Abstract
Purpose
Targeted oncolytic vaccinia virus is an attractive candidate for cancer therapy due to its replication causing lysis of infected tumor cells as well as a delivery vector to overexpress therapeutic transgenes. This study constructed a novel oncolytic vaccinia virus carrying granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-24 (IL-24) double genes to improve efficacy for cancer therapy.
Methods
Vaccinia virus co-expressing GM-CSF and IL-24 based on Chinese Guang9 strain (VG9-GMCSF-IL24) was constructed with disruption of the viral thymidine kinase (TK) gene. The cytotoxicity of VG9-GMCSF-IL24 in various cell lines was assessed by MTT. The synergistic antitumor effect of VG9-GMCSF-IL24 in vivo was assessed on multiple tumor models.
Results
In vitro cytotoxicity assay showed that VG9-GMCSF-IL24 exerted a strongly cytotoxic effect on cancer cells, but with no significant cytotoxicity to normal cells. Significant tumor growth inhibition and prolonged survival were observed in different tumor models treated with VG9-GMCSF-IL24. Additionally, systemic and specific antitumoral immunity was investigated in vivo, and enhanced antitumor immunity was observed in VG9-GMCSF-IL24-treated mice.
Conclusion
Our results indicated that VG9-mediated GM-CSF and IL-24 co-expression performed cooperative and overlapping antitumor effect. As a novel and effective therapeutic strategy for cancer, the combination of oncolysis and immunotherapy with vaccinia virus carrying one or more immunostimulatory genes may have a satisfactory clinical application prospect.
Keywords: oncolytic vaccinia virus, cancer therapy, GM-CSF, IL-24, immunotherfapy
Introduction
Due to a genetically unstable and complex biological system, which has the ability to adapt to and multiply in severe and changing environments, cancer still remains a life-threatening disease in the majority of cases despite therapies having improved significantly. Traditional radiotherapy and chemotherapy have less efficiency owing to resistance after several treatments and side effects for various kinds of limitations. Thus, new cancer therapeutic strategies with novel mechanisms of action that induce high toxicity to tumors and minimal harm to normal tissues are needed, and one such strategy is using oncolytic vaccinia virus.
Oncolytic vaccinia virus has emerged as a promising platform for cancer therapy due to the inherent capacity to infect and replicate within tumor cells, resulting in virus progeny production, tumor cells lysis, and spread to adjacent and distant tumor cells.1 Moreover, oncolytic vaccinia virus can especially kill cancer cells through additional mechanisms including arming therapeutic genes and inducing tumor-specific cytotoxic T lymphocytes (CTL). Therefore, oncolytic vaccinia virus is potent to treat cancers that have become refractory to currently approved treatments.
Another appealing strategy for cancer therapy has been to enhance host antitumor immunity. According to this, oncolytic viruses modified to carry genes coding immunostimulatory molecules can boost the antitumor effect by the combination of oncolysis and immunotherapy. A variety of oncolytic vaccinia viruses armed with immunogenic transgenes, such as granulocyte-macrophage colony-stimulating factor (GM-CSF),2–5 tumor necrosis factor (TNF),6 interferons (IFNs)7 and several interleukins (ILs)8–10 are currently being used in cancer therapy both at preclinical and clinical levels. GM-CSF is most potent in inducing specific and long-lasting antitumor immunity, being able to recruit dendritic cells and natural killer cells and induce tumor-specific cytotoxic T lymphocytes (CTLs).11 JX-594, the vaccinia virus strain Wyeth encoding GM-CSF, has shown promising results and proceeded rapidly in clinical trials.3–5 In our previous study, we also demonstrated that Chinese vaccinia virus strain engineered with GM-CSF induced strong tumoricidal activity and a potent antitumor response.12
Another promising immunostimulatory transgene is interleukin-24 (IL-24), which is a novel member of the IL-10 family that selectively induces apoptosis in a broad spectrum of tumors without harming normal cells or tissues.13,14 Furthermore, IL-24 also possesses “bystander” antitumor activity, along with inhibiting angiogenesis and stimulating an antitumor immune response.15,16 Based on its profound antitumor properties, the IL-24 armed adenovirus (INGN 241) has been evaluated in Phase I clinical trials, in which intratumoral injections of INGN 241 in solid tumors showed evidence of clinical activity with limited toxicity.17,18 However, INGN 241 is nonreplicating and cannot target tumor cells, which limit its further application on cancer gene therapy.
The lysis capacity, wide tumor tropism, large transgene-encoding capacity of vaccinia virus and efficient foreign gene expression using its own enzyme systems make it an ideal gene expression vehicle, by which the sustained expression of target gene can be achieved in tumor tissues with viral replication. To enhance the therapeutic efficacy, the concept of a combined dual gene-virotherapy strategy was devised, which uses the vaccinia virus carrying two transgenes.
Vaccinia strain Guang9 (VG9) is an attenuated vaccinia virus, which was derived from Chinese vaccinia strain Tian Tan (VTT) by consecutive plaque-cloning selection.19 Compared with WR strain, which has been widely used in laboratories and extensively tested in clinical trials, the virulence of VTT strain is about 5000-fold lower,20 whereas the virulence of VG9 in various animal models was found to be lower than its parental virus (VTT).21 Therefore, a recombinant vaccinia virus co-expressing GM-CSF and IL-24 based on the VG9 strain (VG9-GMCSF-IL24) was constructed in this study and its antitumor effects were evaluated in various cancer cell lines and multiple tumor models.
Materials and Methods
Cell Lines
The murine melanoma cell line B16, murine colorectal carcinoma cell line CT26, murine mammary carcinoma cell line 4T1, human colorectal carcinoma cell line HCT116, human mammary carcinoma cell line MDA-MB-231, human lung carcinoma cell line A594, human normal liver cell line L-O2, and human normal breast epithelial cell line MCF-10A were purchased from the Cell Bank of Shanghai Institute for Biological Sciences of the Chinese Academy of Sciences (Shanghai, China). African green monkey kidney epithelial cell lines BSC-40 and Vero were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were cultured under the conditions suggested by the ATCC.
Construction of VG9-GMCSF-IL24
The recombinant vaccinia virus expressing the GM-CSF and IL-24 genes was constructed via homologous recombination between the shuttle plasmid (pCB, gifted from Professor Liu) and vaccinia virus strain VG9 (obtained from National Institutes for Food and Drug Control). Briefly, GM-CSF-IL24 co-expression cassettes linked by a foot-and-mouth-disease virus (FMDV)-derived 2A self-processing peptide (F2A) were inserted into viral thymidine kinase (TK) gene region and was under the control of the vaccinia synthetic early/late promoter (Figure 1A). The generation of recombinant vaccinia viruses based on xanthine-guanine phosphoribosyltransferase (XGPRT) selection was previously described in detail.12,22 VG9-EGFP, VG9-GMCSF and VG9-IL-24 were constructed and conserved in our laboratory. All recombinant vaccinia viruses were purified in sucrose gradient and virus stocks were titrated on BSC-40 cells by plaque assay.
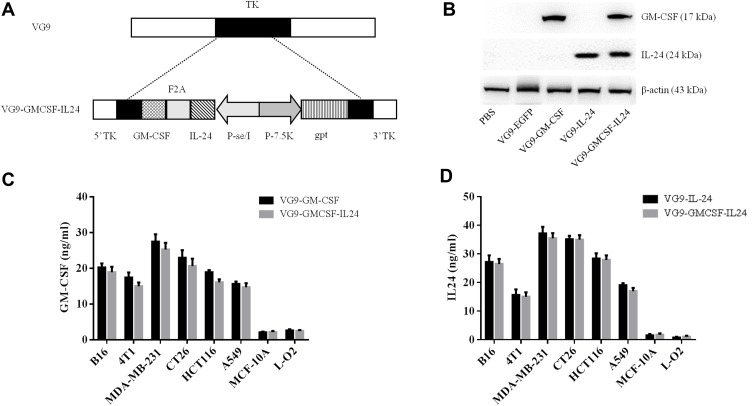
Figure 1.
Characterization of VG9-GMCSF-IL24. (A) Schematic illustration of VG9-GMCSF-IL24 construction. GM-CSF and IL-24 co-expression cassettes linked by F2A were inserted into TK locus of VG9 strain via homologous recombination. (B) Western blotting analysis of GM-CSF and IL-24 proteins from Vero cells treated with PBS, or VG9-EFGP, VG9-GM-CSF, VG9-IL-24, VG9-GMCSF-IL24 at 0.1 MOI for 48 h. (C) Supernatants and lysates from various cell lines treated with VG9-GM-CSF or VG9-GMCSF-IL24 were collected and GM-CSF concentrations were quantified by ELISA. (D) Supernatants and lysates from various cell lines treated with VG9-IL-24 or VG9-GMCSF-IL24 were collected and IL-24 concentrations were quantified by ELISA. Each bar represents the mean ± SD (n=3).
In vitro GM-CSF and IL-24 Production
Vero cells grown in 12-well plates were infected with 0.1 multiplicity of infection (MOI) of VG9, VG9-GM-CSF, VG9-IL-24, VG9-GMCSF-IL24 or PBS for 48 hrs. GM-CSF or IL-24 protein was analyzed by Western blotting. Various cancer cell lines including B16, 4T1, MDA-MB-231, CT26, HCT116, A549 and normal cell lines of MCF-10A and L-O2 grown in 12-well plates were infected with 0.1 MOI of VG9-GMCSF, VG9-IL-24, or VG9-GMCSF-IL24. After infection for 48 hrs, supernatants and lysates were collected and GM-CSF and IL-24 levels were determined by enzyme-linked immunosorbent assay (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s manual.
Cell Viability Assay
The cytotoxic activity of recombinant vaccinia viruses was assessed by 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT, Sigma, USA). Cells (104/well) seeded in 96-well plates were infected with different concentrations of virus (VG9-GMCSF, VG9-IL-24, or VG9-GMCSF-IL24). After infection for 48 hrs, 10 μL of MTT solution (5 mg/mL) was added to each well and then the plates were incubated for 4 hrs. Then, the reaction was stopped by dimethyl sulfoxide (DMSO) and the optical absorbance was measured at a wavelength of 490 nm by SpetraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Animal Studies
The animal experiment was approved by the Institutional Animal Care and Use Committees (IACUC) of Jiangsu Institute of Nuclear Medicine (JSINM2010007). Female C57BL/6 immuno-competent mice (6-week-old) and BALB/c immune-competent mice (6-weeks old) were purchased from Shanghai Laboratory Animals Center (SLAC; Shanghai, China). Mice were housed under standardized conditions with controlled temperature and humidity and a 12-12-h day-night light cycle and were given free access to diet and water.
To generate the murine melanoma B16 model, about 5×105 B16 cells in 100 μL phosphate-buffered saline (PBS) were injected subcutaneously into the right flanks of C57BL/6 mice. The murine CT26 colorectal carcinoma model was established by subcutaneous administration of 5×105 CT26 cells into the right flanks of BALB/c immune-competent mice. For 4T1 mammary carcinoma model, 1×106 4T1 cells were injected subcutaneously into the right flanks of BALB/c immune-competent mice. When tumors reached the size of 3–5 mm in diameter, mice were randomly divided into five groups (n=6 per group). 107 PFU of VG9-EGFP, VG9-GMCSF, VG9-IL-24, or VG9-GMCSF-IL24 were intratumorally injected and PBS treatment worked as control. Tumor growth was followed every other day and the tumor volume curves were limited to the day when the first mice in the corresponding group were sacrificed. The tumor volume was calculated as [(width)2 × length]×0.52.23 Mice were euthanized by CO2 inhalation when tumors reached their maximal permitted size according to the animal regulations, and Kaplan–Meier survival curves were plotted.
CT26 tumors treated with VG9-GMCSF-IL24, VG9-EGFP or PBS were rechallenged by subcutaneous injection of CT26 cells (5×105) or 4T1 cells (1×106) into the opposite flank. Naïve mice that were never treated with tumor cells or virus were also subcutaneously injected with the same amount of tumor cells in the same flank. Tumor growth and survival were followed over time.
Determination of Cytokines
After the injection of virus or PBS for 7 days, serum was obtained from the corresponding group. Cytokine levels of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-4, and IL-6 were measured using an ELISA kit (eBioscience, San Diego, CA, USA).
Statistical Analysis
All data are presented as mean ± standard deviation (SD). The significance of the difference was evaluated by one-way ANOVA analysis for multiple groups. Survival analysis was performed using the method of Kaplan–Meier, and differences between curves were assessed using the Log rank test. Statistical analysis was performed by SPSS 19.0 software (SPSS Statistics, Inc., Chicago, IL, USA).
Results
Characterization of VG9-GMCSF-IL24
Exogenous GM-CSF or IL-24 proteins from Vero cells infected with VG9-EGFP, VG9-GM-CSF, VG9-IL-24, VG9-GMCSF-IL24 orPBS were harvested and analyzed using Western blotting assay (Figure 1B). Results showed that VG9-GMCSF-IL24 could produce both GM-CSF and IL-24 proteins. To further confirm the exogenous expression of GM-CSF or IL-24, proteins in supernatants and lysates from various cancer cell lines and normal cell lines infected with VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24 were harvested and quantified using ELISA. As excepted, all cancer cell lines treated with VG9-GM-CSF or VG9-GMCSF-IL24 produced GM-CSF protein (Figure 1C) and the concentrations of IL-24 protein from all cancer cell lines treated with VG9-IL-24 or VG9-GMCSF-IL24 increased (Figure 1D). The level of GM-CSF in VG9-GM-CSF was not remarkably different from that in VG9-GMCSF-IL24, and the same as IL-24 protein. However, GM-CSF and IL-24 production remained low in normal cells with any treatment.
In vitro Cytotoxicity
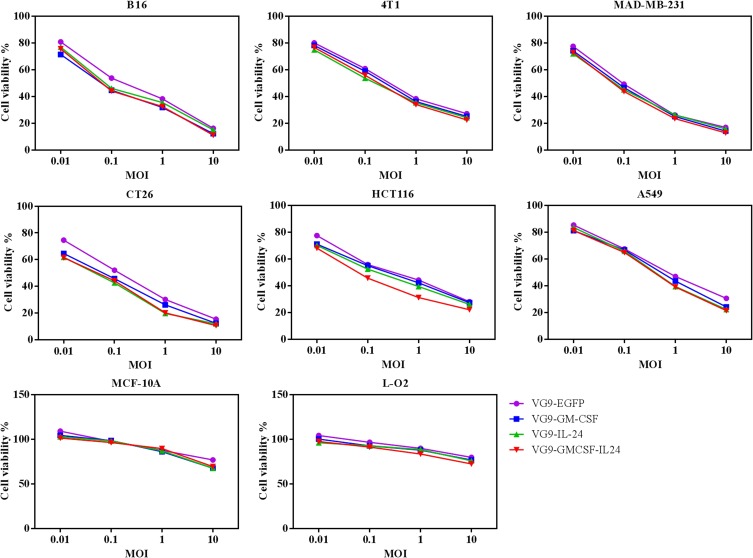
We evaluate the oncolytic efficacy on various cell lines by MTT assay. For all recombinant vaccinia viruses, the increasing of the MOI up to 10 PFU/cell led to notable cytotoxicity in cancer cell lines but not in normal cell lines, indicating that TK-deleted vaccinia viruses are tumor-selective oncolytic viruses which can selectively replicate in cancer cells (Figure 2).
Figure 2.
Cytotoxicity effect in vitro. Various cancer cell lines and normal cell lines were infected with VG9-EFGP, VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24 at different MOIs. After infection for 48 hrs, cell viability was measured by MTT assay.
Antitumor Effect in Multiple Tumor Models
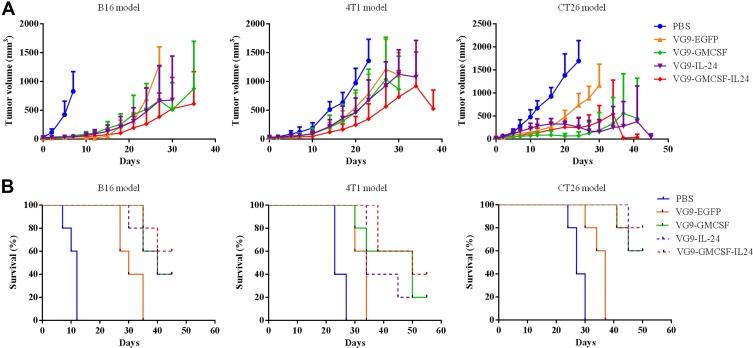
To assess the therapeutic effects of treatment with VG9-GMCSF-IL24 in vivo, three different tumor models were established (Figure 3). In the B16 murine melanoma tumor model, there was a rapid decrease in tumor size of mice that received intratumoral injections of VG9-GM-CSF, VG9-IL-24 or VG9-GMCSF-IL24. All mice treated with PBS died within 12 days, while virus-treated mice lived longer with survival extended up to 45 days. The survival rates of mice treated with VG9-GM-CSF, VG9-IL-24, and VG9-GMCSF-IL24 were 40%, 40%, 60%, respectively, at the end of experiments. The result was similar in the 4T1 mammary carcinoma model. At 3 weeks from the initial treatment, tumors in the PBS-treated group had significantly increased in size, while those in virus-treated groups had stabilized with the survival up to 55 days. VG9-GMCSF-IL24 exhibited more remarkable antitumor efficacy compared to VG9-GM-CSF or VG9-IL-24. In the CT26 murine colorectal carcinoma model, a single intratumoral injection of virus resulted in evident suppression of tumor growth compared to the PBS-treated group. The survival advantage was observed in virus-treated mice with the survival rates of 60%, 60%, and 80% for mice treated with VG9-GM-CSF, VG9-IL-24, and VG9-GMCSF-IL24, respectively.
Figure 3.
Antitumor effect of VG9-GMCSF-IL24 in different tumor models. (A) Mean tumor volumes in mice treated with PBS, VG9-EFGP, VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24. (B) Kaplan–Meier survival curves for tumor-bearing mice treated with PBS, VG9-EFGP, VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24. n=6 per group.
Tumor Cell Rechallenge
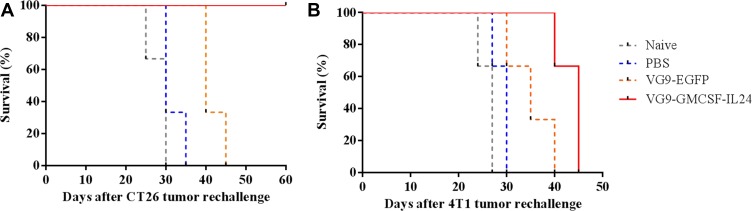
Tumor cell rechallenge study was performed in the CT26 model to observe whether the treatment of primary tumors with VG9-GMCSF-IL24 had developed systemic and long-lasting antitumor immunity. CT26 tumors previously treated with VG9-GMCSF-IL24, VG9-EGFP or PBS were resected, and the contralateral sides were subcutaneously injected with 5×105 CT26 cells. Naïve mice (not tumor-bearing or virus-treated) were also injected with tumor cells. It was observed that naïve mice, PBS-treated, and VG9-EGFP-treated mice all developed tumors, and were eventually euthanized when tumors reached their maximal permitted size. All mice treated with VG9-GMCSF-IL24 were resistant to CT26 cells and survived over the tumor cell rechallenge study (Figure 4A).
Figure 4.
Kaplan–Meier survival curve of tumor cell rechallenge. CT26 tumors treated with PBS, VG9-EGFP or VG9-GMCSF-IL24 previously were resected and the contralateral sides were subcutaneously injected with CT26 (A) or 4T1 (B) cells. Naïve mice were never treated with tumor cells or virus previously.
To determine whether the protection against tumor rechallenge was tumor-specific, syngeneic 4T1 cells were injected into the contralateral side of CT26 tumor-bearing mice. As shown in Figure 4B, all mice inoculated with 4T1 cells developed flank tumors and all died within 45 days. Therefore, not only the treatment with VG9-GMCSF-IL24 was able to develop systemic antitumoral immunity, but also the protection against tumor rechallenge was tumor-specific.
In vivo GM-CSF and IL-24 Production
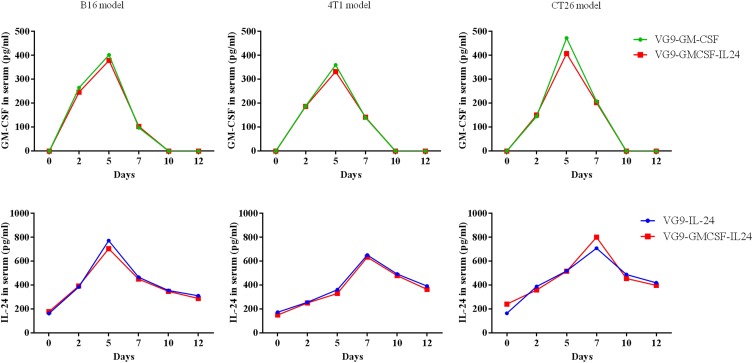
To further confirm the presence of GM-CSF or IL-24 in serum after VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24 treatment, GM-CSF or IL-24 concentrations in serum harvested at different time points were evaluated by ELISA. As shown in Figure 5, the level of GM-CSF or IL-24 in serum was able to be detected on day 2 after the initial intratumoral injections. The level of VG9-GM-CSF reached its peak on day 5 and began to decrease by day 7, while the level of IL-24 was continuously elevated, reaching maximum concentration on day 7 and gradually decreased on day 10 but was still able to be detected on day 12.
Figure 5.
GM-CSF and IL-24 serum concentrations over time following intratumoral injection of VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24 in B16, 4T1, CT26 tumor-bearing mice.
Induction of Antitumor Immunity
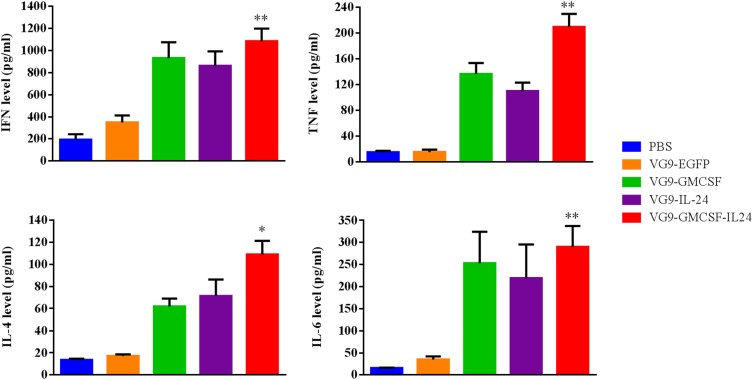
To investigate whether VG9-GMCSF-IL24 produced cytokines that triggered the antitumor immune response, cytokine detection was performed using an ELISA kit. As shown in Figure 6, treatment with VG9-GMCSF-IL24 resulted in higher secretion of IFN-γ, TNF-α, IL-4 and IL-6 compared with VG9-GM-CSF or VG9-IL-24 and was significantly higher than that in PBS or VG9-EGFP group (P<0.01, P<0.05).
Figure 6.
Cytokine levels in serum from CT26 tumor-bearing mice treated with PBS, VG9-EGFP, VG9-GM-CSF, VG9-IL-24, or VG9-GMCSF-IL24 were determined using ELISA kit. Each bar represents the mean ± SD (n=6). *P<0.05; **P <0.01.
Discussion
A wide variety of preclinical or clinical studies have shown the successful application of oncolytic vaccinia virus as a potential strategy for cancer therapy. Unlike traditional cancer therapy, oncolytic vaccinia virus can selectively infect, replicate within and lyse tumor cells and spread to neighboring tumor cells in successive rounds of replication.24,25 Additionally, it also generates antitumor immunity that eradicates metastasized tumor cells with minimum harm to the normal tissue.26 Furthermore, oncolytic vaccinia virus can also be used as a gene transfer vehicle due to its large size and expression using its own enzyme systems. The copies of carried genes are increased with the replication of vaccinia virus, resulting in higher gene expression levels in tumor tissues.
The significant antitumor effect can be obtained by a combination of different therapeutic genes via targeting multiple pathways. GM-CSF, a monomeric glycoprotein, induces myeloid precursor cells to proliferate and differentiate into granulocytes (neutrophils, eosinophils, and basophils) and monocytes, as well as recruits and stimulates dendritic cells.27 Besides, it also has some effects on mature cells of the immune system, for example, inhibiting neutrophil migration and causing an alteration of the receptors expressed on the cell surfaces. Therefore, it is the most potent of a number of cytokines, adhesion molecules and other immunostimulatory molecules for the induction of specific, potent and long-lasting antitumor immunity.28 IL-24 is a broad-spectrum tumor suppressor as well as an important immune mediator,29 which exerts potent antitumor effects by inducing apoptosis, inhibition of tumor cell invasion and metastasis, antiangiogenic activity, immune modulatory activity, and “bystander” antitumor activity.14–16,30 Many previous studies have demonstrated GM-CSF and IL-24 as effective agents for cancer therapy.2–5,17,18 However, the therapeutic effect of the combination treatment of GM-CSF and IL-24 remains unreported. Based on the properties of GM-CSF and IL-24, we speculated that the combination of the double immunostimulatory molecules would exhibit excellent antitumor effects.
Sustained expression of GM-CSF and IL-24 can be obtained by using oncolytic vaccinia virus. Our study showed that the recombinant vaccinia virus co-expressing GM-CSF and IL-24 (VG9-GMCSF-IL24) efficiently infected cancer cells, assuring the high expression of GM-CSF and IL-24 in cancer cells. Results of Western blotting and ELISA demonstrated that VG9-GMCSF-IL24 sufficiently expressed GM-CSF and IL-24 in tumor cells, while the production remained low in normal cells. Additionally, GM-CSF and IL-24 were able to be detected in serum, suggesting that they were secreted into the blood from the tumor. All these data indicated that VG9-GMCSF-IL24 was able to introduce GM-CSF and IL-24 genes directly into tumors, so that cytokines were produced in situ, further enhancing the antitumor effects.
Evidence from in vivo study demonstrated that VG9-mediated GM-CSF and IL-24 co-expression performed cooperative and overlapping antitumor effects. Although single gene expressing oncolytic vaccinia virus VG9-GM-CSF or VG9-IL-24 exhibited potent antitumor effect, the enhanced antitumor effect was observed in the double genes expressing oncolytic vaccinia virus VG9-GMCSF-IL24 might be due to the synergistic effect of GM-CSF and IL-24. Our results showed that the treatment with VG9-GM-CSF or VG9-IL-24 alone inhibited tumor growth and prolonged survival of tumor-bearing mice. However, the strongest antitumor activity and further prolonged survival were observed in VG9-GMCSF-IL24-treated mice. The tumor masses were completely eradicated in ≥60% mice treated with VG9-GMCSF-IL24. The most significant antitumor efficacy of VG9-GMCSF-IL24 was found in the CT26 murine colorectal carcinoma model, with 80% of mice completely healed.
The mechanism involved in VG9-GMCSF-IL24-mediated synergistic antitumor effect was complicated. The antitumor efficacy of VG9-GMCSF-IL24 was outstanding in vivo, while it was similar to VG9-GM-CSF or VG9-IL-24 in vitro, indicating that the immune responses mediated by GM-CSF and IL-24 were superimposed in vivo. A previous study has been demonstrated that IL-24 induces an increase in the secretion of various cytokines including GM-CSF.31 In accordance, GM-CSF can stimulate the expression of IL-24 mRNA and protein.32 Similar to GM-CSF, IL-24 contains AU-rich elements in its 3ʹ untranslated region targeting mRNA for rapid degradation.33 Both GM-CSF and IL-24 can stimulate TH1 type of immune response and play as immunomodulatory and pro-inflammatory,34,35 thus activating antigen-presenting cells to present tumor antigens, and thus triggering an antitumor immune response. Further studies are required to clarify the detailed mechanisms of the synergistic effect of GM-CSF and IL-24.
The combination of oncolysis and immunotherapy is developing as a novel and promising strategy for cancer therapy. Our previous study has demonstrated that even non-gene expressing oncolytic wild type VG9 can induce some immunity against tumor.36 Further promoting such antitumor immune response during oncolytic virotherapy is appealing, due to oncolytic vaccinia virus inhibiting tumor growth directly and immediately during the time required to generate an immune system. In this study, we found that VG9-GM-CSF, VG9-IL-24 and VG9-GMCSF-IL24 all showed excellent antitumor activity and complete regression of tumors were observed. Tumor rechallenge study showed that CT26 tumor-bearing mice healed by VG9-GMCSF-IL24 did not develop tumor with CT26 cells rechallenge, but developed tumors when inoculated with 4T1 cells, implying the ability of VG9-GMCSF-IL24 to induce a specific and lasting immune response. Furthermore, VG9-GMCSF-IL24 treatment upregulated some cytokines which recruited and activated components of the immune system, especially increased IFN-γ production significantly. As a pro-inflammatory cytokine, IFN-γ is important for immunity against intracellular pathogens, including vaccinia virus. VG9-GMCSF-IL24 treatment produced elevated levels of IFN-γ, followed by recruitment of cells of the innate immune system to the inflamed tumor site, thus creating a pro-inflammatory environment, which attribute to the inhibition of growth and regression of the tumor. VG9-EGFP-treated mice delayed the tumor growth and prolonged the survival in rechallenge study further demonstrated that vaccinia virus with nontherapeutic gene-expression could induce immunity against tumor to some extent.
Enhanced antitumor immunity can be achieved by the introduction of immunostimulatory genes. Previous studies combined delivery of oncolytic viruses along with separate vectors for cytokine gene transfer.37–39 The use of a single vector for oncolysis as well as immune stimulation greatly improves and simplifies the strategy by allowing in vivo transduction of tumor cells with genes coding for immunostimulatory molecules. Our study demonstrates that oncolytic vaccinia virus VG9 carrying immunostimulatory genes as multimodality therapy is feasible and efficacious.
Conclusion
The recombinant vaccinia virus VG9-GMCSF-IL24 exhibits notable antitumor efficacy both in vitro and in vivo. GM-CSF and IL-24 co-expression synergistically induced an enhanced effect on tumor growth suppression, survival prolonging and lasting immune response. Our data indicate that the combination of oncolysis and immunotherapy with vaccinia virus carrying one or more immunostimulatory genes may provide a novel and effective therapeutic strategy for cancer.
Acknowledgments
We are grateful to Professor Xinyuan Liu for providing the vaccinia shuttle plasmid (pCB), and the National Institutes for Food and Drug Control (NIFDC) for providing the vaccinia virus of Tian Tan strain VG9.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81703061), Innovation Capacity Development Plan of Jiangsu Province (BM2018023) and Jiangsu Provincial Key Medical Discipline (ZDXKA2016017).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics and Consent Statement
The animal experiment was approved by the Institutional Animal Care and Use Committees (IACUC) of Jiangsu Institute of Nuclear Medicine (JSINM2010007). All procedures were performed in accordance with the Laboratory Animal-Guideline of Welfare Ethical Review of Chinese IACUC.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–1379. doi: 10.1111/cas.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Roh MS, Lee YK, et al. Oncolytic and immunostimulatory efficacy of a targeted oncolytic poxvirus expressing human GM-CSF following intravenous administration in a rabbit tumor model. Cancer Gene Ther. 2010;17(2):73–79. doi: 10.1038/cgt.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14(3):361–370. doi: 10.1016/j.ymthe.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 4.Hwang TH, Moon A, Burke J, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther. 2011;19(10):1913–1922. doi: 10.1038/mt.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–336. doi: 10.1038/nm.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcami A, Khanna A, Paul NL, Smith GL. Vaccinia virus strains lister, USSR and evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80(Pt 4):949–959. doi: 10.1099/0022-1317-80-4-949 [DOI] [PubMed] [Google Scholar]
- 7.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH, McFadden G. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4(12):e353. doi: 10.1371/journal.pmed.0040353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chard LS, Maniati E, Wang P, et al. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res. 2015;21(2):405–416. doi: 10.1158/1078-0432.CCR-14-0464 [DOI] [PubMed] [Google Scholar]
- 9.Meko JB, Yim JH, Tsung K, Norton JA. High cytokine production and effective antitumor activity of a recombinant vaccinia virus encoding murine interleukin 12. Cancer Res. 1995;55(21):4765–4770. [PubMed] [Google Scholar]
- 10.Elkins KL, Ennist DL, Winegar RK, Weir JP. In vivo delivery of interleukin-4 by a recombinant vaccinia virus prevents tumor development in mice. Hum Gene Ther. 1994;5(7):809–820. doi: 10.1089/hum.1994.5.7-809 [DOI] [PubMed] [Google Scholar]
- 11.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22(20):3188–3192. doi: 10.1038/sj.onc.1206459 [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Fan J, Guo M, Huang B. Oncolytic and immunologic cancer therapy with GM-CSF-armed vaccinia virus of Tian Tan strain Guang9. Cancer Lett. 2016;372(2):251–257. doi: 10.1016/j.canlet.2016.01.025 [DOI] [PubMed] [Google Scholar]
- 13.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21(5):708–718. doi: 10.1038/sj.onc.1205116 [DOI] [PubMed] [Google Scholar]
- 14.Sauane M, Gopalkrishnan RV, Sarkar D, et al. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14(1):35–51. doi: 10.1016/S1359-6101(02)00074-6 [DOI] [PubMed] [Google Scholar]
- 15.Lebedeva IV, Emdad L, Su ZZ, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review). Int J Oncol. 2007;31(5):985–1007. [PubMed] [Google Scholar]
- 16.Ramesh R, Mhashilkar AM, Tanaka F, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63(16):5105–5113. [PubMed] [Google Scholar]
- 17.Tong AW, Nemunaitis J, Su D, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11(1):160–172. doi: 10.1016/j.ymthe.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 18.Cunningham CC, Chada S, Merritt JA, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11(1):149–159. doi: 10.1016/j.ymthe.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 19.Zhu R, Liu Q, Huang W, Yu Y, Wang Y. Comparison of the replication characteristics of vaccinia virus strains Guang 9 and Tian Tan in vivo and in vitro. Arch Virol. 2014;159(10):2587–2596. doi: 10.1007/s00705-014-2079-2 [DOI] [PubMed] [Google Scholar]
- 20.Fang Q, Yang L, Zhu W, et al. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tian Tan strain. Virology. 2005;335(2):242–251. doi: 10.1016/j.virol.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Zhu RHW, Yan Z, Wen Z, et al. Studies on the virulence of a novel attenuated vaccinia virus VG9 strain in animals. Chin J Viral Dis. 2011;1:183–187. [Google Scholar]
- 22.Deng L, Fan J, Ding Y, et al. Oncolytic efficacy of thymidine kinase-deleted vaccinia virus strain Guang9. Oncotarget. 2017;8(25):40533–40543. doi: 10.18632/oncotarget.17125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/S0092-8674(00)81848-6 [DOI] [PubMed] [Google Scholar]
- 24.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7(7):781–787. doi: 10.1038/89901 [DOI] [PubMed] [Google Scholar]
- 25.Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12(2):141–161. doi: 10.1038/sj.cgt.7700771 [DOI] [PubMed] [Google Scholar]
- 26.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. doi: 10.1126/science.1317968 [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Carew JF, Kooby DA, et al. Combination gene therapy using multiple immunomodulatory genes transferred by a defective infectious single-cycle herpes virus in squamous cell cancer. Cancer Gene Ther. 2000;7(9):1279–1285. doi: 10.1038/sj.cgt.7700231 [DOI] [PubMed] [Google Scholar]
- 29.Menezes ME, Bhatia S, Bhoopathi P, et al. MDA-7/IL-24: multifunctional cancer killing cytokine. Adv Exp Med Biol. 2014;818:127–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta P, Su ZZ, Lebedeva IV, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111(3):596–628. doi: 10.1016/j.pharmthera.2005.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caudell EG, Mumm JB, Poindexter N, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168(12):6041–6046. doi: 10.4049/jimmunol.168.12.6041 [DOI] [PubMed] [Google Scholar]
- 32.Poindexter NJ, Walch ET, Chada S, Grimm EA. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol. 2005;78(3):745–752. doi: 10.1189/jlb.0205116 [DOI] [PubMed] [Google Scholar]
- 33.Madireddi MT, Dent P, Fisher PB. Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene. 2000;19(10):1362–1368. doi: 10.1038/sj.onc.1203424 [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharya P, Budnick I, Singh M, et al. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J Interferon Cytokine Res. 2015;35(8):585–599. doi: 10.1089/jir.2014.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge H, Farris CM, Tong M, Maina A, Richards AL. Transcriptional profiles of cytokines and chemokines reveal important pro-inflammatory response from endothelial cells during orientia tsutsugamushi infection. Microbes Infect. 2019;21(7):313–320. doi: 10.1016/j.micinf.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 36.Deng L, Fan J, Ding Y, et al. Oncolytic cancer therapy with a vaccinia virus strain. Oncol Rep. 2019;41(1):686–692. doi: 10.3892/or.2018.6801 [DOI] [PubMed] [Google Scholar]
- 37.Lei W, Wang S, Yang C, et al. Combined expression of miR-34a and smac mediated by oncolytic vaccinia virus synergistically promote anti-tumor effects in multiple myeloma. Sci Rep. 2016;6(1):32174. doi: 10.1038/srep32174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao L, Dong A, Gu J, et al. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13(11):1011–1022. doi: 10.1038/sj.cgt.7700969 [DOI] [PubMed] [Google Scholar]
- 39.Scholl S, Squiban P, Bizouarne N, et al. Metastatic breast tumour regression following treatment by a gene-modified vaccinia virus expressing MUC1 and IL-2. J Biomed Biotechnol. 2003;2003(3):194–201. doi: 10.1155/S111072430320704X [DOI] [PMC free article] [PubMed] [Google Scholar]