Abstract
Excessive and hyperactive osteoclast activity causes bone diseases such as osteoporosis and periodontitis. Thus, the regulation of osteoclast differentiation has clinical implications. We recently reported that dehydrocostus lactone (DL) inhibits osteoclast differentiation by regulating a nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), but the underlying mechanism remains to be elucidated. Here we demonstrated that DL inhibits NFATc1 by regulating nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and nuclear factor-erythroid 2-related factor 2 (Nrf2). DL attenuated IκBα phosphorylation and p65 nuclear translocation as well as decreased the expression of NF-κB target genes and c-Fos. It also inhibited c-Jun N-terminal kinase (JNK) but not p38 or extracellular signal-regulated kinase. The reporter assay revealed that DL inhibits NF-κB and AP-1 activation. In addition, DL reduced reactive oxygen species either by scavenging them or by activating Nrf2. The DL inhibition of NFATc1 expression and osteoclast differentiation was less effective in Nrf2-deficient cells. Collectively, these results suggest that DL regulates NFATc1 by inhibiting NF-κB and AP-1 via down-regulation of IκB kinase and JNK as well as by activating Nrf2, and thereby attenuates osteoclast differentiation.
Keywords: AP-1, NFATc1, NF-κB, Nrf2, Osteoclast, ROS
INTRODUCTION
Osteoclasts are multinucleated giant cells that resorb bone (1). Excessive osteoclast activity above that of bone-forming osteoblasts leads to an imbalance between bone synthesis and breakdown, resulting in pathological outcomes, such as osteoporosis, rheumatoid arthritis, and periodontitis (2). Thus, the control of osteoclast differentiation has therapeutic implications.
Osteoclasts are differentiated from bone marrow-derived macrophages (BMM) by macrophage-colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) (3, 4). Binding of RANKL to its receptor, RANK, attracts TNF receptor-associated factor 6 (TRAF6) to RANK and subsequently activates NF-κB and mitogen-activated protein kinases (MAPKs), including c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK) (5). RANKL also stimulates the expression of c-Fos, a major component of activator protein-1 (AP-1) (6). NF-κB and AP-1 mediate the initial induction of the nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), a key determinant of osteoclast differentiation, which regulates the expression of osteoclastogenic genes, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K (CTSK) (5).
RANKL signaling induces reactive oxygen species (ROS) production via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) (7), and the ROS contribute to osteoclast differentiation and bone resorption (8, 9). Antioxidants, Nox inhibitors, and antioxidant enzymes inhibit osteoclast formation and function (10-12), and the loss of nuclear factor-erythroid 2-related factor 2 (Nrf2), a transcriptional regulator of many antioxidant enzymes, promoted osteoclastogenesis (13).
Dehydrocostus lactone (DL) has been reported to possess antioxidant activity (14) and protect osteoblast cell line MC3T3-E1 against oxidative stress and dysfunction (15). Recently, we reported that DL inhibits osteoclast differentiation by regulating NFATc1, and attenuates osteoclast activation by modulating migration and lysosome function (16). However, the mechanism of DL-mediated regulation of RANKL-induced NFATc1 activation has yet to be elucidated. In this study, we demonstrated that DL inhibits NFATc1 via regulation of IKK, JNK, and Nrf2, leading to the attenuation of osteoclast differentiation.
RESULTS
DL inhibits RANKL-induced NF-κB activation by regulating IKK
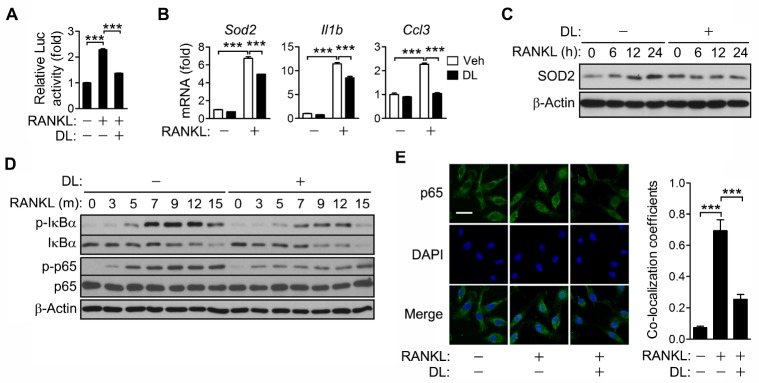
In a recent study, it was reported that DL inhibits RANKL-induced osteoclastogenesis by negatively regulating NFATc1 (16). In order to investigate the DL-mediated inhibition of NFATc1, the effect of DL on NF-κB that plays an essential role in NFATc1 expression was explored. DL strongly inhibited RANKL-induced NF-κB activation as revealed in the luciferase reporter assay (Fig. 1A). DL also inhibited the transcription of NF-κB target genes including superoxide dismutase 2 (Sod2), interleukin-1β (Il1β), and macrophage inflammatory protein 1α (Ccl3), and the expression of SOD2 protein (Fig. 1B, C). In addition, DL decreased the phosphorylation of IκBα and p65 (Fig. 1D), and p65 nuclear translocation (Fig. 1E), indicating that DL inhibits RANKL-induced NF-κB activation via down-regulation of IKK.
Fig. 1.
Inhibition of RANKL-induced NF-κB activation by DL. (A) RAW264.7 cells were transfected for 24 h with 0.45 μg of pNF-κB-Luc (NF-κB reporter plasmid) and 0.15 μg of pRL-SV40 (internal control). The cells were treated with RANKL for 24 h in the presence of 1.5 μM DL. The luciferase activity of each cell lysate was measured using a dual-luciferase assay system. The activity of firefly luciferase was normalized to that of the Renilla enzyme and expressed as fold increase relative to the activity of RANKL-untreated cells. (B-E) BMMs were incubated with RANKL and M-CSF in the presence of 1.5 μM DL for 24 h (B), the indicated times (C, D) or 15 min (E). The mRNA levels of individual genes were assessed by real-time PCR and presented as fold induction (B). Cell lysates were subjected to immunoblotting analysis (C, D). The cells were stained with p65 antibody and DAPI, and then photographed under a fluorescence microscope. Scale bar, 20 mm (E). All values represent means ± SD. N = 3. ***P < 0.001 between the indicated groups.
DL inhibits RANKL-induced c-Fos expression and JNK activation
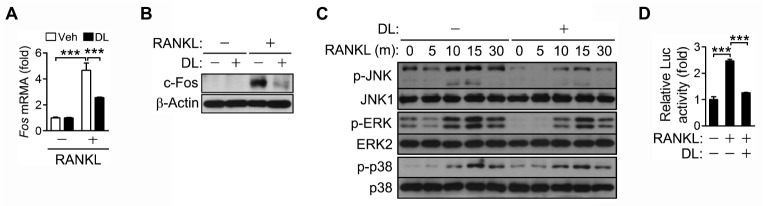
AP-1 is another important regulator of NFATc1 expression. DL inhibited RANKL-induced expression of c-Fos, a major component of AP-1, at both the mRNA and protein levels (Fig. 2A, B). DL also decreased the phosphorylation of JNK, but not that of ERK and p38 (Fig. 2C). As expected, DL inhibited RANKL-induced AP-1 activation as shown in the luciferase reporter assay (Fig. 2D). These results suggest that DL may inhibit AP-1 via down-regulation of c-Fos expression and JNK activation, leading to the attenuation of NFATc1 expression.
Fig. 2.
Inhibition of RANKL-induced AP-1 activation by DL. (A-C) BMMs were incubated with RANKL and M-CSF in the presence of 1.5 μM DL for 24 h (A, B) or the indicated times (C). The mRNA levels of individual genes were assessed by real-time PCR and presented as fold induction (A). Cell lysates were subjected to immunoblotting analysis (B, C). (D) RAW264.7 cells were transfected for 24 h with 0.45 μg of pAP-1-Luc (AP-1 reporter plasmid) and 0.15 μg of pRL-SV40 (internal control). The cells were treated with RANKL for 24 h in the presence of 1.5 μM DL, and the luciferase activity was measured as in Fig. 1A. All values represent means ± SD. N = 3. ***P < 0.001 between the indicated groups.
DL reduces ROS by activating Nrf2 or scavenging them
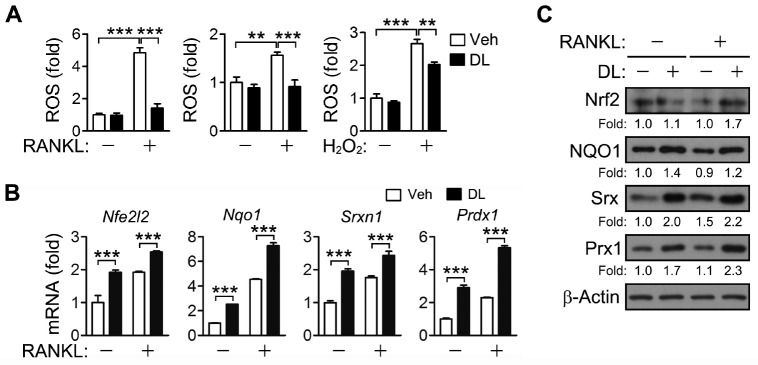
DL has antioxidant activity and activates Nrf2 in HepG2 cells (14). Nrf2 regulates ROS via induction of antioxidant enzymes and plays an important role in osteoclast differentiation and bone resorption (13). Therefore, the effect of DL on RANKL-induced ROS production and Nrf2 activation was investigated. When BMMs were incubated with RANKL for two days in the presence of DL, the ROS level was lower than in vehicle-treated cells (Fig. 3A, left panel). In order to explore the possibility that DL directly eliminates ROS as an antioxidant, ROS were measured after incubating BMMs with RANKL or H2O2 for 15 min, which was inadequate to express antioxidant enzymes. DL significantly decreased the RANKL- and H2O2-induced increases in ROS (Fig. 3A, center and right panels). In addition, DL increased the expression of Nrf2 and its target genes at both the mRNA and protein levels, irrespective of the presence of RANKL (Fig. 3B, C). These results indicate that DL reduces ROS via activation of Nrf2 or by directly scavenging them.
Fig. 3.
ROS removal and Nrf2 activation by DL. (A) BMMs were incubated with RANKL for 2 days (left) and 15 min (center), or with 100 μM H2O2 for 15 min (right) in the presence of 1.5 μM DL. The cells were incubated with 5 μM 5-(and-6-)chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate. The cells were analyzed by a flow cytometer, and the relative levels of fluorescence were presented as fold differences. (B, C) BMMs were treated with RANKL for 24 h in the presence of 1.5 μM DL. The mRNA and protein levels of Nrf2 and its target genes were assessed by real-time PCR (B) and immunoblotting (C), respectively. All values represent means ± SD. N = 3. **P < 0.01; ***P < 0.001 between the indicated groups.
DL inhibits osteoclast differentiation by activating Nrf2
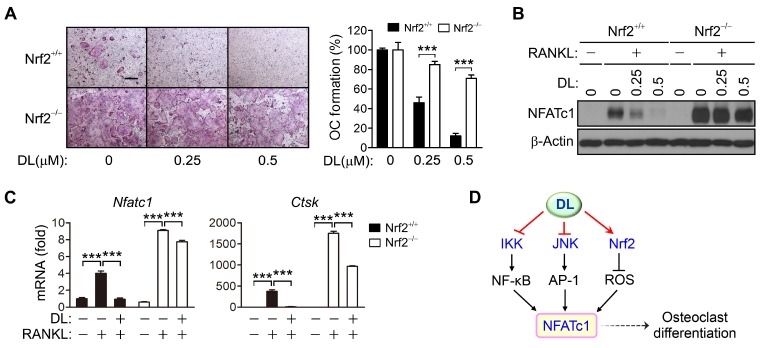
Nrf2 has been known to inhibit osteoclast differentiation (13). In order to investigate whether DL inhibits osteoclast differentiation via Nrf2 activation, the effect of DL on osteoclast differentiation of Nrf2-deficient BMMs was explored. In Nrf2-null cells, RANKL-induced osteoclast differentiation was strongly promoted, and DL was less effective in inhibiting osteoclast formation in Nrf2-deficient BMMs than in wild-type cells (Fig. 4A). The expression of NFATc1 and its target genes at both the mRNA and protein levels was also increased by Nrf2 loss, and the DL inhibition of NFATc1 was much less effective in Nrf2-null cells than in wild-type cells (Fig. 4B, C). Thus, DL may regulate NFATc1 via Nrf2 activation, and thereby inhibit osteoclast differentiation.
Fig. 4.
Less inhibitory effects of DL on the osteoclast differentiation and NFATc1 expression in Nrf2-deficient cells. (A-C) Wild-type and Nrf2-knockout BMMs were incubated with RANKL in the presence of the indicated concentrations of DL for 4 days (A) or 2 days (B), and in the presence of 1.5 μM DL for 2 days (C). The cells were fixed and stained with TRAP. Scale bar, 200 μm. TRAP-positive multinucleated cells containing more than three nuclei were counted and the percentage of osteoclast formation was plotted as a function of the concentration of DL (A). The amount of NFATc1 protein was analyzed by immunoblotting (B) and the expression of NFATc1 and its target gene was assessed by real-time PCR (C). All values represent means ± SD. N = 3. ***P < 0.001 between the indicated groups. (D) Proposed mechanism of DL inhibition of RANKL-induced NFATc1 expression and osteoclast differentiation.
DISCUSSION
A molecular analysis of the inhibitory effect of DL on RANKL-induced osteoclast differentiation indicated that DL suppressed osteoclastogenesis by inhibiting NFATc1 via regulation of IKK, JNK and Nrf2.
NF-κB and AP-1 regulate initial induction of NFATc1 (5). DL inhibited RANKL-induced activation of NF-κB and AP-1 promoters, indicating that DL may suppress NFATc1 expression by inhibiting NF-κB and AP-1. DL inhibited IκBα phosphorylation and p65 nuclear translocation along with decreased expression of NF-κB target genes. In addition, DL inhibits NF-κB by targeting IKKβ (17). Thus, DL appears to suppress NF-κB-dependent NFATc1 expression by inhibiting IKK activity. DL also inhibited RANKL-induced c-Fos expression and JNK activation. Elk-1 is known to regulate the expression of c-Fos, a major component of AP-1 (18). Elk-1 and c-Fos are downstream target genes of NF-κB (19, 20), assuming that NF-κB regulates AP-1 activity. JNK regulates the phosphorylation of Elk-1 as well as of c-Jun, the other major component of AP-1 (21). Thus, DL inhibits RANKL-induced IKK and JNK activation, leading to the suppression of NFATc1 expression via down-regulation of NF-κB and AP-1, resulting in the inhibition of osteoclast differentiation (Fig. 4D).
ROS are generated during osteoclastogenesis and are involved in osteoclast differentiation (7-9). DL protected osteoblastic MC3T3 cells against H2O2-induced oxidative stress (15). In the present study, RANKL stimulation increased ROS, which was attenuated by DL. DL also decreased ROS when cells were exposed to H2O2, indicating that DL may directly eliminate ROS. Nrf2 is known to regulate osteoclast differentiation and function by decreasing ROS via induction of antioxidant enzymes, such as sulfiredoxin (Srx) and peroxiredoxin (Prx) (13). DL has been reported to induce nuclear accumulation of Nrf2 and increase the promoter activity of antioxidant response elements in HepG2 cells (14). DL increased the expression of antioxidant enzymes such as Srx and Prx1 via Nrf2 activation in BMMs. In addition, the previous study showed that Nrf2 loss increases NFATc1 expression via up-regulation of MAPKs (especially JNK) and c-Fos expression (13). Furthermore, the inhibitory effects of DL on NFATc1 expression and osteoclast differentiation were markedly reduced by Nrf2 deficiency, suggesting that DL inhibits RANKL-induced NFATc1 expression and osteoclast differentiation by activating Nrf2 (Fig. 4D). NF-κB and JNK are well-known redox-sensitive molecules (22-24). Therefore, it is likely that DL inhibits RANKL-induced osteoclast differentiation via redox regulation of NF-κB and JNK by scavenging ROS or activating Nrf2.
MATERIALS AND METHODS
Reagents and antibodies
Recombinant mouse RANKL and human M-CSF proteins were purified as previously described (25). A rabbit polyclonal antibody specific for Srx was prepared as previously described (26).
We purchased:
DL from ChemFaces (Wuhan, China).
A rabbit polyclonal antibody against b-actin and a monoclonal antibody against NAD(P)H dehydrogenase (quinone) 1 (NQO1) from Abcam (Cambridge, MA, USA).
Rabbit polyclonal antibodies against phosphorylated (p-) IκBα, p-JNK, p-ERK, and p-p38, and a rabbit monoclonal antibody against p-p65 from Cell Signaling Technology (Danvers, MA, USA).
A goat polyclonal anti-mouse secondary antibody (Alexa Fluor 546 conjugate) from Thermo Fisher Scientific (Waltham, MA, USA).
Rabbit polyclonal antibodies against IκBα, JNK1, p38, c-Fos and Nrf2, and mouse monoclonal antibodies against p65, ERK2 and NFATc1, from Santa Cruz Biotechnology (Dallas, TX, USA).
A rabbit polyclonal antibody against superoxide dismutase 2 (SOD2) from Upstate Biotechnology (Lake Placid, NY, USA).
A rabbit polyclonal antibody against Prx1 from Young In Frontier (Seoul, Korea).
Transfection and luciferase reporter assay
RAW264.7 cells were transfected for 24 h with 0.45 μg of luciferase reporter plasmid and 0.15 μg of pRLSV40 (internal control) in a 24-well plate using Lipofectamine 3000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. A dual luciferase assay (Promega, Fitchburg, WI, USA) was subsequently performed. The activity of firefly luciferase was normalized to that of the Renilla enzyme and was expressed as a fold increase relative to the normalized value of control cells.
Preparation of BMMs
BMMs were prepared as osteoclast precursors from the femurs and tibiae of eight-week-old C57BL/6 male mice, as previously described (27); briefly, bone marrow cells were obtained from the bone marrow cavity using α-minimal essential medium (MEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin, then were incubated at 37°C for 1 day. Non-adherent cells were collected and incubated in Gey’s solution for 10 min. Following clarification, the cells were cultured in the presence of 50 ng/ml M-CSF for 3 days, and then adherent cells were used as osteoclast precursor cells.
Quantification of gene expression
Total RNA was isolated using Trizol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. The first strand cDNA was synthesized by M-MLV reverse transcriptase (Promega) using 2 μg of total RNA as a template in the presence of 0.5 μg of oligo(dT) primers, 0.25 μM of each dNTP, and 24 units of ribonuclease inhibitor. The cDNA was amplified in a reaction mixture containing SYBR Green PCR Master Mix (Bioline, Taunton, MA, USA) and 1 μM gene-specific primer using a StepOnePlus real-time PCR (Applied Biosystems, Foster City, CA, USA) as previously described (28). The primers used in the current study that were not used in the previous report (25, 29) were as follows: Il1β (5’-CTGGTGTGTGACGTTCCCATTA-3’ and 5’-CCGACAGCGAGGCTTT-3’), Ccl3 (5’-ACCATTGCTCAGGATTATGGA-3’ and 5’-GGGGTTCCTCGCTGCCTCCA-3’) and Prdx1 (5’-ACCATTGCTCAGGATTATGGA-3’ and 5’-CAACGGGAAGATCGTTTATTG-3’). Each mRNA level was normalized to the actin level.
Measurement of ROS level
ROS levels were measured as previously described (12, 25). Briefly, cells were washed with HBSS and incubated with 5 μM 5-(and-6-)chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific) for 5 min. The cells were then analyzed using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Osteoclast differentiation and TRAP staining
Osteoclasts were differentiated by incubating precursor cells with 50 ng/ml RANKL and 25 ng/ml M-CSF for 4 days. The cells were washed with phosphate-buffered saline (PBS), fixed for 10 min with 4% paraformaldehyde, and stained for TRAP using a leukocyte acid phosphatase cytochemistry kit (MilliporeSigma, Burlington, MA, USA) according to the manufacturer’s instructions. TRAP-positive multinucleated cells containing three or more nuclei were counted as osteoclasts under a light microscope (Nikon, Shinagawa, Tokyo, Japan).
Animal care
Breeding pairs of Nrf2-knockout (Nrf2−/−) mice were obtained from RIKEN Bio Resource Center (Tsukuba, Japan), and Nrf2+/+ littermates were used as wild-type controls. Mice were allowed free access to food and water, and caged under a 12-h light/12-h dark cycle. All animal studies were conducted in accordance with protocols approved by the International Animal Care and Use Committee of Ewha Womans University.
Statistical analysis
Data are presented as means ± SDs. Statistical significance was assessed by a one-way analysis of variance (ANOVA) using the Prism software version 5.0 (GraphPad, San Diego, CA, USA). A P < 0.05 was considered statistically significant.
ACKNOWLEDGEMENTS
We thank Dr. Masayuki Yamamoto (Tohoku University, Japan) for the Nrf2 knockout mice. This work was supported by the National Research Foundation (NRF) Grants (2017R1A2B2012435, 2019R1C1C1011198 and 2019R1A5A6099645) funded by the Korean Ministry of Science, ICT, and future Planning (MSIP). G.-R. Lee was supported by RP-Grant 2018 of Ewha Womans University.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/er.21.2.115. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 4.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 5.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Grigoriadis AE, Wang ZQ, Cecchini MG, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 7.Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 8.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bax BE, Alam AS, Banerji B, et al. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem Biophys Res Commun. 1992;183:1153–1158. doi: 10.1016/S0006-291X(05)80311-0. [DOI] [PubMed] [Google Scholar]
- 10.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI200318859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JR, Lazarenko OP, Shankar K, et al. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther. 2011;336:734–742. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Huh JE, Lee SY, Shim JK, Rhee SG, Jeong W. TRP14 inhibits osteoclast differentiation via its catalytic activity. Mol Cell Biol. 2014;34:3515–3524. doi: 10.1128/MCB.00293-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyeon S, Lee H, Yang Y, Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Jeong GS, Pae HO, Jeong SO, et al. The alpha-methylene-gamma-butyrolactone moiety in dehydrocostus lactone is responsible for cytoprotective heme oxygenase-1 expression through activation of the nuclear factor E2-related factor 2 in HepG2 cells. Eur J Pharmacol. 2007;565:37–44. doi: 10.1016/j.ejphar.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Choi EM, Kim GH, Lee YS. Protective effects of dehydrocostus lactone against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Toxicol In Vitro. 2009;23:862–867. doi: 10.1016/j.tiv.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Lee HI, Lee J, Hwang D, et al. Dehydrocostus lactone suppresses osteoclast differentiation by regulating NFATc1 and inhibits osteoclast activation through modulating migration and lysosome function. FASEB J. 2019;33:9685–9694. doi: 10.1096/fj.201900862R. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Yu Z, Wang C, et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-kappaB/COX-2 signaling pathway by targeting IKKbeta. Am J Cancer Res. 2017;7:1270–1284. [PMC free article] [PubMed] [Google Scholar]
- 18.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 19.Tu YC, Huang DY, Shiah SG, Wang JS, Lin WW. Regulation of c-Fos gene expression by NF-kappaB: a p65 homodimer binding site in mouse embryonic fibroblasts but not human HEK293 cells. PLoS One. 2013;8:e84062. doi: 10.1371/journal.pone.0084062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitmarsh AJ, Yang SH, Su MS, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/MCB.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y, Kim H, Min SH, Rhee SG, Jeong W. Dynein light chain LC8 negatively regulates NF-kappaB through the redox-dependent interaction with IkappaBalpha. J Biol Chem. 2008;283:23863–23871. doi: 10.1074/jbc.M803072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Hong SE, Lee J, Seo DH, et al. Euphorbia factor L1 inhibits osteoclastogenesis by regulating cellular redox status and induces Fas-mediated apoptosis in osteoclast. Free Radic Biol Med. 2017;112:191–199. doi: 10.1016/j.freeradbiomed.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Hyeon S, Kim H, et al. Dynein light chain LC8 inhibits osteoclast differentiation and prevents bone loss in mice. J Immunol. 2013;190:1312–1318. doi: 10.4049/jimmunol.1202525. [DOI] [PubMed] [Google Scholar]
- 28.Jin SH, Kim H, Gu DR, et al. Actin-binding LIM protein 1 regulates receptor activator of NF-kappaB ligand-mediated osteoclast differentiation and motility. BMB Rep. 2018;51:356–361. doi: 10.5483/BMBRep.2018.51.7.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Son HS, Lee HI, et al. Skullcapflavone II inhibits osteoclastogenesis by regulating reactive oxygen species and attenuates the survival and resorption function of osteoclasts by modulating integrin signaling. FASEB J. 2019;33:2026–2036. doi: 10.1096/fj.201800866RR. [DOI] [PubMed] [Google Scholar]