Abstract
Objective(s):
Pseudomonas aeruginosa is one of the most important nosocomial pathogens causing a high rate of mortality among hospitalized patients. Herein, we report the prevalence of antibiotic resistance genes, class 1 integrons, major virulence genes and clonal relationship among multidrug- resistant (MDR) P. aeruginosa, isolated from four referral hospitals in the southeast of Iran.
Materials and Methods:
In this study, 208 isolates of P. aeruginosa were collected from four referral hospitals in southeast of Iran. Disk diffusion method was used to determine susceptibility to 13 antibacterial agents. AmpC was detected by phenotypic method and β-lactamase genes, virulence genes and class 1 integrons were detected by PCR. Clonal relationship of the isolates was determined by RAPD-PCR.
Results:
All the isolates were susceptible to polymyxin-B and colistin. Overall, 40.4% of the isolates were MDR, among which resistance to third generation cephalosporins, aminoglycosides, and carbapenems was 47.5%, 32.3% and 40%, respectively. None of the isolates was positive for blaNDM-1 genes, while 84.5% and 4.8% were positive for the blaIMP-1 and blaVIM, metallo-β-lactamase genes, respectively. Incidence of class 1 integrons was 95% and AmpC was detected in 33% of the isolates. Prevalence of exoA, exoS, exoU, pilB and nan1 were 98.8%, 44%, 26%, 8.3% and 33.3%, respectively. RAPD profiles identified four large clusters consisting of 77 isolates, and two small clusters and three singletons.
Conclusion: :
The rate of MDR P. aeruginosa isolates was high in different hospitals in this region. High genetic similarity among MDR isolates suggests cross-acquisition of infection in the region.
Key Words: Antibiotic resistance, Beta-lactamases, Class 1 integrons, Pseudomonas aeruginosa, RAPD-PCR, Virulence factors
Introduction
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen and a common source of infections in hospitals, particularly in intensive care units (ICUs), where the organism infects 10-15% of the patients. Rapid increase of multidrug-resistant P. aeruginosa (MDRPA) isolates in clinical settings worldwide has resulted in increased mortality rate (1, 2). Several mechanisms are involved in P. aeruginosa resistance to antimicrobial agents such as β-lactamase production, target mutation, efflux pumps over-expression, decrease in membrane permeability and chromosomal expression of resistance encoding genes (3). Due to the pressure caused by the overuse of β-lactam antibiotics in the hospitals, various forms of β-lactamases such as the extended spectrum β- lactamases (ESBLs), AmpC and metallo-β-lactamases (MBLs) have evolved (4). Integrons are also important factors in dissemination of antibacterial resistance among different bacterial species and the association between integrons and drug resistance has been shown (5). Among the integron classes, class 1 integrons (int1) was the most prevalent among clinical isolates of P. aeruginosa in Ahvaz, Iran (6). In addition to antibiotic resistance, P. aeruginosa produces various virulence factors contributing to pathogenicity of the microorganism. Among these virulence factors are a variety of secreted proteins such as proteases, phospholipases, and exotoxin A (7). In addition, exoenzyme S is a virulence factor encoded by the exoS gene. It is an ADP ribosyltransferase secreted by a type-III secretion system (TTSS), delivered directly into the cytosol of epithelial cells. Another protein, exotoxin U (ExoU), is a necrotizing toxin with phospholipase activity, unique cytotoxic effect, capable of destroying cellular monolayers during short infection periods and plays a role in the development of septic shock (8). In some P. aeruginosa hospital isolates, a gene called nan1 encodes an extracellular neuraminidase that is responsible for the adherence to the respiratory tract and facilitates long term infection in cystic fibrosis patients (9). In recent epidemiological studies, random amplified polymorphic DNA (RAPD) PCR has been extensively used for molecular characterization of bacteria due to its simplicity, rapidity, sensitivity, reproducibility and low cost. It can determine genetic diversity without prior knowledge of the genome under study (10). This study was aimed to investigate the antibiotic resistance profiles, prevalence and production of different type of β-lactamases (ESBL, AmpC and MBL), class 1 integron, and five major virulence genes (exoA, exoS, exoU, pilB, and nan1) in clinical isolates of MDR P. aeruginosa collected from four main referral hospitals in Shiraz and Kerman cities, in southeast of Iran. The clonal relationship of the MDR isolates was also determined.
Materials and Methods
Specimen collection
From September 2013 to November 2014, a total of 208 non-duplicate isolates of P. aeruginosa were recovered from inpatients of four main referral hospitals of A (Namazi with 800 beds), B (Faghihi with 400 beds), C (Ghotbeddin with 80 beds) in Shiraz and D (Shafa with 400 beds) in Kerman, Iran. Samples were taken by expert technicians from hospitalized patients admitted to adult ICUs, NICUs, burn, respiratory care units, general medical and surgical wards, emergency and kidney transplantation wards. The sampling procedure from wound included swabs taken from clinically deep areas of the burn wounds. Blood culture was prepared from patients with suspected bacteremia or sepsis and inoculated into BACTEC™ blood culture medium. The other samples were placed in stuart transport medium (Merck, Darmstadt - Germany) and transferred to the laboratory for identification by standard microbiological tests (11). The bacterial isolates were inoculated into sterile True North TM Cryogenic vials (TNC) containing 1 ml of sterile tryptic soy broth (TSB) (BioMerieux, Marcy-I’Etoile, France), mixed with glycerol (40%) and stored at -70 °C until further examination (11).
Antimicrobial susceptibility testing
Kirby–Bauer disk diffusion method was used for antimicrobial susceptibility test as recommended by Clinical and Laboratory Standards Institute guidelines (CLSI-2014) (12). All antibiotic disks were purchased from Mast Co Ltd, UK and used as per manufacturer’s instructions. The following disks were used for antibiotic sensitivity assay (µg per disk); amikacin (AK 30), tobramycin (TN 10), gentamicin (GM 10), cefepime (CPM 30), ceftazidime (CAZ 30), ceftriaxone (CRO 30), aztreonam (ATM 30), imipenem (IMI 10), meropenem (MEM 10), ciprofloxacin (CIP 5), piperacillin/tazobactam (PTZ 110), polymyxin B (PB 300 units) and colistin (CL 10). Minimum inhibitory concentration (MIC) for imipenem resistance isolates was checked by E-strip test (Liofilchem Co., Italy) as CLSI guideline (13). P. aeruginosa ATCC 27853 was used as the quality control strain for antimicrobial susceptibility testing throughout this study.
Identification of ESBL and MBL enzymes by phenotypic methods
ESBL production was determined by double-disk synergy test (DDST), according to CLSI guidelines and manufacturer’s protocol (MastTM extended spectrum β-lactamase set; CPD10) (12). Cefotaxime (30 μg) and ceftazidime (30 μg) disks alone or in combination with clavulanic acid (30/10 μg) were used for ESBL detection. An increase in zone diameter of ≥5mm around the disks containing clavulanic acid indicated production of ESBL by the test organism. To counteract the effect of high-level expression of the naturally produced AmpC-type β-lactamase, double-disk synergy test was also performed on cloxacillin (200 μg/ml) containing plates (14). Detection of MBL was carried out by MBL-strips (Liofilchem Co, Italy). Briefly, a strip containing imipenem (4-256 µg) on one side and imipenem+EDTA (1-64 µg) on the other side (IMI/IMD) was placed on a Muller Hinton agar plate inoculated with the test organism. The tests were considered positive when the ratio of IMI/IMD was ≥ 8 mm. The results were confirmed by modified Hodges test (15).
Identification of AmpC by phenotypic method
Production of AmpC by the isolates was determined using Muller- Hinton agar (BioMerieux, France) plates, inoculated with Esherichia coli ATCC 25922 cefoxitin-susceptible strains. A cefoxitin disk (FOX 30 µg) was placed between two blank disks each containing 1:1 mixture of saline and Tris-EDTA 100 × solutions. The paper blank was inoculated with P. aeruginosa test isolates. Flattening of the growth inhibition zone toward the paper disk indicates AmpC production (16).
Detection of MBL resistance genes and class 1 integron by PCR
The presence of MBL genes, VIM, IMP, NDM and class 1 integrons (IntΙ1) was assessed by PCR using specific sets of forward and reverse primers, as shown in Table 1. The bacterial cells were grown overnight on Luria-Bertani (LB) agar. The whole genomic DNA was extracted from single colonies using the DNA genomic extraction kit (Thermo Scientific, Vilnius, Lithuania) and used as a template for PCR amplification, as described by the manufacturer. PCR reaction mixtures (25 µl) consisted of 2.5 units of Taq DNA polymerase (Thermo Scientific Co), 10 pmol of each primer, 1 µl of dNTP mix (Thermo Scientific Co), 2 µl of DNA template in the PCR buffer provided by manufacturer (Cinnagen, Iran). DNA amplification was conducted in a temperature gradient thermal cycler (Applied Biosystems, 96 well, Veriti, USA). The PCR was as follows; one cycle pre-denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 sec, annealing at 55 °C for 30 sec (for int1and blaIMP-1 gene temperature was 50 °C), and extension at 72 °C for 30 sec followed by a final extension at 72 °C for 10 min. PCR products (10 µl ) were subjected to gel electrophoresis using 1% agarose gel (Merck, Germany) for 1 hr, stained with UV illuminating dye (Gel Red) and visualized by a UV-gel documentation system (Gel logic200, Kodak, USA) (17).
Table 1.
Primers used in present study to detect genes and integrolls
| target genes | primer sequence (5 ´→ 3 ´ ) |
size
(bps) |
reference |
|---|---|---|---|
| exoS | F: ATC CTC AGG CGT ACA TCC R: ACG ACG GCT ATC TCT CCA C |
328 | (17) |
| exoU | F: GAT TCC ATC ACA GGC TCG R: CTA GCA ATG GCA GTA ATC G |
3308 | (17) |
| pilB | F: TCG AAC TGA TGA TCG TGG R: CTT TCG GAG TGA ACA TCG |
408 | (17) |
| nan1 | F: ACG CTC CGT CCA GCC GGA R: GTC TGG ACG ACG GCG GCA |
221 | (18) |
| exoA | F: GACAACGCCCTCAGCATCACCAGC R: CGCTGGCCCATTCGCTCCAGCGCT |
396 | (18) |
| bla VIM1 | F: CCG ATG GTG TTT GGT CGC AT R: GAA TGC GCA GCA CCA GGA |
391 | (41) |
| bla IMP1 | F: CTA CCG CAG CAG AGT CTT TG R: AAC CAG TTT TGC CTT ACC AT |
587 | (41) |
| bla NDM | F: GGT TTG GCG ATC TGG TTT TC R: CGG AAT GGC TCA TCA CGA TC |
621 | (42) |
| i nt I1 | F: GTT CGG TCA AGG TTC TG R: GCC AAC TTT CAG CAC ATG |
923 | (41) |
Detection of major virulence genes by PCR
Individual colonies of P. aeruginosa isolates were used for detection of exoA, exoS, exoU, pilB, and nan1 genes using the primers listed in Table 1, as described previously (9, 17, 18).
Clonal relationships among MDR isolates by RAPD-PCR
RAPD-PCR was used RAPD primers 208 (5´-ACGGCCGACC-3´) and 272 (5´- AGCGGGCCAA- 3´), as described earlier (19). The amplification protocol consisted of 25 µl RAPD- PCR mixture buffer (10 mM Tris/HCl, 50 mM KCl, 2.5 mM MgCl2, pH 8.3) containing 250 µM of each dNTP, 40 pmol oligonucleotide, 1 U Taq DNA polymerase Invitrogen (Invitrogen, CA, USA) and 40 ng template DNA. A negative control contained all the components except template DNA. PCR products were separated in 1.5% agarose gels and the resulting band patterns were analyzed using unweighted pair-group method with arithmetic averages (UPGMA) clustering (Gel Compare II software, version 4.0, Applied Maths, Sint-Matenslatem, Belgium). Isolates with 96% or greater similarity were considered as identical, and a cut-off value of 80% similarity was used for clustering.
Statistical analyses
All analyses were performed using SPSS, version 16.0 (SPSS Inc, Chicago, IL, USA, 2014). Chi-square test was used to investigate the differences between distributions of categorical data. Two-tailed; P-values of ≤ 0.05 were considered statistically significant.
Results
Sample sources and antibiotic susceptibility
One hundred isolates were from hospital A (31% from urine as the most frequent), 55 from hospital B (44% from sputum of patients with respiratory tract infection as the most frequent), 27 were from hospital C (all from burn exudates) and 26 from hospital D (all from burn exudates) and antibacterial susceptibility profiles of the P. aeruginosa isolates to 13 antibiotics are presented in Table 2. All isolates were susceptible to polymyxin B and colistin. Overall, 40.4% (n=84) of the isolates were MDR and 40% were carbapenem-resistant. From 84 MDRPA isolates, 53 (63%) were resistant to 11 antibiotics, of which 36 isolates were recovered from burn exudates. The rate of MDR P. aeruginosa (defined as resistance to at least 3 antibiotic classes) was 40.4% (in 208 isolates), including 68% from burn exudates, 37.5%, from urinary tract infection and 17.4% from blood samples.
Table 2.
Antibiotic resistant profile of Pseudomonas aeruginosa collected from hospitalized patients of four hospitals in Iran based on source of isolation
| source | number of isolate |
number (%) resistant isolates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTZ | CIP | MEM | IMI | ATM | CRO | CAZ | CPM | GM | TN | AK | ||
| burn exudate | 53 | 37(69.8) | 34(64.15) | 36(67.92) | 35(66.03) | 41(77.35) | 41(77.35) | 31(58.49) | 37(69.81) | 35(66.03) | 35(66.03) | 33(62.26) |
| urine | 48 | 12(25) | 16(33.33) | 17(35.41) | 17(35.41) | 14(29.16) | 30(62.50) | 13(27.08) | 13(27.08) | 17(35.41) | 15(31.25) | 13(27.08) |
| sputum | 29 | 7(24.13) | 9(31.03) | 11(37.93) | 10(34.48) | 8(27.58) | 18(62.06) | 8(27) | 8(27.58) | 8(27.58) | 7(24.13) | 6(20.68) |
| blood | 23 | 4(17.39) | 1(4.34) | 4(17.39) | 3(13.04) | 4(17.39) | 12(52.17) | 3(13.04) | 4(17.39) | 4(17.39) | 2(8.69) | 1(4.34) |
| wound | 22 | 3(13.63) | 6(27.27) | 5(22.72) | 5(22.72) | 7(31.81) | 14(63.63) | 6(27.27) | 5(2.72) | 5(22.72) | 5(22.72) | 5(22.72) |
| eye | 14 | 1(7.14) | 1(7.14) | 6(42.85) | 5(35.71) | 5(35.71) | 8(57.14) | 1(7.14) | 1(7.14) | 1(7.14) | 1(7.14) | 1(7.14) |
| others | 19 | 2(10.52) | 3(15.78) | 7(36.84) | 7(36.84) | 5(26.31) | 11(57.89) | 2(10.52) | 3(15.78) | 3(15.78) | 2(10.52) | 3(15.78) |
| total | 208 | 66(31.73) | 70(33.65) | 86(41.34) | 82(39.42) | 84(40.38) | 134(64.42) | 64(30.76) | 71(34.13) | 73(35.09) | 67(32.21) | 62(29.80) |
Amikacin (AK), Tobramycin (TN), Gentamicin (GM), cefepime (CPM), ceftazidime (CAZ), ceftriaxone (CRO), aztreonam (ATM), imipenem (IMI), meropenem (MEM), ciprofloxacin (CIP), piperacillin/tazobactam (PTZ)
Prevalence of AmpC, ESBL, MBL and class 1 integrons
The rates of ESBL, AmpC and MBL production among the MDRPA are shown in Table 3. The highest rate of AmpC production was detected in isolates recovered from burn exudates of hospitals C and D (n=18, 50%). MBL activity was detected in 64.3% (n=54) of the MDR isolates, and blaIMP-1 and blaVIM genes were detected in 84.5% (n=71) and 4.8% (n=4) of these isolates, respectively. No blaNDM was detected in this study. In addition, class 1 integrons was detected in 95% (n= 80) of the MDR isolates.
Table 3.
Prevalence of multi-drug resistance, β-lactamases production and class 1 integron among Pseudomonas aeruginosa from hospitalized patients of four hospitals of Iran
| source/samples | number of isolates | % MDR | ESBL | MBL(in MDRPA) | AmpC |
IMI
mean MIC (µg/ml) (E-test) |
*MBL genes | |
|---|---|---|---|---|---|---|---|---|
| IMP-1 | VIM | |||||||
| burn exudate | 53 | 36(68) | 46(86.8) | 32(89.9) | 18(50) | 64 | 29(80) | 4(11) |
| urine | 48 | 18(37.5) | 32(66.7) | 9(50) | 5(27.7) | 64 | 14(77) | |
| sputum | 29 | 11(38) | 20(69) | 6(54.5) | 1(9.1) | 128 | 10(90) | - |
| blood | 23 | 4(17.4) | 12(52) | 1(25) | - | 32 | 4(100) | - |
| wound | 22 | 6(27.3) | 15(68) | 2(33.3) | 3(50) | 64 | 6(100) | - |
| eye | 14 | 3(21.5) | 6(42.8) | 3(50) | 1(33.3) | 64 | 3(100) | - |
| others | 19 | 6(31.5) | 14(73.7) | 1(16.7) | - | 32 | 5(83) | - |
| total | 208 | 84(40.4) | 145(70) | 54(64.3) | 28(33) | - | 71(84.5) | 4(4.8) |
MDR= multidrug-resistant, MBL= metallo-β-lactamase, ESBL= extended spectrum-β-lactamase, IMI= imipenem, intΙ1= class 1 integrons integrase gene. Value in bracket indicate % in MDR population.*.NDM gene was not detected in any isolate. Others = fluids/c, Nose/c, etc
Detection of virulence genes
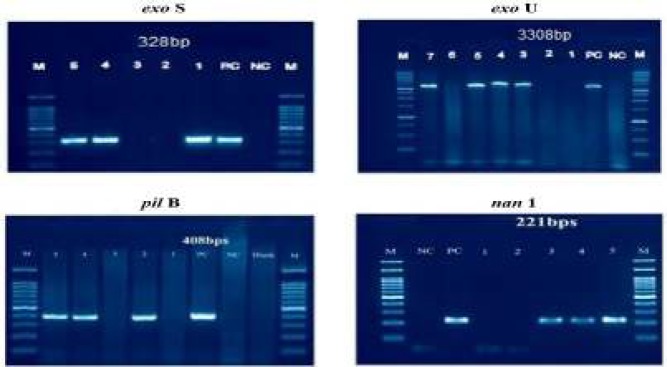
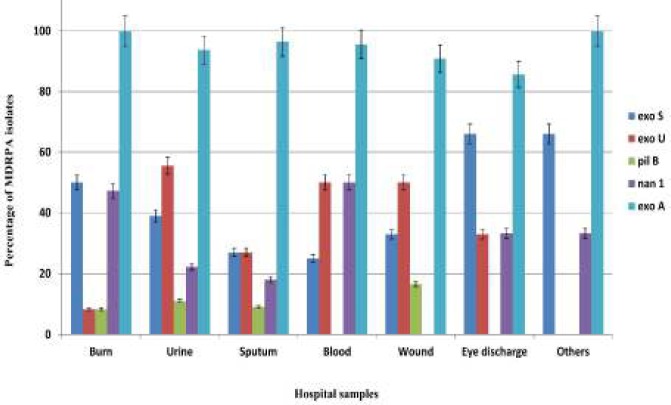
The exoA gene was detected in all 208 isolates and was the most frequent gene in both MDR and non MDR P. aeruginosa isolates with a total frequency of 98.8% and 93.5%, respectively. The second most frequent virulence gene in the 84 MDR isolates was exoS (44%), followed by nan1 (33.3%), exoU (26%) and pilB (8.3%) (Figures 1 and 2).
Figure 1.
Agarose gel electrophoresis of PCR products of virulent genes
M: marker (100 bp); NC: negative control; PC: positive control
Figure 2.
Distribution of exoS, exoU, pilB, nan1 and exoA genes among hospital isolates of MDR Pseudomonas aeruginosa from four hospitals in southeast of Iran. The error bar indicates average of three independent experiments
Clonality relationship among P. aeruginosa MDR isolates
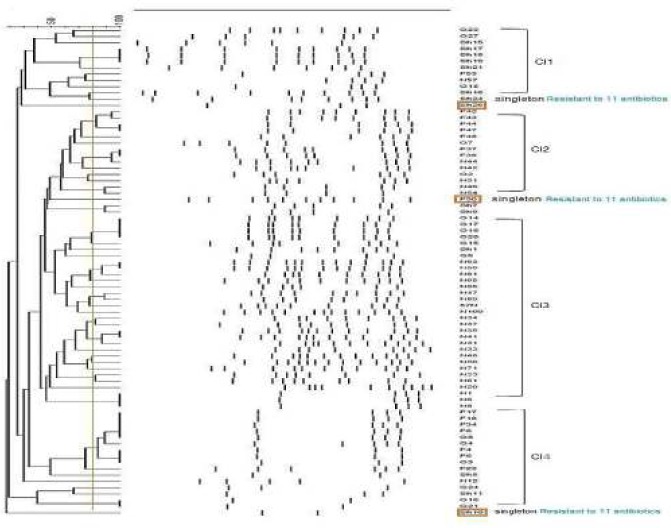
Based on RAPD-PCR profiles, the number of bands ranged from 10 to 19 with sized from 200–2000 bps. As shown in Figure3, four clusters were identified 80% similarity with 95% identical band patterns, indicating similar genetic backgrounds. We also found two small clusters (two member isolates) and three singletons. Most of the cluster 1 isolates were from hospital D in Kerman and most of cluster 2 isolates were recovered from hospitals A (36%), B (50 %) and C (14 %) in Shiraz.
Figure 3.
Clonal relationships among Pseudomonas aeruginosa isolates recovered from hospitals in two geographic locations in Iran shown by RAPD-PCR fingerprints. Banding patterns were analyzed by the unweighted pair-group method with arithmetic averages (UPGMA) clustering using Gel Compare II software, version 4.0 (Applied Maths, Sint-Matenslatem, Belgium). The vertical lines show 80% similarity cut-off. *N= Namazi, F= Faghihi. G= Ghotbeddin (Shiraz, Iran) and Sh= Shafa hospitals (Kerman, Iran)
Discussion
MDRPA has emerged as one of the most frequently observed nosocomial infectious agents causing a high rate of mortality among hospitalized patients (20). According to European Centre for Disease Prevention and Control 2012, point prevalence survey of healthcare-associated infections, it accounted for 8.9% of nosocomial infections, and 17.4% of lower respiratory tract infections in European acute care hospitals (21).
In the present study, and the rate of MDR P. aeruginosa was 40.4% and was highest in the isolates obtained from burn exudates (68%), followed by urinary tract infection isolates (37.5%) and blood samples (17.4%). A report from a surveillance study of 65 laboratories in the United States (1998 to 2001) showed that 7.0% of clinical isolates of P. aeruginosa from non-ICU patients and 9.1% of isolates from ICU patients were MDR. In another surveillance study conducted on MDRPA in an urban tertiary-care teaching hospital in USA in 2002, it was shown that the rate of MDR was 32% and increased by more than 20% over a five-year period (22). In another investigation in a tertiary care hospital in India from February 2012 to January 2013, the rate of MDRPA was 41.3% which is similar to our results (23). Overall, the rates of resistance of our isolates recovered from burn exudates were significantly higher than that of the other sources (P<0.05). Similar rates have been reported from other centers in Iran including 45.3% among burn patients studied in 2012 in Guilan by Nikokar et al. (24), and 45% by Fazeli et al. (25) in 2017 from patients at the university teaching hospital in Iran. Higher rates of MDRPA were reported by Yousefi et al. (2011-2012) in Shiraz (62.8%) and Ghanbarzadeh et al. (2015) from a single center for burn patients in Tehran, Iran (93.1%) (26, 27).
In our study, burn isolates exhibited the highest degree of resistance (>60%) to 11 antibiotics compared to the isolates from other sources (Table 2). Imipenem resistance was detected in 84.5% of (71 out of 84) MDRPA isolates and 39% (82 out of 208) of all P. aeruginosa isolates. As observed, compared to the isolates from other sources MDRPA isolated from burn exudates had the highest rate of ESBL (86.8%), MBL (89.8%) and AmpC (50%) production (Table 3).
In a report from burn patients in Hospital C in 2006, the rate of ESBL production was 4.3%, AmpC was 11.4% and MBL was 0% (28). Excessive use of antibiotics could be associated with the development of more resistant strains as a result of and horizontal gene spread. High frequencies of ESBL production in P. aeruginosa isolates from burn patients were reported from Iran by Rafiee et al. (39.2% ESBL, 37.3% MBL, and 68.6% AmpC production among 51 MDRPA isolates) (29). In a research carried out by Salimi and Eftekhar on 128 imipenem-resistant isolates of P. aeruginosa in Tehran, Iran, 12.5% were capable of producing ESBL, 25% MBL, and 81% produced AmpC β-lactamases (30). MBL blaVIM and blaIMP-1 genes are the common in MDR isolates of P. aeruginosa in Iran (31-33). The rate of blaIMP-1 gene in our study was higher than those reported from other parts of Iran. Also, 95.2% of our MDRPA isolates had IntI1 gene which is similar with the study of Khosravi et al. from Ahvaz and Yousefi et al. from Tehran (6, 34). However, lower rate of IntI1 gene were reported in other parts of Iran (1, 24, 34). Investigating the three MBL genes, blaVIM, blaIMP-1 and blaNDM among MDRPA strains, revealed that all imipenem-resistant isolates harbored blaIMP-1 gene. The common MBL genes in Asia are blaVIM and blaIMP-1 (31). A higher rate of blaIMP-1 gene was detected in the present study in comparison with other Iranian studies: Shahcheraghi et al. 0% in 2010, Kalanter et al. 3% in 2011 and 2012, and Sarhangi et al. 9.75% in 2012 (31, 35, 36). The rate of blaVIM gene was 4.8% detected in 4 MDRPA strains isolated from burn exudates, higher than similar studies from Iran (31, 37). In addition, recently our group identified one strain of P. aeruginosa harboring the blaNDM gene in Kerman, Iran (unpublished result).
Presence of diverse virulence genes in P. aeruginosa hospital isolates has been shown to associate with the intensity and severity of infections (38). The high worldwide prevalence of exotoxin A in P. aeruginosa has resulted in its use for identification of clinical isolates (39). The prevalence rates of exoS and nan1 genes were significantly higher in burn exudates (50% for exoS and 47% for nan1, respectively) than in other samples (P=0.0001). In contrast, the prevalence of exoU gene was significantly lower in burn exudate (16%) (P=0.023). The least virulence gene detected in this study was pilB (n=7, 8.3%), similar in burn exudates and non-burn samples. The higher frequency of nan1in burn patients may be due to its neuraminidase activity which facilitates bacterial attachment to the epithelial surfaces of burns and airways of cystic fibrosis patients resulting in colonization of P. aeruginosa (38).
There are few reports about clonal relationship among hospital isolates of P. aeruginosa in Iran. Our analysis of RAPD data showed four large clusters consisting of 77 isolates out of 84 (91.7%). We also detected the aggregation of isolates related to specific clusters in different hospitals (Figure 3). The high genetic similarity among MDRPA isolates suggests the cross-acquisition of infection. The dendrogram analysis revealed that cluster 3 exhibited the largest fingerprint similarity consisting of 29 isolates. Among the cluster 3 isolates, 76% were from hospital A and 21% from hospital C (Figure 3). Members of this cluster demonstrated close genetic relationships compared to other clusters, suggesting the possible spread of cluster 3 clones in different wards of hospitals A and C in Shiraz. Most members of cluster 4 were from hospitals B and C. As presented in Figure 3, all singletons showed resistance to 11 antibiotics, simultaneously.
Taheri et al. studied the genetic similarity among 73 P. aeruginosa isolates from Tehran referral hospitals by RAPD patterns and showed 67 different patterns, each containing 2-3 isolates, mostly from ICU (40). They concluded that most of the isolates were probably originated from the host.
Conclusion
Based on the results, it can be concluded that our P. aeruginosa hospital isolates are highly resistant to different classes of antibiotics and sensitive to colistin and polymyxin B, which could be used as an empirical therapy in critically ill patients, especially in burn patients and those admitted to ICU. High genetic similarity among MDRPA isolates indicates cross-acquisition of infection, suggesting the importance of infection control in decreasing the prevalence of MDRPA in hospitals.
Acknowledgment
The authors would like to thank the Research Center of Kerman University of Medical Sciences for funding this project which is a part of a PhD thesis at Kerman University of Medical Sciences (Grant No:93/220), and Professor Alborzi Clinical Microbiology Research Center (PACMRC), Shiraz University of Medical Sciences for their help during this research. We also thank Hassan Khajehei, PhD, for copy editing of the manuscript.
Funding
This research was supported by a grant from research council of Kerman University of Medical Sciences (Grant No: 93/220) and Professor Alborzi Clinical Microbiology Research Center (PACMRC).
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Mirahsani M, Khorshidi A, Moniri R, Gilasi HR. Prevalence of class 1 Integron, resistance gene cassettes and antimicrobial susceptibility profiles among Isolates of Pseudomonas aeruginosa in Iran. Open J Med Microbiol. 2016;6:87–96. [Google Scholar]
- 2.Barrios CC, Ciancotti-Oliver L, Bautista-Rentero D, Adán-Tomás C, Zanón-Viguer V. A New treatment choice against multi-drug resistant Pseudomonas aeruginosa: doripenem. J Bacteriol Parasitol. 2014;5:199–203. [Google Scholar]
- 3.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 4.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini SMJ, Naeini NS, Khaledi A, Daymad SF, Esmaeili D. Evaluate the relationship between class 1 integrons and drug resistance genes in clinical isolates of Pseudomonas aeruginosa. Open Microbiol J. 2016;10:188–196. doi: 10.2174/1874285801610010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosravi AD, Motahar M, Montazeri EA. The frequency of class1 and 2 integrons in Pseudomonas aeruginosa strains isolated from burn patients in a burn center of Ahvaz, Iran. PloS one. 2017;12:e0183061. doi: 10.1371/journal.pone.0183061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streeter K, Katouli M. Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect Epidemiol Med. 2016;2:25–32. [Google Scholar]
- 8.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 9.Wolska K, Kot B, Mioduszewska H, Sempruch C, Borkowska L, Rymuza K. Occurrence of the nan1 gene and adhesion of Pseudomonas aeruginosa isolates to human buccal epithelial cells. Biol Lett. 2012;49:59–64. [Google Scholar]
- 10.Nanvazadeh F, Khosravi AD, Zolfaghari MR, Parhizgari N. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39:1409–1413. doi: 10.1016/j.burns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Bekele T, Tesfaye A, Sewunet T, Waktola HD. Pseudomonas aeruginosa isolates and their antimicrobial susceptibility pattern among catheterized patients at Jimma University Teaching Hospital, Jimma, Ethiopia. BMC Res Notes. 2015;8:488–491. doi: 10.1186/s13104-015-1497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne P. Performance standards for antimicrobial susceptibility testing. Ninth informational supplement NCCLS document M100-S9 National Committee for Clinical Laboratory Standards 2008. pp. 120–126. [Google Scholar]
- 13.Ranjan S, Banashankari GS, Babu PRS. Evaluation of phenotypic tests and screening markers for detection of metallo-β-lactamases in clinical isolates of Pseudomonas aeruginosa: A prospective study. Med J DY Patil University. 2015;8:599–605. [Google Scholar]
- 14.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J Clin Microbiol. 2003;41:3542–3547. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K, Chong Y, Shin H, Kim Y, Yong D, Yum J. Modified hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001;7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Mirsalehian A, Kalantar-Neyestanaki D, Nourijelyani K, Asadollahi K, Taherikalani M, Emaneini M, et al. Detection of AmpC-β-lactamases producing isolates among carbapenem resistant P aeruginosa isolated from burn patient. Iran J Microbiol. 2014;6:306–310. [PMC free article] [PubMed] [Google Scholar]
- 17.Finnan S, Morrissey JP, O’gara F, Boyd EF. Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J Clin Microbiol. 2004;42:5783–5792. doi: 10.1128/JCM.42.12.5783-5792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Moore JE, Murphy PG, Millar BC, Elborn JS. Early detection of Pseudomonas aeruginosa–comparison of conventional versus molecular (PCR) detection directly from adult patients with cystic fibrosis (CF) Ann Clin Microbiol Antimicrob. 2004;3:1–5. doi: 10.1186/1476-0711-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahenthiralingam E, Campbell ME, Foster J, Lam JS, Speert DP. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J Clin Microbiol. 1996;34:1129–1135. doi: 10.1128/jcm.34.5.1129-1135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrogi V, Cavalié L, Mantion B, Ghiglia M-J, Cointault O, Dubois D, et al. Transmission of metallo-β-lactamase-producing Pseudomonas aeruginosa in a nephrology-transplant intensive care unit with potential link to the environment. J Hosp Infect. 2016;92:27–29. doi: 10.1016/j.jhin.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Jung R, Fish D, Obritsch M, MacLaren R. Surveillance of multidrug-resistant Pseudomonas aeruginosa in an urban tertiary-care teaching hospital. J Hosp Infect. 2004;57:105–111. doi: 10.1016/j.jhin.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Senthamarai S. Resistance pattern of Pseudomonas aeruginosa in a tertiary care hospital of Kanchipuram, Tamilnadu, India. J Clin Diagn Res. 2014;8:30–32. doi: 10.7860/JCDR/2014/7953.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikokar I, Tishayar A, Flakiyan Z, Alijani K, Rehana-Banisaeed S, Hossinpour M, et al. Antibiotic resistance and frequency of class 1 integrons among Pseudomonas aeruginosa, isolated from burn patients in Guilan, Iran. Iran J Microbiol. 2013;5:36–41. [PMC free article] [PubMed] [Google Scholar]
- 25.Fazeli H, Sadighian H, Esfahani BN, Pourmand MR. Genetic characterization of Pseudomonas aeruginosa resistant isolates at the university teaching hospital in Iran. Adv Biomed Res. 2015;4:156–162. doi: 10.4103/2277-9175.161583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousefi-Avarvand A, Khashei R, Ebrahim-Saraie HS, Emami A, Zomorodian K, Motamedifar M. The Frequency of exotoxin A and exoenzymes S and U genes among clinical isolates of Pseudomonas aeruginosa in Shiraz, Iran. Int J Mol Cell Med. 2015;4:167–173. [PMC free article] [PubMed] [Google Scholar]
- 27.Ghanbarzadeh Corehtash Z, Ahmad Khorshidi FF, Akbari H, Aznaveh AM. Biofilm formation and virulence factors among Pseudomonas aeruginosa isolated from burn patients. Jundishapur J Microbiol. 2015;8:e22345. doi: 10.5812/jjm.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Japoni A, Alborzi A, Kalani M, Nasiri J, Hayati M, Farshad S. Susceptibility patterns and cross-resistance of antibiotics against Pseudomonas aeruginosa isolated from burn patients in the South of Iran. Burns. 2006;32:343–347. doi: 10.1016/j.burns.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Rafiee R, Eftekhar F, Tabatabaei SA, Tehrani DM. Prevalence of extended-spectrum and metallo β-lactamase production in AmpC β-lactamase producing Pseudomonas aeruginosa isolates from burns. Jundishapur J Microbiol. 2014;7:e16436. doi: 10.5812/jjm.16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salimi F, Eftekhar F. Coexistence of AmpC and extended-spectrum β-lactamases in metallo-β-lactamase producing Pseudomonas aeruginosa burn isolates in Tehran. Jundishapur J Microbiol. 2013;6:e7178. doi: 10.5812/jjm.16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahcheraghi F, Nikbin VS, Feizabadi MM. Identification and genetic characterization of metallo-beta-lactamase-producing strains of Pseudomonas aeruginosa in Tehran, Iran. New Microbiol. 2010;33:243–248. [PubMed] [Google Scholar]
- 32.Ghamgosha M, Shahrekizahedani S, Kafilzadeh F, Bameri Z, Taheri RA, Farnoosh G. Metallo-beta-lactamase VIM-1, SPM-1, and IMP-1 genes among clinical Pseudomonas aeruginosa species isolated in Zahedan, Iran. Jundishapur J Microbiol. 2015;8:e17489. doi: 10.5812/jjm.8(4)2015.17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallah F, Noori M, Hashemi A, Goudarzi H, Karimi A, Erfanimanesh S, et al. Prevalence of blaNDM, blaPER, blaVEB, blaIMP, and blaVIM genes among Acinetobacter baumannii isolated from two hospitals of Tehran, Iran. Scientifica. 2014;2014:e245162. doi: 10.1155/2014/245162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yousefi S, Farajnia S, Nahaei MR, Akhi MT, Ghotaslou R, Soroush MH, et al. Detection of metallo-β-lactamase–encoding genes among clinical isolates of Pseudomonas aeruginosa in northwest of Iran. Diagn Microbiol Infect Dis. 2010;68:322–325. doi: 10.1016/j.diagmicrobio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Neyestanaki DK, Mirsalehian A, Rezagholizadeh F, Jabalameli F, Taherikalani M, Emaneini M. Determination of extended spectrum beta-lactamases, metallo-beta-lactamases and AmpC-beta-lactamases among carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. Burns. 2014;40:1556–1561. doi: 10.1016/j.burns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Sarhangi M, Motamedifar M, Sarvari J. Dissemination of Pseudomonas aeruginosa producing blaIMP1, blaVIM2, blaSIM1, blaSPM1 in Shiraz, Iran. Jundishapur J Microbiol. 2013;6:e6920. [Google Scholar]
- 37.Akya A, Salimi A, Nomanpour B, Ahmadi K. Prevalence and clonal dissemination of metallo-beta-lactamase-producing Pseudomonas aeruginosa in Kermanshah. Jundishapur J Microbiol. 2015;8:e20980. doi: 10.5812/jjm.20980v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strateva T, Mitov I. Contribution of an arsenal of virulence factors to pathogenesis of Pseudomonas aeruginosa infections. Ann Microbiol. 2011;61:717–732. [Google Scholar]
- 39.Khan AA, Cerniglia CE. Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Appl Environ Microbiol. 1994;60:3739–3745. doi: 10.1128/aem.60.10.3739-3745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taheri ZM, Shahbazi N, Khoddami M. Genetic diversity of Peudomonas aeruginosa Strains isolated from hospitalized patients. Tanaffos. 2008;7:32–39. [Google Scholar]
- 41.Doosti M, Ramazani A, Garshasbi M. Identification and characterization of metallo-β-lactamases producing Pseudomonas aeruginosa clinical isolates in university hospital from Zanjan Province, Iran. Iran Biomed J. 2013;17:129–133. doi: 10.6091/ibj.1107.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kali A, Srirangaraj S, Kumar S, Divya H, Kalyani A, Umadevi S. Detection of metallo-beta-lactamase producing Pseudomonas aeruginosa in intensive care units. Australas Med J. 2013;6:686–693. doi: 10.4066/AMJ.2013.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]