85–1.44) | 1.03 (0.87–1.22) | 0.95 (0.89–1.02) | 0.94 (0.74–1.20) |
| Obese & former smoker | 761 | 31.0 | 22.3 | 24.3 | 22.3 | 1.24 (1.18–1.31) | 1.28 (1.11–1.47) | 1.28 (1.00–1.64) | 1.14 (1.05–1.24) | 1.09 (0.95–1.26) | 1.03 (0.80–1.31) |
| Obese & current smoker | 379 | 28.0 | 20.3 | 25.6 | 26.1 | 1.20 (1.08–1.35) | 1.45 (1.23–1.71) | 1.55 (1.37–1.76) | 1.00 (0.88–1.14) | 1.06 (0.88–1.27) | 1.05 (0.99–1.11) |
Data are presented as percentage (%) or relative risk ratio (RRR) and their 95% confidence intervals (95% CI) using multinomial logistic regression with a generalised logit link. SURVEYLOGISTIC procedure in SAS was used to incorporate the study cluster into the analyses.
Fully adjusted model included menopausal status, use of menopausal hormone therapy at three-year follow-up, race/ethnicity/region, education, BMI and smoking status at baseline.
VMS, vasomotor menopausal symptoms.
Effect modification by menopausal status
There was a significant interaction between menopausal status and BMI (P<.0001) with VMS risk, but no interaction between menopausal status and smoking (P>.05), indicating the effect of BMI may be modified by menopausal status. After stratifying by menopausal status, in the cross-sectional analyses, the association between overweight, obesity and increased risk of VMS remained in pre- and perimenopause but not in postmenopause (Figure 1). In the prospective analyses, the association between baseline BMI and increased risk of VMS at three-year follow-up among pre- and perimenopausal women disappeared after adjusting for baseline VMS, but higher BMI was associated with reduced risk of VMS among postmenopausal women (Figure 2).
Figure 1.
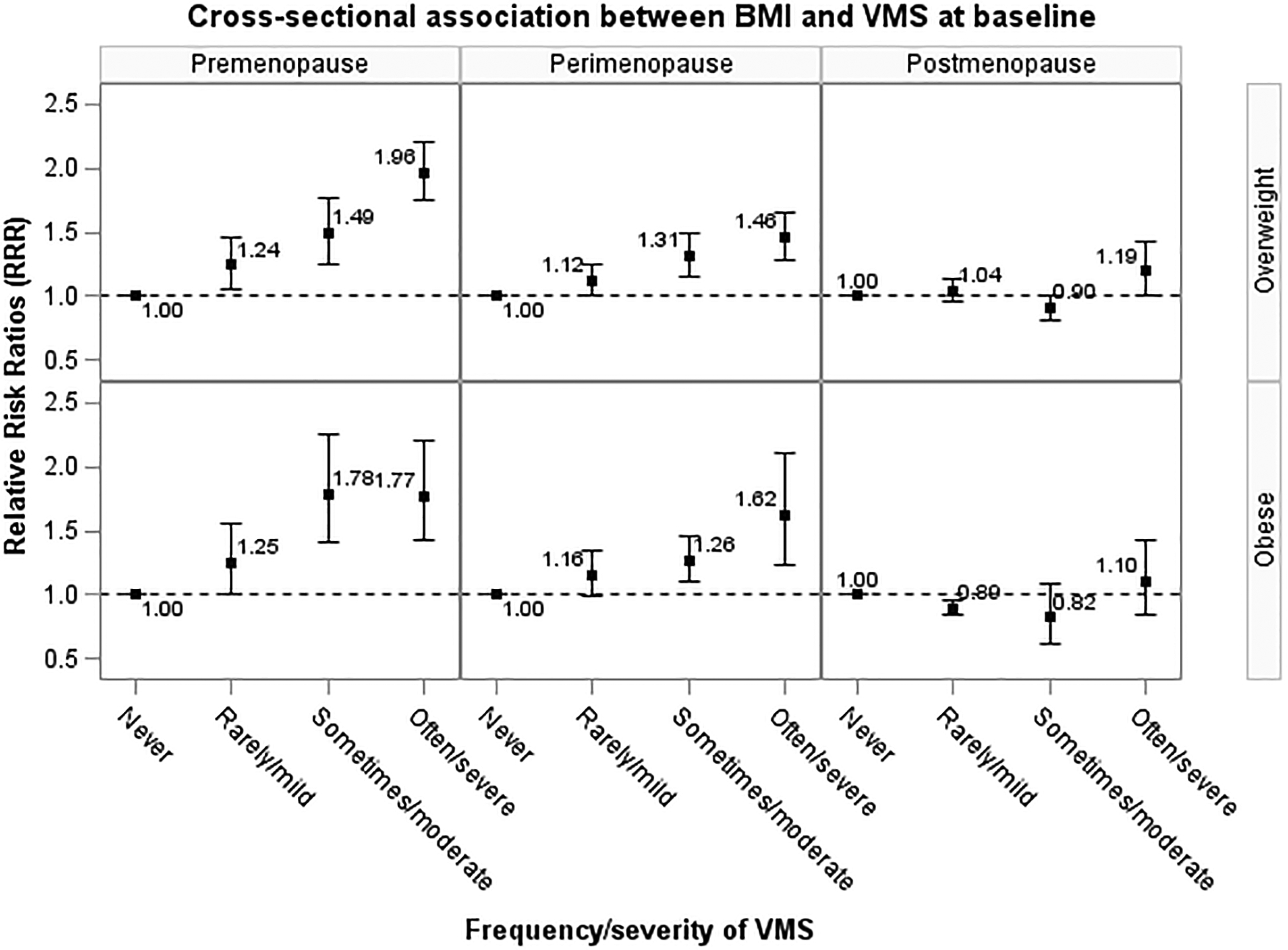
Adjusted cross-sectional association between body mass index and the risk of vasomotor symptoms at baseline, stratified by menopausal status at baseline (premenopause: n=4,169; perimenopause: n=5,881; postmenopause: n=4,109). Relative risk ratio (RRR) and their 95% confidence intervals (95% CI) were adjusted for use of menopausal hormone therapy, race/ethnicity/region, education, and smoking status at baseline.
Figure 2.
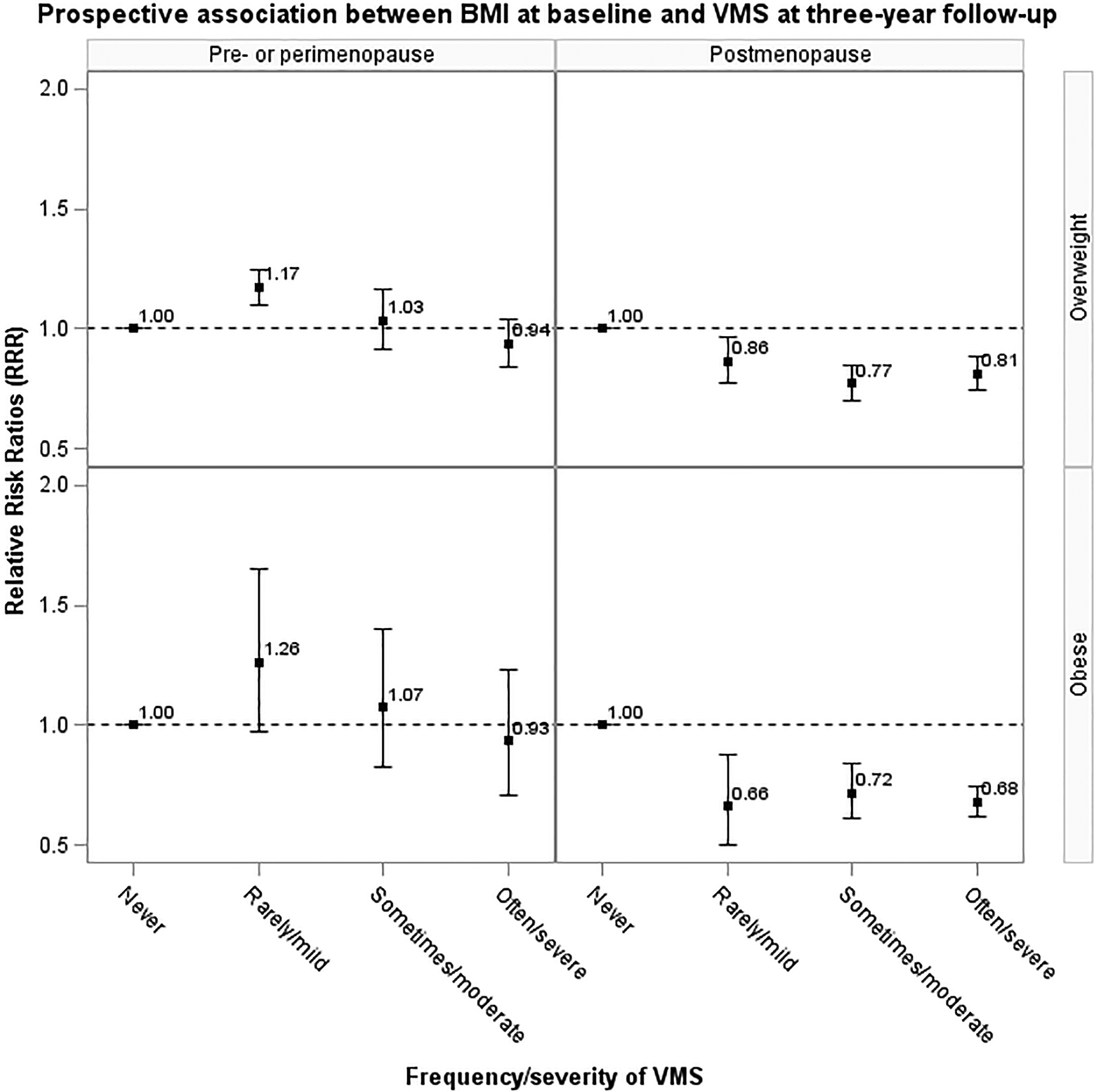
Adjusted prospective association between body mass index at baseline and the risk of vasomotor symptoms at three-year follow-up, stratified by menopausal status at three-year follow-up (data from ALSWH, NSHD, SWAN and WHITEHALL; pre- or perimenopause: n=3,554; postmenopause: n=3,966). Relative risk ratio (RRR) and their 95% confidence intervals (95% CI) were adjusted for use of menopausal hormone therapy at three-year follow-up, race/ethnicity/region, education, smoking status, and vasomotor symptoms at baseline.
COMMENT
Principal findings
This pooled analysis of over 21,000 women from eight studies examined individual and joint associations between two important modifiable factors, BMI and smoking, with frequency/severity of VMS. Results provided robust evidence to indicate that overweight/obesity (BMI≥25 kg/m2) and cigarette smoking were associated with the frequency and severity of VMS, in a dose-dependent manner. These findings are largely consistent with individual InterLACE studies (for example, SWAN13,26) and with other published research.5,27 Most notably, this study also found that smoking intensified the effect of obesity on VMS risk. Smokers who were obese had a particularly high risk of frequent or severe VMS. A significant dose-response was observed for the number of cigarettes, duration of smoking, pack-years, and age at initiation of smoking on risk of VMS in current smokers. Early smoking cessation before the age of 40 years may mitigate the excess risk of VMS. Furthermore, we found that menopausal status modified the association between BMI and VMS. In the cross-sectional analysis, higher BMI was associated with VMS among pre- and perimenopausal women, but not among postmenopause women. In the prospective analysis, baseline BMI was negatively associated with VMS at three-year follow-up among postmenopausal women, even after adjusting for baseline VMS.
Results
Our results are consistent with previous work linking cigarette smoking and elevated BMI with increased frequency and severity of VMS,5,27–31 though the mechanisms behind the relationship between smoking and VMS specifically remain unclear. While it is widely accepted that body fatness is associated with an elevated core body temperature and delayed thermoregulation,32 studies examining the results concerning pathways by which tobacco smoking influences VMS have been inconsistent (some have suggested an anti-estrogenic effect,31 while others have shown the relationship is independent of estrogen levels).29, 30 Alternatively, the chemicals in cigarette smoke affect reproductive function and alter hormone levels and their ratios, for example, higher androstenedione levels, a higher total androgen-to-total estrogen ratio, and lower progesterone levels,33,34 which have been associated with hot flushes.35 Regardless of the exact physiologic mechanisms, however, the particularly increased risk among women who were both obese and current smokers implies that obesity and smoking intensify each other’s effect on frequency/severity of VMS. The mechanisms behind the potential synergistic interaction in relation to VMS were beyond the scope of this study.
Previously, the InterLACE study examining smoking and age at menopause found that the toxic impact of smoking on reproductive function appeared to be cumulative and long-lasting, even former smokers had an increased risk of earlier menopause.36 Only those women who had quit smoking for more than ten years had a similar risk as never smokers. Findings from this study also support that the reversal of negative effects after smoking cessation on VMS may not be immediate. Women who quit smoking for less than five years or quit at more than 40 years still had a significantly higher risk of frequent and severe VMS than never smokers. These results suggest that quitting smoking early is an important part of the routine counselling of women before approaching menopause.
In line with our findings, previous findings from SWAN showed that greater concurrent BMI and waist circumference were associated with increased risk of incident VMS in early menopause but with reduced VMS risk in late menopause, indicating the dominant mechanism of the effect of body fat on VMS differs in pre- and postmenopause.15 Previous NSHD study also found that postmenopausal women with BMI ≥30 kg/m2 were less likely to have severe VMS profile.37 In the early stage of the menopausal transition, overweight and obesity may predispose to increased VMS occurrence (potentially due to greater heat insulation),14 whereas in postmenopausal women increased estrone production from aromatization of androstenedione occurs with increasing weight,38 which may be associated with less symptom reporting. Also, the effect of weight change on VMS is likely to differ in premenopausal and postmenopausal women.15
Clinical implications
This study contributes to the understanding of how unhealthy behaviours, which often co-exist, can interact and increase risk to a greater extent than they would if they occurred alone. Findings also suggested that cigarette smoking conveyed greater risk for VMS than being overweight or obese, consistent with SWAN’s previous results.15 These findings support the opportunity to refer midlife women to health promotion programs and the need to emphasize both early smoking cessation and weight management strategies prior to menopause, as waiting until the menopausal transition and postmenopause is too late to achieve maximum benefit. Encouraging women to stop smoking before the menopausal transition (preferably before age 40 years) is essential. This is particularly important for obese smokers whose risk of experiencing frequent and severe VMS is notably high.
Women with frequent and severe VMS often seek medical advice to manage their symptoms. Hormone therapy is the most common and effective treatment for VMS. However, many women and health-care professionals have concerns about the long-term risks of hormone therapy, in particular on the risk of CVD, based on the results from the Women’s Health Initiative (WHI) trial study.39 The benefits and risks of hormone therapy vary by dosage, regimen, and timing of initiation. According to the NICE guidance,40 women should be informed that taking hormone therapy under 60 years does not increase CVD risk, and the presence of CVD risk factors (e.g. blood pressure, cholesterol) is not a contraindication to hormone therapy as long as they are optimally managed.
Strengths and limitations
To our knowledge, this is the first study to examine the individual and joint associations between BMI and smoking with the risk of VMS. InterLACE consortium draws together individual-level data from a number of large studies and is therefore able to provide precise estimates of the associations. Additionally, the availability of race/ethnicity/regional data, albeit based on self-reports, provides a relatively unique opportunity to examine differences in VMS symptoms in women from Japan, the United States, the United Kingdom, and Australia. Several limitations of these analyses should also be considered. First, data were derived from self-reports and this could have reflected in recall bias. For example, pre- or post-menopausal women, or women who experienced short duration or mild VMS might have been less likely to report their symptoms than women with moderate/severe VMS. Another significant limitation was the differences in the assessment of menopausal symptoms (severity or frequency, over different recall period) across studies, which limited our ability to pool data. Therefore, it is important for the future research to develop standardised measures for menopausal symptoms (e.g., the COMMA initiative – Core Outcome set in Menopause; part of the CROWN project),41 which will enhance the availability of comparable data across different populations. Furthermore, of the four studies that provided longitudinal data on VMS, over 3,500 women with incomplete follow-up data were excluded. These women were more likely to report the exposures (obesity or current smoking), outcome (VMS), or both, which may have led to an underestimation of the frequency/severity of VMS. However, as we observed sufficient variation in the distribution of exposures and outcome, we do not expect the nature of relationships observed in this study to change substantively.
Conclusions
Results from this pooled analysis provided strong evidence that both higher body mass and smoking with higher intensity, longer duration, and earlier initiation were associated with more frequent and severe VMS. Cigarette smoking strengthened the association between obesity and VMS and thus smokers who were obese had a particularly increased risk of VMS. Effective intervention for smoking cessation before age 40 years and maintaining a normal weight before the menopausal transition may have important implications for prevention of VMS in midlife women.
AJOG at a Glance.
Why was this study conducted?
This pooled analysis provided precise estimates of the individual and joint associations between body mass index (BMI) and smoking with the risk of vasomotor menopausal symptoms (VMS).
What are the key findings?
Higher BMI and greater smoking were associated with more frequent/severe VMS in the cross-sectional analysis, and smoking strengthened the effect of obesity. However, women who quit smoking before age 40 years had a similar level of risk as never smokers.
Prospective analyses showed similar results, but the individual and joint effects of BMI and smoking on subsequent VMS at three-year follow-up attenuated markedly after adjustment for baseline VMS.
The effect of BMI on VMS risk differed in pre-/perimenopause and postmenopause.
What does this study add to what is already known?
Being both obese and smoking conferred a much higher risk of frequent/severe VMS than either alone.
Maintaining a normal weight before the menopausal transition and smoking cessation before age 40 years may mitigate the excess risk of frequent/severe VMS.
Acknowledgements
The data on which this research is based were drawn from eight observational studies. The research included data from the Australian Longitudinal Study on Women’s Health (ALSWH), the University of Newcastle, Australia, and the University of Queensland, Australia. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. MRC National Survey of Health Development (NSHD) has core funding from the UK Medical Research Council (MC UU 12019/1). National Child Development Study (NCDS) is funded by the UK Economic and Social Research Council. The Whitehall II study is supported by grants from the Medical Research Council (K013351), British Heart Foundation (BHF RG/16/11/32334) and US National Institutes on Aging (R01AG013196, R01AG034454). Seattle Midlife Women’s Health Study (SMWHS) was supported in part by grants from the National Institute of Nursing Research, P50-NU02323, P30-NR04001, and R01-NR0414. Healthy Ageing of Women Study (HOW) and Japanese Midlife Women’s Health Study (JMWHS) (also called Australian and Japanese Midlife Women’s Health Study) were supported by the Queensland University of Technology Early Career Research Grant and the JSPS Grant-in-aid for Scientific Research.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor - Siobán Harlow, PI 2011 - present, MaryFran Sowers, PI 1994 - 2011; Massachusetts General Hospital, Boston, MA - Joel Finkelstein, PI 1999 - present; Robert Neer, PI 1994 - 1999; Rush University, Rush University Medical Center, Chicago, IL - Howard Kravitz, PI 2009 - present; Lynda Powell, PI 1994 - 2009; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 - 2011; Nanette Santoro, PI 2004 - 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 - 2004; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Chhanda Dutta 2016 - present; Winifred Rossi 2012 - 2016; Sherry Sherman 1994 - 2012; Marcia Ory 1994 - 2001; National Institute of Nursing Research, Bethesda, MD - Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA - Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 - 2012; New England Research Institutes, Watertown, MA-Sonja McKinlay, PI 1995 - 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
All studies would like to thank the participants for volunteering their time to be involved in the respective studies. The findings and views in this paper are not necessarily those of the original studies or their respective funding agencies.
Financial Disclosure Statement
InterLACE project is funded by the Australian National Health and Medical Research Council project grant (APP1027196). GDM is supported by the Australian National Health and Medical Research Council Principal Research Fellowship (APP1121844).
Footnotes
Conflict of Interest
The authors report no conflict of interest.
REFERENCES
- 1.Carpenter J, Gass ML, Maki PM, et al. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of the North American menopause society. Menopause 2015;22:1155–74. [DOI] [PubMed] [Google Scholar]
- 2.Williams RE, Kalilani L, Dibenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas 2007;58:348–58. [DOI] [PubMed] [Google Scholar]
- 3.Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health 2007;10:247–57. [DOI] [PubMed] [Google Scholar]
- 4.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health 2006;96:1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010;17:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantine GD, Graham S, Clerinx C, et al. Behaviours and attitudes influencing treatment decisions for menopausal symptoms in five European countries. Post Reproductive Health 2016;22:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurston R, Johnson BD, Pepine C, et al. Early-onset menopausal vasomotor symptoms are associated with endothelial dysfunction: the National Heart Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol 2015; 65, A1512. [Google Scholar]
- 8.Muka T, Oliver-Williams C, Colpani V, et al. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS One 2016; 11: e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utian W. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health and Quality of Life Outcomes 2005;3:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann GA. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312–S16. [DOI] [PubMed] [Google Scholar]
- 11.Melby MK, Anderson D, Sievert LL, Obermeyer CM. Methods used in cross-cultural comparisons of vasomotor symptoms and their determinants. Maturitas 2011;70:110–19. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RC, Joffe H. Vasomotor symptoms and menopause: Findings from the study of women’s health across the nation. Obstet Gynecol Clin North Am 2011;38:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greendale GA, Gold EB. Lifestyle factors: Are they related to vasomotor symptoms and do they modify the effectiveness or side effects of hormone therapy? Am J Med 2005;118:148–54. [DOI] [PubMed] [Google Scholar]
- 14.Savastano DM, Gorbach AM, Eden HS, Brady SM, Reynolds JC, Yanovski JA. Adiposity and human regional body temperature. Am J Clin Nutr 2009;90:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold EB, Crawford SL, Shelton J, et al. Longitudinal analysis of changes in weight and waist circumference in relation to vasomotor symptoms: the Study of Women’ Health Across the Nation (SWAN). Menopause 2017;24:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra GD, Anderson D, Schoenaker DA, et al. InterLACE: A new international collaboration for a life course approach to women’s reproductive health and chronic disease events. Maturitas 2013;74:235–40. [DOI] [PubMed] [Google Scholar]
- 17.Mishra GD, Chung HF, Pandeya N, et al. The InterLACE study: Design, data harmonization and characteristics across 20 studies on women’s health. Maturitas 2016;92:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson AJ, Hockey R, Brown WJ, et al. Cohort profile update: Australian longitudinal study on women’s health. Int J Epidemiol 2015;44:1547,47a–47f. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: The 1946 national birth cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006;35:49–54. [DOI] [PubMed] [Google Scholar]
- 20.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol 2006;35:34–41. [DOI] [PubMed] [Google Scholar]
- 21.Sowers M, Crawford SL, Sternfeld B, et al. Swan: A multi-center, multiethnic, community-based cohort study of women and the menopausal transition. San Diego, CA: Academic Pres; Number of pages. [Google Scholar]
- 22.Marmot M, Brunner E. Cohort profile: The whitehall ii study. Int J Epidemiol 2005;34:251–6. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric 2011;14:252–61. [DOI] [PubMed] [Google Scholar]
- 24.Anderson D, Yoshizawa T, Gollschewski S, Atogami F, Courtney M. Menopause in Australia and Japan: Effects of country of residence on menopausal status and menopausal symptoms. Climacteric 2004;7:165–74. [DOI] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 26.Thurston RC, Sowers MR, Chang Y, et al. Adiposity and reporting of vasomotor symptoms among midlife women the Study of Women’s Health Across the Nation. Am J Epidemiol 2007;167:78–85. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman MK, Staropoli CA, Langenberg PW, Mccarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol 2003;101:264–72. [DOI] [PubMed] [Google Scholar]
- 28.Gallicchio L, Miller SR, Visvanathan K, et al. Cigarette smoking, estrogen levels, and hot flashes in midlife women. Maturitas 2006;53:133–43. [DOI] [PubMed] [Google Scholar]
- 29.Cleary MP, Grossmann ME. Obesity and breast cancer: The estrogen connection. Endocrinol 2009;150:2537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: Baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol 2004;159:1189–99. [DOI] [PubMed] [Google Scholar]
- 31.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 1986;315:1305–09. [DOI] [PubMed] [Google Scholar]
- 32.Sassarini J, Lumsden MA. Vascular function and cardiovascular risk factors in women with severe flushing. Maturitas 2015;80:379–83. [DOI] [PubMed] [Google Scholar]
- 33.Cochran CJ, Gallicchio L, Miller SR, Zacur H, Flaws JA. Cigarette smoking, androgen levels, and hot flushes in midlife women. Obstet Gynecol 2008;112:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Windham GC, Mitchell P, Anderson M, Lasley BL. Cigarette smoking and effects on hormone function in premenopausal women. Environ Health Perspect 2005: 113; 1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilling C, Gallicchio L, Miller SR, Langenberg P, Zacur H, Flaws JA. Genetic polymorphisms, hormone levels, and hot flashes in midlife women. Maturitas 2007;57:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu D, Chung HF, Pandeya N, et al. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies. PLoS Med 2018;15:e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ 2012;344:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes R, Levrant S. Pharmacology of estrogens In: Lobo R, ed. Treatment of the Postmenopausal Women, third edition. Burlington, MA: Academic Press; 2007:767–777. [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 40.NICE guideline. Menopause: diagnosis and management. 2015. [DOI] [PubMed]
- 41.The CROWN initiative. Core outcome set in Menopause (COMMA). 2016. Available at http://www.crown-initiative.org/core-outcome-sets/ongoing-core-outcome-sets-2 (Accessed 7 May 2019).


