Abstract
Introduction
Parents (PP) of children in primary care clinics previously reported factors influencing their height-related medical decision-making. However, patients seeking height-related care in endocrine subspecialty clinics and their parents (EP) differ demographically from the general population.
Objective
To determine EP height-related medical concerns and expectations, and to compare between EP and PP.
Methods
EP completed a survey assessing their concerns in seeking medical care for their child’s height with identical questions previously asked of PP and two additional questions about growth hormone (GH) treatment.
Results
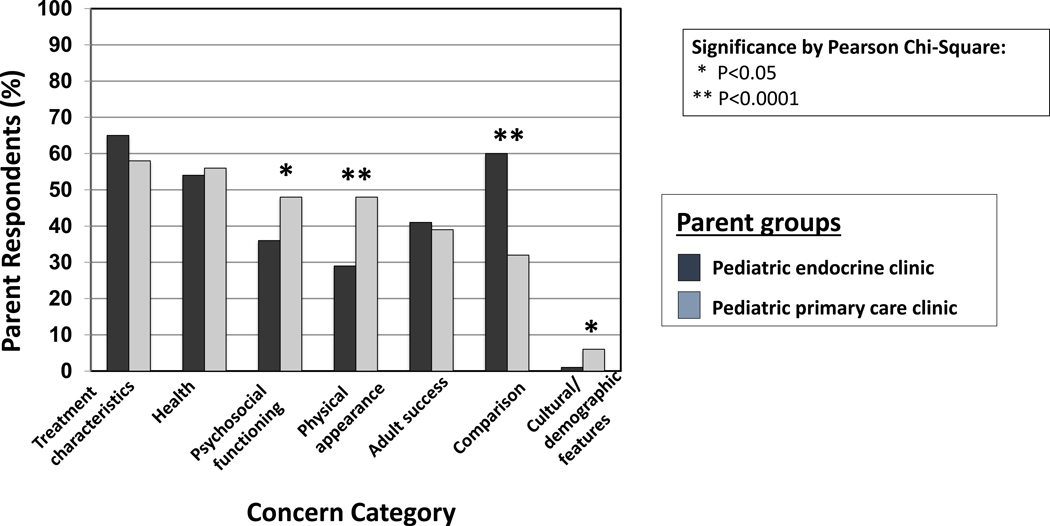
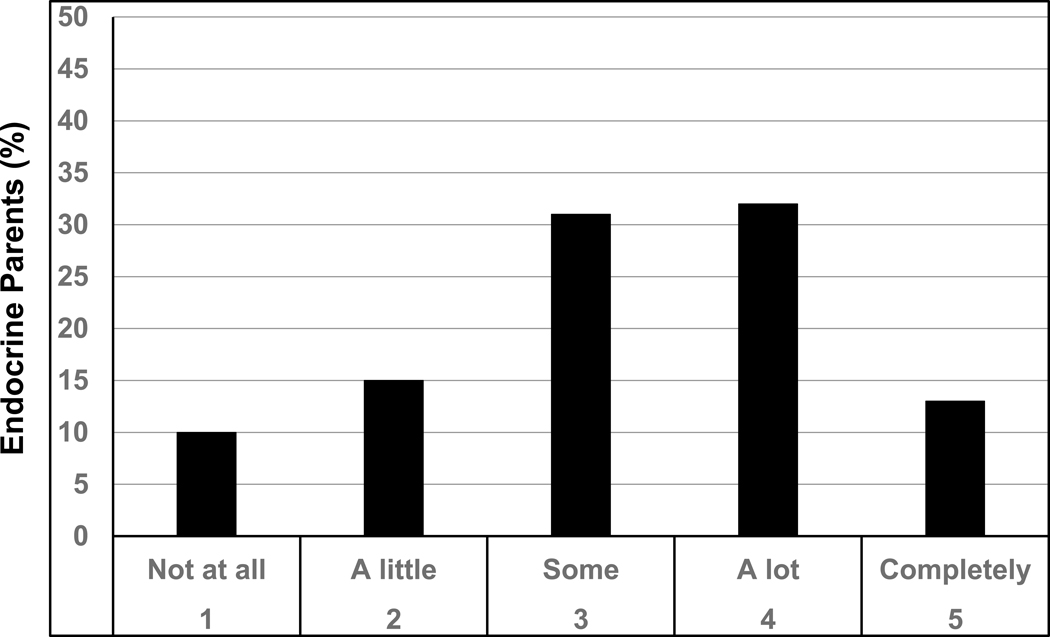
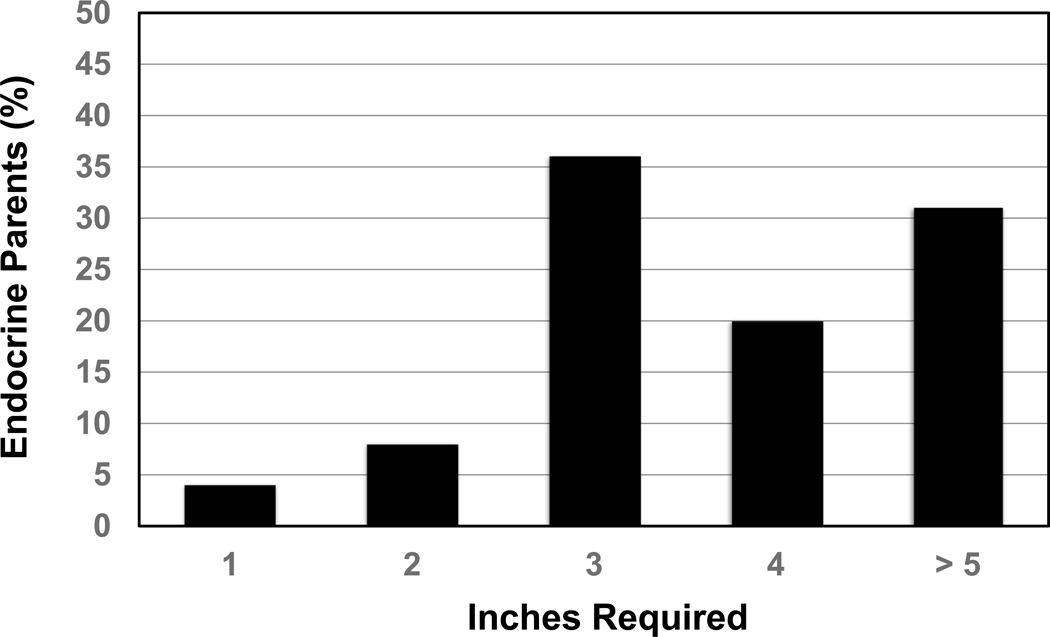
A greater proportion of the 166 EP (80% response rate) than the 1820 PP (83% response rate) surveyed previously was Caucasian (75% EP, 41% PP) and privately insured (80% EP, 58% PP). Both groups rated treatment efficacy and risks most as having a big or extreme impact on decision-making (65% EP, 58% PP). The second most rated concern for EP was comparison of child’s height to peers or growth chart (60% EP, 32% PP) versus child’s health for PP (54% EP, 56% PP). 76% of EP rated GH treatment as potentially improving quality of life (QoL), with 88% reporting a minimum 3-inch height increase as necessary to improve QoL.
Conclusions
Height comparisons were more likely to impact EP than PP in seeking height-related medical care for their children. EP had high expectations of QoL improvement with GH treatment, which are unlikely to be met with treatment of idiopathic short stature. Thus, clinicians should be prepared to support families in other ways that promote positive development in children with short stature.
Keywords: parent, medical decision-making, pediatrics, pediatric endocrinology, height, growth hormone, quality of life, positive youth development
Introduction
Pediatric growth hormone (GH) treatment in the U.S. has increased steadily, fueled in part by the belief that GH therapy will improve quality of life (QoL) for children with short stature even without documented GH deficiency [1–3]. Pediatric endocrinologists continue to debate the benefit to risk balance of GH therapy for patients with idiopathic short stature (ISS) and partial idiopathic isolated GH deficiency (IGHD), for which there is inconsistent evidence for height gain and QoL benefit, yet a high financial cost of GH treatment [2–4]. Despite the controversy, patients with ISS or IGHD comprise the majority of GH-treated patients [5, 6]. In our previous study, we sought to learn what factors influence parental decision-making, both positively and negatively, when considering to seek medical care for a short child among parents in pediatric primary care practices (PP) [7]. We found that PP were concerned most about treatment characteristics (efficacy and side effects), potential health issues, and the child’s current psychosocial functioning in deciding whether to seek medical treatment for a child’s short height [7].
Patients and their families seeking pediatric endocrine subspecialist care (EP) for short stature differ demographically from PP. Children who receive GH therapy for ISS and IGHD are predominantly Caucasian, male and of higher socioeconomic status [3,5,6,8,9]. However, in a regional primary care population of 189,280 patients, girls were equally likely as boys to have short stature sufficient to meet the FDA-approved criterion for GH treatment of ISS [3,8,9]. In a recent survey asking PP, “How short is too short for an adult [male or female]?” higher median height thresholds were reported by parents who were Caucasian, had higher educational and income levels, private insurance, and were from non-urban primary care practices [10]. Based on those results, we cannot assume that the pattern of concerns reported by PP are generalizable to EP.
In this study, we first sought to learn what factors influence the decisions of EP seeking evaluation of their child’s height through a cross-sectional design utilizing a survey with items previously administered to PP [7]. Our second aim was to compare the concerns between EP and PP. We also sought to understand how much height gain and QoL benefits EP expect from GH therapy.
Materials and Methods
The study was reviewed and granted exemption by the Institutional Review Board of the Children’s Hospital of Philadelphia (CHOP; IRB protocol No. 14-011455) per 45 CFR 46.101(b)(2). No clinical trial registration was required.
Participants
A convenience sample of parents of patients age 6–16 years, from the Diagnostic and Research Growth Center of the Children’s Hospital of Philadelphia, was recruited to participate in the study (Table 1). They anonymously completed a brief, one-time survey while awaiting their child’s outpatient appointment during summer 2015. Subject recruitment was sequential for patients within the age criteria who arrived to clinic, and not based on other patient or parent characteristics.
Table 1.
Traits of Survey Respondents
| Parent Group | ||
|---|---|---|
| Endocrinology (EP) | Primary care (PP) | |
| Sample size | 166 | 1,820 |
| Response rate | 80 | 83 |
| PARENT CHARACTERISTICS | ||
| Highest level of education completed | ||
| Some high school | 4 | 6 |
| High school graduate | 9 | 20 |
| Trade school graduate | 7 | 9 |
| Some college | 17 | 15 |
| College graduate | 28 | 29 |
| Master’s or Doctorate degree | 35 | 21 |
| Insurance | ||
| Private | 80 | 58 |
| Government | 19 | 33 |
| Self-Pay | 1 | 7 |
| Race/Ethnicity* | ||
| Asian | 5 | |
| Black or African-American | 47 | |
| Hispanic or Latino | 8 | |
| White or Caucasian | 41 | |
| Other | 3 | |
| Has a child on GH treatment (% yes) | 46 | 1 |
| CHILD CHARACTERISTICS | ||
| Race/Ethnicity* | ||
| Asian | 4 | |
| Black or African-American | 18 | |
| Hispanic or Latino | 8 | |
| White or Caucasian | 75 | |
| Other | 5 | |
| Sex (% male) | 65 | |
| Current age (yrs)** | 12 ± 2.7 | |
| First visit to endocrine clinic | 7 | |
Race/Ethnicity tallies exceed 100% for both parent groups, as parents were instructed to select all that apply (i.e. can be more than one). (PP group replied with respect to themselves).
Child age presented as mean ± SD. All other parent and child characteristics are presented as %.
Survey Design
Survey items were created for a previous mixed-methods study of parents at primary care sites, and full details of the subject recruitment and methodology of the survey were reported [7]. In the previous study, thirteen focus groups explored the broad range of factors that may influence parental height-related decision-making. Subsequently, in ten nominal group technique sessions, parents generated and prioritized the key factors. Tabulation of their rankings provided the most salient factors for the group and became the 22 growth concerns listed in the survey (Table 2) [7].
Table 2.
Discrete Concerns that Compose Concern Categories Rated by Parents in Survey*
| Concern Category | Discrete Concerns Listed in the Surveys |
|---|---|
| Treatment Characteristics | Whether the available treatment has been researched and what were the results. Concerns about side effects or fear of possible outcomes from available treatment. |
| Health | Health issues are causing the child’s short height. The child’s short height is causing health issues. The child is experiencing physical pain or discomfort as a result of their short height. The child’s doctor or nurse believes the child’s height may be a problem. |
| Psychosocial Functioning | The child’s behavior has changed (feeling depressed, acting out). The child is bothered or concerned about their own height. The child is experiencing bullying or teasing. The child is treated differently than their peers because of their height. The child feels isolated or withdrawn from their peers as a result of their height. The child is not able to participate in activities with peers (such as playing sports, going on amusement park rides). The parent wants the child to be well-adjusted and have a positive body image. |
| Physical Appearance | The child is a “midget” or a “dwarf”. The child’s growth is disproportionate (different body parts are growing at different rates). |
| Adult Success | The child’s height would change the child’s behavior, happiness or fulfillment during adulthood. The child’s short height would limit the child’s career choice as an adult. |
| Comparison | The child’s growth chart percentile or their pattern on the growth chart may be worrisome. The child’s height is short compared to their peers. |
| Cultural/Demographic Features | The family’s religious beliefs. Whether the child is a boy or a girl. The child’s race or ethnicity. |
Developed in: Grimberg A, Cousounis P, Cucchiara AJ, Lipman TH, Ginsburg KR. Parental Concerns Influencing Decisions to Seek Medical Care for a Child’s Short Stature. Horm Res Paediatr. 2015 Nov 1;84(5):338–48.
In the previous study, the survey asked: “Parents vary in how much medical care they seek for their children, such as getting testing and treatment. Imagine you have a short child (even if you don’t have one in real life). How much of an impact would each of the following issues make on your decision whether to do something medical for that child’s height?” This was followed by the list of 22distinct concerns generated by the aforementioned process [7]. Using a five-point Likert scale (No impact, little impact, some impact, big impact and extreme impact), respondents scored the degree of impact that each concern would have on their medical decision-making. Research team consensus and factor analysis methods were utilized to group the 22 distinct concerns into seven categories.
In the current study, the survey asked, “Parents vary in how much medical care they seek for their children, such as getting testing and treatment. How much of an impact would each of the following issues make on your decision whether to do something medical for your child’s height?” The same 22 distinct concerns from the previous study were listed, together with the same Likert scale. Summary scores for each of the seven concern categories were calculated as previously reported. Two additional questions were added: “How much do you think growth hormone treatment could improve any quality of life issues possibly related to your child’s short height?” (not at all, a little, some, a lot or completely) and “If you chose some or a lot for [the previous] question, how many inches minimum do you think growth hormone treatment needs to increase height to improve any quality of life issues possibly related to your child’s short height?” The definition of QoL was intentionally left open-ended, rather than defined for the participants, to assess parents’ opinions of how much GH could improve their personal interpetation of QoL.
Data Collection and Analyses
Survey data were managed using REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, Tenn., USA) tools. Probabilistic samples of randomly selected surveys (10% of the 166 surveys) were manually reviewed to test the fidelity of the data entry. Survey results were analyzed statistically using JMP software (SAS Institute Inc., Cary, N.C., USA). EP and PP group responses were compared by Pearson Chi-Square.
Results
Survey Respondent Characteristics
The EP surveys were completed by 166 of the 208 EP approached (80% response rate). The PP surveys were completed by 1,820 of the 2,185 PP approached (83% response rate). The EP surveys were collected at two clinical sites of the CHOP Growth Center, one located in a non-urban area (13%) and the other in an urban area (87%). The PP surveys were collected in practices located in equal distribution of non-urban (51%) and urban (49%) areas. Of EP, 46% reported that they had a child who was treated with GH compared to 1% of the PP survey respondents. Regarding the children of the EP survey respondents, 65% were male and 7% were on their first evaluation by an endocrinologist. Comparison of parent and child traits from EP and PP are shown in Table 1. Of note, EP had a higher proportion of private insurance (80% EP vs. 58% PP) and college degree or above (63% EP vs. 50% PP). A higher proportion of EP described their child’s ancestry as White or Caucasian (75%), whereas 41% of PP described themselves as White or Caucasian.
Parental Concerns
Figure 1 shows the proportion of parents from both EP and PP groups who rated each concern category (Table 2) as having a big or extreme impact on their decision to seek medical treatment for a child’s short stature. Treatment characteristics (efficacy and side effects) was the category rated the highest by the most parents of both groups. The second most frequently rated highly impactful concern category for EP was the Comparison category, which was rated highly by much fewer PP (60% of EP vs. 32% of PP) (p<0.0001). Instead, the second most frequently rated highly impactful category for PP was the Health category (56%), which came in third place for EP (54%). Three other categories were rated highly impactful by significantly fewer EP than PP: Physical Appearance (p<0.0001), Psychosocial Functioning (p<0.05) and Cultural/Demographic Features (p<0.05).
Fig 1.
Parental Concerns Rated as Having a Big or Extreme Impact on Decision Making
Quality of Life Assessment
Of the 166 EP surveyed, 76% rated GH treatment as potentially improving any QoL issues possibly related to their child’s short height by at least some (Figure 2). Of those who selected at least some benefit from GH, the minimum height increase deemed necessary to improve any QoL issues was 1 or 2 inches for only 12% of respondents, 3 inches for 36%, and 4 or more inches for just over half of respondents (Figure 3).
Fig 2.
Endocrine Parents Ratings of Amount of Potential Improvement of QoL by GH Treatment
Fig 3.
Endocrine Parent Rating of Minimum Height Increase Required to Improve QoL
Discussion
This study demonstrates that concerns regarding whether to seek height-related medical care differ between EP and PP. The most notable difference was the significantly higher rating by EP for the Comparison Category, reflecting parental concerns that their child’s height is short relative to their peers or worrisome on the growth chart. Although the Treatment Characteristics Category, regarding treatment efficacy and side effects, was most often ranked as having a big or extreme impact on height-related decision making by both parent groups, the Comparison Category was the second most frequently rated category for the EP assessed, exceeding even the Health Category. This high rating by EP for the Comparison Category demonstrated a strong focus by these parents on external comparisons of their children to others.
Additionally, just over three out of four EP endorsed that GH treatment could improve QoL issues related to their child’s short height. Of these parents, 88% reported 3 or more inches and just over half stated 4 or more inches as the minimum height increase necessary to improve QoL. These expected height gains from GH therapy are unlikely to be met, as three randomized controlled trials have shown that the mean height increase with GH for patients with ISS is ~5cm (2 inches) after an average of 5 years of treatment [4,11,12]. Further, height response to GH therapy was highly variable, including no height gain in some patients and many patients with adult height still below −2 SD, with higher adult height noted in children who initiated treatment earlier (males age <10 years, females age <9 years) and were treated longer than 4 years [4, 12]. These studies demonstrate the burden of GH treatment to achieve greatest height gain: daily injections for > 4 years starting when a child is pre-pubertal. In addition, evidence is inconsistent that the GH-induced increase in height or height velocity is associated with improved QoL [14, 15]. Thus, the findings of the current study show that parents seeking sub-specialist medical care for their child’s short stature have high and unrealistic expectations for QoL and height attainment benefits from GH therapy.
The question that arises then is how EP’s focus on their children’s differences from peers and their unrealistic expectations for benefit from GH therapy may affect their child’s well-being. Adolescence is a critical time for personal development, and successful identity formation (defining “Who am I?”) in adolescence is associated with a smoother transition into a successful adulthood [16]. In addition, a feeling of “authenticity” in adolescence (feeling like “one is acting like their true self”) is associated with improved subjective well-being [17]. The positive youth development (PYD) framework is a prominant and validated developmental science model for supporting successful youth personal development and identity formation; higher scores on PYD outcomes were associated longitudinally with lower rates of depression and at-risk behavior and higher rates of contribution to their communities [16, 18]. The PYD model states that external and internal factors are mutually influential in youth development [16,18, 19]. Despite the increase of peer influence during adolescence, positive social influence from family (including high family support and unconditional acceptance) has been shown to be associated with several positive outcomes, including better school performance, lower rates of substance use, and less depression and anxiety [20].
It is in this context of strong social influences on youth development and during a fundamental time of identify formation, which includes deciding “Am I normal?”, that families are arriving at endocrine clinics for height-related medical care. If, as our study has shown, parental motivation for seeking such care is driven at least in part by concern that their child is physically different from their peers, i.e. shorter stature, we must consider that this parental emphasis on their child’s differences may exert a negative influence on their youth’s development despite the parents’ good intentions. Further, youth often model their perceptions of “normal” vs. “abnormal” based on their parents’ judgements. In a study asking parents “How short is too short for an adult [male or female]?”, parents of children receiving GH treatment reported the highest median acceptable height threshold: 65 inches for an adult male, which is approximately 5th percentile [10]. Thus, at least half of parents of children receiving GH treatment reported that adult male heights in the generally accepted “normal” range were too short. These high parental expectations of “normal” could enhance feelings of “being abnormal” for youth, which may in turn negatively affect their identify formation. Further, unrealistic parental expectations for their child’s height gain and associated QoL improvement from GH therapy may worsen any negative influence of the focus on their child’s differences when these expectations are not met.
As medical authorities, pediatric endocrinologists could play a powerful role in providing a source of social influence in the PYD model. In particular, a pediatric endocrinologist has the unique opportunity to model acceptance and normalization of a child’s external differences. Further, in weighing the risks and benefits of prescribing GH, it may be worth considering the potential negative effects of medicalizing short stature on the personal development and identity process, particularly in patients with ISS and partial IGHD, for whom the treatment benefits for QoL and height gain are uncertain [3,4,11,12,14]. A frank discussion by the pediatric endocrinologist with families seeking height-related medical care of realistic expectations from GH therapy may lessen the potentially negative impact of failing to reach expectations. Clarifying expectations of GH therapy is also particularly important because all parents – EP and PP – rated treatment characteristics (proven efficacy and side effects) as the most impactful concern in considering medical care for a child’s short stature. Additionally, a psychological evaluation with possibly additional psychological support to promote positive development in children with short stature, regardless of GH treatment, may benefit children undergoing a growth evaluation.
Our study demonstrates that these two parent groups have markedly different demographics. The EP surveyed were more likely Caucasian, privately insured, and had higher educational attainment than PP previously surveyed, consistent with previous studies [8,9]. Patient-families seeking growth evaluation in sub-specialist endocrine clinics are a combination of patients referred by primary care physicians and those who seek subspecialist care directly. Primary care referrals are known to be influenced by the level of parental concern independent of the severity of short stature [21,22], but it is unknown the degree that demographic factors related to the social determinants of health (such as lower income level and lower educational achievement) contribute to primary care and self-referrals to growth center evaluations. One possibility may be that PP have a lower financial ability to pay for GH treatment, thereby leading to a higher threshold for seeking evaluation, i.e. only for perceived emergent concerns (such as the Health category) over less urgent categories. Thus, the differences seen in parental height-related concerns between EP and PP are important to note in the context of the demographic differences between them.
A major strength of our study was the development of a survey tool using a mixed qualitative-quantitative method that elicited the concerns of parents themselves [7]. Nonetheless, response bias (the desire to give the socially preferred answer), may have contributed to reduced rating of certain concern categories, such as the surprisingly low ratings of the cultural/demographics category given the pronounced demographic differences between EP and PP. This bias is supported by the prior study, in which parents in several of the explanatory focus groups remarked that the cultural/demographics category was likely under-ratedbecause people would be reluctant to admit on a survey to demographically based biases [7]. Another potential limitation of the current study is that EP had at least one child receiving sub-specialist care for short stature, whereas the PP surveyed included parents of children of all heights who may or may not have had actual concerns about their children’s growth. The PP cohort was not limited to parents of children with short stature or those seeking evaluation for their child’s growth in order to be generalizable to the general population and to capture the factors that impact decison-making of people who do not seek sub-specialist endocrine care. It is possible that some of the differences between PP and EP survey responses may reflect the difference between considering a concern in the hypothetical versus in regards to one’s own child in reality. However, even within the PP population, logistic regression modeling identified higher rating of the Comparison category as significantly associated with higher total household annual income, higher parental educational level, and white race [7], all demographic features that distinguish EP from PP. In addition, 46% of EP surveyed had children already on GH therapy, which may have skewed their responses towards higher expectations for benefits. Lastly, our study design utilized anonymously answered surveys by a convenience sampling of parents in an endocrine clinic. Such design precluded us from collecting data on the diagnosis or indications for GH treatment for those with children on GH therapy to allow comparisons between parental groups of children with different indications for GH treatment. Future studies comparing patient and parent motivations for height-related medical care may be useful to further determine the effect of height-related medical evaluations and GH therapy on the patient’s development.
Conclusions
For EP seeking height-related medical care for their child, comparison of their child to peers or the growth chart was particularly important, and these parents also had high and unrealistic expectations for QoL benefit and height gain with GH therapy. Addressing height in this way could potentially hamper rather than bolster their child’s development and successful social outcomes. During this critical period of identity development, PYD holds the unconditional regard of parents as the key protective factor, so there is potential harm from parental comparison of their child’s height to peers and consideration of short stature as sufficiently unsatisfactory to require medical evaluation and long-term pharmaceutical intervention, especially when this physical feature is beyond the youth’s control. This “risk” may be worth taking if tied to substantial benefit, as in the case of children with classic GH deficiency, but may not be worthwhile for marginal gain in height and uncertain psychosocial benefits of GH therapy, such as for ISS. In order to support positive youth development, pediatric endocrinologists should include a frank discussion of risks and unknown likelihood of benefits from GH treatment of short stature in otherwise healthy children. Such a discussion likely will be impactful, as treatment efficacy and side effects were rated by parents – both in the endocrine clinic and primary care clinic – as highly influential on their height-related medical decision making.
Acknowledgement
We thank Donna T. Gitt, Alexis Hyczko, and Carolyn McAnlis for recruiting parents to complete the surveys; Mariatu Bah and Emma Farrell for entering the data into REDCap; and Donna T. Gitt for testing the fidelity of data entry. We are grateful to all the participating parents for taking the time to complete the survey and share their thoughts.
Funding Sources
This work was supported by grants 1R01 HD57037 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; A.G.) and UL1TR001878 from the National Center for Advancing Translational Sciences (NCATS; A.J.C.) of the National Institutes of Health (NIH). The sponsors did not participate in the preparation of the data or manuscript.
Disclosure Statement
A.G. served on the Steering Committee for the Pfizer International Growth Study Database, and as consultant for the Pediatric Endocrine Society Growth Hormone Deficiency Knowledge Center which is sponsored by Sandoz Inc.
Biography
Talia Hitt: prepared the data for presentation and presented abstract of the data at [Eastern Society for Pediatric Research Annual Meeting, Philadelphia, Pennsylvania, March 2019; Pediatric Endocrine Society Conference, Baltimore, Maryland, April 2019.]; wrote the first draft of the manuscript; edited the manuscript with coauthor feedback; and submitted the final manuscript.
Kenneth R Ginsburg: assisted with design of the survey and organization of the 22 distinct concerns into 7 categories; critically reviewed the manuscript; and approved the final manuscript as submitted.
Pamela Cousounis: assisted with design of the survey and organization of the 22 distinct concerns into 7 categories; critically reviewed the manuscript; and approved the final manuscript as submitted.
Terri H Lipman: assisted with design of the survey and organization of the 22 distinct concerns into 7 categories; critically reviewed the manuscript; and approved the final manuscript as submitted.
Andrew J Cucchiara: assisted with design of the survey and organization of the 22 distinct concerns into 7 categories; critically reviewed the manuscript; and approved the final manuscript as submitted.
Virginia A. Stallings: met regularly with the research assistants; oversaw data management; critically reviewed the manuscript; and approved the final manuscript as submitted.
Adda Grimberg: conceptualized and designed the study; obtained grant funding for the study; assisted with design of the survey and organization of the 22 distinct concerns into 7 categories; met regularly with the research assistants; oversaw data management; performed data analyses; critically reviewed the manuscript; and approved the final manuscript as submitted.
Footnotes
Statements
Statement of Ethics
The study was granted exemption by the Institutional Review Board of the Children’s Hospital of Philadelphia (CHOP; IRB protocol No. 14-011455) per 45 CFR 46.101(b)(2).
All other authors have no conflicts of interest to declare.
References (Numerical)
- 1.Grimberg A, Kanter GP. U.S. growth hormone use in the idiopathic short stature era: Trends in insurer payments and patient financial burden. J Endocr Soc. 2019. August;3(11):2023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimberg A, Allen DB. Growth hormone treatment for growth hormone deficiency and idiopathic short stature: New guidelines shaped by the presence and absence of evidence. Curr Opin Pediatr. 2017. August;29(4): 466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutfield WS, Albert BB. Growth hormone treatment for idiopathic short stature. Pediatr Endocrinol Rev. 2018. Sep;16(Suppl 1):113–22. [DOI] [PubMed] [Google Scholar]
- 4.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: Growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016. November;86(6):361–97. [DOI] [PubMed] [Google Scholar]
- 5.Ranke MB, Lindberg A, Tanaka T, Camacho-Hubner C, Dunger DB, Geffner ME. Baseline characteristics and gender differences in prepubertal children treated with growth hormone in Europe, USA, and Japan: 25 years’ KIGS experience (1987–2012) and review. Horm Res Paediatr. 2017. December;87(1):30–41. [DOI] [PubMed] [Google Scholar]
- 6.Savendahl L, Polak M, Backeljauw P, Blair J, Miller BS, Rohrer TR, et al. Treatment of children with GH in the United States and Europe: Long-term follow-up from NordiNet IOS and ANSWER program. J Clin Endocrinol Metab. 2019. October;104(10):4730–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimberg A, Cousounis P, Cucchiara AJ, Lipman TH, Ginsburg KR. Parental concerns influencing decisions to seek medical care for a child’s short stature. Horm Res Paediatr. 2015. November;84(5):338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimberg A, Lindberg A, Wajnrajch M, Cucchiara AJ, Camacho-Hübner C. Racial/ethnic disparities in US pediatric growth hormone treatment. Horm Res Paediatr. 2018. October;90(2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimberg A, Huerta-Saenz L, Grundmeier R, Ramos MJ, Pati S, Cucchiara AJ, et al. Gender Bias in U.S. Pediatric Growth Hormone Treatment. Sci Rep. DOI: 10.1038/srep11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousounis PA, Lipman TH, Ginsburg K, Cucchiara AJ, Grimberg A. How short is too short according to parents of primary care patients. Endocr Pract. 2014. November;20(11):1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008. November;93(11):4342–50. [DOI] [PubMed] [Google Scholar]
- 12.Deodati A, Cianfarani S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: Systematic review. BMJ. 2011. March;342:c7157. [DOI] [PubMed] [Google Scholar]
- 13.Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res. 2007. January;68(2):53–62. [DOI] [PubMed] [Google Scholar]
- 14.Gardner M, Scerbak T, Sandberg DE. Psychosocial Aspects of Short Stature and rhGH Treatment: Implicit trends over 60+ Years. Pediatr Endocrinol Rev. 2018. Sep;16(Suppl 1):129–141. [DOI] [PubMed] [Google Scholar]
- 15.Sandberg DE, Colsman M. Growth hormone treatment of short stature: Status of the quality of life rationale. Horm Res. 2005. June;63(6):275–83. [DOI] [PubMed] [Google Scholar]
- 16.Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014. Sep;65(1):187–207. [DOI] [PubMed] [Google Scholar]
- 17.Thomaes S, Sedikides C, van den Bos N, Hutteman R, Reijntjes A. Happy to be “me?” Authenticity, psychological need satisfaction, and subjective well-being in adolescence.Child Dev. 2017. July; 88(4):1045–56. [DOI] [PubMed] [Google Scholar]
- 18.Maslow GR, Hill SN, Pollock MD. Comparison of positive youth development for youth with chronic conditions with healthy peers. J Adolesc Heal. 2016. December;59(6):716–21. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg KR, Jablow MM. Building resilience in children and teens: Giving kids roots and wings. 3nd Edition; Elk Grove Village, IL: American Academy of Pediatrics; 2014. [Google Scholar]
- 20.Telzer EH, van Hoorn J, Rogers CR, Do KT. Social influence on positive youth development: A developmental neuroscience perspective In: Benson J, editor. Advances in child development and behavior. Academic Press Inc.; 2018. Vol. 54; p. 215–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein BS, Singh J, Silvers JB, Marrero U, Neuhauser D, Cuttler L. Patient attitudes and preferences regarding treatment: GH therapy for childhood short stature. Horm Res. 1999. June;51(Suppl 1):67–72. [DOI] [PubMed] [Google Scholar]
- 22.Cuttler L, Marinova D, Mercer MB, Connors A, Meehan R, Silvers JB. Patient, physician, and consumer drivers referrals for short stature and access to specialty drugs. Med Care. 2009. Aug;47(8):858–65. [DOI] [PubMed] [Google Scholar]