Abstract
Recombinant DNA technologies have enabled the development of transgenic animal models for use in studying a myriad of diseases and biological states. By placing fluorescent reporters under the direct regulation of the promoter region of specific marker proteins, these models can localize and characterize very specific cell types. One important application of transgenic species is the study of the cytoarchitecture of the nervous system. Neurofluorescent reporters can be used to study the structural patterns of nerves in the central or peripheral nervous system in vivo, as well as phenomena involving embryologic or adult neurogenesis, injury, degeneration, and recovery. Furthermore, crucial molecular factors can also be screened via the transgenic approach, which may eventually play a major role in the development of therapeutic strategies against diseases like Alzheimer’s or Parkinson’s. This review describes currently available reporters and their uses in the literature as well as potential neural markers that can be leveraged to create additional, robust transgenic models for future studies.
Introduction
Detailed analyses of the cytoarchitecture of the nervous system have been accomplished by various means in the past. In 1873, Camillo Golgi introduced the silver stain that enabled the labeling of entire neurons and established the foundation on which modern neuroscience was built [1]. Using this Golgi stain, Cajal was able to reveal the diversity of morphologies within the human nervous system [1], demonstrating that some neurons like select granule cells can have simple processes extending from a small cell body, while others such as Purkinje cells can have more complex projections. The characterization of the morphology and overall network architecture of neurons is a subject heavily covered in the scientific literature, perhaps due to its direct implications in revealing the cells’ underlying functions and connectivity with surrounding tissue. The culmination of efforts to map the global neural connections in the brain (“connectomes”) has greatly expanded our understanding of the organization of the central nervous system [2]. Immunohistochemistry has also been a common tool for identifying neuronal subtypes and distinguishing them based on biochemical charact eristics. Highlighting cells involved in the activity of a certain neurotransmitter and identifying neurons that express a certain protein are tasks enabled by this method. The discovery of neuron-specific enolase and non-neuronal enolase as markers for neuronal and glial cells, respectively [3], has paved the way for immunolabeling to become a widely used tool, with subsequent descriptions of other relevant markers (external glycoprotein, choline acetyltransferase, parvalbumin, neurofilament protein, etc.).
The advent of recombinant DNA technology soon permitted the growing use of transgenic species that express fluorescent reporters developed for use in imaging studies. The elegance of transgenic models can be seen not only in the vibrant images taken via fluorescent microscopic techniques like confocal microscopy but also in their ability to provide visual evidence for physiologic or pathologic phenomena in the nervous system. In 2019 alone, this method was used for research related to Huntington’s disease [4], Alzheimer’s disease (AD) [5, 6], Parkinson’s disease [7], and amyotrophic lateral sclerosis (ALS) [6], and there has been significant effort to innovate novel transgenic species to understand other poorly understood disease states like schizophrenia [8]. Another unique area of study is the eye, which receives input from both the central (CNS) and peripheral nervous system (PNS) [9]. The cornea has become a popular tissue for the visualization and study of peripheral nervous structure, repair, and regeneration due to its accessibility, innate avascularity , and immune privilege [10]. Recently, Bouheraoua et al. compiled a collection of transgenic mice reporters for corneal nerve visualization, including CGRP:GFP BAC and Wnt1:Cre, TAG-1:Cre, En1-Cre, Islet1:Cre, and Ret:CreER, that can be crossed with lines expressing lox-P-containing fluorescent reporters to generate novel reporter lines [11]. Notably, several of the neurofluorescent reporters reviewed herein have been employed in studies of nerve structure and regeneration in the cornea [11].
Thy1-YFP (yellow fluorescent protein) and Nestin-GFP (green fluorescent protein) are two well-described examples of fluorescent genes coupled with neurally expressed genes to enable in vivo imaging of nervous tissue. Thy1 (CD90) is a cell surface glycoprotein that contributes to intercellular communication, particularly in the immune and nervous systems. Various studies have demonstrated that Thy1 expression occurs predominately in the late stages of postnatal development; hence, the Thy1-YFP reporter line can be a tool for studying non-embryonic neural development and response to injury [12]. Nestin, on the other hand, is an intermediate filament expressed in many cell types including nervous tissue. Nestin-GFP allows visualization of neural progenitor cells in the CNS, especially during embryonic development. This also becomes particularly relevant in recent neuroscience research, with attention recently given to describing the unclear nature and mechanism of adult neurogenesis [13]. Thus, in this review, we present an extensive discussion of currently available neurofluorescent reporter mouse models and discuss their possible utilities for investigations of the nervous system.
Biochemical markers and neurofluorescence
The synthesis of fluorescent reporters is made possible by the identification of specific molecular biomarkers that can be engineered to fluoresce via insertion of the gene of a known fluorescent protein (e.g., GFP, YFP, DsRed, mCherry) into the transcriptional site of the biomarker DNA [14]. The identification of biomarkers that represent a specific tissue of interest is crucial for the development of a transgenic neurofluorescence reporter model. Table 1 reviews some key nerve markers and provides examples of their previous experimental use [15].
Table 1.
Common nerve markers and their locations, functions, and cited experimental application
| Biomarker | Experimental Application | ||
|---|---|---|---|
| Thy-1 (CD90) | Subsets of neurons, thymocytes, peripheral T cells, myoblasts, epidermal cells, keratinocytes | Cell interactions | Visualization of nerve structure and regeneration in the cornea [10] |
| Nestin | Neuroepithelial cells, radial glia, ventricular zone progenitor cells, nascent ependyma/subependyma | Class VI Intermediate filament | Visualization of self-renewal and multipotency of CNS stem cells [16] |
| βIII tubulin | Neurons | Structural protein | Expression in the developing PNS and CNS using YFP [17] |
| Neurofilament heavy chain (NF-H) | Neurons (mature myelinated axons) | Axonal phosphorylation and neurofilament transport | Expression of high molecular weight neurofilaments in neurons and their transportation along axons [18] |
| Substance P | Subsets of sensory nerves | Pain transmission in CNS inflammation, wound healing | Understanding the contribution of Substance P to corneal epithelial w ound healing via mechanisms involving the Neurokinin-1 Receptor [19] |
| Neuron-specifice nolase (NSE) | Neurons, peripheral neuroendocrine cells | Neural differentiation and maturation | Expression of β-galactosidase in mature neurons under the control of a neuron-specific enolase promoter [20] |
| Calcitonin gene-related peptide (CGRP) | Subsets of sensory neurons | Vasodilation, smooth muscle relaxation, potentiates excitation caused by noxious stimuli and pronociceptive chemicals | Expression of CGRPα in sensory neurons to integrate pain and itch responses [21] |
Thy1 and Nestin remain amongst the most popular markers used in the generation of transgenic neurofluorescent reporter animal models. Thy1 has been particularly useful in visualizing corneal nerves. On the other hand, Nestin has been useful in retinal imaging due to its strong expression in the CNS [22, 23]. The following sections expand upon the clinical utility of potential nervous tissue markers in more detail.
Thy1
Thymus cell antigen 1 (Thy1 or CD90) is a glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein involved in various cellular processes that include cell adhesion and communication [24]. Thy1 is expressed in various cell types in mice, particularly those involved in the nervous and immune systems. In order to selectively label neuronal Thy1 with YFP, the YFP transgene is directed by modified regulatory elements of the Thy1 gene that has a targeted deletion of exon 3 and its flanking introns [25, 26].
Thy1-YFP reporter mice allow for in vitro and in vivo fluorescent imaging in both the CNS and PNS. Alic et al. found that at day E14.5 in Thy1-YFP-16 transgenic mice, 22% of CNS neurons are positive for Thy1- YFP, and during 1 month of post embryonic development, the percentage of Thy1-YFP-positive neurons in the cortex will more than double to 50% [27, 28]. Thy1-YFP expression thus increases within multiple tissues over the course of embryonic development. Expression of Thy1-YFP also persists in mature neurons, which are characterized by morphological maturation, completion of neuronal migration, and the initiation of dendritic growth [29, 30]. Therefore, Thy1-YFP reporter mice can be used to visualize nerve repair and regeneration following injury after neural development [31].
In Thy1-YFP reporter mice, YFP-labeled axons comprise a minority of the total neuronal population, allowing for clear visualization of individual neurons and their branching patterns. Yu and Rosenblatt first proposed Thy1-YFP-16 reporter mice as a robust model for in vivo nerve imaging in the cornea [10]. Most research in Thy1-YFP mice has utilized two mouse lines-, Thy1-YFP-H and Thy-1-YFP-16 (Table 2), which have variable expression patterns of YFP in nervous tissues [32, 33].
Table 2.
Thy1-YFP expression during embryonic development in Thy1-YFP-16 mice
| Neural system | E12.5 | E13.5 | E14.5 | E15.5 | E16.5 | E17.5 | P0 | W1 | M1 |
|---|---|---|---|---|---|---|---|---|---|
| CNS | |||||||||
| Brain | |||||||||
| Prosencephalon | +/− | +/− | + | + | ++ | ++ | ++ | +++ | +++ |
| Mesencephalon | |||||||||
| Rhombencephalon | + | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ |
| Spinal cord | |||||||||
| Ventral horn | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Dorsal horn | +/− | + | + | ++ | +++ | +++ | +++ | +++ | +++ |
| PNS | |||||||||
| Cranial nerves | + | ++ | ++ | ++ | ++ | +++ | +++ | ||
| Spinal nerves | + | + | ++ | ++ | ++ | ++ | ++ | +++ | +++ |
| Retina | + | ++ | +++ | +++ | |||||
E17.5 has been defined as the referral point and at that stage number of cells has been counted: “−” refers to no signal, “+/−” to signal present in less than 10% of cells, “ +” to signal present in 10–20% of cells, “++” to signal present in 20–35% of cells, “+++” to signal present in 35–50% of cells. Other stages were compared to the referral point and evaluated semi-quantitatively. “PNS” peripheral nervous system, “E” embryonic day, “P” newborn, “W1” 1-week-old pups, “M1” 1-month-old mice. Reproduced with permission from [27].
Many studies investigating peripheral nerve injury have utilized Thy1-YFP expression in a subset of sensory nerves that innervate the transparent cornea to monitor nerve changes and evaluate putative neurotrophic factors [34]. Using Thy1-YFP-16 reporter mice, Namavari et al. demonstrated that surgically transected corneal nerves recover to normal density, albeit with abnormal arrangements, compared to uninjured mouse corneas (Figure 1) [35]. In another recent study, Sarkar et al. disrupted Thy1-YFP mouse stromal nerve trunks in situ using a far infrared laser, which allowed the assessment of nerve regeneration after point, rather than planar, transections [36]. This 2019 study reinforces the utility of a Thy1-YFP reporter line for studying corneal nerve repair and healing processes in vivo [36].
Figure 1.
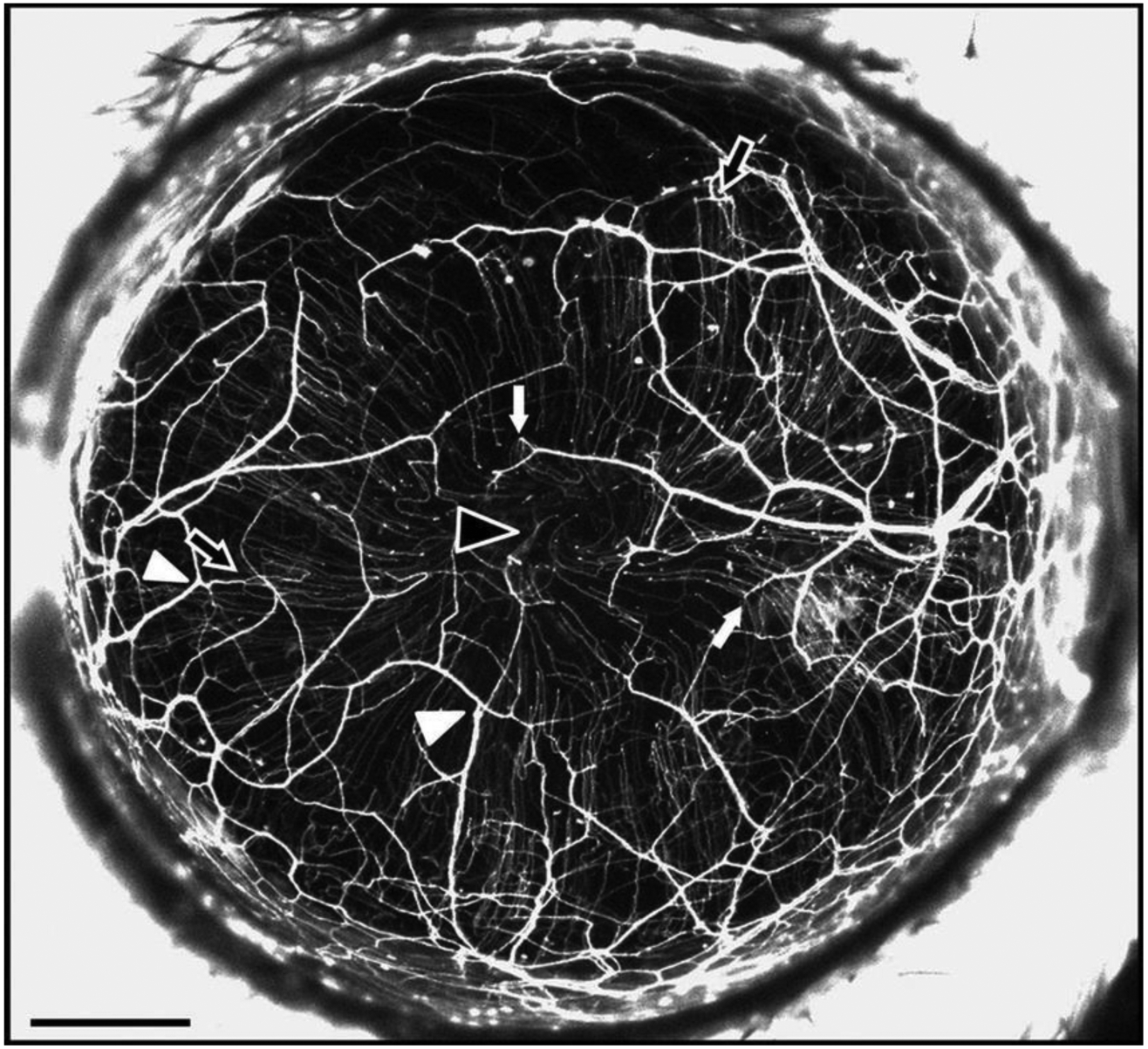
In vivo image of fluorescent nerves in the healthy Thy1-YFP-16 mouse cornea focusing particularly on the stromal plexus. Following surgical transection, nerves display disorganized regenerated fibers Scale bar, 500 μm. Reproduced with permission from [35].
Other studies have demonstrated that neurotrophins such as brain-derived nerve factor (BDNF), small proline-rich repeat protein 1A (Sprr1a), semaphorin 3A (Sema3A), and vascular endothelial growth factors A and B (VEGFA and VEGFB) are expressed post injury and contribute to corneal nerve regeneration in vivo [37–39], while the immunosuppressant cyclosporine inhibits corneal nerve growth [40]. Another study found that in Thy1-YFP mice, YFP+ bone marrow cells (YFP+ BMCs) infiltrate the cornea after excimer laser annular keratectomy and have the ability to promote trigeminal ganglion neurite growth through secretion of growth factors, like such as neural growth factor (NGF) [22].
The Thy1-YFP reporter line has been particularly useful in studies of peripheral neuropathy caused by diabetes mellitus. Corneal nerves in Thy1-YFP-16 mice treated with streptozocin (STZ) were viewed by high-resolution in vivo imaging using two types of confocal microscopy: laser scanning microscopy (CLSM) and two-photon microscopy (TPM) (Figure 2). CLSM is a non-invasive method for assessing nerve fiber changes and, helping determine the extent of nerve damage in diabetic patients. TPM is a form of nonlinear microscopy possessing a high signal-to-noise ratio and capable of producing can produce multimodal in vivo images. Both CLSM and TPM support a statistically significant decrease in corneal nerve fiber density in STZ-diabetic mice compared to the control mice [23]. According to the wide variety of potential imaging applications for Thy1-YFP reporter mice, we expect such imaging approaches to be useful for visualizing nervous structures in tissues beyond the cornea [41].
Figure 2.
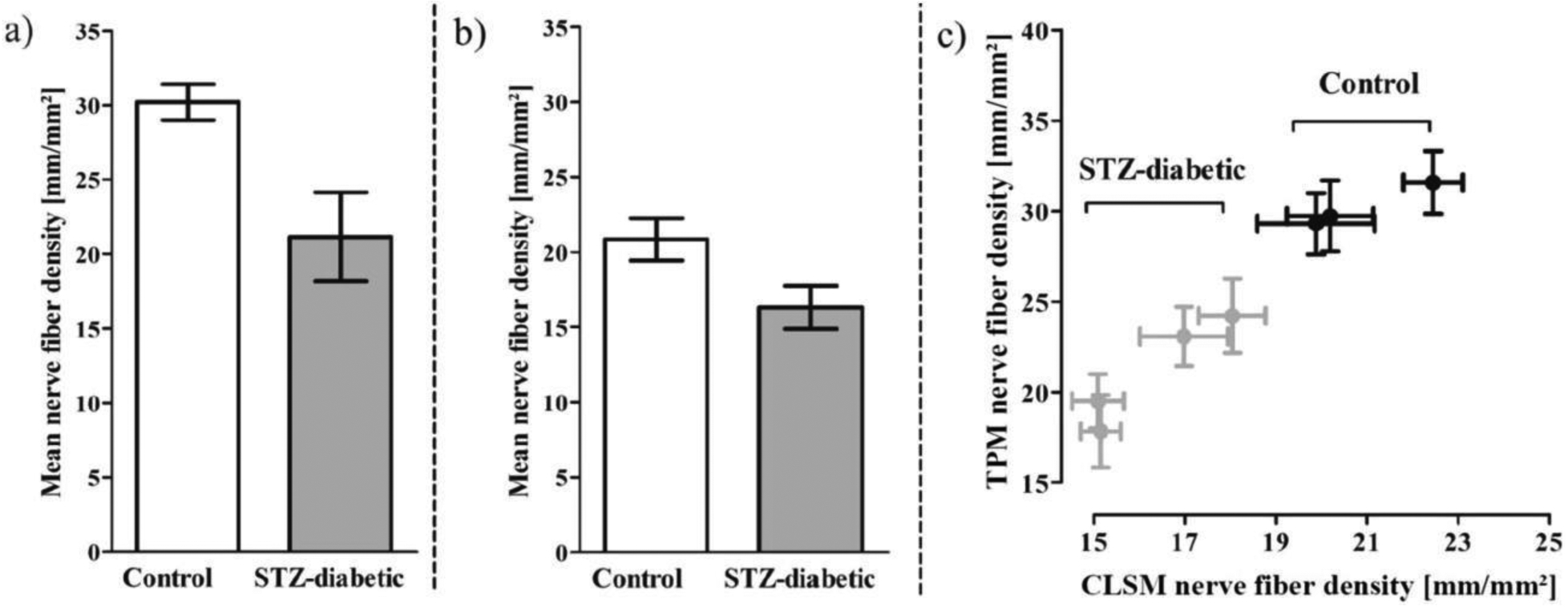
Mean nerve corneal subbasal fiber density in four STZ-treated and three control Thy1-YFP mice. Based on quantitative results from confocal laser scanning microscopy (CLSM) and two-photon microscopy (TPM) (both of which are forms of confocal microscopy) of corneal nerves in vivo, STZ treatment caused significant reductions in corneal nerve fiber density. Reproduced with permission from [23].
Outside of the cornea, a number of studies have used the Thy1-YFP reporter model to characterize peripheral and central nerve morphology and response to injury. Bierowski et al. observed Wallerian degeneration of distal tibial nerve axons in Thy1-YFP-H mice following sciatic nerve axotomy and validated a delay in degeneration in WldS-overexpressing mutants [42]. In a seminal paper that assessed nerve injury and regeneration in vivo, Kerschensteiner et al. characterized nerves undergoing acute axonal degeneration in contrast to the relatively delayed Wallerian degeneration. This was achieved by utilizing time-lapse imaging of a Thy1-GFP-S fluorescent reporter mouse line after nerve transection of centrally-projecting dorsal root ganglia (DRG) neurons. This study demonstrated that although many transected central neurons attempt to regenerate within 24 hours of injury, they are unable to meet their original, pre-injury tissue targets [43]. Alternatively, Carter et al. used a thoracic dorsal column crush spinal cord injury model in Thy1-YFP-H mice to characterize CNS changes within the cortex layer V pyramidal neurons and demonstrate the protective effects of chondroitinase ABC intracerebroventircular infusions, demonstrated as reduction in cell atrophy, increased axonal sprouting, upregulation of ERK1 and other kinases thought to be important to neuronal survival [44]. This study verifiedthat the responses of intrinsically YFP-labeled neurons were comparable with those of neurons labeled with retrograde tracing methods, such as Fast Blue cell soma labeling. Additionally, patterns of fragmentation in Thy1-YFP+ neurons paralleled patterns of Wallerian degeneration as assessed by classical light microscopy and electron microscopy techniques (Figure 3) [42]. Therefore, although Comley et al. raised valid concerns that the fluorescent proteins expressed in Thy1-YFP mice are not biologically inert in degeneration studies and may have diverse unpredictable effects on neurodegeneration [45], the aforementioned findings by Carter et al. and Bierowski et al. reinforce the appropriateness of this transgenic reporter for the study of neuronal responses to injury.
Figure 3.
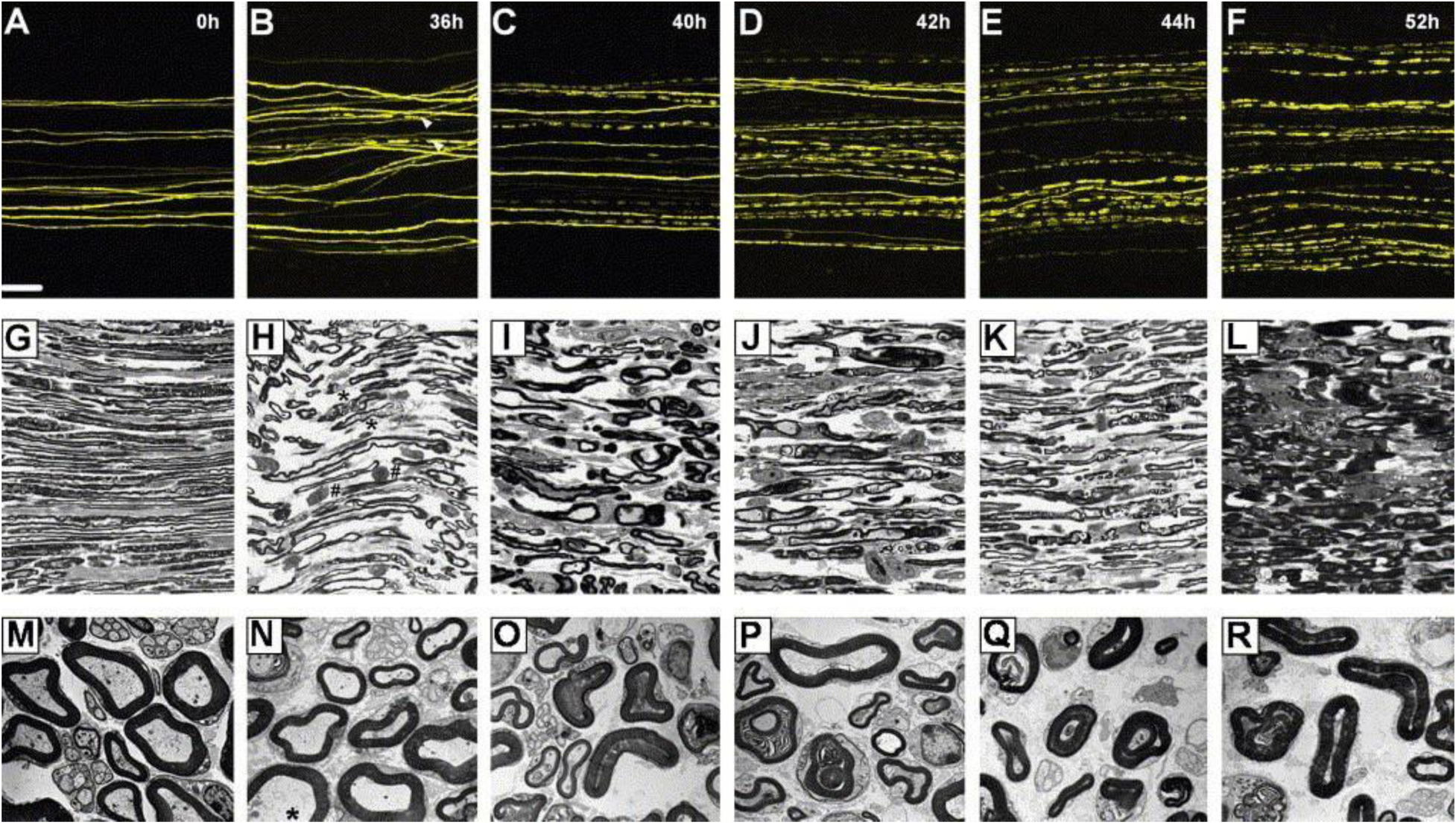
Imaging of Thy1-YFP+ neurons revealed that YFP axons could be successfully used to display nerve changes following Wallerian degeneration. Such imaging was achieved through light and electron microscopy techniques. Scale bars, (A–F) 100 μm, magnification: (G–L) 1000×, magnification: (M–R) 4400×. Reproduced with permission from [42].
Beyond mice, recently developed transgenic Thy1-GFP rat reporters have allowed for the study of nerve injury in a larger and longer-living animal model. For example, facial nerve re-innervation of the zygomaticus muscle was assessed 4 weeks after crush injury via confocal microscopic imaging of Thy1GFP labeled neurons in rats [46, 47].
In summary, Thy1 reporter lines demonstrate fluorescence only in a subset of central and peripheral neurons. However, this limited expression has proven to be particularly useful for in vivo imaging studies that track individual neuronal responses to injury. Use of Thy1 reporter lines has led to a number of breakthroughs in our understanding of nerve injury, regeneration, and putative therapeutic targets for promoting nerve repair.
Nestin
Nestin is a class VI intermediate filament (IF) protein and marker of neural stem cells that is expressed in many cell types. Through co-expression with GFP, Nestin has been used to identify progenitor cells in the CNS. Neural stem cells express Nestin during early embryonic nervous system development, but the protein is downregulated as these cells differentiate into mature cells. The differentiated cells in turn express either glial fibrillary acidic protein (GFAP) or neurofilaments. Therefore, the expression of Nestin can be used to assess the undifferentiated state of neural progenitor cells, whereas downregulation of Nestin correlates with differentiation of these cells in the developing CNS (Figure 4) [27, 48]. Mignone et al. confirmed this finding by using Nestin-GFP to identify undifferentiated neural stem cells [49].
Figure 4.
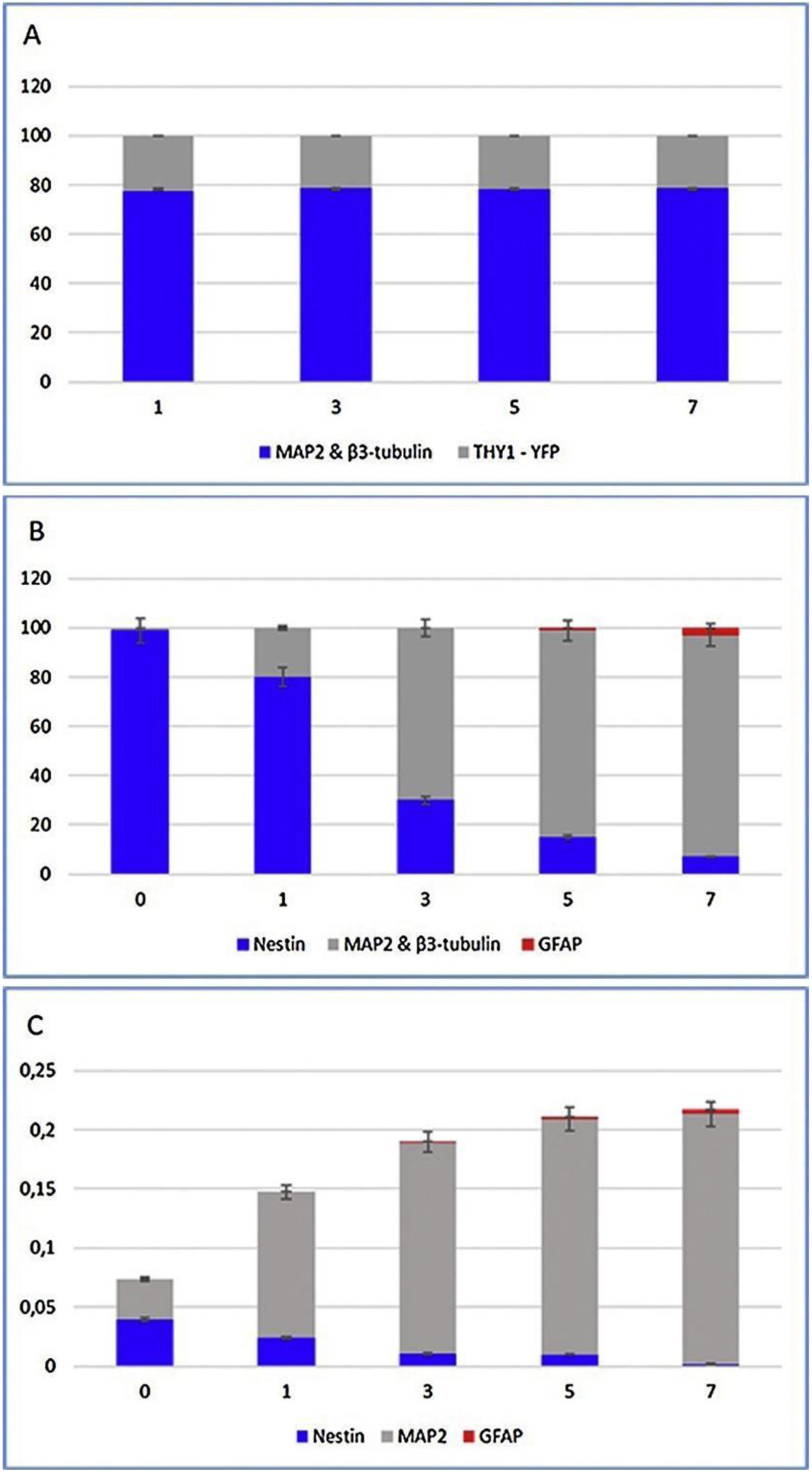
Quantitative analyses of various cellular markers by immunocytochemistry (A–B) and RT-PCR (C). Notably, the percentage of cells expressing Nestin decreased from 99% to 7% during in vitro differentiation, because Nestin is preferentially expressed in undifferentiated neural progenitors. However, the expression of MAP2 and β3-tubulin conversely increased over time. Legend: x-axis – days in differentiation conditions; y-axis – number of counted cells (A), % of counted cells (B), and relative expression in RNA (C). Reproduced with permission from [27].
Nestin deficiency leads to reduced self-renewal ability in neural stem cells, but surprisingly, such deficiency has no measurable effect on cytoskeletal integrity [50]. Moon et al. found that Nestin is expressed in Müller cells (glial cells of the retina) following acute injury, such as in experimentally induced glaucoma and pharmaceutically induced retinal degeneration. Upon inducing retinal degeneration using N-methyl-N-nitrosourea (MNU), cellular localization and temporal patterns were studied. Nestin expression on days 3, 5, 7 and 21 following MNU treatment was analyzed by Western blotting (Figure 5). The Nestin expression levels sharply increased following MNU treatment before returning to baseline, suggesting that this protein could be used as an immediate marker of acute retinal injury [51].
Figure 5.
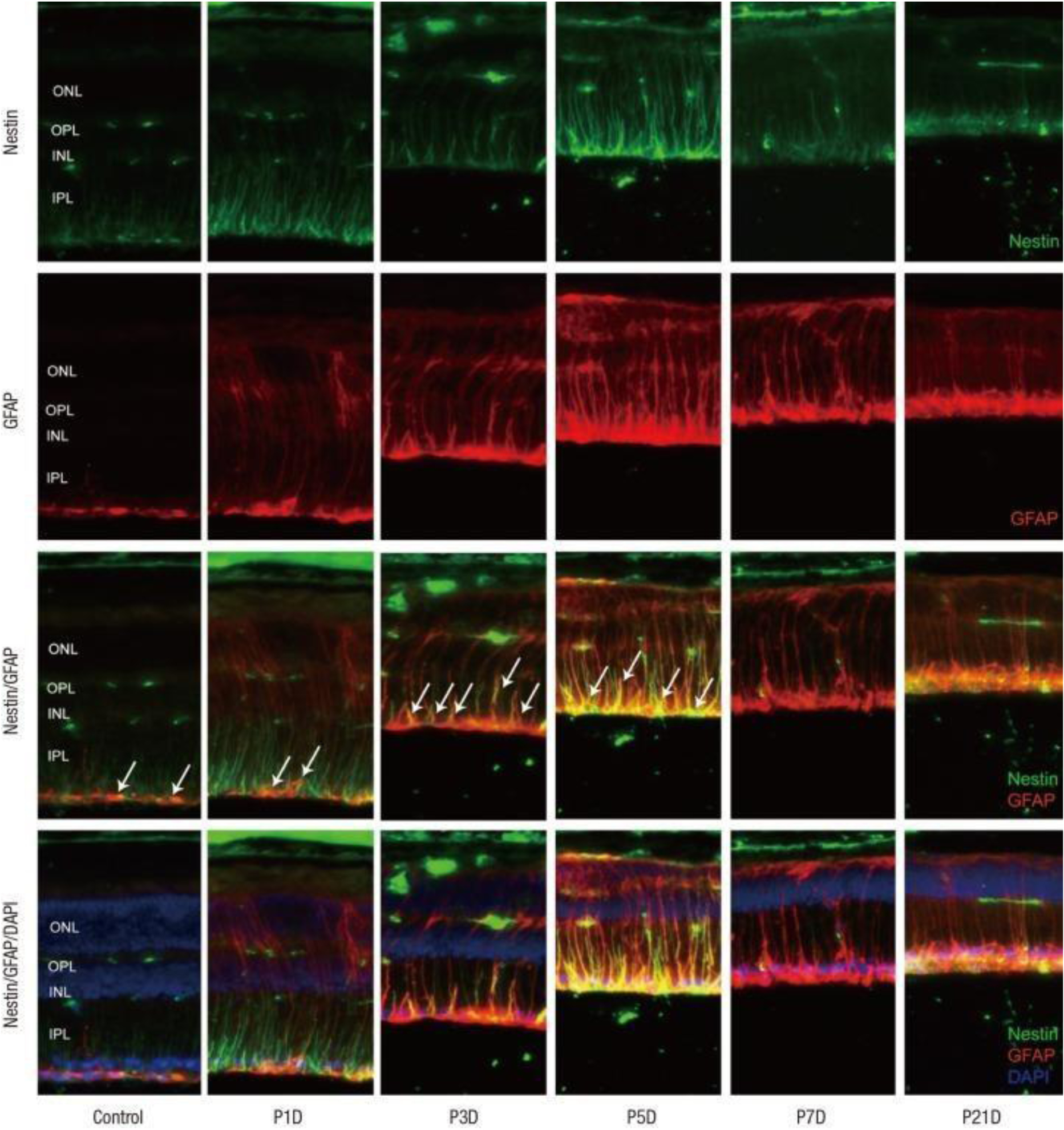
Images showing Nestin, GFAP, and DAPI staining as green, red, and blue fluorescence, respectively. Following MNU-induced retinal degeneration, marker expression was assessed on days 1, 3, 5, 7, and 21 by Western blotting. Nestin expression in Müller cells progressively increased for 5 days and then decreased thereafter. Image labels: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer. Reproduced with permission from [51].
Nestin-GFP models have been used to analyze neuronal and glial markers in various parts of the gastrointestinal tract of newborn and adult mice under homeostatic conditions. Grundmann et al. found that the myenteric plexus houses a specific Nestin-GFP–positive neuron population with both marker expression and neuronal morphology (nNGFP). It was concluded that Nestin-GFP can serve as a marker of neuronal plasticity in enteric neurons [52].
Nestin-GFP models have also been used in vivo to follow mouse mammary tumor (MMT) cells and the subsequent nuclear-cytoplasmic deformation and partition during cancer cell death following immune rejection. Nestin is found beyond the nervous system in tumors, and the protein localizes to the nucleus in tumor cells. MMT cells were dual stained with red fluorescent protein (RFP) in the cytoplasm and Nestin-GFP in the nucleus, and at 6 days after their transplantation, GFP-expressing nuclei partitioned from RFP-expressing cytoplasm [53].
Although Nestin may not be a useful marker for neurons of the PNS, it represents a particularly useful marker for tracking early CNS development and tumor progression.
βIII-tubulin
βIII-tubulin is a moderately sensitive and specific nerve marker. Liu et al. synthesized a transgenic βIII-tubulin-YFP mouse reporter and demonstrated that βIII-tubulin is expressed in the developing CNS and PNS from embryonic day 9 onwards, as well as postnatally in structures that exhibit adult neurogenesis, such as the subgranular layer and hilus of the dentate gyrus (Figure 6) [17].
Figure 6.
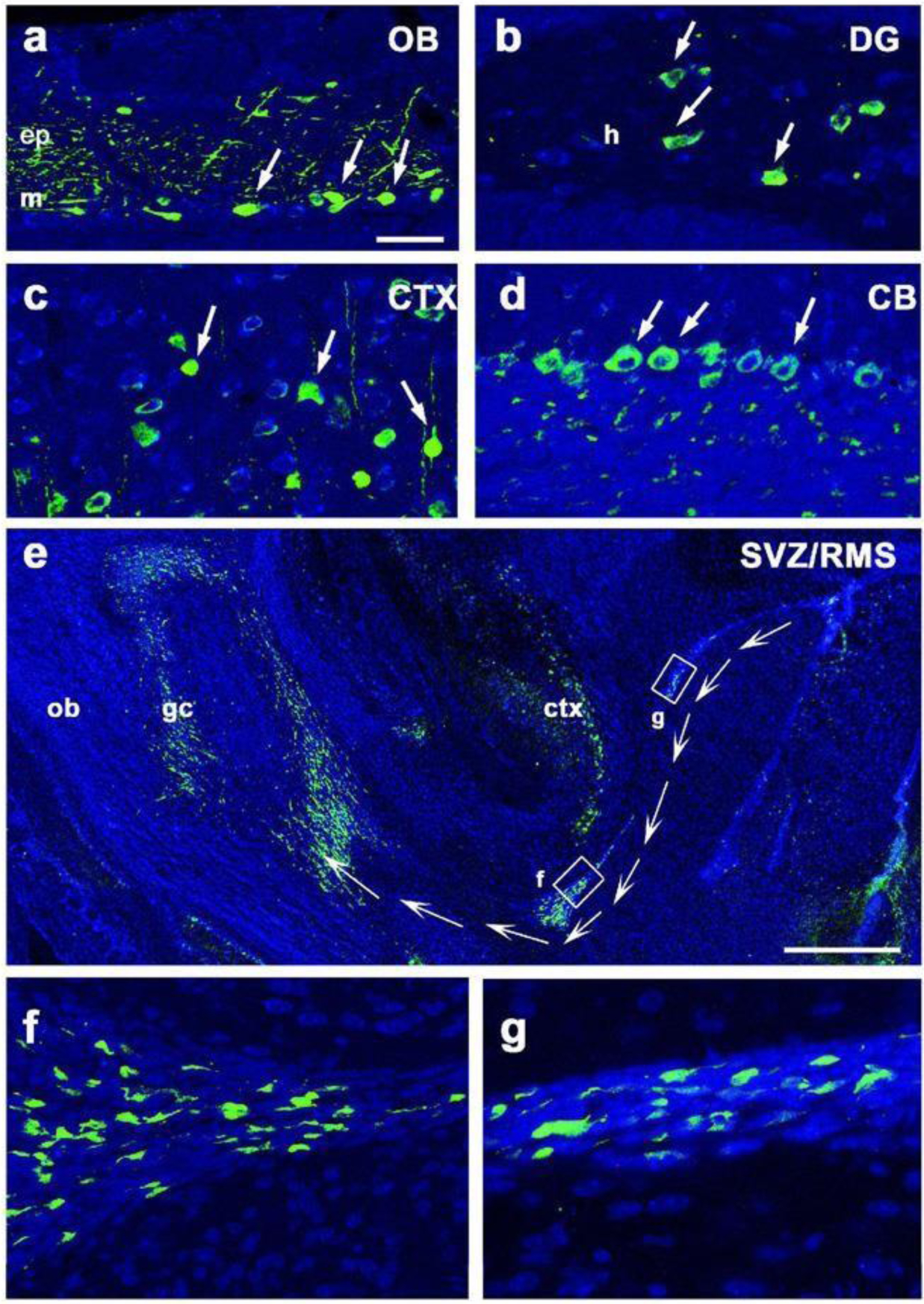
Cellular and dendritic expression of YFP in a transgenic βIII-tubulin-YFP reporter. YFP+ cells were observed in the (A) mitral cell (m) and external plexiform (ep) layers of the olfactory bulb (OB), (B) hilus (h) and surrounding structures of the dentate gyrus (DG), (C) cortex (CTX), (D) cerebellum (CB), and (E-G) rostral migratory stream (RMS). Scale bars, 50 μm. Reproduced with permission from [17].
Another study conducted by Hwang et al. found that βIII-tubulin also serves as a strongly predictive marker for tracking the response to chemotherapy in metastatic gastric cancer, suggesting a clinical use for βIII-tubulin that extends beyond the nervous system [54].
Neurofilament heavy chain (NF-H)
Neurofilament (NF) chains generally consist of three filament proteins: light (L), medium (M), and heavy (H). These proteins play a major role in the maintenance of neuronal caliber. NF-H (Neurofilament-200) is specifically involved in axonal phosphorylation and neurofilament transport processes in mature axons; the two smaller NF proteins (NF-L and NF-M) are not known to have these functions. Furthermore, NF-H is thought to be involved in abnormal neurofilament accumulation during neurodegenerative disease processes; for example, mutations in the C-terminal of NF-H have been discovered in patients with ALS [18]. Within the sensory system, NF-H has classically been used as a marker of larger myelinated sensory neurons [55].
In another study exploring the degeneration and regeneration of corneal nerves, C57BL/6J mice were infected with herpes simplex virus type 1 (HSV-1) [56]. Corneas were harvested at predetermined time points following infection, and their nerves were assessed for expression of different biomarkers such as βIII-tubulin and NF-H by immunohistochemistry [57]. Analysis of corneal whole mounts revealed a regression of sensory fibers (and NF-H+ and βIII-tubulin+ staining) by day 8 post-infection. Moreover, this was followed by a pathological response characterized by abnormal nerve regeneration (with an abnormal presence of NF-H+ nerves) [58].
S100
The S100 group of proteins consists of 24 members and is expressed only in vertebrates. These members are functionally categorized into three subgroups: those with intracellular regulatory effects, those with extracellular regulatory effects, and those with both. Their expression is linked to the refinement of cell-specific gene expression and responses to external stimuli [59].
Certain cancers have been shown to express S100, including schwannoma and melanoma. S100A1 is abundantly expressed in skeletal muscle fibers, cardiomyocytes, and certain neuronal populations. S100A2 expression is downregulated in many cancers, where a loss of nuclear expression is associated with poor prognosis [59]. Extracellular S100A4 and S100B interact with epidermal growth factor and basic fibroblast growth factor, respectively. These interactions enhance the ability of the growth factors to affect the downstream activity of their corresponding receptors. In certain cases, the increased expression of an S100 protein may be an indicator of the cell’s response to a stressor. For example, S100B is not typically expressed in cardiomyocytes but has been observed after an infarct, as it functions to limit the hypertrophic response of cardiomyocytes [59]. By using a S100B-EGFP transgenic reporter, Vives et al. found that S100B is expressed in both CNS and PNS glial cells, as well as certain neuronal populations. However, the diffuse expression and unknown precise physiological function of S100B in nervous tissue reduce its utility in monitoring individual neuronal growth and repair patterns following injury [60].
Substance P (SP)
Substance P (SP) is an 11-amino acid neuropeptide involved in pain transmission, inflammation, and wound healing. SP exerts its actions by interacting with neurokinin receptors, like NK-1. SP is expressedin naïve corneal nerve fibers and mobilizes stem cells for corneal repair [61]. SP can also activate epidermal growth factor receptor (EGFR), mitogen-activated protein kinases (MAPKs), extracellular signal–regulated kinases (ERKs), and phosphoinositide 3-kinase–Akt (PI3K-Akt) signaling pathways. Yang et al. analyzed the mechanism of SP in corneal epithelial wound healing in type 1 diabetic mice. After induction of type 1 diabetes in adult male C57BL/6 mice, topical SP was administered to the eye [62]. The effects of SP on corneal sensitivity and epithelial wound healing were assessed in control, diabetic, and NK-1 receptor antagonist-injected mice. SP application attenuated corneal sensitivity and significantly improved corneal epithelial wound healing in diabetic mice. In mice injected with NK-1 antagonist, SP did not alter the activation of the Akt, EGFR, and Sirt1 signaling cascades, and thus, epithelial wound healing was not observed [19].
Neuron-specific enolase (NSE)
Neuron-specific enolase (NSE) is an isoenzyme of the glycolytic enzyme enolase that is found in terminally differentiated neurons and neuroendocrine cells. As CNS neurons increase in number, levels of NSE also increase. In adults, NSE is one of the most abundant brain-specific proteins [20]. The utility of NSE in imaging mouse neurons when paired to a fluorescent marker is relatively unknown. However, one study that utilized a rat gamma protein kinase C-GFP reporter (which was under the control of an NSE promoter) confirmed that GFP expression was present in the majority of neurons and at significantly higher levels in pups than in adult rats.
Piezo2
Piezo channels represent a novel class of mechanically sensitive channels. A study on Piezo2 expression in guinea pig corneal afferent neurons found that approximately 26% of trigeminal ganglion neurons (Figure 7) and 30% of corneal afferent neurons express Piezo2. These neurons are neurochemically distinct from corneal polymodal nociceptors or cold-sensing neurons, suggesting that Piezo2 may solely contribute to the transduction of noxious mechanical stimuli. Therefore, Piezo2 could be a useful marker for analyzing the effects of physical injury and trauma in the cornea [63].
Figure 7.
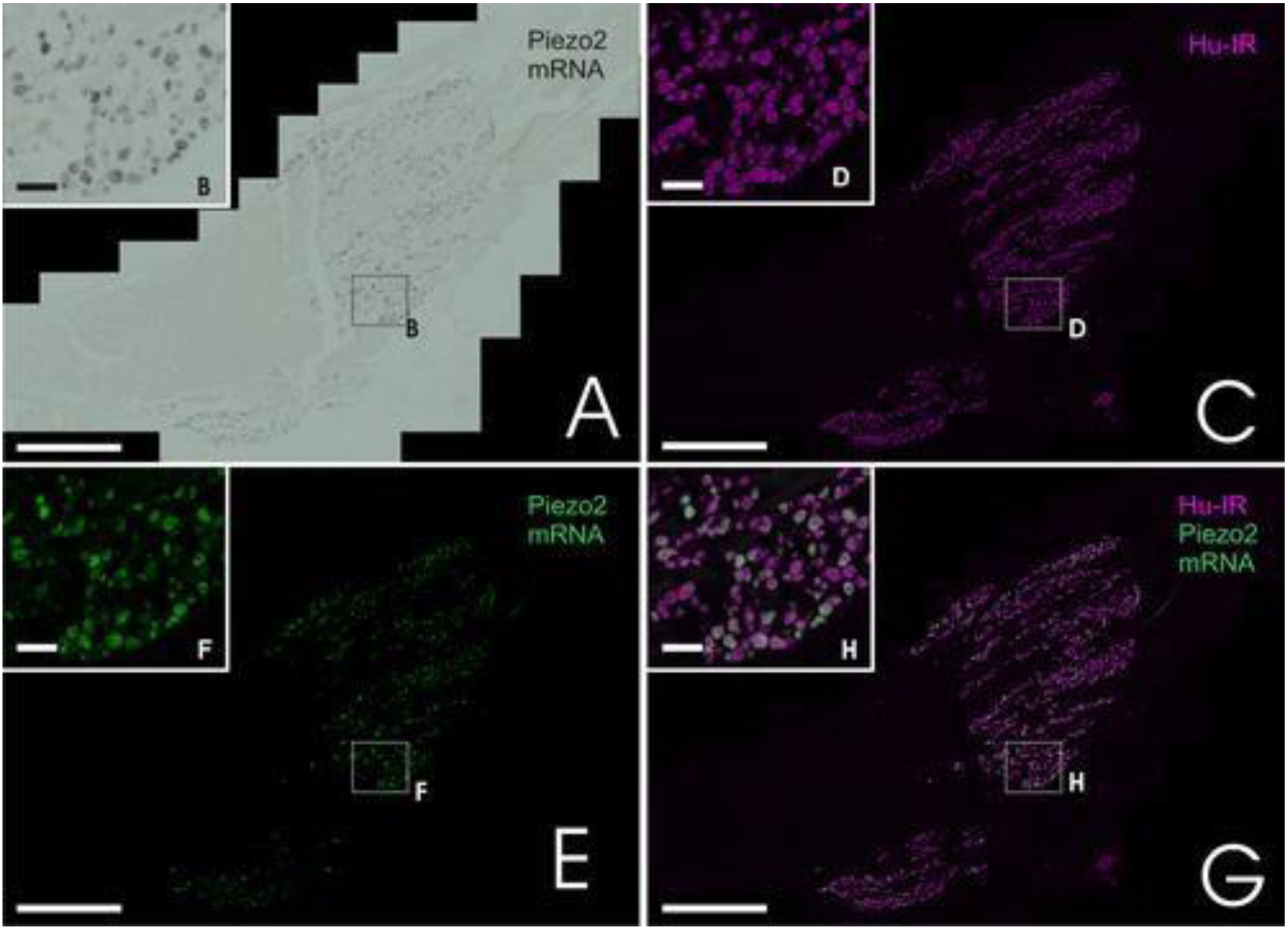
Piezo2 expression in trigeminal ganglion neurons of guinea pigs. Approximately one-quarter of neurons were found to express Piezo2, as indicated by green fluorescence in image (G). Scale bars, 200 μm for main images, 20 μm for insets. Reproduced with permission from [63].
Calcitonin gene-related peptide (CGRP)
Calcitonin gene-related peptide (CGRP) is a well-documented marker of nociceptive DRG neurons. A 2012 study that used CGRPα-GFP reporter mice and confocal microscopy imaging found that CGRPα(+) DRG neurons contribute to responses in a pathway that is distinct from nonpeptidergic and cool temperature nociceptive pathways (Figure 8). A wide range of nociceptive ligands induced mostly small-to medium-diameter CGRPα-GFP+ neurons; these neurons innervate various cutaneous and visceral tissues, such as the submucosal layer of the small intestine. CGRPα-GFP expression was additionally present in a small subset of motor neurons, neurons intrinsic to the dorsal spinal cord, and in regions of the brain (which includes the visual cortex) [21].
Figure 8.
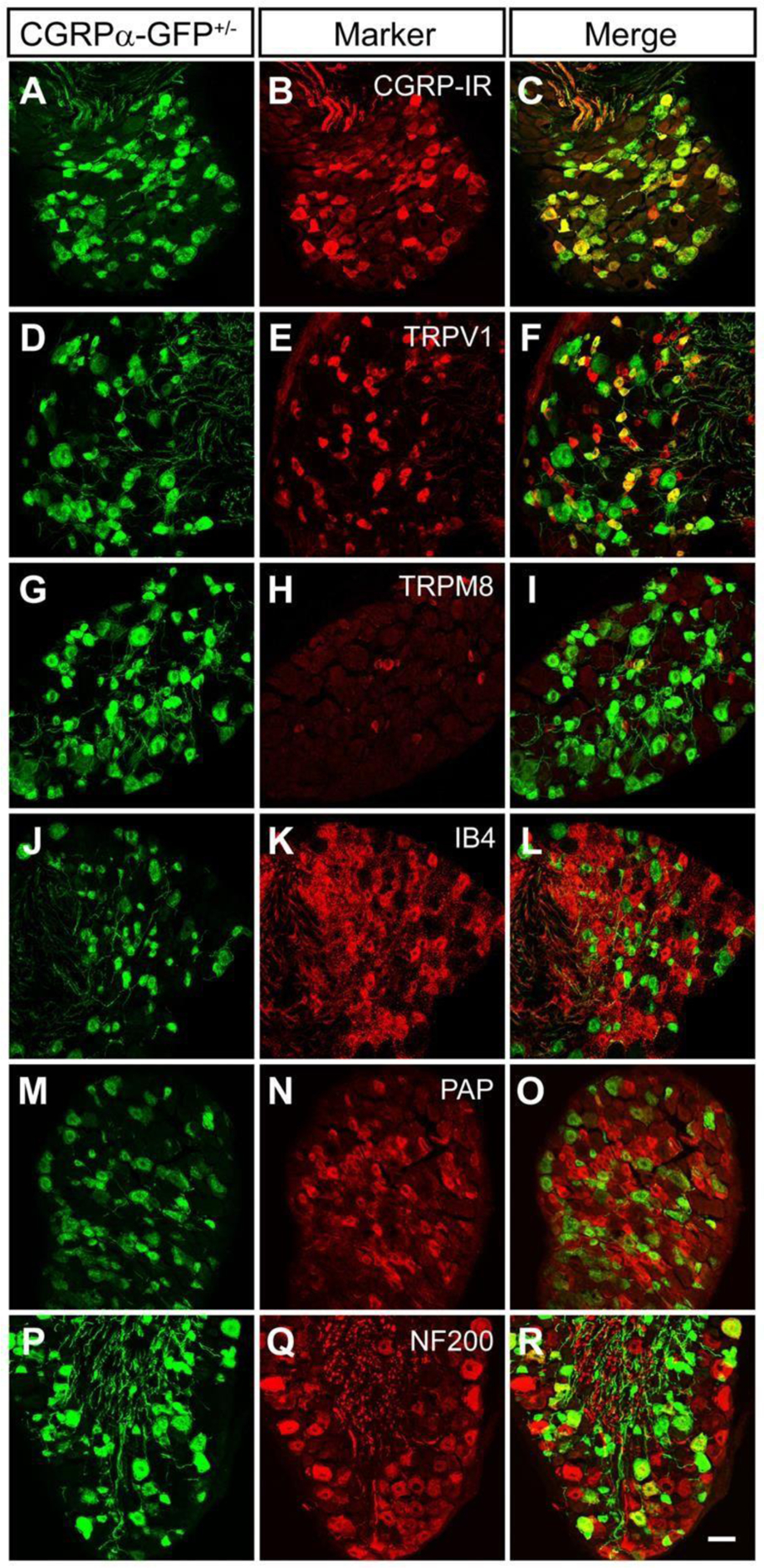
Through confocal imaging of CGRPα-GFP reporter mice, sections of DRG from L4-L6 were stained with GFP-targeting antibodies to view CGRPα expression. CGRPα typically colocalized with peptidergic nociceptive neuronal markers. Scale bar, 50 μm. Reproduced with permission from [21].
Transient Receptor Potential Ankyrin 1 (TRPA1)
Transient Receptor Potential Ankyrin 1 (TRPA1) is a member of the structurally related TRP family, which includes seven channel groups: TRPA (ankyrin), TRPC (canonical), TRPN (no mechanoreceptor potential C), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPV (vanilloid). Rec ent research on TRPV1 and TRPA1 demonstrated a correlation between these two channels. A study conducted by Fernandes et al. showed that 97% of TRPA1-expressing sensory neurons concomitantly express TRPV1. On the other hand, 30% of TRPV1-expressing neurons also express TRPA1. TRPA1 was shown to play an important role in pain and neurogenic inflammation via sensory nerve activation, aiding in the integration and processing of noxious stimuli [64] . As such, there may be a role for TRPA1 in further understanding the pathology of disease states where sensation to noxious stimuli is attenuated or diminished.
Transient Receptor Potential Vanilloid type 1 (TRPV1)
Transient Receptor Potential Vanilloid type 1 (TRPV1) is a non-specific cation channel that reacts to noxious stimuli, making it a mediator of inflammation. The expression of TRPV1 in the nervous system has become a recent topic of discussion. Some studies have reported that TRPV1 expression is limited to extrinsic afferent fibers, while others have argued for a role of TRPV1 in intrinsic afferent fibers. In a study by Buckinx et al., the distribution pattern of TRPV1 expression was shown to be dependent on the type of antibody used in immunohistochemical staining. This study was carried out using two antibodies directed against different epitopes of TRPV1. One primarily stained neuronal fibers, while the other stained perikarya of enteric neurons [65]. This suggests that different modulated forms of TRPV1 exist, and that each displays a unique imaging distribution pattern based on the specific antibody used.
Cavanaugh et al. used two separate lines of TRPV1 reporter mice by crossing TRPV1-Cre mice with multiple Cre-dependent reporter lines to investigate primary afferent expression of TRPV1. They found that TRPV1 is transiently expressed in peptidergic and nonpeptidergic C-fibers as well as in some myelinated DRG neurons during development (Figure 9). This expression ultimately narrows over time to a specific group of peptidergic sensory neurons. The study also emphasized that TRPV1 is more extensively expressed in primary pain afferents than previously thought [66]. An additional study found that 45% of corneal afferent neurons expressed TRPV1 in a guinea pig model, of which most neurons serve as polymodal nociceptors. This supports the notion that TRPV1 serves as a potential modulator of corneal sensation processes [67].
Figure 9.
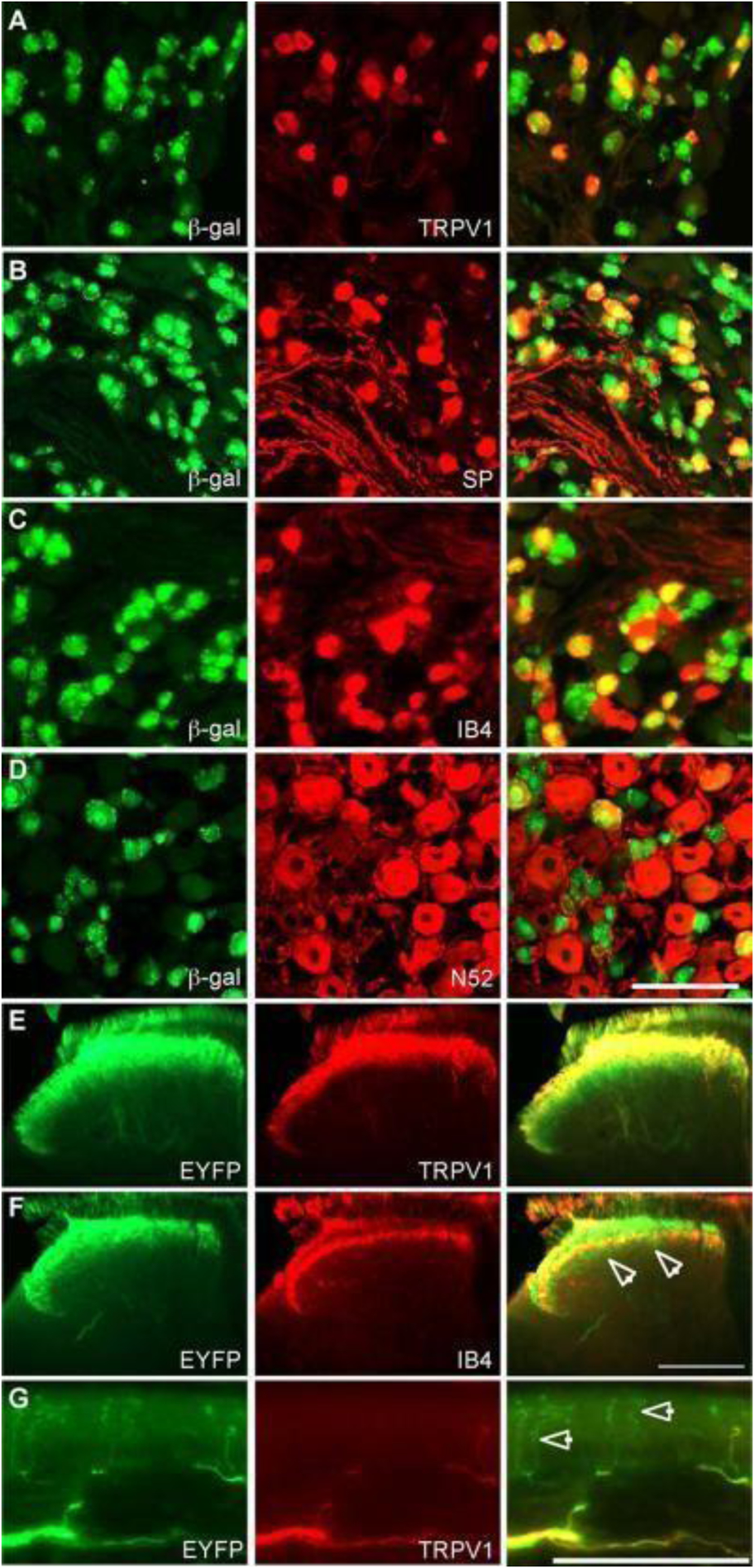
Staining of DRG and spinal cord from TRPV1Cre mice crossed with multiple Cre-dependent reporter lines. Immunohistochemical staining revealed TRPV1 expression in peptidergic and nonpeptidergic C-fibers, as well as myelinated DRG neurons during development. Scale bars, (D) and (G) 100 μm, (F) 200 μm. Reproduced with permission from [66].
Additional neurofluorescent reporter mouse lines
Table 3 and the figures that follow serve to reinforce and further add to the list of transgenic mouse reporter lines that have been previously used in the context of in vivo imaging studies.
Table 3.
Previously used transgenic mouse reporters
| Mouse Reporter Line | Other Associated References |
|---|---|
| Thy1-YFP | [68–84] |
| Thy1-EGFP | [85–87] |
| Thy1-CFP | [88–111] |
| Thy1-RFP | [112] |
| Nestin-GFP | [113–202] |
| ChAT-ChR2-EYFP BAC | [203–205] |
| UCHL1-EGFP | [206, 207] |
| ChATBAC-EGFP | [208, 209] |
| Mapttm1(EGFP)kit | [210–212] |
| CNP-EGFP | [213–219] |
| Peripherin-EGFP (hPRPH1-G) | [220] |
| Hoxb4-ENE-GFP-cre | [221] |
| Math1-GFP | [222] |
| CNPase-GFP | [105, 223] |
| NGF-EGFP | [224–226] |
| SOX2-EGFP | [227–240] |
| Drd1a- & Drd2-EGFP, Drd1a-tdTomato | [241–244] |
| GABASnFR | [245, 246] |
Discussion
In the last decade alone, there has been rapid development in tissue biology research, which can be partially attributed to the advent of transgenic fluorescent reporter mice. Specifically, an extensive literature search demonstrates a handful of useful neurofluorescent reporters now available for nerve assay-related studies. Each mouse reporter described in this review has distinctive features that make it applicable to specific neuroscience investigations. Collectively, we can appreciate the advantages that fluorescent mice provide for the in vivo study of both sensory and motor nerves in the CNS and PNS.
These mice have demonstrated stable and robust fluorescence along their axons, often extending to their terminals.[248] With these advantages, neurofluorescent reporter mice have been used extensively in brain imaging, especially for neurofluorescence using the promoter region of Thy1. [27, 68, 71, 74–76, 80, 83, 86, 87, 93, 112, 249–252] Some transgenic neurofluorescent reporters can additionally express multiple colored fluorescent proteins in different subsets of neurons via the Brainbow system, which uniquely labels neurons with distinct colors using Cre/lox recombination to create a random expression between multiple fluorescent proteins. Brainbow elevates the utilization of neurofluorescent reporter mice for strategies such as for neuronal mapping, cellular dynamics, lineage tracing, and in-depth genomic and genetic analysis.[253, 254]. Nestin, another robust promoter for neurofluorescent, along with co-expression of GFP, has also been extensively used for investigating the nervous system, specifically in early CNS embryonic development. Nestin expression correlates with the undifferentiated state of neural progenitor cells, and its downregulation correlates with the differentiation of these cells [27, 49, 50, 52, 131, 255–258]. To further enhance the capability for deep tissue imaging and to preserve the quality and fluorescence of the specimens, several techniques of tissue preparation have been introduced, such as using dibenzyl ether clearing medium and tetrahydrofuran dehydration medium or utilizing the CLARITY or uDISCO clearing protocol.[259–262]
The ability to combine fluorescence has also permitted discoveries relating to the physiological competition and elimination of neonatal synapses that occurs in neuromuscular junctions [263]. Recently, transgenic reporter mice have been used to assess nerve regeneration as well as the performance of bioengineered nerve guidance conduits [264]. Fluorescent reporters optimize the time and cost associated with in vivo imaging, and ultimately, serve as a very useful tool in the detailed study of nerve physiology and pathophysiology.
Given that research using transgenic mice is becoming increasingly popular, novel reporter subtypes beyond those discussed in this review are continuously being generated. Transgenic reporters have been valuable in studies of nervous system development [265], physiology, and poorly understood pathologies, such as neurodegenerative diseases like Alzheimer’s [266] and prion disease [267], as well as ethanol-induced neurodegeneration during fetal development [268]. This approach has also shown promise in ophthalmology research that extends beyond the cornea; one recent study successfully implemented spatiotemporal mapping to quantified patterns of photoreceptor degeneration in retinitis pigmentosa [269], while another explored potential neuroprotective features of mitochondrial uncoupling protein 2 (UCP2) in a glaucoma mouse model [270].
Though transgenic animal models have fundamentally changed modern approaches to understanding nervous system function and disease states, the generation of appropriate visualization models is a challenging and time-consuming process. One of the limitations to this process occurs in the pronuclear promoter transgene assembly injection. For a gene that is expressed in many cell types, the regulatory mechanism for that gene within a specific system is difficult to ascertain from imaging studies alone. Additionally, cis-acting elements that serve as enhancers to promote the specificity of expression could be located too far from the gene’s recorded start site and may not have been fully identified yet [271]. During this process, the expected repetition of specific gene patterns by a relatively short promoter is not guaranteed. To date, only a small number of such promoters have successfully driven cell-specific expression in transgenic mice neurons [272]. Moreover, the integration process of these transgenes is a random process, and thus, their expression can be vastly influenced by uncontrolled activation/inactivation effects. This results in an expression that is ectopic and impertinent to the promoter in question or suppressed in targeted cells.
Transgene synthesis may also fail due to incomplete, erratic, and sometimes variable expression across the targeted cell region of an individual animal. For example, in the Thy1.2 YFP-16 model, it was found that YFP is only expressed in a select portion of non-nociceptive sensory neurons, while a significant portion of large, NF200-positive sensory neurons fail to express YFP [273]. Inherent variability among animal subjects being treated is also a significant limitation. These methods require laborious validation and verification of the expression of the transgenes for each animal. Interpreting data variation within mice groups can thus be a difficult task. To overcome these limitations, it seems necessary to develop transgenic lines of mice that have genetically encoded actuators and indicators that can produce regulated and inducible expressions. Additionally, there are conflicting data regarding the biological inertness of fluorescent proteins in vivo. Feng et al. claimed that repeated imaging is neither toxic nor phototoxic [32]. However, Comley et al. claimed that neuronal cells expressing YFP exhibit cell stress at the RNA and protein levels, leading to diverse and unpredictable effects on neurodegeneration pathways [45]. Furthermore, it is difficult to predict the variance of transgene expression due to the effects of genomic integration sites and transgene copy numbers, as mice used to exhibit a high expression level of transgenes are usually infertile and not viable.
The purpose of this review was to provide an in-depth exploration and discussion of the major transgenic fluorescent reporters used in nerve tissue visualization, including important considerations of their limitations and local expressions. For instance, Nestin has been used in studies of neural progenitor cell development, while S100 expression has been used to assess neoplastic growth and longitudinal therapeutic responses. These markers can be further used in studies that aim to uncover the molecular regulators driving nerve development following injury. For example, studies investigating the effects of NGF on corneal nerve regeneration and patterning may utilize a Thy1 transgenic mouse model in order to track the changes in nerve structure over time. Alternatively, investigations of the interactions of nerve patterning with vasculature (or other structures) following injury could bolster our understanding of how nerve regeneration may involve signaling pathways that extend beyond the nervous system. Future studies may also directly compare the relative success of IVCM versus transgenic mouse reporters in studies of neuronal regeneration and repair.
The application of transgenic mouse reporters extends beyond studies of just the nervous system. For example, one recent study used a type 1 collagen promoter-driven GFP reporter to study osteoblastic activity during skeletal regeneration [274], while another used Rip1Tag2 transgenic mice to monitor the progression of pancreatic neuroendocrine tumors [275]. The application of Flt1-dsRed and Prox1-GFP transgenic reporters in our lab at the University of Illinois have further enabled visualization of blood and lymphatic vessels, respectively. Such reporters have been important when analyzing patterns of neovascularization following corneal injury and how knockout of selected growth factors and their receptors may reduce unwanted vascular proliferation that results from hypoxic disease states. These transgenic reporters have also been bred to generate a Prox1-GFP/Flt1-dsRed (PGFD) dual transgenic reporter that permits simultaneous visualization of both vessel types[276] [277]. Similarly, we have bred Thy1-YFP with Flt1-dsRed mice to establish a dual blood vessel and nerve reporter, useful in imaging neurovasculature in tissues including spinal dorsal root ganglia and the nervous plexus of the gastrointestinal tract. The generation of dual transgenic reporters serves to improve knowledge of the mechanistic interactions shared between multiple organ systems (rather than one system in isolation), and how the pathophysiology of one system may influence and/or be influenced by the other.
Sümbül et al. has introduced a reproducible, objective approach to classify neuronal cell types, by using dendritic arbor distribution and density as well as molecular characteristics of the cells.[278, 279] This algorithm was tested using the retina as the study bed, which is known to contain diverse types of neuronal cells,[280, 281] specifically the retinal ganglion cells (RGCs).[278, 279] It would be very compelling to combine this classification technique with the available neurofluorescent reporter transgenic mice. This could create a more robust and accurate neuronal cell classification system that may further impact the development of therapies for many nervous system-related diseases, such as those in the cornea and retina.[282]
Beyond injury models, transgenic reporter mice enable us to observe the anatomical evolution of neurons and vascular structures that occur because of development or normal aging. This serves to improve knowledge of normal physiologic functioning and isolate age-related structural changes from potentially pathologic ones. Ultimately, the application of this experimental approach can extend across multiple lines of research that take advantage of in vivo tissue imaging techniques. With the emergence of advanced imaging approaches such as those discussed in this paper, a clearer concept of the interrelationship between structure and function, as well as the mechanisms that drive inflammation and healing may pave the way for the development of improved therapeutic interventions for relevant pathologies.
Figure 10.
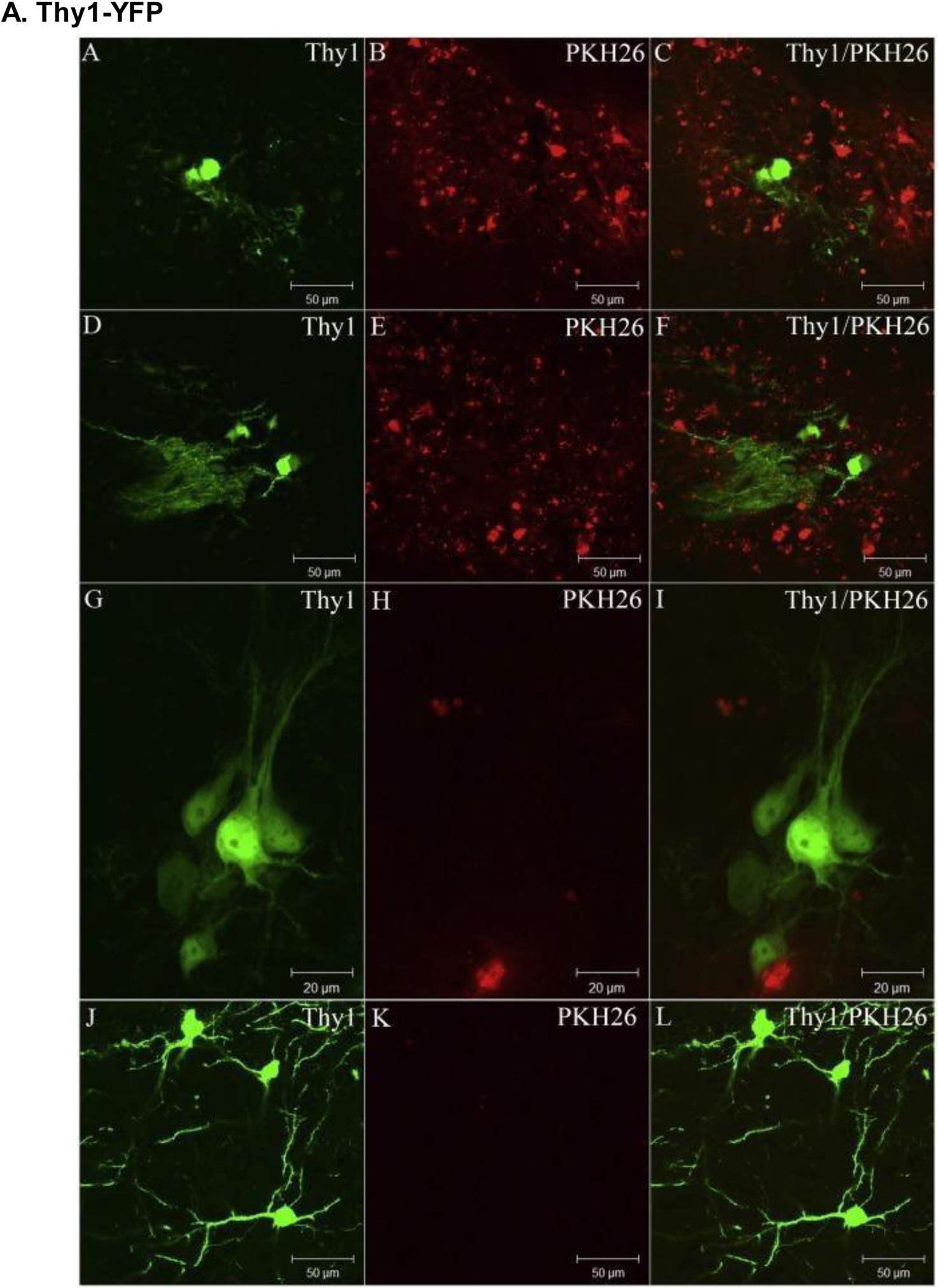
Changes in Thy1-YFP expression in the mouse brain following PKH26-labeled cell transplantation over a 14-week period; (A-C) 2 weeks, (D-F) 4 weeks, (G-I) 8 weeks, and (J-L) 14 weeks. Thy1 expression consistently increased as time progressed, while PKH26 expression mostly regressed within 1 month of transplantation. Reproduced with permission from [27].
Figure 11.
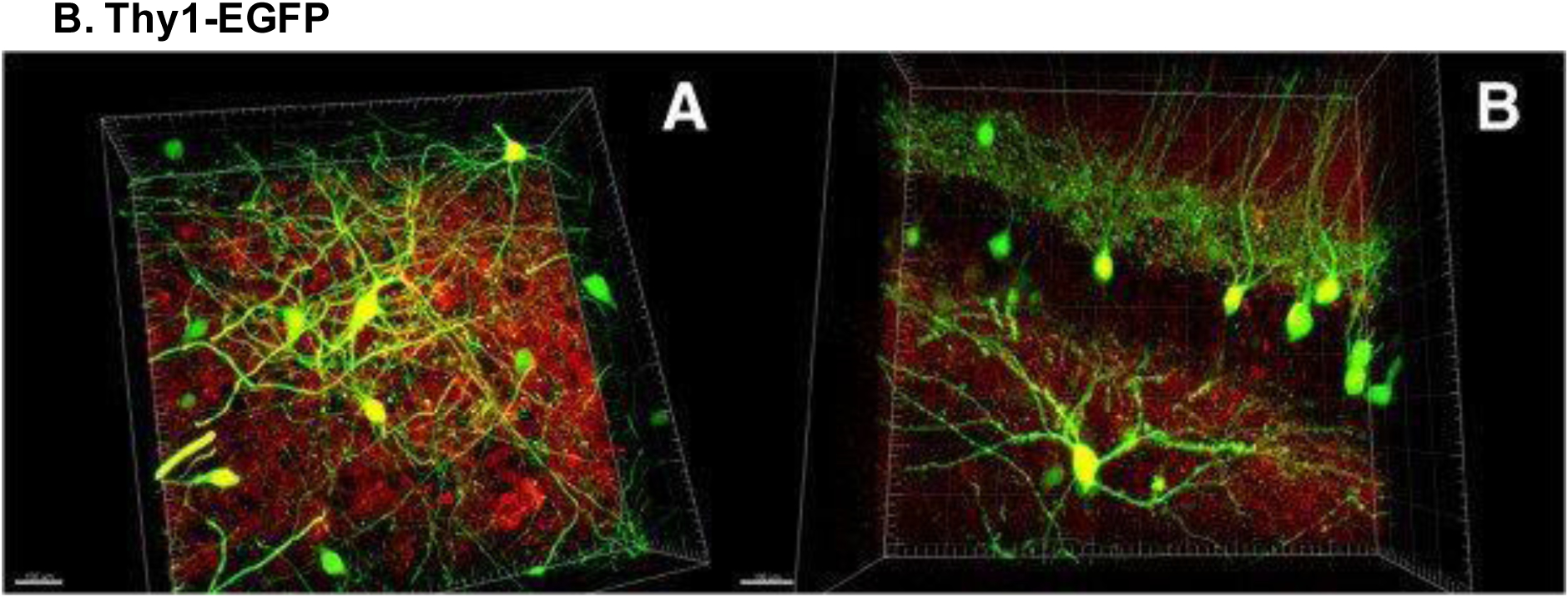
Confocal images displaying Thy1 expression in striatal and hippocampal neurons of a Thy1-EGFP transgenic reporter mouse (GFP+ neurons in green, PSD95+ neurons in red). Scale bars, 100 μm. Reproduced with permission from [87].
Figure 12.
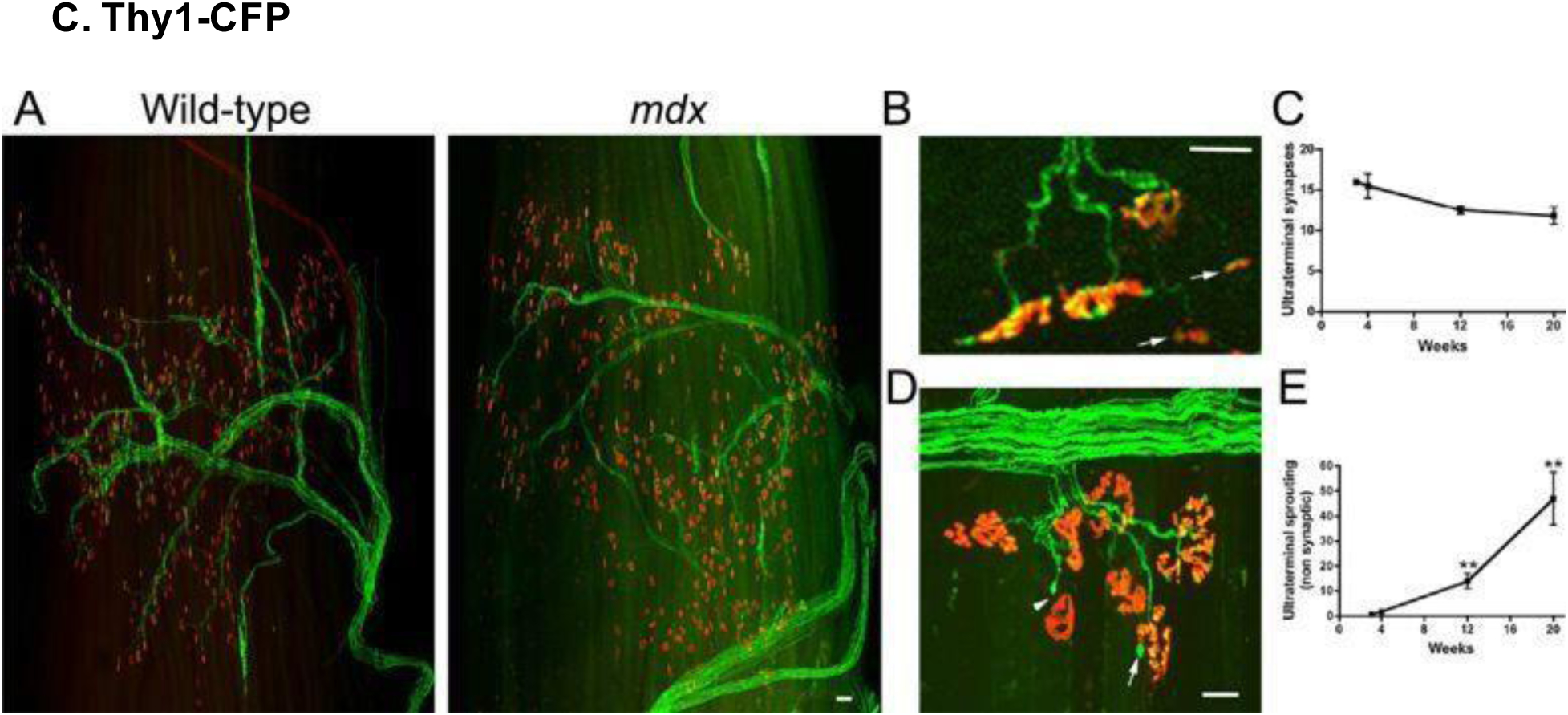
Transgenic cyan fluorescence protein (CFP) expression in neurons of the third extensor digitorum longus (EDL) compartment in wild-type and mdx mice (for neuronal visualization in Duchenne muscular dystrophy mice); mdx muscles demonstrated profound infiltration of cells expressing acetylcholine receptors (AChR). Scale bars, (A) 100 μm, (B) and (D) 30 μm. Reproduced with permission from [110].
Figure 13.
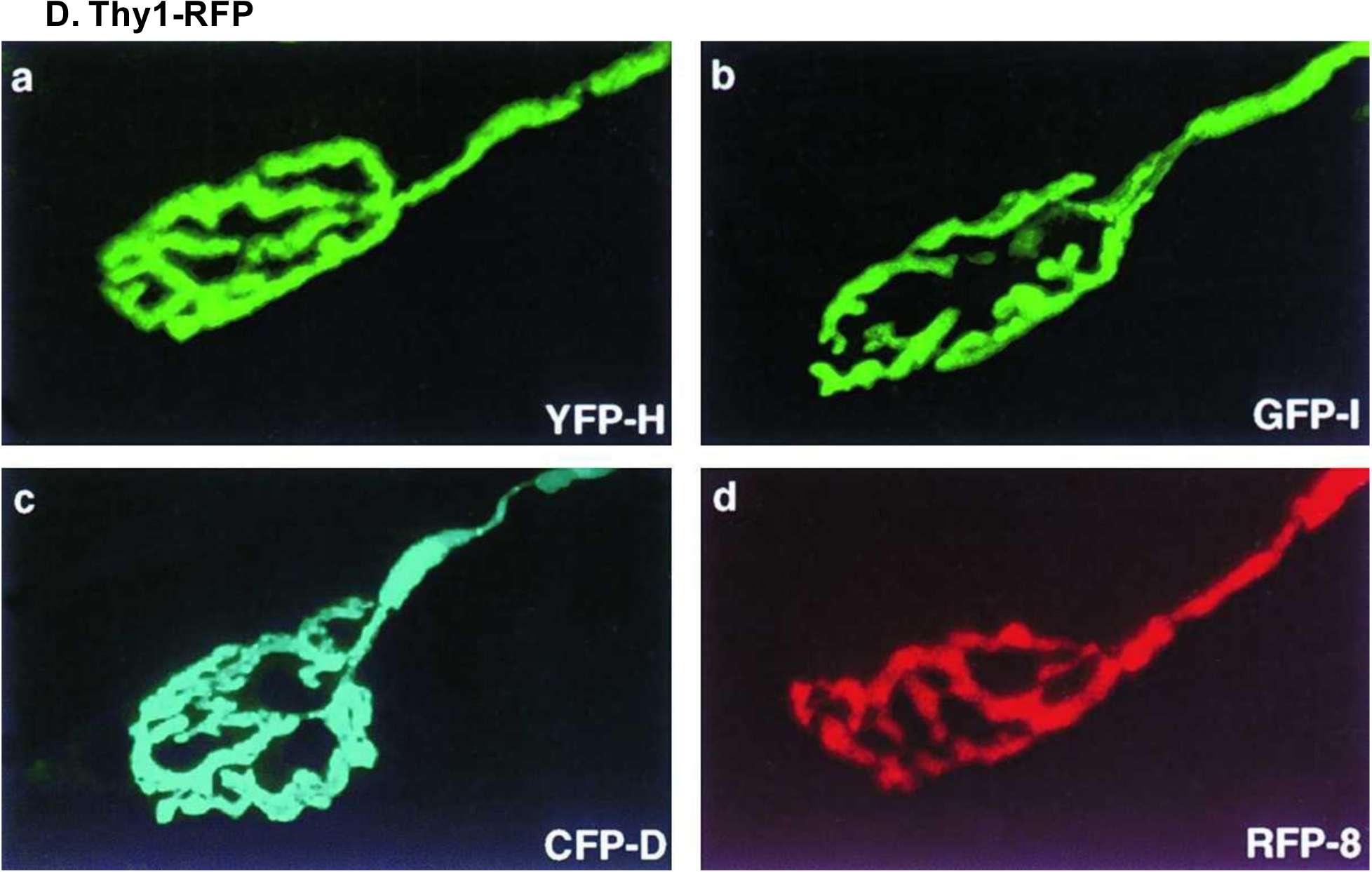
Expression of four fluorescent proteins important for transgenic reporter staining. Muscles were labeled with bungarotoxin, and neuromuscular junctions were imaged in (A) Thy1-YFP line H, (B) Thy1-GFP line H, (C) Thy1-CFP line D, and (D) Thy1-RFP line 8 transgenic mice. Reproduced with permission from [32].
Figure 14.
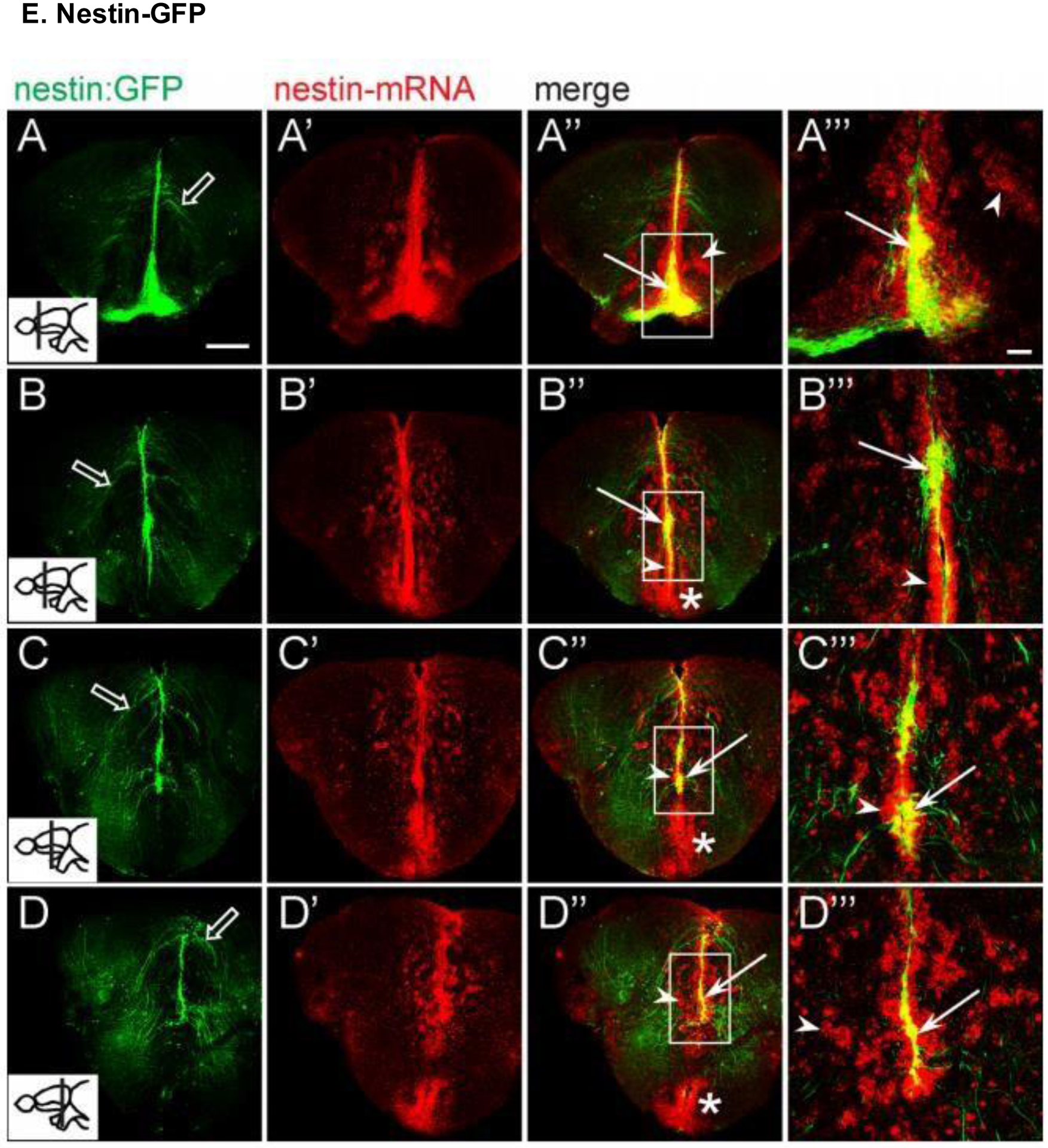
Anterior to posterior expression of Nestin-GFP and endogenous Nestin mRNA in trigeminal neurons of the mouse cerebrum in confocal single optical sections (insets indicate the plane of section for each row). GFP+ cells emanated in projections from the ventricular zone; endogenous Nestin expression was most prominent in the ventricular zone as well as in scattered cells of the brain parenchyma. Scale bars, (A) 100 μm, (A’) 50 μm. Reproduced with permission from [202].
Figure 15.
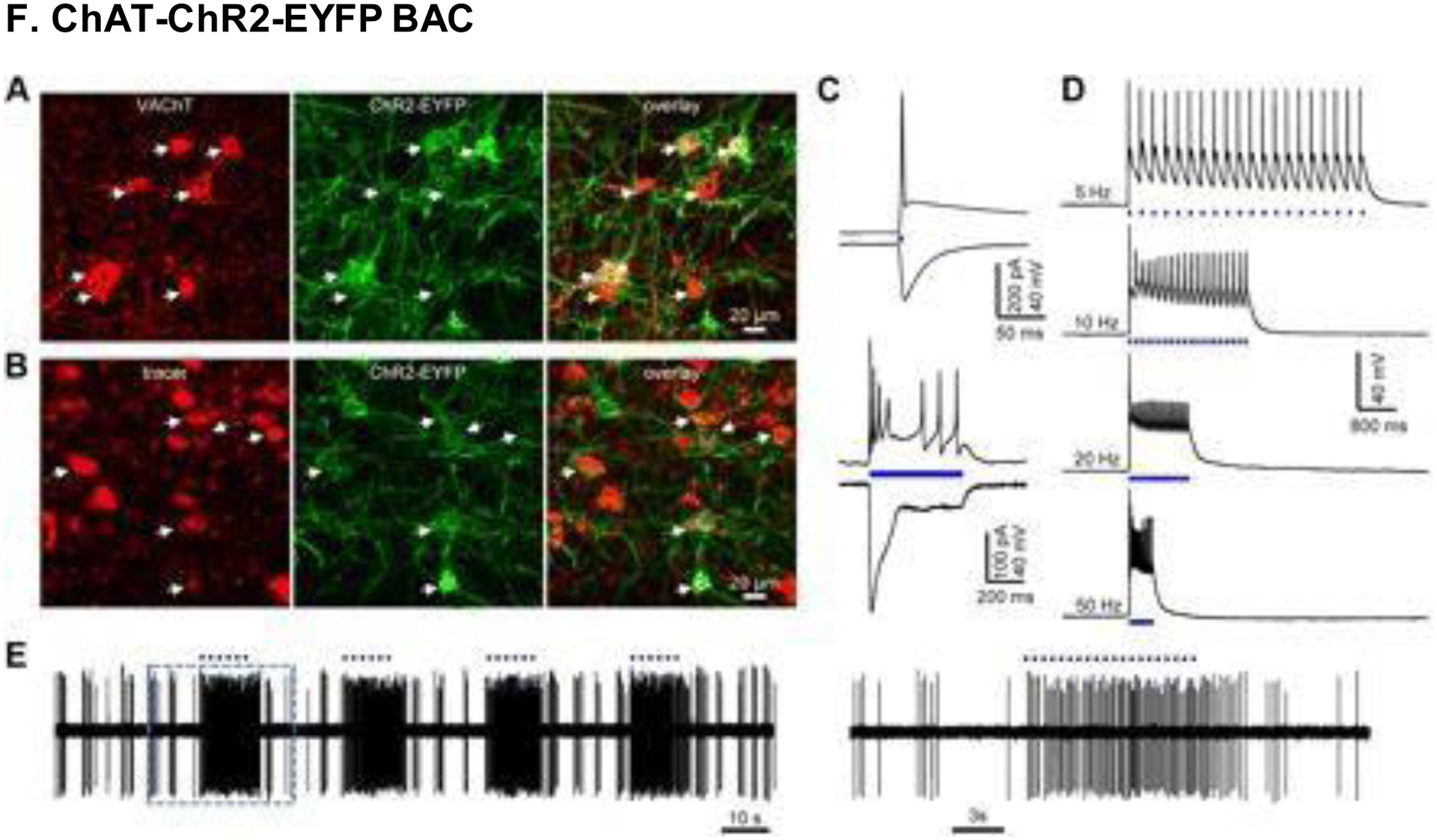
Immunostaining against VAChT (red) revealed the expression of ChAT–ChR2–EYFP+ cells, with an affinity towards cholinergic transgenic mouse neurons. (A) Images taken from the horizontal diagonal band of Broca (HDB). EYFP expression was weak in somata, because ChR2 protein tends to localize to neuronal membranes. (B) Retrograde tracing confirmed that HDB neurons feed into the main olfactory bulb (MOB). Some neurons lacked EYFP expression, since the MOB is only partially innervated by cholinergic neurons. (C-D) Blue light stimulation up to 50 Hz led to activation and firing of ChR2– EYFP+ neurons in the HDB. (E) Light pulses produced vigorous subsequent firing of HDB neurons. Reproduced with permission from [204].
Figure 16.
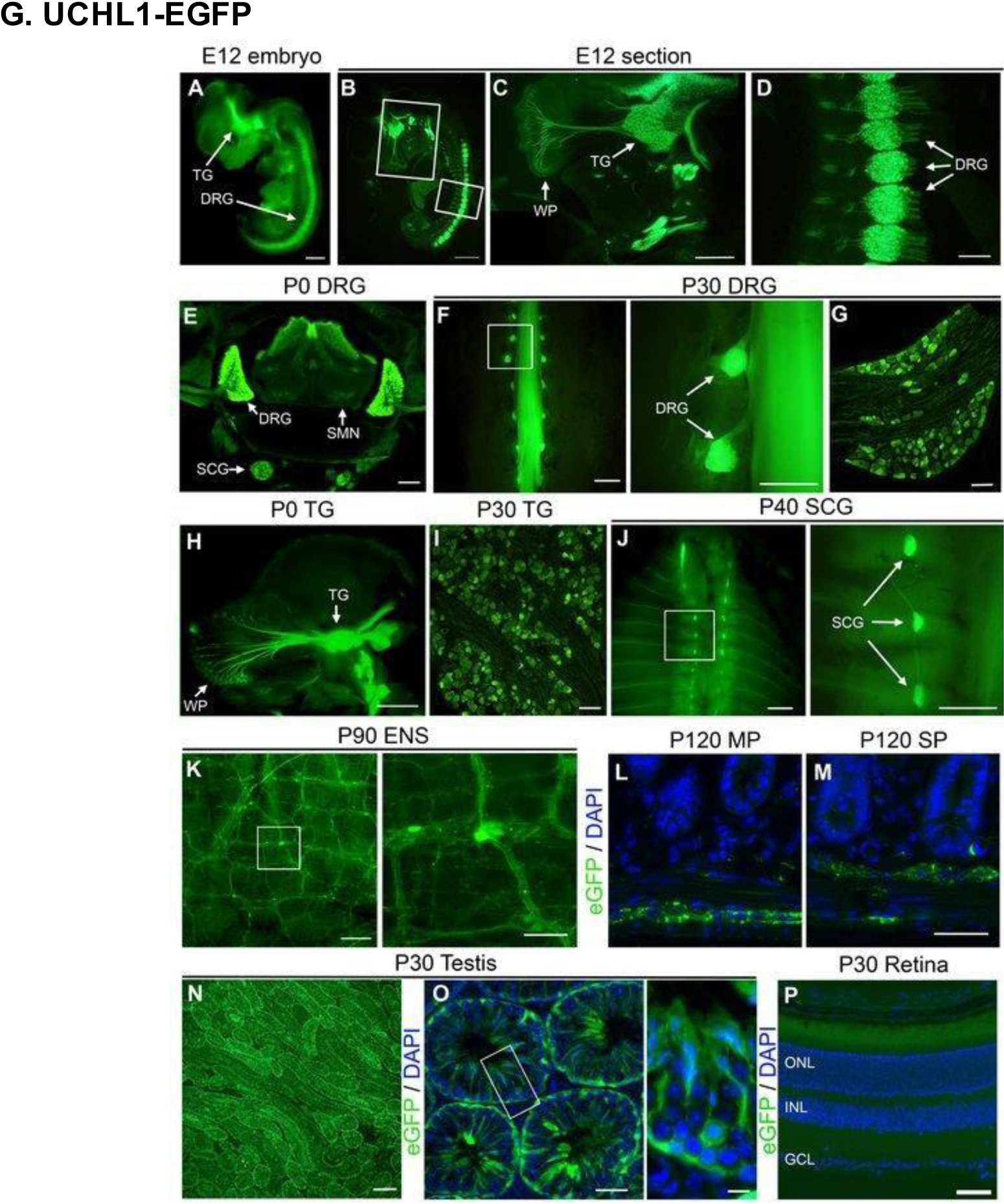
The use of a UCHL1-EGFP transgenic reporter model enabled visualization of peripheral nerves in vivo starting from embryonic day (E) 12 (A). Levels of EGFP expression across various time points and tissues are displayed in this figure. (B-D) TG and DRG neurons at E12. (E) DRG neurons, sympathetic chain ganglia (SCG), and spinal motor neurons (SMN) of the ventral spinal cord on PD0. (F-G) Adult DRG neurons (with zoomed inset). (H) TG neurons on PD0. (I) Adult TG neurons. (J) SCG neurons (with zoomed inset). (K) Enteric nervous system (ENS) neurons (with zoomed inset). (L-M) Myenteric plexus (MP) and submucosal plexus (SP) of the gastrointestinal tract. (N-0) Adult testis neurons. (P) Lack of EGFP expression in the retina. Scale bars, (A, B, F inset and J inset) 1 mm; (C) 500 μm; (D, E, K, N) 200 μm; (F, H, J) 2 mm; (G, I, K inset, L, M, O) 50 μm; (O inset) 10 μm; (P) 100 μm. Reproduced with permission from [207].
Figure 17.
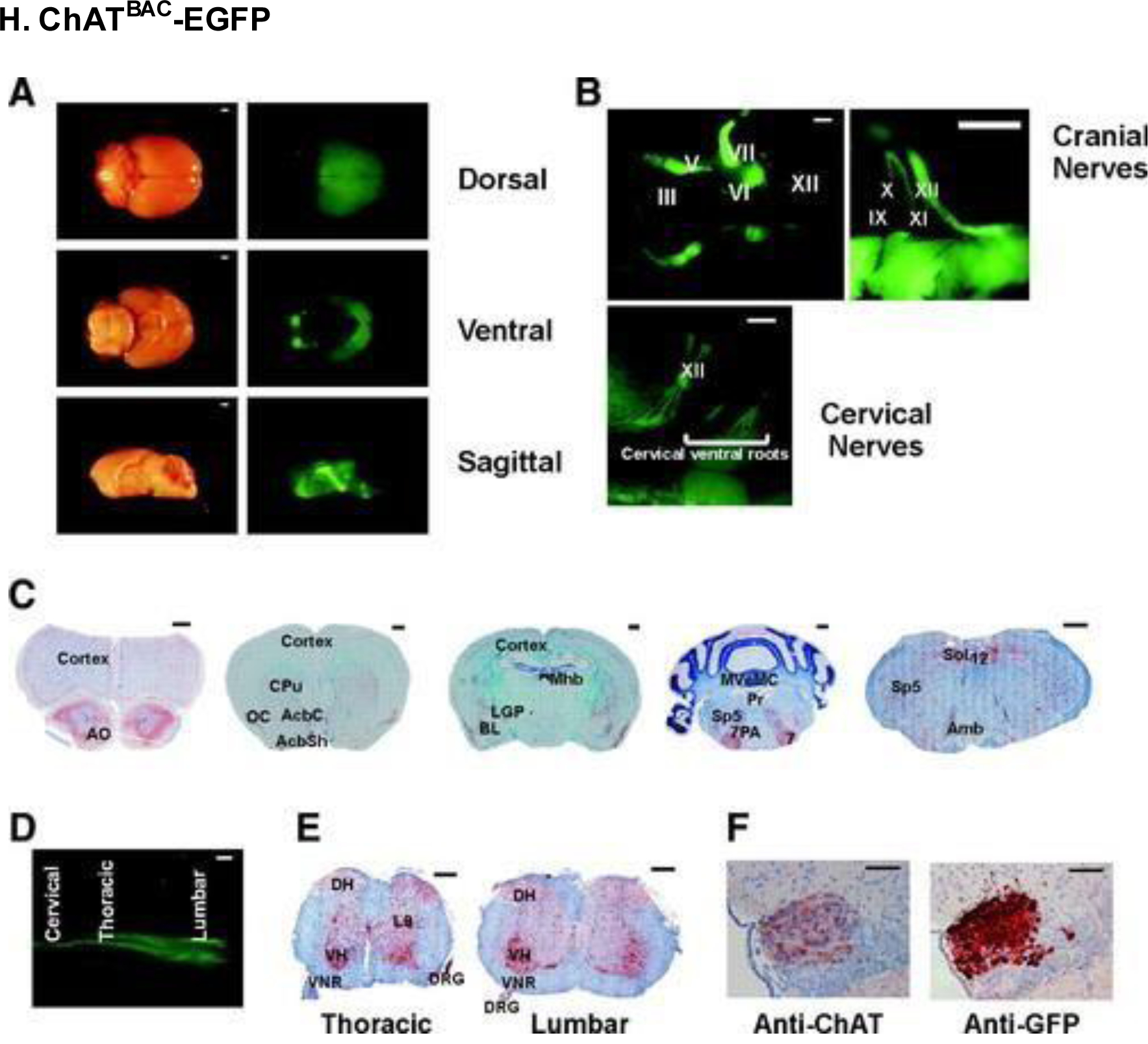
ChATBAC-EGFP mice were used to visualize neurons of the central nervous system. (A) Light (left) and fluorescent (right) ex vivo microscopy images of adult mice. (B) Magnified in vivo images from the brainstem and rostral spinal cord. (C) Immunostaining for anti-EGFP antibody in various cross-sections of the brain. (D) Ventral spinal cord view on PD2. (E) Immunostaining for anti-EGFP antibody in various spinal cord segments. (F) Sections of the medial habenular nucleus of the brain showing expression of anti-ChAT (left) and anti-EGFP (right). Roman numerals III-XII correspond to their respective cranial nerves (e.g., III is oculomotor). Other abbreviations: AO, anterior olfactory nucleus; CPu, caudate putamen; AcbC, accumbens nucleus core; AcbSh, accumbens nucleus shell; OC, olfactory cortex; LGP, lateral globus pallidus; Mnb, medial habenular nucleus; BL, basolateral amygdaloid nucleus; 7, facial nucleus; Sp5, spinal 5 nucleus; MVeMC, medial vestibular nucleus; Pr, prepositus hypoglossal nucleus; 7PA, facial nucleus proximal axons; 12, hypoglossal nucleus; Sol, solitary tract nucleus; Amb, ambiguus nucleus; VH, ventral horn; L9, lamina 9; DH, dorsal horn; VNR, ventral nerve root. Scale bars, (A and B cranial left) 1 mm; (B cranial right) 500 μm; (B cervical nerves) 1 mm; (C) 500 μm; (D) 1 mm; (E) 250 μm; (F) 50 μm. Reproduced with permission from [208].
Figure 18.
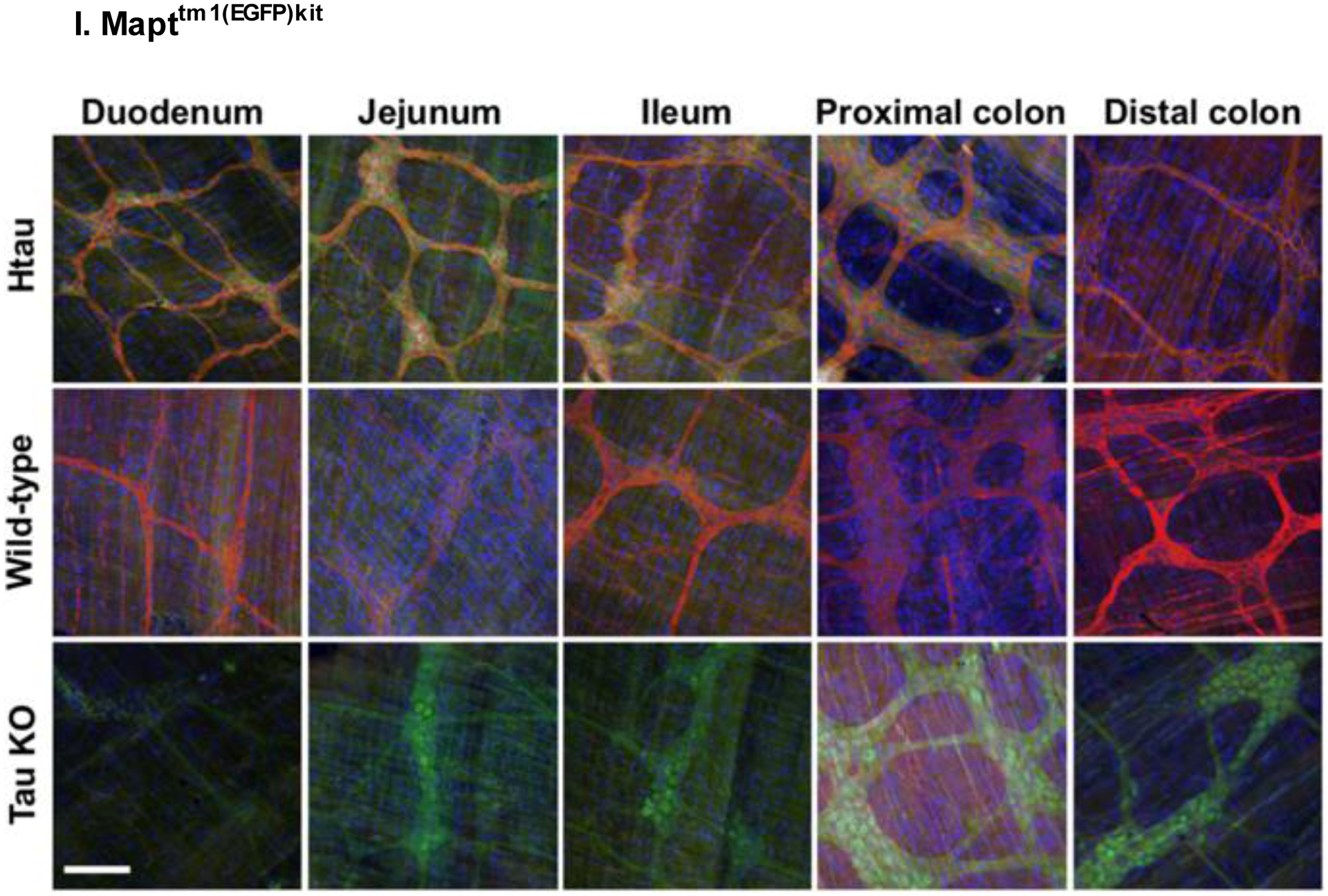
Expression of tau in the myenteric plexus of various segments of the gastrointestinal system of 2-month-old htau (Mapttm1(EGFP)kit), wild-type (WT), and tau knockout (KO) mice. Images are merged showing tau in red, EGFP in green, and nucleic acid labels in blue. Scale bar, 100 μm. Reproduced with permission from [211].
Figure 19.
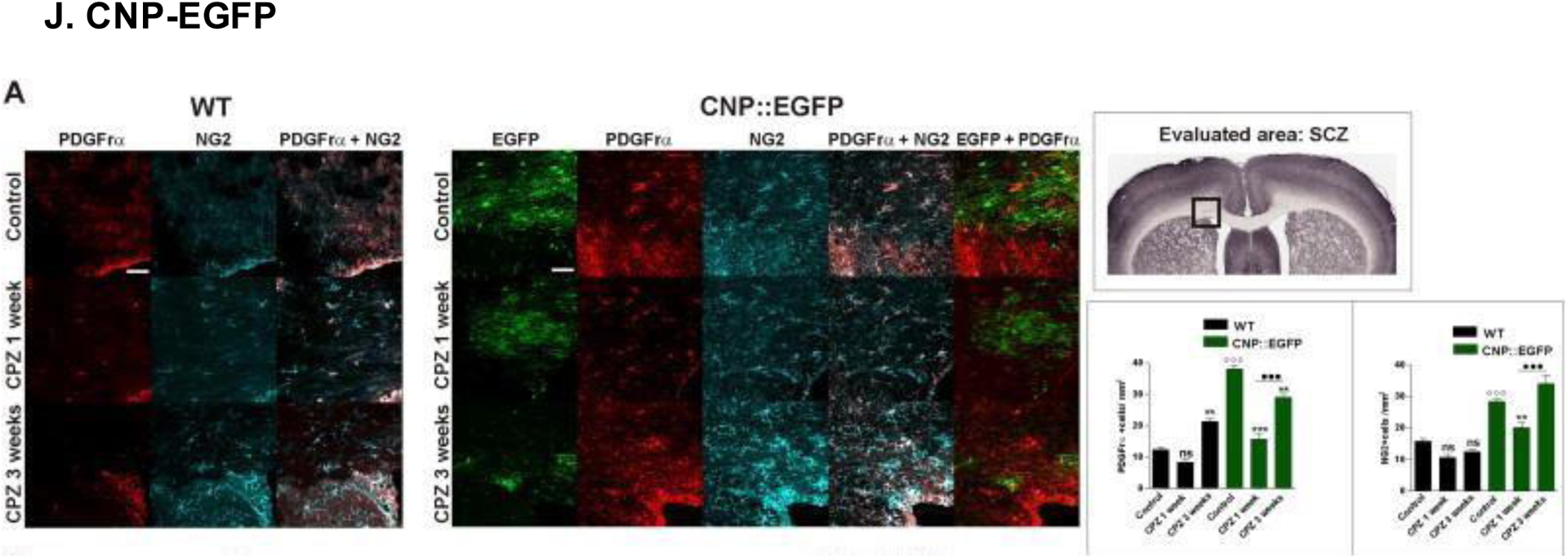
(A) Confocal microscopy images of oligodendrocyte progenitors (OPCs) in CNP-EGFP and WT mice following 1 or 3 weeks of treatment with cuprizone (CPZ). OPC expression was markedly elevated in CNP-EGFP mice compared to WT mice, as confirmed by analysis of variance (ANOVA) and Bonferroni post-hoc statistical tests (ns = non-significant, **p<0.01, ***p<0.001). Scale bars, 50 μm. Reproduced with permission from [213].
Figure 20.
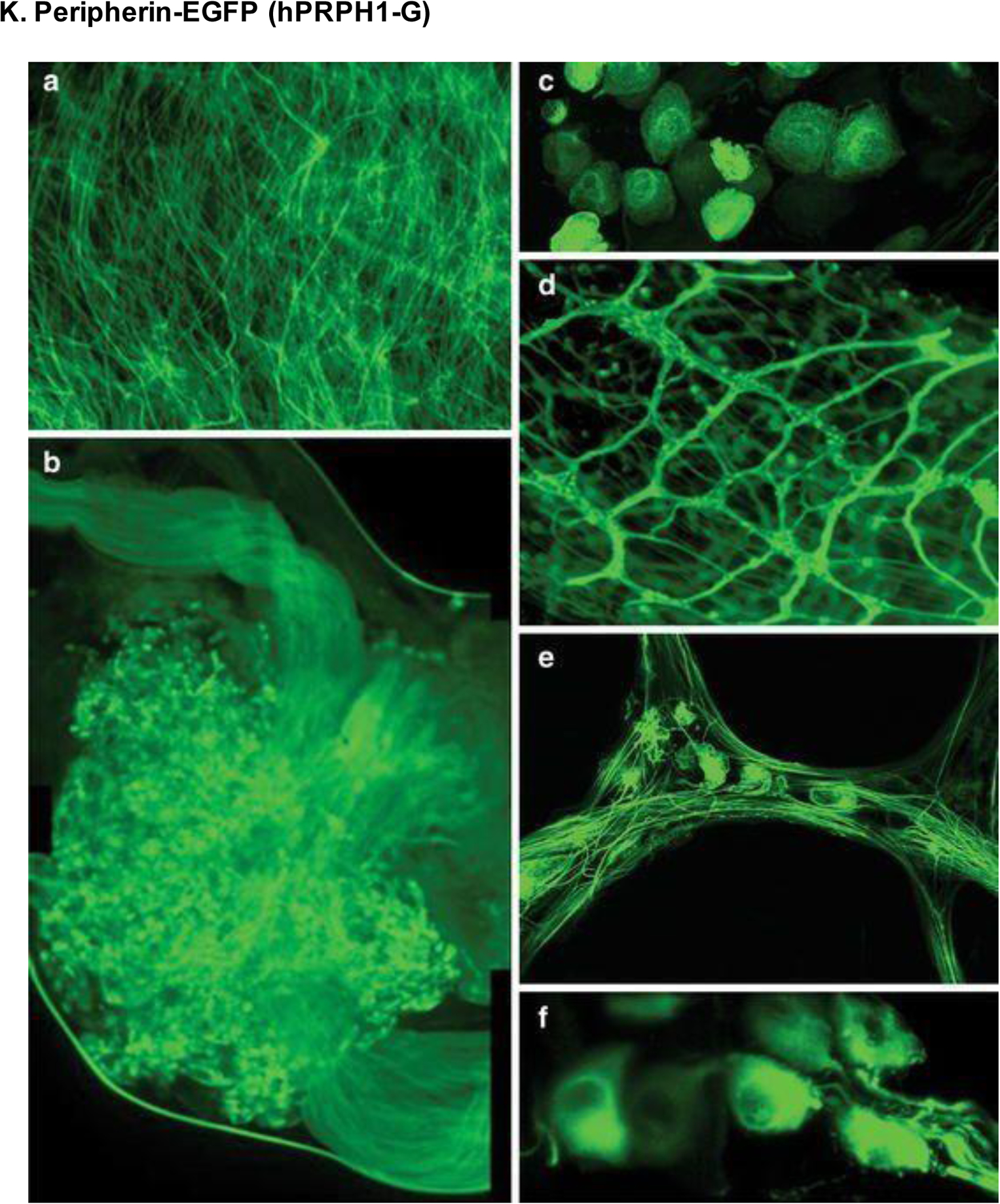
Expression of peripherin-EGFP (hPRPH1-G) in unfixed tissues viewed by multiphoton and confocal microscopy. (A) Cervical spinal cord at 200× magnification. (B) Images combined to show the DRG. (C) DRG sensory neuron cell bodies. (D-E) Small intestine seen by fluorescence (D) and confocal (E) microscopy at 100× and 400× magnification, respectively. (F) Retinal images showing RGC bodies at 1000× magnification. Reproduced with permission from [220].
Figure 21.
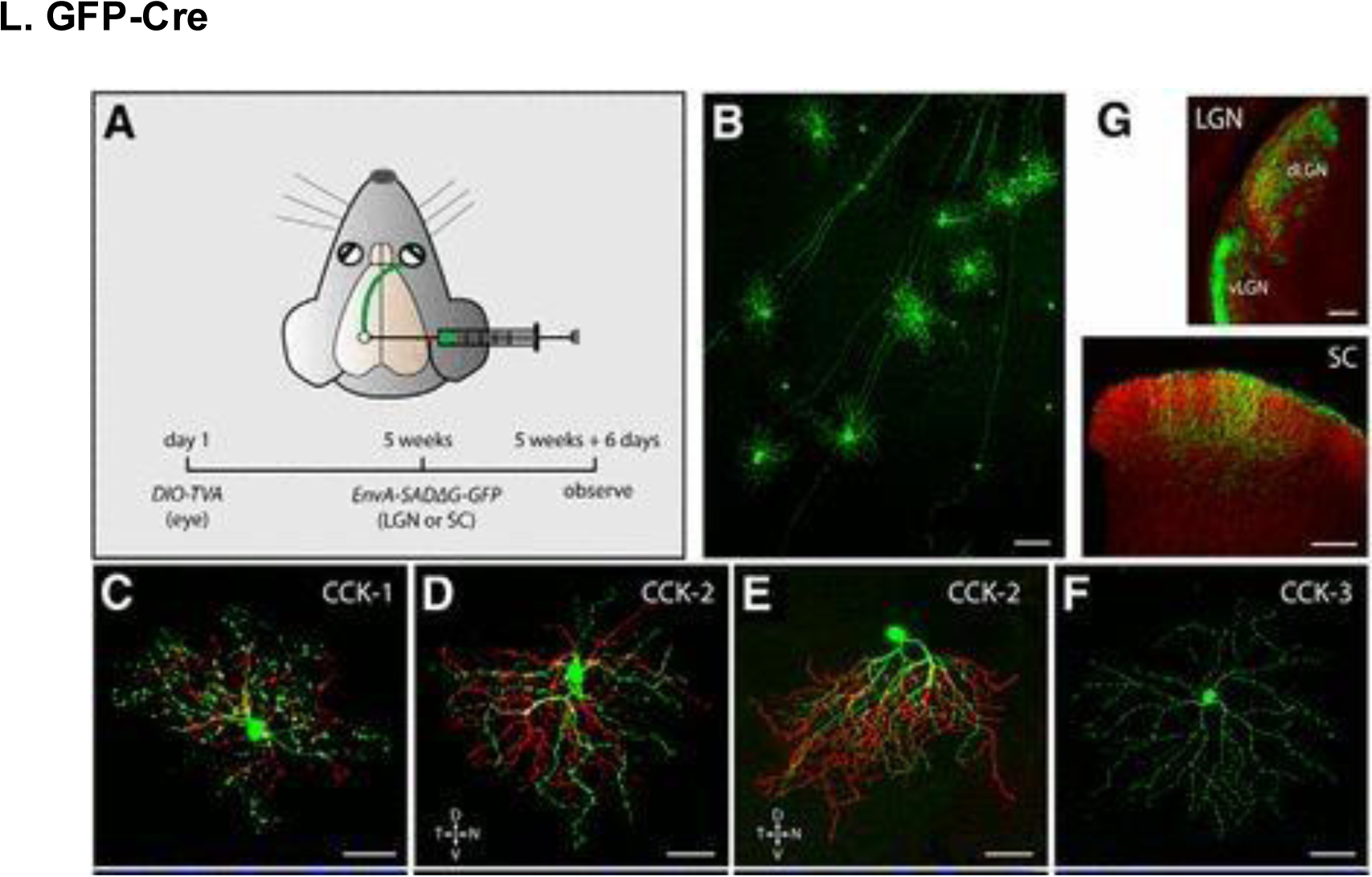
GFP-Cre expression in RGCs. (A) Experimentation strategy for labeling CCK -RGCs at axon terminals. (B) GFP-labeled CCK-RGCs in the retina of CCKires-Cre mice. (C-F) Neuronal morphology of various CCK-RGCs. (G) CCK-RGC axon terminals in the spinal cord, dLGN, and vLGN (red corresponds to CTb-594 labeling of all RGC axons). Scale bars, (B) 100 μm; (C-F) 50 μm; (G) 200 μm. Reproduced with permission from [247].
Figure 22.
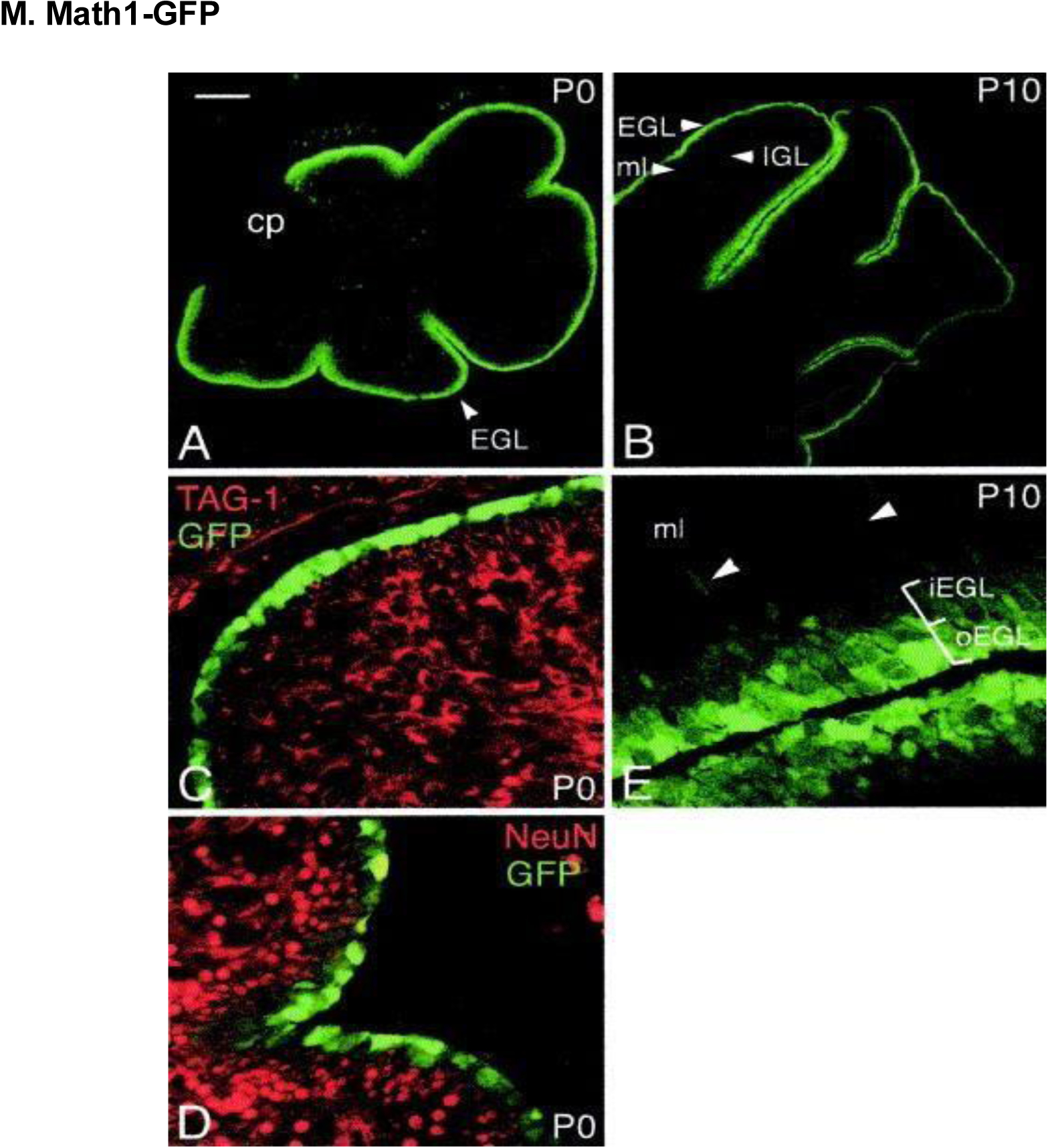
Images of cerebellar granule cells on either P0 (A, C, D) or P10 (B, E). Math1-GFP expression was most prominent in the outer cerebellar EGL, which corresponds to normal expression of Math1. At P0, a lack of GFP expression overlap with markers of differentiating granule cells [TAG-1 and Neu-N in (C) and (D), respectively] was noted. (B) The majority of Math1-GFP–expressing cells were observed in the EGL, with minimal Math1-GFP expression in the IGL at P10. (E) Higher magnification images of the outer/inner EGL, with more intense Math1-GFP expression in the outer EGL; low Math1-GFP expression was also noted in the molecular layer (ml). Scale bars, (A) 150 μm; (B) 300 μm; (C-D) 25 μm; (E) 30 μm. Reproduced with permission from [222].
Figure 23.
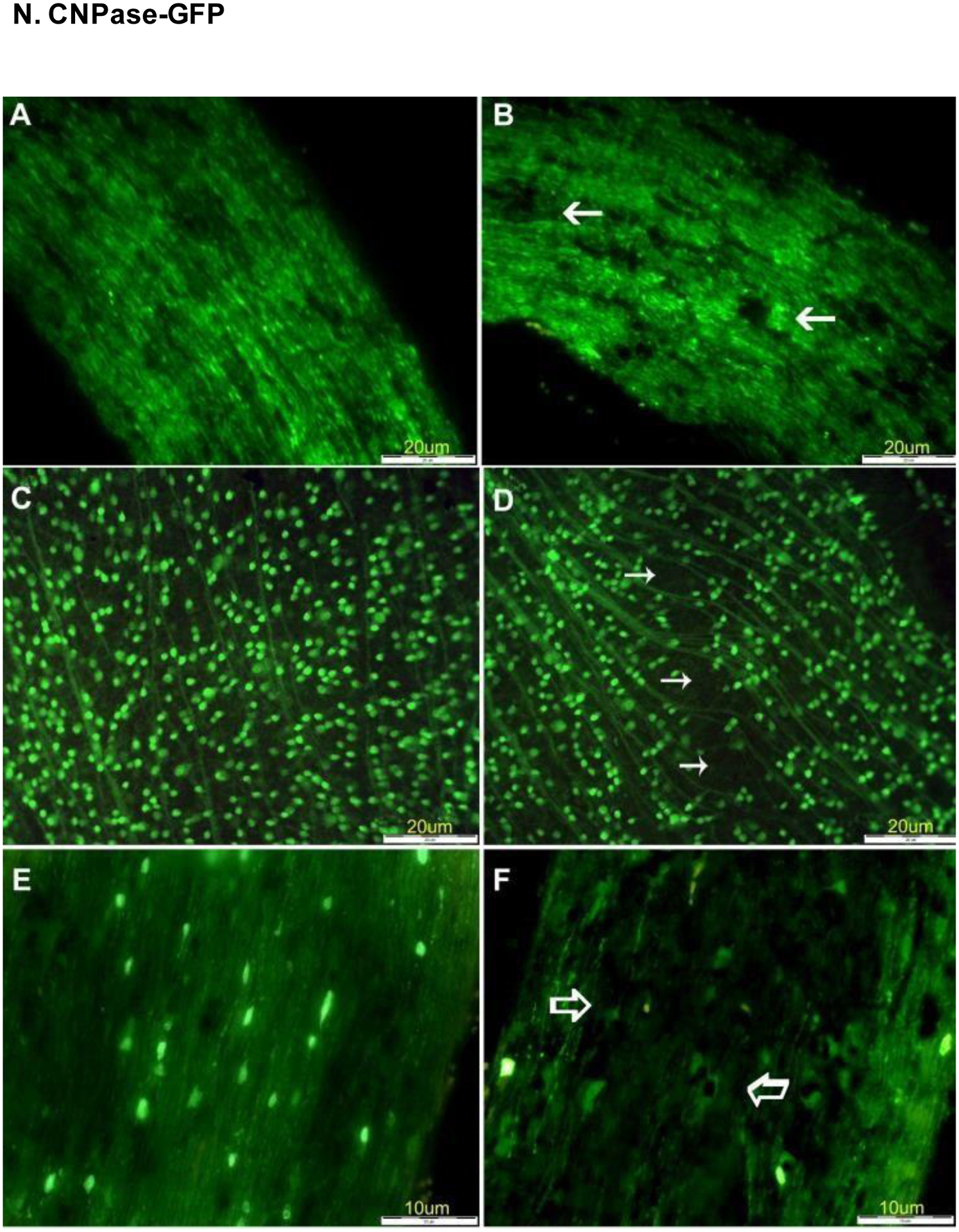
Expression of Thy1-CFP and CNPase-GFP in the retina and optic nerve of a mouse model of experimentally-induced rodent anterior ischemic optic neuropathy (rAION). (A) Optic nerve images in control Thy1-CFP mice before induction of rAION. (B) View of optic nerve 21 days after induction of rAION, with arrows noting regions of significant axon loss. (C) Retina of control mouse before induction of rAION. (D) Optic nerve of CNPase-GFP mouse before induction of rAION. (F) Loss of oligodendrocyte expression 21 days after induction of rAION. Reproduced with permission from [105].
Figure 24.
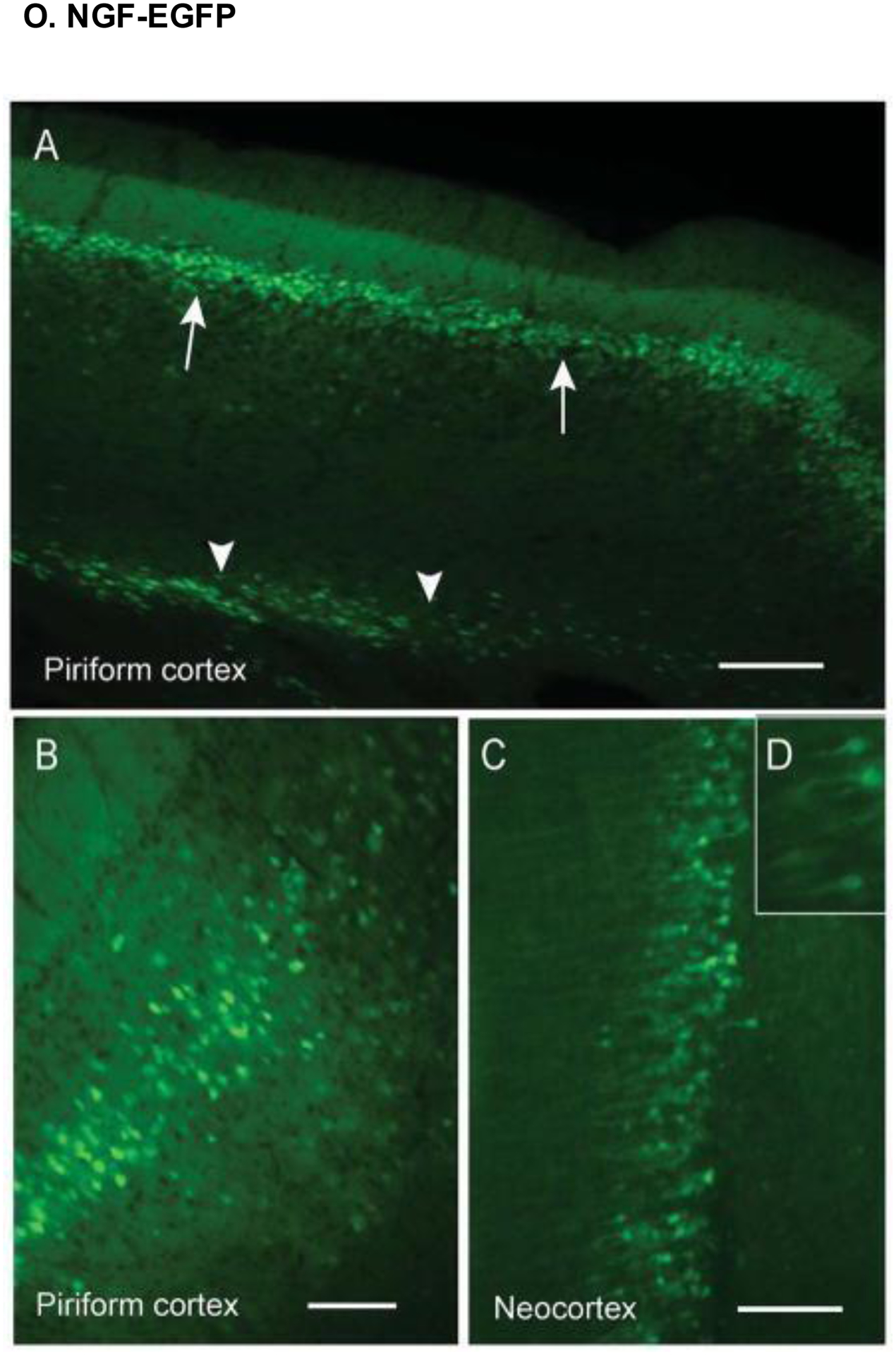
EGFP expression in the cortex (axial sections) of NGF-EGFP transgenic mice. (A) The piriform cortex displayed prominent EGFP expression, especially in layer III. (B) Magnified image of the piriform cortex, showing both larger and smaller neurons. (C-D) The neocortex also displayed prominent EGFP expression, particularly in layer V. Scale bars, (A) 200 μm; (B & C) 100 μm. Reproduced with permission from [225].
Figure 25.
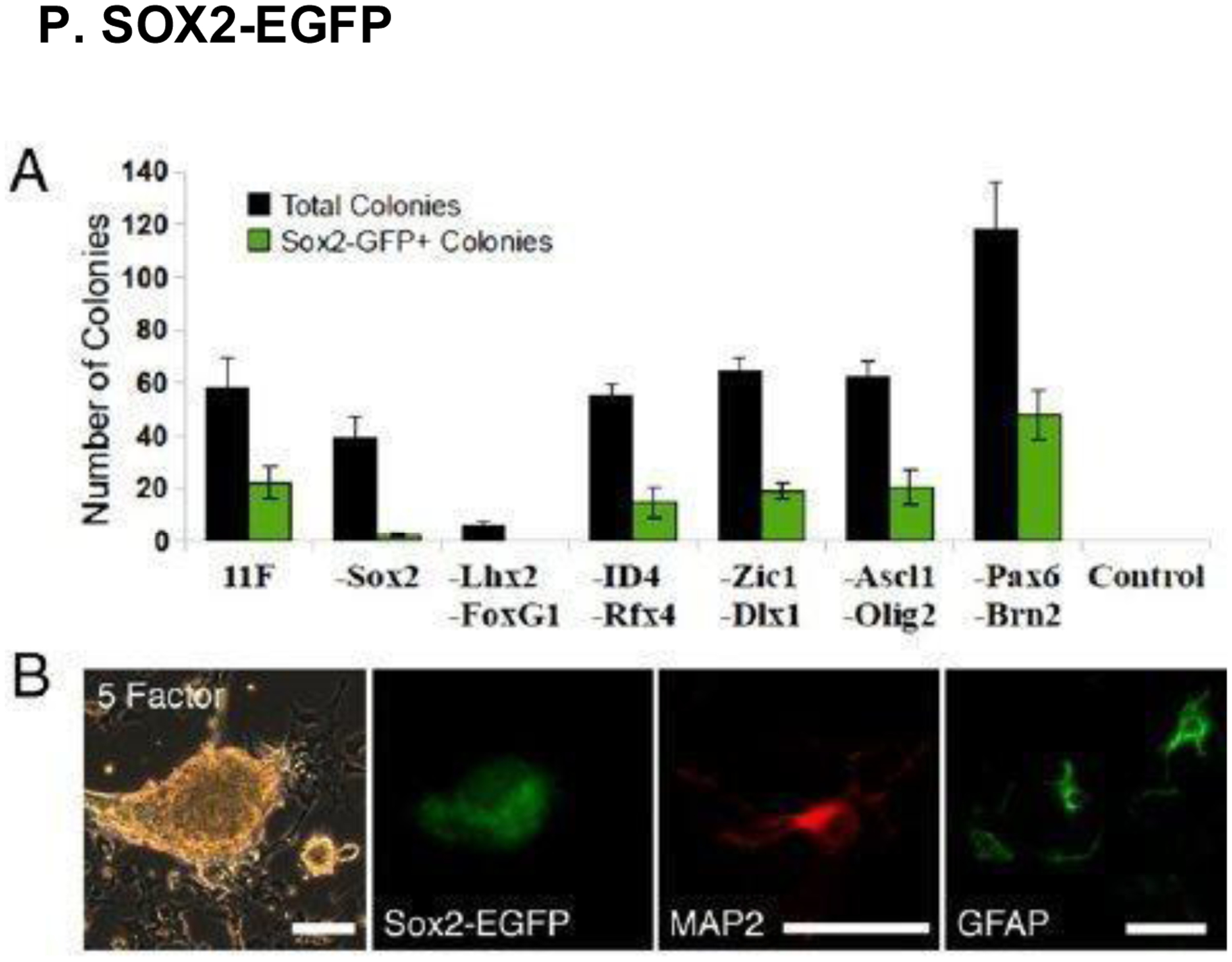
(A) Comparison of total mouse colonies vs. colonies that express SOX2-EGFP 24 days after experimental transgene induction. (B) SOX2-EGFP expression in brain tissue, imaged 25 days after 5-Factor transgene induction, compared with MAP2+ (neuronal) and GFAP+ (astrocyte) cell expression. Scale bars, 50 μm. Reproduced with permission from [232].
Figure 26.
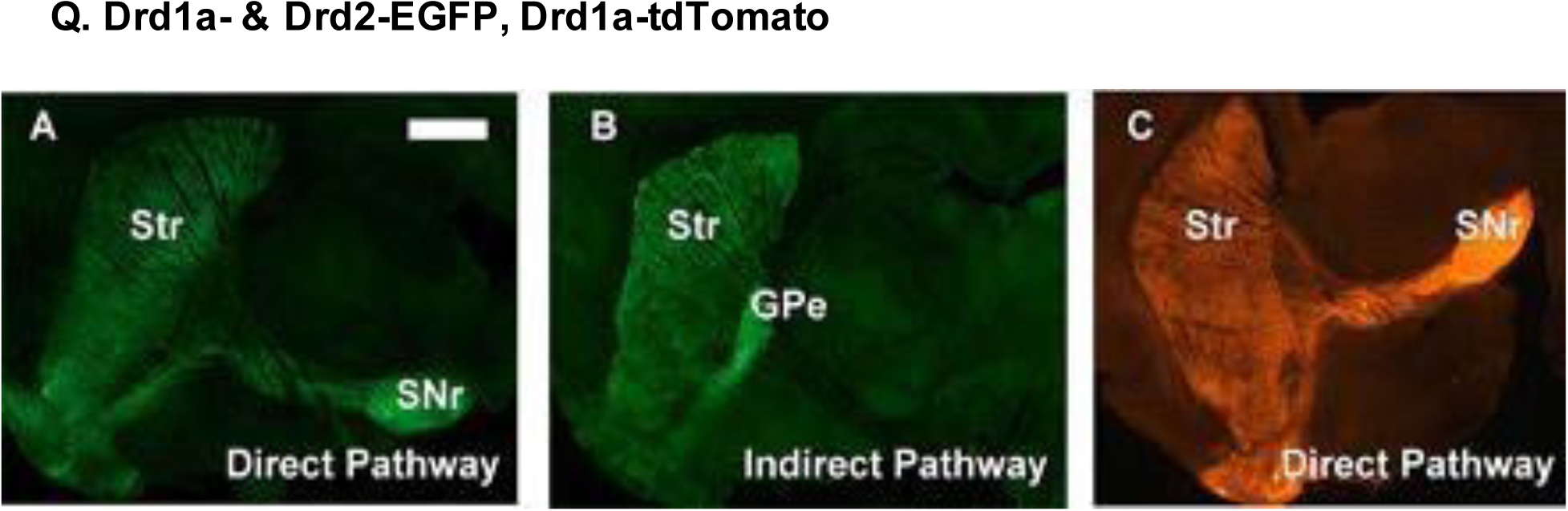
Expression patterns in key basal ganglia structures of BAC transgenic mice. (A) Drd1a-EGFP, (B) Drd2-EGFP, and (C) Drd1a-tdTomato BAC lines. Fluorescence was most prominent in striatal soma and axonal projections. Scale bar, 1 mm. Other abbreviations: GPe, globus pallidus externa; SNr, substantia nigra pars reticulata; Str, striatum. Reproduced with permission from [243].
Figure 27.
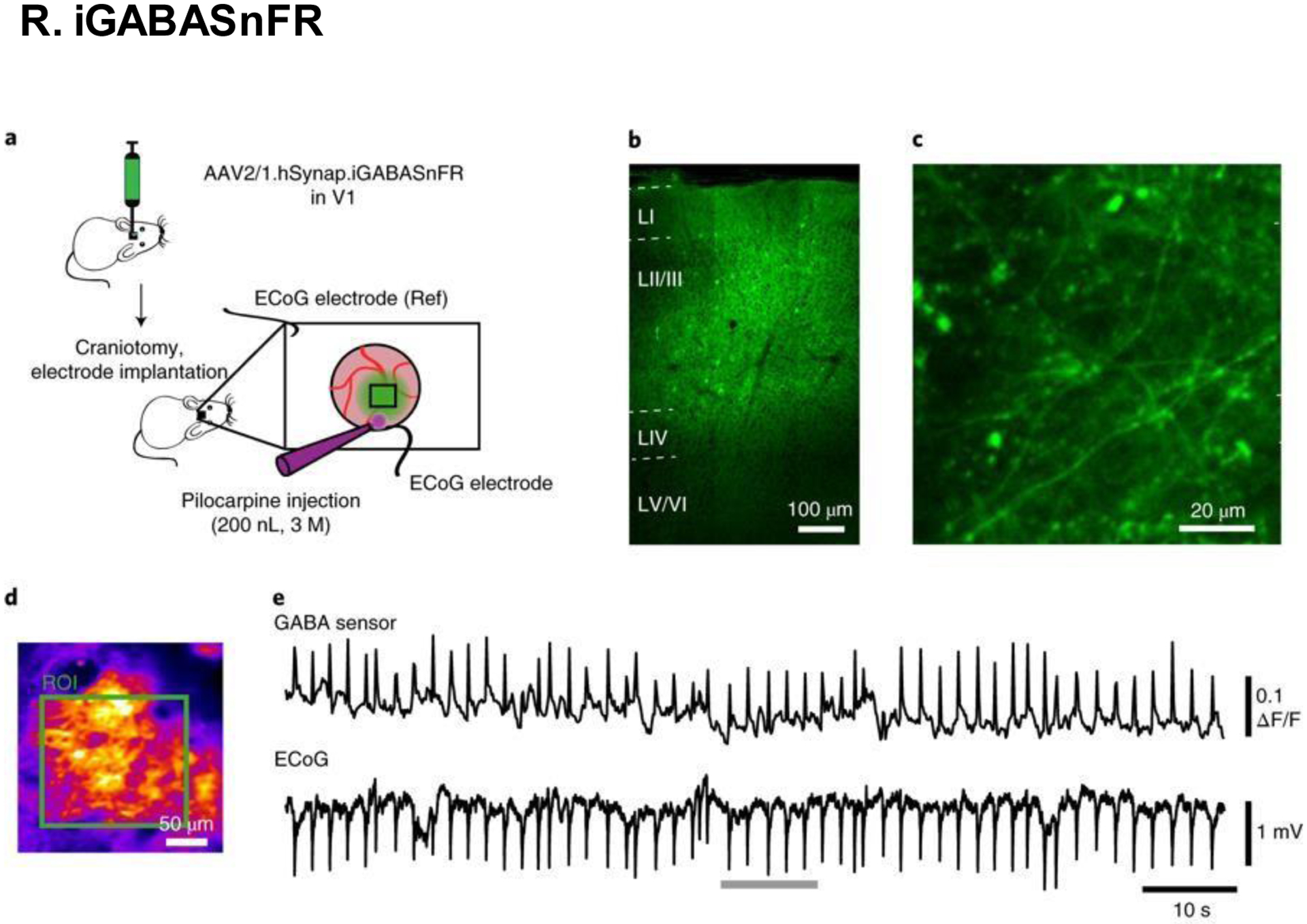
Cortical iGABASnFR expression viewed under immunofluorescence microscopy. (A) Experiment diagram and design. (B) Images of cortical layers I-VI. (C) Images of cortical neurons proximal to the inferior pia. (D) Average intensity of interictal iGABASnFR fluorescence. (E) Interictal neuronal activity, as measured by electrocorticography. Scale bars, (B) 100 μm; (C) 20 μm; (D) 50 μm. Reproduced with permission from [245].
Highlights.
In neurofluorescent reporter mice, nerve cells are viewable via fluorescence imaging.
In these models, fluorescent proteins are co-expressed with specific marker proteins.
This fluorescence allows for studies of the structural patterns of nerves in vivo.
These mice are useful for examining nerve formation, injury, repair, and degeneration.
Marker proteins Thy1 and Nestin are used to visualize different subsets of neurons.
Acknowledgments
Publication of this article was supported by National Institutes of Health grants EY10101 (D.T.A.), I01 BX002386, and I01 BX004234; the Eversight, Midwest Eye Bank Award (J.H.C), EY01792, and EY027912 (MIR); and an unrestricted grant from Research to Prevent Blindness, New York, NY.
List of Abbreviations
- 5-HT
5-Hydroxytryptamine (serotonin)
- ACh
Acetylcholine
- Akt
Protein kinase B (PKB)
- Mapttm1(EGFP)kit
Transgenic mouse reporter for microtubule-associated protein tau
- βIII-tubulin-YFP
Transgenic mouse reporter for βIII-tubulin neuronal marker
- BAC
Bacterial artificial chromosome (DNA construct)
- BGEM
Brain gene expression map
- BMC
Bone marrow concentrate
- CCK-RGC
Cholecystokinin-retinal ganglion cell
- CFP
Cyan fluorescent protein
- CGRP
Calcitonin gene-related peptide
- CGX
Cingulate cortex
- ChATBAC-EGFP
Transgenic mouse reporter for choline acetyltransferase expression
- ChAT-ChR2-EYFP BAC
Channel rhodopsin-expressing fluorescent mouse reporter
- CLSM
Confocal laser scanning microscopy
- CNPase
2’,3’-Cyclic-nucleotide 3’-phosphodiesterase (myelin-associated enzyme)
- CNPase-GFP
Transgenic mouse reporter
- CNP-EGFP
Transgenic mouse reporter
- CNS
Central nervous system
- Cp
Choroid plexus
- CPZ
Cuprizone (copper-chelating agent)
- Cre
Protein used in site-specific recombinant technology
- CRISPR-Cas9
Clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9, a DNA editing technology
- CST
Corticospinal tract
- CTb-594
Cholera toxin beta subunit coupled to alexa 594 (axon tracer)
- D1-MSN
Direct dopamine pathway medium spiny neuron
- DAPI
4′,6-Diamidino-2-phenylindole (blue-fluorescent DNA stain)dLGN: Dorsolateral geniculate nucleus of the thalamus
- Drd1a-EGFP
Transgenic mouse reporter for type 1a dopamine receptor gene
- Drd1a-tdTomato
Transgenic mouse reporter for type 1a dopamine receptor gene
- Drd2-EGFP
Transgenic mouse reporter for dopamine receptor D2 expression
- DRG
Dorsal root ganglia
- ED
Embryonic day
- EAE
Experimental autoimmune encephalomyelitis (used in transgenic mouse models)
- EDL
Extensor digitorum longus
- EGFP
Enhanced green fluorescent protein
- EGFR
Epidermal growth factor receptor
- EGL
External granular layer of the developing cerebellum
- En1-Cre
Knock-in Cre expression mouse reporter line for the engrailed gene
- ENS
Enteric nervous system
- EPC
Endothelial progenitor cell
- ERCC1
Excision repair cross-complementation group 1
- FRAP
Fluoride-resistant acid phosphatase
- GCL
Ganglion cell layer
- GFAP
Glial fibrillary acidic protein
- GFP
Green fluorescent protein
- GFP-Cre
Transgenic mice reporter construct where GFP fluorescence is coupled to Cre expression
- GPe
Globus pallidus externa
- HDB
Horizontal diagonal band of Broca
- Hoxb4
Homeobox B4 gene
- hPRPH1-G
Peripherin-EGFP genomic reporter construct
- HSV-1
Herpes simplex virus type 1
- IBA
Ionized calcium binding adaptor molecule
- IF
Immunofluorescence
- iGABASnFR
GABA-sensing fluorescence reporter
- INL
Inner nuclear layer of the retina
- IVCM
In vivo confocal microscopy
- LASIK
Laser-assisted in situ keratomileusis
- CLSM
Laser scanning microscopy
- MAP2
Microtubule-associated protein 2
- MAPK
Mitogen-activated protein kinases
- Math1
Basic helix-loop-helix (bHLH) transcription factor (in neural progenitor cells)
- Math1-GFP
Transgenic mouse reporter
- MIP
Maximum intensity projection
- ml
Molecular layer
- MNU
N-methyl-N-nitrosourea
- MOB
Main olfactory bulb
- MP
Myenteric plexus of the gastrointestinal tract
- mRNA
Messenger RNA
- NE
Norepinephrine
- Nestin-GFP
Transgenic mouse reporter
- Neu-N
Neuronal nuclei specific marker
- NF
Neurofilament
- NF-H
Neurofilament heavy chain
- NF-200
Neurofilament 200
- NGF
Nerve growth factor
- NGF-EGFP
Transgenic mouse reporter
- NGFR
Mouse nerve growth factor receptor
- NGFpr
Nerve growth factor promoter
- NK
Neurotrophic keratitis
- NK-1
Neurokinin-1 receptor that binds Substance P
- NMPP1
Cell-permeable protein phosphatase 1 (PP1) analog
- nNGFP
Neuronal Nestin-GFP
- NPC
Neural precursor cell
- NPY
Neuropeptide Y
- NSE
Neuron-specific enolase
- OCT
Optical coherence tomography
- OEC
Olfactory ensheathing cell
- ONL
Outer nuclear layer of the retina
- OVA
Ovalbumin
- p75NTR
p75 neurotrophin receptor
- PACAP
Pituitary adenylate cyclase-activating peptide
- PD
Postnatal day
- Peripherin-EGFP
Transgenic mouse reporter
- PI3K
Phosphoinositide 3-kinase
- Piezo2
Piezo type mechanosensitive ion channel component 2
- PKH26
Red fluorescent dye
- PNS
Peripheral nervous system
- PSD95
Postsynaptic density protein 95 (membrane associated guanylate kinase)
- rAAV/EnvA
Recombinant adeno-associated virus/avian virus envelope protein
- RAG
Recombination-activating gene
- rAION
rodent Anterior ischemic optic neuropathy
- Ret-CreER
Transgenic mouse reporter
- RFP
Red fluorescent protein
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SC
Sympathetic chain
- SCG
Sympathetic chain ganglia
- Sirt1
NAD-dependent deacetylase sirtuin-1 protein
- SMN
Spinal motor neurons
- SNr
Substantia nigra pars reticulata
- SOX2-EGFP
Transgenic mouse reporter
- SP
Substance P or Submucosal plexus of the gastrointestinal tract
- Sprr1a
Small proline rich protein 1A
- Str
Striatum
- STZ
Streptozotocin
- TAG-1
Transient axonal glycoprotein-1 or contactin-2
- TAG1-Cre
Transgenic mouse reporter
- TG
Trigeminal ganglia
- Thy1
Thy-1 cell surface antigen (Thy1 or CD90)
- TPM
Two-photon microscopy
- TrKA
Tropomyosin receptor kinase A
- TRPA1
Transient receptor potential ankyrin 1
- TRPC
Transient receptor potential channel
- TRPM
Transient receptor potential melastatin channels
- TRPM8
Transient receptor potential cation channel, subfamily M member 8
- TRPML
Transient receptor potential cation channel, mucolipin subfamily
- TRPP
Transient receptor potential polycystic
- TRPV
Transient receptor potential cation channel, vanilloid subfamily
- TRPV1
Transient receptor potential cation channel, subfamily V member 1
- TPM
Two-photon microscopy
- UCHL1-EGFP
Ubiquitin carboxy-terminal hydrolase isozyme L1 transgenic mouse reporter
- VAChT
Vesicular acetylcholine transporter
- VEGF
Vascular endothelial growth factor
- VIP
Vasoactive intestinal peptide
- vLGN
Ventral lateral geniculate nucleus
- Wnt1
Wnt family member 1
- WP
Whisker pad
- XFP
Generic abbreviation for fluorescent proteins (i.e. CFP, GFP, YFP, RFP, etc.)
- YFP
Yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jones EG, Neuroanatomy: Cajal and after Cajal, Brain Res Rev 55(2) (2007) 248–55. [DOI] [PubMed] [Google Scholar]
- [2].Ohno N, Katoh M, Saitoh Y, Saitoh S, Recent advancement in the challenges to connectomics, Microscopy (Oxf) 65(2) (2016) 97–107. [DOI] [PubMed] [Google Scholar]
- [3].Schmechel D, Marangos PJ, Zis AP, Brightman M, Goodwin FK, Brain endolases as specific markers of neuronal and glial cells, Science 199(4326) (1978) 313–5. [DOI] [PubMed] [Google Scholar]
- [4].Koch ET, Woodard CL, Raymond LA, Direct assessment of presynaptic modulation of cortico-striatal glutamate release in a Huntington’s disease mouse model, Journal of neurophysiology 120(6) (2018) 3077–3084. [DOI] [PubMed] [Google Scholar]
- [5].Aulston B, Liu Q, Reilly P, Yuan SH, An In Vitro Model for Studying Tau Aggregation Using Lentiviral-mediated Transduction of Human Neurons, Journal of visualized experiments : JoVE (147) (2019). [DOI] [PubMed] [Google Scholar]
- [6].Piekna-Przybylska D, Reporter Assays for BER Pathway, Methods Mol Biol 1999 (2019) 145–160. [DOI] [PubMed] [Google Scholar]
- [7].Hong H, Daadi MM, Generating Neural Stem Cells from iPSCs with Dopaminergic Neurons Reporter Gene, Methods Mol Biol 1919 (2019) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McGonigle P, Animal models of CNS disorders, Biochemical pharmacology 87(1) (2014) 140–9. [DOI] [PubMed] [Google Scholar]
- [9].Rozsa AJ, Beuerman RW, Density and organization of free nerve endings in the corneal epithelium of the rabbit, Pain 14(2) (1982) 105–20. [DOI] [PubMed] [Google Scholar]
- [10].Yu CQ, Rosenblatt MI, Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration, Investigative ophthalmology & visual science 48(4) (2007) 1535–42. [DOI] [PubMed] [Google Scholar]
- [11].Bouheraoua N, Fouquet S, Marcos-Almaraz MT, Karagogeos D, Laroche L, Chedotal A, Genetic Analysis of the Organization, Development, and Plasticity of Corneal Innervation in Mice, The Journal of neuroscience : the official journal of the Society for Neuroscience 39(7) (2019) 1150–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G, Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice, Nat Neurosci 11(6) (2008) 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Semerci F, Maletic-Savatic M, Transgenic mouse models for studying adult neurogenesis, Front Biol (Beijing) 11(3) (2016) 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson RJ, Drake JC, Cui D, Zhang M, Perry HM, Kashatus JA, Kusminski CM, Scherer PE, Kashatus DF, Okusa MD, Yan Z, Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy, Mitochondrion 44 (2019) 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Forstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D, Rempfler M, Xavier ALR, Kress BT, Benakis C, Steinke H, Liebscher S, Bechmann I, Liesz A, Menze B, Kerschensteiner M, Nedergaard M, Erturk A, Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections, Nat Neurosci 22(2) (2019) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, Okabe M, Tano Y, Goldman SA, Okano H, Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells, Molecular and cellular neurosciences 17(2) (2001) 259–73. [DOI] [PubMed] [Google Scholar]
- [17].Liu L, Geisert EE, Frankfurter A, Spano AJ, Jiang CX, Yue J, Dragatsis I, Goldowitz D, A transgenic mouse class-III beta tubulin reporter using yellow fluorescent protein, Genesis 45(9) (2007) 560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Letournel F, Bocquet A, Perrot R, Dechaume A, Guinut F, Eyer J, Barthelaix A, Neurofilament high molecular weight-green fluorescent protein fusion is normally expressed in neurons and transported in axons: a neuronal marker to investigate the biology of neurofilaments, Neuroscience 137(1) (2006) 103–11. [DOI] [PubMed] [Google Scholar]
- [19].Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, Danielson P, Xie L, Zhou Q, Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor, Diabetes 63(12) (2014) 4262–74. [DOI] [PubMed] [Google Scholar]
- [20].Forss-Petter S, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe JG, Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control, Neuron 5(2) (1990) 187–97. [DOI] [PubMed] [Google Scholar]
- [21].McCoy ES, Taylor-Blake B, Zylka MJ, CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch, PloS one 7(5) (2012) e36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sarkar J, Chaudhary S, Jassim SH, Ozturk O, Chamon W, Ganesh B, Tibrewal S, Gandhi S, Byun YS, Hallak J, Mahmud DL, Mahmud N, Rondelli D, Jain S, CD11b+GR1+ myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration, Investigative ophthalmology & visual science 54(9) (2013) 5920–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ehmke T, Leckelt J, Reichard M, Weiss H, Hovakimyan M, Heisterkamp A, Stachs O, Baltrusch S, In vivo nonlinear imaging of corneal structures with special focus on BALB/c and streptozotocin-diabetic Thy1-YFP mice, Experimental eye research 146 (2016) 137–44. [DOI] [PubMed] [Google Scholar]
- [24].Dando SJ, Kazanis R, Chinnery HR, McMenamin PG, Regional and functional heterogeneity of antigen presenting cells in the mouse brain and meninges, Glia 67(5) (2019) 935–949. [DOI] [PubMed] [Google Scholar]
- [25].Vidal M, Morris R, Grosveld F, Spanopoulou E, Tissue-specific control elements of the Thy-1 gene, The EMBO journal 9(3) (1990) 833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Josvay K, Winter Z, Katona RL, Pecze L, Marton A, Buhala A, Szakonyi G, Olah Z, Vizler C, Besides neuro-imaging, the Thy1-YFP mouse could serve for visualizing experimental tumours, inflammation and wound-healing, Scientific reports 4 (2014) 6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alic I, Kosi N, Kapuralin K, Gorup D, Gajovic S, Pochet R, Mitrecic D, Neural stem cells from mous e strain Thy1 YFP-16 are a valuable tool to monitor and evaluate neuronal differentiation and morphology, Neuroscience letters 634 (2016) 32–41. [DOI] [PubMed] [Google Scholar]
- [28].Leckenby JI, Chacon MA, Grobbelaar AO, Lichtman JW, Imaging Peripheral Nerve Regeneration: A New Technique for 3D Visualization of Axonal Behavior, J Surg Res 242 (2019) 207–213. [DOI] [PubMed] [Google Scholar]
- [29].Rege TA, Hagood JS, Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses, Biochimica et biophysica acta 1763(10) (2006) 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xue GP, Calvert RA, Morris RJ, Expression of the neuronal surface glycoprotein Thy −1 is under post-transcriptional control, and is spatially regulated, in the developing olfactory system, Development 109(4) (1990) 851–64. [DOI] [PubMed] [Google Scholar]
- [31].Yin X, Yu T, Chen B, Xu J, Chen W, Qi Y, Zhang P, Li Y, Kou Y, Ma Y, Han N, Wan P, Luo Q, Zhu D, Jiang B, Spatial Distribution of Motor Endplates and its Adaptive Change in Skeletal Muscle, Theranostics 9(3) (2019) 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR, Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP, Neuron 28(1) (2000) 41–51. [DOI] [PubMed] [Google Scholar]
- [33].Leckenby JI, Chacon MA, Rolfe K, Lichtman JW, Grobbelaar AO, Development of the interscutularis model as an outcome measure for facial nerve surgery, Ann Anat 223 (2019) 127–135. [DOI] [PubMed] [Google Scholar]
- [34].Blandford SN, Hooper ML, Yabana T, Chauhan BC, Baldridge WH, Farrell SRM, Retinal Characterization of the Thy1-GCaMP3 Transgenic Mouse Line After Optic Nerve Transection, Investigative ophthalmology & visual science 60(1) (2019) 183–191. [DOI] [PubMed] [Google Scholar]
- [35].Namavari A, Chaudhary S, Sarkar J, Yco L, Patel K, Han KY, Yue BY, Chang JH, Jain S, In vivo serial imaging of regenerating corneal nerves after surgical transection in transgenic thy1-YFP mice, Investigative ophthalmology & visual science 52(11) (2011) 8025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sarkar J, Milani B, Kim E, An S, Kwon J, Jain S, Corneal nerve healing after in situ laser nerve transection, PloS one 14(6) (2019) e0218879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pan Z, Fukuoka S, Karagianni N, Guaiquil VH, Rosenblatt MI, Vascular endothelial growth factor promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27(7) (2013) 2756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chaudhary S, Namavari A, Yco L, Chang JH, Sonawane S, Khanolkar V, Sarkar J, Jain S, Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery, Cornea 31(12) (2012) 1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang M, Zhou Q, Luo Y, Nguyen T, Rosenblatt MI, Guaiquil VH, Semaphorin3A induces nerve regeneration in the adult cornea-a switch from its repulsive role in development, PloS one 13(1) (2018) e0191962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Namavari A, Chaudhary S, Chang JH, Yco L, Sonawane S, Khanolkar V, Yue BY, Sarkar J, Jain S, Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves, Investigative ophthalmology & visual science 53(2) (2012) 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nichols M, Pavlov EV, Robertson GS, Tamoxifen-induced knockdown of the mitochondrial calcium uniporter in Thy1-expressing neurons protects mice from hypoxic/ischemic brain injury, Cell Death Dis 9(6) (2018) 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP, Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice, Journal of neuroscience methods 134(1) (2004) 23–35. [DOI] [PubMed] [Google Scholar]
- [43].Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T, In vivo imaging of axonal degeneration and regeneration in the injured spinal cord, Nature medicine 11(5) (2005) 572–7. [DOI] [PubMed] [Google Scholar]
- [44].Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ, The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(52) (2008) 14107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Comley LH, Wishart TM, Baxter B, Murray LM, Nimmo A, Thomson D, Parson SH, Gillingwater TH, Induction of cell stress in neurons from transgenic mice expressing yellow fluorescent protein: implications for neurodegeneration research, PloS one 6(3) (2011) e17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Magill CK, Moore AM, Borschel GH, Mackinnon SE, A new model for facial nerve research: the novel transgenic Thy1-GFP rat, Archives of facial plastic surgery 12(5) (2010) 315–20. [DOI] [PubMed] [Google Scholar]
- [47].Placheta E, Wood MD, Lafontaine C, Frey M, Gordon T, Borschel GH, Macroscopic in vivo imaging of facial nerve regeneration in Thy1-GFP rats, JAMA facial plastic surgery 17(1) (2015) 8–15. [DOI] [PubMed] [Google Scholar]
- [48].Espana EM, Kawakita T, Di Pascuale MA, Li W, Yeh LK, Parel JM, Liu CY, Tseng SC, The heterogeneous murine corneal stromal cell populations in vitro, Investigative ophthalmology & visual science 46(12) (2005) 4528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G, Neural stem and progenitor cells in nestin-GFP transgenic mice, The Journal of comparative neurology 469(3) (2004) 311–24. [DOI] [PubMed] [Google Scholar]
- [50].Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, Walton N, Lahn BT, Nestin is required for the proper self-renewal of neural stem cells, Stem Cells 28(12) (2010) 2162–71. [DOI] [PubMed] [Google Scholar]
- [51].Moon CH, Cho H, Kim YK, Park TK, Nestin Expression in the Adult Mouse Retina with Pharmaceutically Induced Retinal Degeneration, Journal of Korean medical science 32(2) (2017) 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grundmann D, Markwart F, Scheller A, Kirchhoff F, Schafer KH, Phenotype and distribution pattern of nestin-GFP-expressing cells in murine myenteric plexus, Cell and tissue research 366(3) (2016) 573–586. [DOI] [PubMed] [Google Scholar]
- [53].Amoh Y, Hamada Y, Katsuoka K, Hoffman RM, In vivo imaging of nuclear-cytoplasmic deformation and partition during cancer cell death due to immune rejection, Journal of cellular biochemistry 113(2) (2012) 465–72. [DOI] [PubMed] [Google Scholar]
- [54].Hwang JE, Hong JY, Kim K, Kim SH, Choi WY, Kim MJ, Jung SH, Shim HJ, Bae WK, Hwang EC, Lee KH, Lee JH, Cho SH, Chung IJ, Class III beta-tubulin is a predictive marker for taxane-based chemotherapy in recurrent and metastatic gastric cancer, BMC cancer 13 (2013) 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bhattacherjee A, Mu Y, Winter MK, Knapp JR, Eggimann LS, Gunewardena SS, Kobayashi K, Kato S, Krizsan-Agbas D, Smith PG, Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome, Proceedings of the National Academy of Sciences of the United States of America 114(33) (2017) E6952–E6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ballester-Lopez C, Conlon TM, Ertuz Z, Greiffo FR, Irmler M, Verleden SE, Beckers J, Fernandez IE, Eickelberg O, Yildirim AO, The Notch ligand DNER regulates macrophage IFNgamma release in chronic obstructive pulmonary disease, EBioMedicine 43 (2019) 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boccella S, Guida F, De Logu F, De Gregorio D, Mazzitelli M, Belardo C, Iannotta M, Serra N, Nassini R, de Novellis V, Geppetti P, Maione S, Luongo L, Ketones and pain: unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33(1) (2019) 1062–1073. [DOI] [PubMed] [Google Scholar]
- [58].Chucair-Elliott AJ, Zheng M, Carr DJ, Degeneration and regeneration of corneal nerves in response to HSV-1 infection, Investigative ophthalmology & visual science 56(2) (2015) 1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL, Functions of S100 proteins, Current molecular medicine 13(1) (2013) 24–57. [PMC free article] [PubMed] [Google Scholar]
- [60].Vives V, Alonso G, Solal AC, Joubert D, Legraverend C, Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice, The Journal of comparative neurology 457(4) (2003) 404–19. [DOI] [PubMed] [Google Scholar]
- [61].Stone RA, Kuwayama Y, Substance P-like immunoreactive nerves in the human eye, Arch Ophthalmol 103(8) (1985) 1207–11. [DOI] [PubMed] [Google Scholar]
- [62].Wu YF, Wang CY, Yang TL, Tsao PN, Lin SJ, Tan HY, Intravital multiphoton microscopic imaging platform for ocular surface imaging, Experimental eye research 182 (2019) 194–201. [DOI] [PubMed] [Google Scholar]
- [63].Bron R, Wood RJ, Brock JA, Ivanusic JJ, Piezo2 expression in corneal afferent neurons, The Journal of comparative neurology 522(13) (2014) 2967–79. [DOI] [PubMed] [Google Scholar]
- [64].Fernandes ES, Fernandes MA, Keeble JE, The functions of TRPA1 and TRPV1: moving away from sensory nerves, British journal of pharmacology 166(2) (2012) 510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buckinx R, Van Nassauw L, Avula LR, Alpaerts K, Adriaensen D, Timmermans JP, Transient receptor potential vanilloid type 1 channel (TRPV1) immunolocalization in the murine enteric nervous system is affected by the targeted C-terminal epitope of the applied antibody, The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 61(6) (2013) 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI, Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(28) (2011) 10119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alamri A, Bron R, Brock JA, Ivanusic JJ, Transient receptor potential cation channel subfamily V member 1 expressing corneal sensory neurons can be subdivided into at least three subpopulations, Frontiers in neuroanatomy 9 (2015) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Richter M, Negro-Demontel ML, Blanco-Ocampo D, Taranto E, Lago N, Peluffo H, Thy1-YFP-H Mice and the Parallel Rod Floor Test to Evaluate Short- and Long-Term Progression of Traumatic Brain Injury, Current protocols in immunology 120 (2018) 24 1 1–24 1 25. [DOI] [PubMed] [Google Scholar]
- [69].Ren J, Li X, Sun G, Li S, Liang S, Li Z, Li B, Xia M, Protective effect of leptin-mediated caveolin-1 expression on neurons after spinal cord injury, Cell calcium 76 (2018) 122–128. [DOI] [PubMed] [Google Scholar]
- [70].Clark JA, Blizzard CA, Breslin MC, Yeaman EJ, Lee KM, Chuckowree JA, Dickson TC, Epothilone D accelerates disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis, Neuropathology and applied neurobiology 44(6) (2018) 590–605. [DOI] [PubMed] [Google Scholar]
- [71].Kosi N, Alic I, Salamon I, Mitrecic D, Stroke promotes survival of nearby transplanted neural stem cells by decreasing their activation of caspase 3 while not affecting their differentiation, Neuroscience letters 666 (2018) 111–119. [DOI] [PubMed] [Google Scholar]
- [72].Groman-Lupa S, Adewumi J, Park KU, Brzezinski JI, The Transcription Factor Prdm16 Marks a Single Retinal Ganglion Cell Subtype in the Mouse Retina, Investigative ophthalmology & visual science 58(12) (2017) 5421–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dailey WA, Drenser KA, Wong SC, Cheng M, Vercellone J, Roumayah KK, Feeney EV, Deshpande M, Guzman AE, Trese M, Mitton KP, Norrin treatment improves ganglion cell survival in an oxygen-induced retinopathy model of retinal ischemia, Experimental eye research 164 (2017) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P, Evidence for newly generated interneurons in the basolateral amygdala of adult mice, Molecular psychiatry 23(3) (2018) 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhao R, Hu W, Tsai J, Li W, Gan WB, Microglia limit the expansion of beta-amyloid plaques in a mouse model of Alzheimer’s disease, Molecular neurodegeneration 12(1) (2017) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mitrecic D, Alic I, Gorup D, Stem cells and stroke-how glowing neurons illuminate new paths, Neurogenesis 4(1) (2017) e1304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nadal L, Garcia N, Hurtado E, Simo A, Tomas M, Lanuza MA, Cilleros V, Tomas J, Presynaptic Muscarinic Acetylcholine Receptors and TrkB Receptor Cooperate in the Elimination of Redundant Motor Nerve Terminals during Development, Frontiers in aging neuroscience 9 (2017) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yan Y, Wood MD, Moore AM, Snyder-Warwick AK, Hunter DA, Newton P, Poppler L, Tung TH, Johnson PJ, Mackinnon SE, Robust Axonal Regeneration in a Mouse Vascularized Composite Allotransplant Model Undergoing Delayed Tissue Rejection, Hand 11(4) (2016) 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Baltrusch S, [Ophthalmological Monitoring of Diabetic Neuropathy in a Mouse Model], Klinische Monatsblatter fur Augenheilkunde 233(12) (2016) 1313–1319. [DOI] [PubMed] [Google Scholar]
- [80].Yu F, Shukla DK, Armstrong RC, Marion CM, Radomski KL, Selwyn RG, Dardzinski BJ, Repetitive Model of Mild Traumatic Brain Injury Produces Cortical Abnormalities Detectable by Magnetic Resonance Diffusion Imaging, Histopathology, and Behavior, Journal of neurotrauma 34(7) (2017) 1364–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leckelt J, Guimaraes P, Kott A, Ruggeri A, Stachs O, Baltrusch S, Early detection of diabetic neuropathy by investigating CNFL and IENFD in thy1-YFP mice, The Journal of endocrinology 231(2) (2016) 147–157. [DOI] [PubMed] [Google Scholar]
- [82].McNabb RP, Blanco T, Bomze HM, Tseng HC, Saban DR, Izatt JA, Kuo AN, Method for single illumination source combined optical coherence tomography and fluorescence imaging of fluorescently labeled ocular structures in transgenic mice, Experimental eye research 151 (2016) 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cui L, Wang D, McGillis S, Kyle M, Zhao LR, Repairing the Brain by SCF+G-CSF Treatment at 6 Months Postexperimental Stroke: Mechanistic Determination of the Causal Link Between Neurovascular Regeneration and Motor Functional Recovery, ASN neuro 8(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hayes LR, Asress SA, Li Y, Galkin A, Stepanova A, Kawamata H, Manfredi G, Glass JD, Distal denervation in the SOD1 knockout mouse correlates with loss of mitochondria at the motor nerve terminal, Experimental neurology 318 (2019) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kobayashi W, Onishi A, Tu HY, Takihara Y, Matsumura M, Tsujimoto K, Inatani M, Nakazawa T, Takahashi M, Culture Systems of Dissociated Mouse and Human Pluripotent Stem Cell-Derived Retinal Ganglion Cells Purified by Two-Step Immunopanning, Investigative ophthalmology & visual science 59(2) (2018) 776–787. [DOI] [PubMed] [Google Scholar]
- [86].Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, Bergstrom HC, Holmes A, Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior, Alcoholism, clinical and experimental research 41(7) (2017) 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Poguzhelskaya E, Artamonov D, Bolshakova A, Vlasova O, Bezprozvanny I, Simplified method to perform CLARITY imaging, Molecular neurodegeneration 9 (2014) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sone K, Mori A, Sakamoto K, Nakahara T, GYY4137, an Extended-Release Hydrogen Sulfide Donor, Reduces NMDA-Induced Neuronal Injury in the Murine Retina, Biological & pharmaceutical bulletin 41(4) (2018) 657–660. [DOI] [PubMed] [Google Scholar]
- [89].Caravagna C, Jaouen A, Desplat-Jego S, Fenrich KK, Bergot E, Luche H, Grenot P, Rougon G, Malissen M, Debarbieux F, Diversity of innate immune cell subsets across spatial and temporal scales in an EAE mouse model, Scientific reports 8(1) (2018) 5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liu H, Ding C, Establishment of an experimental glaucoma animal model: A comparison of microbead injection with or without hydroxypropyl methylcellulose, Experimental and therapeutic medicine 14(3) (2017) 1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sakamoto K, Murakami Y, Sawada S, Ushikubo H, Mori A, Nakahara T, Ishii K, Apelin-36 is protective against N-methyl-D-aspartic-acid-induced retinal ganglion cell death in the mice, European journal of pharmacology 791 (2016) 213–220. [DOI] [PubMed] [Google Scholar]
- [92].Ju WK, Kim KY, Noh YH, Hoshijima M, Lukas TJ, Ellisman MH, Weinreb RN, Perkins GA, Increased mitochondrial fission and volume density by blocking glutamate excitotoxicity protect glaucomatous optic nerve head astrocytes, Glia 63(5) (2015) 736–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ricard C, Stanchi F, Rougon G, Debarbieux F, An orthotopic glioblastoma mouse model maintaining brain parenchymal physical constraints and suitable for intravital two-photon microscopy, Journal of visualized experiments : JoVE (86) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sakamoto K, Kuroki T, Okuno Y, Sekiya H, Watanabe A, Sagawa T, Ito H, Mizuta A, Mori A, Nakahara T, Ishii K, Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina, European journal of pharmacology 733 (2014) 13–22. [DOI] [PubMed] [Google Scholar]
- [95].Fenrich KK, Weber P, Rougon G, Debarbieux F, Implanting glass spinal cord windows in adult mice with experimental autoimmune encephalomyelitis, Journal of visualized experiments : JoVE (82) (2013) e50826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rodriguez AR, de Sevilla Muller LP, Brecha NC, The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina, The Journal of comparative neurology 522(6) (2014) 1411–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lindsey JD, Duong-Polk KX, Dai Y, Nguyen DH, Leung CK, Weinreb RN, Protection by an oral disubstituted hydroxylamine derivative against loss of retinal ganglion cell differentiation following optic nerve crush, PloS one 8(8) (2013) e65966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dai Y, Lindsey JD, Duong-Polk KX, Chindasub P, Leung CK, Weinreb RN, Brimonidine protects against loss of Thy-1 promoter activation following optic nerve crush, BMC ophthalmology 13(1) (2013) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang Q, Vuong H, Huang X, Wang Y, Brecha NC, Pu M, Gao J, Melanopsin-expressing retinal ganglion cell loss and behavioral analysis in the Thy1-CFP-DBA/2J mouse model of glaucoma, Science China. Life sciences 56(8) (2013) 720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tsuruga H, Murata H, Araie M, Aihara M, A model for the easy assessment of pressure-dependent damageto retinal ganglion cells using cyan fluorescent protein-expressing transgenic mice, Molecular vision 18 (2012) 2468–78. [PMC free article] [PubMed] [Google Scholar]
- [101].Chauhan BC, Stevens KT, Levesque JM, Nuschke AC, Sharpe GP, O’Leary N, Archibald ML, Wang X, Longitudinal in vivo imaging of retinal ganglion cells and retinal thickness changes following optic nerve injury in mice, PloS one 7(6) (2012) e40352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tosi J, Wang NK, Zhao J, Chou CL, Kasanuki JM, Tsang SH, Nagasaki T, Rapid and noninvasive imaging of retinal ganglion cells in live mouse models of glaucoma, Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 12(4) (2010) 386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Raymond ID, Pool AL, Vila A, Brecha NC, A Thy1-CFP DBA/2J mouse line with cyan fluorescent protein expression in retinal ganglion cells, Visual neuroscience 26(5–6) (2009) 453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang X, Archibald ML, Stevens K, Baldridge WH, Chauhan BC, Cyan fluorescent protein (CFP) expressing cells in the retina of Thy1-CFP transgenic mice before and after optic nerve injury, Neuroscience letters 468(2) (2010) 110–4. [DOI] [PubMed] [Google Scholar]
- [105].Dratviman-Storobinsky O, Hasanreisoglu M, Offen D, Barhum Y, Weinberger D, Goldenberg-Cohen N, Progressive damage along the optic nerve following induction of crush injury or rodent anterior ischemic optic neuropathy in transgenic mice, Molecular vision 14 (2008) 2171–9. [PMC free article] [PubMed] [Google Scholar]
- [106].Raymond ID, Vila A, Huynh UC, Brecha NC, Cyan fluorescent protein expression in ganglion andamacrine cells in a thy1-CFP transgenic mouse retina, Molecular vision 14 (2008) 1559–74. [PMC free article] [PubMed] [Google Scholar]
- [107].Murata H, Aihara M, Chen YN, Ota T, Numaga J, Araie M, Imaging mouse retinal ganglion cells and their loss in vivo by a fundus camera in the normal and ischemia-reperfusion model, Investigative ophthalmology & visual science 49(12) (2008) 5546–52. [DOI] [PubMed] [Google Scholar]
- [108].Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ, Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice, Investigative ophthalmology & visual science 49(6) (2008) 2635–42. [DOI] [PubMed] [Google Scholar]
- [109].Hayashi A, Koob JW, Liu DZ, Tong AY, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM, A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves, Experimental neurology 207(1) (2007) 128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Faber RM, Hall JK, Chamberlain JS, Banks GB, Myofiber branching rather than myofiber hyperplasia contributes to muscle hypertrophy in mdx mice, Skeletal muscle 4 (2014) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Walker CL, Uchida A, Li Y, Trivedi N, Fenn JD, Monsma PC, Lariviere RC, Julien JP, Jung P, Brown A, Local Acceleration of Neurofilament Transport at Nodes of Ranvier, The Journal of neuroscience : the official journal of the Society for Neuroscience 39(4) (2019) 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lee JH, Rao MV, Yang DS, Stavrides P, Im E, Pensalfini A, Huo C, Sarkar P, Yoshimori T, Nixon RA, Transgenic expression of a ratiometric autophagy probe specifically in neurons enables the interrogation of brain autophagy in vivo, Autophagy 15(3) (2019) 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kunisaki Y, Pericytes in Bone Marrow, Advances in experimental medicine and biology 1122 (2019) 101–114. [DOI] [PubMed] [Google Scholar]
- [114].Hain EG, Sparenberg M, Rasinska J, Klein C, Akyuz L, Steiner B, Indomethacin promotes survival of new neurons in the adult murine hippocampus accompanied by anti-inflammatory effects following MPTP-induced dopamine depletion, Journal of neuroinflammation 15(1) (2018) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nakatomi M, Quispe-Salcedo A, Sakaguchi M, Ida-Yonemochi H, Okano H, Ohshima H, Nestin expression is differently regulated between odontoblasts and the subodontoblastic layer in mice, Histochemistry and cell biology 149(4) (2018) 383–391. [DOI] [PubMed] [Google Scholar]
- [116].Jimenez-Gonzalez A, Garcia-Concejo A, Leon-Lobera F, Rodriguez RE, Morphine delays neural stem cells differentiation by facilitating Nestin overexpression, Biochimica et biophysica acta. General subjects 1862(3) (2018) 474–484. [DOI] [PubMed] [Google Scholar]
- [117].Whoolery CW, Walker AK, Richardson DR, Lucero MJ, Reynolds RP, Beddow DH, Clark KL, Shih HY, LeBlanc JA, Cole MG, Amaral WZ, Mukherjee S, Zhang S, Ahn F, Bulin SE, DeCarolis NA, Rivera PD, Chen BPC, Yun S, Eisch AJ, Whole-Body Exposure to (28)Si-Radiation Dose-Dependently Disrupts Dentate Gyrus Neurogenesis and Proliferation in the Short Term and New Neuron Survival and Contextual Fear Conditioning in the Long Term, Radiation research 188(5) (2017) 532–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, Hinoi E, Yoneda Y, Takarada T, Disruption of Bmal1 Impairs Blood-Brain Barrier Integrity via Pericyte Dysfunction, The Journal of neuroscience : the official journal of the Society for Neuroscience 37(42) (2017) 10052–10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nirwane A, Gautam J, Yao Y, Isolation of Type I and Type II Pericytes from Mouse Skeletal Muscles, Journal of visualized experiments : JoVE (123) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Roelofs AJ, Zupan J, Riemen AHK, Kania K, Ansboro S, White N, Clark SM, De Bari C, Joint morphogenetic cells in the adult mammalian synovium, Nature communications 8 (2017) 15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Stuelsatz P, Keire P, Yablonka-Reuveni Z, Isolation, Culture, and Immunostaining of Skeletal Muscle Myofibers from Wildtype and Nestin-GFP Mice as a Means to Analyze Satellite Cell, Methods Mol Biol 1556 (2017) 51–102. [DOI] [PubMed] [Google Scholar]
- [122].Vadodaria KC, Yanpallewar SU, Vadhvani M, Toshniwal D, Liles LC, Rommelfanger KS, Weinshenker D, Vaidya VA, Noradrenergic regulation of plasticity marker expression in the adult rodent piriform cortex, Neuroscience letters 644 (2017) 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS, Differential cytokine contributions of perivascular haematopoietic stem cell niches, Nature cell biology 19(3) (2017) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gautam J, Nirwane A, Yao Y, Laminin differentially regulates the stemness of type I and type II pericytes, Stem cell research & therapy 8(1) (2017) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Stuelsatz P, Keire P, Yablonka-Reuveni Z, Erratum to: Isolation, Culture, and Immunostaining of Skeletal Muscle Myofibers from Wildtype and Nestin-GFP Mice as a Means to Analyze Satellite Cells, Methods Mol Biol 1556 (2017) E1. [DOI] [PubMed] [Google Scholar]
- [126].Wang X, Seekaew P, Gao X, Chen J, Traumatic Brain Injury Stimulates Neural Stem Cell Proliferation via Mammalian Target of Rapamycin Signaling Pathway Activation, eNeuro 3(5) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Liu W, Dan X, Wang T, Lu WW, Pan H, A Bone-Implant Interaction Mouse Model for Evaluating Molecular Mechanism of Biomaterials/Bone Interaction, Tissue engineering. Part C, Methods 22(11) (2016) 1018–1027. [DOI] [PubMed] [Google Scholar]
- [128].Steenblock C, Rubin de Celis MF, Androutsellis-Theotokis A, Sue M, Delgadillo Silva LF, Eisenhofer G, Andoniadou CL, Bornstein SR, Adrenal cortical and chromaffin stem cells: Is there a common progeny related to stress adaptation?, Molecular and cellular endocrinology 441 (2017) 156–163. [DOI] [PubMed] [Google Scholar]
- [129].Koning JJ, Konijn T, Lakeman KA, O’Toole T, Kenswil KJ, Raaijmakers MH, Michurina TV, Enikolopov G, Mebius RE, Nestin-Expressing Precursors Give Rise to Both Endothelial as well as Nonendothelial Lymph Node Stromal Cells, J Immunol 197(7) (2016) 2686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cao W, Liu F, Amoh Y, Hoffman RM, Protocols for Gelfoam((R)) Histoculture of Hair-Shaft-Producing Mouse Whisker Follicles Containing Nestin-GFP-Expressing Hair-Follicle-Associated Pluripotent (HAP) Stem Cells for Long Time Periods, Methods Mol Biol 1453 (2016) 145–50. [DOI] [PubMed] [Google Scholar]
- [131].Mignone J, Peunova N, Enikolopov G, Nestin-Based Reporter Transgenic Mouse Lines, Methods Mol Biol 1453 (2016) 7–14. [DOI] [PubMed] [Google Scholar]
- [132].Moss J, Gebara E, Bushong EA, Sanchez-Pascual I, O’Laoi R, El M’Ghari I, Kocher-Braissant J, Ellisman MH, Toni N, Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature, Proceedings of the National Academy of Sciences of the United States of America 113(18) (2016) E2536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Krityakiarana W, Zhao PM, Nguyen K, Gomez-Pinilla F, Kotchabhakdi N, de Vellis J, Espinosa-Jeffrey A, Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration, Neurochemical research 41(1–2) (2016) 431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Saboor F, Reckmann AN, Tomczyk CU, Peters DM, Weissmann N, Kaschtanow A, Schermuly RT, Michurina TV, Enikolopov G, Muller D, Mietens A, Middendorff R, Nestin-expressing vascular wall cells drive development of pulmonary hypertension, The European respiratory journal 47(3) (2016) 876–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Xu W, Jiang S, Chen Q, Ye Y, Chen J, Heng BC, Jiang Q, Wu B, Ding Z, Zhang C, Systemically Transplanted Bone Marrow-derived Cells Contribute to Dental Pulp Regeneration in a Chimeric Mouse Model, Journal of endodontics 42(2) (2016) 263–8. [DOI] [PubMed] [Google Scholar]
- [136].Zeng Q, Zheng M, Zhang T, He G, Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer’s disease, Neuroscience 314 (2016) 64–74. [DOI] [PubMed] [Google Scholar]
- [137].Jhaveri DJ, O’Keeffe I, Robinson GJ, Zhao QY, Zhang ZH, Nink V, Narayanan RK, Osborne GW, Wray NR, Bartlett PF, Purification of neural precursor cells reveals the presence of distinct, stimulus-specific subpopulations of quiescent precursors in the adult mouse hippocampus, The Journal of neuroscience : the official journal of the Society for Neuroscience 35(21) (2015) 8132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE, PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity, Genes & development 29(11) (2015) 1106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Belkind-Gerson J, Hotta R, Nagy N, Thomas AR, Graham H, Cheng L, Solorzano J, Nguyen D, Kamionek M, Dietrich J, Cherayil BJ, Goldstein AM, Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism, Inflammatory bowel diseases 21(4) (2015) 870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kara N, Narayanan S, Belmaker RH, Einat H, Vaidya VA, Agam G, Chronic Lithium Treatment Enhances the Number of Quiescent Neural Progenitors but Not the Number of DCX-Positive Immature Neurons, The international journal of neuropsychopharmacology 18(7) (2015) pyv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O, Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner, Stem cell research & therapy 5(6) (2014) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S, The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function, eLife 3 (2014) e03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stuelsatz P, Shearer A, Li Y, Muir LA, Ieronimakis N, Shen QW, Kirillova I, Yablonka-Reuveni Z, Extraocular muscle satellite cells are high performance myo-engines retaining efficient regenerative capacity in dystrophin deficiency, Developmental biology 397(1) (2015) 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].DeCarolis NA, Rivera PD, Ahn F, Amaral WZ, LeBlanc JA, Malhotra S, Shih HY, Petrik D, Melvin N, Chen BP, Eisch AJ, (56)Fe Particle Exposure Results in a Long-Lasting Increase in a Cellular Index of Genomic Instability and Transiently Suppresses Adult Hippocampal Neurogenesis in Vivo, Life sciences in space research 2 (2014) 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Klein D, Meissner N, Kleff V, Jastrow H, Yamaguchi M, Ergun S, Jendrossek V, Nestin(+) tissue-resident multipotent stem cells contribute to tumor progression by differentiating into pericytes and smooth muscle cells resulting in blood vessel remodeling, Frontiers in oncology 4 (2014) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Guha SK, Tillu R, Sood A, Patgaonkar M, Nanavaty IN, Sengupta A, Sharma S, Vaidya VA, Pathak S, Single episode of mild murine malaria induces neuroinflammation, alters microglial profile, impairs adult neurogenesis, and causes deficits in social and anxiety-like behavior, Brain, behavior, and immunity 42 (2014) 123–37. [DOI] [PubMed] [Google Scholar]
- [147].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ, Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow, Cell stem cell 15(2) (2014) 154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jhaveri DJ, Nanavaty I, Prosper BW, Marathe S, Husain BF, Kernie SG, Bartlett PF, Vaidya VA, Opposing effects of alpha2- and beta-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis, PloS one 9(6) (2014) e98736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS, Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development, Developmental cell 29(3) (2014) 340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O, Type-2 pericytes participate in normal and tumoral angiogenesis, American journal of physiology. Cell physiology 307(1) (2014) C25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Thakker-Varia S, Behnke J, Doobin D, Dalal V, Thakkar K, Khadim F, Wilson E, Palmieri A, Antila H, Rantamaki T, Alder J, VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling, Stem cell research 12(3) (2014) 762–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Macas J, Ku MC, Nern C, Xu Y, Buhler H, Remke M, Synowitz M, Franz K, Seifert V, Plate KH, Kettenmann H, Glass R, Momma S, Generation of neuronal progenitor cells in response to tumors in the human brain, Stem Cells 32(1) (2014) 244–57. [DOI] [PubMed] [Google Scholar]
- [153].Mii S, Amoh Y, Katsuoka K, Hoffman RM, Comparison of nestin-expressing multipotent stem cells in the tongue fungiform papilla and vibrissa hair follicle, Journal of cellular biochemistry 115(6) (2014) 1070–6. [DOI] [PubMed] [Google Scholar]
- [154].Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O, Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle, American journal of physiology. Cell physiology 305(11) (2013) C1098–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Park JH, Glass Z, Sayed K, Michurina TV, Lazutkin A, Mineyeva O, Velmeshev D, Ward WF, Richardson A, Enikolopov G, Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain, The European journal of neuroscience 37(12) (2013) 1987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Tuo Y, Jiang MH, Cai B, Deng CH, Xiang P, [Expression of P75NTR in the testis of nestin-GFP transgenic mice], Zhonghua nan ke xue = National journal of andrology 19(5) (2013) 392–7. [PubMed] [Google Scholar]
- [157].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Role of pericytes in skeletal muscle regeneration and fat accumulation, Stem cells and development 22(16) (2013) 2298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z, Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice, The FEBS journal 280(17) (2013) 4063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Abla AA, Sanai N, GFP+ cells in nestin-GFP adult mouse hippocampus are radial glia-like quiescent neural stem cells capable of gamma-aminobutyric acid-mediated regulation by parvalbumin-expressing interneurons, World neurosurgery 79(2) (2013) 216–7. [DOI] [PubMed] [Google Scholar]
- [160].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Skeletal muscle pericyte subtypes differ in their differentiation potential, Stem cell research 10(1) (2013) 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Skeletal muscle neural progenitor cells exhibit properties of NG2-glia, Experimental cell research 319(1) (2013) 45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J, Goldstein AM, Nestin-expressing cells in the gut give rise to enteric neurons and glial cells, Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 25(1) (2013) 61–9 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mendes-Jorge L, Llombart C, Ramos D, Lopez-Luppo M, Valenca A, Nacher V, Navarro M, Carretero A, Mendez-Ferrer S, Rodriguez-Baeza A, Ruberte J, Intercapillary bridging cells: immunocytochemical characteristics of cells that connect blood vessels in the retina, Experimental eye research 98 (2012) 79–87. [DOI] [PubMed] [Google Scholar]
- [164].Yang CP, Gilley JA, Zhang G, Kernie SG, ApoE is required for maintenance of the dentate gyrus neural progenitor pool, Development 138(20) (2011) 4351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Marinaro C, Butti E, Bergamaschi A, Papale A, Furlan R, Comi G, Martino G, Muzio L, In vivo fate analysis reveals the multipotent and self-renewal features of embryonic AspM expressing cells, PloS one 6(4) (2011) e19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O, Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle, PloS one 6(2) (2011) e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hoffman RM, Nestin-driven green fluorescent protein as an imaging marker for nascent blood vessels in mouse models of cancer, Methods Mol Biol 689 (2011) 183–204. [DOI] [PubMed] [Google Scholar]
- [168].Wolf SA, Melnik A, Kempermann G, Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia, Brain, behavior, and immunity 25(5) (2011) 971–80. [DOI] [PubMed] [Google Scholar]
- [169].Smeti I, Savary E, Capelle V, Hugnot JP, Uziel A, Zine A, Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice, Gene expression patterns : GEP 11(1–2) (2011) 22–32. [DOI] [PubMed] [Google Scholar]
- [170].Khoury M, Bransky A, Korin N, Konak LC, Enikolopov G, Tzchori I, Levenberg S, A microfluidic traps system supporting prolonged culture of human embryonic stem cells aggregates, Biomedical microdevices 12(6) (2010) 1001–8. [DOI] [PubMed] [Google Scholar]
- [171].Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Muller A, Melnik A, Waltinger TP, Ullrich O, Kempermann G, Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis, Cell communication and signaling : CCS 8 (2010) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Krityakiarana W, Espinosa-Jeffrey A, Ghiani CA, Zhao PM, Topaldjikian N, Gomez-Pinilla F, Yamaguchi M, Kotchabhakdi N, de Vellis J, Voluntary exercise increases oligodendrogenesis in spinal cord, The International journal of neuroscience 120(4) (2010) 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Bennett LB, Cai J, Enikolopov G, Iacovitti L, Heterotopically transplanted CVO neural stem cells generate neurons and migrate with SVZ cells in the adult mouse brain, Neuroscience letters 475(1) (2010) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Marz M, Chapouton P, Diotel N, Vaillant C, Hesl B, Takamiya M, Lam CS, Kah O, Bally-Cuif L, Strahle U, Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon, Glia 58(7) (2010) 870–88. [DOI] [PubMed] [Google Scholar]
- [175].Day K, Shefer G, Shearer A, Yablonka-Reuveni Z, The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny, Developmental biology 340(2) (2010) 330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kim Y, Comte I, Szabo G, Hockberger P, Szele FG, Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration, PloS one 4(12) (2009) e8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Muller D, Hida B, Guidone G, Speth RC, Michurina TV, Enikolopov G, Middendorff R, Expression of guanylyl cyclase (GC)-A and GC-B during brain development: evidence for a role of GC-B in perinatal neurogenesis, Endocrinology 150(12) (2009) 5520–9. [DOI] [PubMed] [Google Scholar]
- [178].Gao X, Enikolopov G, Chen J, Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus, Experimental neurology 219(2) (2009) 516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Petrus DS, Fabel K, Kronenberg G, Winter C, Steiner B, Kempermann G, NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis, The European journal of neuroscience 29(2) (2009) 244–52. [DOI] [PubMed] [Google Scholar]
- [180].Amoh Y, Katsuoka K, Hoffman RM, Color-coded fluorescent protein imaging of angiogenesis: the AngioMouse models, Current pharmaceutical design 14(36) (2008) 3810–9. [DOI] [PubMed] [Google Scholar]
- [181].Hayashi A, Moradzadeh A, Tong A, Wei C, Tuffaha SH, Hunter DA, Tung TH, Parsadanian A, Mackinnon SE, Myckatyn TM, Treatment modality affects allograft-derived Schwann cell phenotype and myelinating capacity, Experimental neurology 212(2) (2008) 324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M, Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis, The American journal of pathology 172(6) (2008) 1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Murdoch B, Roskams AJ, A novel embryonic nestin-expressing radial glia-like progenitor gives rise to zonally restricted olfactory and vomeronasal neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(16) (2008) 4271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Donovan MH, Yamaguchi M, Eisch AJ, Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus, Hippocampus 18(5) (2008) 435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, Keyes WM, Mills AA, Gleiberman A, Zhang MQ, Enikolopov G, Neural potential of a stem cell population in the hair follicle, Cell Cycle 6(17) (2007) 2161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kronenberg G, Wang LP, Geraerts M, Babu H, Synowitz M, Vicens P, Lutsch G, Glass R, Yamaguchi M, Baekelandt V, Debyser Z, Kettenmann H, Kempermann G, Local origin and activity -dependent generation of nestin-expressing protoplasmic astrocytes in CA1, Brain structure & function 212(1) (2007) 19–35. [DOI] [PubMed] [Google Scholar]
- [187].Park JH, Ahn JI, Kim SY, Park KS, Lee YD, Yamaguchi M, Chung HJ, Genetic modification does not affect the stemness of neural stem cells in nestin promoter-GFP transgenic mice, Neuroscience letters 421(3) (2007) 185–90. [DOI] [PubMed] [Google Scholar]
- [188].Patschan D, Michurina T, Shi HK, Dolff S, Brodsky SV, Vasilieva T, Cohen-Gould L, Winaver J, Chander PN, Enikolopov G, Goligorsky MS, Normal distribution and medullary-to-cortical shift of Nestin-expressing cells in acute renal ischemia, Kidney international 71(8) (2007) 744–54. [DOI] [PubMed] [Google Scholar]
- [189].Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z, Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells, Developmental biology 304(1) (2007) 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA, Neurocytoma is a tumor of adult neuronal progenitor cells, The Journal of neuroscience : the official journal of the Society for Neuroscience 26(48) (2006) 12544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T, GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells, Neuron 47(6) (2005) 803–15. [DOI] [PubMed] [Google Scholar]
- [192].Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, Enikolopov G, Expression of nestin-green fluorescent protein transgene marks oval cells in the adult liver, Developmental dynamics : an official publication of the American Association of Anatomists 234(2) (2005) 413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kronenberg G, Wang LP, Synowitz M, Gertz K, Katchanov J, Glass R, Harms C, Kempermann G, Kettenmann H, Endres M, Nestin-expressing cells divide and adopt a complex electrophysiologic phenotype after transient brain ischemia, Journal of cerebral blood flow and metabolism : official journal of the International Societyof Cerebral Blood Flow and Metabolism 25(12) (2005) 1613–24. [DOI] [PubMed] [Google Scholar]
- [194].Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H, Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival, The Journal of neuroscience : the official journal of the Society for Neuroscience 25(10) (2005) 2637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Hoffman RM, Imaging tumor angiogenesis with fluorescent proteins, APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 112(7–8) (2004) 441–9. [DOI] [PubMed] [Google Scholar]
- [196].Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G, Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli, The Journal of comparative neurology 467(4) (2003) 455–63. [DOI] [PubMed] [Google Scholar]
- [197].Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM, Nestin expression in hair follicle sheath progenitor cells, Proceedings of the National Academy of Sciences of the United States of America 100(17) (2003) 9958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G, Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes, Molecular and cellular neurosciences 23(3) (2003) 373–82. [DOI] [PubMed] [Google Scholar]
- [199].Yoshida N, Hishiyama S, Yamaguchi M, Hashiguchi M, Miyamoto Y, Kaminogawa S, Hisatsune T, Decrease in expression of alpha 5 beta 1 integrin during neuronal differentiation of cortical progenitor cells, Experimental cell research 287(2) (2003) 262–71. [DOI] [PubMed] [Google Scholar]
- [200].Sawamoto K, Nakao N, Kakishita K, Ogawa Y, Toyama Y, Yamamoto A, Yamaguchi M, Mori K, Goldman SA, Itakura T, Okano H, Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-GFP transgene, The Journal of neuroscience : the official journal of the Society for Neuroscience 21(11) (2001) 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yamaguchi M, Saito H, Suzuki M, Mori K, Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice, Neuroreport 11(9) (2000) 1991–6. [DOI] [PubMed] [Google Scholar]
- [202].Lam CS, Marz M, Strahle U, gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain, Developmental dynamics : an official publication of the American Association of Anatomists 238(2) (2009) 475–86. [DOI] [PubMed] [Google Scholar]
- [203].Jiang Y, Dong H, Eckmann L, Hanson EM, Ihn KC, Mittal RK, Visualizing the enteric nervous system using genetically engineered double reporter mice: Comparison with immunofluorescence, PloS one 12(2) (2017) e0171239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ma M, Luo M, Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb, The Journal of neuroscience : the official journal of the Society for Neuroscience 32(30) (2012) 10105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Perez-Medina AL, Galligan JJ, Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice, Am J Physiol Gastrointest Liver Physiol 317(5) (2019) G569–G579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Wiese CB, Fleming N, Buehler DP, Southard-Smith EM, A Uchl1-Histone2BmCherry:GFP-gpi BAC transgene for imaging neuronal progenitors, Genesis 51(12) (2013) 852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Genc B, Lagrimas AK, Kuru P, Hess R, Tu MW, Menichella DM, Miller RJ, Paller AS, Ozdinler PH, Visualization of Sensory Neurons and Their Projections in an Upper Motor Neuron Reporter Line, PloS one 10(7) (2015) e0132815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI, BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons, Physiological genomics 27(3) (2006) 391–7. [DOI] [PubMed] [Google Scholar]
- [209].Brown TC, Bond CE, Hoover DB, Variable expression of GFP in different populations of peripheral cholinergic neurons of ChAT(BAC)-eGFP transgenic mice, Autonomic neuroscience : basic & clinical 210 (2018) 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].van Hummel A, Bi M, Ippati S, van der Hoven J, Volkerling A, Lee WS, Tan DC, Bongers A, Ittner A, Ke YD, Ittner LM, No Overt Deficits in Aged Tau-Deficient C57Bl/6.Mapttm1(EGFP)Kit GFP Knockin Mice, PloS one 11(10) (2016) e0163236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Lionnet A, Wade MA, Corbille AG, Prigent A, Paillusson S, Tasselli M, Gonzales J, Durieu E, Rolli-Derkinderen M, Coron E, Duchalais E, Neunlist M, Perkinton MS, Hanger DP, Noble W, Derkinderen P, Characterisation of tau in the human and rodent enteric nervous system under physiological conditions and in tauopathy, Acta neuropathologica communications 6(1) (2018) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Joshi SS, Tandukar B, Pan L, Huang JM, Livak F, Smith BJ, Hodges T, Mahurkar AA, Hornyak TJ, CD34 defines melanocyte stem cell subpopulations with distinct regenerative properties, PLoS Genet 15(4) (2019) e1008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Millet V, Marder M, Pasquini LA, Adult CNP::EGFP transgenic mouse shows pronounced hypomyelination and an increased vulnerability to cuprizone-induced demyelination, Experimental neurology 233(1) (2012) 490–504. [DOI] [PubMed] [Google Scholar]
- [214].Lytle JM, Chittajallu R, Wrathall JR, Gallo V, NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury, Glia 57(3) (2009) 270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sohn J, Natale J, Chew LJ, Belachew S, Cheng Y, Aguirre A, Lytle J, Nait-Oumesmar B, Kerninon C, Kanai-Azuma M, Kanai Y, Gallo V, Identification of Sox17 as a transcription factor that regulates oligodendrocyte development, The Journal of neuroscience : the official journal of the Society for Neuroscience 26(38) (2006) 9722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Thallmair M, Ray J, Stallcup WB, Gage FH, Functional and morphological effects of NG2 proteoglycan deletion on hippocampal neurogenesis, Experimental neurology 202(1) (2006) 167–78. [DOI] [PubMed] [Google Scholar]
- [217].Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C, Spatial and temporal expression of S100B in cells of oligodendrocyte lineage, Glia 51(2) (2005) 81–97. [DOI] [PubMed] [Google Scholar]
- [218].Aguirre A, Gallo V, Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone, The Journal of neuroscience : the official journal of the Society for Neuroscience 24(46) (2004) 10530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V, Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies, Journal of neuroscience research 70(4) (2002) 529–45. [DOI] [PubMed] [Google Scholar]
- [220].McLenachan S, Goldshmit Y, Fowler KJ, Voullaire L, Holloway TP, Turnley AM, Ioannou PA, Sarsero JP, Transgenic mice expressing the Peripherin-EGFP genomic reporter display intrinsic peripheral nervous system fluorescence, Transgenic research 17(6) (2008) 1103–16. [DOI] [PubMed] [Google Scholar]
- [221].Rivkin E, Cordes SP, Generation of a transgenic mouse line expressing GFP-Cre protein from a Hoxb4 neural enhancer, Genesis 46(2) (2008) 119–24. [DOI] [PubMed] [Google Scholar]
- [222].Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE, Math1-driven GFP expression in the developing nervous system of transgenic mice, Gene expression patterns : GEP 3(4) (2003) 389–95. [DOI] [PubMed] [Google Scholar]
- [223].Yang J, Yang J, Li Y, Xu Y, Ran C, Near-infrared Fluorescence Ocular Imaging (NIRFOI) of Alzheimer’s Disease, Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 21(1) (2019) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Li BN, Li WD, Feng HG, Lin JT, Yuan ZQ, Construction and identification of pIRES(2)-NGFVEGF(1)(6)(5) bicistronic eukaryotic expression vector, Genetics and molecular research : GMR 13(3) (2014) 5674–85. [DOI] [PubMed] [Google Scholar]
- [225].Kawaja MD, Smithson LJ, Elliott J, Trinh G, Crotty AM, Michalski B, Fahnestock M, Nerve growth factor promoter activity revealed in mice expressing enhanced green fluorescent protein, The Journal of comparative neurology 519(13) (2011) 2522–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Tozaki-Saitoh H, Masuda J, Kawada R, Kojima C, Yoneda S, Masuda T, Inoue K, Tsuda M, Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development, Glia 67(4) (2019) 729–740. [DOI] [PubMed] [Google Scholar]
- [227].Meas SJ, Nishimura K, Scheibinger M, Dabdoub A, In vitro Methods to Cultivate Spiral Ganglion Cells, and Purification of Cellular Subtypes for Induced Neuronal Reprogramming, Frontiers in neuroscience 12 (2018) 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Stoltz K, Sinyuk M, Hale JS, Wu Q, Otvos B, Walker K, Vasanji A, Rich JN, Hjelmeland AB, Lathia JD, Development of a Sox2 reporter system modeling cellular heterogeneity in glioma, Neuro-oncology 17(3) (2015) 361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Lee HJ, Wu J, Chung J, Wrathall JR, SOX2 expression is upregulated in adult spinal cord after c ontusion injury in both oligodendrocyte lineage and ependymal cells, Journal of neuroscience research 91(2) (2013) 196–210. [DOI] [PubMed] [Google Scholar]
- [230].Agrawal V, Siu BF, Chao H, Hirschi KK, Raborn E, Johnson SA, Tottey S, Hurley KB, Medberry CJ, Badylak SF, Partial characterization of the Sox2+ cell population in an adult murine model of digit amputation, Tissue engineering. Part A 18(13–14) (2012) 1454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Langer L, Taranova O, Sulik K, Pevny L, SOX2 hypomorphism disrupts development of the prechordal floor and optic cup, Mechanisms of development 129(1–4) (2012) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M, Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells, Proceedings of the National Academy of Sciences of the United States of America 109(7) (2012) 2527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Hutton SR, Pevny LH, SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon, Developmental biology 352(1) (2011) 40–7. [DOI] [PubMed] [Google Scholar]
- [234].Wang S, Chandler-Militello D, Lu G, Roy NS, Zielke A, Auvergne R, Stanwood N, Geschwind D, Coppola G, Nicolis SK, Sim FJ, Goldman SA, Prospective identification, isolation, and profiling of a telomerase-expressing subpopulation of human neural stem cells, using sox2 enhancer-directed fluorescence-activated cell sorting, The Journal of neuroscience : the official journal of the Society for Neuroscience 30(44) (2010) 14635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL, Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm, Development 134(13) (2007) 2521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Okubo T, Pevny LH, Hogan BL, Sox2 is required for development of taste bud sensory cells, Genes & development 20(19) (2006) 2654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS, Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain, Aging cell 4(4) (2005) 197–207. [DOI] [PubMed] [Google Scholar]
- [238].Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L, SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult, Developmental neuroscience 26(2–4) (2004) 148–65. [DOI] [PubMed] [Google Scholar]
- [239].Papanikolaou M, Lewis A, Butt AM, Glial and neuronal expression of the Inward Rectifying Potassium Channel Kir7.1 in the adult mouse brain, J Anat 235(5) (2019) 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Matsuo K, Ji S, Miya A, Yoda M, Hamada Y, Tanaka T, Takao-Kawabata R, Kawaai K, Kuroda Y, Shibata S, Innervation of the tibial epiphysis through the intercondylar foramen, Bone 120 (2019) 297–304. [DOI] [PubMed] [Google Scholar]
- [241].Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA, Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(1) (2011) 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Chan CS, Peterson JD, Gertler TS, Glajch KE, Quintana RE, Cui Q, Sebel LE, Plotkin JL, Shen W, Heiman M, Heintz N, Greengard P, Surmeier DJ, Strain-specific regulation of striatal phenotype in Drd2-eGFP BAC transgenic mice, The Journal of neuroscience : the official journal of the Society for Neuroscience 32(27) (2012) 9124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Shuen JA, Chen M, Gloss B, Calakos N, Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(11) (2008) 2681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Ade KK, Wan Y, Chen M, Gloss B, Calakos N, An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons, Frontiers in systems neuroscience 5 (2011) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, Leidenheimer NJ, Rusakov DA, Ahrens MB, Kullmann DM, Looger LL, A genetically encoded fluorescent sensor for in vivo imaging of GABA, Nature methods (2019). [DOI] [PubMed] [Google Scholar]
- [246].Agostinelli LJ, Geerling JC, Scammell TE, Basal forebrain subcortical projections, Brain structure & function 224(3) (2019) 1097–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Zhu Y, Xu J, Hauswirth WW, DeVries SH, Genetically targeted binary labeling of retinal neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 34(23) (2014) 7845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Okabe S, Fluorescence imaging of synapse dynamics in normal circuit maturation and in developmental disorders, Proceedings of the Japan Academy. Series B, Physical and biological sciences 93(7) (2017) 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, Osten P, Serial two-photon tomography for automated ex vivo mouse brain imaging, Nature methods 9(3) (2012) 255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, Kim DS, Svoboda K, Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo, PloS one 9(9) (2014) e108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Chen Q, Cichon J, Wang W, Qiu L, Lee SJ, Campbell NR, Destefino N, Goard MJ, Fu Z, Yas uda R, Looger LL, Arenkiel BR, Gan WB, Feng G, Imaging neural activity using Thy1-GCaMP transgenic mice, Neuron 76(2) (2012) 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Stefaniuk M, Gualda EJ, Pawlowska M, Legutko D, Matryba P, Koza P, Konopka W, Owczarek D, Wawrzyniak M, Loza-Alvarez P, Kaczmarek L, Light-sheet microscopy imaging of a whole cleared rat brain with Thy1-GFP transgene, Scientific reports 6 (2016) 28209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Weissman TA, Pan YA, Brainbow: new resources and emerging biological applications for multic olor genetic labeling and analysis, Genetics 199(2) (2015) 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW, Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system, Nature 450(7166) (2007) 56–62. [DOI] [PubMed] [Google Scholar]
- [255].Krishnasamy S, Weng YC, Thammisetty SS, Phaneuf D, Lalancette-Hebert M, Kriz J, Molecular imagingof nestin in neuroinflammatory conditions reveals marked signal induction in activated microglia, Journal of neuroinflammation 14(1) (2017) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Bernal A, Arranz L, Nestin-expressing progenitor cells: function, identity and therapeutic implications, Cellular and molecular life sciences : CMLS 75(12) (2018) 2177–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE, Expression of nestin by neural cells in the adult rat and human brain, PloS one 6(4) (2011) e18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Sunabori T, Tokunaga A, Nagai T, Sawamoto K, Okabe M, Miyawaki A, Matsuzaki Y, Miyata T, Okano H, Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors, Journal of cell science 121(Pt 8) (2008) 1204–12. [DOI] [PubMed] [Google Scholar]
- [259].Becker K, Jahrling N, Saghafi S, Weiler R, Dodt HU, Chemical clearing and dehydration of GFP expressing mouse brains, PloS one 7(3) (2012) e33916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Magliaro C, Callara AL, Mattei G, Morcinelli M, Viaggi C, Vaglini F, Ahluwalia A, Clarifying CLARITY: Quantitative Optimization of the Diffusion Based Delipidation Protocol for Genetically Labeled Tissue, Frontiers in neuroscience 10 (2016) 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Li Y, Xu J, Wan P, Yu T, Zhu D, Optimization of GFP Fluorescence Preservation by a Modified uDISCO Clearing Protocol, Frontiers in neuroanatomy 12 (2018) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Scott DJ, Gunn NJ, Yong KJ, Wimmer VC, Veldhuis NA, Challis LM, Haidar M, Petrou S, Bathgate RAD, Griffin MDW, A Novel Ultra-Stable, Monomeric Green Fluorescent Protein For Direct Volumetric Imaging of Whole Organs Using CLARITY, Scientific reports 8(1) (2018) 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Walsh MK, Lichtman JW, In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination, Neuron 37(1) (2003) 67–73. [DOI] [PubMed] [Google Scholar]
- [264].Mohan S, Hernandez IC, Wang W, Yin K, Sundback CA, Wegst UGK, Jowett N, Fluorescent Reporter Mice for Nerve Guidance Conduit Assessment: A High-Throughput in vivo Model, The Laryngoscope 128(11) (2018) E386–E392. [DOI] [PubMed] [Google Scholar]
- [265].Hirrlinger J, Marx G, Besser S, Sicker M, Kohler S, Hirrlinger PG, Wojcik SM, Eulenburg V, Winkler U, Hulsmann S, GABA-Glycine Cotransmitting Neurons in the Ventrolateral Medulla: Development and Functional Relevance for Breathing, Front Cell Neurosci 13 (2019) 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Herzer S, Hagan C, von Gerichten J, Dieterle V, Munteanu B, Sandhoff R, Hopf C, Nordstrom V, Deletion of Specific Sphingolipids in Distinct Neurons Improves Spatial Memory in a Mouse Model of Alzheimer’s Disease, Front Mol Neurosci 11 (2018) 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Cardona SM, Dunphy JM, Das AS, Lynch CR, Lynch WP, Astrocyte Infection Is Required for Retrovirus-Induced Spongiform Neurodegeneration Despite Suppressed Viral Protein Expression, Frontiers in neuroscience 13 (2019) 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Bird CW, Barber MJ, Post HR, Jacquez B, Chavez GJ, Faturos NG, Valenzuela CF, Neonatal ethanol exposure triggers apoptosis in the murine retrosplenial cortex: Role of inhibition of NMDA receptor-driven action potential firing, Neuropharmacology 162 (2020) 107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Orlans HO, Barnard AR, MacLaren RE, Dynamic in vivo quantification of rod photoreceptor degeneration using fluorescent reporter mouse models of retinitis pigmentosa, Experimental eye research 190 (2019) 107895. [DOI] [PubMed] [Google Scholar]
- [270].Hass DT, Barnstable CJ, Cell Autonomous Neuroprotection by the Mitochondrial Uncoupling Protein 2 in a Mouse Model of Glaucoma, Frontiers in neuroscience 13 (2019) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Hernandez-Garcia CM, Finer JJ, Identification and validation of promoters and cis-acting regulatory elements, Plant Sci 217–218 (2014) 109–19. [DOI] [PubMed] [Google Scholar]
- [272].Blankvoort S, Witter MP, Noonan J, Cotney J, Kentros C, Marked Diversity of Unique Cortical Enhancers Enables Neuron-Specific Tools by Enhancer-Driven Gene Expression, Curr Biol 28(13) (2018) 2103–2114 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Taylor-Clark TE, Wu KY, Thompson JA, Yang K, Bahia PK, Ajmo JM, Thy1.2 YFP-16 transgenic mouse labels a subset of large-diameter sensory neurons that lack TRPV1 expression, PloS one 10(3) (2015) e0119538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Zhu H, Swami S, Yang P, Shapiro F, Wu J, Direct reprogramming of mouse fibroblasts into functional osteoblasts, J Bone Miner Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Balan M, Trusohamn M, Ning FC, Jacob S, Pietras K, Eriksson U, Berggren PO, Nyqvist D, Noninvasive intravital high-resolution imaging of pancreatic neuroendocrine tumours, Scientific reports 9(1) (2019) 14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Gao X, Guo K, Santosa SM, Montana M, Yamakawa M, Hallak JA, Han KY, Doh SJ, Rosenblatt MI, Chang JH, Azar DT, Application of corneal injury models in dual fluorescent reporter transgenic mice to understand the roles of the cornea and limbus in angiogenic and lymphangiogenic privilege, Scientific reports 9(1) (2019) 12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Zhong W, Gao X, Wang S, Han K, Ema M, Adams S, Adams RH, Rosenblatt MI, Chang JH, Azar DT, Prox1-GFP/Flt1-DsRed transgenic mice: an animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis, Angiogenesis 20(4) (2017) 581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Sumbul U, Zlateski A, Vishwanathan A, Masland RH, Seung HS, Automated computation of arbor densities: a step toward identifying neuronal cell types, Frontiers in neuroanatomy 8 (2014) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Sumbul U, Song S, McCulloch K, Becker M, Lin B, Sanes JR, Masland RH, Seung HS, A genetic and computational approach to structurally classify neuronal types, Nature communications 5 (2014) 3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Sanes JR, Masland RH, The types of retinal ganglion cells: current status and implications for neuronal classification, Annual review of neuroscience 38 (2015) 221–46. [DOI] [PubMed] [Google Scholar]
- [281].Masland RH, Systems neuroscience: Diversity in sight, Nature 542(7642) (2017) 418–419. [DOI] [PubMed] [Google Scholar]
- [282].Marc R, Pfeiffer R, Jones B, Retinal prosthetics, optogenetics, and chemical photoswitches, ACS chemical neuroscience 5(10) (2014) 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]


