Abstract
The present study was conducted to analyze bacterial diversity profile of Cholistan desert located in Pakistan. The study investigates the influence of physicochemical parameters of soil on distribution of different bacteria at all taxonomic levels and also study the distribution pattern between different desert environments, particularly rhizospheric and bulk desert sands. Species richness showed phyla Proteobacteria and Chloroflexi as the dominant OTUs in all the samples. Besides the two phyla, the rhizospheric soils with root remnants were dominated by Firmicutes, Deinococcus-Thermus, Actinobacteria and Acidobacteri, while phylum Thermotogae was present in significant quantity in rhizosheaths devoid of roots. In non-rhizospheric desert soils, a considerable number of OTUs belonged to phyla Proteobacteria, Chloroflexi, Bacteroidetes and Acidobacteria. An important finding from this study is that a bulk portion of the OTUs were assigned to unclassified taxa, indicating a large repertoire of unexplored taxa in the desert ecology of Pakistan. Distribution of taxonomic groups among various regions of the desert was collaborating well with the physicochemical parameters of the sites. The findings of this study establish the fundamental relationships between desert ecosystem, specific native plant and the total bacterial flora. This is the first study of microbial community analysis of any desert in Pakistan and thus, will serve as a future platform to explore further on desert ecosystem functioning by employing the ever-changing biotechnological tools.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02204-6) contains supplementary material, which is available to authorized users.
Keywords: Bacterial community, Desert sand, Pyrosequencing, Rhizosphere, 16S rRNA
Highlights
| First report of bacterial diversity profile and distribution pattern of any Desert from Pakistani ecology |
| High level of species richness with large portion of the OTUs as unclassified taxa |
| Distribution of the taxonomic groups among the various regions of the deserts |
| Collaborated well with the physicochemical parameters of the sites |
| This study will serve as a basis to explore further on the desert ecosystem functioning |
Introduction
Microbes, especially archaea and bacteria, are an indispensable component of life forms (Fierer and Lennon 2011; Kuske et al. 2002; Roesch et al. 2007). These organisms are ubiquitously present and more prominently on soil through complex intermolecular interactions (Young et al. 2008). In fact, the high functionalities of soils are maintained by these complex interactions (Costanza et al. 1997) and unravelling this ecosystem functioning will require the understanding of relationships between biotic and abiotic constituents (Loreau et al. 2001). The recent development of new techniques, such as next-generation multiplexed pyrosequencing (Hamady et al. 2008) coupled with geostatistical analysis has enabled us new insights in the spatial distribution of microbes (Lauber et al. 2009; Roesch et al. 2007; Amin et al. 2017). Orsi et al. (2016) reported that pyrosequencing technique and -omics should be utilized for easy understanding of bacterial diversity from habitats like deserts. The diversity and distribution of microbes in different ecosystems are; therefore, influenced by overbearing environmental parameters (Kuske et al. 2002).
In rhizospheric ecosystems, the intermolecular interactions become more complex with the establishment of a three-way relationship between plants, soil and microbes. Between plant and soils, plant communities influence spatial structure of soil either physically through development of root system or chemically through release of exudates (Angers and Caron 1998; Philippot et al. 2013). These interactions, subsequently, enhance rhizopheric mineral flows and create a rich and dynamic environment (Lu et al. 2004; Zhang and Wienhold 2002). Although the resulting ecosystem was supposed to serve as treasure-trove for microbial activities (Parniske 2005), the recalcitrant nature of many microbes hindered the elucidation of plant–microbe interaction (Berg and Smalla 2009; Buée et al. 2009). In this way, soil microorganisms signify the world’s largest reservoir of biological diversity. They also constitute the most vital and sensitive bioactive factor in soil, where they perform an exceptional role in the maintenance of healthy desert ecosystems and restoration of vegetation. Increased soil bacterial diversity, not only improves the soil ecosystem stability, but also helps to mitigate deterioration of the soil ecological environment. However, almost none of the studies have been reported concerning 16S rRNA gene sequence-based deep bacterial diversity in deserts of Pakistan, which let us to study effects of different non-rhizospheric and rhizospheric bacterial communities in desert steppes (Nimaichand et al. 2016). Few arid zones faced alterations in annual rainfall and eventually whole new climatic variation altered bacterial flora too unlike the Cholistan region (Amin et al. 2016). During one such project to explore and characterize bacterial diversity, a novel bacterium Microvirga pakistanensis sp. nov. has been isolated previously and described from these desert samples in Cholistan, Pakistan (Amin et al. 2016).
Concerning the desert ecosystems, environmental stressors, such as high aridity, elevated UV radiations and low moistures restrict the occurrence of life to typically the poikilohydric style (Pointing and Belnap 2012). Under such conditions, plant diversity is even more limited and confine mostly to halophytes. Since deserts represent the largest terrestrial ecosystem by surface area, it is expected that the limited plant–microbe interaction plays a major role in desert ecosystem functioning. Therefore, in order to plan and further hypothesized on the possible mechanisms of soil–plant–microbe interactions, we initially target to understand the microbial diversity in desert samples of Cholistan as our source. Several earlier hypotheses have suggested that desert plants may exert direct influences on microbial communities by providing valuable nutritive resources (Herman et al. 1995; Schlesinger et al. 1996). We, therefore, hypothesized that more microbial diversity is expected on the rhizospheric samples as compared to non-rhizospheric soil.
In this study, we employed a multiplexed pyrosequencing approach to examine the dominant bacterial communities. Comparison was made between bulk desert soil and rhizosphere based on the abundance of operational taxonomic units (OTUs) assigned from the pyrosequencing data. Two representative plants were selected and effects of presence or absence of root materials to phase-out endophytic bacteria prevalence was also observed. We further analyzed the influence of physicochemical parameters of soil on distribution of different bacteria at all taxonomic levels.
Materials and methods
Study site and sample collection
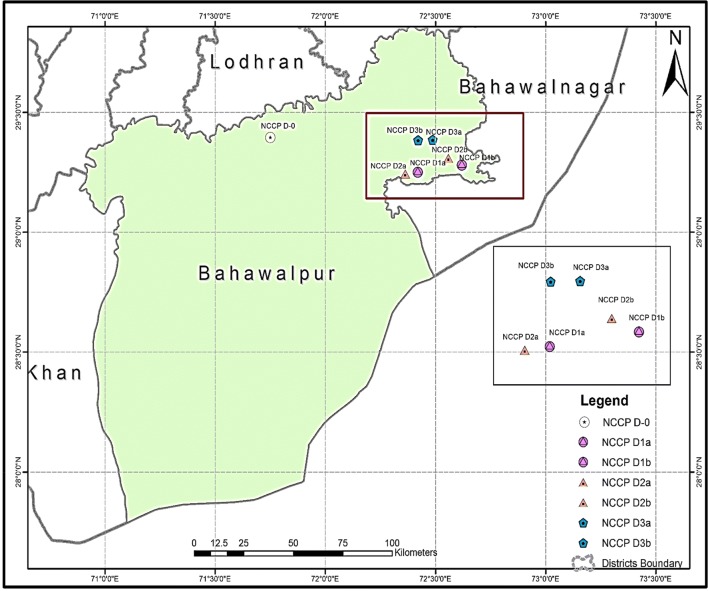
Samples for the present study were collected randomly in Cholistan desert, Bahawalpur, Pakistan (Fig. 1) using a random sampling technique as described earlier (Wang et al. 2013). The Cholistan desert is a subtropical and harsh region characterized by lack of precipitation and extended dearth season. The rate of evaporation is generally high, making it a low humid region. The Cholistan desert spreads over 0.44 million hectares, there are salt-affected low levelled and clayey segments which are locally known as 'dhars'. These are ideal spots for growing salt-tolerant grasses and low height plants because of rainwater and saline groundwater. These salt-tolerant plants produce significant biomass and also have potential to grow equally well in the presence of under upland and submerged saline soil environment. Such 'dhars' have flat and hard surface with thick salt deposition and surrounded by sand dunes. Dhars are shallow to moderately deep, have low vegetation, have calcifications and saline sodic fine to medium textured clayey soils.
Fig. 1.
Map indicating sampling sites in the Cholistan desert, Bahawalpur, Pakistan
There are various plant communities recognized in Cholistan desert, Haloxylon recurvum indigenous in smaller Cholistan and Larrea tridentate a part of stable plant community in greater Cholistan. Except these plants, other plant species cannot survive due to the salinity, compaction of soil and complete asphyxiated conditions during rainy season. Haloxylon recurvum belongs to the Amaranthaceae plant family which is a densely branched pale shrub locally known as Sajji or Khar. Larrea tridentate is an evergreen shrub that belongs to the Zygophallaceae plant family.
Haloxylon recurvum and Larrea tridentate being two of the native plant varieties were selected. Former is prevalent in high-salt-rich domes with no soil covers and latter in low salt concentration areas. Their roots penetrate at depth of 20–30 cm. Using Google Map images, sites were preset for sampling. Then, two depth levels were selected at sites based on the depths of root penetrations. These samples included (i) rhizosheaths of two desert halophytes viz. Haloxylon recurvum and Larrea tridentata, D1-a/D1-b; D2-a/D2-b and (ii) bulk desert sands D3-a/D3-b. In each case, samples were collected from depths between 20 and 30 cm, below the top surface layer rhizosheath soil (with root remnants of plants Haloxylon recurvum and Larrea tridentata), NCCP-D1-a and D1-b, respectively, whereas rhizosheath soil around Haloxylon recurvum and Larrea tridentata (without plant roots) collected from the same depths (NCCP-D2-a and NCCP-D2-b, respectively). Root remnants were eliminated to screen possible prevalent endophytic bacteria in D2 a and b samples. Samples of dry soil from vegetation-free desert area were collected (NCCP-D3-a at depth of 100 cm and NCCP-D3-b at depth of 50 cm). Three control samples were also collected at the vicinity of the desert from wet soil around desert area and mixed to get a composite sample (NCCP-D-0). Detailed profiles of these samples are listed in Table 1.
Table 1.
Description of the samples
| Sample | Sample type | Coordinates | Sequence read archives | Accession number | |
|---|---|---|---|---|---|
| Latitude | Longitude | ||||
| D-0 | Wet soil around desert area | 29°23′44.002″N | 71°45′1.001″E | SRS822652 | SAMN03284242 |
| D1-a | Rhizosheath soil (with root remnants of plant Haloxylon recurvum) from 20–30 cm depth | 29°15′0.478″N | 72°25′9.939″E | SRS825421 | SAMN03284243 |
| D1-b | Rhizosheath soil, with root remnants of plant Larrea tridentata collected at 20–30 cm depth | 29°16′49.426″N | 72°37′11.437″E | SRS825422 | SAMN03284244 |
| D2-a | Rhizosheath soil around Larrea tridentata (without plant roots) collected from depth of 20–30 cm | 29°14′33.721″N | 72°21′47.039″E | SRS825423 | SAMN03284245 |
| D2-b | Rhizosheath soil around Haloxylon recurvum (without plant roots) collected from depth of 20–30 cm | 29°18′29.257″N | 72°33′30.988″E | SRS825424 | SAMN03284246 |
| D3-a | Dry soil from vegetation-free desert area collected at depth of 100 cm | 29°23′8.740″N | 72°29′15.713″E | SRS825425 | SAMN03284247 |
| D3-b | Dry soil from vegetation-free desert area collected at depth of 50 cm | 29°23′4.164″N | 72°25′17.127″E | SRS825426 | SAMN03284248 |
Sample IDs were designated with the suffix a, b to indicate the two different depths with a being less deep and b being deeper. In Literature sample description NCCP Prefix is also added with samples (NCCP—stands for National Culture Collection of Pakistan)
All the samples were collected aseptically in triplicates in a sterilized sealed plastic zipped bags and immediately transported to the laboratory (~ 8 h). The collected samples were transported to the laboratory to preserve the physicochemical constituent of the samples and the samples were processed upon arrival All samples were sieved into 2-mm sieve to remove stones and plant roots, and triplicates were merged together to get a composite sample. A portion of each sample was stored at − 80 °C and used to extract metagenomic DNA. The other part was air-dried and used for measuring chemical properties.
Physicochemical analysis
The soil–pH values and electrical conductivity were measured by dissolving the soil sample in water at a 1:1 (w/v) ratio. A second measurement of pH was done using a 1:1 (w/v) suspension in a 0.01 M CaCl2 solution. The use of 0.01 M CaCl2 tend to mask small differences in salt contents accumulated under limited rainfall in arid regions and under restricted drainage (McLean 1982). Percent carbon and nitrogen and the cation exchange capacity (CEC) were determined by the standard protocol described earlier (Andrew et al. 2012; Rhoades 1982b, a). In wet soil around the desert area (NCCP-D-0) the carbon content was measured in the rhizosheath samples containing plant roots (NCCP-D1-a/b) and the bulk desert sands (NCCP-D3-a/b).
DNA extraction, amplification and pyrosequencing
DNAs were extracted using PowerSoil® DNA extraction kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instruction. Described procedures of Hur et al. (2011) were adopted for pyrosequencing analyses of the genomic DNA extracts. Amplifications of the V1–V3 region of the bacterial 16S rRNA gene were done using a C1000 Touch Thermal Cycler (Bio-Rad, CA, USA) and bar-coded fusion primers (8 nucleotide long barcodes) (Table S1). The PCR mix contained 100 ng template DNA, 5 μL 10 × ExTaq buffer, 0.2 mM of each dNTPs, 0.5 μΜ of each primer and 2 units ExTaq DNA Polymerase (Takara, Japan) in a 50 μL reaction. After initial denaturation at 94 ºC for 5 min, the amplification reaction was carried out using a Touchdown program for 10 cycles of denaturation (94 ºC, 30 s), annealing (60 ºC, 45 s) and extension (72 ºC, 90 s) with subsequent decrease in annealing temperature by 0.5 ºC, followed by an additional 20 cycles of denaturation (94 ºC, 30 s), annealing (55 ºC, 45 s), extension (72 ºC, 90 s). The amplified products were checked on 2% agarose gel electrophoresis and visualized using the Gel Doc system (Bio-Rad, USA). Amplicons were purified using a QIAquick PCR purification kit (Qiagen, CA, USA) and quantified using a PicoGreen dsDNA Assay kit (Invitrogen, CA, USA). Equimolar concentrations of each amplicon from different samples were pooled, purified using AMPure bead kit (Agencourt Bioscience, MA, USA) and amplified on sequencing beads by emulsion PCR. Pyrosequencing reactions were performed using a Roche GS FLX Titanium system at ChunLab, Inc (Seoul, Korea) according to the manufacturer’s instructions.
Metagenome sequence and statistical analyses
Pyrosequencing data from Roche GS FLX Titanium platform were analysed in the CLcommunity™ bioinformatics pipeline v3.45 from ChunLab, Inc (Seoul, Korea). To achieve the quality, reads shorter than 300 bp or those containing any ambiguous base or incorrect primer sequences were excluded from the dataset. The resulting sequences were assembled into contigs using the inbuilt setup within the pipeline. When the number of sequences assembled was five or more, nucleotide positions that were found in at least two reads were included in the final contig sequence. This method helped in correcting the 0.5% mismatch errors, which are often generated in Roche 454 pyrosequencing. For the purpose of diversity analysis, OTUs were assigned on the contigs representing multiple sequences and unique individual read by using the CD-HIT program (Li and Godzik 2006) at a sequence similarity cutoff level of ≥ 97%, while the taxonomic assignments were done based on the pairwise similarity comparison on EzTaxon-e server (Kim et al. 2012), coupled with the BLASTN search tool (Altschul et al. 1990). OTUs that were not matched to EzTaxon-e database at the species level (> 97%), were re-checked for chimeras using UCHIME program. SeqPrep and cutadapt were used to strip the adapter sequences from the 3′ and 5′ ends of the paired-end Illumina reads. Low-quality reads (quality value < 20 or contain N bases) and short reads were removed by sickle.
Alpha-diversity indices were calculated with the Mothur package v.1.8.0 (Schloss et al. 2009). Among the diversity parameters, species richness was estimated by employing Chao1 and ACE methods, while quantitative species richness/evenness by non-parametric Shannon and Simpson indices. Rarefaction curves to standardize and compare species richness were generated with SigmaPlot (Systat Software, San Jose, CA). Relative abundance of each taxon was clustered by using Bionumerics version 6.0 software (Applied Maths, Sint Martens Latem, Belgium). For the beta-diversity analyses, an unweighted UniFrac Distance Matrix (Hamady et al. 2010) was calculated, and based on the analysis, an UPGMA dendrogram was generated to represent the clustering of bacterial communities. Two-way analysis of variance (ANOVA) on microbial diversity indices and relative sequence abundances of important phyla against physicochemical properties was performed. For significant difference among the different sites, pairwise test was performed using SPSS statistics (IBM SPSS version 20.0 software, IBM Corp). Correlation between the bacterial community and the environmental parameters were represented in a canonical correspondence analysis (CCA) plot. Best-fit modelling of Faith’s phylogenetic diversity (PD) and individual phyla against the pH of the samples were performed in SigmaPlot, using linear, polynomial (quadratic) and power law functions.
Results
Cholistan desert is one of the threatened ecosystems, where most of the biodiversity is depleting. Several plants have faced extinction due to anthropogenic activities in addition to several natural threats. As far as the studies on microorganisms are concerned, there are only three to four reports on isolation of pure microbial species from the soils of Cholistan desert or from the rhizosphere of its vegetation, that gave a very limited data and researchers were unable to find in depth distribution of microbial species in these areas. The situation compelled us to explore unculturable bacterial flora in specific regions of two selective native plant species at the depth of their root penetrations.
Physicochemical characteristics of the soil vs soil bacterial communities in the Desert
Detailed description of the physicochemical parameters of the samples is provided in Table 2. All the samples showed a low concentration of C and K. In wet soil around desert area (NCCP-D-0), the carbon content was 0.10 ± 0.006%. The rhizosheaths samples containing plant roots (NCCP-D1-a/b) and bulk desert sands (NCCP-D3-a/b), the carbon contents were 0.15 and 0.14%, respectively, irrespective of the sampling depths. Rhizosheaths without root remnants (NCCP-D2-a and NCCP-D2-b) had different carbon contents in the two different depths (0.15 and 0.12% respectively) (Table 2). The carbon content in various soil samples showed slight variations except for the carbon content in wet soil around desert area showing a significant difference of 0.05% from rest of the samples. Ammonium concentration is the lowest in control (0.1 mg kg−1) and the highest in NCCP-D1-a/b. Nitrite concentration is the highest in NCCP-D1-a/b and the lowest in control (NCCP-D-0). Potassium and magnesium concentration are the highest in NCCP-D3-a/b and NCCP-D2-b, respectively. The pH values for all the samples are slightly alkaline and ranged from 7.1 in NCCP-D3-b to 7.7 in NCCP-D2-b.
Table 2.
Physicochemical parameters of the sampling sites
| Sample | C % | NH4-N mg kg−1 dry soil | NO2—N mg kg−1 dry soil | Mg mg kg−1 | K mg kg−1 | pH | Na+ µM | Cl− µM |
|---|---|---|---|---|---|---|---|---|
| D-0 | 0.10 ± 0.006 | 0.1 ± 0.4 | 0.4 ± 0.4 | 2.1 ± 0.05 | 33 ± 0.09 | 7.1 ± 0.06 | 541.0 ± 0.1 | 249.1 ± 0.08 |
| D1-a | 0.15 ± 0.010 | 2.3 ± 0.04 | 2.6 ± 0.6 | 2.0 ± 0.07 | 44 ± 0.05 | 7.5 ± 0.05 | 5216.1 ± 0.10 | 1614.1 ± 0.1 |
| D1-b | 0.15 ± 0.004 | 2.3 ± 0.02 | 2.6 ± 0.1 | 2.0 ± 0.03 | 44 ± 0.18 | 7.4 ± 0.10 | 5610.1 ± 0.02 | 2611.0 ± 0.8 |
| D2-a | 0.15 ± 0.009 | 1.3 ± 0.05 | 1.6 ± 0.1 | 2.0 ± 0.02 | 44 ± 0.04 | 7.5 ± 0.09 | 3611.1 ± 0.04 | 2694.1 ± 0.3 |
| D2-b | 0.12 ± 0.014 | 1.1 ± 0.02 | 2.5 ± 0.5 | 3.0 ± 0.04 | 50 ± 0.09 | 7.7 ± 0.10 | 3613.8 ± 0.18 | 2171.1 ± 0.10 |
| D3-a | 0.14 ± 0.005 | 1.1 ± 0.08 | 1.4 ± 0.3 | 1.7 ± 0.18 | 52 ± 0.30 | 7.4 ± 0.05 | 4671.4 ± 0.04 | 2610.8 ± 0.3 |
| D3-b | 0.14 ± 0.009 | 1.2 ± 0.09 | 1.6 ± 0.1 | 1.5 ± 0.04 | 52 ± 0.10 | 7.1 ± 0.11 | 4618.5 ± 0.02 | 2416.1 ± 0.09 |
Pyrosequencing data and rarefaction analysis
Since three samples were collected from each of the seven locations, and for each samples, DNAs was extracted in triplicates, a total of 63 DNAs were obtained from the desert samples of Cholistan. These DNAs were pooled into 7 groups based on their origin and pyrosequencing data following amplifications of V1–V3 region of 16S rRNA genes was generated. After excluding low quality reads and dropped reads, a total number of sequences obtained from the seven samples were 20,952 (Table S1) with an average length ranging between 470 and 560 bases after trimming. If the control is excluded, the highest (NCCP-D1-a, 2912 sequences) and the lowest (NCCP-D2-a, 2677) numbers of sequences were obtained from rhizosheath samples of Haloxylan recurvum with and without root remnants, respectively. Rarefaction curves for all the samples estimated at 97% sequence similarity fail to reach an asymptote (Fig. 2).
Fig. 2.
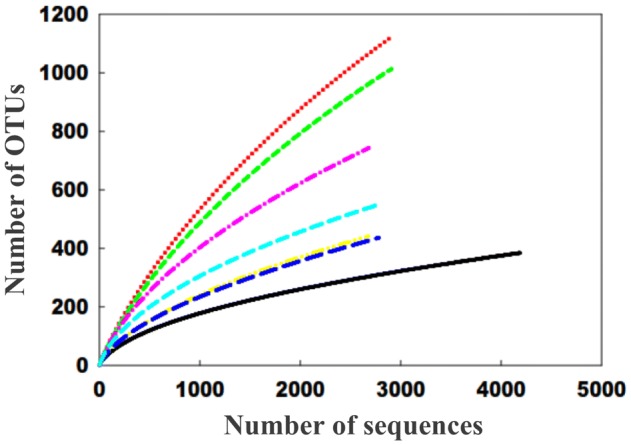
Rarefaction curve determined by CD-HIT method showing sampling accuracy. Pooled samples showed relatively even sampling of rhizospheric soils (NCCP-D1-a/b; NCCP-D2-a/b) and desert soil samples (NCCP-D3-a/b). Low diversity profile is observed in plot ( ) denote NCCP-D1-0, (
) denote NCCP-D1-0, ( ) NCCP-D1-a, (
) NCCP-D1-a, ( ) NCCP-D1-b, (
) NCCP-D1-b, ( ) NCCP-D2-a, (
) NCCP-D2-a, ( ) NCCP-D2-b, (
) NCCP-D2-b, ( ) NCCP-D3-a and (
) NCCP-D3-a and ( ) NCCP-D3-b (color figure online)
) NCCP-D3-b (color figure online)
Species richness
Measurement of sample richness was based on OTUs that represent the potential number of species present in a community. The number of OTUs in the six tested samples ranged from 437 to 1124, as compared to 385 for the control (Table 3). Species richness, assessed with the Chao1 estimator showed that bacterial diversity can vary significantly among rhizospheric and non-rhizospheric samples within a single desert. Richness, estimated by Chao1, ranged from 976.234 (NCCP-D2-a, rhizosheath without plant roots remnant collected at depth of 20–30 cm) to 2,482.953 (NCCP-D1-a, rhizosheath with plant roots at depth of 20–30 cm). The highest diversity level according to Shannon indices is observed in NCCP-D1-a (6.14) followed by NCCP-D1-b (5.93) indicating maximum diversity in the rhizospheric samples with root remnants.
Table 3.
Alpha diversity indices of the Cholistan desert samples
| Samples | Valid reads | OTUs | Chao 1 | Jackknife | ACE | Shannon | Simpson |
|---|---|---|---|---|---|---|---|
| D-0 | 4190 | 385 | 799.306 | 1036.922 | 1122.164 | 3.96 | 0.06 |
| D1-a | 2912 | 1124 | 2482.953 | 3684.148 | 4044.267 | 6.14 | 0.01 |
| D1-b | 2911 | 1014 | 2235.706 | 2806.511 | 3474.43 | 5.93 | 0.01 |
| D2-a | 2678 | 442 | 976.234 | 1201.685 | 1584.542 | 4.55 | 0.03 |
| D2-b | 2784 | 437 | 1102 | 1453.315 | 1702.534 | 4.50 | 0.03 |
| D3-a | 2685 | 744 | 1642.193 | 2277.132 | 2505.786 | 5.66 | 0.01 |
| D3-b | 2795 | 552 | 1182.373 | 1504.435 | 1613.255 | 5.18 | 0.01 |
Bacterial community profiles
In all the samples, the most dominant bacterial group were the phyla Proteobacteria and Chloroflexi. Besides these two phyla, the prominent phyla present in rhizosheath samples with roots (NCCP-D1-a/b) were Firmicutes, Deinococcus-Thermus, Actinobacteria and Acidobacteria (Fig. 3). In the rhizospheric samples without root remains (NCCP-D2-a/b) and non-rhizospheric samples (NCCP-D3-a/b), the proportion of phyla Firmicutes and Actinobacteria were comparatively less while Thermotogae was significantly present. Phyla Bacteroidetes and Acidobacteria were also found in considerable numbers in the non-rhizospheric samples (NCCP-D3-a/b) (Fig. 3).
Fig. 3.
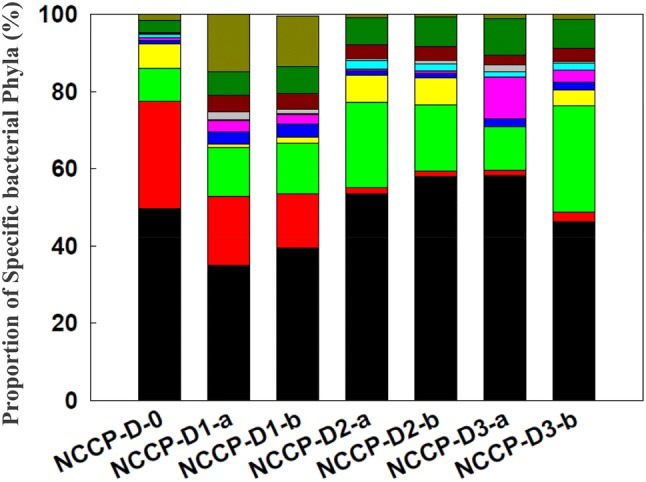
Proportional taxonomic classification of different sites at phylum level ( ) Proteobacteria, (
) Proteobacteria, ( ) Firmicutes, (
) Firmicutes, ( ) Chloroflexi, (
) Chloroflexi, ( ) Thermotogae, (
) Thermotogae, ( ) Acidobacteria, (
) Acidobacteria, ( ) Bacteroidetes, (
) Bacteroidetes, ( ) Chlorobi, (
) Chlorobi, ( ) Planctomycetes, (
) Planctomycetes, ( ) Deinococcus-Thermus, (
) Deinococcus-Thermus, ( ) Actinobacteria and (
) Actinobacteria and ( ) remaining phyla (< 1%) (color figure online)
) remaining phyla (< 1%) (color figure online)
Pairwise comparison of the samples showed significant differences in the diversity between different sites (Table S2). Phyla Acidobacteria, Actinobacteria, Deinococcus-Thermus and Planctomycetes were the most dominant in rhizosheath soil samples with plant roots (NCCP-D1-a/b). However, in rhizosheath soil samples without root remnants (NCCP-D2-a/b), the dominant groups were represented by phyla Thermotogae, Chlorobi, Deinococcus-Thermus, Spirochaetes, Proteobacteria and Chloroflexi (Fig. 3). For the vegetation-free desert soils, distribution of dominant phyla except Spirochaetes, Chlorobi and Deinococcus-Thermus were not the same. At bulk desert soil sample (NCCP-D3-a), phyla Proteobacteria, Bacteroidetes and Planctomycetes were present in considerable number, while Chloroflexi and Thermotogae were present abundantly in the other bulk soil sample NCCP-D3-b.
Gradient heat map generated for dominant taxa at the species level (Fig. 4) indicated that rhizosheath soil with root remnants (NCCP-D1-a/b) were dominated by Rhizobium daejeonensis, Microvirga lotonidis and another OTU belonging to Deinococcus_uc. While bulk desert sand NCCP-D3-a possessed the unclassified species HQ902253_s as the most dominant OTU, the other bulk soil from 50 cm depth (NCCP-D3-b) was represented by Anaerolinea thermophila, Anarolinaceae_uc_s, Thiobacillus thioparus and unclassified species GQ500701_g_uc. The species Meiothermus hypogaeus, Thiobacillus_uc and an unclassified species GQ50071_g_uc were common between both the two bulk desert samples. Unlike the other two sites, rhizosheaths without root remnants (NCCP-D2-a/b) possessed various common dominant species including Anaerolinea thermophila, Arenimonas malthae, Fervidobacterium gondwanense, Fervidobacterium riparium, Halothiobacillus neapolitanus, Hydrogenophaga electricum, Meiothermus hypogaeus and Thiobacillus_uc (Fig. 4).
Fig. 4.

Heat map analysis at species level normalized by rows showing dominant species at each site, by Unifrac clustering, green colour represents maximum dominance while red colour represents minimum number. Genus name with uc sign represents unclassified/uncultured species. Map is presenting differences in the dominance of different phyla at individual sites in the gradients generated by UPGMA algorithm
Variability in the dominance of different phyla
Differences in the dominance of different phyla at individual sites were demonstrated in the gradient heat maps generated with UPGMA algorithm (Fig. 4). The results indicated that OTUs were significantly associated with the principal location of samples. As for example, OTUs associated with nitrogen-fixing ability, such as Rhizobium daejeonense were abundant in rhizospheric soil samples with root-remnants particularly NCCP-D1-a/b, but not in the other locations where roots were excluded or totally absent. Similar observation was recorded by Andrew et al. (2012) where local soil characteristics shaped microbial communities in the bulk soil and rhizosphere. Species variations were also detected with variation in depths of sampling site, e.g. OTUs belonging to Anaerolinea thermophila, Anaerolinaceae_uc_s, Thiobacillus thioparus and unclassified GQ500701_g_uc were present in significant number at lower depth (50 cm, NCCP-D3-b) as compared to higher depth (100 cm, NCCP-D3-a). In contrast, OTUs for species Hydrogenophaga electricum and unclassified Anaerolinaecea_uc_s and GQ500701_g_uc were more in NCCP-D2-a than in NCCP-D2-b.
Distribution of phylum Actinobacteria in Cholistan desert samples
The phylum Actinobacteria, a dominant soil bacteria, was considered for further analysis as there was a major fluctuation in its distribution among the different desert samples. The highest number of OTUs for phylum Actinobacteria was observed in the rhizospheric samples NCCP-D1-a and NCCP-D1-b, representing 14.73% and 13.26% of total OTUs respectively. The distribution was however negligible in the remaining samples, i.e. 1.4% in NCCP-D3-a, 1.3% in NCCP-D3-b and 0.5% each in NCCP-D2-a, NCCP-D2-b. A review of the actinobacterial taxonomic groups distributed in sample NCCP-D1-a (with the highest number of OTUs for phylum Actinobacteria) indicated that the major groups representing phylum Actinobacteria were from the class Rubrobacteria (183 OTUs) followed by class Actinobacteria (149) (Table S3). Among the class Rubrobacteria, the highest number of OTUs was represented by genus Rubrobacter (94 OTUs) which are all unclassified at species level. The remaining OTUs of class Rubrobacteria are unclassified at various levels of bacterial hierarchy. Among the class Actinobacteria, majority of the OTUs belonged to the order Micrococcales (61 OTUs) followed closely by Propionibacteriales (57). Unlike the class Rubrobacter, less number of OTUs in class Actinobacteria was unclassified. The unclassified Actinobacteria, however, constituted a major bulk of the total OTUs within this sample (> 50%) (Fig. 5).
Fig. 5.
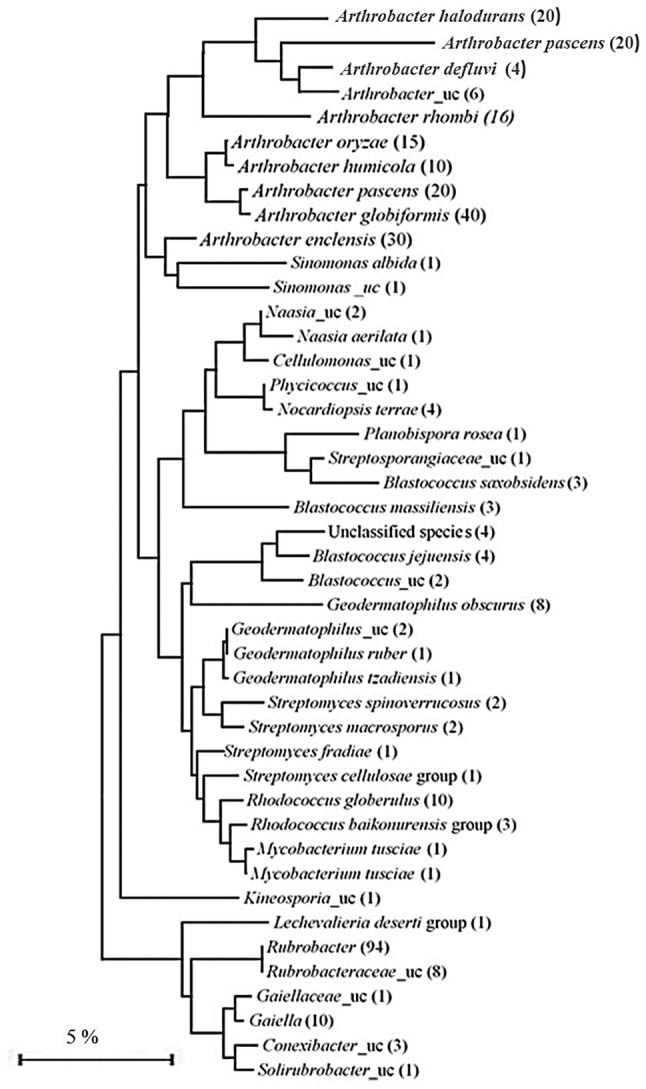
Neighbour joining phylogenetic tree inferred by using metagenome sequences (OTUs/429 total OTUs based on a comparison of 450 nucleotides) showing actinobacterial taxonomic groups in the rhizospheric desert soil of Cholistan, Pakistan. Numbers within the parentheses indicate the number of OTUs obtained. Sign _uc stands for OTUs belonging to uncultured and unclassified species. Scale bar (5%) represents as sequence divergence
Discussion
General characteristics of soil bacterial communities
Despite generation of more than 2600 quality sequences per site, the sequencing depths were insufficient to capture the full extent of bacterial diversity in each of the sampling site. This finding is in congruent with that of Lauber et al. (2009) who indicated that the use of pyrosequencing was unable to tap full bacterial diversity of soils. However as stated by Lauber et al. (2009), we can only speculate from our findings that phylotypes of soil were infrequent and its actual number per site were higher than 2600. However, unlike some of the previous studies on deserts (Connon et al. 2007; Garcia-Pichel et al. 2003; Nagy et al. 2005; Navarro-Gonzalez et al. 2003; Price et al. 2010), both alpha and beta diversity indices were much higher in our study. However, the reason may lie with the technique and method adopted for estimating the diversity.
The earlier studies on desert microbial diversity (Garcia-Pichel et al. 2003; Navarro-Gonzalez et al. 2003; Price et al. 2010) rely on standard cloning methods that possibly was giving an unassertive diversity indices, while our pyrosequencing technique were able to provide a moderate sequencing depth of the environmental samples. Few recent studies have also indicated that desert ecosystems hold abundant and diverse bacterial diversity in a way similar to major extreme environments (An et al. 2013; Andrew et al. 2012; Prashar et al. 2014). The diversity indices in our samples comprised less than half of the actual species diversity as indicated by the rarefaction curve (Fig. 2). This low diversity profile might be due to different factors: (i) low sequencing depths, (ii) amplification constraints, (iii) sampling errors, (iv) handling errors and most importantly (v) environmental factors. Although some of these factors could be corrected or improved through proper designing, others require advancement in analyzing techniques. Furthermore, pathogen invasion of soil could also be a vital factor for defining low microbial diversity in delicate soil structures, such as desert (van Elsas et al. 2012).
Variability in the dominance of different phyla
Differences in the dominance of different phyla at individual sites were demonstrated in the gradient heat maps generated with UPGMA algorithm (Fig. 4). These results also indicated that OTUs were significantly associated with the principal location of samples (Fig. 6). As for example, OTUs associated with nitrogen-fixing ability, such as Rhizobium daejeonensis were abundant in rhizospheric soil samples with root remnants; particularly, NCCP-D1-a/b, but not in the other locations where roots were excluded or totally absent. Similar observation was recorded by Andrew et al. (2012) where local soil characteristics shaped microbial communities in the bulk soil and rhizosphere. Species variations were also detected with variation in depths of sampling site, e.g. OTUs belonging to Anaerolinae thermophila, Anaerolinaceae_uc_s Thiobacillus thioparus and unclassified GQ500701_g_uc were present in significant number at lower depth (50 cm, NCCP-D3-b) as compared to higher depth (100 cm, NCCP-D3-a). In contrast, OTUs for species Hydrogenophaga electricum and unclassified Anaerolinaecea_uc_s and GQ500701_g_uc were more in NCCP-D2-a (30 cm depth) than in NCCP-D2-b (20 cm depth).
Fig. 6.

Three-dimensional PCoA analysis (PC1 148.653%, PC2 18.991% and PC3 16.063%) showing clustering of sampling sites according to OTUs present at species level. Samples collected from rhizospheric soil with root remains (NCCP-D1-a/b) do not share the same cluster with rhizospheric soil without root remains (NCCP-D2-a/b) or with native soil of desert (NCCP-D3-a/b). Control does not fall near any samples in the three-dimensional space
Bacterial community structure with relation to physicochemical characteristics
Our strategy to sample the soil at different depths proved to be very supportive in indicating the main physiological driver for specific microbial diversity under local environments (Figs. 2 and 3). In Cholistan Desert samples, the most dominant bacterial group was found to be the phylum Proteobacteria, which in the case of Canadian, Alaskan and Siberian Arctic soils was phylum Acidobacteria (Campbell et al. 2010; Chu et al. 2010; Mannisto et al. 2013; Neufeld and Mohn 2005; Wallenstein et al. 2009). Dominance of phylum Proteobacteria over Acidobacteria was also reported in the polar Arctic deserts (Lee et al. 2013; McCann et al. 2016). This slight variation in the species distribution might be related to the same intensity of pH variations of soil. Our samples have a neutral to slightly alkaline pH as compared to the acidic condition in the Canadian, Alaskan and Siberian soil. Positive correlation of pH and bacterial diversity has been deliberated by Chu et al. (2010) and Rao et al. (2016), and this relation is represented at low intensity in our Faith’s PD plot (Fig. 7). pH variation was reported, but changes were not significant and soil types could not be categorized as acidic to alkaline in our case. Because of this reason, this strong correlation could not be determined from our findings. According to Lauber et al. (2009), dependence of soil bacterial community on hydrogen-ion concentration (pH) can be through direct or indirect interference. As for instance, pH can enforce a physiological constraint on soil bacteria resulting in a viability shift, most probably toward a specific group of bacteria that can survive at specific hydrogen ion concentration while reducing the growth of other taxa (direct effect). Soil pH may influence the soil characteristics, e.g. nutrient availability, cationic exchange capacity, organic C characteristics and soil moisture content which are often directly or indirectly related to soil pH. This, in turn, alters the bacterial community structure (indirect effect).
Fig. 7.

Relationship between soil pH and soil bacterial diversity. a Faith’s PD (b) Number of phylotypes with phylotypes at the 97% sequence similarity level. Lines represent the best-fit quadratic model to the data. Diversity indices were calculated using 2000 sequences per soil sample. The plot is representing slightly positive influence of pH indicator on distribution of related genera ( ) denote NCCP-D1-a, (
) denote NCCP-D1-a, ( ) NCCP-D1-b, (
) NCCP-D1-b, ( ) NCCP-D2-a, (
) NCCP-D2-a, ( ) NCCP-D2-b, (
) NCCP-D2-b, ( ) NCCP-D3-a and (
) NCCP-D3-a and ( ) NCCP-D3-b (color figure online)
) NCCP-D3-b (color figure online)
Proteobacteria are globally distributed and were thought to be prominent members of desert soil bacterial communities. In a study comparing desert soil with agricultural soil bacterial communities, Proteobacteria phylotypes were twofold higher (retrieved by 16S rRNA gene amplicon pyrosequencing) in the desert soil communities, with the genus Ochrobactrum (Alphaproteobacteria) being most prevalent. Alpha-, Beta- and Gammaproteobacteria are often associated with soils receiving higher rates of organic carbon inputs. However, Proteobacteria may be functionally important in nutrient-limited arid environments because members of this phylum are implicated in bacteriochlorophyll-dependent photosynthesis. Members of the phylum Firmicutes are also well represented in desert soils. Certain Firmicutes spp. (Bacillus, Paenibacillus, etc.) can form endospores, which facilitate survival under desiccating conditions. The rapid spore germination, non-fastidious growth requirements and short doubling times of these aerobic taxa means that members of the Firmicutes are some of the most readily isolated microbial ‘weeds’ from arid soils. Bacteroidetes are also common in desert soils, which is surprising given the proposed copiotrophic phenotype of members of this phylum. Desert soil microbial isolation studies have shown an abundance of Pontibacter sp. from the family Cytophagaceae. Interestingly, isolates from Bacteroidetes often show optimum growth at high pH values, which is consistent with the generally alkaline character of desert soils.
Environmental richness refers to the interconnectedness of various life forms on Earth. It involves the ecosystem diversity, species diversity and genetic diversity from around the world. Not all bacterial taxa appeared to be well correlated with pH, indicating that distribution of phylotypes within a particular environment involved additional factors. The correlation between the bacterial community and the environmental parameters can be deduced from the CCA plot (Fig. 8). In our study, the most significant impact for the rhizospheric soils (NCCP-D1-a/b, NCCP-D2-a/b) was inserted by NO2 and NH4. Bacterial diversity at non-rhizospheric desert soils (NCCP-D3-a/b) was influenced by K+ and Mg++ (Fig. 8). Unlike the above parameters, percent carbon did not influence the selection of microbial population on either of the rhizospheric or non-rhizospheric samples. In other words, nitrogen did not have any significant influence in designing bacterial community of native soil of desert (NCCP-D3-a/b) while K+ and Mg++ did not have significant impact on soil communities of rhizospheric soils (NCCP-D1-a/b or NCCP-D2-a/b). Edaphic factors, such as moisture content, temperature and pH of the soil have also been correlated with the richness of the environment (Reth et al. 2005; Qu et al., 2020).
Fig. 8.

CCA plot showing effect of physicochemical parameters (pH, Mg, K, NH4, NO2 and C on distribution of different bacterial genera’s). Plot shows the most significant impact for the rhizospheric soils (NCCP-D1-a/b, NCCP-D2-a/b) was inserted by NO2 and NH4. Bacterial diversity at non-rhizospheric desert soils (NCCP-D3-a/b) was influenced by K+ and Mg++
Though temperature and water retention capacity of the soil were not measured during our study, variations in bacterial community in different depths within similar sampling sites give an indirect hint about the influence of these factors. As for example, the diversity of phylum Actinobacteria was not significantly different at the different depth levels, while there was variation in the distribution of certain Proteobacteria. These findings indicated that the influence of other physicochemical factors, apart from the major driver which in our case is pH, need to be examined for developing a suitable model that can predict an accurate soil microbial community structure across larger spatial scales. Like plant and animal communities, the diversity and relative abundances of major soil bacterial taxa can be related to broad-scale gradients in biotic and abiotic characteristics. Diversity was significantly correlated with phylogenetic and taxonomic diversity across the soils. The bacterial metagenomes obtained from the two desert soils were relatively similar to one another, suggesting that the composition and functional attributes of the microbial communities in these two types may be more comparable than often assumed because of shared habitat. However, habitat is defined by edaphic factors too and in many reports all edaphic factors, including soil pH, total organic carbon, total nitrogen, total phosphorus and soil moisture were negatively related to the plant–microbial relationship (Liu et al., 2020).
Desert soil is dominated by ubiquitous phyla that includes Actinobacteria, Bacteroidetes and considered as predominant components of soil microbiota. They can tolerate solute stress and desiccation, which makes them stand out among the rest of the bacteria, and they have been isolated from hostile environments, such as arid deserts. Actinobacteria recovered from extreme ecosystems characterized by severe conditions of radiations and desiccation-like deserts are representative of the deepest clads Acidimicrobidae, Rubrobacteridae. The stringent desiccating desert condition is one of the main force responsible for the evolution of DNA repair mechanisms responsible for generating resistance to ultraviolent and gamma radiations, which is one of the main characteristics of many desert Actinobacteria. The strains of Deinococcus and Geodermatophilus are among the most resistant genera in such ecosystems that have the ability to tolerate irradiation upto 30 Gy. The Geodermatophilaceae contains only two other genera of Blastococcus and Modestobacter, which thrive in the conditions of low availability of water and nutrients. Geodermatophilus prefers arid soils as natural habitats and out of 15 species described in this genus, at least nine species are isolated from the desert area, whereas Blastococcus and Modestobacter are inhabitants of rock surfaces. Members of the Terrabacteria genus are also characterized by adaptations to desiccation, radiation and high salinity. The communities of arid and semiarid zones have a profound influence on ecosystem functioning, including photosynthetic carbon fixation, nitrogen inputs and soil fertility and also efficiently controls annual and perennial plant colonization (Li et al., 2020; Bowker et al., 2018).
Conclusion
Cholistan desert being one of the most ignored sites with only few studies of local plant varieties and isolation of selective strains. It made us explore these sites with a more comprehensive analyses of soil metagenomes for a thorough analysis of bacterial flora. However, even at these sequencing depths, we have not surveyed the full extent of bacterial taxonomic and phylogenetic diversity within individual soil samples, we have not described the full range of soil microbial community types found across the globe. Nevertheless, we were still able to highlight the predictability of soil microbial community attributes across biomes. Within the Cholistan desert several different dominant plant species and soil types are found and their distribution is based on the high or low saline domes.
This study arrived at the conclusion that there is unexpectedly high bacterial diversity in different arid zones, which were driven by environmental heterogeneity. Modest to positive influence of edaphic factors was observed. Keeping the findings from this study as the platform, future studies should involve identification of key factors influencing species distribution under controlled experiments. Further, genomic and metabolic-based studies might help in determining the interconnectivity between different bacterial populations (e.g. methane cycle-related microorganisms, sulphate-reducing bacteria) and their functional roles in environments especially with relation to biogeochemical cycles.
Electronic supplementary material
Supplementary Materials: The following are available online, Table S1: Metadata for total number of reads and dropped reads of all samples, Table S2: Pair wise comparison between studied sites at phylum level, Table S3: List of actinobacterial taxonomic groups (out of 429 OTUs) in the rhizospheric desert soil of Cholistan, Pakistan. Numbers within the parentheses indicate the number of OTUs obtained. Difference in total sum of OTUs is due to the omission of few OTUs which belonged to unclassified taxonomic group.
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Science & Technology Basic Resources Investigation Program of China (No. 2017FY100300), Xinjiang Uygur Autonomous Region regional coordinated innovation project (Shanghai Cooperation Organization Science and Technology Partnership Program, No. 2017E01031) and China Biodiversity Observation Networks (Sino BON).
Author contributions
“Conceptualization, IA and WJL; Data curation, IA and WJL; Formal analysis, AA and AA; Funding acquisition, WJL; Investigation, AA and IUK; Methodology, AA, IA, NK and WJL; Project administration, IA and WJL; Resources, SMD and WJL; Software, AA; Supervision, IA and WJL; Writing—original draft, AA, IA and NK; Writing—review and editing, IA, IUK, AA, SMD and WJL”.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Ethical statement
It is to state that all the authors agreed to the contents of the manuscript and also agreed that we (Dr. Iftikhar Ahmed from Pakistan and Professor Dr. Wen-Jun Li from China) would act as corresponding authors for the publication. It is also declared that there is no conflict of interest with anyone and we do not have choice for any Editor and /or Reviewer to be included or excluded for reviewing this manuscript.
Contributor Information
Iftikhar Ahmed, Email: iftikhar.ahmed@parc.gov.pk.
Wen-Jun Li, Email: liwenjun3@mail.sysu.edu.cn.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/s0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amin A, Ahmed I, Habib N, Abbas S, Hasan F, Xiao M, Hozzein WN, Li WJ. Microvirga pakistanensis sp. nov., a novel bacterium isolated from desert soil of Cholistan. Pakistan Arch Microbiol. 2016;198:933–939. doi: 10.1007/s00203-016-1251-3. [DOI] [PubMed] [Google Scholar]
- Amin A, Ahmed I, Salam N, Kim BY, Singh D, Zhi XY, Xiao M, Li WJ. Diversity and distribution of thermophilic bacteria in hot springs of Pakistan. Microb Ecol. 2017;74:116–127. doi: 10.1007/s00248-017-0930-1. [DOI] [PubMed] [Google Scholar]
- An S, Couteau C, Luo F, Neveu J, DuBow MS. Bacterial diversity of surface sand samples from the Gobi and Taklamaken deserts. Microb Ecol. 2013;66(4):850–860. doi: 10.1007/s00248-013-0276-2. [DOI] [PubMed] [Google Scholar]
- Andrew DR, Fitak RR, Munguia-Vega A, Racolta A, Martinson VG, Dontsova K. Abiotic factors shape microbial diversity in Sonoran Desert soils. Appl Environ Microbiol. 2012;78(21):7527–7537. doi: 10.1128/aem.01459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers DA, Caron J. Plant-induced changes in soil structure: processes and feedbacks. Biogeochemistry. 1998;42(1/2):55–72. doi: 10.1023/A:1005944025343. [DOI] [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009;68(1):1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Bowker MA, Reed SC, Maestre FT, et al. Biocrusts: the living skin of the earth. Plant Soil. 2018;429:1–7. doi: 10.1007/s11104-018-3735-1. [DOI] [Google Scholar]
- Buée M, De Boer W, Martin F, van Overbeek L, Jurkevitch E. The rhizosphere zoo: an overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil. 2009;321(1):189–212. doi: 10.1007/s11104-009-9991-3. [DOI] [Google Scholar]
- Campbell BJ, Polson SW, Hanson TE, Mack MC, Schuur EA. The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ Microbiol. 2010;12(7):1842–1854. doi: 10.1111/j.1462-2920.2010.02189.x. [DOI] [PubMed] [Google Scholar]
- Chu H, Fierer N, Lauber CL, Caporaso JG, Knight R, Grogan P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ Microbiol. 2010;12(11):2998–3006. doi: 10.1111/j.1462-2920.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- Connon SA, Lester ED, Shafaat HS, Obenhuber DC, Ponce A (2007) Bacterial diversity in hyperarid atacama desert soils. J Geophys Res 112 (G4): n/a–n/a. doi: 10.1029/2006JG000311
- Costanza R, d’Arge R, De Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’neill RV, Paruelo J (1997) The value of the world's ecosystem services and natural capital. Nature 387 (6630):253-260
- Fierer N, Lennon JT. The generation and maintenance of diversity in microbial communities. Am J Bot. 2011;98(3):439–448. doi: 10.3732/ajb.1000498. [DOI] [PubMed] [Google Scholar]
- Garcia-Pichel F, Johnson SL, Youngkin D, Belnap J. Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado plateau. Microb Ecol. 2003;46(3):312–321. doi: 10.1007/s00248-003-1004-0. [DOI] [PubMed] [Google Scholar]
- Hamady M, Lozupone C, Knight R. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. The ISME journal. 2010;4(1):17–27. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers allow hundreds of samples to be pyrosequenced in multiplex. Nat Methods. 2008;5(3):235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman RP, Provencio KR, Herrera-Matos J, Torrez RJ. Resource islands predict the distribution of heterotrophic bacteria in chihuahuan desert soils. Appl Environ Microbiol. 1995;61(5):1816–1821. doi: 10.1128/AEM.61.5.1816-1821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur M, Kim Y, Song HR, Kim JM, Choi YI, Yi H. Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol. 2011;77(21):7611–7619. doi: 10.1128/aem.06102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62(Pt 3):716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- Kuske CR, Ticknor LO, Miller ME, Dunbar JM, Davis JA, Barns SM, Belnap J. Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Appl Environ Microbiol. 2002;68(4):1854–1863. doi: 10.1128/AEM.68.4.1854-1863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber CL, Hamady M, Knight R, Fierer N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol. 2009;75(15):5111–5120. doi: 10.1128/aem.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Jin XY, Zhang XC, Chen L, Liu JL, Zhang HM, Jin D. Comparative metagenomics of two distinct biological soil crusts in the tengger desert. China Soil Biol Biochem. 2020;140:107637. doi: 10.1016/j.soilbio.2019.107637. [DOI] [Google Scholar]
- Lee SH, Jang I, Chae N, Choi T, Kang H. Organic layer serves as a hotspot of microbial activity and abundance in Arctic tundra soils. Microb Ecol. 2013;65(2):405–414. doi: 10.1007/s00248-012-0125-8. [DOI] [PubMed] [Google Scholar]
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics (Oxf Engl) 2006;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu K, Wurzburger N, Zhang J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere. 2020;11(1):e02999. [Google Scholar]
- Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime J, Hector A, Hooper D, Huston M, Raffaelli D, Schmid B. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294(5543):804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- Lu Y, Murase J, Watanabe A, Sugimoto A, Kimura M. Linking microbial community dynamics to rhizosphere carbon flow in a wetland rice soil. FEMS Microbiol Ecol. 2004;48(2):179–186. doi: 10.1016/j.femsec.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Mannisto MK, Kurhela E, Tiirola M, Haggblom MM. Acidobacteria dominate the active bacterial communities of Arctic tundra with widely divergent winter-time snow accumulation and soil temperatures. FEMS Microbiol Ecol. 2013;84(1):47–59. doi: 10.1111/1574-6941.12035. [DOI] [PubMed] [Google Scholar]
- McCann CM, Wade MJ, Gray ND, Roberts JA, Hubert CR, Graham DW. Microbial communities in a high arctic polar desert landscape. Front Microbiol. 2016;7:419. doi: 10.3389/fmicb.2016.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean E (1982) Soil pH and lime requirement. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, WI, USA
- Nagy ML, Perez A, Garcia-Pichel F. The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ) FEMS Microbiol Ecol. 2005;54(2):233–245. doi: 10.1016/j.femsec.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Navarro-Gonzalez R, Rainey FA, Molina P, Bagaley DR, Hollen BJ, de la Rosa J, Small AM, Quinn RC, Grunthaner FJ, Caceres L, Gomez-Silva B, McKay CP. Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science. 2003;302(5647):1018–1021. doi: 10.1126/science.1089143. [DOI] [PubMed] [Google Scholar]
- Neufeld JD, Mohn WW. Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl Environ Microbiol. 2005;71(10):5710–5718. doi: 10.1128/aem.71.10.5710-5718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimaichand S, Devi AM, Li W-J (2016) Direct plant growth-promoting ability of actinobacteria in grain legumes. In: Plant growth promoting actinobacteria, Springer, pp 1–16
- Orsi WD, Smith JM, Liu S, Liu Z, Sakamoto CM, Wilken S, Poirier C, Richards TA, Keeling PJ, Worden AZ, Santoro AE. Diverse, uncultivated bacteria and archaea underlying the cycling of dissolved protein in the ocean. ISME J. 2016;10(9):2158–2173. doi: 10.1038/ismej.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. Plant–fungal associations: cue for the branching connection. Nature. 2005;435(7043):750–751. doi: 10.1038/435750a. [DOI] [PubMed] [Google Scholar]
- Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11(11):789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- Pointing SB, Belnap J. Microbial colonization and controls in dryland systems. Nat Rev Microbiol. 2012;10(8):551–562. doi: 10.1038/nrmicro2831. [DOI] [PubMed] [Google Scholar]
- Prashar P, Kapoor N, Sachdeva S. Rhizosphere: its structure, bacterial diversity and significance. Rev Environ Sci Biotechnol. 2014;13(1):63–77. doi: 10.1007/s11157-013-9317-z. [DOI] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Li WJ, Dastager SG, Hozzein WN. Editorial: actinobacteria in special and extreme habitats: diversity, function roles, and environmental adaptations. Front Microbiol. 2016;7:1415. doi: 10.3389/fmicb.2016.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu EB, Omelon CR, Oren A, Meslier V, Cowan DA, Maggs-Kölling G, DiRuggiero J. Trophic selective pressures organize the composition of endolithic microbial communities from global deserts. Front Microbiol. 2020;10:2952. doi: 10.3389/fmicb.2019.02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Chan Y, Bugler-Lacap DC, Bhatnagar A, Bhatnagar M, Pointing SB. Microbial diversity in soil, sand dune and rock substrates of the Thar Monsoon Desert. India Indian J Microbiol. 2016;56(1):35–45. doi: 10.1007/s12088-015-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth S, Reichstein M, Falge E. The effect of soil water content, soil temperature, soil pH-value and the root mass on soil CO 2 efflux–a modified model. Plant Soil. 2005;268(1):21–33. doi: 10.1007/s11104-005-0175-5. [DOI] [Google Scholar]
- Rhoades J (1982a) Cation exchange capacity. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, WI, USA
- Rhoades J (1982b) Soluble salts. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, WI, USA
- Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AK, Kent AD, Daroub SH, Camargo FA, Farmerie WG, Triplett EW. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1(4):283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger WH, Raikes JA, Hartley AE, Cross AF. Erratum: on the spatial pattern of soil nutrients in desert ecosystems. Ecology. 1996;77(4):1270–1270. doi: 10.2307/2265595. [DOI] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/aem.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas JD, Chiurazzi M, Mallon CA, Elhottovā D, Krištůfek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci. 2012;109(4):1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein MD, McMahon SK, Schimel JP. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol. 2009;15(7):1631–1639. doi: 10.1111/j.1365-2486.2008.01819.x. [DOI] [Google Scholar]
- Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G, Zhang C, Song Z, Zhang Y, Ren H, Zhang J, Zhang L. Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS ONE. 2013;8(5):e62901. doi: 10.1371/journal.pone.0062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young IM, Crawford JW, Nunan N, Otten W, Spiers A. Microbial distribution in soils: physics and scaling. Adv Agron. 2008;100:81–121. doi: 10.1016/S0065-2113(08)00604-4. [DOI] [Google Scholar]
- Zhang R, Wienhold BJ. The effect of soil moisture on mineral nitrogen, soil electrical conductivity, and pH. Nutr Cycl Agroecosyst. 2002;63(2–3):251–254. doi: 10.1023/A:1021115227884. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.