Abstract
Recent studies show that neutrophils mediate both tissue damage and host protection in response to multicellular parasites. In this issue of Cell Host & Microbe, Bouchery et al. demonstrate the importance of neutrophil extracellular traps in helminth damage after primary infections.
Pick up a recent Immunology textbook and more often than not, myeloid cells are distinctly separated according to the particular infectious or stimulating agent; within these groupings, eosinophils, basophils, and mast cells are linked to type 2 responses to allergens or parasitic worms (helminths) while macrophages and neutrophils are linked to type 1 responses triggered by microbial infections, including viruses and bacteria. Increasingly, it is clear that, indeed, myeloid cells show considerable heterogeneity and distinct functions in response to a broad range of infecting pathogens, including helminths. These large multicellular parasites stimulate alternatively activated (M2) macrophages, which can promote tissue repair in response to the considerable damage these multicellular helminths may cause as they traffick through vital organs. Furthermore, recent studies indicate that helminth-primed M2 macrophages can also mediate acquired resistance to helminths in the skin, lung, and intestine, often through arginase-1-dependent mechanisms, and can directly kill parasites in vitro (Chen et al., 2014; Yap and Gause, 2018). It should also be noted that M2 macrophages themselves show heterogeneity exhibiting varying phenotypes and requirements for differentiation depending on the particular pathogen or other activating insult and the specific tissue microenvironment in which they are stimulated. It now appears that neutrophils are also alternatively activated (N2) during helminth infection, expressing many genes associated with type 2 immunity and in some cases also expressed by M2 macrophages (Chen et al., 2014). Shortly after helminth invasion of the lung, neutrophils are rapidly recruited to this tissue and, when there, contribute to lung tissue damage and hemorrhaging (Chen et al., 2012). However, shortly thereafter, these N2 neutrophils can provide essential help to macrophages promoting their alternative activation state (Bosurgi et al., 2017; Chen et al., 2014). It remains unclear whether distinct subsets of neutrophils contribute to tissue damage and macrophage help.
During a type 1 response to microbial pathogens, neutrophils play a crucial role through phagocytosis and intracellular killing of pathogens. In this context, neutrophils also release cytokines or other factors into their surroundings that attract and activate other innate and adaptive immune cells that can help orchestrate effective immunity. In addition, neutrophils may release a chromatin mesh filled with proteinases and peptidases, termed neutrophil extracellular traps (NETs), to trap and kill extracellular bacteria, viruses, and fungi (Kolaczkowska and Kubes, 2013). These NETs appear to provide an important defense against invading pathogens that may be too large to easily phagocytose. In contrast, helminth infections induce a potent type 2 immune response, which is initiated by the release of thymic stromal lymphopoietin (TSLP), inter-leukin 25 (IL-25), and IL-33 by epithelial and myeloid cells. These cytokine alar-mins activate innate myeloid and lymphoid cells, which can together secrete type 2 cytokines including IL-4, IL-5, and IL-13, resulting in profound changes in host physiology and immunity such as goblet cells hyperplasia and mucus accumulation, increased epithelial cell turnover and smooth muscle hyper contractility, and activation of dendritic cells and T and B cells. These innate and adaptive immune components together orchestrate mitigation of tissue damage and host resistance during helminth infection. The helminths also produce excretory and secretory (ES) products that have previously been shown to include a number of distinct molecules that can specifically modulate the type 2 immune response (Maizels et al., 2018). Overall, the actual mechanisms contributing to host protection against helminth remain little understood. As these parasites are a major cause of morbidity in developing countries and as there are currently no successful vaccines, elucidating immune mechanisms of host protection is of considerable significance.
A widely used experimental model for studying type 2 immune responses elicited by helminth involves infection of mice with the rodent intestinal nematode parasite, Nippostrongylus brasiliensis (Nb). This helminth has a similar life cycle to hookworms, invading the skin and passing through the lungs on its way to the small intestine, where the intestinal nematodes breed and produce eggs, which are excreted in the feces. Duringprimary infection, adult worms are primarily expelled by “weep and sweep” mechanisms in the small intestine, including increased luminal fluids, mucous production, and muscle contractility. However, during secondary infection, the majority of the migrating larvae are trapped and damaged in the skin and in the lung, with previous studies showing that alternatively activated (M2) macrophages, eosinophils, and basophils play a significant role.
Neutrophils also now appear to be major players in the host response to Nb, actually serving multiple functions (see Figure 1). When the larvae first invade the lung, local TCRgd T cells are activated to produce IL-17, which then recruits neutrophils into the lung (Sutherland et al., 2014). These neutrophils contribute to acute lung injury, which is controlled and mitigated as the type 2 immune response kicks in, in part through the reduction in IL-17 elevations and associated neutrophil infiltration (Yap and Gause, 2018). However, these neutrophils also swarm the invading larvae in the lung shortly after primary infection, and their depletion not only reduces tissue damage but also results in more parasites making it to the small intestine (Sutherland et al., 2014), indicating a role for neutrophils in controlling the parasite burden at this early stage of the primary response. Neutrophils also differentiate in the context of the developing type 2 response upregulating expression of various type 2 related genes, including IL-13 IL-33, Retnla, and Chi3l3. At these early stages of the response, neutrophils play an essential role in driving activation of M2 macrophages, which then contribute to both tissue repair and acquired resistance (Bosurgi et al., 2017; Chen et al., 2014).
Figure 1. The Role of Neutrophils in Host Protective Responses to Helminths.
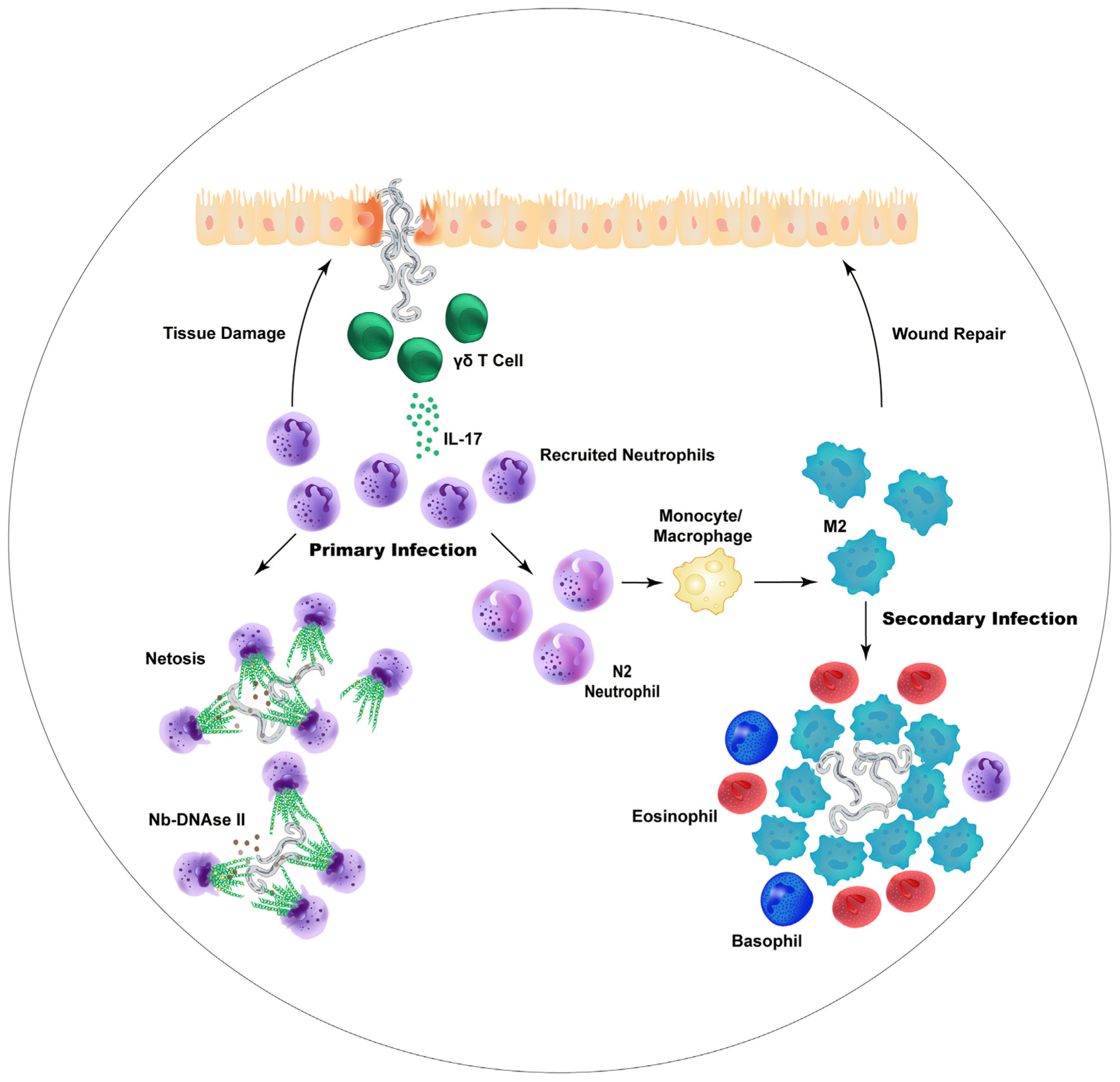
In a primary infection with Nippostrongylus brasiliensis (Nb), the infective third-stage larvae penetrating the skin induce the rapid recruitment of neutrophils, which deploy neutrophil extracellular traps (NETs) that are capable of damaging the larvae. The parasites mitigate the effects of the NETs by secreting Nb-deoxyribonuclease (Nb-DNase II) that can degrade the NET DNA backbone. In addition, larvae entering the lungs stimulate the production of IL-17 by local TCRgd T cells which triggers neutrophil recruitment, contributing to lung injury. However, as the type 2 response develops, neutrophils promote the maturation of macrophages into alternatively activated (M2) macrophages that mediate wound repair and can also kill the parasites. During a secondary infection, the neutrophil-trained M2 macrophages and eosinophils primarily surround the larvae, contributing to acquired resistance.
In the accompanying paper published in this issue, Bouchery et al. (2020) provide evidence for how these neutrophils may reduce the worm burden after a primary infection. In these studies, the authors examined the response in the skin just after penetration of the larvae. Within an hour after penetrating the skin, either by natural infection or intradermal injection, the larvae were surrounded by myeloid cells. Further analyses revealed that neutrophils were immediately surrounding the invading larvae, while monocytes were more peripheral, essentially surrounding the neutrophils. In parallel in vitro cultures, neutrophils also swarmed the larvae in a partly complement-dependent manner. Identifying what factors actually attract the neutrophils to the invading larvae is an area of significant interest. Depletion of neutrophils in vivo increased the number of gut larvae, consistent with previous studies (Sutherland et al., 2014), and in the skin the presence of neutrophils caused a delay in cuticle shedding (exsheathment). One possible mechanism by which these neutrophils might negatively impact these large multicellular parasites could be through production of NETs. In further in vitro studies, the authors showed that, indeed, NETs were forming but were rapidly degrading in the presence of live but not dead larvae. Nb ES (NES) products were then tested and shown to be capable of directly degrading NETs. Searching previously published databases, the authors discovered a DNase II motif that they named NB-DNase II, which was highly expressed in invading larvae but not adults. Recombinant Nb-DNase II could rapidly degrade NETs, and a specific anti-serum could block NES-mediated NET degradation and also increases in vitro parasite killing in the presence of neutrophils. Intriguingly, the human hookworm, Necator ancylostoma, was also found to produce a DNase II capable of degrading NETs, indicating that secretion of this DNase II may be a conserved mechanism of evading neutrophil-derived NET killing.
Taken together, these studies demonstrate an important role for neutrophils, and more specifically NETosis, in host protective responses against helminths. They further provide evidence for a host-parasite co-evolutionary dynamic, where the helminth has developed an immuno-evasion mechanism limiting the deleterious effects of the neutrophil-derived NETs. Although previous studies have suggested that NETs may contribute to helminth killing through a more passive trapping mechanism (Bonne-Année et al., 2014), these studies indicate that, in vitro, the NETs are sufficient to directly kill helminths. Now, the question that remains is regarding how the NETs actually harm these large multi-cellular parasites. In future studies, it will be interesting to examine whether eosinophils, which, along with basophils, appears to be more important in secondary infections (Inclan-Rico and Siracusa, 2018), may also secrete eosinophil extracellular traps (EETs) that contribute to worm damage. In the lung, although the neutrophil is heavily recruited shortly after primary infection, it is scarcer after secondary infection, perhaps because of reduced lung damage resulting from rapid macrophage and eosinophil killing of the invading larvae. As such, the role of EETs in acquired resistance would be an intriguing area of further study.
ACKNOWLEDGMENTS
This work was supported by NIH R01 grants AI131634-01A1, DK113790, and AI131634 to W.C.G. and T32AI125185 to D.W.E.-N.
REFERENCES
- Bonne-Année S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, Lok JB, Nolan TJ, and Abraham D(2014). Extracellular traps are associatedwithhuman and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 16, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery T, Moyat M, Sotillo J, Silverstein S, Volpe B, Coakley G, Tsourouktsoglou TD, Becker L, Shah K, Kulagin M, et al. (2020). Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe 27, this issue, 277–289. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF Jr., Wynn TA, and Gause WC (2012). An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med 18, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, Boucher JL, Urban JF Jr., Kim CC, and Gause WC (2014). Neutrophils prime long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol 15, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inclan-Rico JM, and Siracusa MC (2018). First Responders: Innate Immunity to Helminths. Trends Parasitol. 34, 861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, and Kubes P (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 13, 159–175. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Smits HH, and McSorley HJ (2018). Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 49, 801–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger B, Maizels RM, and Allen JE (2014). Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol 15, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap GS, and Gause WC (2018). Helminth Infections Induce Tissue Tolerance Mitigating Immunopathology but Enhancing Microbial Pathogen Susceptibility. Front. Immunol 9, 2135. [DOI] [PMC free article] [PubMed] [Google Scholar]


