Abstract
The unexpected pandemic set off by the novel coronavirus 2019 (COVID-19) has caused severe panic among people worldwide. COVID-19 has created havoc, and scientists and physicians are urged to test the efficiency and safety of drugs used to treat this disease. In such a pandemic situation, various steps have been taken by the government to control and prevent the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2). This pandemic situation has forced scientists to rework strategies to combat infectious diseases through drugs, treatment, and control measures. COVID-19 treatment requires both limiting viral multiplication and neutralizing tissue damage induced by an inappropriate immune reaction. Currently, various diagnostic kits to test for COVID-19 are available, and repurposing therapeutics for COVID-19 has shown to be clinically effective. As the global demand for diagnostics and therapeutics continues to rise, it is essential to rapidly develop various algorithms to successfully identify and contain the virus. This review discusses the updates on specimens/samples, recent efficient diagnostics, and therapeutic approaches to control the disease and repurposed drugs mainly focusing on chloroquine/hydroxychloroquine and convalescent plasma (CP). More research is required for further understanding of the influence of diagnostics and therapeutic approaches to develop vaccines and drugs for COVID-19.
Keywords: Chloroquine, Convalescent plasma (CP), COVID-19, Current diagnostics, SARS-CoV-2
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19), an outbreak caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), continues to spread, and as per the World Health Organization (WHO) data on April 21, 2020, it has reached 213 countries, with 23,56,414 confirmed cases and 160,120 deaths (1). At present, the COVID-19 pandemic has entered a dangerous new phase. The spread of COVID-19 is more severe than that of the previous Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS), because of the increased industrialization, which led to virus evolution (2). This is the third coronavirus epidemic after the SARS and MERS coronavirus outbreaks. Structural studies revealed that there is a close relationship between the receptor-binding domains of SARS-CoV-2 and SARS-CoV. SARS-CoV-2, which causes COVID-19, is spherical, and the viral envelope entails a bilipid layer, which has the membrane (M), envelope (E), spike (S) proteins, and positive-sense RNA as genome, with an RNA-dependent RNA polymerase sequence. SARS-CoV-2 can be viewed as a self-assembled nanostructure in which the most vulnerable and weakest part is the lipid bilayer envelope composed of phospholipid molecules. In addition, SARS-CoV-2 infects host cells through ACE2 receptors, leading to COVID-19 related pneumonia, while also causing acute myocardial injury and chronic damage to the cardiovascular system (3). The transmission of COVID-19 is primarily caused by human coming into contact with the viral droplets, which suggests that keeping a one-meter distance from an infected person will be beneficial.
According to WHO (1), COVID-19 has caused a major concern among public health throughout the world. Many countries have taken precautionary measures against the virus, and government officials in all countries continue to try to minimize human contact by facilitating countrywide shutdowns of public places, and various steps have been started to safeguard the people, like social distancing and self-quarantine, which limits our social interactions (4). This will decrease the risk of spreading COVID-19 to people, by breaking the transmission chain and the influx of new COVID-19 cases in a given time period. Further, the COVID-19 pandemic has forced scientists to rework strategies to combat infectious diseases through drugs, treatment, and control measures. The entire world is taking necessary steps to develop a vaccine for this dreadful virus, and for this kind of research governments are providing copious funds for scientists and institutions. The South Korean government and the Korean Centers for Disease Control and Prevention (KCDC) have announced urgent research projects for the development of vaccines and drugs against COVID-19. In the Republic of Korea, since the first confirmed case of SARS-CoV-2 on January 20, 2020, as per the KCDC report on April 21, 2020, the confirmed cases are 10,683, released from isolation 8213, isolated 2233, and deceased 237. The KCDC is rapidly taking the necessary steps to minimize the infection. The Republic of Korea has implemented several measures to effectively flatten the curve and provide timely medical care to the infected patient. India’s first case of COVID-19 was reported on January 30, 2020, in Kerala. As per WHO’s report on April 21, 2020, in India 18,601 were confirmed cases, 3252 had recovered, and 590 had died. The Indian government is using compulsory measures to ensure that people are well prepared to conquer this deadly infection of COVID-19.
The government of India has announced the call for research projects from its various funding sources and has embarked on some major research projects on COVID-19 that are relevant to national needs. This promotes the new areas of Science and Technology with special emphasis on emerging needs by providing funds for the development of vaccines and drugs in this pandemic situation. In light of this, it will become increasingly necessary to maintain systemic and coordinated efforts between the public, clinical, commercial, and industrial sectors to establish a cohesive body of knowledge and ensure robust diagnostics and treatment supply lines in the midst of this pandemic. Hence, clinical laboratories play a major role in this crisis, contributing to patient screening, diagnosis, monitoring, and treatment. Hence, cooperation with various institutions, academics, governments, and pharmaceutical companies is inevitably necessary to control the virus, combat the situation, and provide solutions for any future pandemic outbreaks. In this review, we aim to discuss the updates of current diagnostics and therapeutic approaches to COVID-19 patients. As rapid diagnostics and development of vaccines and drugs for this dreadful virus are important interventions in the management of the COVID-19 outbreak, the updates on this topic are essential in the current scenario for the betterment of patients. In addition, we discuss the types of sample/specimens collected and rapid diagnostics methods for accurate results to guide treatment options. Currently, chloroquine and hydroxychloroquine are the most-used drugs, which have been discussed along with their mechanism of action on the virus. In addition, Convalescent plasma therapy, which is promising as a way to improve the clinical outcomes of COVID-19 patients, is also discussed.
TYPES OF SPECIMENS COLLECTION FOR COVID-19
The dramatic COVID-19 pandemic has surged at an increasing rate, where the characteristic transmissibility can only be defined at commencement because confirmed cases of COVID-19 may be presented with either asymptomatic or symptomatic infection, which could range from mild to severe or life-threatening pneumonia (5). Particularly, COVID-19 seems to be transmitted mostly during the incubation period, when most of the patients either lack the symptoms or have very mild non-specific symptoms (6). The common symptoms associated with this disease are high fever, cough, difficulty in breathing, and lesions in the lungs; in the worst cases, it can cause severe pneumonia, acute respiratory distress syndrome (ARDS), and risk for life (7). Individuals affected with such symptoms or who have had any International travel history or contact with a person who is ill or been quarantine should approach the government and health-care officials to monitor and check their health status for the safety of their family as well as society. The Centre for Disease Control and Prevention (CDC) (8), the World Health Organization (WHO) (9), and the Indian Council of Medical Research (ICMR) (10) has recommended a few guidelines for the collection of the specimens from the affected or suspected COVID-19 patients. It is mandatory that the affected or suspected individuals should co-operate with the state or local health-care departments for the collection, the further process like storage, and shipment of the specimens appropriately. It is highly mandatory and recommended that the specimens should be collected only in a BSL-3 laboratory for the safety of the clinicians and researchers. Based on the recommended guidelines of WHO, the sample will be isolated from two major sources, which are the lower respiratory tract and the upper respiratory tract. The specimens, such as a nasopharyngeal swab (NP) or the oropharyngeal swab (OP), will be collected from the upper respiratory tract, whereas the bronchoalveolar lavage, tracheal aspirate, or sputum will be collected from the lower respiratory tract (Table 1).
Table 1.
Details about the specimens/samples used for the COVID-19 detection
| Specimen | Form of sample collection | Volume Required | Collection Material | Storage Temperature | Disease Condition | Test can be done |
|---|---|---|---|---|---|---|
| Nasopharyngeal Swab | Frozen | 0.8-1.4 ml | Synthetic fibre swabs with plastic shafts | 2-8°C | Recommended for both symptomatic and asymptomatic | RT-PCR |
| RDT | ||||||
| Oropharyngeal Swab | Frozen | 0.8-1.4 ml | Synthetic fibre swabs with plastic shafts | 2-8°C | Symptomatic Condition | RT-PCR |
| RDT | ||||||
| Bronchoalveolar lavage | Frozen | 0.8-1.4 ml | Sterile Container | 2-8°C | Severe Condition | RT-PCR |
| RDT | ||||||
| Tracheal aspirate | Frozen | 0.8-1.4 ml | Sterile Container | 2-8°C | Severe Condition | RT-PCR |
| RDT | ||||||
| Sputum | Frozen | 0.8-1.4 ml | Sputum collection cup or sterile dry container | 2-8°C | Severe Condition | RT-PCR |
| RDT | ||||||
| Urine | Normal | 0.8-1.4 ml | Urine collection container | 2-8°C | To confirm the viral infection | RT-PCR |
| Blood | Normal | 0.8-1.4 ml | EDTA & ACT tubes | 2-8°C | To confirm the viral infection | RT-PCR |
| RDT | ||||||
| ELISA | ||||||
| Neutralization assay | ||||||
| Stool | Normal | - | Stool container | 2-8°C | To confirm the viral infection | RT-PCR |
RT-PCR: Reverse Transcription-Polymerase Chain Reaction; RDT: Rapid Diagnostic Test; ELISA: Enzyme-linked immunosorbent assay.
Nasopharyngeal swabs: The NP specimen is a vital and sensitive sample to test the SARS-CoV-2 virus, as suggested by the CDC. This sample could be used to analyze an asymptomatic patient with the disease. For this type of sample collection, only synthetic fibre swabs with plastic shafts are recommended, because calcium alginate or wooden-shaft swabs might inactivate the virus and could provide a negative result for the nucleic-acid assay. After the collection of the swab, it should be placed immediately into a 2-3 mL of sterile medium or saline for proper viral transport. If the NP swabs are not available, then the anterior nares and mid-turbinate specimen can also be collected from the symptomatic patients for the detection of the viral infection.
Oropharyngeal swabs: Another important specimen recommended by the WHO and CDC to detect SARS-CoV-2 infection is the OP. This swab is collected from the posterior pharynx region, avoiding contact with the tongue. According to the CDC, if the NP and OP specimens are collected together, then both specimens should be placed in a single vial for more sensitive and appropriate results of the SARS-CoV-2 infection.
Bronchoalveolar lavage and tracheal aspirate: For patients who are severely ill or having severe symptoms for the COVID-19 disease, the bronchoalveolar lavage (BAL) and tracheal aspirate could be used from the lower respiratory tract. The BAL sample is acquired using a bronchoscope or catheter into the bronchus, where the aspirated fluid with the virus will be collected for the testing of the COVID-19 infection.
Sputum: The patients who have severe coughing symptoms will be asked to collect their sputum as the sample to detect the viral infection. The patients will be asked to rinse the mouth with sterile water and further expel the deep cough sputum straight away into a sterile, leak-proof, screw-cap-tightened sputum collection cup or sterile dry container.
Other samples: Since SARS-CoV-2 is present in blood and stool, these specimens can be collected in addition to the respiratory specimens. However, the efficacy of these tests remains unclear, because the data on viral shedding is still preliminary. In addition, for patients who have died, autopsy material and lung tissue may be used to test for the presence of the virus. Based on the samples collected, the diagnostic techniques will be carried out to verify the presence of the viral infection.
AN UPDATE ON DIAGNOSIS FOR COVID-19
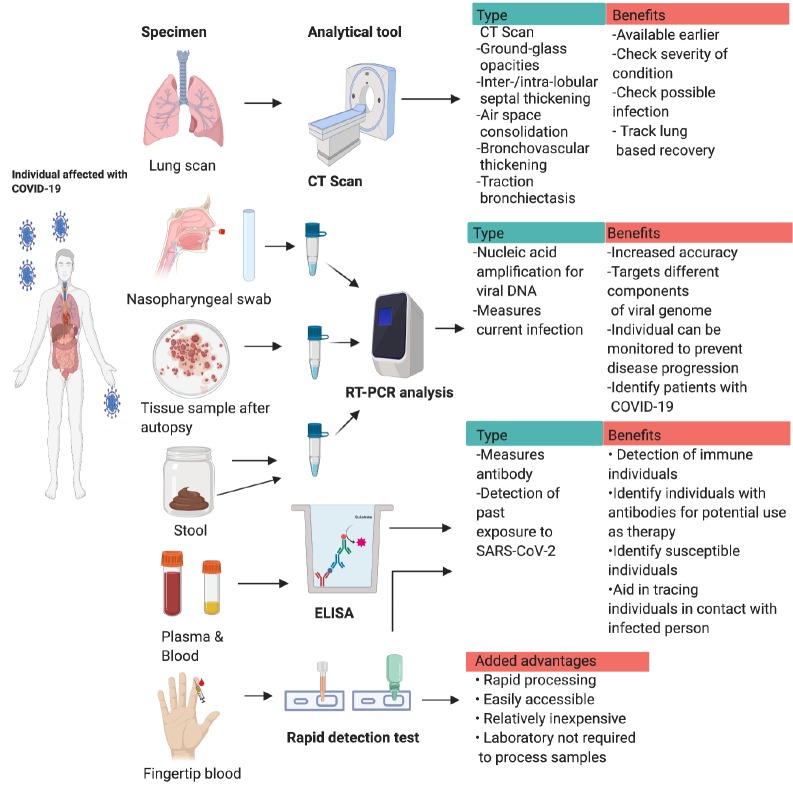
With the number of COVID-19 infections flying off the charts, accurate and rapid diagnosis has evolved as a tool to detect and control the virus (Fig. 1). Currently, various diagnostic tools have been approved by the WHO, which has recommended the collection of upper respiratory specimens using NP swabs. If unavailable, the CDC and WHO recommend the collection of OP specimens, sputum, endotracheal aspirate, bronchoalveolar lavage, blood, stool, and autopsy material and tissue from lungs (8). Recently, the WHO has approved the emergency use of qSARS-CoV-2 IgG/IgM rapid serological tests, in which venipuncture blood collected by medical professionals can be used. However, the use of this test is limited to authorized laboratories alone. This test identifies the IgM and IgG antibodies produced by the patient in response to SARS-CoV-2 infection (9). Likewise, ICMR has also issued some guidelines for the diagnosis of COVID-19. The ICMR has recommended the use of RT-PCR probes from the USA that are distributed to laboratories in the country. In addition, commercial kits and US FDA EUA/CE IVD-approved kits may be used for diagnostic purposes in India. Further, kits with 100% concordance will also be fast-tracked for use in India; 20 non-US/FDA/EUA/CE/IVD kits have completed evaluation in this regard (10). Moreover, the tests available predominantly check for the presence of viral nucleic acid in the specimen through RT-PCR. In order to confirm the diagnosis, a minimum of two targets must be chosen on the SARS-CoV-2 genome, with one being specific for SARS-CoV-2. Otherwise, the test can include a primer specific for beta coronavirus, and the presence of SARS-CoV-2 must be confirmed by sequencing of the genome. Various viral proteins like orf1ab, N, RdRp, S, E, ORF1b, and nsp14, have been targeted for diagnosis in laboratories worldwide. At this point, it is essential to point out that a negative result does not exclude the probability of COVID-19, because such a result can be caused by poor-quality specimens, late or very early collection, improper handling of the sample, and inaccurate testing methods. The commonly available RT-PCR tests that are widely used for detection of COVID-19 take a significant amount of time to give the results and also require trained personnel and well-equipped laboratories for their use. As rapid detection tests appear to be a break-through in COVID-19 diagnosis, fine-tuning of this technique is required to use them as regular diagnostic kits. Although these tests are quick and relatively inexpensive, their accuracy has not been established yet. Another drawback is that antibodies develop only a few weeks after infection, and the levels may be more pronounced in severe cases, increasing the chances of a false negative in patients with mild or asymptomatic COVID-19. For now, we recommend the use of RT-PCR as diagnostic tools for the detection of COVID-19. In addition to these methods, CT scans may aid in the diagnosis of COVID-19, but this method is not recommended for routine screening, because it may not be precise in all patients (11). It is also possible that biochemical assays and blood counts, including lymphocyte levels and C‐reactive protein (CRP) proteins, may serve as useful biomarkers for COVID-19, and these tests can be used as preliminary screening for COVID-19. More research is required to ascertain the value of these tests in this domain. As the global demand for diagnostic tools continues to rise, it is essential to rapidly develop diagnostic kits using various algorithms to successfully identify and contain the virus.
Fig. 1.
Collection of Specimens/samples and diagnostics methods for COVID-19: Depiction of various diagnostic methods for COVID-19 infection. CT scans can be utilized to find lung abnormalities in patients with infection, this can be a serious tool to determine severity and track progress. The NP swab taken from the patient is collected and transported to a laboratory. Here, RT-PCR analysis is conducted using specific viral gene probes targeting viral specific genes. If the viral nucleic acid is present in the specimens, the patients is diagnosed with COVID-19. Similar RT-PCR techniques are utilized to detect presence of the virus in tissue samples after autopsy and stool samples of patients exhibiting symptoms. The plasma and blood collected by venipuncture is used to detect virus specific antibodies in the blood using ELISA method using a reporter antibody. As antibodies will be present in the blood after infection, this can be utilized as a tool to detect exposure to virus. The rapid detection kits use blood from a finger prick to detect 1gG and IgM antibodies. These tests are easily accessible and do not need a laboratory for further processing.
BLOOD PROFILING IN COVID-19
Blood profiles and biochemical assays are the most commonly recommended test by physicians in case of any disease conditions (Supplementary Table 1). Various blood and biochemical abnormalities have been witnessed in patients with COVID-19. The most commonly found irregularities include leucocytosis, leukopenia, lymphopenia, increased CRP, lactate dehydrogenase (LDH), and erythrocyte sedimentation rate (ESR) (12). Other observed discrepancies include abnormal procalcitonin, and albumin and liver enzyme levels. On viral entry into the body, the virus attacks the throat, causing the initial symptoms of COVID-19, further progresses to the lungs, and causes distress, following which it reaches the bloodstream (13), resulting in the progression of the condition. In healthy individuals, white blood cells (WBCs) circulate in the blood and are attacked by foreign particles, such as bacteria and viruses. Leukocytosis occurs when there is an abnormal increase in WBCs above the normal range (3,400 to 9,600 cells/μL). This is a common sign of inflammation induced by viral entry. Similarly, leukopenia is the anomalous decrease of WBCs (less than 4,000 cells/μL) in the blood and can also result from various viral infections in which the WBCs are used more quickly than they are made. Likewise, lymphocytes are cells produced by the bone marrow involved with immunity; a dynamic balance is maintained in the number of lymphocytes in healthy individuals. Lymphopenia or reduction of lymphocytes (less than 1,000 lymphocytes/μL) is another abnormality commonly observed in COVID-19 patients. Lymphopenia is a factor that is positively correlated with disease severity (14) and may be caused by the surge in cytokines mediated by COVID-19 or by direct inactivation by SARS-CoV-2. Correspondingly, the inflammatory response triggered by SARS-CoV-2 results in the modification of levels of CRP and ESR in the blood. In COVID-19, CRP concentration rises following IL-6 secretion caused by the cytokine storm after infection. It binds to markers present on dying cells and consequently activates the complement system to mediate phagocytosis of the dead cells. As a result, CRP (normal 3.0 mg/L) is increased in patients with COVID-19. Likewise, after infection, the cytokine storm increases the fibrinogen levels in the blood; this makes the erythrocytes adhere together, increasing the ESR during times of infection. Aside from the changes in the blood portfolio, LDH, a common component of cells of various organs, is increased in COVID-19. The normal blood LDH level is 140-280 units/L, but when the cells or tissues are damaged, LDH is released into the serum, increasing its levels. Hence LDH is used as a test to detect cell or tissue injury. Like LDH, procalcitonin is normally negligible in the blood (0.01 μg/L) but increases once stimulated by cytokines. The lungs and liver also produce procalcitonin as a response to inflammatory cytokines. These processes increase the levels of procalcitonin in the blood upon infection or injury. This is concomitant with increased levels of AST and ALT, indicating liver damage. Since many reports have observed kidney and liver injury in patients with COVID-19, these markers could thus be elevated (Supplementary Table 2). The changes in the levels of these components suggest that COVID-19 may be involved with damage to various tissues and cells in the body. It is imperative to closely monitor the changes in these parameters to uncover early biomarkers of COVID-19. Changes in levels of these cells and molecules may happen well in advance of the onset of typical COVID-19 symptoms. Moreover, a deeper understanding of the changes in these parameters will aid in the development of therapies for COVID-19. Further investigation is needed in this area for a better understanding of these abnormalities in blood parameters to provide accurate therapeutic drugs for COVID-19.
CURRENT TREATMENT OPTIONS UNDER STUDY
According to WHO, based on evidence from laboratory, animal, and clinical studies, the drugs which are advised for treatment of COVID-19 are Remdesivir, Lopinavir/Ritonavir, Lopinavir/Ritonavir with interferon beta-1a, chloroquine, and hydroxychloroquine. Remdesivir has been previously tested for Ebola treatment (15). According to a recent study, an inflammatory drug, baricitinib, when used in combination with anti-viral drugs like Remidesivir, increases the potential of the drug to reduce viral infection (16). The Lopinavir/Ritonavir drug is currently provided to treat HIV infection; from the laboratory experiments, it is evident that these combinational drugs could be used to treat the COVID-19 infections. According to the (15), the interferon beta-1a, which is used to treat multiple sclerosis, can also be used as a remedial approach for COVID-19 disease. Ongoing clinical trials used to treat COVID-19 (Table 2) and drugs in use have been updated (Table 3).
Table 2.
Updated details on Ongoing Clinical Trials in COVID-19
| Study | Drug | Status | Organization |
|---|---|---|---|
| Ongoing Clinical Trials for the Management of the COVID-19 Pandemic | Umifenovir, triazavirin, baloxavir marboxil, danoprevir/ritonavir, azvudine, sofosbuvir/ledipasvir, sofosbuvir/ daclatasvir, darunavir/cobicistat, and emtricitabine/tenofovir; dexamethasone; methylprednisolone; ASC09 and oseltamivir ixekizumab; bevacizumab | Recruiting | China, Iran, Spain, UK |
| Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in Participants with Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | Remdesivir | Recruiting | 1. Hoag Memorial Hospital Presbyterian Newport Beach, California, United States |
| 2. Stanford Hospital, Stanford, California, United States | |||
| 3. Providence Regional Medical left Everett, Everett, Washington, United States | |||
| Fingolimod in COVID-19 | Fingolimod 0.5 mg | Recruiting | Wan-Jin Chen Fuzhou, China |
| The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | 1. Carrimycin | Not yet recruiting | - |
| 2. Lopinavir/ritonavir tablets or Arbidol or Chloroquine phosphate | |||
| Efficacy and Safety of Corticosteroids in COVID-19 | Methylprednisolone | Recruiting | 1. Hubei province hospital of integrated Chinese & Western Medicine Wuhan, Hubei, China |
| 2. Yichang first people's Hospital Yichang, Hubei, China | |||
| 3. Renmin Hospital of Wuhan UniversityWuhan, China | |||
| Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | Sarilumab | Recruiting | Regeneron Study Site |
| New York, New York, United States | |||
| Mild/Moderate 2019-nCoV Remdesivir RCT | Remdesivir | Recruiting | Jin Yin-tan hospital |
| Wu Han, Hubei, China | |||
| Adaptive COVID-19 Treatment Trial | Remdesivir | Recruiting | 1. National Institutes of Health - Clinical left, National Institute of Allergy and Infectious Diseases Laboratory of Immunoregulation, Clinical Research SectionBethesda, Maryland, United States |
| 2. University of Nebraska Medical left - Infectious DiseasesOmaha, Nebraska, United States | |||
| 3. University of Texas Medical Branch - Division of Infectious DiseaseGalveston, Texas, United States | |||
| 4. Providence Sacred Heart Medical leftSpokane, Washington, United States | |||
| Ongoing Clinical Trials for the Management of the COVID-19 Pandemic | Thymosin; suramin; conventional treatment + adalimumab | Not yet recruiting | China |
| Severe 2019-nCoV Remdesivir RCT | Remdesivir | Recruiting | Bin Cao; Beijing, Beijing, China |
| Nitric Oxide Gas Inhalation for Severe Acute Respiratory Syndrome in COVID-19 | Nitric Oxide Gas | Not yet recruiting | - |
| Efficacy and Safety of IFN-a2b in the Treatment of Novel Coronavirus Patients | Recombinant human interferon α1β | Not yet recruiting | - |
| Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | 1. ASC09/ritonavir group | Not yet recruiting | - |
| 2. Lopinavir/ritonavir group | |||
| Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection | mRNA-1273 | Not yet recruiting | Kaiser Permanente Washington Health Research Institute - Vaccines and Infectious DiseasesSeattle, Washington, United States |
| Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in Participants With Severe Coronavirus Disease (COVID-19) | Remdesivir | Recruiting | 1. Hoag Memorial Hospital PresbyterianNewport Beach, California, United States |
| 2. Stanford Hospital, Stanford, California, United States | |||
| 3. Providence Regional Medical left Everett, Everett, Washington, United States | |||
| Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | 1. Lopinavir/ritonavir | Recruiting | University of Hong Kong, Queen Mary Hospital |
| 2. Ribavirin | Hong Kong, Hong Kong | ||
| 3. Interferon Beta-1B | |||
| Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multileft randomized controlled clinical study | 1. Chloroquine | - | The Fifth Affiliated Hospital Sun Yat-Sen University |
| 2. Lopinavir/ Ritonavir | |||
| A prospective, randomized, open-label, parallel controlled trial for the preventive effect of hydroxychloroquine on medical personnel after exposure to COVID-19 | Hydroxychloroquine | - | Renmin Hospital of Wuhan University |
| Glucocorticoid Therapy for Novel Coronavirus Critically Ill Patients with Severe Acute Respiratory Failure | Methylprednisolone | Recruiting | Medical ICU,Peking Union Medical College HospitalBeijing, Beijing, China |
| The efficacy and safety of carrimycin treatment in patients with novel coronavirus infectious disease (COVID-19): a multileft, randomized, open- label controlled trial | Carrimycin | - | Beijing You'an Hospital, Capital Medical University |
| A prospective clinical study for recombinant human interferon alpha 1b spray in the prevention of novel coronavirus (COVID-19) infection in highly exposed medical staffs | recombinant human interferon alpha 1b | - | Chinese PLA General Hospital |
| A Pilot Study of Sildenafil in COVID-19 | Sildenafil citrate | Recruiting | Department and Institute of Infectious Disease, Wuhan, Hubei, China |
| A study for the efficacy of hydroxychloroquine for mild and moderate COVID-19 infectious diseases | Hydroxychloroquine | - | The Second Affiliated Hospital of Chongqing Medical University |
| The Efficacy and Safety of Thalidomide Combined with Low-dose Hormones in the Treatment of Severe COVID-19 | Thalidomide | Not yet recruiting | - |
| Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Chloroquine for Treatment of COVID19: A Randomized Control Trial | Oral | Not yet recruiting | Subsai Kongsaengdao, Bangkok, Thailand |
| Randomized Controlled Trial of Losartan for Patients With COVID-19 Not Requiring Hospitalization | Losartan | Not yet recruiting | Hennepin County Medical left, Minneapolis, Minnesota, United States |
| M Health Fairview University of Minnesota Medical left, Minneapolis, Minnesota, United States | |||
| University of Minnesota, Minneapolis, Minnesota, United States | |||
| Chloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting | Chloroquine | Not yet recruiting | - |
| Favipiravir Combined with Tocilizumab in the Treatment of Corona Virus Disease 2019 | Favipiravir Combined with Tocilizumab | Recruiting | Anhui Medical University Affiliated First Hospital, Hefei, Anhui, China |
| Guiqiang Wang, Beijing, Beijing, China | |||
| Peking University First Hospital, Beijing, Beijing, China | |||
| Trial of Treatments for COVID-19 in Hospitalized Adults | 1. Remdesivir | Not yet recruiting | - |
| 2. Lopinavir/ritonavir | |||
| 3. Interferon Beta-1A | |||
| Randomized Controlled Trial of Losartan for Patients With COVID-19 Requiring Hospitalization | Losartan | Not yet recruiting | Hennepin County Medical left, Minneapolis, Minnesota, United States |
| M Health Fairview University of Minnesota Medical left, Minneapolis, Minnesota, United States | |||
| University of Minnesota, Minneapolis, Minnesota, United States | |||
| Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients with Mild Coronavirus Disease (COVID-19) | 1. Lopinavir/ritonavir Hydroxychloroquine sulfate | Recruiting | Asan Medical left, University of Ulsan College of Medicine, Seoul, Korea, Republic of Korea |
| Evaluation of Ganovo (Danoprevir) Combined with Ritonavir in the Treatment of Novel Coronavirus Infection | Ganovo with ritonavir +/-Interferon | Recruiting | The Ninth Hospital of Nanchang |
| Nanchang, Jiangxi, China | |||
| Eculizumab (Soliris) in Covid-19 Infected Patients | Eculizumab | Initiated | - |
| Norwegian Coronavirus Disease 2019 Study | Hydroxychloroquine Sulfate | Not yet recruiting | - |
| Post-exposure Prophylaxis for SARS-Coronavirus-2 | Hydroxychloroquine | Recruiting | University of Minnesota, Minneapolis, Minnesota, United States |
| The efficacy and safety of pirfenidone capsules in the treatment of severe new coronavirus pneumonia (COVID-19) | Pirfenidone | - | Third Xiangya Hospital of Central South University |
| Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China | Oseltamivir | Recruiting | Zhongnan Hospital of Wuhan University in Wuhan, China |
| Expanded Access Remdesivir (RDV; GS-5734TM) | Remdesivir | Initiated | - |
The table represents a list of selected clinical trials for the amelioration of COVID-19 specific drugs and vaccines.
Table 3.
Details of currently available drugs in the treatment of COVID-19
| Name of Drug | Illnesses treated |
|---|---|
| Ribavirin | RSV and RSV pneumonia |
| Reverse transcriptase inhibitors: zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir and emtricitabine | SARS |
| Cathespin L | SARS |
| Methylprednisolone | SARS, MERS |
| Protease Inhibitors (PIs): saquinavir, ritonavir, indinavir, nelfinavir, amprenavir, lopinavir, atazanavir and fosamprenavir | SARS |
| Fusion inhibitor: enfuvirtide. Lamivudine and adefovir dipivoxil | SARS |
| Baricitinib | COVID-19 |
| Umifenovir | ARVI, influenza, rhinovirus, adenovirus, parainfluenza, respiratory syncytial virus, coronavirus, including the causative agent of atypical pneumonia |
| Used in the phase III trials of 2019-nCoV virus, SARS, MERS | |
| 3-chymotrypsin-like protease | SARS, MERS |
| Capsid spike glycoprotein (hCoV-EMC) | SARS, Human Coronavirus |
| Guanosine-analog RNA synthesis inhibitors | Coronavirus |
| Ritonavir and lopinavir | SARS, MERS |
| Interferon Subtypes of β-1b, α-n1, α-n3, and human leukocyte interferon α | SARS |
| Acyclovir | SARS, MERS, Coronavirus 229E and COVID-19 |
| Nitazoxanide | SARS, MERSand Influenza |
| Influenza drugs | MERS |
| Remdesivir | COVID-19, SARS, MERS |
| Favipiravir | COVID-19 |
| Darunavir | COVID-19 |
| Lopinavir | COVID-19, SARS, MERS |
| Alcohol Vaporization/Nebulization Inhalation Therapy | COVID-19 |
| Chloroquine | SARS, Human Coronavirus OC43 |
| Chloroquine phosphate; Arbidol | COVID-19 |
| ASC09 | ARDS, Respiratory distress syndrome, SARS, MERS |
| TMPRSS2 inhibitor Camostat mesylate | SARS, MERSCoronavirus 229E and COVID-19 |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs): nevirapine, delavirdine and efavirenz | SARS |
| Ruxolitinib | COVID-19 |
| Saquinavir | SARS and Feline Coronavirus |
| Relenza, Tamiflu, Amantadine | Influenza virus |
| Indinavir | SARS and COVID-19 |
| Carfilzomib | COVID-19 |
| Oseltamivir | COVID-19 |
| Azvudine | COVID-19 |
| Baloxavir marboxil | COVID-19 |
| Thymosin α1 | MERS |
| Nucleotide reverse transcriptase inhibitor: tenofovir disoproxil fumarate. | SARS |
| Papain-like protease | SARS, MERSand Human Coronavirus NL63 |
| RNA-dependent RNA polymerase | SARS, Murine Coronavirus |
| Tocilizumab | COVID-19 |
| α-interferon | Spectrum of respiratory infectionsΈ RSV and SARS |
Table 3 represents the commercially available drugs used for the treatment of the various forms of coronaviruses. The viral infections discussed in the table are SARS - Severe Acute Respiratory Syndrome, MERS-Middle East Respiratory Syndrome, RSV - Respiratory Syncytial Virus, ARVI - Acute respiratory viral infections.
CHLOROQUINE AS A THERAPEUTIC DRUG FOR HUMAN DISEASES
Chloroquine was first developed in the 1940s, as an aminoquinolone derivative developed for the treatment of malaria (17). The chemical formula of chloroquine is C18H6CIN3. Chloroquine was granted FDA approval on October 31, 1949 (FDA, Approved Drug Products: Aralen Chloroquine Oral Tablets). Chloroquine and its derivative, hydroxychloroquine, are very closely related and used to treat malaria and rheumatologic conditions, respectively. Hydroxychloroquine is a disease-modifying anti-rheumatic drug, and it regulates the activity of the immune system, which may be overactive in some conditions including rheumatoid arthritis, discoid and systemic lupus erythematosus, and juvenile idiopathic arthritis. Chloroquine and hydroxychloroquine have been repurposed for the treatment of several disease conditions, including HIV, Systemic lupus erythematosus, and rheumatoid arthritis (18). Chloroquine is classified as an anti-malarial drug and has shown potential in the treatment of avian influenza A (19). Chloroquine has been indicated to treat infections caused by P. vivax, P. malariae, P. ovale, and P. falciparum. It can also be used to treat extraintestinal amebiosis (FDA; Approved Drug Products: Chloroquine Phosphate Oral tablets) and prophylaxis caused by Zika virus (20, 21).
The anti-malarial drugs hydroxychloroquine and chloroquine have demonstrated antiviral activity SARS-CoV-2 in vitro (22). A Chinese study found that chloroquine will reduce the symptom duration, exacerbation of pneumonia, including radiological improvement, and promotes virus-negative seroconversion with no side effects (23). Because of the COVID-19 pandemic, the FDA has approved an emergency authorization for use of chloroquine and hydroxychloroquine (FDA: Emergency Use Authorization Information). Chloroquine is currently undergoing clinical trials for the treatment of COVID-19 (23).
CHLOROQUINE/HYDROXYCHLOROQUINE: IS IT PROMISING FOR COVID-19?
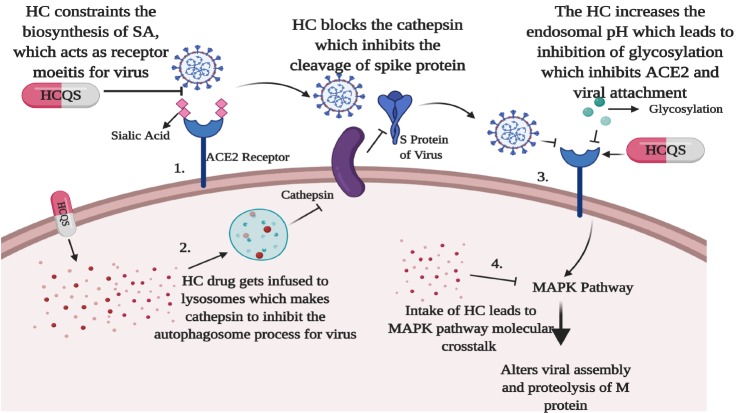
Chloroquine, which is categorized as an anti-viral drug, is principally used as an anti-malarial and autoimmune-treating drug (19, 24). Chloroquine and its combination of drugs used in the treatment of COVID-19 has been listed in Table 4. Hydroxychloroquine and chloroquine are currently undergoing clinical trials to ensure their preventive action for SARS-CoV-2 infected patients (8). Chloroquine diffuses to the cell passively through the cell membranes and to the endosomes, lysosomes, and Golgi vesicles, where it becomes protonated, trapping the chloroquine in the organelle and raising the surrounding pH (25, 26). The raised pH in the endosomes prevent virus particles from using their activity for fusion and entry into the cell. Chloroquine does not affect the expression of ACE2 on cell surfaces but inhibits terminal glycosylation of the ACE2 receptor for cell entry targeted by SARS-CoV and SARS-CoV-2 (26-28). The chloroquine and hydroxychloroquine have various way to inhibit SARS-CoV-2 action. Also, because of chloroquine, there is a change in the pH of lysosomes that leads to inhibition of the cathepsins, which is mandatory for the formation of autophagosomes to cleave the SARS-CoV-2 spike (S) protein and blocks the viral attachment to the human host receptors. Additionally, the chloroquine can constrain the quinone reductase-2, which is an essential agent required for the biosynthesis of sialic acid, which is generally used as the receptor moieties by the SARS-CoV-2. Moreover, the chloroquine obstructs the MAP-kinase, which results in SARS-CoV-2 virus molecular crosstalk, further alters the viral assembly, and intrudes into the proteolytic process of the M protein of the virus (26). Savarino et al. (29) hypothesized that chloroquine might block the production of pro-inflammatory cytokines like interleukin-6 by blocking the pathway leading to ARDS.
Table 4.
Chloroquine and its combination of drugs used in the treatment of COVID-19
| Study Particulars | Drugs | Dosage | Reference |
|---|---|---|---|
| Chloroquine (C) phosphate against pneumonia caused by COVID-19 but need confirmation through randomized trials | Chloroquine phosphate | Not specified | 15 |
| SARS-CoV-2 and blocked viral infection by increasing the endosomal pH required for viral fusion | Chloroquine | 1.13 μM at half maximal concentration | 27 (Nichol et al., 2020) |
| Chloroquine (C) and Hydroxychloroquine (HC) are under investigation in clinical trials for pre-exposure or post-exposure prophylaxis of SARS-CoV-2 infection, and treatment of patients with mild, moderate, and severe COVID-19 | Chloroquine and Hydroxychloroquine | Not specified | 8 |
| C and HC inhibit in vitro replication of viruses which envelope fuses with that of the acidified endosome. The in vitro antiviral activity of C and HC against SARS-CoV-2 reported | Chloroquine and Hydroxychloroquine | Not specified | 27 (Nichol et al., 2020); 43 (Liu et al., 2020) |
| Prescribing Hydroxychloroquine treatment on a large scale, and decided to perform a study aimed at demonstrating that HC is effective in vivo against SARS-CoV-2 | Hydroxychloroquine | Not specified | 31 (Parola et al., 2020) |
| Azithromycin–suggested to act in combination with C/HC against SARs-CoV-2 | Azithromycin + Chloroquine + Hydroxychloroquine | Not specified | 31 (Parola et al., 2020) |
| Two drugs, remdesivir and chloroquine phosphate, efficiently inhibited SARS-CoV-2 infection in vitro | Remdesivir + chloroquine phosphate | Not specified | 27 (Nichol et al., 2020) |
| COVID-19 pneumonia and without contraindications to chloroquine, be treated with 500 mg chloroquine twice a day for ten days | Chloroquine | 500 mg twice a day | 31 (Parola et al., 2020) |
| Hydroxychloroquine is recommended for high-risk COVID-19 cases | Hydroxychloroquine | Not specified | 32 (Hoffman et al., 2006) |
| HC and C demonstrated antiviral activity against SARS–CoV-2 in vitro and in small, poorly controlled or uncontrolled clinical studies | Chloroquine and Hydroxychloroquine | Not specified | 22 (Zhang et al., 2020) |
| Reducing symptom duration, exacerbation of pneumonia including radiological improvement and promoting virus-negative seroconversion without any severe side effects in COVID-19 | Chloroquine | Not specified | 23 (Yang et al., 2020) |
Additional assays demonstrated that chloroquine functioned at both entries and post-entry stages of the 2019-nCoV infection in Vero E6 cells. The in vitro study findings revealed that remdesivir and chloroquine are highly effective in the control of COVID-19. Because these compounds have already passed the safety test against various diseases, they could be used to treat the patients suffering from COVID-19 infection (30). The patients affected with COVID-19 infection and who have no complications with the chloroquine drug are advised to take 500 mg of chloroquine two times a day for 10 days (31). The ICMR recommends the use of hydroxychloroquine for high-risk COVID-19 cases (32).
In China and France, small studies provide some indications of possible benefits of chloroquine phosphate against pneumonia caused by COVID-19, but need confirmation through randomized trials (9). Chloroquine also has been shown to have anti-viral as well as immune-modulating properties. This drug also showed 1.13 μM at half-maximal concentration against SARS-CoV-2 and blocked viral infection by increasing the endosomal pH required for viral fusion (Fig. 2) (30). Azithromycin has been suggested to act in combination with Chloroquine/hydroxychloroquine against SARs-CoV-2 (31). Several in vitro studies reported the anti-viral activity of Chloroquine and hydroxychloroquine against SARS-CoV-2. At present, there is insufficient in vivo evidence to recommend their use for the current pandemic outside of clinical trials. Further high-quality studies are urgently needed to provide guidance to clinicians and policymakers.
Fig. 2.
Probable mechanism of hydroxychloroquine (HC) against SARS-CoV-2: The figure depicts the mechanism of action of hydroxychloroquine targeting the SARS-CoV-2 through many ways. (1) The hydroxychloroquine has the ability to constrain the quinone reductase-2 which is an essential agent required for the biosynthesis of sialic acid (SA) which is generally used as the receptor moieties by the SARS-CoV-2. (2) The hydroxychloroquine could change the pH of lysosomes that leads to inhibition of the cathepsins which is mandatory for the formation of autophagosomes to cleave the SARS-CoV-2 spike (S1 and S2) protein and blocks the viral attachment to the human host receptors. (3) The drug also targets the virus through increasing the endosomal pH and hinders the glycosylation process of the cellular receptors of SARS-CoV-2, which eventually blocks the viral attachment to the ACE2 receptors and inhibits the viral infection. (4) Moreover, the hydroxychloroquine obstructs the MAP-kinase pathway which results to SARS-CoV-2 virus molecular crosstalk resulting into alteration of viral assembly and also intrude the proteolytic process of the M protein of the virus.
CONVALESCENT PLASMA (CP) THERAPY SPELLS A HOPE FOR COVID-19
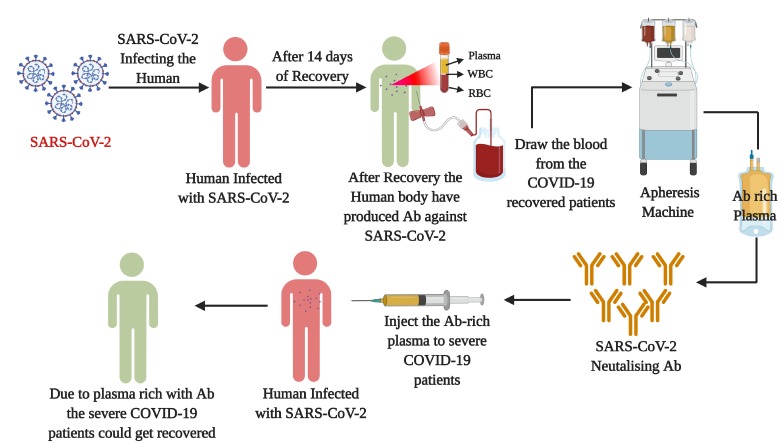
Although a definitive treatment or specific vaccine for this deadly viral infection is still a question to be answered, an experimental study related to convalescent plasma (CP) therapy appears as a shaft of light to combat the SARS-CoV-2 infection. Doctors and scientists are claiming that CP therapy was effective during the past epidemics; it could be a potential treatment option in this current scenario as well. Even the ICMR has given approval for the state hospitals to register for a CP trail protocol, to initiate the clinical trials of CP therapy for the COVID-19 patients. The CP therapy is a common adaptive immunotherapy, where the patients recovered from a viral disease with high neutralising antibody titer against the virus and would be used as plasma donors to treat the affected individuals. In this therapy, when our body gets infected with the SARS-CoV-2, our immune system produces antibodies against it. These antibodies reach out to identify and mark the invading virus as the intruding foreign body inside the human system (Fig. 3). This, in future, triggers the white blood cells to attack the identified intruders (SARS-CoV-2), which leads to the deactivation of the viral infection. The CP therapy has been used to treat several infectious diseases, including Spanish flu (1917-1918) (32), the Ebola epidemic in West Africa (33), human coronaviruses (34), influenza A (H1N1) and A(H5N1) (35). During the H1N1 viral infection, the CP therapy gave positive results, where the respiratory tract viral load, serum cytokine levels, and mortality levels were significantly reduced in the infected groups compared to their control group (36).
Fig. 3.
Convalescent plasma (CP) therapy: The figure illustrates the process and importance of CP therapy to treat COVID-19 disease. CP therapy is an immunotherapy where the humoral antibody (Ab) from the recovered patients to the severely affected diseased patients. In CP therapy, as the SARS-CoV-2 affected is infected the Ab spans out and marks the virus as an intruding agent into the human system. This in future triggers the White blood cells to identify the SARS-CoV-2 virus which deactivates the viral function in the human body. In this procedure almost 1ltr of blood will be collected from the recovered patients and approximately 250 ml of plasma will be injected to the COVID-19 diseased patients. This might reduce the COVID-19 disease symptoms, give relief to the patients and would get recovered from this dreadful infection.
During the SARS and MERS pandemics, CP had provided several satisfactory and successful therapeutic outcomes (37, 38). In a meta-analysis from 32 studies conducted on SARS-CoV infection, the mortality rate was significantly decreased when compared to their placebo group (35). Since there is a high sequence as well as virological homology between the SARS-CoV and SARS-CoV-2, the CP therapy could be a promising therapy to treat the severely affected COVID-19 patients (39, 40). In CP therapy the antibody-rich plasma will be isolated from the COVID-19 recovered patients using an apheresis machine; the plasma-rich antibody against the SARS-CoV-2 would be injected, which could neutralise the viral load in the severely affected COVID-19 patients. For this procedure, around one litre of plasma will be collected, where approximately only 250 mL of plasma is required for one patient (41). According to a recent study in China, the researchers found that CP therapy could rescue COVID-19 patients from disease severity. During this experimental assay, one dose of 200 ml CP transfusion was readily accepted, which also reduced several symptomatic conditions of the COVID-19 patients. The researchers found that after the introduction of CP therapy, the patients showed negative for the SARS-CoV-2 nucleic-acid test, increased the oxygen saturation levels and lymphocyte counts, and also improved the functions of organs (42). These studies demonstrate that CP therapy could be a promising therapy to improve the clinical outcomes of COVID-19 patients. Since this therapy is in its pipeline stage, more investigations are required in larger cohorts to make it a global and standardised therapy to treat COVID-19 infection.
RECOMMENDATIONS
Through this review, we recommend a few guidelines which could help to speed up the process of viral deactivation and production of the vaccine. Also, these recommendations will help the COVID-19 patients to recover soon and help the recovered patients be safe against this virus in the future. The guidelines are as follows.
Based on the global data available, it is very unfortunate that the spread of the virus is still ongoing and the impact of the infection is still on the rise, despite the various preventive and precautionary interventions carried out by us.
It is mandatory that the infected or possibly infected SARS-CoV-2 patients should immediately contact the nearby health-care professional to check on their health status to safeguard both their families and their societies.
It is necessary to follow the rules and regulations provided by the health-care professional and government officials, which include quarantine, national lockdown, and social distancing as a measure to control the spread of the infection.
It is highly encouraged and recommended that only vigorous obedience to the guidelines amended by the government and maintaining the preventive and precautionary measures will yield the desired result of containing this viral infection.
We should co-operate with the health-care officials if they need to test our samples by providing them with the desired specimens to confirm the presence or absence of the infection in a particular individual.
Based on the efficiency of the hydroxychloroquine and chloroquine drugs, it should be approved soon as the one-stop remedy to treat the SARS-CoV-2 infection.
As per the government order, more state hospitals should take up the charge to carry out the clinical trials on convalescent plasma therapy as a remedial approach for this viral infection.
The recovered patients should come forward to provide their blood samples to extract the antibody-rich plasma as a therapy to serve the severely affected COVID-19 patients.
Home quarantine should be strictly followed, as it will be easy to combat the disease more expediently.
People who are infected or may have been infected must drink plenty of water to stay hydrated and also should be encouraged to have more nutritious food to improve their immunity and stamina to fight this infection.
Individuals should follow the guidelines of the health-care and government professionals and should reduce their intake of smoking and alcohol consumption in order to improve will improve their immunity.
These social strategies could be a game-changer in rapidly controlling the spread of this pandemic, where a unified and strict co-operation and co-ordination from us as citizens are very important for combatting the COVID-19 disease.
During this dreadful situation, it is highly suggested that any travel to the international sectors should be avoided, because it could increase the spread of the viral load among societies.
It is evident that the spread of infection is mainly through human-to-human transmission; hence it is mandatory to monitor our health status regularly if we have even a mild symptom associated with this disease.
Containing the spread of SARS-CoV-2 infection will potentially reduce the stress and strain on the health-care professionals as well as on the researchers/scientist, which will enable them to concentrate more on the development of the specific vaccine for this disease.
CONCLUSION
COVID-19 is a global pandemic, and currently it has emerged as the most intense and petrifying viral infection to be handled by the human race. Many countries have taken precautionary measures against the virus, and government officials in all countries continue to make efforts to minimize human contact by facilitating countrywide shutdowns of public places. Several steps have been initiated to ensure the safety of the people, like social distancing and self-quarantine, which limits our social interactions. Since the long-term effects of COVID-19 remain unknown, we still need to figure out the exact mechanism of the virus for the preventive, diagnostic and therapeutic approaches to battle the situation.
This will help to diagnose diseases at earlier stages, allowing medical intervention at this time. Early medical intervention will help to prevent the conditions altogether or at least ameliorate its prognosis. As the number of individuals infected with SARS-CoV-2 continues to rise globally, health-care systems become increasingly stressed. It is clear that the clinical laboratory plays a major role in this crisis, contributing to patient screening. As COVID-19 has triggered human casualties and a serious economic crisis posing a global threat, an understanding of the current scenario and developing a plan of action to contain the spread of the virus are urgently needed. Rapid diagnostics, vaccines, and therapeutics are important interventions for the management of the COVID-19 outbreak. It is essential to move forward with all the information necessary to be more prepared for a pandemic of its kind in the future. At present, scientists are working rigorously to find the solution to treat the SARS-CoV-2 infection at a rapid pace. Hence, more research is needed to adequately care for patients post recovery and to provide a framework of possible physical manifestations of the disease.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGEMENTS
The author Dr. VB would like to thank Bharathiar University for providing the necessary infrastructure facility and the Science and Engineering Research Board (SERB) (ECR/2016/001688), Government of India, New Delhi, for providing necessary help in carrying out this review of diagnostic and therapeutic approaches. Dr. SMD would like to thank the Science and Engineering Research Board (SERB) (ECR/2018/000718), Government of India, New Delhi, for providing necessary help in carrying out this review process. The study was supported by a grant from the National Research Foundation (NRF) funded by the Korean Government (Grant no: 2019M3A9H1030682).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.World Health Organization. [accessed on 21 April, 2020];2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Balachandar V, Kaavya J, Mahalaxmi I, et al. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138277. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng F, Tang W, Li H, Huang Y, Xie Y, Zhou Z. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 4.Balachandar V, Mahalaxmi I, Kaavya J, et al. COVID-19: Emerging protective measures. Eur Rev Med Pharmaco. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- 5.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway E, Cyranoski D. China coronavirus: six questions scientists are asking. Nature. 2020;577:605–607. doi: 10.1038/d41586-020-00166-6. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Prevention and Control (CDC) [accessed on 16 April, 2020];Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) 2020 https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html.
- 9.World Health Organization (WHO) [accessed on 21 April, 2020];Advice on the use of point-of-care immunodiagnostic tests for COVID-19. 2020 https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19.
- 10.Indian Council of Medical Research (ICMR) [accessed on 16 April, 2020];2020 https://icmr.nic.in/sites/default/files/upload_documents/Validation_of_Commercial_Kits_02042020.pdf.
- 11.World Health Organization (WHO) [accessed on 21 April, 2020];Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical guidance/laboratory-guidance.
- 12.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐ CoV‐2: a systematic review and meta‐analysis. J Med Virol. 2020 doi: 10.1002/jmv.25822. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao J, Tu WJ, Cheng W, Yu L, Tu WJ, Liu Q. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) [accessed on 16 April, 2020];"Solidarity" clinical trial for COVID-19 treatments. 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments.
- 16.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethesda, LiverTox: Clinical and Research Information on Drug-Induced liver injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012-. [2017 Feb 2]. [PubMed] [Google Scholar]
- 18.Plantone D, Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic and neurological diseases: A Mini-Review. Clin Drug Investig. 2018;38:653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 19.Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine. 2017;24:189–194. doi: 10.1016/j.ebiom.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiryaev SA, Mesci P, Pinto A, et al. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci Rep. 2017;7:15771. doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 24.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996;31:257–274. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effectd of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LS, Wang YR, Ye DW, Liu QQ. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105948. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke T, Kilbane E, Jackson J, Hoffman S. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 33.Van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 35.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Z, Lu Y, Cao Q, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. Am J Roentgenol. 2020 doi: 10.2214/AJR.20.22959. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 39.Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.02.001. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobati SS, Naik KR. Therapeutic plasma exchange - an emerging treatment modality in patients with neurologic and non-neurologic diseases. J Clin Diagn Res. 2017;11:EC35–EC37. doi: 10.7860/JCDR/2017/27073.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2004168117. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.