Abstract
Objective(s):
Artemisia species are important medicinal plants throughout the world. Some species are traditionally used for their anti-inflammatory effect. The present study was designed to isolate sesquiterpene fractions from several Artemisia species and evaluate their anti-inflammatory activities on key mediators and signaling molecules involved in regulation of inflammation.
Materials and Methods:
Sesquiterpene fractions were prepared from several Artemisia species using the Herz-Högenauer technique. Lipopolysaccharide (LPS)-stimulated J774A.1 macrophages were exposed to isolated fractions. Their possible cytotoxic effect was examined using MTT assay. In addition, nitric oxide (NO) release was measured using Griess method and prostaglandin E2 (PGE2) level was determined by enzyme-linked immunosorbent assay (ELISA). Moreover, protein expression of pro-inflammatory enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) were investigated using Western blot analysis.
Results:
Nitric oxide level produced by LPS-primed macrophages was significantly decreased with all prepared fractions in a dose-dependent manner. Saturated sesquiterpene lactones-rich species (Artemisia kopetdaghensis, Artemisia santolina, Artemisia sieberi) showed the highest suppressive activity on NO and PGE2 production via suppression of iNOS and COX-2 expression. Fractions bearing unusual (Artemisia fragrans and Artemisia absinthium) and unsaturated sesquiterpene lactones (Artemisia ciniformis) possess less modulatory effect on PGE2 production and COX-2 expression.
Conclusion:
It can be concluded that some of the medicinally beneficial effects attributed to Artemisia plants may be associated with the inhibition of pro-inflammatory signaling pathways. However, these effects could be dependent on the type of their sesquiterpene content. These findings also introduce new Artemis species cultivated in Iran as a useful anti-inflammatory agents.
Key Words: Artemisia, Asteraceae, Inflammation, Macrophage, Sesquiterpene lactone - fraction
Introduction
The genus Artemisia belongs the family Asteraceae, which are comprises about 550 species distributed in Europe, Asia, and North America (1). The leaves are very aromatic, alternate, often with long, narrow segments, usually grayish or silvery, hairy. The flower heads are greenish or brownish, without rise. The fruits are seeded, usually flattened or ribbed, without a pappus. The genus in Iran has 34 species which two of them are endemic (2).
Some Artemisia species including Artemisia absinthium L. (Afsantin), Artemisia maritima L. (Shih), Artemisia abrotanum L. (Qaysum Zakar) and Artemisia dracunculus L. (Tarkhun) have been used extensively in folk medicine to alleviate several ailments (3). Artemisia species exhibit a wide range of leishmanicidal (4), cytotoxic (5-7), anti-microbial (8) and anti-oxidant activities (8). More importantly, the anti-inflammatory effects of Artemisia species have been reported (9-11).
A number of bioactive compounds including acetylenes, coumarins, terpenes, monoterpenes, monoterpene lactones, sesquiterpenes, sesquiterpene lactones, flavonoids, dipeptides, phenolics, coumarin, ethers, esters, esterols and polysaccharides are commonly found in members of the genus (12). In recent years, several sesquiterpenes derived from Artemisia including artemisinin and dihydroarteannuin (13), artemisolide and eupatilin (14), scoparone and capillarisin (15), scopoletin (16) have received special attention due to their pharmacological activity on inflammatory processes and other illnesses.
Nitric oxide (NO) is mainly synthesized by inducible NO synthase (iNOS) which is largely involved in the pathophysiology of many inflammatory diseases (17). Another key enzyme in inflammatory responses is cyclooxygenase-2 (COX-2) which is responsible for prostaglandin E2 (PGE2) production (18). Several inflammatory stimuli such as bacterial lipopolysachharide (LPS) could activate iNOS and COX-2 expression. Various agents could serve as an important therapeutic target in the treatment of various inflammation-based pathologies (19, 20).
Many medicines commonly used for the treatment of inflammatory diseases could impart various adverse side-effects (21). Numerous researches have focused on herbal cures with lower adverse effects and improved efficacy.
Taking into consideration the above facts, present study aimed to evaluate and compare the anti-inflammatory effects of sesquiterpene fractions isolated from various Artemisia species through effects on the production of NO and PGE2 as well as on the expression of iNOS and COX-2 by LPS-primed J774A.1 macrophages. To our knowledge, no other study has been carried out to compare the anti-inflammatory effect of Artemisia species in regard to their sesquiterpene contents. Aside from this, essentially nothing is known regarding the potential anti-inflammatory property of some Artemisia species tested here.
Materials and Methods
Chemicals
Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), penicillin and streptomycin were purchased from Gibco Laboratories (Detroit, MI). Escherichia coli lipopolysaccharide (LPS, serotype 0111:B4), sodium nitrite, N-(1-naphtyl) ethylenediamine, sulfanilamide and 3-(4, 5 dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO). ELISA kit for PGE2 measurement was bought from Assay Designs (Farmingdale, NY). The protein bands for iNOS and COX-2 were detected with monoclonal antibodies were from Panomics, Inc (Redwood City, CA). Goat anti-rabbit-horseradish peroxide-conjugated antibody was purchased from KOMA Biotech (Seoul, South Korea). ECL-detection reagent was obtained from Amersham (Cardiff, UK).
Plant materials and isolation of sesquiterpene fractions
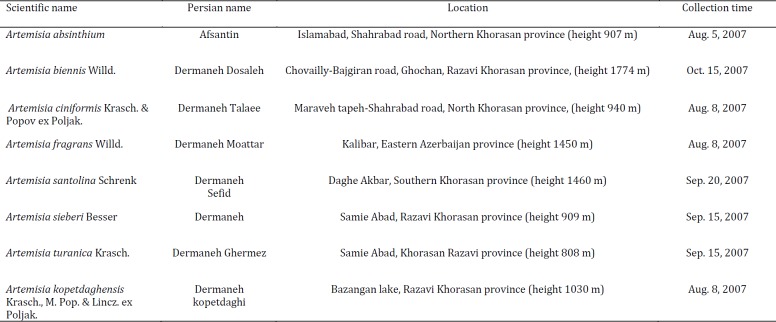
Aerial parts of Artemisia species (Table 1) collected from different regions of Khorasan Province, Iran and identified by Dr V Mozaffarian (Research Institute of Forest and Rangelands, Ministry of Jahad Keshavarzi, Iran). Voucher specimens were deposited in the Herbarium of National Botanical Garden of Iran (TARI). The shade dried and powdered plant samples were preserved for further experimentations.
Table 1.
Tested Artemisia species
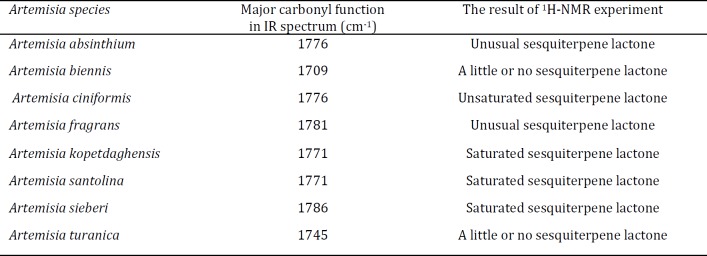
Table 2.
1H-NMR key data for the sesquiterpene lactone fractions of tested Artemisia species
Sesquiterpene fractions were prepared using Herz-Högenauer technique (22). The chlorophyll and common phenolic were removed by lead-(Π)-acetate precipitation and preparing crude sesquiterpene samples for further chromatographic and spectral investigations. Dried and ground plant materials (20 g) were soaked in dichloromethane (DCM; ≈ 100 ml) overnight. The slurry products were then filtered and evaporated in vacuo. The gummy residue was dissolved in 96% ethanol (≈ 50 ml) and heated to improve solubility. The aqueous solutions of lead acetate (5%) were included for precipitation of fatty acids, phenolics, and chlorophyll; precipitates were eliminated using filtration through a pad of silica gel (230-400 mesh (Merck, Germany). Lastly, the filtrates were concentrated in a water bath (40-50 °C) until a viscous mass was developed. IR spectra were recorded as KBr disks and in methylene chloride on a Unicam dp 110 spectrometer (Shimidzu Scientific, Japan). 1H-NMR (500 MHz) spectra were assessed in CDCl3 by a DRX 500 spectrometer (Bruker, Germany). All fractions were dissolved in dimethyl sulfoxide (DMSO) with the final concentration of less than 0.1% in culture medium.
Cell culture and treatment
J774A.1 murine macrophage cell line was obtained from the National Cell Bank of Iran (Tehran, Iran). Cells were cultured in DMEM supplemented with 100 U penicillin/ml and 100 μg streptomycin/ml and 10% heat inactivated FBS at 37 °C in 5% CO2 humidified incubator. Cell viability was determined by Trypan blue (0.4% in phosphate-buffered saline [PBS, pH 7.4]) exclusion method. In order to verify their in vitro anti-inflammatory effects, J774A.1 cells (1.25 × 105/well) were treated with/without indicated concentrations of fractions (10-100 μg/ml) for 2 hr prior to inflammatory stimulation of LPS (1 μg/ml). The plates were then incubated for additional 24 hr before being used for the cytotoxicity, NO release and PGE2 production assays.
Determination of cell viability
Cytotoxicity was examined to verify the possible toxic effect of fractions on macrophages using colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay (23). Briefly, MTT (5 mg/ml) reagent was added to each well and the plates were then incubated for 3 hr at 37 °C. The formazan crystals formed as a result of MTT reduction in living cells were dissolved in DMSO. The optical density was measured using a microplate reader (Convergent Technologies, Germany) at 545 nm.
Measurement of nitric oxide
Nitrite level, as an indicator of NO synthesis, released into the supernatants of cultured cells was determined using colorimetric Griess assay (24, 25). Briefly, aliquots of cell culture supernatants were incubated with equal volume of Griess reagent, consisting of (1% sulphanilamide in 5% phosphoric acid, 0.1% naphtylenediamine dihydrochloride). The optical density of the mixture was measured after 10 min at room temperature using a microplate reader (Convergent Technologies, Germany) at 545 nm. Nitrite concentrations were calculated by extrapolation from a sodium nitrite standard curve obtained in parallel with sodium nitrite standard solutions.
Determination of PGE 2 production
The amount of PGE2 secreted into the culture supernatants was determined using Enzyme-linked immunosorbent assay (ELISA) kit, following manufacturer instructions. Sensitivity of the kit was 16 pg PGE2/ml.
Preparation of whole cellular extract
Cells were washed twice with cold PBS and lysed in freshly prepared buffer (10 mM HEPES [pH 7.5], 10 mM KCl, 0.1 mM EDTA, 1 mM dithiotheritol (DTT), 0.5% IGEPAL, along with the protease inhibitor cocktail). Cell lysates were then centrifuged at 14,000 g for 3 min at 4° C to obtain the supernatants as total cell extracts. Samples were stored at -70 °C. The protein content in each sample was quantified by use of Bradford assay.
Western blot analysis
Equal amounts of proteins were denaturated in Laemmli sample buffer, separated on 12% SDS-polyacrylamide gel and were then transferred to polyvinylidene difluoride (PVDF) membranes. Non-specific binding sites were blocked with 5% non-fat milk buffer (10 mM Tris-HCl and 100 mM NaCl, pH 7.5) at 4 °C overnight. The blots were incubated sequentially with primary antibodies (anti-mouse iNOS or COX-2 polyclonal antibodies; 1:200 or 1:500 dilution, respectively) overnight at 4 °C. After gentle washing, the blots were incubated with secondary goat anti-rabbit-horseradish peroxide-conjugated antibody (1:10,000 dilution). Immunoreactive protein bands were developed using enhanced chemiluminescence (ECL) detection reagent (Pierce, Rockford, IL).
Statistical analysis
All data were reported as mean±SD of at least three independent experiments. Statistical analysis were conducted using SPSS 11.0 software (Chicago, IL). Significant differences between the groups were determined using one-way analysis of variance (ANOVA). A P<0.05 was considered statistically significant.
Results
Characterization of isolated sesquiterpene fractions from Artemisia species
The various IR spectra revealed that all tested samples had notable absorptions between 1730 and 1780 cm-1 that point out the existence of carbonyl functional groups. A γ-lactone moiety present as the absorptions > 1760 cm-1. Fractions from A. absinthium, A. ciniformis, A. fragrans, A. kopetdaghensis, A. santolina, and A. sieberi exhibited significant absorption peaks at 1776, 1776, 1781, 1771, 1771 m and 1786 cm-1, respectively. This could be due to the presence of high amount of sesquiterpene lactones in obtained fractions. Maximum absorption peaks of carbonyl groups in A. biennis and A.turanica samples emerged at 1709 and 1745 cm-1 which could represent the low content of sesquiterpene lactones. The 1H-NMR spectra confirmed the presence of unsaturated, saturated and unusual sesquiterpene lactones in the fractions (Table 2).
Sesquiterpene fractions did not affect cell viability J774A.1 cells were exposed to different concentrations of fractions in order to exclude the possibility that the observed anti-inflammatory effects were due to their toxicity. None of the fractions at tested concentrations (10-100 μg/ml) significantly altered cell viability. The maximum percentage of growth inhibition of cells following by exposure to the highest tested concentration (100 µg/ml) of fractions of studied Artemisia species were A. absinthium 92.49±0.86, A. biennis 92.74±1.51, A. ciniformis 90.97±1.70, A. fragrans 93.25±0.56, A. kopetdaghensis 90.40±2.60, A. santolina 90.00±2.15, A. sieberi 91.54±0.56, A. turanica 90.97±1.70.
Effect of sesquiterpene fractions on macrophage NO and PGE 2 production
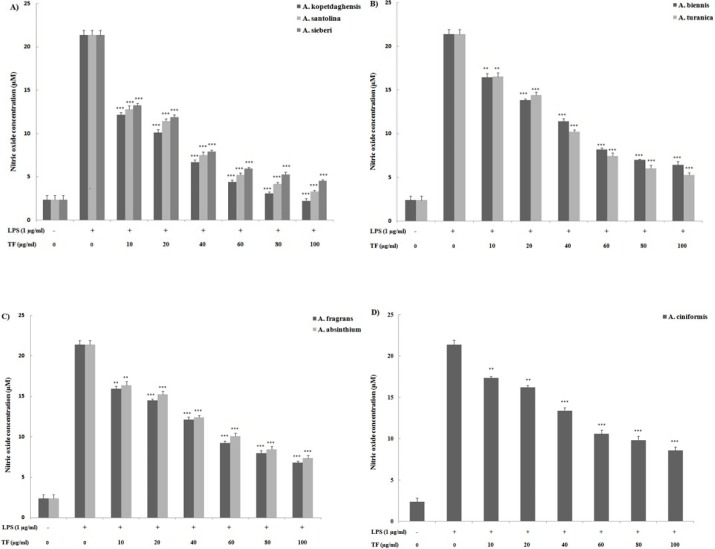
To investigate whether sesquiterpene fractions regulate NO production, J774A.1 macrophages were pre-treated with fractions for 2 hr before stimulation with LPS for 24 hr, and nitrite content was measured using Griess reagent assay (Figure 1). Stimulation of J774A.1 macrophages with LPS alone resulted in noticeable upregulation of NO production (21.4±0.5 µM), compared to the unstimulated control (2.4±0.4 µM). However, J774A.1 macrophages pre-treated with sesquiterpene fractions (10-100 μg/ml) displayed a marked dose-dependent reduction in inflammatory LPS-induced NO production. Saturated sesquiterpene lactones-rich species including A. kopetdaghensis, A. santolina, A. sieberi most effectively reduced NO release by LPS-activated J774A.1 macrophages.
Figure 1.
Effect of sesquiterpene lactone fractions of different Artemisia species including A) Artemisia kopetdaghensis, Artemisia santolina, Artemisia sieberi, B) Artemisia biennis, Artemisia turanica, C) Artemisia fragrans, Artemisia absinthium, D) Artemisia ciniformis on LPS-stimulated J774A.1 macrophages production of NO. Cells were treated for a total of 24 hr with LPS alone or after an initial 2-hr treatment with the sesquiterpene fractions (10-100 μg/ml). Values shown are means (± SD) of at least three determinations. *P<0.05, **P<0.01 vs LPS alone
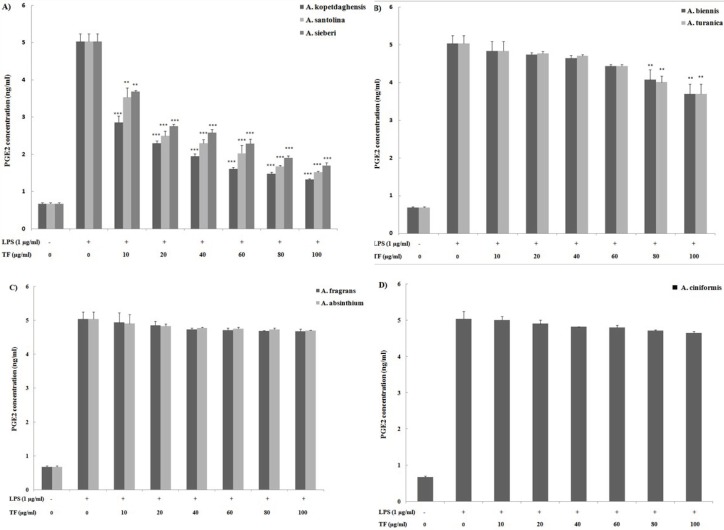
We next compared the inhibitory effects of fractions on PGE2 production as a key mediator of inflammatory responses. The immune response to LPS as an inflammatory stimuli is associated with PGE2 production therefore, the effect of sesquiterpene fractions on PGE2 production was measured in the supernatants of inflammatory LPS-primed J774A.1 macrophages using ELISA (Figure 2). Under unstimulated condition, macrophages release low levels of PGE2 (0.7±0.2 ng/ml); however LPS induced a substantive release of PGE2 (5.0±0.2 ng/ml). Sesquiterpene fractions containing saturated sesquiterpene lactones (A. kopetdaghensis, A. santolina, and A. sieberi) and fractions containing a little sesquiterpene lactones (A. biennis and A. turanica) markedly suppressed the LPS-induced PGE2 formation.
Figure 2.
Activitiy of sesquiterpene lactone fractions of various Artemisia species including A) Artemisia kopetdaghensis, Artemisia santolina, Artemisia sieberi, B) Artemisia biennis, Artemisia turanica, C) Artemisia fragrans, Artemisia absinthium, D) Artemisia ciniformis on LPS-stimulated J774A.1 macrophages production of PGE2. Cells were treated for a total of 24 hr with LPS alone or after an initial 2-hr treatment with the sesquiterpene fractions (10-100 μg/ml). Values shown are means (± SD) of at least three determinations. *P<0.05, **P<0.01 vs. LPS alone
Effect of sesquiterpene fractions on expression of pro-inflammatory iNOS and COX-2
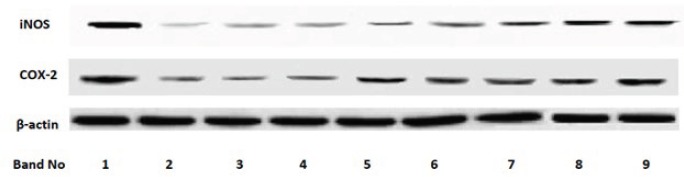
We further evaluated the effect of sesquiterpene fractions on LPS-induced iNOS and COX-2 expression in J774A.1 cells at the protein level by Western blot analysis (Figure 3). Stimulation with LPS led to increased protein expression (Band 1) in comparison with unstimulated macrophages. However, pre-treatment with tested fractions decreased the levels of iNOS protein expression in LPS-stimulated J774A.1 macrophages (Bands 2-9). Saturated sesquiterpene lactone-rich fractions of A. kopetdaghensis, A. santolina, and A. sieberi (bands 2-4) exhibited inhibitory activity on the expression of COX-2 protein. This finding revealed that the inhibitory pattern of fractions on iNOS and COX-2 expression overlap with their suppressive effects on LPS-induced NO and PGE2 production.
Figure 3.
Modulatory effect of sesquiterpene lactone fractions from different Artemisia species on LPS-stimulated iNOS and COX-2 protein expression. J774A.1 macrophages were pre-treated (2 hr) with/without 80 μg/ml of fractions and then stimulated for 22 hr with LPS (1 μg/ml). After the end of the 24 hr exposure period, whole cell lysates were prepared and underwent Western blot analysis with antibodies to detect iNOS, and COX-2. Antibody to β-actin was also used to monitor for protein loading. A representative blot is shown as bands 1-9 including: band 1) LPS-stimulated macrophages alone, band 2) A. kopetdaghensis, band 3) Artemisia santolina, band 4) Artemisia sieberi, band 5) Artemisia biennis, band 6) Artemisia turanica, band 7) Artemisia fragrans, band 8) Artemisia absinthium, band 9) Artemisia ciniformis
Discussion
There is growing attention to natural products derived from medicinal plants. In particular, there is a great interest in their potential use in prevention of inflammatory responses. The use of natural anti-inflammatory products could provide an attractive safe alternative to many common widely-used pharmaceuticals. Along these lines of thought, there are numerous rationale for development of dual iNOS/COX-2 inhibitors as effective anti-inflammatory and analgesic drugs to block the symptoms of inflammation related to nitric oxide and prostaglandin(s) formation due to their increased levels mediate inflammatory responses (26).
Previous phytochemical investigations of Artemisia species resulted in the isolation of various classes of chemical compositions (11). Among them, inhibitory effects against inflammatory mediators by several triterpenoids and sesquiterpenes derivatives present in Artemisia were widely reported. Although the traditional use of Artemisia species as anti-inflammatory agents is supported by scientific reports, to our knowledge, there is little evidence showing specific biological activities of these species. We attempted here to verify the in vitro anti-inflammatory potential of sesquiterpene fractions that were isolated from eight Artemisia species common to Iran. After preparing the fractions, their anti-inflammatory impact was assessed by measuring any changes in the release of pro-inflammatory mediators, i.e., NO and PGE2, produced in response to LPS activation.
The present results demonstrated that each tested sesquiterpene fractions from the isolated Artemisia species significantly suppressed LPS-induced NO production in a dose-dependent manner. A. kopetdaghensis, A. santolina, and A. sieberi were revealed to mainly contain saturated sesquiterpene lactones that seemed to be the most potent inhibitors of NO production. Samples from A. biennis and A. turanica proved to have little or no sesquiterpene lactones in their fractions also dramatically inhibited NO production in the stimulated macrophages. Although A. absinthium and A. fragrans contained unusual sesquiterpene lactones, they still imparted considerable inhibitory effects as well. Lastly, the sesquiterpene fraction of A. ciniformis that mainly contained unsaturated sesquiterpene lactones was also able to inhibit NO production. The Western blot data of iNOS induction were found to be comparable to those from the NO production assays. Taken together, this data suggested to us that the potent anti-inflammatory properties of A. kopetdaghensis, A. santolina, and A. sieberi are mediated in part through the inhibition of the NO synthesis pathway and that this could likely be attributed to the presence of saturated sesquiterpene lactones. However, even though the medium containing fractions was replaced by medium containing LPS, the possibility still also remains that the treatments might have affected subsequent LPS binding itself, i.e., impacting TLR sites on cells.
While the inflammatory mediator prostaglandin E2 (PGE2) is generated via the cyclooxygenase pathway, it has been reported that NO can directly activate cyclooxygenase pathways and that NO production does not seem regulated by mediators generated by those pathways (17, 18). Assessing the amount of PGE2 produced by the treated cells revealed that most of the sesquiterpene fractions failed to inhibit PGE2 synthesis or modulate the expression of COX-2 protein. This could be explained, in part, by the fact that changes in PGE2 release is most often associated with alterations in COX-2 expression. However, PGE2 release was remarkably inhibited by the saturated sesquiterpene lactone-rich fractions of A. kopetdaghensis, A. santolina, and A. sieberi at levels akin to their effects on the production of NO. When both endpoints are examined in tandem, it appeared that the sesquiterpene fractions of A. kopetdaghensis, A. santolina and A. sieberi were apparently the most potent fractions for suppressing NO and PGE2 production.
The current findings showed that both iNOS and COX-2 pathways were maximally inhibited by extracts prepared from A. kopetdaghensis, A. santolina and A. sieberi. By comparing these results with those of a previous study (11) in which we reported the anti-inflammatory activity of a sesquiterpene lactone-bearing fraction prepared from A. khorassanica (SLAK), we surmise that saturated sesquiterpene lactones in various Artemisia can impart potent anti-inflammatory effects - in part - through the modulation of both iNOS and COX-2 expression, and that these agents might be classified as dual inhibitors.
Conclusion
The present study suggested sesquiterpene fractions from Artemisia species could be further investigated to isolate/identify bioactive compounds that may function as potential anti-inflammatory agents. Studying their modulatory effects in inflammatory animal models would also further validate their potential beneficial properties as natural health products.
Acknowledgment
This study is financially supported by Research Council of Mashhad University of Medical Sciences (MUMS) vice president (grant numbers 85448 and 89399), Mashhad University of Medical Sciences, Mashhad, Iran.
Conflicts of Interest
The Authors declare no conflicts of interest.
References
- 1.Salmaki Y, Bendiskby M, Heubl G. Molecular phylogeny confirms the placement of enigmatic Stachys persepolitana in Lamium (Lamiaceae; subfam. Lamioideae) 2015;192:13. [Google Scholar]
- 2.Emami SA, Aghazari F, Johartchi MR. Les phanérogames endémiques de la flore d’Iran. Téhéran: L’Université de Téhéran des Sciences Médicales, Institut des Études d’Histoire de la Médecine, de Médecine Islamique et de Médecine Complémentaire . 2011. [Google Scholar]
- 3.Zargaran A, Mehdizadeh A, Zarshenas MM, Mohagheghzadeh A. Avicenna (980-1037 AD) J Neurol. 2012;259:389–390. doi: 10.1007/s00415-011-6219-2. [DOI] [PubMed] [Google Scholar]
- 4.Emami SA, Zamanai Taghizadeh Rabe S, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against leishmania major parasites. Iran J Basic Med Sci. 2012;15:807–811. [PMC free article] [PubMed] [Google Scholar]
- 5.Emami S, Zamani Taghizadeh Rabe S, Ahi A, Mahmoudi M, Tabasi N. Study the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacologyonline. 2009;3:1062–1069. [Google Scholar]
- 6.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm Biol. 2011;49:962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoudi M, Zamani Taghizadeh Rabe S, Ahi A, Emami SA. Evaluation of the cytotoxic activity of different Artemisia khorassanica samples on cancer cell lines. Pharmacologyonline. 2009;2:778–286. [Google Scholar]
- 8.Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem. 2005;53:1408–1416. doi: 10.1021/jf048429n. [DOI] [PubMed] [Google Scholar]
- 9.Ahn H, Kim JY, Lee HJ, Kim YK, Ryu JH. Inhibitors of inducible nitric oxide synthase expression fromartemisia iwayomogi. Arch Pharm Res. 2003;26:301–305. doi: 10.1007/BF02976959. [DOI] [PubMed] [Google Scholar]
- 10.Noori S, Naderi GA, Hassan ZM, Habibi Z, Bathaie SZ, Hashemi SMM. Immunosuppressive activity of a molecule isolated from Artemisia annua on DTH responses compared with cyclosporin A. Int Immunopharmacol. 2004;4:1301–1306. doi: 10.1016/j.intimp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Emami SA, Taghizadeh Rabe SZ, Iranshahi M, Ahi A, Mahmoudi M. Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacol Immunotoxicol. 2010;32:688–695. doi: 10.3109/08923971003677808. [DOI] [PubMed] [Google Scholar]
- 12.Rustaiyan A, Masoudi S. Chemical constituents and biological activities of Iranian Artemisia species. Phytochem Lett. 2011;4:440–447. [Google Scholar]
- 13.Konkimalla VB, Blunder M, Korn B, Soomro SA, Jansen H, Chang W, et al. Effect of artemisinins and other endoperoxides on nitric oxide-related signaling pathway in RAW 2647 mouse macrophage cells. Nitric Oxide. 2008;19:184–191. doi: 10.1016/j.niox.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011;88:1121–1126. doi: 10.1016/j.lfs.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Han S, Lee JH, Kim C, Nam D, Chung WS, Lee SG, et al. Capillarisin inhibits iNOS, COX-2 expression, and proinflammatory cytokines in LPS-induced RAW 2647 macrophages via the suppression of ERK JNK and NF-κB activation. Immunopharmacol Immunotoxicol. 2013;35:34–42. doi: 10.3109/08923973.2012.736522. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Jang SI, Kim YJ, Chung HT, Yun YG, Kang TH, et al. Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line RAW 2647 cells. Fitoterapia. 2004;75:261–266. doi: 10.1016/j.fitote.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Kröncke K, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 19.Spitzer JA, Zheng M, Kolls JK, Vande Stouwe C, Spitzer JJ. Ethanol and LPS modulate NF-kappaB activation, inducible NO synthase and COX-2 gene expression in rat liver cells in vivo. Front Biosci. 2002;7:99–108. doi: 10.2741/A744. [DOI] [PubMed] [Google Scholar]
- 20.Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, et al. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol. 2001;280:930–938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- 21.Suthar SK, Sharma M. Recent developments in chimeric NSAIDs as safer anti-inflammatory agents. Med Res Rev. 2015;35:341–407. doi: 10.1002/med.21331. [DOI] [PubMed] [Google Scholar]
- 22.Iranshahi M, Emami SA, Mahmoud-Soltani M. Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iran J Basic Med Sci. 2007;10:183–188. [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.Zamanai Taghizadeh Rabe S, Iranshahi M, Rastin M, Tabasi N, Mahmoudi M. In vitro immunomodulatory properties of a sesquiterpene lactone-bearing fraction from Artemisia khorassanica. J Immunotoxicol. 2015;12:223–230. doi: 10.3109/1547691X.2014.930079. [DOI] [PubMed] [Google Scholar]
- 26.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]