Abstract
Background
Dispersion in ventricular repolarization is relevant for arrhythmogenesis.
Objective
To determine the spatiotemporal effects of sympathetic stimulation on ventricular repolarization.
Methods
In five anesthetized female open chested pigs ventricular repolarization was measured from the anterior, lateral and posterior wall of the left (LV) and right ventricle (RV) using up to 40 transmural plunge needles (4 electrodes each), before and after left and right stellate stimulation (LSGS and RSGS). In addition, LSGS was performed in 3 pigs (2 male, 1 female) before and after verapamil (5–10 mg/hr) administration.
Results
LSGS yielded a biphasic response in repolarization in the lateral and posterior wall of the LV, with prolongation at ~5 seconds (+10±1.5 ms) and a shortening at 20–30 seconds of stimulation (–28.9±4.4 ms) during a monotonic pressure increase. While the initial prolongation was abolished by verapamil, the late shortening was augmented. Sequential transections of the vagal nerve and stellate ganglia augmented repolarization dispersion responses to LSGS in 2 out of 5 hearts. An equal pressure increase by aortic occlusion resulted in a homogeneous shortening of repolarization in the LV and the effects were smaller than during LSGS. RSGS shortened repolarization mainly in the anterior LV wall, but the effects were smaller than those of LSGS.
Conclusion
LSGS first prolongs (through the L-type calcium current) and then shortens repolarization. The effect of LSGS was prominent in the posterior and lateral, not the anterior, LV wall.
Keywords: Repolarization, T-wave, stellate ganglion, autonomic nervous system, heterogeneity
Introduction
Life-threatening ventricular arrhythmias are commonly initiated by autonomic triggers like exercise or emotional arousal 1–3. Antiarrhythmic therapy targeting the autonomic nervous system (ANS) is effective but the exact role of ANS in arrhythmogenesis is unclear 4–6.
Sympathetic stimulation shortens ventricular action potential duration, activation-recovery intervals, or refractory periods 7–12, although a lengthening has been observed as well 12–14. These changes may be time-dependent, or dependent on distribution of the autonomic nerves 12. Histological data point to a transmural distribution in innervation 15, but whether this dispersion in innervation leads to transmural dispersion in repolarization is unknown. The heterogeneity in cardiac innervation and the time dependence of its effects may lead to dispersion in repolarization and thus promote reentrant arrhythmias.12,16 In this study we aim to document the spatiotemporal ventricular repolarization changes following stellate stimulation.
In view of the complex feedback system that constitutes the autonomic nervous system we reasoned that the loss of neural connections to the central neural system would enhance the response of the heart to sympathetic stimulation 17, and increase the dispersion in repolarization. We therefore compared the ventricular repolarization changes before and after the removal of the afferent connections from the heart to the brain.
Methods
Animal handling and care were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the University of California, Los Angeles, Institutional Animal Care and Use Committees. Animal protocols were approved by the Chancellor’s Animal Research Committee, University of California, Los Angeles.
Animal preparation
Female Yorkshire pigs (Group 1, n=5, weighing 60–64 kg) were premedicated with intramuscular telazol (8–10 mg/kg), intubated and ventilated. A midsternal thoracotomy was performed and the stellate ganglia were prepared free (as published previously, see Supplementary Methods) 16. Left ventricular (LV) pressures were assessed by using a 5-F pigtail pressure catheter connected to a MPVS Ultra processor (Millar Instruments, Inc, Houston, TX) placed in the LV via a carotid artery. Umbilical tape (1/2”) was loosely placed around the descending aorta. Pulling the umbilical tape through tubing resulted in constriction of the aorta and a temporary increase in aortic and left ventricular pressure.
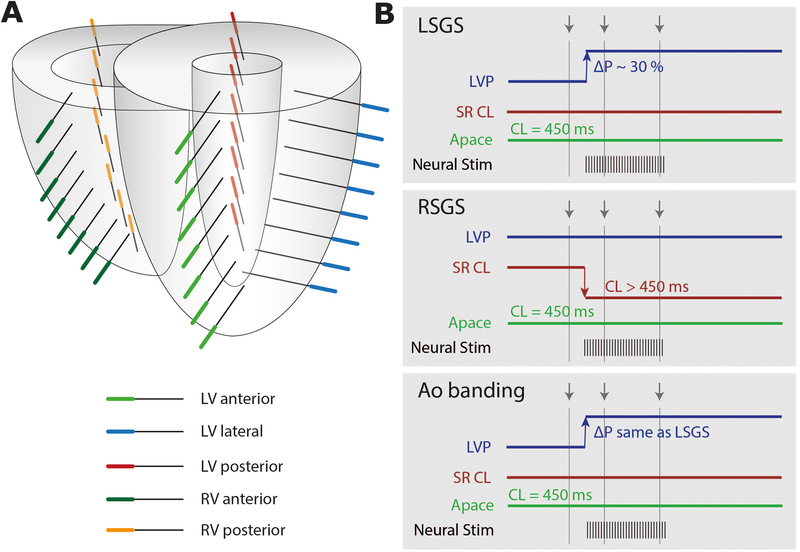
Transmural plunge needles (diameter 0.5 mm; n=40) with 4 electrodes each (4mm interelectrode distance) were inserted into the heart according to the diagram in Figure 1A: within the LV in the anterior part (8 needles), lateral part (8 needles) and posterior part (8 needles), and within the right ventricle in the posterior part (8 needles), and anterior part (8 needles) (Figure 1A). The reference electrode was positioned subcutaneously at the thoracic incision site. A bipolar stimulation electrode was attached to the right atrium. In 3 additional experiments (Group 2, 2 male, 1 female) LSGS was performed before and after verapamil (continuous IV infusion 5–10 mg/hr).
Figure 1.
A) Needle configuration. Three sets of 8 needles were inserted in the left ventricle (LV) in anterior, lateral and posterior wall. Two sets of 8 needles were inserted in the right ventricle (RV) in the anterior and posterior wall. B) Diagram of the autonomic manipulations: left stellate ganglion stimulation (LSGS), right stellate ganglion stimulation (RSGS), Aortic banding (AoBanding). Grey arrows indicate time points of analyses. LVP= left ventricular pressure, ΔP= change in pressure, SR= sinus rhythm, CL= cycle length, Apace= atrial pacing.
Stellate Ganglion Stimulation (SGS)
Right (RSG) or left (LSG) stellate ganglia were electrically stimulated through a bipolar platinum electrode connected to a Grass stimulator (S88; Grass Technologies, West Warwick, RI) via SIU6 constant current stimulus isolation units as published previously (see Supplementary Methods) 16. Figure 1B schematically shows the protocol. For LSGS a pressure increase by 30% and for RSGS a cycle length decrease to reach a cycle length of 450 ms was targeted.
Aortic constriction
During Aortic constriction we aimed at reaching the same LV pressure increase as obtained during LSGS.
After each of these interventions at least 5 minutes were allowed for normalization of the arterial pressure and heart rate to baseline values. The autonomic manipulations were performed before and after bilateral vagotomy (i.e. cut vagal nerves cranial to pacing site) and following bilateral decentralization of the stellate ganglia (i.e. cut stellate ganglion caudal to stimulation site). These procedures interrupt vagal and sympathetic afferent cardiac input to the central neural system, respectively.
Electrophysiological recordings
Recordings were made from a total of 160 electrode terminals (Group 1, 40 needles with 4 terminals each) and from 128 epicardial electrodes in Group 2. Unipolar electrograms were recorded via a 256-channel acquisition system (24 bit dynamic range, 122.07 nV LSB, total noise 0.5 microV [BioSemi], sampling frequency 2048 Hz (bandwidth [−3dB] DC-400 Hz)). Surface electrocardiograms (lead I, II and III) were recorded. Recordings were made during atrial pacing (cycle length 450 ms) before and during stellate ganglion stimulation and before and after aortic constriction prior to and following vagotomy and stellate decentralization.
Electrical signal analysis was performed offline using custom-made data analysis software based on MATLAB2016a (Mathworks Inc., Natick, Ma, USA) as published previously 18. Repolarization times (RT) were defined as the interval between the beginning of the QRS complex and the maximum positive slope of the T wave in the local electrograms.
Statistics
Data are presented as mean±SEM. A linear mixed model for repeated measurements was used to test the effect LSGS and RSGS on the repolarization and to explore interactions with spatial and temporal factors (see Supplementary Methods).
Results
LSGS: Biphasic changes in repolarization
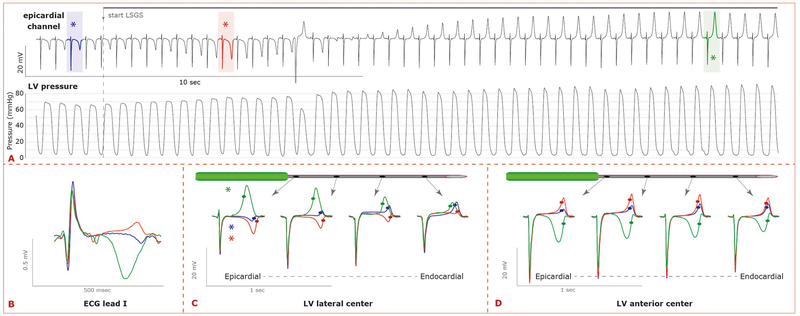
Figure 2 shows an example of the pressure and T-wave changes during LSGS. Pressure increased monotonically following the onset of LSGS, while the T-wave initially became increasingly more negative (EARLY phase of LSGS) and subsequently became positive (LATE phase of LSGS). This was reflected by the T-wave in the ECG (Figure 2B). The biphasic response was present in both male and female pigs (Supplemental Figure 1).
Figure 2.
A) Typical example of the changes in repolarization during LSGS. Superimposition of ECG lead I at the same time points as indicated in B and C. B) The changes in repolarization resulted in large T-wave changes in the ECG C+D) Superimposition of local electrograms recorded along a needle in the LV lateral (C) or LV anterior wall (D) before LSGS (PRE, blue), and during LSGS (EARLY, red; LATE, green). During the early phase of LSGS repolarization prolongs and during the late phase of LSGS repolarization shortens. The LSGS effects were largest in the left ventricular lateral and posterior wall. Note that in the left anterior wall the configuration of the local T-wave changes, but the moments of repolarization do not. The moments of repolarization are indicated by dots (maximum positive slope of the local T-wave).
Figures 2C and 2D show three superimposed electrograms (pre: blue, early: red, late: green) recorded at 4 electrode terminals on each of two needles, one in the left lateral (Figure 2C), the other in the left anterior wall (Figure 2D). T-wave changes occur at both needle positions, but local repolarization time (indicated by dots) changes in the left lateral but not in the left anterior sites. At all sites, the T-wave morphology changed in a biphasic pattern. Only at the lateral site (Figure 2C) prolongation of the repolarization time was followed by marked shortening of the repolarization time. More pronounced changes occurred at the LV subepicardium than in the LV subendocardium (Figure 2C).
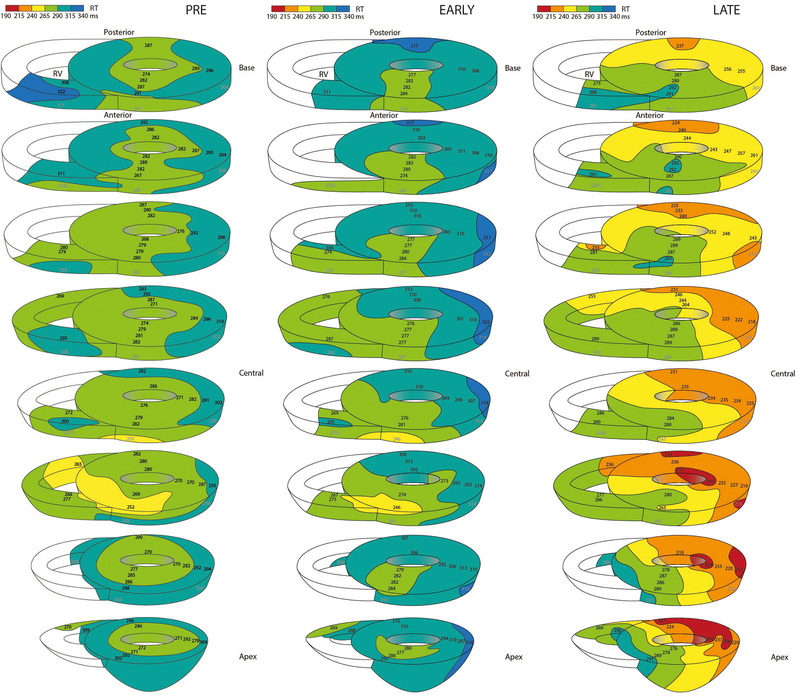
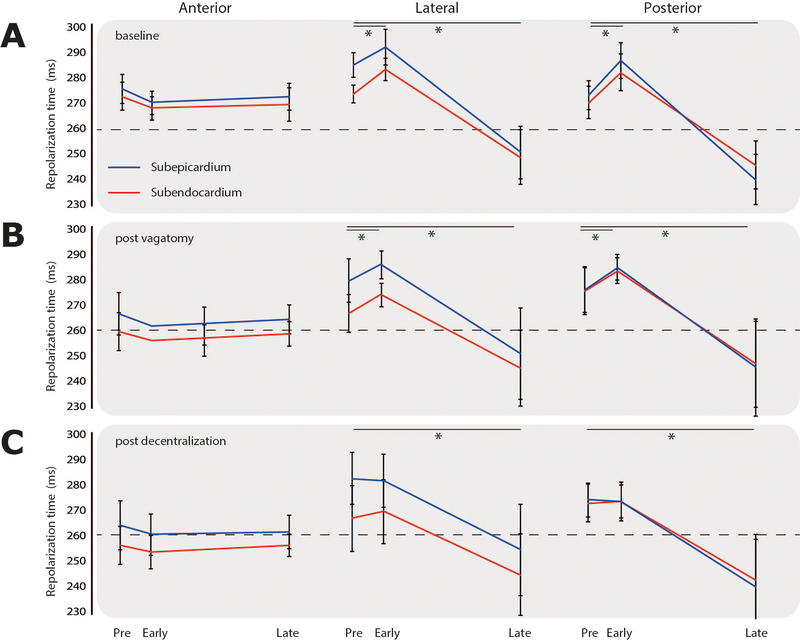
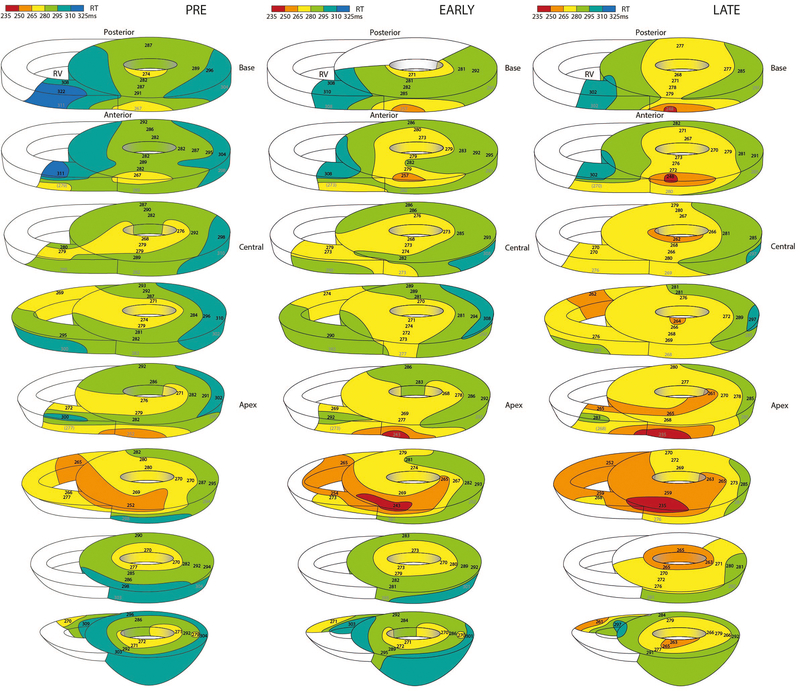
Figure 3 shows three-dimensional repolarization maps before, and at the maximum early and late response of LSGS in the same heart. As a result of LSGS, spatial dispersion in repolarization increased during the early phase (lateral posterior prolongation), but especially during the late phase of LSGS (lateral posterior shortening). Figure 4 summarizes the results of all group 1 experiments. LSGS had effect in the LV lateral and posterior regions (p=0.006 and p=0.003, respectively), but not in the LV anterior region (p>0.05). In the posterior LV wall, the LSGS effect was larger in the subepicardium than subendocardium (p=0.043), with a significant biphasic response in epicardial as well as endocardial sites. In the lateral wall the response to LSGS in the subepicardium and subendocardium was almost significantly different (p=0.056). In 3 out of 5 pigs the repolarization gradient reversed (PRE: RT LV anterior < RT lateral/posterior, LATE: RT LV posterior < RT LV anterior). In the other 2 pigs the direction of the gradient was unchanged. On average the total RT dispersion (defined as 95th-5th percentile) increased from 40±5 to 52±8 ms (pre vs late), although this reached no statistical significance.
Figure 3.
Examples of repolarization maps represented by 8 parallel transverse slices in the same heart before LSGS, early phase during LSGS, and late phase during LSGS. Especially in the lateral and posterior wall of the left ventricle repolarization prolongs during the early phase and shortens during the late phase. Note that the direction of the spatial repolarization gradient is inverted between early (RT anterior is shorter than RT posterior) and late during LSGS (RT posterior is shorter than RT anterior).
Figure 4.
Accumulated repolarization changes in the anterior, lateral and posterior left ventricular wall (columns), of subepicardial (blue) and subendocardial (red) recording sites during left stellate ganglion stimulation, at baseline and, after vagotomy and after decentralization (rows). Significance was tested using a 2-way ANOVA for each condition (Region and LSGS).
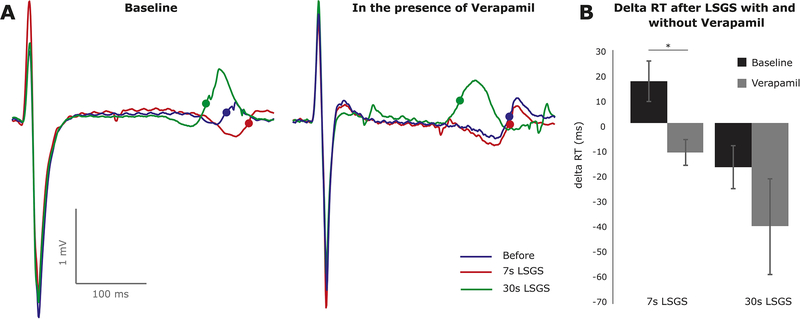
To the test the hypothesis that enhanced L-type calcium current explained the LSGS-induced prolongation of repolarization we administered verapamil (Figure 5A). Overall, Verapamil abolished the initial prolongation (+17.4±8.2 vs −11.8±5.3, p=0.047) and tended to increase the late shortening in repolarization (−18.0±8.8 vs −41.8±19.4, p=0.165).
Figure 5.
The initial prolongation of repolarization after 7 s of LSGS is caused by L-type calcium current. A) typical examples of changes in repolarization during LSGS with (right) and without (left) Verapamil. B) The bar graphs illustrate the average (n=3) prolongation and shortening of local repolarization after 7 s and 30 s of LSGS, respectively. LSGS; Left Stellate Ganglion Stimulation.
RSGS shortens repolarization in the anterior LV
The effects of RSGS were smaller than those of LSGS (Figure 6, Supplemental Table 1). In the entire LV the repolarization changes were −4±2 and −12±1 ms (early and late phase respectively, compared to +10±1.5 ms and–28.9±4.4 ms with LSGS). The effect was larger in the LV anterior wall than in the LV lateral and LV posterior wall (p=0.03). We only detected a biphasic response in 2 pigs (Supplemental Figure 2). Transmural differences in repolarization were absent.
Figure 6.
Repolarization maps represented by 8 parallel transverse slices, in the same heart as in Figure 5, before RSGS, early phase during RSGS, and late phase during RSGS. In contrast to LSGS, no prolongation of repolarization was present in the early phase during RSGS. However, in the anterior wall of the left ventricle and lateral wall of the right ventricle repolarization shortened during the late phase.
LSGS: effects following Vagotomy and Stellate Decentralization
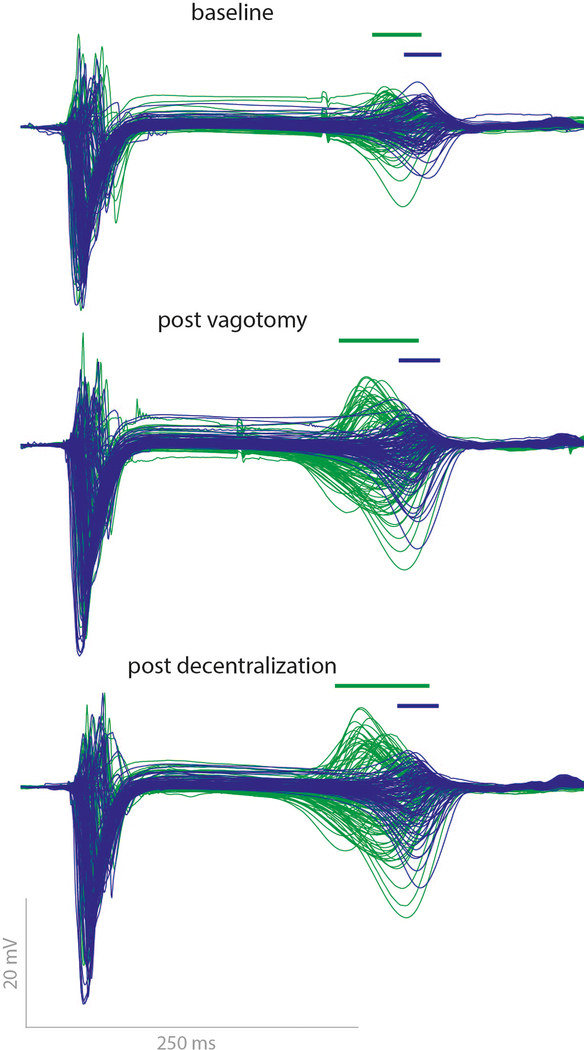
In 2 out of 5 pigs the late phase shortening was markedly enhanced after vagotomy and decentralization. In these two animals the current required to reach the target increase in left ventricular pressure was much higher than in the other animals (see Methods). Figure 7 shows the superimposition of all electrograms recorded in one of these pigs before LSGS (blue) and during late LSGS (green) at baseline, after vagotomy and after subsequent decentralization. The bars above the electrograms show dispersion (5th to 95th percentile) of repolarization during late LSGS. After vagotomy the shortening was larger and the dispersion of repolarization was increased. Following subsequent decentralization this effect was even more pronounced. In these 2 pigs, maximal shortening of repolarization in the late phase of LSGS was 48 and 49 ms at baseline, 63 and 59 ms after vagotomy, and 71 and 62 ms after decentralization.
Figure 7.
Superimposition of all LV electrograms in one heart before LSGS (blue, pre) versus late phase during LSGS (green, late) at baseline (upper), after vagotomy (middle) and after decentralization (lower). Bars indicate the dispersion repolarization times (5th to 95th percentile). Note that the dispersion of the local T-waves increases more after vagotomy and decentralization.
Premature beats and left ventricular pressure increase
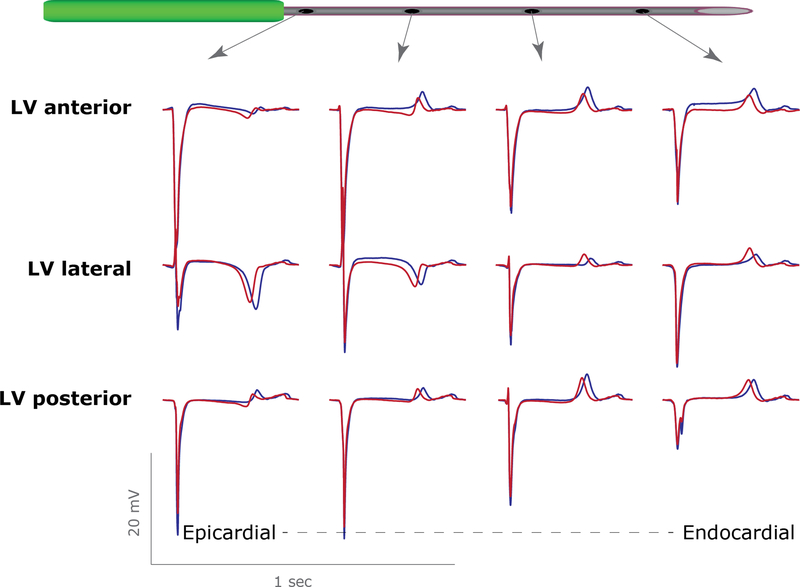
LV pressure increased monotonically during LSGS by 29 ± 6 % (n=5) and was not related to the occurrence of premature ventricular beats (VPB, Figure 2A). In 3 experiments none or a single VPB occurred while in the 2 other experiments VPBs occurred both in the periods of repolarization prolongation and shortening. However, the increase in LV pressure during LSGS could explain part of the changes in repolarization via mechanoelectrical feedback alone. Therefore, we applied transient aortic banding to generate a LV pressure change, after full decentralization. Left ventricular pressure increased by 41±9%, equivalent to that during late phase LSGS. Figure 8 shows typical examples of electrograms from 3 needles in the anterior, lateral and posterior wall of the left ventricle in one pig after decentralization. The pressure increase resulted in a global shortening of the repolarization times in the LV wall (269±7 ms to 255±7ms, before vs during pressure increase, n=5, p= 0.023), without a regional effect (RT changes were −14±1,−14±1 and −13±1 for LV anterior, lateral or posterior wall, respectively).
Figure 8.
Local electrograms from the left anterior (upper), left lateral (middle) and left posterior (lower) ventricular wall before (blue) and during (red) transient aortic occlusion after decentralization. Note that the morphology of the local T-waves remains the same, indicating that the repolarization shortens similarly in all three areas (compare with figure 1).
Discussion
Our data show that 1) LSGS causes a biphasic response in repolarization in the LV posterior and lateral wall with prolongation followed by shortening in repolarization, 2) the effect of LSGS on repolarization is more pronounced in the sub-epicardium than in the sub-endocardium but only in the posterior LV wall, 3) the overall response to LSGS on repolarization did not change after decentralization but the late phase shortening was more pronounced in 2 out of 5 pigs, 4) shortening of repolarization can partly be explained by the increase in LV pressure and 5) right stellate ganglion stimulation shortens repolarization mainly in the anterior LV wall, but the effects are smaller than those of LSGS.
Mechanism underlying the biphasic response in repolarization due to LSGS
While previous studies showed that LSGS can cause alternation of T wave morphologies 19 and dynamic changes of QT times, 20 so far no studies have shown biphasic changes of local repolarization times at a constant heart rate during LSGS. Our experiments show that the initial prolongation following LSGS is caused by activation of the L-type calcium current. A possible clinical implication is that Verapamil enhances dispersion in repolarization when the sympathetic system is activated and may be arrhythmogenic.
The most likely explanation for the subsequent shortening in repolarization during LSGS is that at low neurotransmitter concentrations, the calcium current is activated, which would prolong the action potential, while at high concentrations this would be overcome by activation of the IKs leading to shortening 21,22. Alternatively, a different time course of response per type of channel following exposure even at a constant concentration may lead to a biphasic response. Liu et al 23 described that the averaged time constant for ICa2+ in response to administration of isoprenaline is ~10 seconds (causing APD prolongation), and for IKs about 40 seconds (causing APD shortening). The late shortening in repolarization during LSGS could also result from the opening of stretch activated channels following the LV pressure increase 24,25. However, the last explanation is unlikely since repolarization shortens in the posterior and lateral wall but not in the anterior wall despite being exposed to similar increase in intraventricular pressure. In addition, the repolarization changes following aortic constriction in decentralized animals were immediate, not biphasic (shortening only) and were homogeneous throughout the LV 26. However, there is a possibility that cardiopulmonary baroreceptors responding to LV pressure may continue to mediate local cardio-cardiac responses via intrinsic cardiac autonomic nerves even after decentralization. Therefore, the results of aortic constriction are complex and the contribution of myocardial stretch receptors cannot be totally eliminated by vagosympathetic deafferentation.
Effect of LSGS on transmural heterogeneity in repolarization
We show that the effects of LSGS were larger on sub-epicardial than on the sub-endocardial myocardium, although the differences were small. The differences in effect in the transmural plane are consistent with histological observation that the sympathetic fibers insert at the epicardium15. An alternative – functional – explanation for the transmurally different effect of LSGS on repolarization is that the density of the ion channels carrying IKs is larger in the epicardium than in the endocardium 27. IKs is particularly sensitive to catecholamines. The observation that the shortening of repolarization follows an initial prolongation is less easy to explain with this mechanism.
The transmural gradients in repolarization times induced by LSGS are minor (i.e. about 13 ms), especially compared to the regional gradients (i.e. anterior versus posterior about 52 ms, figure 3). This is in line with our earlier observations 28. Therefore, we suggest that the T-wave changes observed during LSGS (figure 2) most likely represent the observed regional (anterior-posterior) rather than transmural changes in repolarization gradients. No midmural regions with prolonged repolarization were found before nor during LSGS.
Role of parasympathetic and sympathetic feedback loops
The overall response to LSGS on repolarization did not change after decentralization but the late phase shortening became more pronounced in 2 out of 5 pigs. In these 2 pigs the required amplitude of LSGS stimulation was higher than in the other pigs (5.8 and 10 mA respectively versus 1.6 – 2mA). In these hearts an increase in heart rate of about 50% was also observed during LSGS in sinus rhythm, while this was absent in the other three pigs. We speculate that the more intense LSGS stimulation likely had activated both afferent and efferent projections leading to contralateral effects. Previous work has indicated that with an intact innervation, the response to efferent stimulation of the cardiac nervous system is dampened by afferent activation and resultant alterations in central autonomic drive 29,30. This idea is supported by our observations that the LSGS response showed a stepwise increase after vagotomy and decentralization (Figure 4).
Clinical relevance
There has been considerable controversy over the antiarrhythmic effects of left vs bilateral stellectomy 6,31. Left cardiac sympathetic denervation has been successful in patients with cardiac channelopathies 32. However, bilateral cardiac sympathetic denervation has been shown to be superior to left denervation alone in patients with structural heart disease 31,33. Our data confirm and extend the results of earlier studies showing that LSGS-effects are dominant in the posterior and lateral LV, and those of RSGS in the anterior LV but also that overlap exists 7,12,16,34–36.
Limitations
We obtained very few reliable transmural data from the RV wall, because of its smaller thickness. Moreover, stimulation intensity of LSGS was chosen based on a LV pressure rise of about 30%, whereas the intensity for RSGS was based on the increase in heart rate (max 450 ms interval). The latter may hamper direct comparisons between LSGS and RSGS. Finally, this study was performed on normal hearts and – therefore – the results may not be entirely valid for pathologic situations such as hearts with a myocardial infarction or heart failure.
Conclusion
LSGS results in lengthening, followed by shortening of repolarization. The lengthening is caused by activation of the L-type calcium current. The effects are most prominent in the posterior and lateral wall of the LV. Repolarization at the epicardium shortens more than at the endocardium. Removal of feedback loops enhances the response to LSGS in some hearts. RSGS also results in shortening of LV repolarization although the effect is smaller than that of left stellate stimulation and is most prominent in the anterior wall of the LV.
Supplementary Material
Acknowledgements
We thank Joseph Hadaya for help during experiments. This study was supported by grant 16CVD02 (RHYTHM) from the Leducq Foundation and by grant OT2OD023848 and UO1EB025138 from the NIH (to KS & JAA). BJB received support from the Dutch Heart Foundation (2016T047).
Footnotes
Conflicts of interest: none
References
- 1.Zipes DP, Levy MN, Cobb LA, Julius S, Kaufman PG, Miller NE, Verrier RL: Sudden cardiac death. Neural-cardiac interactions. Circulation 1987; 76:I202––7. [PubMed] [Google Scholar]
- 2.Shen MJ, Zipes DP: Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 2014; 114:1004–1021. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Priori SG, Spazzolini C, et al. : Genotype-Phenotype Correlation in the Long-QT Syndrome : Gene-Specific Triggers for Life-Threatening Arrhythmias. Circulation 2001; 103:89–95. [DOI] [PubMed] [Google Scholar]
- 4.Blocker Heart Attack Trial Research Group: A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982; 247:1707–1714. [DOI] [PubMed] [Google Scholar]
- 5.Ajijola OA, Vaseghi M, Mahajan A, Shivkumar K: Bilateral cardiac sympathetic denervation: why, who and when? Expert Rev Cardiovasc Ther 2012; 10:947–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz PJ: Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol 2014; 11:346–353. [DOI] [PubMed] [Google Scholar]
- 7.Haws CW, Burgess MJ: Effects of bilateral and unilateral stellate stimulation on canine ventricular refractory periods at sites overlapping innervation. Circ Res 1978; 42:195–198. [DOI] [PubMed] [Google Scholar]
- 8.Millar CK, Kralios F a., Lux RL: Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 1985; 72:1372–1379. [DOI] [PubMed] [Google Scholar]
- 9.Vaseghi M, Zhou W, Shi J, Ajijola OA, Hadaya J, Shivkumar K, Mahajan A: Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart Rhythm, 2012; 9:1303–1309. [DOI] [PubMed] [Google Scholar]
- 10.Winter J, Tanko AS, Brack KE, Coote JH, Ng GA: Differential cardiac responses to unilateral sympathetic nerve stimulation in the isolated innervated rabbit heart. Auton Neurosci, 2012; 166:4–14. [DOI] [PubMed] [Google Scholar]
- 11.Martins JB, Zipes DP: Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res 1980; 46:100–110. [DOI] [PubMed] [Google Scholar]
- 12.Opthof T, Ramdat Misier AR, Coronel R, Vermeulen JT, Verberne HJ, Frank RG, Moulijn a. C, van Capelle FJ, Janse MJ: Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ Res 1991; 68:1204–1215. [DOI] [PubMed] [Google Scholar]
- 13.Veldkamp MW, Verkerk AO, Van Ginneken ACG, Baartscheer A, Schumacher C, de Jonge N, de Bakker JMT, Opthof T: Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts. Eur Hear J 2001; 22:955–963. [DOI] [PubMed] [Google Scholar]
- 14.Murayama M, Harumi K, Mashima S, Shimomura K, Murao S: Prolongation of ventricular action potential due to sympathetic stimulation. Jpn Heart J 1977; 18:259–265. [DOI] [PubMed] [Google Scholar]
- 15.Zipes DP, Jalife J: Cardiac electrophysiology from cell to bedside: The autonomic nervous system and the heart In Saunders WB, ed: Cardiac electrophysiology from cell to bedside. Philadelphia: WB Saunders, 1990, pp. 316–317. [Google Scholar]
- 16.Vaseghi M, Yamakawa K, Sinha A, et al. : Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am J Physiol Heart Circ Physiol 2013; 305:H1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamakawa K, Howard-Quijano K, Zhou W, Rajendran P, Yagishita D, Vaseghi M, Ajijola OA, Armour JA, Shivkumar K, Ardell JL, Mahajan A: Central vs. peripheral neuraxial sympathetic control of porcine ventricular electrophysiology. Am J Physiol Integr Comp Physiol 2016; 310:R414––R421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potse M, Linnenbank AC, Grimbergen CA: Software design for analysis of multichannel intracardial and body surface electrocardiograms. Comput Methods Programs Biomed 2002; 69:225–236. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz PJ, Malliani A: Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J 1975; 89:45–50. [DOI] [PubMed] [Google Scholar]
- 20.Coumel P: Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol 1993; 4:338–355. [DOI] [PubMed] [Google Scholar]
- 21.Kass RS, Wiegers SE: The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac purkinje fibres. J Physiol 1982; 322:541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volders PGA, Stengl M, van Opstal JM, Gerlach U, Spätjens RLHMG, Beekman JDM, Sipido KR, Vos MA: Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation 2003; 107:2753–2760. [DOI] [PubMed] [Google Scholar]
- 23.Liu GX, Choi BR, Ziv O, Li W, de Lange E, Qu Z, Koren G: Differential conditions for early after-depolarizations and triggered activity in cardiomyocytes derived from transgenic LQT1 and LQT2 rabbits. J Physiol 2012; 590:1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taggart P, Sutton PM: Cardiac mechano-electric feedback in man: clinical relevance. Prog Biophys Mol Biol 1999; 71:139–154. [DOI] [PubMed] [Google Scholar]
- 25.Meijborg VMF, Belterman CNW, de Bakker JMT, Coronel R, Conrath CE: Mechano-electric coupling, heterogeneity in repolarization and the electrocardiographic T-wave. Prog Biophys Mol Biol 2017; 130:356–364. [DOI] [PubMed] [Google Scholar]
- 26.Taggart P, Sutton P, Lab M, Runnalls M, O’Brien W, Treasure T: Effect of abrupt changes in ventricular loading on repolarization induced by transient aortic occlusion in humans. Am J Physiol 1992; 263:H816––23. [DOI] [PubMed] [Google Scholar]
- 27.Gintant GA: Regional differences in IK density in canine left ventricle: role of IK,s in electrical heterogeneity. Am J Physiol 1995; 268:H604––13. [DOI] [PubMed] [Google Scholar]
- 28.Opthof T, Coronel R, Wilms-Schopman FJG, Plotnikov AN, Shlapakova IN, Danilo P, Rosen MR, Janse MJ: Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 2007; 4:341–348. [DOI] [PubMed] [Google Scholar]
- 29.Ardell JL, Ragendran PS, Nier HA, KenKnight BH, Armour JA, Rajendran PS, Nier HA, KenKnight BH, Armour JA: Central-Peripheral Neural Network Interactions Evoked by Vagus Nerve Stimulation: Functional Consequences on Control of Cardiac Function. Am J Physiol - Hear Circ Physiol 2015; 309:ajpheart.00557.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM: A cardiocardiac sympathovagal reflex in the cat. Circ Res 1973; 32:215–220. [DOI] [PubMed] [Google Scholar]
- 31.Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, Mahajan A, Shivkumar K: Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol, 2012; 59:91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olde Nordkamp LRA, Driessen AHG, Odero A, Blom NA, Koolbergen DR, Schwartz PJ, Wilde AAM: Left cardiac sympathetic denervation in the Netherlands for the treatment of inherited arrhythmia syndromes. Netherlands Heart J 2014; 22:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaseghi M, Barwad P, Corrales FJM, et al. : Cardiac Sympathetic Denervation for Refractory Ventricular Arrhythmias. J Am Coll Cardiol 2017; 69:3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanowitz F, Preston JB, Abildskov J: Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res 1966; 18:416–428. [DOI] [PubMed] [Google Scholar]
- 35.Kralios FA, Martin L, Burgess MJ, Millar K: Local ventricular repolarization changes due to sympathetic nerve-branch stimulation. Am J Physiol 1975; 228:1621–1626. [DOI] [PubMed] [Google Scholar]
- 36.Randall WC, Armour JA, Geis WP, Lippincott DB: Regional cardiac distribution of the sympathetic nerves. Fed Proc 1972; 31:1199–1208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.