Abstract
Cell polarity plays an important role in a wide range of biological processes in plant growth and development. Cell polarity is manifested as the asymmetric distribution of molecules, e.g. proteins and lipids, at the plasma membrane and/or inside of a cell. Here, we summarize a few polarized proteins that have been characterized in plants and we review recent advances towards understanding the molecular mechanism for them to polarize at the plasma membrane. Multiple mechanisms, including membrane trafficking, cytoskeletal activities, and protein phosphorylation, etc., define the polarized plasma membrane domains. Recent discoveries suggest that the polar positioning of the proteo-lipid membrane domain may instruct the formation of polarity complexes in plants. In this review, we highlight the factors and regulators for their functions in establishing the membrane asymmetries in plant development. Furthermore, we discuss a few outstanding questions to be addressed to better understand the mechanisms by which cell polarity is regulated in plants.
INTRODUCTION
Cell polarity, referring to the asymmetric distribution of cellular components, structure and function within a cell, is a fundamental feature of all living organisms and plays critical roles in almost all aspects of cellular function, e.g. expansion, division, differentiation, growth and morphogenesis (Campanale et al. 2017; Chiou et al. 2017). The plant cells display a diverse array of polarity underlying growth and patterning in development (Yang 2008; Qi and Greb 2017). For example, the pollen tube and root hair are formed by extremely polarized tip growth (Guan et al. 2013; Mendrinna and Persson 2015). The puzzle-shaped pavement cells require diffused polar growth for morphogenesis (Guimil and Dunand 2007; Qian et al. 2009). Specialized cell function, including directional movement of nutrient or phytohormones, can be achieved by directional enrichment and/or activity of the transporters (Yoshinari and Takano 2017). Cell polarity also plays important roles in the regulation of asymmetric cell division (ACD) (Shao and Dong 2016; Zhang and Dong 2018; Muroyama and Bergmann 2019), an important biological process that generates two daughter cells that differ in cell fates and is essential for the development of multicellularity while maintaining the stem cell population in plants.
The plant cells possess numerous unique features, including the cell walls, that function to assist in the establishment of cellular asymmetry (De Smet and Beeckman 2011). One of the major mechanisms is to place key regulators, e.g. proteins or lipids, to one side of the cell and this process often requires highly coordinated activities of cell signaling, membrane trafficking and cytoskeleton reorganization. With regards to polarly localized proteins, they can be integral to the plasma membrane (PM) or associated with the PM. For the integral membrane proteins, to reach the PM, they are first synthesized in the endoplasmic reticulum (ER), followed by vesicle delivery along the secretory pathway through the Golgi apparatus and the trans-Golgi network (TGN), and finally reach to the PM by exocytosis and vesicle fusion (Wang et al. 2017b). Many proteins are dynamically regulated at the plasma membrane where they play their biological function, while are also endocytosed via the clathrine-dependent and/or -independent pathways (Chen et al. 2011; Zhang et al. 2019). The destinations of the endocytosed PM proteins include being recycled back to the PM and/or delivered to the lytic vacuole for degradation (Jurgens 2004). The polarization of the PM proteins involves combined activities of targeted protein secretion, endocytosis, and/or endosomal recycling with the direction guided by external cues (Luschnig and Vert 2014; Langowski et al. 2016). On the other hand, the polarization of membrane-associated proteins requires the establishment of local membrane domain with distinct signatures that can be defined by specific biochemical or unique mechanical features (Hepler et al. 2013; Mangano et al. 2016). Also, the endosomes and their coordinated activities seem to be tightly integrated into the polarization machinery to polarize both membrane-embedded and -associated proteins.
In this review, we summarize the identified polarity factors and the key regulators in the establishment and maintenance of polarized membrane domains in plant cells. We give significant consideration of the endomembrane system and try to understand how dynamic membrane trafficking drives protein polarization in plants.
MAJOR POLARITY PROTEINS AND THE CELL SYSTEMS
The asymmetric distribution of proteins at the PM is an important feature of cell polarity in plants (Dettmer and Friml 2011). A few well-recognized such proteins include the auxin efflux carrier PIN-FORMED (PIN) proteins and some of their regulators (Wisniewska et al. 2006), the boron transporters NIP5;1 and BOR1 for nutrient uptake in the roots (Yoshinari and Takano 2017), the small GTPase ROPs in polarized cell growth (Yang 2008), and the scaffold proteins BASL and POLAR in stomatal development (Guo and Dong 2019). By specialized subcellular localization, they play important roles to regulate specific biological processes in plant development and growth.
Polarized PINs drive directional auxin flow
Auxin is unique among all phytohormones because it regulates numerous aspects of plant growth and development via its polar transport (Leyser 2018; Gallei et al. 2019). Based on molecular genetic studies in the model plant Arabidopsis, the tightly controlled auxin flow and distribution control the embryonic axes (Friml et al. 2003), the formation of primary and lateral roots (Sabatini et al. 1999; Benkova et al. 2003), shoot-derived organ generation (Benkova et al. 2003), and fruit development (Sorefan et al. 2009). At the cellular level, auxin signaling is also important for cell division patterning and morphogenesis (Xu et al. 2014; Smit and Weijers 2015). Auxin gradients are established and maintained mainly by the PIN efflux carriers that are polarly localized to drive directional auxin flow (Wisniewska et al. 2006; Adamowski and Friml 2015). The Arabidopsis genome encodes eight PIN proteins and five of them, including PIN1, PIN2, PIN3, PIN4 and PIN7, showed polarization at the PM in a cell type-specific manner and associated with specific developmental stages (Vieten et al. 2007; Adamowski and Friml 2015). Polarization of PIN proteins have been well characterized in a few cell systems in plant development. During early embryogenesis in Arabidopsis, the polarization of PIN1, PIN4 and PIN7 directs the auxin accumulation towards distinct parts of the developing embryo that results in the specification of the apical–basal plant axis and the division patterning (Figure 1A). In the root, the PIN proteins are differentially polarized at different cell layers and the coordinated directions of PIN polarization drives directional auxin flow (Kleine-Vehn et al. 2008)(Figure 1B, C). PIN3 and PIN7 are expressed in the columella cells, where they regulate root gravitropism (Kleine-Vehn et al. 2010).
Figure 1. PIN distribution and polarization in the plant development.
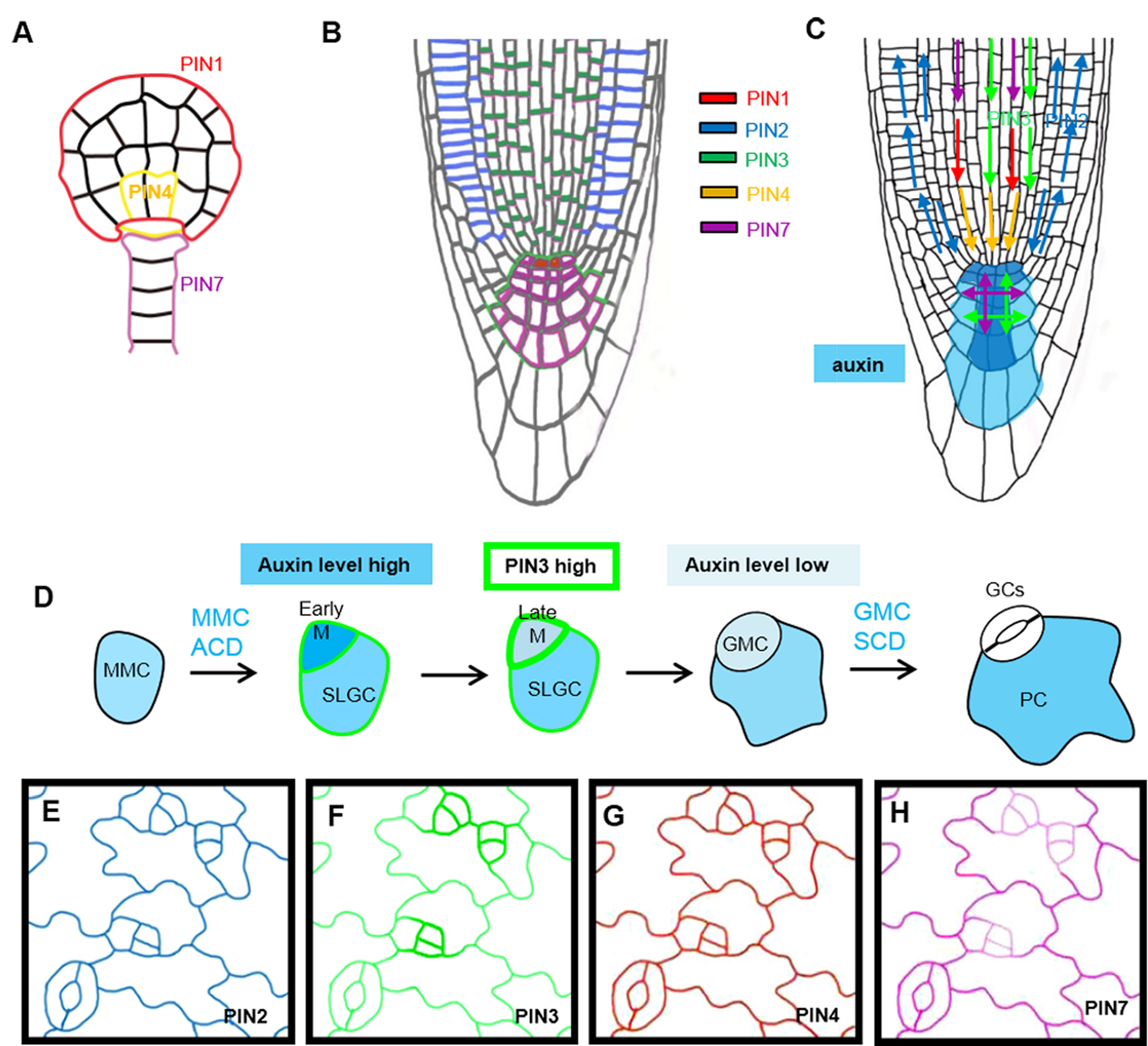
(A) PINs expression and polarization pattern in the globular stage of Arabidopsis embryo. (B) PINs expression and polarization pattern in the Arabidopsis root tip. (C) Distribution of PIN proteins contributes to the auxin gradients in the root tip. (D) Differential PIN3 and auxin activity levels during the process of stomatal development. (E-H) Differential expression patterns of PIN2, PIN3, PIN4 and PIN7 in the epidermis of Arabidopsis cotyledon. Note, high levels of PIN3 and low levels of PIN7 in the M cells. E-H, confocal images processed by photoshop to demonstrate differential expression levels of PIN proteins.
Polarized proteins and asymmetric cell signaling in stomatal development
Stomata are epidermal pores that allow gas exchange between plants and the atmosphere. Although monocots and dicots have distinctly structured stomatal complexes, 4-celled vs. 2-celled, respectively, the formation of both types requires highly regulated asymmetric cell division (ACD) that generates daughter cells with distinct cell fates. In Arabidopsis, the initiation of stomatal cell lineage begins with the asymmetric division of meristemoid mother cells (MMCs), each of which divides to create a smaller meristemoid (M) and a larger stomatal lineage ground cell (SLGC) (Dong et al. 2009). After an ACD, the two daughter cells acquire different cell fate: the meristemoid ultimately turns into a pair of guard cells, whilst the SLGC retrieves from stomatal differentiation, but undergoes spacing divisions before becoming a pavement cell (Lau and Bergmann 2012; Shao and Dong 2016).
By using Arabidopsis as a genetic model system, both auxin signaling and protein expression showed asymmetries that regulate stomatal division and cell fate determination. First of all, auxin levels were found to dynamically fluctuate at different stages of stomatal development (Le et al. 2014). Auxin signaling peaks in the young meristemoids, whereas drops when a meristemoid is differentiating into a guard mother cell (GMC) (Le et al. 2014). The depletion of auxin levels in the Ms was believed to trigger the transition of cell division from stem cell-like ACD to the GMC symmetric division followed by terminal guard cell differentiation. Interestingly, among the four PIN proteins expressed in the leaf epidermal cells (PIN2, 3, 4, and 7), the dynamic expression levels of PIN3 are most closely correlated with the dynamic auxin signaling (Le et al. 2014). Although the other PIN protein show differential expression patterns (Figure 1D–G), their combined function is critical for stomatal development and patterning because in pin higher-order mutants, pin2;3;4;7, stomata form clusters (Le et al. 2014).
During the past decade or so, significant progress has been made regarding to the identification of polarized proteins and their function in the regulation of stomatal ACD in Arabidopsis. The plant-specific protein, BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL), was first identified for its function in stomatal ACD by cortical polarization (Dong et al. 2009). The loss-of-function basl mutants produce stomatal divisions being pronouncedly symmetric, leading to both daughter cells become stomata that are in direct contact. Before an ACD, the GFP-tagged BASL protein accumulates in the nucleus and polarizes at the cortical PM to direct division orientation. After an ACD, the BASL polarity is only inherited to the SLGC where it assists in the daughter cell fate specification (Dong et al. 2009) (Figure 2A). The functions of polarized BASL was connected to MAPK signaling by locally concentrating the components of the signaling cassette, the MAPKKK YODA (YDA) and MAPK 3 and 6, for suppressing SPCH thus stomatal fate differentiation (Zhang et al. 2015; Zhang et al. 2016). The BREVIS RADIX (BRX) proteins were identified as physical partners of BASL and their cortical polarization recapitulated that of BASL in the stomatal lineage cells (Bringmann and Bergmann 2017). Previously, the BRX genes were found to regulate root development by basally polarizing in the root phloem vasculature (Scacchi et al. 2009), where BRX may suppress the D6 protein kinase-related kinase PAX and its activation of PIN-mediated auxin efflux (Marhava et al. 2018). However, how the BRX family functions in stomatal development remains unknow. In addition, POLAR LOCALIZATION DURING ASYMMETRIC DIVISION AND REDISTRIBUTION (POLAR) is another plant-specific protein in the stomata lineage and its polarization depends on the presence of BASL (Pillitteri et al. 2011). With no evidence supporting that BASL and POLAR directly interact, POLAR appears to regulate ACDs by recruiting BIN2 and other GSK3-like kinases to the polarity crescent, where BIN2 suppresses the YDA MAPK module, so that stomatal ACD can be enabled (Houbaert et al. 2018). Thus, the BASL-centered polarity complex, by recruiting POLAR and BIN2, may help to promote the division potential before an ACD and, by elevating the YDA MAPK signaling, may specify the SLGC to become a non-stomatal pavement cell after an ACD (Figure 2B). Interestingly, all of the polarity proteins identified in the stomatal lineage cells so far are associated with the PM, therefore their polarization was hypothesized to rely on protein-protein and/or protein-lipid interactions.
Figure 2. Cell polarity drives asymmetric cell division during stomatal formation in Arabidopsis cotyledon.

(A) Confocal image to show GFP-BASL expression in the Arabidopsis cotyledon. MMC: meristemoid mother cell; M: meristemoid; CG: Guard cell; SLGC: Stomatal lineage ground cell; Green indicates GFP-BASL and magenta marks cell outines. Yellow arrowheads indicate the sites of GFP-BASL polarization. (B) Molecular components and genetic interactions of the BASL/POLAR polarity module in the regulation of stomatal asymmetric cell division.
In monocots, a stomatal complex consists of four cells including two GCs and two subsidiary cells. The formation of such a stomatal complex involves a series of coordinated ACD. A stomatal precursor cell undergoes an ACD to generate a GMC, which may release unknown signals to induce the neighboring cells in contact to become the subsidiary mother cells (SMCs) (Facette and Smith 2012). The SMCs subsequently divide asymmetrically to generate subsidiary cells flanking the GMC, and the GMC then divides longitudinally to form a pair of guard cells. Much knowledge has been gained through the studies of the SMC asymmetric divisions in Zea mays (Figure 3) (Facette and Smith 2012; Pillitteri and Torii 2012). Genetic screens identified Pangloss1 (PAN1) and PAN2, the two genes encoding leucine-rich repeat receptor-like proteins (LRR-RLKs) that function cooperatively to polarize the SMC divisions (Cartwright et al. 2009; Sutimantanapi et al. 2014). Mutations of either gene resulted in similar phenotypes of defective SMC divisions and abnormal subsidiary cells. The PAN1 and PAN2 proteins co-polarize in the premitotic SMCs at the site of GMC contact to promote the formation of actin patches, which seemed to drive directional migration of the SMC nuclei (Sutimantanapi et al. 2014). PAN1 interacts with the type I Rho of Plants (ROP) GTPases to promote SMC polarization (Humphries et al. 2011). Downstream of the ROP signaling, the Brk1 gene encodes a small subunit of the SCAR/WAVE regulatory complex (WRC) that may activate the ARP2/3-mediated actin nucleation (Facette et al. 2015). But interestingly, the polarization of SCAR/WAVE appears prior to that of PANs and ROPs, suggesting asymmetric organization of the actin network might occurs early (Facette et al. 2015). Thus, the crosstalk between PAN1/2 and the actin cytoskeleton establishes the SMC division to take place asymmetrically, but the detailed mechanism needs to further investigated (Facette et al. 2015).
Figure 3. The PAN1/2 polarity module in the maize SMC asymmetric cell division.

In maize, before the subsidiary mother cell (SMC) asymmetric division, the SCAR/WAVE regulatory complex first polarizes to the membrane adjacent to the GMC contact sites, where PAN2 polarizes subsequently. After PAN2 polarization, PAN1 and ROP proteins are polarized, followed by the formation of an actin patch and the directional migration of the pre-mitotic SMC nucleus. Finally, the SMC undergoes asymmetric cell division. Purple lines indicates the site of SCAR/WAVE complex; green lines indicate where PAN1 and PAN2 proteins are polarized. The Orange lines indicate the locally enriched ROP6 molecules.
The establishment of cell fate asymmetry in the SMC ACD in monocots was found to be regulated by mobile transcription factors, e.g. the bHLH MUTE in Brachypodium (Raissig et al. 2017). Interestingly, ZmMUTE is also mobile in maize, with the initial expression in the GMCs, and its function is required for both SMC polarization, differentiation and GMC divisions (Wang et al. 2019).
Rop GTPases are master regulators of cell polarity in plants
The Rho family of small GTPases are conserved master regulators in the establishment of cell polarity in all eukaryotic organisms. In plants, there is a single Rho-GTPase subfamily called Rho-like GTPases from plants (ROPs) (Craddock et al. 2012; Yang and Fu 2007) and their functions have been tightly associated with the cytoskeleton and vesicular trafficking (see reviews in Ying Gu 2003; Craddock et al. 2012). Plants have also evolved specific regulators, including ROP-Guanine Exchange Factors (GEFs) and the Rop-interactive CRIB motif-containing protein (RIC) effectors (Craddock et al. 2012). ROPs cycle between membrane-bound and cytosolic locations and are only active when associated with membranes via lipid modifications (Yalovsky 2015).
Arabidopsis genome encodes 11 ROPs. Members of the ROP subfamily have diverged to regulate distinct cellular functions (Vernoud, 2003 #133). ROP1–6 retain the conserved Rho-GTPase function of spatially controlling a variety of cellular processes by regulating the cytoskeleton and vesicular trafficking. ROP1, ROP3, and ROP5 may act redundantly in the modulation of tip growth in Arabidopsis pollen tubes (Li et al. 2001; Gu et al. 2006), whereas ROP2, ROP4, and ROP6 regulate polar cell growth and cell polarity in vegetative cells (Fu et al. 2005; Craddock et al. 2012).
NIP5;1 and BOR1 regulates boron homeostasis in plants
Boron is an essential element for plants but is toxic in excess. Therefore, plants must adapt to both limiting and excess boron conditions for normal growth (Yoshinari and Takano 2017). Under boron-limiting conditions, plants use boric acid channels of the major intrinsic protein (MIP) family and the BOR family of borate exporters for uptake and translocation of boron to support growth of various plant species (Takano et al. 2008). In Arabidopsis, NIP5;1 and BOR1 are located in the plasma membrane and polarized toward soil and stele, respectively, in various root cells for efficient transport of boron from the soil to the stele (Wang et al. 2017a; Yoshinari and Takano 2017) (Figure 4A).
Figure 4. Polarized proteins driving directional nutrient uptake and Casparian strip formation, respectively, in the root.

(A) Nutrient uptake (Boron) in the Arabidopsis root is driven by polarized transmembrane transporters, BOR1 and NIP5;1. Blue lines indicate Casparian strip. (B) The working model for key regulators in the generation of Casparian strip in the Arabidopsis root. CASP1 localizes at the plasma membrane precisely coincided with the Casparian strip. MYB36 directly and positively regulates the expression of the other Casparian strip genes CASP1, PER64, and ESB1. EXO70A1 affects the local enrichment of CASP1. The receptor-like kinase (SGN3/GASSHO1) receives the signals from the Casparian strip integrity factor 1 (CIF1) and CIF2 for the Casparian strip formation.
Casparian strip polarized proteins
Casparian strip plays a critical role in sealing endodermal cells in the root to block uncontrolled extracellular uptake of nutrients and water (Doblas et al. 2017). In Arabidopsis, there are five Casparian strip membrane domain proteins 1 to 5 (CASP1–5) (Roppolo et al. 2011). The CASP1–GFP signal localizes at the plasma membrane precisely coincided with the Casparian strip itself. EXO70A1 plays a central role in the spatial organization of Casparian strip because exo70a1 mutations specifically affected the localization, but not the secretion of CASP1-GFP (Kalmbach et al. 2017). The receptor-like kinase SGN3/GASSHO1 receives the signals from the Casparian strip integrity factor 1 (CIF1) and CIF2 to regulate the Casparian strip formation (Figure 4B) (Nakayama et al. 2017).
MOLECULAR MECHANISMS UNDERLYING PROTEIN POLARIZATION AT THE PM
The organization of the plasma membrane is highly complex and ever-changing. Polarized proteins residing at or in the PM of the cell, instead of statically staying, are often tightly regulated by dynamic membrane activities, including endocytosis, exocytosis, endocytic recycling that send the internalized proteins back to the PM, and/or vacuolar delivery for protein degradation (Figure 5). Endocytosis and endosomal trafficking are essential processes that control the dynamic turnover of plasma membrane proteins, which include transporters, cell surface receptors and cell wall regulators, etc. The PM proteins enter into the cell though endocytosis that is mediated mainly by clathrin-dependent and clathrin-independent mechanisms (Fan et al. 2015). Endocytic recycling events rely on small GTPases-dependent and retromer-dependent pathways (Paez Valencia et al. 2016), by which cellular polarization, hormone and metal ion transport, and specialized developmental programs can be regulated.
Figure 5. Membrane trafficking in the regulation of protein polarization.

The plasma membrane (PM) proteins are internalized by the clathrine-mediated endocytosis (CME). Once endocytosed, they travel to the trans-Golgi-Network (TGN)/early endosomes (EE) (1) where they can be directed to the vacuole for degradation (2 and 4) or from TGN recycled back to the plasma membrane (3).
The necessity of endocytosis for protein polarization
As in animal cells, the clathhrin-mediated endocytosis (CME) is the main entry point for extracellular materials and the PM proteins in plant cells (Paez Valencia et al. 2016). Also, the CME has a great influence on the polarization of PM proteins in plants that can be well exemplified by how the PIN proteins are polarized. The AP-2 (ADAPTOR PROTEIN2) complex is implicated in the plant CME process (McMahon and Boucrot 2011). In plants, when endocytosis is blocked by chemical inhibitors, the polarized PIN proteins were found to spread laterally (Kleine-Vehn et al. 2008). The laterally diffused PIN proteins outside of the polar domains can be recycled by the CME internalization followed by directional transcytosis for the recovery (Kleine-Vehn et al. 2008). Indeed, the polar PIN1-GFP localization during embryogenesis or the PIN2-GFP localization in the male reproductive organ development can be disrupted because of the impaired endocytosis caused by mutations in the AP-2 subunits, such as the σ adaptin (ap2σ) or the μ adaptin (ap2m) (Fan et al. 2013; Kim et al. 2013). In addition, both boron (B) transporters NIP5;1 and BOR1 require the AP2-controlled CME pathway to maintain their polarization at the PM (Wang et al. 2017a; Yoshinari et al. 2016). Interestingly, the BOR1 protein associates with the AP2 complex and under the low-B conditions, the AP2-dependent CME maintains the polar localization of BOR1 to support plant growth and face the challenge of boron deficiency (Yoshinari et al. 2019).
The SNX and VPS29 retromer complexes regulate cell polarity
Not all endocytosed membrane proteins are degraded in the vacuole. Some proteins escape vacuolar degradation from the endosomes by returning to the PM via the recycling pathways. The retromer complex with the core subunits Vps35, Vps29, and Vps26 and SORTING NEXINS (SNXs) plays central roles in this process (Paez Valencia et al. 2016). In Arabidopsis, AtSNX1 and VPS29 were found essential for this recycling pathway. AtSNX1 defines a particular endosomal population, distinct from many other endosomal compartments, and PIN2 was found to accumulate to the AtSNX1 endosomal compartments under certain conditions (Jaillais et al. 2006). The functions of the retromer protein VPS29 was also linked to the regulation of cell polarity. The Arabidopsis mutant vps29 displayed severe developmental defects in the formation of new axes in embryogenesis (Jaillais et al. 2007). Furthermore, VPS29 associates with the SNX1 endosomes and functions in the regulation of endosome homeostasis, PIN function in polar auxin transport and specifically required for PIN1 and PIN2 trafficking (Jaillais et al. 2007).
GNOM-mediated endocytic recycling
The GNOM/EMB30 gene encodes a guanine nucleotide exchange factor (GEF) that activates the ADP-ribosylation factors (ARF) and is sensitive to Brefeldin A (BFA) (Niko Geldner et al. 2003). GNOM mediates auxin transport in both embryogenesis and post-embryonic organ development (Geldner et al. 2004). The loss-of-function gnom mutants bear aberrant cell shape and mis-orientation cell division planes (Shevell et al. 1994). The well-established role for the ARF GEF GNOM is to regulate the polar localization of PIN1 (Thomas Steinmann et al. 1999), because randomized PIN1 polarity in gnom mutant embryos was found (Geldner et al. 2004). Knocking out multiple PIN genes led to the phenotypes that very much resembled those of gnom mutants (Friml et al. 2003). GNOM was subcellularly localized to the Golgi apparatus and, upon the BFA disturbance, it translocated to the abnormally aggregated endosomal compartments (Naramoto et al. 2014). Considering that GNOM functions as an ARF GEF, some ARF GTPases are the possible candidates participating in this process. Indeed, in Arabidopsis, ARF1 was found to localize to the Golgi apparatus and endocytic organelles and, when ARF1 is absent, the polar localization of PIN2 was affected (Xu and Scheres 2005). A mechanistic understanding about how GNOM and ARF function at the Golgi to control the PIN proteins to polarize at the plasma membrane is still lacking that should be further pursued in future studies.
The RAB GEF TRAPPII at the trans-Golgi network (TGN)
The TGN in plant cells is seen as an independent organelle that rapidly associates and disassociates with the Golgi bodies. The TGN provides a protein sorting station where it functions as early endosomes (EE) to receive endocytosed materials from the PM that will be either transported to the vacuole or recycle back to the PM (Paez Valencia et al. 2016). Recently, the TGN has been identified as a key organelle for protein polar transportation to the PM in plants (Viotti et al. 2010). Around the TGN, the highly conserved TRAPPII complex, a tethering factor for vesicle trafficking, acts as a GEF for Rab11 (homolog of yeast Ypt31/32) in early Golgi trafficking (Thomas and Fromme 2016). In Arabidopsis, TRAPPII was localized to the TGN and functionally linked to RabA/Rab11 to regulate polar targeting of both the auxin efflux carrier PIN2 and the auxin influx carrier AUX1 in root tip cells (Qi et al. 2011).
Small GTPases are a group of hydrolase enzymes implicated in a broad range of cellular signaling events, including protein and cell polarization in plants (Kania et al. 2014). To establish cell polarity, the small GTPases, such as RAB and ARF, may regulate vesicular trafficking between intracellular compartments by recruiting coat protein complexes to the vesicle formation sites, organizing the cytoskeleton and docking vesicles to the destination membranes (Nielsen et al. 2008). The conserved Rab GTPases organize intracellular membrane trafficking at three consecutive stages of vesicular transport: vesicle formation, vesicle motility, followed by the tethering of vesicles to target compartments preceding the membrane-fusion events (Molendijk et al. 2004). Different isoforms of Rab GTPases assemble specific Rab domains on organelle membranes to define the identity of the membrane compartments (Zerial and McBride 2001). The plant Rab family consists of eight subfamilies, designated AtRabA-H with six of them present in yeast (Rutherford and Moore 2002). The RabA branch contains almost half of the total Rab GTPases in Arabidopsis and the closest mammalian homologues are localized to apical recycling endosomes to assist in polarizing epithelial cells (Rutherford and Moore 2002). Consistently, the RabA2 subgroup was found to be associated with the recycling endosomes that regulate polarized PIN proteins at the PM (Rutherford and Moore 2002). Additionally, AtRabA4b was found to label a TGN compartment that is polarly distributed in the growing root hairs, where RabA4b promotes polarized secretion of cell wall components to support tip growth (Preuss et al. 2006).
Secretion and exocytosis for PM proteins to polarize
In exocytosis, the PM proteins and membrane-bound secretory vesicles are delivered to the cell membrane or the extracellular matrix through membrane fusion. The delivery of either newly synthesized or recycled materials back to the PM is connected to the maintenance of protein homeostasis at the PM. In this process, the exocyst, an octameric protein complex, mediates vesicle trafficking by tethering and spatially targeting of post Golgi vesicles to the PM. Each exocyst complex contains eight subunits that are conserved in yeast and mammals and functions in targeted vesicular trafficking for the establishment of cellular polarity (Polgar and Fogelgren 2018). All of the eight subunits were found in the Arabidopsis genome and some of them were found to contribute to protein polarization. The EXO84B subunit regulates the secretion of polarized NIP5;1 (Xu et al. 2014; Mao et al. 2016). Furthermore, the EXO70 family (23 members in Arabidopsis) is important for polar growth and plant development (Synek et al. 2006). EXO70A1 was shown to regulate the recycling of internalized PIN1 and PIN2 to polarize at the PM (Drdova et al. 2013). In addition, EXO70A1 generates transient positional information to polarize the key factor CASP1 and plays a central role in the formation of Casparian strip (Kalmbach et al. 2017).
The cytoskeleton networks
In plants, the cytoskeleton is the intracellular scaffolding on which polarity can be framed (Wasteneys 2000). Trafficking of vesicles and their polar deposition to the PM takes place along the cytoskeleton. Treatment of actin and microtubules with depolymerizing chemicals revealed that the cytoskeleton targets polarly localized proteins, such as PIN proteins (Kleine-Vehn et al. 2008). The MT associated protein CLASP interacts with SNX1 to retrieve PIN2 from the vacuolar degradation to the recycling pathway in Arabidopsis (Ambrose et al. 2013). However, recent results also showed that the polarized PIN2 distribution is likely independent of cytoskeleton-guided endomembrane trafficking (Glanc et al. 2019).
The polarization of ROPs was evident in a number of cell systems, e.g. the pollen tube, root hair and pavement cells (Craddock et al. 2012). The ROP effectors and feedback functioning in actin and microtubule dynamics have been extensively studied and reviewed (Gu et al. 2004; Yang 2008; Craddock et al. 2012; Oda and Fukuda 2013), therefore are not further discussed here. Recent progress also suggested that ROP GEF, the DOCK family of SPIKE1, has sophisticated feedback regulations with both actin and microtubule networks to enable polarized growth in the trichome (Yanagisawa et al. 2018).
Polar positioning of the proteo-lipid membrane
In yeast and animal cells, the sterol composition regulates the asymmetric localization of some PM proteins (Makushok et al. 2016). In plants, the sterol-rich, detergent-resistant membrane domains, also called “lipid rafts”, were found to provide mechanisms for protein localization and polarized function (Fischer et al. 2004). Previous studies showed that the mutants displaying cell and tissue polarity have defects in sterol composition, glycosylphosphatidylinositol-anchored proteins, glycosylphosphatidylinositol biosynthesis and phospholipid signaling (Fischer et al. 2004). Correct sterol composition is required for both auxin transport polarity at the organ level and polar positioning of the efflux carriers at the subcellular level (Willemsen et al. 2003).
Lipid rafts have long been proposed to functionally segregate proteins and lipids within different local membrane compartments, thereby regulating their interactions (Simons and Toomre 2000; Lingwood and Simons 2010). Lipid rafts may provide dynamic scaffolding for a variety of cellular processes, including cell signaling, stress responses, and membrane trafficking. In 2000, the first plant “lipid rafts” was isolated from tobacco leaf cells (Peskan et al. 2000). Recent studies showed that sterols and sphingolipids in lipid rafts function to regulate nanoclustering of small GTPases, such as Ras, Cdc42 and ROP6 (Platre et al. 2019; Xue Pan 2019). It was shown that the levels of the phospholipid phosphatidylserine modulate the number of ROP6 nanoclusters, so that ROP6 can be immobilized in the membranes (Platre et al. 2019). The regulation of this process was also linked to auxin signaling in root epidermal cells (Platre et al. 2019). Consistently, in leaf epidermal cells, auxin triggers the TMK1 receptor protein to form nanoclusters with sterols at the plasma membrane, which in turn promotes its downstream effector ROP6 (Xu et al. 2014) to cluster together and be activated (Figure 6). The activation of ROP6 signaling in the lipid rafts cross talks with the cortical microtubule, so that the protein nanoclusters are stabilized (Xue Pan 2019).
Figures 6. Working model for auxin-mediated multi-polarity establishment.

Under normal conditions, the TMK receptor and sterols are randomly distributed in the PM. The TMK proteins are first induced by auxin to form large nanoclusters. The enlarged nanoclusters lead to the formation of sterol-rich ordered lipid nanodomains that promote the formation and activation of ROP6 nanoclusters. Active ROP6 interacts with downstream effectors and promotes the cortical microtubule (CMT) ordering, which in turn promotes larger TMK1 nanoclusters and lipid nanodomains. The positive feedback loop between lipid nanodomain-ROP6 signaling and CMT ordering eventually leads to the establishment cell polarity.
Besides sterol, phospholipid signaling also has a role in regulating plant cell polarity. Phospholipids may contribute to the establishment and maintenance of cell polarity by providing either local docking places or substrates for the local generation of secondary signaling molecules (Fischer et al. 2004). The Arabidopsis thaliana serine/threonine kinase D6 PROTEIN KINASE (D6PK) colocalizes with PINs and activates PIN-mediated auxin efflux in regulating plant development (Zourelidou et al. 2009; Willige et al. 2013; Barbosa et al. 2014). The association of D6PK with PINs is dependent on the phospholipid composition of the plasma membrane, as well as the phosphatidylinositol phosphate 5-kinases PIP5K1 and PIP5K2 in epidermal cells of the primary root (Stanislas et al. 2015). D6PK binds the poly-acidic phospholipids through a poly-basic lysine-rich motif that is required for proper PIN3 phosphorylation (Barbosa et al. 2016).
Protein phosphorylation
Protein phosphorylation catalyzed by kinase enzymes is a post-translational modification that occurs on serine, threonine or tyrosine residues. The reverse process of phosphate groups removal is mediated by protein phosphatases. In addition to many other regulations, the phosphorylation status of proteins also serves as an intrinsic cue for protein polar delivery, including both membrane-embedded and membrane-associated proteins in plants.
The PIN protein polarization is regulated by the PINOID (PID) kinase in Arabidopsis (Friml et al. 2004; Huang et al. 2010). An antagonistic function of the serine/threonine PID kinase and protein phosphatase 2A (PP2A) in the polar PIN trafficking was demonstrated by a genetic study of pp2a and pid mutants in embryo and root development (Michniewicz et al. 2007). An evolutionarily conserved phosphorylation site within the central hydrophilic loop of PIN proteins is important for the apical and basal polar PIN localizations, consequently, the redirection of auxin fluxes between the cells (Dhonukshe et al. 2010; Huang et al. 2010; Zhang et al. 2010). In addition, the polar localization of the transmembrane transporter NIP1;5 is also dependent on phosphorylation in the TPG repeat that is necessary for the efficient transport of boron in roots (Wang et al. 2017a).
Protein phosphorylation is also influential for peripheral membrane proteins to polarize. One prominent example is that the MAPK scaffold protein BASL needs to be phosphorylated to translocate from the nucleus to the PM polarity site (Zhang et al. 2015). On the other hand, the BIN2 kinase also phosphorylates BASL and POLAR to regulate their subcellular localization (Houbaert et al. 2018), but the molecular machinery and mechanisms by which BASL and POLAR are polarized remain unknown.
Others
Plant cells have a complex extracellular matrix, the cell wall, which seems to provide a plant-specific mechanism for cell polarity maintenance (Feraru et al. 2011). The PIN polarization in plant cells is maintained by the connections between the polar domains at the PM and the cell wall (Feraru et al. 2011).
The plant hormones auxin and cytokinin mutually coordinate their activities to control various aspects of development (Vanstraelen and Benkova 2012). Cytokinin modulates endocytic trafficking of PIN1 to control plant organogenesis (Marhavy et al. 2011). Specifically, cytokinin enhances the PIN1 depletion at specific polar domains, thus rearranging the cellular PIN polarities directly regulating the auxin flow direction. Cytokinin signaling also regulates pavement cell morphogenesis in Arabidopsis, likely acting through the ROP signaling (Li et al. 2013).
Calcium, a second messenger, is an organizer of cell polarity in plants. Calcium signaling plays multiple roles by 1) providing a hallmark for cell polarity; 2) coordinating differential membrane trafficking; 3) controlling cytoskeleton dynamics; 4) interconnecting with ROP polarization; 5) integrating mechanical polarity signals (see review in Himschoot et al. 2015). It has been well known that the tip-focused [Ca2+] gradient is an important factor for localizing growth of the elongating root hair and pollen tube to the apex (Bibikova et al. 1997). More recently, it was found that calcium signals are necessary to establish auxin transporter polarity in plant stem cell niche (Li et al. 2019).
CONCLUSION
During the past years, research has been mainly focused on revealing the regulatory mechanisms for the transmembrane proteins to be polarized, such as the PIN auxin effluxers and the boron transporters. Despite the knowledge obtained to explain ROP polarization, there was almost nothing known about how membrane peripheral proteins are polarized in plant cells. Many outstanding questions remain in the field of plant cell polarity to address how protein polarization is initiated, maintained and regulated in plants. For example, what are the orientation cues for cell polarity or protein/lipid polarization in plant cells? With regards to PIN polarization, auxin itself with its local source directs its transportation and the orientation of developmental axis in Arabidopsis (Robert et al. 2013). The finding that auxin induces nanoclustering of the PM proteins was exciting (Xue Pan 2019), but the identity of the cell surface receptor remains to be established. With regards to the polarization of membrane associated proteins, such as D6PK, BASL and POLAR, the identification of their binding partners (proteins or lipids) will likely provide insights suggesting how they can be possibly delivered and maintained at the polarity site. Also, it was noted that the TGN as a membrane sorting platform in plant cells plays critical roles in the establishment of the PM polarity domains. Among the few key regulators discussed earlier for their roles in establishment PIN polarization, the ARF GEF GNOM appeared to control both auxin transport and auxin signaling to establish tissue polarity (Verna et al. 2019). How GNOM functions at the Golgi/TGN to instruct PM polarity is a major mystery. In the future, more studies are desired to identify new regulators, which are dependent- or independent-of the membrane systems, towards understanding the molecular mechanisms by which cell polarity is initiated and maintained in plant cells.
ACKNOWLEDGEMENT
This work was supported by grants from the National Natural Science Foundation of China program (31828006, 31871377, 30971652 and 31271463). Research program in J.D.’s group is supported by the National Institute of Health R01GM109080 and R35GM131827.
REFERENCES
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO (2013) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell 24: 649–659 [DOI] [PubMed] [Google Scholar]
- Barbosa IC, Shikata H, Zourelidou M, Heilmann M, Heilmann I, Schwechheimer C (2016) Phospholipid composition and a polybasic motif determine D6 PROTEIN KINASE polar association with the plasma membrane and tropic responses. Development 143: 4687–4700 [DOI] [PubMed] [Google Scholar]
- Barbosa ICR, Zourelidou M, Willige BC, Weller B, Schwechheimer C (2014) D6 PROTEIN KINASE activates auxin transport-dependent growth and PIN-FORMED phosphorylation at the plasma membrane. Dev Cell 29: 674–685 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Zhigilei A, Gilroy S (1997) Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203: 495–505 [DOI] [PubMed] [Google Scholar]
- Bringmann M, Bergmann DC (2017) Tissue-wide mechanical forces influence the polarity of stomatal stem cells in Arabidopsis. Curr Biol 27: 877–883 [DOI] [PubMed] [Google Scholar]
- Campanale JP, Sun TY, Montell DJ (2017) Development and dynamics of cell polarity at a glance. J Cell Sci 130: 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright HN, Humphries JA, Smith LG (2009) PAN1: A receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science 323: 649–651 [DOI] [PubMed] [Google Scholar]
- Chen X, Irani NG, Friml J (2011) Clathrin-mediated endocytosis: The gateway into plant cells. Curr Opin Plant Biol 14: 674–682 [DOI] [PubMed] [Google Scholar]
- Chiou JG, Balasubramanian MK, Lew DJ (2017) Cell polarity in yeast. Ann Rev Cell Dev Biol 33: 77–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C, Lavagi I, Yang Z (2012) New insights into Rho signaling from plant ROP/Rac GTPases. Trends in cell biology 22: 492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Beeckman T (2011) Asymmetric cell division in land plants and algae: The driving force for differentiation. Nat Rev Mol Cell Biol 12: 177–188 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Friml J (2011) Cell polarity in plants: When two do the same, it is not the same. Curr Opin Cell Biol 23: 686–696 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Huang F, Galvan-Ampudia CS, Mahonen AP, Kleine-Vehn J, Xu J, Quint A, Prasad K, Friml J, Scheres B, Offringa R (2010) Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137: 3245–3255 [DOI] [PubMed] [Google Scholar]
- Doblas VG, Geldner N, Barberon M (2017) The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39: 136–143 [DOI] [PubMed] [Google Scholar]
- Dong J, MacAlister CA, Bergmann DC (2009) BASL controls asymmetric cell division in Arabidopsis. Cell 137: 1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drdova EJ, Synek L, Pecenkova T, Hala M, Kulich I, Fowler JE, Murphy AS, Zarsky V (2013) The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J 73: 709–719 [DOI] [PubMed] [Google Scholar]
- Facette MR, Park Y, Sutimantanapi D, Luo A, Cartwright HN, Yang B, Bennett EJ, Sylvester AW, Smith LG (2015) The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat Plants 1: 14024. [DOI] [PubMed] [Google Scholar]
- Facette MR, Smith LG (2012) Division polarity in developing stomata. Curr Opin Plant Biol 15: 585–592 [DOI] [PubMed] [Google Scholar]
- Fan L, Hao H, Xue Y, Zhang L, Song K, Ding Z, Botella MA, Wang H, Lin J (2013) Dynamic analysis of Arabidopsis AP2 sigma subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development 140: 3826–3837 [DOI] [PubMed] [Google Scholar]
- Fan L, Li R, Pan J, Ding Z, Lin J (2015) Endocytosis and its regulation in plants. Trends Plant Sci 20: 388–397 [DOI] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Kleine-Vehn J, Martiniere A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J (2011) PIN polarity maintenance by the cell wall in Arabidopsis. Curr Biol 21: 338–343 [DOI] [PubMed] [Google Scholar]
- Fischer U, Men S, Grebe M (2004) Lipid function in plant cell polarity. Curr Opin Plant Biol 7: 670–676 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, Hooykaas PJ, Palme K, Offringa R (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Gallei M, Luschnig C, Friml J (2019) Auxin signalling in growth: Schrodinger’s cat out of the bag. Curr Opin Plant Biol 53: 43–49 [DOI] [PubMed] [Google Scholar]
- Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jurgens G (2004) Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400 [DOI] [PubMed] [Google Scholar]
- Glanc M, Fendrych M, Friml J (2019) PIN2 polarity establishment in Arabidopsis in the absence of an intact cytoskeleton. Biomolecules 9: doi: 10.3390/biom9060222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z (2006) Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 18: 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z (2004) ROP/RAC GTPase: An old new master regulator for plant signaling. Curr Opin Plant Biol 7: 527–536 [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo J, Li H, Yang Z (2013) Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol Plant 6: 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimil S, Dunand C (2007) Cell growth and differentiation in Arabidopsis epidermal cells. J Exp Bot 58: 3829–3840 [DOI] [PubMed] [Google Scholar]
- Guo X, Dong J (2019) To divide or differentiate: It Is about scaffolding. Trends Plant Sci 24: 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Rounds CM, Winship LJ (2013) Control of cell wall extensibility during pollen tube growth. Mol Plant 6: 998–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himschoot E, Beeckman T, Friml J, Vanneste S (2015) Calcium is an organizer of cell polarity in plants. Biochim Biophys Acta 1853: 2168–2172 [DOI] [PubMed] [Google Scholar]
- Houbaert A, Zhang C, Tiwari M, Wang K, de Marcos Serrano A, Savatin DV, Urs MJ, Zhiponova MK, Gudesblat GE, Vanhoutte I, Eeckhout D, Boeren S, Karimi M, Betti C, Jacobs T, Fenoll C, Mena M, de Vries S, De Jaeger G, Russinova E (2018) POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature 563: 574–578 [DOI] [PubMed] [Google Scholar]
- Huang F, Zago MK, Abas L, van Marion A, Galvan-Ampudia CS, Offringa R (2010) Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22: 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG (2011) ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 23: 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T (2006) AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miege C, Gaude T (2007) The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Jurgens G (2004) Membrane trafficking in plants. Ann Rev Cell Dev Biol 20: 481–504 [DOI] [PubMed] [Google Scholar]
- Kalmbach L, Hematy K, De Bellis D, Barberon M, Fujita S, Ursache R, Daraspe J, Geldner N (2017) Transient cell-specific EXO70A1 activity in the CASP domain and Casparian strip localization. Nat Plants 3: 17058. [DOI] [PubMed] [Google Scholar]
- Kania U, Fendrych M, Friml J (2014) Polar delivery in plants; commonalities and differences to animal epithelial cells. Open biology 4: 140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Pan W, Jones SA, Zhang Y, Zhuang X, Wu D (2013) Clathrin and AP2 are required for PtdIns(4,5)P2-mediated formation of LRP6 signalosomes. J Cell Biol 200: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J (2010) Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Prof Natl Acad Sci USA 107: 22344–22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Langowski L, Wisniewska J, Dhonukshe P, Brewer PB, Friml J (2008) Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol Plant 1: 1056–1066 [DOI] [PubMed] [Google Scholar]
- Langowski L, Wabnik K, Li H, Vanneste S, Naramoto S, Tanaka H, Friml J (2016) Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov 2: 16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Bergmann DC (2012) Stomatal development: A plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 139: 3683–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M, Ding ZJ, Friml J, Beeckman T, Sack F (2014) Auxin transport and activity regulate stomatal patterning and development. Nat Commun 5: 3090. [DOI] [PubMed] [Google Scholar]
- Leyser O (2018) Auxin Signaling. Plant Physiol 176: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z (2001) The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol 126: 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu T, Lin D, Wen M, Xie M, Duclercq J, Bielach A, Kim J, Reddy GV, Zuo J, Benkova E, Friml J, Guo H, Yang Z (2013) Cytokinin signaling regulates pavement cell morphogenesis in Arabidopsis. Cell Res 23: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yan A, Bhatia N, Altinok A, Afik E, Durand-Smet P, Tarr PT, Schroeder JI, Heisler MG, Meyerowitz EM (2019) Calcium signals are necessary to establish auxin transporter polarity in a plant stem cell niche. Nat Commun 10: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327: 46–50 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Vert G (2014) The dynamics of plant plasma membrane proteins: PINs and beyond. Development 141: 2924–2938 [DOI] [PubMed] [Google Scholar]
- Makushok T, Alves P, Huisman SM, Kijowski AR, Brunner D (2016) Sterol-Rich membrane domains define fission yeast cell polarity. Cell 165: 1182–1196 [DOI] [PubMed] [Google Scholar]
- Mangano S, Juarez SP, Estevez JM (2016) ROS regulation of polar growth in plant cells. Plant Physiol 171: 1593–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Nakamura M, Viotti C, Grebe M (2016) A framework for lateral membrane trafficking and polar tethering of the PEN3 ATP-Binding cassette transporter. Plant Physiol 172: 2245–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhava P, Bassukas AEL, Zourelidou M, Kolb M, Moret B, Fastner A, Schulze WX, Cattaneo P, Hammes UZ, Schwechheimer C, Hardtke CS (2018) A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature 558: 297–300 [DOI] [PubMed] [Google Scholar]
- Marhavy P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, Parezova M, Petrasek J, Friml J, Kleine-Vehn J, Benkova E (2011) Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell 21: 796–804 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533 [DOI] [PubMed] [Google Scholar]
- Mendrinna A, Persson S (2015) Root hair growth: It’s a one way street. F1000Prime Rep 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C, Offringa R, Friml J (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Ruperti B, Palme K (2004) Small GTPases in vesicle trafficking. Curr Opin Plant Biol 7: 694–700 [DOI] [PubMed] [Google Scholar]
- Muroyama A, Bergmann D (2019) Plant Cell Polarity: Creating Diversity from Inside the Box. Ann Rev Cell Dev Biol 35: 309–336 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa-Ohnishi M, Matsubayashi Y (2017) A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 355: 284–286 [DOI] [PubMed] [Google Scholar]
- Naramoto S, Otegui MS, Kutsuna N, de Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H, Nakano A, Friml J (2014) Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell 26: 3062–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Cheung AY, Ueda T (2008) The regulatory RAB and ARF GTPases for vesicular trafficking. Plant Physiol 147: 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niko Geldner NA, Hanno Wolters,, Jutta Keicher WK, Philippe Muller AD, Takashi Ueda AN, rgens aGJ (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 11. [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2013) Spatial organization of xylem cell walls by ROP GTPases and microtubule-associated proteins. Curr Opin Plant Biol 16: 743–748 [DOI] [PubMed] [Google Scholar]
- Paez Valencia J, Goodman K, Otegui MS (2016) Endocytosis and endosomal trafficking in plants. Annu Rev Plant Biol 67: 309–335 [DOI] [PubMed] [Google Scholar]
- Peskan T, Westermann M, Oelmuller R (2000) Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur J Biochem 267: 6989–6995 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU (2011) Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell 23: 3260–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Torii KU (2012) Mechanisms of stomatal development. Annu Rev Plant Biol 63: 591–614 [DOI] [PubMed] [Google Scholar]
- Platre MP, Bayle V, Armengot L, Bareille J, Marques-Bueno MDM, Creff A, Maneta-Peyret L, Fiche JB, Nollmann M, Miege C, Moreau P, Martiniere A, Jaillais Y (2019) Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 364: 57–62 [DOI] [PubMed] [Google Scholar]
- Polgar N, Fogelgren B (2018) Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb Perspect Biol 10: doi: 10.1101/cshperspect.a031401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E (2006) A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol 172: 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Greb T (2017) Cell polarity in plants: The Yin and Yang of cellular functions. Curr Opin Plant Biol 35: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Kaneda M, Chen J, Geitmann A, Zheng H (2011) A specific role for Arabidopsis TRAPPII in post-Golgi trafficking that is crucial for cytokinesis and cell polarity. Plant J 68: 234–248 [DOI] [PubMed] [Google Scholar]
- Qian P, Hou S, Guo G (2009) Molecular mechanisms controlling pavement cell shape in Arabidopsis leaves. Plant Cell Rep 28: 1147–1157 [DOI] [PubMed] [Google Scholar]
- Raissig MT, Matos JL, Anleu Gil MX, Kornfeld A, Bettadapur A, Abrash E, Allison HR, Badgley G, Vogel JP, Berry JA, Bergmann DC (2017) Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355: 1215–1218 [DOI] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J (2013) Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr Biol 23: 2506–2512 [DOI] [PubMed] [Google Scholar]
- Roppolo D, De Rybel B, Denervaud Tendon V, Pfister A, Alassimone J, Vermeer JE, Yamazaki M, Stierhof YD, Beeckman T, Geldner N (2011) A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383 [DOI] [PubMed] [Google Scholar]
- Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family: Another enigma variation. Curr Opin Plant Biol 5: 518–528 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Scacchi E, Osmont KS, Beuchat J, Salinas P, Navarrete-Gomez M, Trigueros M, Ferrandiz C, Hardtke CS (2009) Dynamic, auxin-responsive plasma membrane-to-nucleus movement of Arabidopsis BRX. Development 136: 2059–2067 [DOI] [PubMed] [Google Scholar]
- Shao W, Dong J (2016) Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation. Developmental biology 419: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell DE, Leu WM, Gillmor CS, Xia G, Feldmann KA, Chua NH (1994) EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77: 1051–1062 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Smit ME, Weijers D (2015) The role of auxin signaling in early embryo pattern formation. Curr Opin Plant Biol 28: 99–105 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galvan-Ampudia CS, Offringa R, Friml J, Yanofsky MF, Ostergaard L (2009) A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459: 583–586 [DOI] [PubMed] [Google Scholar]
- Stanislas T, Huser A, Barbosa ICR, Kiefer CS, Brackmann K, Pietra S, Gustavsson A, Zourelidou M, Schwechheimer C, Grebe M (2015) Arabidopsis D6PK is a lipid domain-dependent mediator of root epidermal planar polarity. Nat Plants 1: 15162. [DOI] [PubMed] [Google Scholar]
- Sutimantanapi D, Pater D, Smith LG (2014) Divergent roles for maize PAN1 and PAN2 receptor-like proteins in cytokinesis and cell morphogenesis. Plant Physiol 164: 1905–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Elias M, Quentin M, Hauser MT, Zarsky V (2006) AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J 48: 54–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Miwa K, Fujiwara T (2008) Boron transport mechanisms: Collaboration of channels and transporters. Trends Plant Sci 13: 451–457 [DOI] [PubMed] [Google Scholar]
- Thomas LL, Fromme JC (2016) GTPase cross talk regulates TRAPPII activation of Rab11 homologues during vesicle biogenesis. J Cell Biol 215: 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 3. [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Benkova E (2012) Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 28: 463–487 [DOI] [PubMed] [Google Scholar]
- Verna C, Ravichandran SJ, Sawchuk MG, Linh NM, Scarpella E (2019) Coordination of tissue cell polarity by auxin transport and signaling. Elife 8: doi: 10.7554/eLife.51061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12: 160–168 [DOI] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, Jurgens G, de Vries SC, Robinson DG, Schumacher K (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo S, Qiao X, Guo J, Li Z, Zhou Y, Bai S, Gao Z, Wang D, Wang P, Galbraith DW, Song CP (2019) BZU2/ZmMUTE controls symmetrical division of guard mother cell and specifies neighbor cell fate in maize. PLoS Genet 15: e1008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yoshinari A, Shimada T, Hara-Nishimura I, Mitani-Ueno N, Feng Ma J, Naito S, Takano J (2017a) Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates boron transport in Arabidopsis roots. Plant Cell 29: 824–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chung KP, Lin W, Jiang L (2017b) Protein secretion in plants: Conventional and unconventional pathways and new techniques. J Exp Bot 69: 21–37 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO (2000) The cytoskeleton and growth polarity. Curr Opin Plant Biol 3: 503–511 [DOI] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15: 612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ahlers S, Zourelidou M, Barbosa IC, Demarsy E, Trevisan M, Davis PA, Roelfsema MR, Hangarter R, Fankhauser C, Schwechheimer C (2013) D6PK AGCVIII kinases are required for auxin transport and phototropic hypocotyl bending in Arabidopsis. Plant Cell 25: 1674–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusova H, Wang W, Jones AM, Friml J, Patterson SE, Bleecker AB, Yang Z (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Pan LF, Jianfeng Liu, Betul Senay-Aras, Wenwei Lin, Shuan Zheng, Tong Zhang, Uri Manor, Weitao Chen, Zhenbiao Yang (2019) Auxin-induced nanoclustering of membrane signaling complexes underlies cell polarity establishment in Arabidopsis. BioRxiv: [Google Scholar]
- Yalovsky S (2015) Protein lipid modifications and the regulation of ROP GTPase function. J Exp Bot 66: 1617–1624 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Alonso JM, Szymanski DB (2018) Microtubule-dependent confinement of a cell signaling and actin polymerization control module regulates polarized cell growth. Curr Biol 28: 2459–2466 [DOI] [PubMed] [Google Scholar]
- Yang Z (2008) Cell polarity signaling in Arabidopsis. Ann Rev Cell Dev Biol 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Fu Y (2007) ROP/RAC GTPase signaling. Curr Opin Plant Biol 10: 490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Gu VV, Ying Fu and Zhenbiao Yang (2003) ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot 54: 8. [DOI] [PubMed] [Google Scholar]
- Yoshinari A, Fujimoto M, Ueda T, Inada N, Naito S, Takano J (2016) DRP1-dependent endocytosis is essential for polar localization and boron-induced degradation of the borate transporter BOR1 in Arabidopsis thaliana. Plant Cell Physiol 57: 1985–2000 [DOI] [PubMed] [Google Scholar]
- Yoshinari A, Hosokawa T, Amano T, Beier MP, Kunieda T, Shimada T, Hara-Nishimura I, Naito S, Takano J (2019) Polar localization of the borate exporter BOR1 requires AP2-dependent endocytosis. Plant Physiol 179: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari A, Takano J (2017) Insights into the mechanisms underlying boron homeostasis in plants. Front Plant Sci 8: 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Bio 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nodzynski T, Pencik A, Rolcik J, Friml J (2010) PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci USA 107: 918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xing J, Lin J (2019) At the intersection of exocytosis and endocytosis in plants. New Phytol 224: 1479–1489 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong J (2018) Cell polarity: Compassing cell division and differentiation in plants. Curr Opin Plant Biol 45: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo X, Dong J (2016) Phosphorylation of the polarity protein BASL differentiates asymmetric cell fate through MAPKs and SPCH. Curr Biol 26: 2957–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang P, Shao W, Zhu JK, Dong J (2015) The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev Cell 33: 136–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M, Muller I, Willige BC, Nill C, Jikumaru Y, Li H, Schwechheimer C (2009) The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development 136: 627–636 [DOI] [PubMed] [Google Scholar]


