Abstract
Galectin-3 is a carbohydrate-binding protein and regulates diverse functions, including cell proliferation and differentiation, mRNA splicing, apoptosis induction, immune surveillance and inflammation, cell adhesion, angiogenesis, and cancer-cell metastasis. Galectin-3 is also recommended as a diagnostic or prognostic biomarker of various diseases, including heart disease, kidney disease, and cancer. Galectin-3 exists as a cytosol, is secreted in extracellular spaces on cells, and is also detected in nuclei. It has been found that galectin-3 has different functions in cellular localization: (i) Extracellular galectin-3 mediates cell attachment and detachment. (ii) cytosolic galectin-3 regulates cell survival by blocking the intrinsic apoptotic pathway, and (iii) nuclear galectin-3 supports the ability of the transcriptional factor for target gene expression. In this review, we focused on the role of galectin-3 on translocation from cytosol to nucleus, because it happens in a way independent of carbohydrate recognition and accelerates cancer progression. We also suggested here that intracellular galecin-3 could be a potent therapeutic target in cancer therapy.
Keywords: Cancer progression, Carbohydrate-binding, Cellular localization, Galectin-3
INTRODUCTION
Galectin-3, as a member of the galectin family, which are recognized β-galactoside-containing glycoconjugates by means of carbohydrate-recognition domain (CRD) (1, 2). Based on molecular structure, galectin family consists of the 15 members and these family are divided into three main groups: 1) prototype group (Galectin-1, -2, -5, -7, -10, -11,-13, -14, and -15), 2) tandem repeat group (galectin-4, -6, -8, -9, and -12), and 3) chimera type (galectin-3) (1,3-6). Whereas prototype galectins are mostly homodimers with two polypeptides each containing a CRD, the tandem repeat galectins have two CRDs, connected by a linker region (1, 4, 5). Chimeric galectin-3 consist of one CRD connected to an extended Proline-Glycine-Tyrosine tandem repeats region and an N-terminal proline and glycine rich domain (3, 5). Galectin family are present in a various tissues, whereas others have a more specific location (1). Galectins have many functions, such as cell proliferation and differentiation, immune response, apoptosis, cancer progression, and metastasis (4, 7). The mechanisms underlying these aspects are currently the focus of massive research projects.
Galectin-3 is the only chimera type in animal lectins. Also, galectin-3 is one of the most studied of the galectin family, (8-10). It is a versatile 29–35 kDa protein, as an involved in multiful biological processes followed in cellular location: cell adhesion, cell growth and differentiation, the cell cycle, and apoptosis (1, 11, 12). Galectin-3 is situated on chromosome 14, locus q21–q22 which is coded by a single gene LGALS3 (13). LGALS3 gene promoter region have a several regulatory elements, like a Sp1 binding sites, AP-1 complex, cAMP-dependent response element (CRE) motifs, and two NF-kB-like sites (10, 11). Galectin-3 mainly exists in the cytosol and is secreted out to the extracellular membrane (ECM) (14), but galectin-3 is also reported in the nucleus and mitochondria (9, 15).
In galectin family, it is known that there is no signal peptide to guide you through the classical secretion pathway. In particular, galectin-3 to go in extracellular space can interact with multiple binding partners or generality polylactosamine-rich molecules in the extracellular matrix (ECM) or on the surface of cells, and plays a major role in the extracellular regulation of various cancer progression (5, 16, 17). The non-classical secretion mechanism for galectin-3 remains unclear, but recently acquired data show that the secreted galactin-3 is regulated by exosomes (18) and that the N-terminal domain serves to position the galactin-3 in these structures (6, 10, 19).
Galectin-3 is also present in the nucleus and cytosol. Especially, depending on the various cell types and specific experimental conditions, galectin-3 has been reported to be predominantly located in the cytosol and nuclei or distributed between the two subcellular compartments (1, 20). Many articles have supported galectin-3 localization, transport, and association with the interaction of distinct subcellular components (1, 20).
Through the in this review, we were described brief overview of the intracellular galectin-3 functions in cancer progression that are independent of carbohydrate recognition and nucleus or cytoplasmic shuttling.
REGULATION OF GALECTIN-3 EXPRESSION IN CANCERS
Despite of expression of galectin-3 in various of tissues and cell types, and their involvement in various human diseases, this molecule is of particular interest due to its remarkable role in controlling cancer progression (21, 22). Galectin-3 is often high expressed in various solid and malignant tumors, and this case is generally correlated with the progression of cancer, suggesting that this molecule plays an important role in disease outcome (4, 5). In particular, the expression of galectin-3 in cells is characterized by the following malignant cell transformation (23), tumor growth (24), cell adhesion (25), anoikis resistance (26, 27), pro- or anti-apoptosis (28-30), angiogenesis (31-33), and cell motility (34-36) have been reported. Galectin-3 expression may also be a potential biomarker of various cancers (37). Interestingly, expression of galectin-3 was implicated in many cancers (16, 38). Especially, highly expression of galectin-3 was detected in stomach, liver, esophagus, thyroid, and pancreas cancers (23,39-44). This highly expressed galectin-3 is correlated with cancer progression or metastatic potential in various cancers (38, 45). However, contradictory results have also been reported, in which the expression of galectin-3 was significantly reduced in breast, prostate and endometrial cancers (46-49). In addition, expression of galectin-3 has also been reported to be up-regulated at an early stage of intrahepatic cholangiocarcinoma and down-regulated at later stage of intrahepatic cholangiocarcinoma (50). Also, galectin-3 translocation from the nucleus to the cytoplasm during prostate carcinoma was observed (51). This implies that decreased galectin-3 expression may be associated with alterations in cytoplasm / nucleus expression patterns and provides a reason why studies on translocation as well as the expression of galectin-3 in various carcinomas should be continued.
According to many reports, galectin-3 is not a common and obvious marker for various cancers, but it can be a useful parameter for diagnosis many tumors. Also, both transcriptional and translational galectin-3 expression was regulated by various stimulations and ligands. In addition, numerous factors have an effect on the complex regulatory mechanism of galectin-3 (1, 7). For example, the expression of galectin-3 in adenoma that prolactin and adrenocorticotropic hormone (ACTH) in the pituitary gland and other tumors is associated with the galectin-3’s promoter methylation status of the galectin-3 (52). Also, regulatory mechanism of galectin-3 expression is not directly induced by certain factors, but the cellular differentiation state or tissue type has been involved. Moreover, various transcription factors, as a RUNX (rent-related protein) family, nuclear factor kB (NF-kB), homeodomain-interacting protein kinase 2 (HIPK2), and many intracellular signal pathways, such as Wnt and Notch signaling, are regulated in the regulation of galectin-3 expression (11,53-56).
MECHANISM OF NUCLEUS AND CYTOPLASMIC GALECTIN-3 SHUTTLING
Nucleus and cytoplasmic shuttling is generally reported as the repeated bi-directional movement of proteins across nuclear pore complex (7, 20, 57). Both nucleus and cytoplasmic shuttling of galectin-3 reported by means of the many articles (57, 58). The galectin-3 movement between the nucleus and the cytoplasm has been the focus of attention for years (7, 17, 20). This is because the shuttle of galectin-3 from the nucleus to the cytoplasm is necessary because it protects certain cells from stress challenges (20, 57). Especially, the N-terminal area containing the phosphorylated Ser6 site, plays an important role in nuclear transport because the mutation in the Ser6 interferes with the export of galectin-3 by cytoplasm (1, 12, 59). CRD of galectin-3 is important for carbohydrate bonding, but this structural domain is also important for galectin-3 localization in cells (1, 7, 12). Recently, galectin-3 is seen as an important nuclear protein, which may be evidenced by the discovery of both nuclear import sequences (nuclear localization sequence; NLS) and nuclear export sequences (NES) sequences within the CRD (Fig. 1) (1, 4, 20). The combination with Importin α is very important for the movement of galectin-3 but the Importin α/β complex is necessary for the transport of galecin-3 (60, 61). Also, export of galectin-3 is known to rely on the binding to Nucleoporin 98 (Nup98), and the Nup98:XPO1 complex is involved in the nuclear migration of galectin-3 (62).
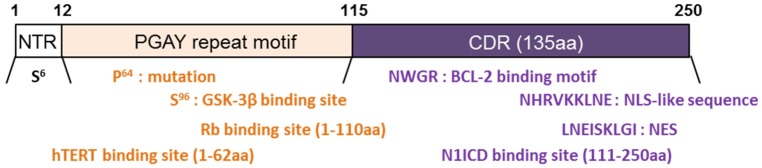
Fig. 1.
Structure of galectin-3. Galectin-3 consists of an N-terminal Domain (NTD), which has an N-terminal Region of 12 amino acids (aa) and a PGAY repeat motif (12-115aa). The carbohydrate-recognition domain (CRD) 130 aa comprises the C-terminal. Each domain describes a binding motif and signaling pathway.
Also, a mutation of galectin-3 at position 64 of amino-acid (rs4644) substituting proline for histidine (gal-3H64) increased nuclear galectin-3 in breast and gastric cancers (63, 64). Moreover, gal-3H64 enhances gastric cancer progression more than wild type galectin-3 (gal-3P64) does. gal-3H64 also increased both nuclear accumulation of β-catenin and expression of TCF-4 target genes, such as fascin-1 and c-Myc, by means of the augmented promoter-binding activity of TCF-4 more than did gal-3P64 (63). Thus, galectin-3 shuttling was regulated by the domain or mutation in various cancers and was involved in cancer progression.
Apoptosis regulated by galectin-3 interacts with carbohydrate recognition independent manner
Galectin-3 involved in diverse signal-transduction cascades and pro- or anti-survival processes. Actually, phosphorylated galectin-3 is required for anti-apoptotic activity and for phosphorylation regulated by carbohydrate recognition (9, 12, 17). However, those anti-apoptotic functions of galectin-3 were regulated by non-carbohydrate recognition. It was proved that galectin-3 interacts Bcl-2 (28). Although galectin-3 is not a member included in the Bcl-2 family, interestingly, these galectin-3 and Bcl-2 genes have significant sequence similarity (48% protein sequence similarity) (28). Especially, CRD region of galectin-3 have a four-amino-acid motif, as an Asn-Trp-Gly-Arg (NWGR) (amino-acid residues 180–183), which motif is highly conserved in Bcl-2 family’s BH-1 domain (amino-acid residues 143–146 in Bcl-2 gene) (12, 28). Moreover, galectin-3 is the only member that contains the NWGR motif in the galectin family and that acts as an anti-apoptotic molecule in intracellular localization. Therefore, the NWGR motif in CRD of galectin-3 is closely involved in anti-apoptotic process through interaction with Bcl-2. However, galectin-3 regulates apoptosis by means of the cytochrome c release and cell-cycle regulation.
Cancer progression and cell motility regulated by galectin-3 interacts with carbohydrate recognition independent manner
As mentioned in the introduction, galectin-3 has many roles in various cancers, such as splicing (65), cell proliferation (66), regulation of the cell cycle (26), angiogenesis (33), tumorigenesis (67), and cancer metastasis (68). It has also been demonstrated to be highly expressed in various primary and metastatic tumors (69) associated with increased cancer progression, cell motility, and metastasis (4, 70). Actually, galectin-3 binding with transcription factor (TF) in the nucleus by means of the following signaling pathway, such as Wnt, Ras, or MEK, as followed various cancer progression (1, 4). In this part, we showed that the role of galectin-3 in cancer progression and cell motility with regulation of gene transcription focuses on our galectin-3-related story.
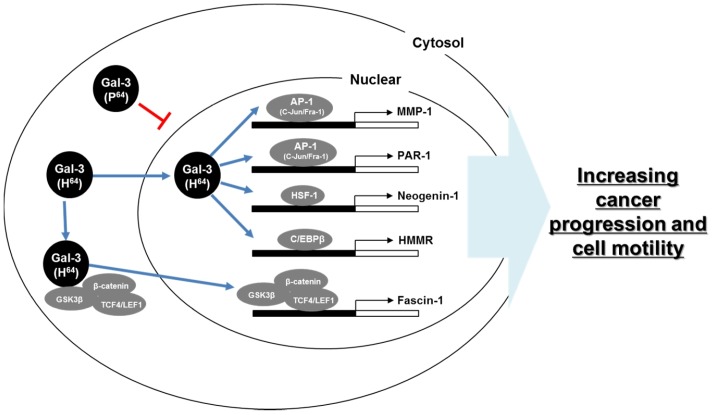
Galectin-3 and Fascin-1: For the cancer progression and cell motility with galectin-3, we focus on motility-related genes, among which fascin-1, an actin-bundling protein, is located along the entire length of filopodia in cells (71, 72). Because (a) a highly expressed fascin-1 was reported in various cancers including gastric, lung, and esophagus cancers (73-75). (b) Increased fascin-1 induces membrane protrusions and increases cancer cell motility (76). (c) Depletion of fascin-1 leads to a substantially reduced number of filopodia and overexpression of fascin-1, significantly increasing cell migration (74, 77, 78). Especially, fascin-1 expression is regulated by the Wnt-signaling pathway. As previously reported, galectin-3 was also reported to interact with GSK-3b and b-catenin which is regulate Wnt signaling (79). Therefore, galectin-3 regulates b-catenin nuclear accumulation via strongly interacting with GSK-3b and the binding of b-catenin/TCF-4 to promoter region of fascin-1 (Fig. 2) (35). Those studies propose that galectin-3 is involved in gastric-cancer metastasis and a critical therapeutic target for the cancer prevention.
Fig. 2.
Schematic model of mechanism of galectin-3 in cancer progression and metastasis. Galectin-3 bound various transcription factors, such as AP-1, HSF-1, C/EBPβ, and TCF4/LEF1, and regulates cancer progression and cell motility.
Galectin-3 and Protease-activated receptor-1 (PAR-1), Matrix Metalloprotease (MMP)-1: PAR-1, a cell-surface receptor, is a member of the family of transmembrane G-protein-coupled receptors (80). Activated PAR-1 is initiated by cleavage at its N terminus exodomain between Arg41 and Ser42, and auto-phosphorylates to trigger amplification of downstream signaling by proteases (80-83), such as thrombin or MMP-1 derived from stroma (84). PAR-1 and MMP-1 were related in cancer progression and cell motility. Interestingly, up-regulation of PAR-1 and MMP-1 via c-Jun and Fra-1 over-expression also followed, AP-1 complex, as a c-Jun and Fra-1, were direct interaction of galectin-3 (Fig. 2). It was previously reported that galectin-3 regulates MUC2 expression via interaction with AP-1, leading to its activation, and the site of formation of complexes was hypothesized to be AP-1 on the MUC2 promoter (85). Those results also support about galectin-3 regulates cancer progression and motility.
Galectin-3 and Neogenin-1: Neogenin-1 is a transmembrane receptor, as a member of immunoglobulin superfamily (86). Although neogenin-1 has significant sequence similarity (50% amino-acid identity) with the tumor suppressor molecule deleted in colon cancer (DCC) (86), but the expression is increased in gastric cancer patients (87). Also, neogenin-1 is enhanced in cancer proliferation and cell motility (87). Actually, expression of neogenin-1 is regulated by heat shock factor-1 (HSF-1). Moreover, galectin-3 promotes gastric-cancer cell motility by means of up-regulation of neogenin-1 expression by means of the induced phosphorylation of ROCK1(87). Additionally, galectin-3 induced accumulation of HSF-1 in nuclei by means of direct binding (Fig. 2). Those data suggest that galectin-3 is involved in cancer progression.
Galectin-3 and Hyaluronan-mediated motility receptor (HMMR): At last, HMMR binds with hyaluronan on the cell surface, where it activates a signal-transduction cascade causing intracellular protein tyrosine phosphorylation (88, 89). In addition, HMMR interacts with actin filaments, microtubules, and mitotic spindle assemblies, necessary for the organization of the cytoskeletal network (88,90-92). These HMMRs are regulated by interaction between the transcriptional factor CCAAT/enhancer-binding protein b (C/EBPb) and galectin-3 (36). Also, C/EBPb is regulated by galectin-3BP (galectin-3 binding protein) and promotes tumor progression in various cancers (Fig. 2) (93-95). These data mean galectin-3 binds with transcription factor in nuclei and regulates cancer progression and metastasis.
Senescence regulated by galectin-3 interacts with carbohydrate recognition independent manner
By means of the aberrant activation of oncogenes, such as Ras and Myc, or excessive mitogenic signals, can enhance senescence by means of two different pathways, p14ARF/p53/p21 or p16INK4A/pRB (96). However, galectin-3 knock-out (KO) mouse embryo fibroblasts (MEF) and silenced galectin-3 gastric-cancer cells showed that galectin-3 relies on p27KIP1, not p21wAF1/CIP1, to regulate premature senescence without oncogenic stress (97). Actually, the N-terminal of galectin-3 (amino-acid residues 1–110), bound with Rb, E2F1, as a transcription factor of SKP2, was released from Rb and initiated its transcriptional functions (Fig. 1) (97). Increased SKP2 regulates p27KIP1 degradation. Based on the research, galectin-3 prevents premature senescence by means of interaction and phosphorylation of Rb and consequent regulation of SKP2 and p27KIP1 expression. Moreover, galectin-3 bound to human Telomerase Reverse Transcriptase (hTERT) is an important factor of tumorigenesis and senescence (98). Especially, hTERT plays an important role in the regulation of telomerase activity in cell division, which is responsible for immortalized cell growth. The hTERT was binding with the N-terminal of galectin-3 (amino-acid residues 1–62) and increased telomeric activity (Fig. 1) (98). This evidence will show that galectin-3 regulates cellular senescence by means of the related genes interaction.
Cancer stemness regulated by galectin-3 interacts with carbohydrate recognition independent manner
Cancer Stem Cells (CSCs) are a malignant and aggressive cancer phenotype and have been increasingly studied over the last decade. These cells are derived from more differentiated cancer cells, potentially acquiring self-renewal properties and the ability to undergo epithelial-mesenchymal metastasis (EMT). Recently, galectin-3 expression increased in tumor sphere formation in cancer cells, together with stem-cell markers Oct4, Sox2, Nanog, CD133, and CXCR4 (99). Those mean galectin-3 is involved in the stemness of cancer cells. Especially, galectin-3 binds with EGF and bFGF (99) as a component of stem-cell culture medium and regulates KLF4 expression with miRNA-152 (100). These results support that galectin-3 is involved in cancer stemness. Also, galectin-3 supports stemness by signaling pathway regulation. Galectin-3 was essential for cluster formation using αvβ3 integrin and KRAS to activate the NF-kB pathway and stemness (101). Also, the Wnt, Notch, and SHH signaling pathways can help CSCs properties from normal stem cells. Among the signaling pathways, the Notch 1 intra-cellular domain (N1ICD) interacts with CRD of galectin-3 (amino-acid residues 111-250) (Fig. 1) in the cytoplasm of ovarian cancer cells (55). Moreover, galectin-3 is interaction with β-catenin, as a component Wnt signaling pathway, in the cytoplasm and help to nuclear accumulation in the gastric cancer cells (35, 63). The above results suggest that galectin-3 regulates cancer stemness by means of the various interaction molecules, not only carbohydrates.
PERSPECTIVES: GALECTIN-3 IS A POTENTIAL THERAPEUTIC TARGET FOR CANCER PROGRESSION
Among current research on the role of galectin-3, the prognosis value of cancer patients still needs to be discussed (5, 102). Nevertheless, the expression of galectin-3 was proved to be a useful parameter for the diagnosis and/or prognosis of various cancers. (21, 38, 103). Therefore, many researchers have attempted to develop new approaches for the diagnosis and treatment of cancer by galectin-3 targeting. First, transfected galectin-3 antisense cDNA decreases in the malignant phenotype of thyroid gland cancer cells (104). Numerous studies have focused on the galectin-3 targeting inhibitors, including peptide antagonists (105) and galactose-based inhibitors (106, 107). Also, in addition to these synthetic molecules, a natural product, pectin, has emerged as a good source for generating high-affinity galectin-3 inhibitors with low toxicity. Recently, a wide range of sub-molecular inhibitors have been considered (108). Among these, a new kind of galectin-3 inhibitor, which contains only one residue of sugar (109) that constitutes membrane permeability and oral available powerful galectin-3 inhibitor has been developed.
CONCLUSION
Highly expressed galectin-3 is detected in various cancers and tissues, and is involved in many biological processes, like a cell proliferation, adhesion, anti- or pro-apoptosis, cancer progression, and metastasis. Therefore, galectin-3 is enhanced in cancer progression and metastasis by means of different mechanisms. Also, the biological function of galectin-3 were attributed to each carbohydrate-binding activity. However, many articles showed that galectin-3 interacts with many molecules by non-carbohydrate binding. In this review, we focus on the regulation mechanism with nucleus and cytoplasmic shuttling and the role of nuclear galectin-3 in cancer progression. Especially, galectin-3 has an NLS and NES; however, galectin-3 mutation also helps the galectin-3 shuttling nucleus and cytosol. As follows, galectin-3 has many functions in each location. Additionally, galectin-3 is involved in cancer progression and metastasis by means of the binding with transcription factor, such as AP-1 complex (c-Jun/Fra-1), HSF-1, C/EBPβ, and TCF4/LEF1. Also, recently many articles supported about multi-function of galectin-3 in various cancers and other diseases. Those increased understanding evidences give to the galectin-3 expression or activity regulation mechanism for therapeutic purposes. Given these results, we propose that galectin-3 is a core protein in cancer progression and metastasis. In addition, the function of galectin-3 and the mechanisms by which it can be regulated should be understood in detail.
ACKNOWLEDGEMENTS
This work was supported by from the National Research Foundation (NRF) of Korea grants, funded by the Korean government (NRF-2017R1C1B2005265, NRF-2017R1A2B200 6238, NRF-2019R1A2C2089237), the Bio & Medical Technology Development Program, MSIP (NRF-2015M3A9B6073835), KBRI basic research program through Korea Brain Research Institute funded by Ministry of Science and ICT(20-BR-03-02), and the International Research & Development Program of the NRF, funded by the Ministry of Education, Science and Technology (MEST) of Korea (NRF-2016K1A3A1A47921595).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2002;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 3.Drickamer K, Fadden AJ. Genomic analysis of C-type lectins. Biochem Soc Symp. 2002;69:59–72. doi: 10.1042/bss0690059. [DOI] [PubMed] [Google Scholar]
- 4.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 5.Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavao MS. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 2014;4:138. doi: 10.3389/fonc.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirabayashi J, Kasai K. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 7.Johannes L, Jacob R, Leffler H. Galectins at a glance. J Cell Sci. 2018;131 doi: 10.1242/jcs.208884. [DOI] [PubMed] [Google Scholar]
- 8.Argueso P, Panjwani N. Focus on molecules: galectin-3. Exp Eye Res. 2011;92:2–3. doi: 10.1016/j.exer.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863:427–437. doi: 10.1016/j.bbamcr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7–20. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Guo XL. Molecular regulation of galectin-3 expression and therapeutic implication in cancer progression. Biomed Pharmacother. 2016;78:165–171. doi: 10.1016/j.biopha.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004;9:305–328. [PubMed] [Google Scholar]
- 13.Raimond J, Zimonjic DB, Mignon C, et al. Mapping of the galectin-3 gene (LGALS3) to human chromosome 14 at region 14q21-22. Mamm Genome. 1997;8:706–707. doi: 10.1007/s003359900548. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/S0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 15.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 16.van den Brule F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2002;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 17.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2002;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 18.Inohara H, Raz A. Identification of human melanoma cellular and secreted ligands for galectin-3. Biochem Biophys Res Commun. 1994;201:1366–1375. doi: 10.1006/bbrc.1994.1854. [DOI] [PubMed] [Google Scholar]
- 19.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 20.Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta. 2010;1800:181–189. doi: 10.1016/j.bbagen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahim AH, Alalawi Z, Mirandola L, et al. Galectins in cancer: carcinogenesis, diagnosis and therapy. Ann Transl Med. 2014;2:88. doi: 10.3978/j.issn.2305-5839.2014.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka Y, Inohara H, Yoshii T, et al. Malignant transformation of thyroid follicular cells by galectin-3. Cancer Lett. 2003;195:111–119. doi: 10.1016/S0304-3835(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 24.Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/S0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 27.Zhao Q, Barclay M, Hilkens J, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harazono Y, Kho DH, Balan V, et al. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget. 2014;5:9992–10001. doi: 10.18632/oncotarget.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 31.Nangia-Makker P, Wang Y, Raz T, et al. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LF, Liu YS, Yang B, et al. The extracellular matrix protein mindin attenuates colon cancer progression by blocking angiogenesis via Egr-1-mediated regulation. Oncogene. 2018;37:601–615. doi: 10.1038/onc.2017.359. [DOI] [PubMed] [Google Scholar]
- 33.Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SJ, Shin JY, Lee KD, et al. Galectin-3 facilitates cell motility in gastric cancer by up-regulating protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) PLoS One. 2011;6:e25103. doi: 10.1371/journal.pone.0025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SJ, Choi IJ, Cheong TC, et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–1045. e1031–e1032. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 36.Kang HG, Kim WJ, Kang HG, Chun KH, Kim SJ. Galectin-3 interacts with C/EBPbeta and upregulates hyaluronan-mediated motility receptor expression in gastric cancer. Mol Cancer Res. 2020;18:403–413. doi: 10.1158/1541-7786.MCR-19-0811. [DOI] [PubMed] [Google Scholar]
- 37.Dong R, Zhang M, Hu Q, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review) Int J Mol Med. 2018;41:599–614. doi: 10.3892/ijmm.2017.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) Int J Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- 39.Baldus SE, Zirbes TK, Weingarten M, et al. Increased galectin-3 expression in gastric cancer: correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 2000;21:258–266. doi: 10.1159/000030131. [DOI] [PubMed] [Google Scholar]
- 40.Volante M, Bozzalla-Cassione F, Orlandi F, Papotti M. Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch. 2004;444:309–312. doi: 10.1007/s00428-004-0993-5. [DOI] [PubMed] [Google Scholar]
- 41.Gudowska M, Gruszewska E, Cylwik B, et al. Galectin-3 Concentration in Liver Diseases. Ann Clin Lab Sci. 2015;45:669–673. [PubMed] [Google Scholar]
- 42.Inufusa H, Nakamura M, Adachi T, et al. Role of galectin-3 in adenocarcinoma liver metastasis. Int J Oncol. 2001;19:913–919. doi: 10.3892/ijo.19.5.913. [DOI] [PubMed] [Google Scholar]
- 43.Honjo Y, Inohara H, Akahani S, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6:4635–4640. [PubMed] [Google Scholar]
- 44.Shimamura T, Sakamoto M, Ino Y, et al. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–2575. [PubMed] [Google Scholar]
- 45.Sciacchitano S, Lavra L, Morgante A, et al. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int J Mol Sci. 2018;19:pii: E379. doi: 10.3390/ijms19020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castronovo V, Van Den Brule FA, Jackers P, et al. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996;179:43–48. doi: 10.1002/(SICI)1096-9896(199605)179:1<43::AID-PATH541>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 47.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- 48.Pacis RA, Pilat MJ, Pienta KJ, et al. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118–123. doi: 10.1002/1097-0045(20000701)44:2<118::AID-PROS4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 49.Brustmann H, Riss D, Naude S. Galectin-3 expression in normal, hyperplastic, and neoplastic endometrial tissues. Pathol Res Pract. 2003;199:151–158. doi: 10.1078/0344-0338-00368. [DOI] [PubMed] [Google Scholar]
- 50.Shimonishi T, Miyazaki K, Kono N, et al. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001;32:302–310. doi: 10.1053/hupa.2001.22767. [DOI] [PubMed] [Google Scholar]
- 51.van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361–367. doi: 10.1002/1097-0215(20000720)89:4<361::AID-IJC8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 52.Jin L, Riss D, Ruebel K, et al. Galectin-3 Expression in Functioning and Silent ACTH-Producing Adenomas. Endocr Pathol. 2005;16:107–114. doi: 10.1385/EP:16:2:107. [DOI] [PubMed] [Google Scholar]
- 53.Shimura T, Takenaka Y, Fukumori T, et al. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–3537. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- 54.Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang HG, Kim DH, Kim SJ, et al. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget. 2016;7:68229–68241. doi: 10.18632/oncotarget.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 57.Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12:329–337. doi: 10.1093/glycob/12.5.329. [DOI] [PubMed] [Google Scholar]
- 58.Arnoys EJ, Ackerman CM, Wang JL. Nucleocytoplasmic shuttling of galectin-3. Methods Mol Biol. 2015;1207:465–483. doi: 10.1007/978-1-4939-1396-1_30. [DOI] [PubMed] [Google Scholar]
- 59.Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Res. 2006;66:9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- 60.Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. J Biol Chem. 2006;281:39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 61.Davidson PJ, Li SY, Lohse AG, et al. Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import. Glycobiology. 2006;16:602–611. doi: 10.1093/glycob/cwj088. [DOI] [PubMed] [Google Scholar]
- 62.Li SY, Davidson PJ, Lin NY, Patterson RJ, Wang JL, Arnoys EJ. Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology. 2006;16:612–622. doi: 10.1093/glycob/cwj089. [DOI] [PubMed] [Google Scholar]
- 63.Kim SJ, Shin JY, Cheong TC, et al. Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of beta-catenin in gastric cancer. Clin Exp Metastasis. 2011;28:743–750. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 64.Balan V, Nangia-Makker P, Schwartz AG, et al. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008;68:10045–10050. doi: 10.1158/0008-5472.CAN-08-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995;92:1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res. 1998;245:294–302. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- 67.Bresalier RS, Mazurek N, Sternberg LR, et al. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology. 1998;115:287–296. doi: 10.1016/S0016-5085(98)70195-7. [DOI] [PubMed] [Google Scholar]
- 68.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19:543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 69.Raz A, Zhu DG, Hogan V, et al. Evidence for the role of 34-kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer. 1990;46:871–877. doi: 10.1002/ijc.2910460520. [DOI] [PubMed] [Google Scholar]
- 70.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 71.Yoder BJ, Tso E, Skacel M, et al. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res. 2005;11:186–192. [PubMed] [Google Scholar]
- 72.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 73.Hashimoto Y, Ito T, Inoue H, et al. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:2597–2605. doi: 10.1158/1078-0432.CCR-04-1378. [DOI] [PubMed] [Google Scholar]
- 74.Jawhari AU, Buda A, Jenkins M, et al. Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol. 2003;162:69–80. doi: 10.1016/S0002-9440(10)63799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grothey A, Hashizume R, Ji H, et al. C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864–4875. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67:262–270. doi: 10.1159/000081327. [DOI] [PubMed] [Google Scholar]
- 77.Yamashiro S, Yamakita Y, Ono S, Matsumura F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol Biol Cell. 1998;9:993–1006. doi: 10.1091/mbc.9.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B. Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene. 2003;22:3616–3623. doi: 10.1038/sj.onc.1206561. [DOI] [PubMed] [Google Scholar]
- 79.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 80.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 81.Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–3137. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- 82.Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–928. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- 83.Pei D. Matrix metalloproteinases target protease-activated receptors on the tumor cell surface. Cancer Cell. 2005;7:207–208. doi: 10.1016/j.ccr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 84.Blackburn JS, Liu I, Coon CI, Brinckerhoff CE. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene. 2009;28:4237–4248. doi: 10.1038/onc.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song S, Byrd JC, Mazurek N, Liu K, Koo JS, Bresalier RS. Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology. 2005;129:1581–1591. doi: 10.1053/j.gastro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Wilson NH, Key B. Neogenin: one receptor, many functions. Int J Biochem Cell Biol. 2007;39:874–878. doi: 10.1016/j.biocel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 87.Kim SJ, Wang YG, Lee HW, et al. Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget. 2014;5:3386–3398. doi: 10.18632/oncotarget.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J, Shima H, Nishizawa H, et al. Phosphorylation of BACH1 switches its function from transcription factor to mitotic chromosome regulator and promotes its interaction with HMMR. Biochem J. 2018;475:981–1002. doi: 10.1042/BCJ20170520. [DOI] [PubMed] [Google Scholar]
- 89.Yeh MH, Tzeng YJ, Fu TY, et al. Extracellular Matrix-receptor Interaction Signaling Genes Associated with Inferior Breast Cancer Survival. Anticancer Res. 2018;38:4593–4605. doi: 10.21873/anticanres.12764. [DOI] [PubMed] [Google Scholar]
- 90.Casini P, Nardi I, Ori M. RHAMM mRNA expression in proliferating and migrating cells of the developing central nervous system. Gene Expr Patterns. 2010;10:93–97. doi: 10.1016/j.gep.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Bahrami SB, Tolg C, Peart T, et al. Receptor for hyaluronan mediated motility (RHAMM/HMMR) is a novel target for promoting subcutaneous adipogenesis. Integr Biol (Camb) 2017;9:223–237. doi: 10.1039/C7IB00002B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Connell M, Chen H, Jiang J, et al. HMMR acts in the PLK1-dependent spindle positioning pathway and supports neural development. Elife. 2017;6:pii: e28672. doi: 10.7554/eLife.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silverman AM, Nakata R, Shimada H, Sposto R, DeClerck YA. A galectin-3-dependent pathway upregulates interleukin-6 in the microenvironment of human neuroblastoma. Cancer Res. 2012;72:2228–2238. doi: 10.1158/0008-5472.CAN-11-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Regalo G, Forster S, Resende C, et al. C/EBPbeta regulates homeostatic and oncogenic gastric cell proliferation. J Mol Med (Berl) 2016;94:1385–1395. doi: 10.1007/s00109-016-1447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaught JB. Biorepository and biospecimen science: a new focus for CEBP. Cancer Epidemiol Biomarkers Prev. 2006;15:1572–1573. doi: 10.1158/1055-9965.EPI-06-0632. [DOI] [PubMed] [Google Scholar]
- 96.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SJ, Lee HW, Gu Kang H, et al. Ablation of galectin-3 induces p27(KIP1)-dependent premature senescence without oncogenic stress. Cell Death Differ. 2014;21:1769–1779. doi: 10.1038/cdd.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.La SH, Kim SJ, Kang HG, Lee HW, Chun KH. Ablation of human telomerase reverse transcriptase (hTERT) induces cellular senescence in gastric cancer through a galectin-3 dependent mechanism. Oncotarget. 2016;7:57117–57130. doi: 10.18632/oncotarget.10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nangia-Makker P, Hogan V, Raz A. Galectin-3 and cancer stemness. Glycobiology. 2018;28:172–181. doi: 10.1093/glycob/cwy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma J, Yao Y, Wang P, et al. MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Kruppel-like factor 4. Cancer Lett. 2014;355:85–95. doi: 10.1016/j.canlet.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Seguin L, Kato S, Franovic A, et al. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–1941. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El Gendy H, Madkour B, Abdelaty S, et al. Galectin 3 for the diagnosis of bladder cancer. Arab J Urol. 2014;12:178–181. doi: 10.1016/j.aju.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshii T, Inohara H, Takenaka Y, et al. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol. 2001;18:787–792. doi: 10.3892/ijo.18.4.787. [DOI] [PubMed] [Google Scholar]
- 105.Nangia-Makker P, Balan V, Raz A. Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 2008;1:43–51. doi: 10.1007/s12307-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen WS, Cao Z, Leffler H, Nilsson UJ, Panjwani N. Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis. Invest. Ophthalmol Vis Sci. 2017;58:9–20. doi: 10.1167/iovs.16-20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glinskii OV, Sud S, Mossine VV, et al. Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-L-leucine. Neoplasia. 2012;14:65–73. doi: 10.1593/neo.111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campo VL, Marchiori MF, Rodrigues LC, Dias-Baruffi M. Synthetic glycoconjugates inhibitors of tumor-related galectin-3: an update. Glycoconj J. 2016;33:853–876. doi: 10.1007/s10719-016-9721-z. [DOI] [PubMed] [Google Scholar]
- 109.Zetterberg FR, Peterson K, Johnsson RE, et al. Monosaccharide Derivatives with Low-Nanomolar Lectin Affinity and High Selectivity Based on Combined Fluorine-Amide, Phenyl-Arginine, Sulfur-pi, and Halogen Bond Interactions. Chem Med Chem. 2018;13:133–137. doi: 10.1002/cmdc.201700744. [DOI] [PubMed] [Google Scholar]