Abstract
Activation of peroxisome proliferator-activated receptor γ (PPARγ) serves as a key factor in the proliferation and invasion of breast cancer cells and is a potential therapeutic target for breast cancer. However, the mechanisms underlying this effect remain largely unknown. Heme oxygenase-1 (HO-1) is induced and over-expressed in various cancers and is associated with features of tumor aggressiveness. Recent studies have shown that HO-1 is a major downstream target of PPARγ. In this study, we investigated the effects of induction of HO-1 by PPARγ on TPA-induced MMP-9 expression and cell invasion using MCF-7 breast cancer cells. TPA treatment increased NF-μB /AP-1 DNA binding as well as MMP-9 expression. These effects were significantly blocked by 15d-PGJ2, a natural PPARγ ligand. 15d-PGJ2 induced HO-1 expression in a dose-dependent manner. Interestingly, HO-1 siRNA significantly attenuated the inhibition of TPA-induced MMP-9 protein expression and cell invasion by 15d-PGJ2. These results suggest that 15d-PGJ2 inhibits TPA-induced MMP-9 expression and invasion of MCF-7 cells by means of a heme oxygenase-1-dependent mechanism. Therefore, PPARγ/HO-1 signaling-pathway inhibition may be beneficial for prevention and treatment of breast cancer.
Keywords: Heme oxygenase-1, MCF-7, MMP-9, PPARγ, 15d-PGJ2
INTRODUCTION
Breast cancer is the major cause of cancer death in women worldwide. The high prevalence of breast cancer and the limited options for treatment provide an obvious rationale for discovering new molecular targets that can be pharmacologically modulated. Recent evidence suggests that matrix metalloproteinases (MMPs) may play a role in breast cancer initiation and growth (1-3). Key genes involved in breast cancer metastasis, such as MMP, have been the focus of research into targets for cancer breast treatment.
Phorbol esters bind to protein kinase C (PKC) in a way similar to that of its natural ligand, diacylglycerol, and activate the kinase (4, 5). The phorbol ester is 12-O-tetradecanoylphorbol- 13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical tool for research. Recently it has been found that TPA activates integrin signaling pathway (6, 7), which may be activated by some carcinogens.
PPARγ is one of nuclear receptor subfamily that includes receptors for thyroid, steroid, and retinoid hormones. PPARγ from heterodimers with retinoid receptors and these dimers regulate various genes (8). Many recent papers have reported that modulations of PPARγ control the growth of human cancers, such as breast cancer (9-11). One of the earliest events in the metastasis of cancer cells is expression of the g isoform of PPAR. Thus, PPARγ control may have significant promise for breast cancer prevention. Recently, PPARγ ligands were shown to inhibit the growth of a variety of transformed cells (9, 12, 13); hence signals that modulate PPARγ activity may serve a primary role in regulating breast cancer metastasis and may be major targets for treatment of breast cancer.
Endogeneous 15-Deoxy-D12,14-prostaglandin J2 (15d-PGJ2) has been identified as a ligand of PPARγ. 15d-PGJ2 inhibited the invasiveness of breast cancer cells by upregulating a tissue inhibitor of MMP-1 (14). A recent study has shown that heme oxygenase-1 (HO-1) overexpression in MCF-7 cells inhibits MMP expression, indicating that HO-1 plays a pivotal role in the invasion of breast cancer cells (15). These results suggest that PPARγ ligands control invasion and MMP expression of human breast cancer cells by means of HO-1. In the present study, we examined the role of HO-1 in the action of 15d-PGJ2 on the invasion and MMP expression of breast cancer cells.
RESULTS
Effect of 15d-PGJ2 on MMP-9 expression in MCF-7 cells
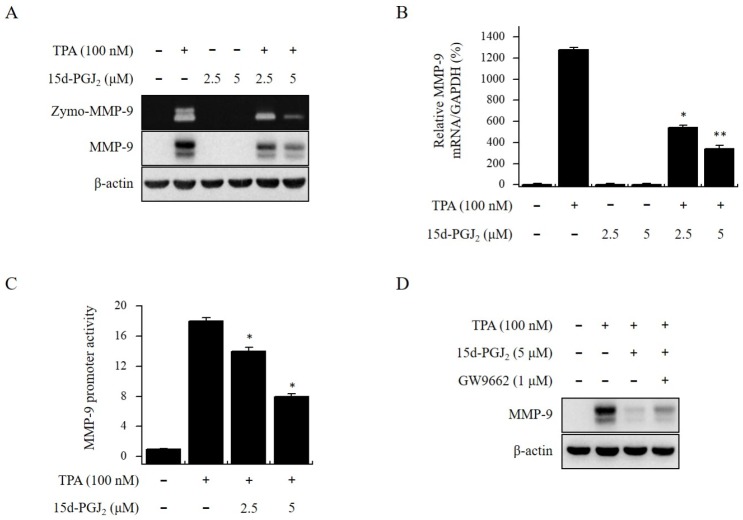
We treated MCF-7 cells with 15d-PGJ2 (0-5 μM) for 24 h, and toxicity was detected using an MTT assay. Treatment with 15d-PGJ2 did not change MCF-7 cell viability (data not shown). Therefore, we used non-toxic concentrations of 2.5 and 5 μM in the experiments. The range of non-toxic concentrations was applied in all subsequent experiments. Gelatin zymography showed that 15d-PGJ2 suppressed TPA-induced MMP-9 secretion in a dose-dependent manner. Western blotting and real-time PCR revealed that 15d-PGJ2 suppressed TPA-induced MMP-9 expression at both mRNA and protein levels (Fig. 1A and B). The luciferase assay showed that 15d-PGJ2, a known PPARγ agonist, suppressed TPA-induced MMP-9 promoter activity in MCF-7 cells (Fig. 1C). We next examined whether the inhibitory effect of 15d-PGJ2 on MMP-9 expression depended on PPARγ. In MCF-7 cells treated with 15d-PGJ2, inhibition of TPA- induced MMP-9 expression was recovered by the PPARγ antagonist GW9662 (Fig. 1D). These results indicate that the inhibition of TPA-induced MMP-9 expression by 15d-PGJ2 does depend on PPARγ.
Fig. 1.
15d-PGJ2 inhibits TPA-induced MMP-9 expression in MCF-7 cells. We pretreated MCF-7 cells with 15d-PGJ2 and then added TPA for 24 h. (A) We analyzed MMP-9 secretion by gelatin zymography (Zymo). MMP-9 protein expression was analyzed by Western blot. (B) We analyzed MMP-9 mRNA levels by real-time PCR using GAPDH mRNA as an internal control. (C) Wild type MMP-9-luc reporters were co-transfected with TK (Renilla) reporter into the MCF-7 cells. We then treated cells with 15d-PGJ2 in the presence of TPA, and measured the MMP-9 promoter activity with a dual- luciferase reporter assay. (D) PPARγ antagonist GW9662 was added to cells for 1 h before the 15d-PGJ2 treatment and were analyzed by Western blot. Each value represents the mean ± SEM of three independent experiments. Data are presented as means ± SE of three independent experiments. **P < 0.01 vs. TPA. *P < 0.05 vs. TPA.
Effect of 15d-PGJ2 on TPA-induced activation of NF-kB and AP-1 in MCF-7 cells
To investigate the signaling and transcription factors by which 15d-PGJ2 inhibited MMP-9 expression, we used gelatin zymography (zymo) and Western blotting to evaluate the effects of 15d-PGJ2 on the TPA-induced activation of NF-κB and AP-1. TPA-induced MMP-9 expression was inhibited by NF-κB (Bay 11-7082) and AP-1 (SR 11302) inhibitors in a dose-dependent manner (Fig. 2A and B). Pretreatment with 15d-PGJ2 inhibited the transfer of p50, p65, and p-c-Fos to the nucleus by TPA, and 15d-PGJ2 also inhibited phosphorylation of IKKα/β and IκBα (Fig. 2C). In addition, an EMSA assay showed that treatment with 15d-PGJ2 suppressed TPA-induced NF-κB and AP-1 DNA binding activity (Fig. 2D and E). These results indicate that 15d-PGJ2 inhibits TPA‐induced MMP-9 expression by regulating NF-κB/AP-1 activation in MCF-7 cells.
Fig. 2.
15d-PGJ2 inhibits TPA-induced MMP-9 activation by means of NF-κB/AP-1 signaling pathway in MCF-7 cells. (A, B) We treated MCF-7 cells with inhibitors of NF-κB (Bay 11-7082), AP-1 (SR 11302) for 1 h, and then with TPA for 24 h. The MMP-9 protein expression was analyzed by Western blot. We analyzed MMP- 9 secretion by gelatin zymography (Zymo). (C) We treated cells with 15d-PGJ2 in the presence of TPA for 3 h and then prepared nuclear extracts. PCNA were used as loading controls for nuclear proteins. (D, E) We treated cells with 15d-PGJ2 in the presence of TPA for 3 h and then prepared nuclear extracts. NF-κB and AP-1 DNA binding was analyzed by EMSA. Data are presented as means ± SE of three independent experiments.
Role of HO-1 on MMP-9 expression and cell invasion inhibition by 15d-PGJ2 in MCF-7 cells
We investigated whether HO-1 plays a role in the inhibition of TPA-induced MMP-9 expression by 15d-PGJ2. Western blotting and real-time PCR revealed that 15d-PGJ2 increased HO-1 expression at both protein and mRNA levels in a dose-dependent manner (Fig. 3A and B). To verify the involvement of HO-1 in inhibition of MMP-9 expression, we used HO-1 siRNA to knock down HO-1 expression. 15d-PGJ2 inhibitory effects on TPA-induced MMP-9 expression were significantly decreased in cells transfected with HO-1 siRNA (Fig. 3C). We used a matrigel invasion assay to confirm the inhibition of MCF-7 cell invasion by 15d-PGJ2. Treatment with 15d-PGJ2 (5 μM) effectively inhibited the invasion of MCF-7 cells by approximately 65% compared to untreated control cells. To verify the involvement of HO-1 in inhibition of cell invasion, we used siRNA to knock down HO-1 gene expression. Treatment of cells transfected with HO-1 siRNA with 15d-PGJ2 showed 50% inhibition, indicating that 15d-PGJ2 inhibits the invasion of MCF-7 cells by means of HO-1 activation (Fig. 3D). These results suggest that 15d-PGJ2 inhibits TPA-induced MMP-9 expression and cell invasion by means of the HO-1 pathway in MCF-7 cells.
Fig. 3.
15d-PGJ2 inhibits TPA-induced MMP-9 activation and cell invasion by means of the Nrf2/HO-1 signaling pathway in MCF-7 cells. (A, B) We treated MCF-7 cells with 15d-PGJ2 for 24 h. HO-1 protein expression was analyzed by Western blot. HO-1 mRNA levels were analyzed by RT-PCR using GAPDH mRNA as an internal control. (C) Cells were transfected with HO-1 siRNA, and then treated with 15d-PGJ2 and TPA for 24 h. HO-1 siRNA attenuated the inhibitory effects of 15d-PGJ2. MMP-9 secretion was analyzed by gelatin zymography (Zymo). We analyzed MMP-9 and HO-1 protein expression by Western blot. (D) Cells were transfected with HO-1 siRNA for 24 h. We carried out matrigel invasion assays on cells treated with TPA and 15d-PGJ2 (5 μM) for 24 h. The data presented as the mean number of migrated cells. Data are presented as means ± SE of three independent experiments. **P < 0.01 vs. control. *P < 0.05 vs. control.
Effect of HO-1 in 15d-PGJ2-induced PPARγ expression
15d-PGJ2 is known as a PPARγ agonist. We investigated whether HO-1 is involved in PPARγ expression induced by 15d-PGJ2. When cells were treated with 15d-PGJ2 at 0.5, 1, 2, 3, and 4 h, 15d-PGJ2 induced time-dependent PPARγ nuclear translocation (Fig. 4A). In addition, 15d-PGJ2 induced Nrf2 nuclear translocation, a major transcription factor for HO-1 expression (Fig. 4B). The pretreatment with ZnPP, an HO-1 inhibitor, inhibited the increased expression of PPARγ by 15d-PGJ2 (Fig. 4C). To confirm this more clearly, when the cells were transfected with Nrf2 siRNA and HO-1 siRNA for 24 h, Western blotting showed that the increased PPARγ expression by 15d-PGJ2 was inhibited by Nrf2 and HO-1 knockdown (Fig. 4D and E). When cells were treated with 15d-PGJ2 for 3, 6, 12, and 24 h, HO-1 siRNA also inhibited 15d-PGJ2-induced PPARγ mRNA levels (Fig. 4F). Inhibition of Nrf2 and HO-1 also decreased PPARγ mRNA levels (Fig. 4G). These results suggest that PPARγ expression depends on the activation of Nrf2 and HO-1.
Fig. 4.
HO-1 regulation affected PPARγ expression in MCF-7 cells. (A, B) We treated cells with 15d-PGJ2 for 0.5, 1, 2, 3, 4 h, followed by cytosol, and then prepared nuclear extracts. Protein expression was analyzed by Western blot. (C) We treated cells with 15d-PGJ2 in the presence of ZnPP for 4 h and then prepared nuclear extracts. (D, E) Cells were transfected with Nrf2 siRNA and HO-1 siRNA for 24 h, then treated with 15d-PGJ2 for 4 h and then d nuclear extracts and whole cell lysates were prepared. PCNA was used as loading controls for nuclear and β-actin were used as internal control for the whole cell. (F) Cells were transfected with HO-1 siRNA for 24 h, then treated for 3, 6, 12, and 24 h with 15d-PGJ2. (G) Cells were transfected with Nrf2 siRNA and HO-1 siRNA for 24 h. We analyzed PPARγ mRNA levels by real-time PCR using GAPDH mRNA as an internal control. Data are presented as means ± SE of three independent experiments. **P < 0.01 vs. control. *P < 0.05 vs. control.
DISCUSSION
Activation of peroxisome proliferator activated receptor g (PPARγ) during carcinogenesis is known to increase as part of normal physiological metabolic factors that affect growth and survival of cancer cells. Furthermore, the potential effect of PPARγ expression on patient survival has been the focus of breast cancer treatment strategies, suggesting the importance of PPARγ regulation in breast cancer treatment. In this study, we evaluated the relationship between physiologic activation of PPARγ and MMP expression and invasion of breast cancer cells. Thus, we focused on the role of HO-1 in PPARγ activation in the carcinogenesis of breast cancer. Our results showed that 15-Deoxy-D12,14-prostaglandin J2 (15d-PGJ2), an endogenous PPARγ ligand, blocks TPA-induced MMP-9 expression and cell invasion by means of an HO-1-mediated PPARγ signaling mechanism in MCF-7 cells. This suggests that HO-1 may be a novel member of the PPARγ signaling cascade to target for prevention and treatment of breast cancer.
The promoter region of the matrix metalloproteinase-9 (MMP- 9) gene has binding sites for nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), and TPA is known to induce MMP-9 by means of NF-kB and AP-1 signaling (16, 17). We confirmed that TPA-induced MMP-9 was inhibited by treatment with NF-kB inhibitor BAY11-7082 and AP-1 inhibitor SR11302, and that 15d-PGJ2 inhibits TPA-induced NF-kB signal proteins and p-c-fos (AP-1 subunit). 15d-PGJ2 also inhibited the TPA-induced NF-kB and AP-1 promoter activities. These results indicate that 15d-PGJ2 inhibits MMP-9 by means of TPA-induced NF-kB/AP-1 signal inhibition.
Heme oxygenase (HO) is a rate-limiting enzyme in the degradation of heme. Three HO isoforms have been identified. HO-2 and HO-3 are constitutively expressed, whereas HO-1 is a potent antioxidant enzyme induced by a variety of factors, such as heme and oxidants (18). HO-1 is associated with pathogenesis in human diseases (19). In breast cancer cells, HO-1 inhibit proliferation and induces apoptosis in human cancer cells (20, 21). Although HO-1 has been studied in various cancer cells, the roles of HO-1 in breast cancer cells are still undefined. Here we found that 15d-PGJ2 inhibited TPA-induced MMP-9 expression in a PPARγ-dependent mechanism. We confirmed that 15d-PGJ2 increased HO-1 expression at both protein and mRNA levels in a dose-dependent manner, and that 15d-PGJ2 inhibitory effects on TPA-induced MMP-9 expression were significantly decreased in cells transfected with HO-1 siRNA. 15d-PGJ2 also has inhibitory effects on TPA- induced MMP-9 expression that were significantly decreased in cells transfected with HO-1 siRNA. These results indicate that HO-1 plays a role in the inhibition of TPA-induced MMP-9 expression by the 15d-PGJ2-PPARγ signaling cascade.
Activation of PPARγ is reported to induce HO-1 expression in cells (22). Conversely, reports that HO-1 influences PPARγ activity are not well known (23, 24). In particular, the effect of HO-1 on PPARγ in breast cancer is still unclear. Our study showed that HO-1 directly induces PPARγ expression in breast cancer cells. We confirmed that 15d-PGJ2 increased Nrf2 nuclear translocation, a major transcription factor for HO-1 expression, in a time-dependent manner. The pretreatment with ZnPP, a HO-1 inhibitor, inhibited the increased expression of PPARγ by 15d-PGJ2. In addition, when the cells were transfected with Nrf2 siRNA and HO-1 siRNA, the increased PPARγ expression by 15d-PGJ2 was inhibited by Nrf2 and HO-1 knockdown. Inhibition of Nrf2 and HO-1 also decreased PPARγ mRNA levels. These results indicate that PPARγ expression depends on the activation of Nrf2 and HO-1 in MCF-7 cells. Collectively, these findings suggest that HO-1 is a major regulator of PPARγ expression in the pathogenesis of breast cancer.
In conclusion, this study demonstrates that MMP-9 expression and invasion of ER-positive breast cancer cells may be regulated by means of an HO-1-induced PPARγ signaling mechanism. Our data showed that HO-1 may be a novel target in the PPARγ signaling cascade for prevention and treatment of breast cancer. However, additional experiments are necessary to confirm this relationship between HO-1 and PPARγ at the transcription factor level and to further delineate its molecular basis.
MATERIALS AND METHODS
Cells and materials
We purchased:
MCF-7 cells from the American Type Culture Collection (ATCC, Manassas. VA, USA); these were cultured at 37°C in a 5% CO2 incubator in DMEM (Gibco, Gaithersburg, MD, USA) with 1% antibiotics and 10% FBS (Gibco).
15d-PGJ2 from Cayman Chemical (Ann Arbor, MI, USA).
Anti-β-actin antibody, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and 12-O-tetradecanoylphorbol- 13-acetate (TPA) from Sigma-Aldrich (St Louis, MO, USA).
Primary antibodies to p-c-Fos, p-IκBα, and p-IKKα/β from Cell Signaling Technology (Danvers, MA, USA).
MMP-9, p65, p50, PPARγ, Nrf2, PCNA antibodies, NF-κB inhibitor BAY 11-7082, and AP-1 inhibitor SR 11302 from Santa Cruz Biotechnology (Dallas, TX, USA).
Heme oxygenase-1 (HO-1) antibody and PPARγ inhibitor GW9662 from Enzo Life Sciences (New York, NY, USA).
NF-κB and AP-1 consensus oligonucleotides from Promega (Fitchburg, WI, USA).
Cell viability
We confirmed the effect of 15d-PGJ2 on cell viability of MCF-7 using an MTT assay. Cells were seeded in each well of 96-well plates at a density of 3 × 104 cells/well. After 24 h, cells were stimulated with 2.5, 5, 10, or 20 μM 15d-PGJ2 for 24 h at 37°C. The cells were then washed with PBS, and MTT (0.5 mg/ml of PBS) was added; we then incubated the treated cells at 37°C for 30 min. We dissolved Formazan crystals with DMSO. OD was detected at 570 nm using a microplate reader (Bio- Rad, Richmond, CA, USA).
Preparation of nuclear extract
We treated MCF-7 cells with 15d-PGJ2 in the presence or absence of TPA (100 nM) for 3 h. Cells were washed twice, scraped into 1.5 ml of PBS, and pelleted at 4,000 × g for 4 min. We prepared cytoplasmic and nuclear extracts using NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA).
Western blot analysis
We pretreated MCF-7 cells with 15d-PGJ2 for 1 h and then incubated them with TPA for 24 h at 37°C. Cells were lysed using M-PER Mammalian Protein Extraction Reagent (Thermo Fisher). We separated samples (10 μg) by SDS-PAGE gels, and the protein loaded-gel were transferred to Hybond-polyvinylidene fluoride membranes (GE Healthcare Life Sciences, Pittsburgh, PA, USA) for 1 h. Each membrane was blocked for 2 h with 5% skim milk or 5% bovine serum albumin and was then incubated with a primary antibody overnight at 4°C. We detected protein levels with an image analyzer (Fuji-Film, Tokyo, Japan). We did densitometry using Image J software (NIH, Bethesda, MD, USA).
Gelatin zymography assay
We pretreated cells with 15d-PGJ2, BAY 11-7082, and SR 11302 for 1 h and then incubated them with TPA for 24 h at 37°C. After that, we collected conditioned media and separated them in a polyacrylamide gel containing 0.1% (w/v) gelatin. The gel was washed with 2.5% Triton X-100, incubated at 37°C for 15 h in developing buffer, subsequently stained with 0.25% (w/v) Coomassie blue buffer and photographed on an image analyzer (Fuji-Film).
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using RNAiso Plus (Takara, Shiga, Japan). We detected the RNA purity and concentration by absorbance at 260/280 nm. We synthesized cDNA using a PrimeScript RT reagent Kit (Takara). GAPDH (NM002046), PPARγ (NM 001330615), HO-1 (NM 002133), and MMP-9 (NM 004994) mRNA expression was detected by real-time PCR using a SYBR Green and ABI PRISM 7900 sequence detection system (Applied Biosystems, Foster City, CA, USA).
Luciferase assay
MCF-7 cells were seeded on 24-well plates until the cells reached 60-70% confluence and were then transfected with MMP-9 reporter plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). We pretreated the transfected cells with 15d-PGJ2 for 1 h and then incubated them with TPA for 24 h. We did Luciferase reporter assays using the Dual-Luciferase Reporter Assay System (Promega) and detected them using a luminometer (EG & G Berthold, Gaithersburg, MD, USA).
Electrophoretic mobility shift assay (EMSA)
An oligonucleotide containing the AP-1 (5’CGCTTGATGAGTCAGCCGGAA-3’) or NF-κB (5’CCGGTTAACAGAGGGGGCTTTCCGAG-3’) binding sites were synthesized and used as a probe for the gel retardation assay. The two complementary strands were annealed and labeled with [α-32P]dCTP (Amersham, UK). Binding buffer, 10 μg of nuclear extracts, and labeled oligonucleotides (10,000 cpm) were then incubated for 30 min at room temperature. The reaction mixtures underwent electrophoresis on 4% polyacrylamide gels in 0.5X Tris-borate buffer, and then the gels were dried and examined by autoradiography.
siRNA transfection
We transfected MCF-7 cells with human Nrf2 siRNA (sc- 37030, Santa Cruz), human HO-1 siRNA (sc-35554, Santa Cruz) using Opti-MEM medium (Gibco) and Lipofectamine 2000. Negative control siRNA was purchased from Genotech (Daejeon, Korea). We seeded MCF-7 cells on 6-well plates at 40-50% confluence 24 h before transfection. Opti-MEM, Lipofectamine 2000, and siRNA were mixed and incubated at room temperature for 20 min. Lipofectamine 2000-siRNA complexes were added and transfected for 6 h. We used siRNA at a concentration of 10 nM, and knockdown of Nrf2, HO-1 was confirmed by RT-PCR and Western blot.
Invasion assay
We did the invasion assay in 24-well chambers (8-μm pore size) coated with Matrigel (Corning Life sciences, Tewksbury, MA, USA). We added cells to the upper chamber; the lower compartment of the invasion chamber contained conditioned medium. After the cells were incubated for 24 h, the migrated cells were fixed with 3.8% formaldehyde solution for 10 min and stained with a crystal violet solution for 30 min. Cells on the upper side of the chamber were removed using a cotton swab, and we counted migrated cells in five random areas of the membrane. Analyzed data are the mean ± SE from three independent experiments performed in triplicate.
Statistical analysis
We did statistical analysis using ANOVA and Duncan’s test. Differences with P < 0.05 were considered statistically significant.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGEMENTS
This paper was supported by the Fund of Biomedical Research Institute, Chonbuk National University Hospital and National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2016R1D1A1B03930499).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzy Inhi Med Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 2.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 3.Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono Y, Fujii T, Igarashi K, et al. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Z, Song J, Wang Z, et al. Tumor promoter TPA activates Wnt/beta-catenin signaling in a casein kinase 1-dependent manner. Proc Natl Acad Sci U S A. 2018;115:E7522–E7531. doi: 10.1073/pnas.1802422115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Park SY, Park OJ, Kim YM. Curcumin suppresses migration and proliferation of Hep3B hepatocarcinoma cells through inhibition of the Wnt signaling pathway. Mol Med Rep. 2013;8:282–286. doi: 10.3892/mmr.2013.1497. [DOI] [PubMed] [Google Scholar]
- 8.Diradourian C, Girard J, Pegorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87:33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Sun Y, Wong J, Conklin DS. PPARγamma maintains ERBB2-positive breast cancer stem cells. Oncogene. 2013;32:5512–5521. doi: 10.1038/onc.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins GT, Nie D. PPAR gamma, bioactive lipids, and cancer progression. Front Biosci (Landmark Ed) 2012;17:1816–1834. doi: 10.2741/4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourtidis A, Srinivasaiah R, Carkner RD, Brosnan MJ, Conklin DS. Peroxisome proliferator-activated receptor-gamma protects ERBB2-positive breast cancer cells from palmitate toxicity. Breast Cancer Res. 2009;11:R16. doi: 10.1186/bcr2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava N, Kollipara RK, Singh DK, et al. Inhibition of cancer cell proliferation by PPARγamma is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014;20:650–661. doi: 10.1016/j.cmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubaki M, Takeda T, Tomonari Y, et al. Pioglitazone inhibits cancer cell growth through STAT3 inhibition and enhanced AIF expression via a PPARγamma-independent pathway. J Cell Physiol. 2018;233:3638–3647. doi: 10.1002/jcp.26225. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Zang C, Fenner MH, Possinger K, Elstner E. PPARγamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat. 2003;79:63–74. doi: 10.1023/A:1023366117157. [DOI] [PubMed] [Google Scholar]
- 15.Lin CW, Shen SC, Hou WC, Yang LY, Chen YC. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol Cancer Ther. 2008;7:1195–1206. doi: 10.1158/1535-7163.MCT-07-2199. [DOI] [PubMed] [Google Scholar]
- 16.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Jeong YJ, Park KK, et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-kappaB and AP-1-dependent MMP-9 expression. Mol Cells. 2010;29:209–215. doi: 10.1007/s10059-010-0028-9. [DOI] [PubMed] [Google Scholar]
- 18.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. doi: 10.1096/fasebj.2.10.3290025. [DOI] [PubMed] [Google Scholar]
- 19.Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Am J Cardiovasc Dis. 2011;1:150–158. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HN, Jin HO, Park JA, et al. Heme oxygenase-1 determines the differential response of breast cancer and normal cells to piperlongumine. Mol Cells. 2015;38:327–335. doi: 10.14348/molcells.2015.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WY, Chen YC, Shih CM, et al. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol Appl Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Kronke G, Kadl A, Ikonomu E, et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Bae JU, Hong KW, Kim CD. HO-1 Induced by cilostazol protects against TNF-alpha-associated cytotoxicity via a PPAR-gamma-dependent pathway in human endothelial cells. Korean J Physiol Pharmacol. 2011;15:83–88. doi: 10.4196/kjpp.2011.15.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanella L, Kim DH, Asprinio D, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.