Abstract
Introduction
The aim of this study was to analyse the relationship between exposure to residential radon and chronic obstructive pulmonary disease (COPD) by means of a systematic review.
Material and Methods
A search was conducted in PubMed and OVID for papers making reference to the radon–COPD relationship. No search filters were applied, whether by date of publication, study type or sample size. All studies not written in English or Spanish were discarded.
Results
A total of 174 and 57 papers were found in PubMed and OVID, respectively: of these, 13 (11 on miners and 2 on the general population) fulfilled the inclusion criteria. Only four of the studies on cohorts of miners analysed COPD as a specific disease, and only one reported statistically significant results. In addition, many of these studies lacked information on tobacco use among miners. In contrast, studies conducted on the general public showed an association between mortality and hospital admissions, on the one hand, and residential radon on the other.
Conclusion
There are not enough studies to provide a basis for confirming or ruling out an association between radon exposure and COPD. Nonetheless, the most recent general population studies point to evidence of a possible association. In view of the heterogeneity of available studies, it is impossible to say whether this gas may or may not affect COPD morbidity and mortality, until such a time as further studies are carried out.
Keywords: radon, COPD, chronic obstructive pulmonary disease, systematic review
Introduction
Epidemiology of COPD
Chronic obstructive pulmonary disease (COPD) is a disorder of major importance by reason of its related mortality and morbidity. It is usually defined as a chronic airflow limitation during breathing, which develops progressively and irreversibly. According to the World Health Organization (WHO) data, 65 million persons have moderate-to-severe COPD,1 but in total it is estimated that some 175 million persons around the world suffered from COPD in 2015.2 In 2010, 2.8 million persons died from this disease3 (5% of total deaths),1 with COPD currently ranked as the 3rd leading cause of mortality worldwide.3
While the Epidemiological Study of COPD in Spain (EPISCAN) originally reported a COPD prevalence in Spain of 10.2% (15.1% in men and 5.7% in women)4 in the 40- to 80-year age group, the preliminary EPISCAN II data indicate that this prevalence has increased to 12.4% (16.9% in men and 9.5% in women), a percentage that rises in both sexes with age.5 This said, however, even in developed countries there is important degree of underdiagnosis, estimated at 73% in the EPISCAN study.4
Radon, and the Radon–COPD Relationship
Radon is a colourless, odourless, tasteless noble gas that comes from the disintegration of uranium contained in rocks forming part of the Earth’s crust.6 Exposure to radon is ubiquitous, and although the gas is swiftly diluted in the atmosphere, it can nevertheless accumulate in large quantities in closed spaces such as dwellings and workplaces.7,8 The concentration at any given site fundamentally depends on the uranium content of rocks in the subsoil, since radon is a product of the radioactive decay chain in which uranium is the parent element. Being a gas, it leaks into interior spaces through cracks or fissures, gradually accumulating in the process.9 The harmful effect of radon really comes from its short half-life daughters (polonium-214 and polonium-218), which release alpha radiation that causes molecular changes in pulmonary epithelial cells on impact.10
The relationship between lung cancer and radon has been exhaustively documented. Exposure to radon is the second known leading risk factor for lung cancer after tobacco, and the first in never-smokers, as is recognised by both, the WHO8 and the United States Environmental Protection Agency (USEPA).7
In contrast, the relationship between radon and COPD is unclear, with few studies published. Essentially, the same studies that examined lung cancer mortality showed an increase in non-malignant respiratory-disease mortality, which, due to the coexistence of other risk factors for these diseases (silica dust, tobacco, etc.), might cause the above relationship to go unnoticed. However, it cannot be ruled out that prolonged exposure to radon in high concentrations may generate a permanent sub-inflammatory lung microenvironment, which would, in turn, facilitate the appearance of COPD. Even so, this is no more than an hypothesis, and at the date of writing, the mechanisms that might underlie such a possible COPD-radon relationship are still unknown.
Accordingly, the designated aim of this study was to analyse the relationship between exposure to radon and appearance of COPD by means of a systematic review of the scientific literature.
Materials and Methods
Study Design
To respond to the research question of whether exposure to radon is the cause of COPD-related morbidity and mortality, we conducted a systematic review of the scientific literature up until the present, using the PICO (Patients, Intervention, Comparison and Outcome) system. The study participants were individuals exposed to radon (Patients and Intervention) who were tested for appearance of COPD (mortality or morbidity) (Outcome) and compared to individuals with a different exposure to this gas (Comparison), with the aim of demonstrating or ruling out a relationship between the radon and COPD. The review was performed using the PRISMA methodology.11
The database used was PubMed (Medline). No search filters were applied, whether by date of publication, study type or sample size. The following search query was performed:
(((((((((((Pulmonary Disease, Chronic Obstructive [Mesh]) OR COPD) OR Chronic Obstructive Pulmonary Disease) OR COAD) OR Obstructive Airway Disease) OR Obstructive Lung Disease) OR Airflow Obstruction) OR Airflow Obstructions)) AND radon))
(“radon”[All Fields] OR “Actinon”[All Fields] OR “Thoron” [All Fields]) AND (“copd”[All Fields] OR “Pulmonary Disease, Chronic Obstructive”[All Fields] OR “Chronic Obstructive”[All Fields] OR “Pulmonary disease”[All Fields] OR (non-malignant[All Fields] AND respiratory[All Fields]) OR (nonmalignant[All Fields] AND respiratory[All Fields]) OR “Chronic Obstructive Pulmonary Disease”[All Fields] OR “Chronic Obstructive Lung Disease”[All Fields] OR “Chronic Obstructive Airway Disease”[All Fields] OR “Chronic Airflow Obstruction”[All Fields] OR “COAD”[All Fields] OR (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR (“airflow”[All Fields] AND “obstruction”[All Fields] AND “chronic”[All Fields])) OR “Airflow Obstruction”[All Fields])
Radon AND (COPD OR Respiratory diseases)
To ascertain the existence of any other paper of interest outside PubMed, the following search was made in OVID:
((“radon” or “Actinon” or “Thoron” or “radon220”) and (“COPD” or “Chronic Obstructive Pulmonary Disease” or “COAD” or “non-malignant respiratory” or “non malignant respiratory” or “Chronic Obstructive Airway Disease” or “Chronic Airflow Obstruction” or “pulmonary disease” or “pulmonary diseases” or “respiratory disease” or “respiratory diseases”))
This search was restricted to abstracts, and covered all years of publication and all types of study.
The first search was made on 4 November 2017. For updating purposes, on 20 July 2019 we conducted a last search of both PubMed and OVID for possible new papers.
Inclusion and Exclusion Criteria
Due to the heterogeneity of the concept of COPD, our study included papers which, while not addressing COPD as such, made reference to non-malignant respiratory diseases (though these studies do not have the same capacity to respond to the research question as those which evaluate COPD as an individual entity).
Insofar as the variable radon was concerned, our study included papers that used dosimetric measures, both individual and collective, in work or residential settings. The fact that papers reported exposure to other risk factors (suspended dust particles, silica, etc.) was not grounds for exclusion but was nonetheless taken into account.
To reduce the relevance of studies being missed for language reasons, we applied no restrictions to the language field in the initial search. Subsequently, however, all studies that were not written in Spanish or English were eliminated manually. Due to the low expected number of papers analysing the relationship under study, no restriction was applied to the date of publication of the studies analysed. Likewise, no restrictions were placed on the searches in PubMed in terms of study type, sample size, or the fact that the search for a radon–COPD relationship might constitute the study’s main or secondary aim.
After a perusal of the records obtained, we excluded all papers which included the concept of radon solely in the Introduction section or which, focusing on the study of radon’s relationship with lung cancer, discussed the radon–COPD relationship superficially without furnishing any quantitative data on it.
Results
Search Results
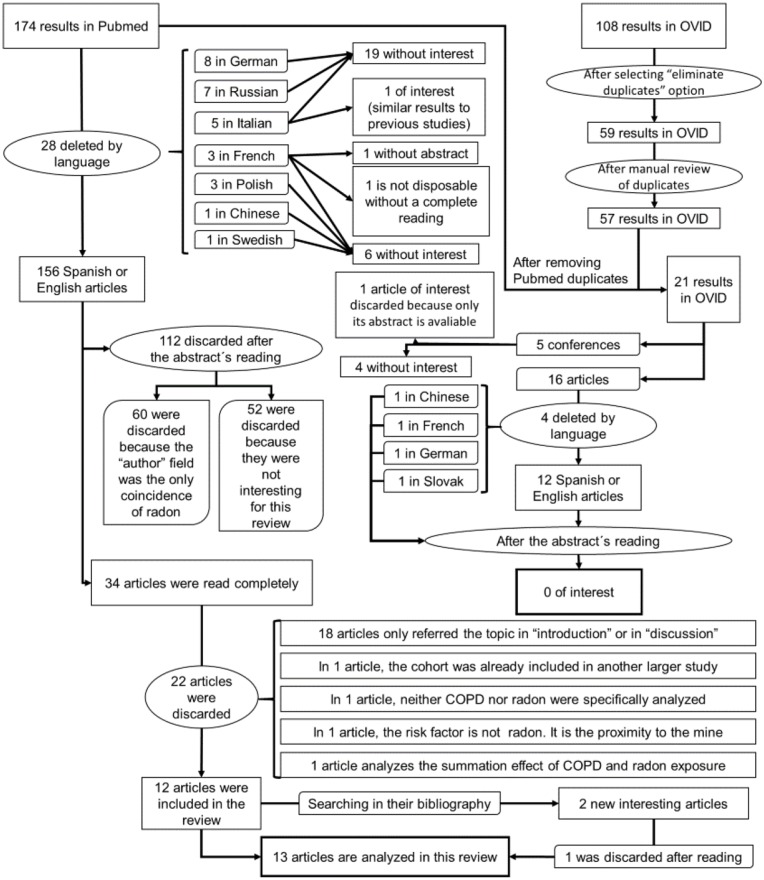
The PubMed search yielded 174 results, 28 of which were discarded for language reasons. The only paper that appeared to furnish information relating to this review described a study conducted on a mine in Italy, which found a relationship between lung cancer and high radon levels, as well as a higher incidence of pulmonary disease among miners.12
Of the above 146 papers, 112 were discarded after reading their abstracts and ascertaining that they did not examine the radon–COPD relationship. A search of the references cited in the remaining 34 articles yielded 2 more papers, thereby leaving a total of 37 for a reading of the full text. Following the full-text reading, a further 24 were discarded.
After elimination of duplicates, the search in OVID yielded 57 results. A reading of the Abstracts showed that only one conference paper provided new data on the radon–COPD relationship.
Figure 1 uses a flow chart to depict the paper-selection and -elimination process.
Figure 1.
Flow chart.
Results in Miners
A total of 11 studies were conducted on miners between 1988 and 2014. All of these were cohort studies, which analysed subjects with a minimum employment period of one month13,14 to 4 years,15 with 6 months being the most frequently used minimum time of exposure.16–18 The sole exceptions were 2 studies which made no reference to a minimum employment period.19,20
In 4 papers, the term COPD was used for analysis of mortality.13,14,16,17 The remaining studies only analysed this type of mortality as that due to non-malignant respiratory diseases, among which COPD is a principal component.
Only 3 of the studies analysed tobacco use in the cohort sample itself.14,19,21 In 2 papers, information on tobacco use in the mines under study was obtained from data reported by earlier cross-sectional studies.20,22 In a number of studies that failed to report specific data on tobacco use among their workers, reference was made to a high prevalence of smoking among local mine workers.15,18,23 One such study made reference to cancers of the larynx and bladder and, taking these as a proxy, reported no differences with respect to the general population.16 In the remaining studies, there were either no data, or the data were discarded in view of their inaccuracy when it came to including findings on tobacco use.
Of the 4 studies that studied COPD, three reported observing no statistically significant relationship13,16,17 versus one that did observe it.14
In studies that analysed non-malignant respiratory diseases or different variants that were not precisely COPD, a statistically significant relationship was reported by four15,18-20 but was not found to exist by two.21,23
Results in the General Population
There were only 2 studies24,25 located which had been conducted on the general population, and both were recent. The first was a cohort study, in which the relationship between COPD and the concentration of environmental radon was analysed in the Cancer Prevention Study II cohort, with 1,184,881 participants.24 While confounding factors such as tobacco use were taken into account, in this study radon concentration was measured on an aggregate basis (concentration assigned to participants according to county of residence). The other study was an ecological study which analysed COPD prevalence along with a possible increase in COPD-related complications, e.g., as shown by hospital admissions.25 Although individualised data on tobacco use were shown, prevalence of bladder cancer, a type of cancer strongly associated with tobacco use, was used as a proxy, with stress also being laid on the fact that tobacco use cannot be a confounding factor since it is not linked to radon concentrations in the home. It should be noted that this study did not find a link between tobacco use and radon exposure in the home, so it cannot be a confounding factor.
Both studies showed an association between radon and COPD. In the cohort study,24 COPD mortality registered an increase of 13% (95% CI: 5–21%) for every increase of 100 Bq/m3 (100 becquerels per cubic metre) in radon concentrations. The ecological study25 observed a statistically significant relationship with hospital admissions (RR: 1.04 (95% CI: 1.00–1.10) for every increase of 100 Bq/m3, whereas there was no association with prevalence (RR: 0.95 (95% CI: 0.92–0.97) for an increase of 100 Bq/m3, and an RR of 1.06 (95% CI: 1.02–1.10) with a cut point of 50 Bq/m3. While neither age nor rural-urban setting proved relevant in this study, the single exception was sex, with women being found to register a higher risk. These results can be seen in Table 1.
Table 1.
Breakdown of Studies Included
| Title | Study | No | Population | Minimum Employment Period | The Study Itself Analyses Tobacco Consumption | Measuring | Results | |
|---|---|---|---|---|---|---|---|---|
| 1 | Residential radon and COPD. An ecological study in Galicia, Spain25 | Ecological | 313 Galician municipalities | General population | Disregarded | No. A proxy is used. It is estimated that there is no confusion because there is no radon-tobacco relationship | COPD (prevalence and admissions due to exacerbation) | No association prevalence: 0.95, CI95: (0.92–0.97) per 100 Bq/m3 Association risk admission: 1.04, CI95 (1.00–1.10) 100 Bq/m3 |
| 2 | Mortality from internal and external radiation exposure in a cohort of male German uranium millers, 1946–200816 | Cohorts | 4054 | Miners | 6 months | No | COPD | No association. SMR: 0.77, CI95: (0.54; 0.99) |
| 3 | Silica dust, radon and death from non-malignant respiratory diseases in German uranium miners17 | Cohorts | 58,982 | Miners | 6 months | No | COPD | No association. RER: 0.007 (p: 0.41) |
| 4 | Mortality and ionising radiation exposures among workers employed at the Fernald Feed Materials Production Center (1951–1985)13 | Cohorts | 6409 | Miners | 1 month | No | COPD | No association. Men working by hours: SMR 1.01, CI95 (0.81–1.25). Male employees: SMR 0.43, CI95 (0.25–0.69). Female employees 1.29, CI95 (0.64 to 2.31) |
| 5 | Radon and COPD mortality in the American Cancer Society Cohort24 | Cohorts | 811,961 | General population | Disregarded | Yes | COPD | Association: HR per 100 Bq/m3 1.13. CI95: (1.05–1.21) |
| 6 | A cohort study of uranium millers and miners of Grants, New Mexico, 1979–200518,22 | Cohorts | 2745 | Miners | 6 months | No. It is reported that high numbers of smokers were found in other studies conducted in miners in the country | NMRD and chronic bronchitis, emphysema and asthma subgroup | Association: NMRD 1.42 CI95(1.14–1.76) and chronic bronchitis, emphysema and asthma subgroup 1.78 CI95 (1.24–2.48) The stratification shows that the increase in mortality depends on the underground miners |
| 7 | An update of mortality from all causes among white uranium miners from the Colorado Plateau Study Group14 | Cohorts | 3238 | Miners | 1 month | Yes | COPD, only in text, data from the tables are differently categorized | Association of SMR 2.7, CI95 (2.0–3.5) in the 1980s–1990s remaining high in the last decade of monitoring. No increase was observed with the increase in years worked. |
| 8 | Mortality of Sardinian lead and zinc miners: 1960–8822 | Cohorts | 4740 | Miners | 2 consecutive months | No. Although they refer to the study (21), in which their population is included in it | NMRD | Association. SMR 3.08 CI95 (2.74–3.45) |
| 9 | Mortality in uranium miners in west Bohemia: a long-term cohort study15 | Cohorts | 4320 | Miners | 4 years | No. A percentage of smokers from a Czech mine is provided to indicate that it is likely to be higher than in the general population | NMRD | Association: Observed/Expected: 1.21 with a non-significant p until 25 years after first employment, from which it becomes significant |
| 10 | Lung cancer mortality and airways obstruction among metal miners exposed to silica and low levels of radon daughters19 | Cohorts | 1741 | Miners | Not known | Yes | NMRD | Association. Mine A SMR 1.64 CI95 (0.93–2.65) and mine B with SMR 3.51 CI95 (2.22–5.21). Mine with higher radon levels: Mine A |
| 11 | Mortality experience of haematite mine workers in China21 | Cohorts | 6444 | Miners | 1 year | Yes | NMRD | No numerical data are provided, only indicates increased mortality for silicosis and other non-malignant respiratory disease |
| 12 | Mortality among pyrite miners with low-level exposure to radon daughters20 | Cohorts | 1899 | Miners | Not known | No. It is commented that cases of bladder and laryngeal cancer, intimately related to tobacco, are not different from those of the general population | NMRD | Association. SMR: 1.73 CI95 (1.35–2.31) |
| 13 | Mortality risk in the French cohort of uranium miners: extended follow-up 1946–199923 | Cohorts | 5086 | Miners | 1 year | Data of their own cohort are not provided. A case-control study of miners in France is used as reference | NMRD | No association. SMR: 0.98 CI95 (0.74–1.27) |
Abbreviations: COPD, chronic obstructive pulmonary disease; NMRD, non-malignant respiratory disease; SMR, standardized mortality ratio; CI, confidence interval; HR, hazard ratio; RER, relative excess risk.
While the Abstract located in OVID reported a positive relationship between highest radon levels and COPD, the differences failed to prove statistically significant (OR: 1.19 (95% CI: 0.98–1.43)).26
Discussion
This is the first systematic review to analyse the possible relationship between radon and COPD. Based on the papers analysed, no definite conclusion can be drawn about the relationship between exposure to radon and appearance of COPD. Even so, most of the studies, whether conducted on miners or the general population, observe an association or a trend towards the existence of an association. However, separate conclusions cannot be specifically drawn for miners and the general population.
Despite their advantage of making it possible to analyse individuals exposed to very high radon concentrations, when it comes to extrapolating such data to the general population, studies on miners have important potential biases, e.g., the so-called “healthy worker” bias. In the miner cohort in France, emphasis is laid on the importance of this effect not being found,23 in contrast to other studies conducted on these types of workers.
At the opposite end of the scale from the “healthy worker” effect, there is another important characteristic of studies on miners in terms of the mix of diverse risk factors for different diseases, and for lung-related diseases in particular. In mines, in addition to exposure to radon there are other types of exposure, some intrinsic to the work, such as silica dust or other components that are unrelated to uranium (which also releases gamma radiation), e.g., in the Fernald Feed mine study13 the authors report that both effects cannot be separated, with the result that the dust of some compound could act as a confounder. Another risk exposure that may affect extrapolation of results to the general population could be greater tobacco use by miners vis-à-vis the general population.14,15
Moreover, though mines might furnish a great deal of information about persons with high exposure to radon, these concentrations are not the same as those normally experienced by the general population. Consequently, the harm that they cause may be different to what is expected, if the association between radon concentration and disease is not linear.
Lastly, mine working conditions have improved in recent decades, with the advent of individual protection measures and better air-extraction and -renewal systems. Hence, studies undertaken some time ago may not be comparable to more recent studies, particularly with regard to the presence of suspended dust particles, or even exposure to radon if the mine is equipped with extraction and ventilation systems that are effective in terms of reducing radon concentrations.
One of the principal problems in the cohort studies on miners is the lack of adjustment for tobacco use, since only 3 of the studies collected individualised data on tobacco use.14,19,21 The papers in which tobacco use could not be analysed mention the importance of controlling for this confounding factor. As a result, a number of studies endeavoured to obtain data on tobacco use among workers in these mines as reported in other studies, or in mines in the area or across the country, so as to have these as reference,15,18,20,22,23 or alternatively resorted to some proxy.16,20 In other studies, data on the miners’ smoking habit could not be collected, thus posing a serious problem when it came to analysing and combining the results. As discussed in Darby et al,27 the excess relative risk of lung cancer from radon exposure was only significant if adjustment for confounders, among them smoking, by a priori stratification was made. When this adjustment was not made, the excess relative risk of lung cancer per 100 Bq/m3 was not statistically significant and was even slightly negative. Therefore, the lack of data on smoking may have contributed to the fact that no statistically significant association was found in the studies on miners.
The two studies conducted on the general population open up avenues for new research. On the one hand, the American Cancer Society cohort24 shows a relationship between residing in areas with higher radon measurements and a higher probability of dying of COPD (adjusted for variables such as educational level, body mass index, cigarette smoking, passive smokers, etc.). On the other hand, the ecological study on indoor radon and COPD in Galicia25 views the radon–COPD relationship from another angle, i.e., rather than studying COPD mortality, it studied COPD prevalence or the need for hospitalisation, and found statistically significant differences. Accordingly, more studies should analyse this possible increase in COPD prevalence or COPD-related complications among patients with exposure to settings with higher radon concentrations. It should be stressed here that the fact of conducting studies in areas with a high presence of radon, such as Galicia,28,29 may well facilitate the detection of this association, should it in fact exist.
Currently, a case–control study is being undertaken in Galicia to ascertain whether radon may influence the appearance of COPD. To this end, it applies a methodology similar to that used by case–control studies which have analysed the radon-lung cancer relationship, namely, selection of COPD cases who are aged under 75 years and have been living for 15 years or more in the same home, with their disease diagnosed less than 10 years previously, and comparison of their radon concentrations against those of controls sharing the same characteristics.
When it comes to evaluating these studies, there is the handicap of diagnosis and classification of COPD, something that has changed considerably in recent years. Hence, many of the older studies make no mention of COPD: instead they talk of obstructive diseases, though these diseases were mostly brought under the wider umbrella of an even larger and more heterogeneous group such as non-malignant respiratory diseases (in some cases, due to the importance of silicosis and pneumoconiosis in these workers, special mention is made of non-malignant respiratory diseases other than silicosis/tuberculosis, which encompassed all the other diseases, including COPD).23
Among the strengths of this study is the fact that it is a systematic review which summarises the available literature on the COPD-radon relationship, thereby allowing us to assess which aspects have been evaluated and which still remain to be studied. The search design used means that there is very little likelihood of having missed papers that might have fulfilled the inclusion criteria, or studies that were published in languages other than English and Spanish.
Among the disadvantages, it should be stressed that the relationship between radon and COPD is difficult to analyse due to the heterogeneity of existing studies. The fact that tobacco use was not analysed in a number of these poses a problem when it comes to interpreting the data, particularly in the studies on miners. In the latter, aside from healthy user bias, one may encounter biases such as exposure to other risk factors, some intrinsic to the work and others not, such as greater tobacco use among miners. Furthermore, since not many studies have measured radon concentrations individually, there is a risk of misclassification of such exposure.
Conclusion
In conclusion, the fact that it has not yet proved possible to clearly demonstrate the relationship between COPD and radon means that there is a need for more studies which make this their prime objective, evaluate any possible confounding factors, and ideally – where feasible – use individual evaluations of exposure. Apart from being few in number, the studies undertaken to date are extremely heterogeneous and, though the most recent of these show a trend that points to a relationship between radon and COPD, there is nevertheless a need for studies with a robust methodology which would enable this association to be confirmed or discarded.
Disclosure
The authors have no conflicts of interest in this work.
References
- 1.WHO. Chronic obstructive pulmonary disease (COPCD). Burden of COPD. Available from: http://www.who.int/respiratory/copd/burden/en/. Accessed April21, 2020.
- 2.Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burney PGJ, Patel J, Newson R, Minelli C, Naghavi M. Global and regional trends in COPD mortality, 1990–2010. Eur Respir J. 2015;45(5):1239–1247. doi: 10.1183/09031936.00142414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi: 10.1136/thx.2009.115725 [DOI] [PubMed] [Google Scholar]
- 5.SEPAR. El infradiagnóstico de la EPOC asciende hasta el 81,7%, según datos preliminares del estudio EPISCAN II puesto en marcha por GSK en colaboración con SEPAR [Internet]. 2018. Available from: https://separ.es/?q=node/1139. Accessed April21, 2020.
- 6.United Nations Environment Programme (UNEP). Radiation: Effects and Sources. Vienna, United Nations: United Nations Environment Programme; 2016. [Google Scholar]
- 7.U.S. Environmental Protection Agency. A Citizen’s Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon. U.S. Environmental Protection Agency; 2012. [Google Scholar]
- 8.World Health Organization. Handbook on Indoor Radon: A Public Health Perspective 2009. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- 9.Ruano-Ravina A, Barros-Dios JM. Radon and lung cancer. Implications for health workers, citizens and public administrators. Med Clínica. 2007;128(14):545–549. doi: 10.1157/13101166 [DOI] [PubMed] [Google Scholar]
- 10.National Research Council. Committee on Health Risks of Exposure to Radon (BEIR VI). Health Effects of Exposure to Radon. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 11.Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin (Barc). 2010;135(11):507–511. doi: 10.1016/j.medcli.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 12.Belli S, Comba P, Germani D, et al. Mortality among lead-zinc miners in Val Seriana. Med Lav. 1989;80(6):467–478. [PubMed] [Google Scholar]
- 13.Silver SR, Bertke SJ, Hein MJ, et al. Mortality and ionising radiation exposures among workers employed at the fernald feed materials production center (1951–1985). Occup Environ Med. 2013;70(7):453–463. doi: 10.1136/oemed-2012-100768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roscoe RJ. An update of mortality from all causes among white uranium miners from the colorado plateau study group. Am J Ind Med. 1997;31(2):211–222. doi: [DOI] [PubMed] [Google Scholar]
- 15.Tomásek L, Swerdlow AJ, Darby SC, Placek V, Kunz E. Mortality in uranium miners in west Bohemia: a long-term cohort study. Occup Environ Med. 1994;51(5):308–315. doi: 10.1136/oem.51.5.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreuzer M, Dufey F, Laurier D, et al. Mortality from internal and external radiation exposure in a cohort of male German uranium millers, 1946–2008. Int Arch Occup Environ Health. 2015;88(4):431–441. doi: 10.1007/s00420-014-0973-2 [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer M, Sogl M, Brüske I, et al. Silica dust, radon and death from non-malignant respiratory diseases in German uranium miners. Occup Environ Med. 2013;70(12):869–875. doi: 10.1136/oemed-2013-101582 [DOI] [PubMed] [Google Scholar]
- 18.Boice JD, Cohen SS, Mumma MT, Chadda B, Blot WJ. A cohort study of uranium millers and miners of Grants, New Mexico, 1979–2005. J Radiol Prot. 2008;28(3):303–325. doi: 10.1088/0952-4746/28/3/002 [DOI] [PubMed] [Google Scholar]
- 19.Carta P, Cocco P, Picchiri G. Lung cancer mortality and airways obstruction among metal miners exposed to silica and low levels of radon daughters. Am J Ind Med. 1994;25(4):489–506. doi: 10.1002/ajim.4700250404 [DOI] [PubMed] [Google Scholar]
- 20.Battista G, Belli S, Carboncini F, et al. Mortality among pyrite miners with low-level exposure to radon daughters. Scand J Work Environ Health. 1988;14(5):280–285. doi: 10.5271/sjweh.1919 [DOI] [PubMed] [Google Scholar]
- 21.Chen SY, Hayes RB, Liang SR, Li QG, Stewart PA, Blair A. Mortality experience of haematite mine workers in China. Br J Ind Med. 1990;47(3):175–181. doi: 10.1136/oem.47.3.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocco PL, Carta P, Belli S, Picchiri GF, Flore MV. Mortality of Sardinian lead and zinc miners: 1960-88. Occup Environ Med. 1994;51(10):674–682. doi: 10.1136/oem.51.10.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vacquier B, Caer S, Rogel A, et al. Mortality risk in the French cohort of uranium miners: extended follow-up 1946–1999. Occup Environ Med. 2008;65(9):597–604. doi: 10.1136/oem.2007.034959 [DOI] [PubMed] [Google Scholar]
- 24.Turner MC, Krewski D, Chen Y, Pope CA 3rd, Gapstur SD, Thun MJ. Radon and COPD mortality in the American Cancer Society Cohort. Eur Respir J. 2012;39(5):1113–1119. doi: 10.1183/09031936.00058211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbosa-Lorenzo R, Ruano-Ravina A, Ramis R, et al. Residential radon and COPD. An ecological study in Galicia, Spain. Int J Radiat Biol. 2017;93(2):222–230. doi: 10.1080/09553002.2017.1238526 [DOI] [PubMed] [Google Scholar]
- 26.Stacy SL, Robertson L, Wang R, et al. Abstract 3240: radon exposure, lung cancer, and respiratory outcomes in a cohort of former and current smokers: an ecologic analysis. Cancer Res. 2018;78(13 Supplement):3240. [Google Scholar]
- 27.Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health. 2006;32(Suppl 1):1–83. [PubMed] [Google Scholar]
- 28.Lorenzo-González M, Ruano-Ravina A, Peón J, Piñeiro M, Barros-Dios JM. Residential radon in Galicia: a cross-sectional study in a radon-prone area. J Radiol Prot. 2017;37(3):728–741. doi: 10.1088/1361-6498/aa7922 [DOI] [PubMed] [Google Scholar]
- 29.Barros-Dios JM, Ruano-Ravina A, Gastelu-Iturri J, Figueiras A. Factors underlying residential radon concentration: results from Galicia, Spain. Environ Res. 2007;103(2):185–190. doi: 10.1016/j.envres.2006.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Chronic obstructive pulmonary disease (COPCD). Burden of COPD. Available from: http://www.who.int/respiratory/copd/burden/en/. Accessed April21, 2020.
- SEPAR. El infradiagnóstico de la EPOC asciende hasta el 81,7%, según datos preliminares del estudio EPISCAN II puesto en marcha por GSK en colaboración con SEPAR [Internet]. 2018. Available from: https://separ.es/?q=node/1139. Accessed April21, 2020.