Abstract
Recent identification of platelet/megakaryocyte-biased hematopoietic stem/repopulating cells requires revision of the intermediate pathway for megakaryopoiesis. Here, we show a unipotent megakaryopoietic pathway bypassing the bipotent megakaryocyte/erythroid progenitors (biEMPs). Cells purified from mouse bone marrow by CD42b (GPIbα) marking were demon-strated to be unipotent megakaryocytic progenitors (MKPs) by culture and transplantation. A subpopulation of freshly isolated CD41+ cells in the lineage Sca1+cKit+ (LSK) fraction (subCD41+LSK) differentiated only into MKP and mature megakaryocytes in culture. Although CD41+LSK cells as a whole were capable of differentiating into all myeloid and lymphoid cells in vivo, they produced unipotent MKP, mature megakaryocytes, and platelets in vitro and in vivo much more efficiently than Flt3+CD41−LSK cells, especially at the early phase after trans-plantation. In single cell polymerase chain reaction and thrombopoietin (TPO) signaling analyses, the MKP and a fraction of CD41+LSK, but not the biEMP, showed the similarities in mRNA expression profile and visible TPO-mediated phosphorylation. On increased demand of platelet production after 5-FU treatment, a part of CD41+LSK population expressed CD42b on the surface, and 90% of them showed unipotent megakaryopoietic capacity in single cell culture and predominantly produced platelets in vivo at the early phase after transplantation. These results suggest that the CD41+CD42b+LSK are straightforward progenies of megakaryocytes/platelet-biased stem/repopulating cells, but not progenies of biEMP. Consequently, we show a unipotent/highly biased megakaryopoietic pathway interconnecting stem/repopulating cells and mature megakaryocytes, the one that may play physiologic roles especially in emergency mega-karyopoiesis.
Keywords: Adult hematopoietic stem cells, Megakaryocyte, Thrombopoietin, Hematopoietic progenitors, Thrombopoiesis
INTRODUCTION
Megakaryocytes has been thought to be generated downstream to bipotent megakaryocyte/erythroid progenitors (biEMPs) that have been variably identified, such as megakaryocyte–erythroid progenitors (MEPs defined as lineage (Lin)−Sca1−cKit+CD34−FcγRII/IIIlow/- [1] and PreMegE as Lin−Sca1−cKit+CD41− CD150+Endoglin−FcγRII/III−) [2]), although the presence of a megakaryopoietic pathway that bypasses the biEMP has also been proposed [3–6]. Recently, hematopoietic stem cells (HSCs) that were biased to platelet production (platelet-biased HSC, PbHSC) [7] were identified at the top of the hematopoietic hierarchy. In a line with substantial similarities with this, megakaryocyte-restricted repopulating cells (MkRP) have been demonstrated by extensive single cell transplantation experiments [8].These latest studies provided a concept that the commitment to the megakaryocytic lineage could be made at the much higher hierarchical level than that previously thought. However, the intermediate pathway bridging the stem/repopulating cells and mature megakaryocytes/platelets has been elusive.
To that end, the prospective isolation of progenitors that can explain the intermediate megakaryopoietic pathway is inevitable. Several immunophenotypic markers such as CD9 (a member of tetraspan transmembrane protein), CD41 (an integrin aIIb subunit), and CD150 (a SLAM surface marker) have been used in various combinations to isolate unipotent progenitors for megakaryocytic progenitor (MKP) from the Lin−Sca1−cKit+ progenitor compartment [2, 9, 10]. However, none of those surface markers were unique to MKP or megakaryocytic lineage cells [10–12].
Precise understanding of the usage of thrombopoietin (TPO)/cMpl signaling is another issue to solve questions on megakaryopoiesis. TPO was originally identified as a growth and differentiation factor for megakaryocytes [13, 14], but subsequently proved to also have an essential role in the self-renewal of HSC [15, 16]. Because of the paucity of information about the intermediate pathway, TPO/cMpl signaling has been differently discussed for HSC regulation and megakaryopoiesis [17]. Consequently, it has been unclear whether biEMPs are among central targets of TPO and whether they actually give rise to MKP.
In this study, we show that CD42b (also called as glycoprotein [GP] Ibα), previously thought to be a specific surface marker of mature megakaryocytes and platelets, is expressed on unipotent MKP in the Lin−Sca1−cKit+ population. Furthermore, expression of CD42b can be conveniently used to isolate MKP. In single cell analyses, we found that a subpopulation of the CD41+ fraction in the Lin−Sca1+cKit+ (LSK) compartment (designated CD41+LSK cells) is highly biased to the megakaryocyte/platelet lineage and actually represents direct progenitors of the CD42b+MKP. Furthermore, CD42b is expressed even in the LSK compartment upon increased demand of platelet production. Our results imply the TPO/cMpl signaling in HSC through megakaryopoiesis in a continuous manner, and ultimately, a previously unraveled intermediate pathway for megakaryocyte/platelet production.
MATERIALS AND METHODS
Mice
Eight to 12 weeks old C57BL/6 (purchased from Japan SLC, Hamamatsu, Japan) and CAG promoter-green fluorescent protein (GFP) transgenic mice (from Dr S. Takahashi, University of Tsukuba, Ibaraki, Japan) and cMpl-deficient mice (from Dr F.de Sauvage Genentech, San Francisco, CA) were used in the study. All mice procedures were performed according to the Guide for Care and Use of Laboratory Animals at the University of Tsukuba.
Purification of HSC/Progenitor Cells Including Pure Megakaryocyte Progenitors
HSCs and progenitor cells were isolated by staining of unfractionated bone marrow cells (BMCs) with biotin-conjugated antibodies against CD3, CD4, CD8, B220, TER119, Gr1, and Mac1, and eliminated by anti-biotin beads (Miltenyi) using the magnetic sorting method. Lineage-eliminated cells were stained with allophycocyanin (APC)-conjugated anti-cKit, phycoerythrin-cyanin 7 (PECy7)-conjugated anti-Sca1, APC or fluorescein isothiocyanate (FITC)-conjugated anti-CD34, allophycocyanin-cyanin 7 (APCCy7)-conjugated anti-CD16/32 (FcγRII/III), PE or FITC or PECy7-conjugated anti-CD41, PE or FITC-conjugated anti-CD150, phycoerythrin (PE)-conjugated anti-Flt3, FITC-conjugated anti-Endoglin, CD9, and PE-conjugated anti-CD42b (Emfret), and streptoavidin-PERCP. Propidium ionide (molecular probes) was used to eliminate the dead cells. All antibodies were purchased from BD Bioscience unless otherwise indicated. The viability of the BMCs was >95%. The data obtained from 5–10 × 105 live cells were used to estimate the frequencies of each progenitor fraction [18]. Quantification of CD42b positive MKP or mature megakaryocytes was performed by Trucount Beads (BD Bioscience). The gating strategy for quantification of the cell in the culture and the standard curve were shown in Supporting Information Figure S3A. The number of the cells in the sample was calculated by the following equation: “(The number of events of the cells of interest/the number of events of the beads) × the absolute number of the beads in the tube = the absolute number of the cells of interest in the sample”. Cells were sorted on fluorescence-activated cell sorting (FACS) Aria (BD Bioscience) and all flow cytometry data were analyzed with FlowJo software (Tree Star).
In Vitro Culture Assay
Freshly isolated unfractionated BMCs and sorted HSC/progenitor cells were cultured with semisolid culture using Methocult M3234 media or liquid culture using StemSpan SFEM (Stem cell Technologies, at 37°C, 5% CO2) in 96-well round-bottom dishes to evaluate lineage potentials of isolated cell populations. The additional cytokine condition was 50 ng/ml human TPO, 50 ng/ml stem cell factor (SCF), 20 ng/ml interleukin 6 (IL-6), and 20 ng/ml IL-11. Cytokines were purchased from PeproTech. Single cell culture sorted by FACS was also performed in the same conditions. For evaluation of lineage commitment, differentiated colonies or cells in the dish were picked up and stained with May-Giemsa staining on a poly-L-lysine-coated glass slide. For immunostaining, anti vWF (DAKO), CD42b, and TER119 antibodies were used for megakaryocyte–erythroid differentiation. Secondary antibodies and nuclear staining were performed with goat anti-rat Alexa 488, goat anti rabbit Alexa 547, and Vector shield with 4’ 6-diamidino-2-pheylindole (DAPI) (Vector). Same antibodies were used for the immunostaining using fresh frozen bone sample. For the cytokine signal analysis, the cells on the poly-L-lysine coated glass slides were stained with anti-Y694-phosphorylated STAT5, S473-phosphorylated AKT, and T1007-phosphorylated JAK2 antibodies (Cell Signaling Technology) 30 minutes after cytokine stimulation. Fluorescence was visualized by a confocal microscopy (TCS SP5, Leica).
Transplantation
Cells from CAG promoter-GFP mice were transplanted at indicated cell numbers into sublethally (4.5Gy) or lethally (9.5Gy) irradiated syngeneic recipient mice to evaluate the platelet production capacities in vivo. At indicated time points, peripheral blood was collected and evaluated by flow cytometry for CD41+GFP+ platelets. Peripheral blood was mixed with 3% fetal bovine serum/phosphate-buffered saline and centrifuged at 900 rpm for 5 minutes. Supernatant was used for platelet analysis and the pellets were used for leukocyte analysis after removal of red blood cells. The absolute number of blood cells was counted by auto cytometer (MEK-6400, Nihon Kohden).
5-FU Treatment
To evaluate hematopoietic reconstitution after 5-FU treatment, 150 mg/kg 5-FU (gifted from Kyowa Hakko Kirin Pharma) was intraperitoneally injected into the mice. BMCs and peripheral blood were obtained at the indicated time points and stained with indicated antibodies.
Single Cell RT-PCR Analysis
Single hematopoietic progenitor cells were sorted into round-bottomed 96-well plates. The template cDNA was generated from the cell lysates using CellsDirect One-Step quantitative real-time polymerase chain reaction (qRT-PCR) kits (Invitrogen) and the specific Taqman probe for the each gene. The cDNA was preamplified by 20 cycles of PCR, before real-time RT-PCR using the BioMark HD system (Fluidigm). In all progenitor fractions, the experiment was repeated twice and at least 48 cells were analyzed in each experiment. Actin beta mRNA (endogenous control) was detected in >95% of samples.
Single Cell mRNA Imaging
Sorted cells on the poly-L-lysine coated glass slides were fixed and permeabilized with 4% paraformaldehyde and cold methanol. Then, a mouse CD42b mRNA probe was directly hybridized in situ. The probe was visualized with QuantiGene FlowRNA system (eBioscience) by a confocal microscopy.
RESULTS
CD42b Marks Unipotent MKP in the Hematopoietic Progenitor Fraction
We found that 6.6% (range, 6.0%–7.0%) of the common myeloid progenitors (CMPs, Lin−Sca1−cKit+CD34+FcγRII/IIIlow/-) [1] expressed CD42b, the receptor of von Willebrand factor (vWF) [19], which has thus far been identified only in mature megakaryocytes and platelets (Fig. 1A) [19, 20]. CD42b expression was not detected in LSK, granulocyte–monocyte progenitors (GMPs; Lin− Sca1− cKit+CD34+FcγRII/III+), and MEPs (Fig. 1A). The CD42b+ fraction in CMP also expressed CD150, CD41high, and CD9high on the cell surface (Supporting Information Fig. S1A). CD42c (GPIbβ), CD42d (GPV), and CD42a (GPIX), which are the components of GPIb-V-IX complex, were also expressed on this fraction (Supporting Information Fig. S1A). In Wright-Giemsa staining, these cells showed morphology distinct from mature megakaryocytes, featuring mononuclear and basophilic immature morphology (Supporting Information Fig. S1A(ii)). These cells may correspond to the small round-shaped cells expressing CD42b and vWF, and CD42b and CD34, identified on the bone marrow section at low frequencies (Supporting Information Fig. S1A(iii)).
Figure 1.

CD34+CD42b+ cells have a restricted capacity of megakaryocyte differentiation in vitro and platelet production in vivo. (A): Identification of CD34+CD42b+ population in bone marrow cells (BMCs). Adult mouse BMCs were stained with antibodies for cKit, Sca1, lineage marker (Lin), CD34, CD16/32 (FccRII/III), CD42b, CD41, CD150, and CD9. Note that only CD34+ fraction of the Lin− population expressed CD42b (3rd figure from the left in the upper panels) and that the CD34+CD42b+ population was confined to the Sca1−cKit+ population (right in the upper panels), mainly in the common myeloid progenitor (CMP) fraction (the lower panels). A representative result from five independent experiments is shown. (B): (i) The representative morphologies of the colonies derived from indicated cell types in 96-well-plate liquid culture. The number of cells seeded in one well was 500 for LSK/CMP (CD42b-), 2000 for megakaryocyte–erythroid progenitor (MEP)/megakaryocytic progenitor (MKP). Arrowheads indicate mature megakaryocytes. (ii) Frequencies of vWF+ and TER119+ cells in the liquid culture shown in (i). (C): Capacity of CD34+CD42b+ cells (MKP) and MEP to generate platelet in vivo. Sublethally irradiated (4.5 Gy) mice were transplanted with 1 × 104 CD34+CD42b+ cells or MEP from green fluorescent protein transgenic mice. On days 4, 7, 11, and 14 after transplantation, peripheral blood was collected and analyzed for platelet differentiation using CD41+ platelet-sized cells (n 5 4). Abbreviations: CMP, common myeloid progenitor; FSC, forward scatter; GFP, green fluorescent protein; GMP, granulocyte–monocyte progenitor; LSK, lineage−Sca1+cKit+; MEP, megakaryocyte–erythroid progenitor; MKP, megakaryocytic progenitor; SSC, side scatter.
To investigate whether the population identified as Lin−Sca1−cKit+CD34+CD42b+ cells represents MKP, we cultured them in semisolid and liquid medium in the presence of SCF and TPO. Despite the cell-surface antigen expression pattern compatible with CMP, the cells proliferated on average less than twofold, even after 7 days in liquid culture (Supporting Information Fig. S1B(i)) and 14 days in semisolid cultures (Supporting Information Fig. S1B(ii)). Remarkably, all of the cells in a 5-day liquid culture showed a large multi-nucleated morphology with positive staining of vWF (Fig. 1B(i), 1B(ii); Supporting Information Fig. S1B(iii)), compatible with mature megakaryocytes. CD34 and CD42b double-positive cells were confined to the Lin−Sca1−cKit+ fraction (Fig. 1A) and the previously identified MKP (CD9highCD41+ cells [9], CD150+CD41+ cells [2], and CD9highCD150+ cells [10]) also expressed CD42b (Supporting Information Fig. S1A(i)). Indeed, the absolute numbers of variously identified MKP were very similar to each other (Supporting Information Fig. S1C). All this data indicated that CD34 and CD42b double-positivity represented an excellent marker for MKP, and thus was used to isolate MKP. Megakaryocyte-producing activities were also evaluated for LSK, CD42b-negative CMP, GMP, and MEP in the same liquid and semisolid culture systems. All these fractions, except for GMP, produced large, vWF-positive megakaryocytes, but only as a minor component (Fig. 1B(i), 1B(ii)). As expected, the major products from MEP were TER119+ and/or CD71+ erythroid cells, and vWF staining-positive cells were produced at a lower frequency (Fig. 1B(i), Fig. 1B(ii); Supporting Information Fig. S1B(iii)).
We next investigated the platelet production capacity of CD34+CD42b+ MKP in vivo using transgenic mice that express GFP under the CAG promoter. After injection of CD34+CD42b+ MKP into sublethally irradiated mice, GFP+ platelets emerged in the peripheral blood as early as 4 days after the injection (Fig. 1C). GFP+ platelets were also generated in recipients injected with MEP, but to a much lesser extent compared with the MKP injection on a per-cell basis (Fig. 1C; Supporting Information Fig. S1D(i)). GFP+ leukocytes were detected in the mice injected with LSK (Supporting Information Fig. S1D(ii)), but not in the mice injected with CD34+CD42b+ MKP. This finding confirms unipotency of CD34+CD42b+ MKP and capability of producing platelets in vivo.
Subpopulation of CD41+LSK Is the Source of Unipotent Megakaryopoiesis Through MKP
In liquid culture, LSK gave rise to cells with the Lin−Sca1−cKit+CD34+CD42b+ MKP phenotype after a 2-day culture at the approximate frequency of 4% (Fig. 2A; Supporting Information Fig. S2A). Cells with the same phenotype were not identified or identified only at very low frequencies after the culture of CMP, GMP, MEP, and PreMegE (<0.5%; Fig. 2A; Supporting Information Fig. S2A, S2B), despite the fact that biEMP gave rise to mature megakaryocytes, although at relatively low frequencies (Fig. 1B(i), 1B(ii); Supporting Information Fig. S2B, S2C). We therefore hypothesized that MKP could be generated directly from a fraction of LSK, bypassing biEMP.
Figure 2.
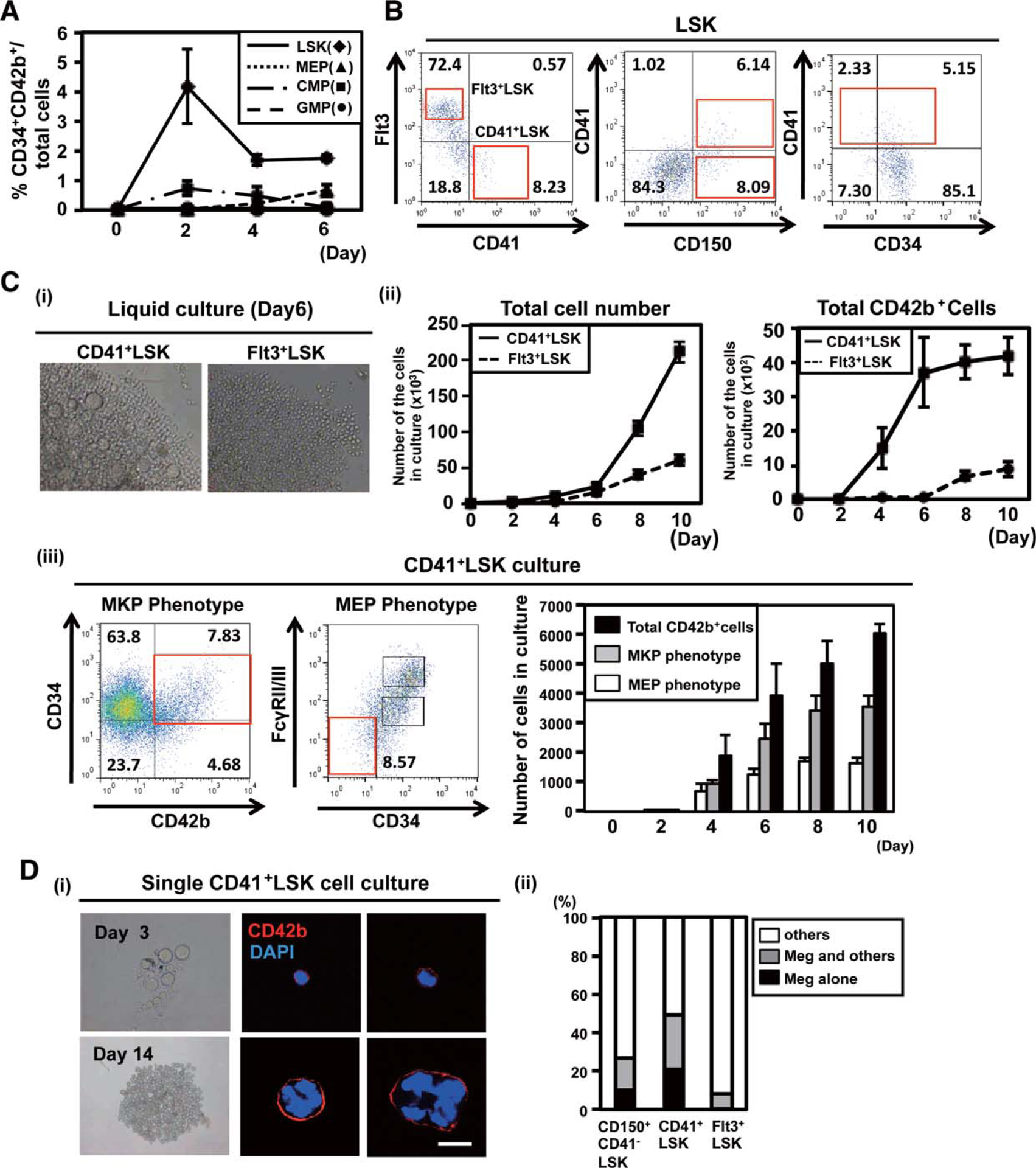
A subpopulation of CD41+LSK is the source of unipotent megakaryopoiesis through megakaryocytic progenitor (MKP). (A):Frequencies of CD34+CD42b+ cells after liquid culture of each of the progenitor cells. Two thousand Lineage−Sca1+cKit+ (LSK), megakaryocyte–erythroid progenitor (MEP), common myeloid progenitor (CMP) depleted with MKP, and granulocyte–monocyte progenitor (GMP) were sorted from mouse bone marrow and cultured in the presence of stem cell factor (SCF), thrombopoietin (TPO), interleukin (IL)26, and IL-11. Cells in culture were stained with antibodies for CD34 and CD42b, and analyzed by flow cytometry. The means of triplicate were shown. (B): Expression pattern of CD150, CD41, CD34, and Flt3 in the LSK population. Note that CD41 and Flt3 are expressed in an almost mutually exclusive manner. (C): (i) A representative morphology of CD41+LSK-and Flt3+LSK-derived colonyforming cells (day 6; 3200, left panel). (ii) Numbers of total CD42b+ cells after culturing 500 CD41+LSK or Flt3+LSK were calculated on flow cytometry, using the fixed numbers of beads (left). (iii) The numbers of total CD42b+, Lin−Sca1−cKit+CD34+CD42b+ (MKP phenotype), and Lin− Sca1−cKit+FcγRII/III−CD34− cells (MEP phenotype) after culturing 500 CD41+LSK. Representative results of day 10 were shown in left panel. The cell numbers were calculated using the same methods (right graph). (D): (i) Typical morphologies of pure megakaryocyte-lineage colonies derived from a single CD41+LSK in 96-well (upper panels, day 3; lower panels, day 14 of culture). The right panels show immunostaining of the cells from the pure megakaryocytes-lineage colonies (red, CD42b; blue, DAPI). Scalebar 5 20 lm. (ii) Preferential differentiation of CD41+LSK into megakaryocytes assessed by single cell culture. CD150+CD41−LSK (HSC-enriched population), CD41+LSK, and Flt3+LSK were single cell-sorted into each well of 96-well dishes and cultured in the presence of SCF, TPO, IL-6, and IL-11. Colonies formed in each well were classified into those containing megakaryocytes alone, containing megakaryocytes and others, and without megakaryocytes. The results of three independent experiments are shown. The total number of colonies from each progenitor was 376 in CD41+LSK, 204 in Flt3+LSK, and 446 in CD150+CD41−LSK. Abbreviations: CMP, common myeloid progenitor; DAPI, 4’6-diamidino-2-phenylindole; GMP, granulocyte–monocyte progenitor; LSK, lineage−Sca1+cKit+; MEP, megakaryocyte–erythroid progenitor; MKP, megakaryocytic progenitor; SSC, side scatter.
The LSK population has been divided into at least five fractions phenotypically and biologically (CD34− HSC and CD34+ multi-potent progenitors 1–4 (MPP1–4)) [21]. We focused on the CD41+ fraction in LSK (CD41+LSK), because CD41 is a well-known marker expressed during megakaryopoiesis and also reported to be expressed on a portion of HSC [8, 22, 23] and MPP [11, 24]. The expression of CD41 was confined to the CD150+ and Flt3− fraction in the LSK compartments (Fig. 2B) as predicted from the previous report [25], while both CD34+ and CD34− LSK contained CD41+ cells (Fig. 2B). These results indicated that CD41 was expressed on fractions of HSC, MPP1, and MPP2, but not on MPP3 and MPP4.
CD41+LSK gave rise to mature megakaryocytes at a much higher efficiency than Flt3high fraction in LSK (Flt3+LSK, Fig. 2B) representing MPP3 and MPP4, from which very few megakaryocytes were produced (Fig. 2C(i), 2C(ii)). CD41+LSK also produced Lin−Sca1−cKit+CD34+CD42b+ cells (MKP phenotype) at a high efficiency, while Lin−Sca1−cKit+ FcγRII/III−CD34− cells (MEP phenotype) were also produced (Fig. 2C(iii)). When resorted and cultured, Lin−Sca1− cKit+CD34+CD42b+ cells derived from CD41+LSK showed low-proliferative potential and unipotency to differentiate only into megakaryocytes (Supporting Information Fig. S2D), similar to the MKP isolated from bone marrow.
To get insight into whether the CD41+LSK-derived Lin− Sca1−cKit+CD34+CD42b+ cells were direct progeny of a sub-population of CD41+LSK (subCD41+LSK), we cultured CD41+LSK in a single cell and observed that only blast-like cells and megakaryocytes were generated in approximately 20% of wells (Fig. 2D). When stained, all the mononuclear blast-like cells and multinucleated megakaryocytes were positive in CD42b (Fig. 2D). In the culture of CD150+CD41−LSK, which contained HSC [24], 10% of wells contained only blast-like cells and megakaryocytes (Fig. 2D(ii), see the most left column). Large numbers (1000–10,000) of blasts and megakaryocytes, without other kinds of cells, were generated in 1% of wells seeded with both CD41+LSK and CD150+CD41−LSK (Fig. 6C, see the first two columns from the left in the right panel). We routinely used a cytokine condition with SCF, TPO, IL-6, and IL-11 in the single cell culture (see Methods), and there was a possibility that the cytokine condition affected the results. Nevertheless, a different cytokine combination with SCF, TPO, IL-3, and erythropoietin (EPO) [26] did not significantly affect the frequencies of wells with only blasts and megakaryocytes from both CD41+LSK and CD150+CD41−LSK (Supporting Information Fig. S2E). This indicated that the megakaryocytic unipotency in fractions of these HSC and MPP populations is intrinsically determined rather than influenced by the culture condition.
Figure 6.
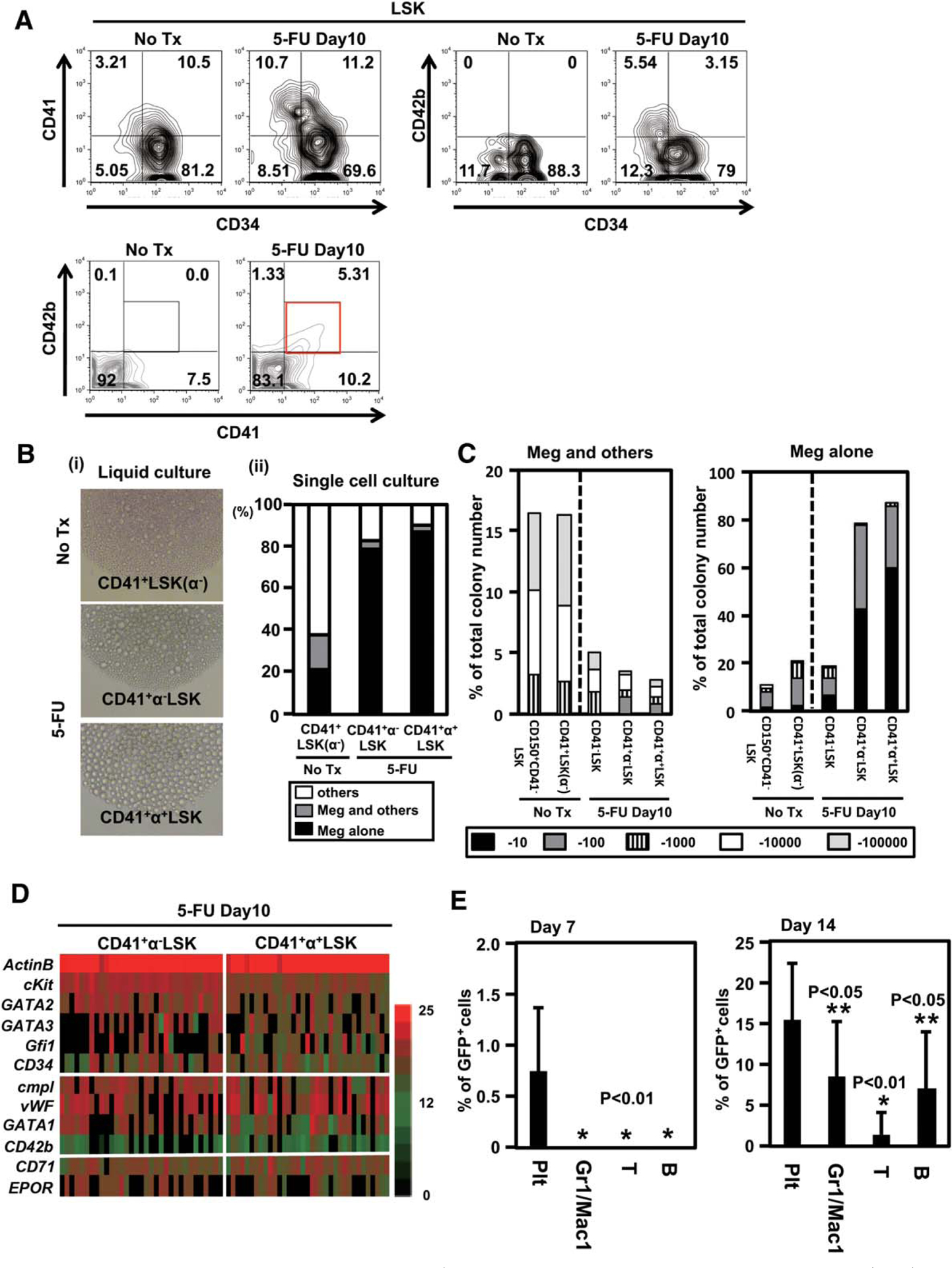
Emergency megakaryopoiesis is driven at the CD41+LSK stage. (A): Expression of CD42b in lineage−Sca1+cKit+ (LSK) compartment during the recovery phase after 5-FU treatment. Expression of CD42b in LSK was analyzed 10 days after a single 5-FU (150 mg/kg) treatment. A representative result of CD34, CD41, and CD42b staining from five independent experiments are shown. (B): (i) Sorted 500 CD41+CD42b−LSK [CD41+LSK (α−)] from untreated mice and CD41+CD42b− LSK cells (CD41+α−LSK) and CD41+CD42b+LSK (CD41+α+LSK) from 5-FU-treated mice were cultured in 96-well plates to assess their capacity for megakaryopoiesis. The pictures show representative morphologies of differentiated cells. (No Tx, no treatment). (ii) Preferential megakaryocyte differentiation from CD41+α+LSK and CD41+α−LSK after 5-FU treatment, compared with CD41+LSK (α−) at steady-state condition, assessed by single cell culture. Indicated progenitor cells were single cell-sorted into each well of 96-well dishes and cultured in the presence of stem cell factor (SCF), thrombopoietin (TPO), interleukin (IL)26, and IL-11. Colonies formed in each well were classified into those containing megakaryocytes alone, containing megakaryocytes and others, and without megakaryocytes. Three separated experiments were combined. (C): Proliferative capacity of indicated progenitors in single cell culture. Clone-sorted cells were cultured in 96 wells in the presence of TPO, SCF, IL-6, and IL-11. The frequency of cell proliferation was assessed on day 10. The numbers of megakaryocyte-containing (Meg1others, left) and megakaryocytes (Meg Alone, right) colonies were classified into five categories. (D): The results of single cell PCR using the samples from CD41+α−LSK and CD41+α+LSK from 5-FU treated mice. Expression of 11 genes in 38 representative cells from each population is shown. The levels of transcripts in individual cells are quantified by cycle threshold values and shown by indicated colors (red, highest; black, lowest). (E): Five hundred CD41+LSK from 5-FU-treated green fluorescent protein (GFP) transgenic mice were sorted and transplanted into lethally irradiated wild type mice. The frequencies of GFP+ cells in peripheral blood at very early time points are shown (n 5 10, each group). Abbreviations: GFP, green fluorescent protein; LSK, lineage−Sca1+cKit+; Meg, megakaryocyte.
Given the unique expression profile of CD42b as demonstrated, these results collectively supported our hypothesis that MKP are a direct progeny of a subCD41+LSK and can bypass biEMP. It was further speculated that subCD41+LSK might be an intermediate progenitor between PbHSC/MkRP and MKP.
To analyze the in vivo platelet-producing capacity, CD41+LSK or Flt3+LSK derived from GFP transgenic mice were transplanted (Fig. 3A; Supporting Information Fig. S2F). Peripheral blood was analyzed by flow cytometry at the several time points. CD41+LSK produced GFP+ platelets, together with Gr1/Mac1+ granulocytes, B220+ B cells, and CD3e+ T cells. In contrast, Flt3+LSK did not produce platelets. Notably, GFP+ platelets derived from CD41+LSK were the earliest to emerge among other GFP+ cells (see Day 7, Fig. 3B)
Figure 3.
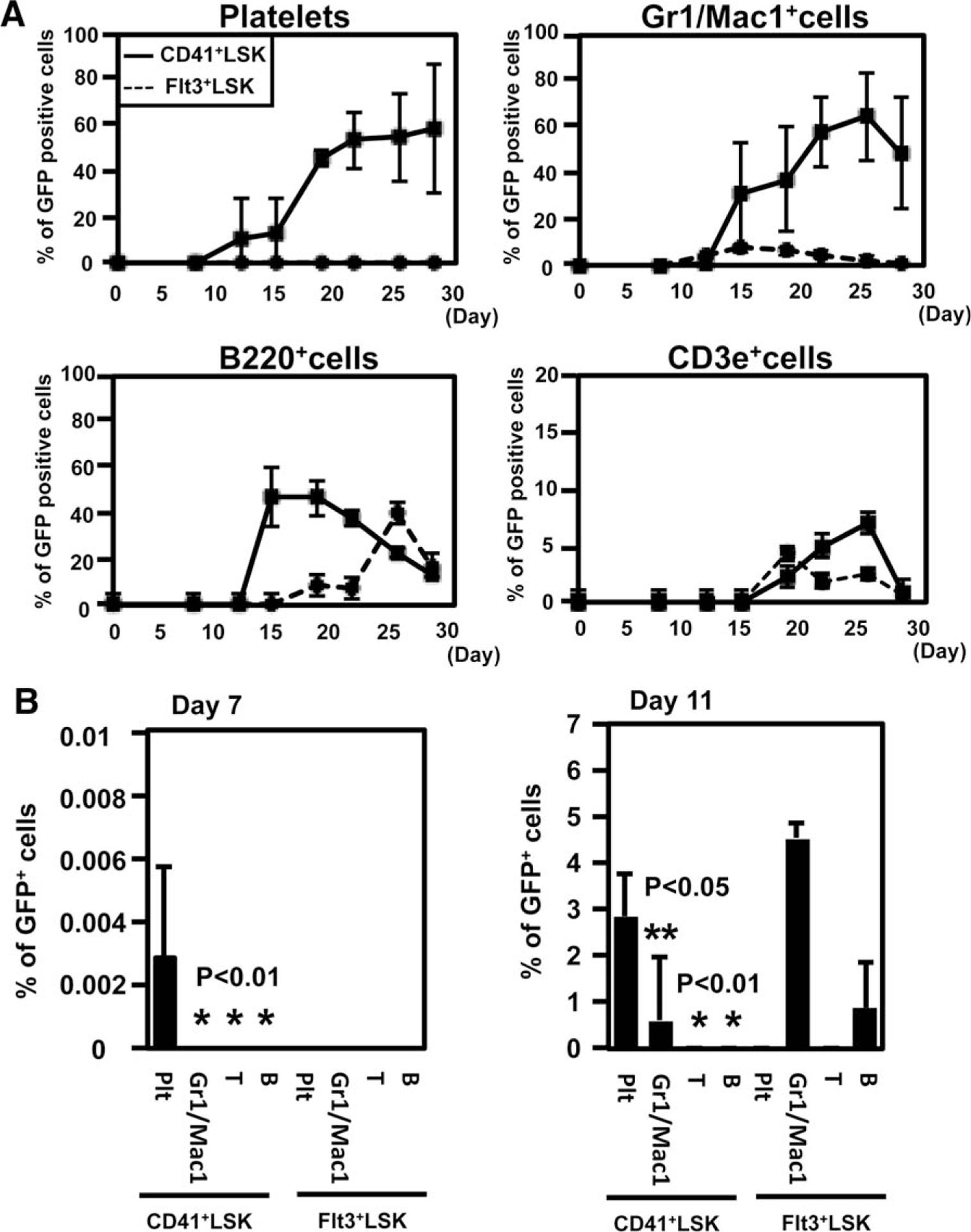
CD41+LSK efficiently differentiate into megakaryocytic progenitor (MKP) and platelets in vivo. (A): (i) Five hundred CD41+LSK or Flt3+LSK from green fluorescent protein (GFP) transgenic mice were sorted and transplanted into lethally irradiated wild type B6 mice. Peripheral blood was analyzed by flow cytometry at the indicated time points after transplantation (n 5 5, each group). The frequency of GFP-positive cells in each lineage is shown. CD41+ Gr1/Mac1+, B220+, and CD3e+ represent platelets, granulocytes/monocytes, B cells, and T cells, respectively. (B):The frequencies of donor-derived cells at the early time point after transplantation were shown (right panel, day 7; left panel, day 11). Abbreviations: GFP, green fluorescent protein; LSK, lineage−Sca1+cKit+.
Single Cell Gene Expression and TPO/cMpl Signaling Analyses Suggest the Proximity Between SubCD41+LSK and MKP
To gain further insight into the proximity of subCD41+LSK and MKP, we evaluated the expression of 11 genes by the single-cell RT-PCR method for six progenitor fractions (Fig. 4A). The expression of CD34, CD42b, cMpl, and vWF was detected in all the single cells consisting of the MKP population, with very rare exceptions. The expression pattern of these genes was similar to that of a fraction of CD41+LSK compared with other progenitor populations. In particular, 77% of the samples from CD41+LSK showed detectable cMpl expression. CD150+CD41−LSK, in which HSC are enriched [24], also showed the expression of cMpl and vWF at substantial frequencies. In contrast, only a minority or none of CD150−LSK (MPP3 and MPP4) showed CD42b, cMpl, and vWF expression. Surprisingly, all of these genes were expressed only at very low frequencies in MEP and PreMegE populations. It is of note that the expression of GATA1 is detected in most of MKP and MEP and PreMegE, and a substantial fraction of CD41+LSK [27]. We also confirmed the CD42b mRNA expression by in situ hybridization with a specific probe (Fig. 4B). Multiple CD42b mRNA signals were detected in the cytoplasm of MKP at high frequencies. These mRNA signals were also detected in CD41+LSK and CD150+CD41−LSK, but they were mainly localized to the nucleus and the frequency of signals was lower than in MKP (Fig. 4B). This observation was consistent with the data from single cell RT-PCR (Fig. 4A), and the fact that CD42b protein was detected only in MKP but not in CD41+LSK and CD150+CD41−LSK by flow cytometry (Fig. 1A).
Figure 4.
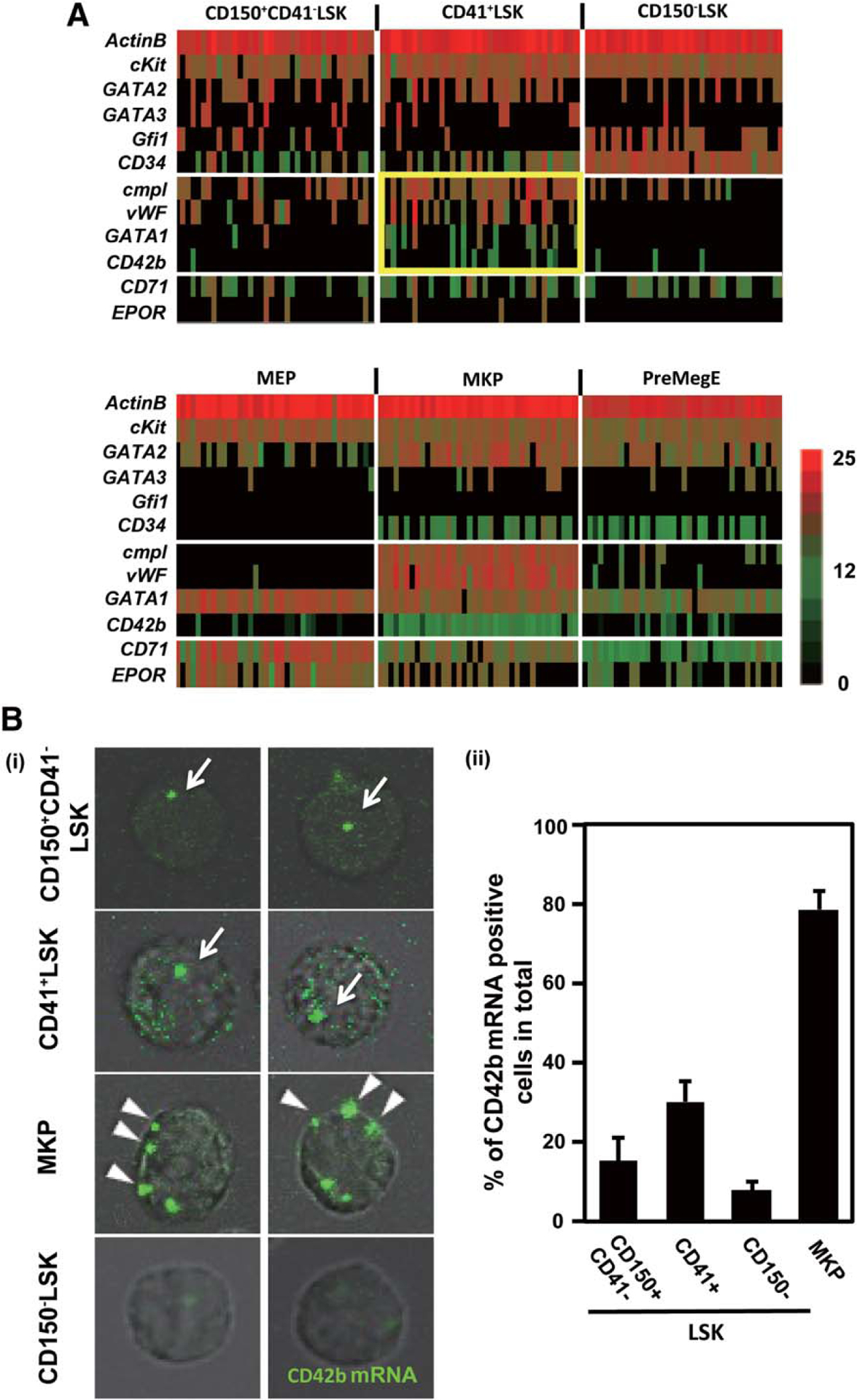
A single cell gene expression revealed the proximity between a subpopulation of CD41+LSK and MKP. (A):Single cell gene expression. Single cell real-time polymerase chain reaction was applied to analyze gene expression in single cells constituting CD150+CD41−LSK, CD41+LSK, CD150−LSK, megakaryocyte–erythroid progenitor, MKP, and PreMegE. Expression of 11 genes in 38 representative cells from each population was shown. The levels of transcripts in individual cells quantified by cycle threshold values and shown by colors indicated (red, highest; black, lowest). Note that cMpl, vWF, and CD42b were densely expressed in MKP, and that the closest pattern is observed in CD411LSK (yellow gate). (B): CD42b mRNA imaging at a single cell level. Sorted CD150+CD41−LSK, CD41+LSK, MKP, and CD150−CD41−LSK from wild type bone marrow are fixed on the poly-L-lysine coated glass slides and stained with a CD42b mRNA specific probe. Typical single cell images were shown in (i), green signals, CD42b mRNA. arrow, nuclear-localized CD42b mRNA; arrowhead, cytoplasm-localized CD42b mRNA. The frequencies of CD42b mRNA-positive cells in each progenitor population are shown in (ii). Each graph shows the result of three independent experiments. Abbreviations: LSK, lineage−Sca1+cKit+; MEP, megakaryocyte–erythroid progenitor; MKP, megakaryocytic progenitor; PreMegE, pre-megakaryocyte/erythrocyte progenitors.
To evaluate whether cMpl-expressing cells respond to TPO stimulation, Y694-phosphorylated STAT5, S473-phosphorylated AKT, and T1007-phosphorylated JAK2 were stained at the single cell level (Fig. 5) [28]. Phospho-STAT5, AKT, and JAK2 were detected in 80%–90% of MKP after the TPO stimulation. In contrast, they were much more weakly or not stained after stimulation with EPO. TPO-dependent, rather than EPO-dependent, phospho-STAT5, AKT, and JAK2 were detected again in >80% of CD41+LSK. In sharp contrast, 72%, 18%, and 81% of MEP demonstrated EPO-dependent phosphorylation of STAT5, AKT, and JAK2, respectively, but phosphorylation of these substrates was rarely detected after TPO stimulation in biMEP (MEP and PreMegE; Fig. 5; Supporting Information Fig. S4). Given the recent identification of MkRP, self-renewing HSC-like cells with unilineage megakaryopoietic potential [8], and the proximity of CD41+LSK to HSC, our observations from single cell analyses collectively indicate that subCD41+LSK and MKP constitute a pathway continuously using TPO-cMpl signaling, presumably in continuity from HSC. In contrast, biEMP may stand in a position independent of this pathway.
Figure 5.

Thrombopoietin (TPO)/cMpl signaling in single cell in subCD41+LSK and megakaryocytic progenitor (MKP). (A): (i) Sorted CD41+LSK, MKP, and megakaryocyte–erythroid progenitor (MEP) from bone marrow were stimulated with TPO or EPO on a glass slide and stained with anti-phospho-STAT5 (left), AKT (middle), and JAK2 (right) antibody. Representative pictures from each progenitor are shown (green; p-STAT5/P-AKT/p-JAK2). Scale bar 5 50 lm. (B): Frequencies of phospho-STAT5, AKT, and JAK2-positive cells in each progenitor population. Each graph shows the result from three independent experiments. Abbreviations: DAPI, 4ʹ 6-diamidino-2-phenylindole; EPO, erythropoetin; LSK, lineage−Sca1+cKit+; MEP, megakaryocyte–erythroid progenitor; MKP, megakaryocytic progenitor; TPO, thrombopoietin.
CD42b Marks Megakaryopoietic Commitment in the CD34−LSK Compartment During Recovery After 5-FU Treatment
To investigate the mechanism of the platelet generation during the hematopoietic recovery phase, we next analyzed megakaryocyte/platelet production after 5-fluorouracil (5-FU) treatment (Fig. 6). After injection, the platelet count is known to be decreased and to recover peaking at day 10 (Supporting Information Fig. S5A) [29]. On day 10, the LSK population in bone marrow was expanded up to 7.6% (range, 6.1%–9.0%) in Lin− cells (Supporting Information Fig. S5B), as previously reported [15, 30]. The frequencies of CD41+LSK in the total LSK fraction and MKP in the total Sca1− progenitors were statistically increased and significantly reduced, respectively (Supporting Information Fig. S5C). The frequency of MEP in the total Sca1− progenitors was increased 1.7-fold (Supporting Information Fig. S5C). The total number of MEP in bone marrow was also increased after 5-FU treatment (Supporting Information Fig. S5D), but the absolute number of CD42b+ megakaryocytes produced from MEP on a per-cell basis was decreased to 60% compared with the MEP prepared from untreated mice (Supporting Information Fig. S5E). The frequency of PreMegE was slightly decreased (Supporting Information Fig. S5C). Unexpectedly, the absolute number of MKP did not recover by day 10 after 5-FU treatment (Supporting Information Fig. S5D). These observations obscured the actual source of emergency platelet production. Intriguingly, we found that a fraction of CD41+LSK expressed CD42b on the cell surface at detectable levels (CD41+α+LSK; Fig. 6A) and that the majority of CD41+α+LSK resided in the CD34− fraction (Fig. 6A). After sorting (Supporting Information Fig. S6A), cultured CD41+α+LSK produced mature megakaryocytes at much higher efficiencies compared with the CD41+LSK isolated from steady-state mice and CD41+α−LSK isolated from 5-FU-treated mice (Fig. 6B(i); Supporting Information Fig. S6A, S6B). In a single cell culture, >90% of CD41+a+LSK-generated and >80% of CD41+α−LSK-generated colonies consisted of only vWF+ cells including abundant mature megakaryocytes, implying extreme bias in CD41+LSK to megakaryocytic lineage differentiation during the recovery period (Fig. 6B(ii)). Single cell RT-PCR for CD41+α−LSK and CD41+α+LSK during recovery after the 5-FU injection also showed that both populations included cells expressing cMpl, vWF, and CD42b at higher frequencies than CD41+LSK from untreated mice, with slightly more prominent frequencies in CD41+α+LSK (Fig. 6D, compare with Fig. 4A). Despite such an extreme bias to megakaryopoiesis, proliferative potential of CD41+α+LSK was much higher than that of MKP (Fig. 6C, compare with Supporting Information Fig. S1B). To evaluate in vivo platelet generation capacity, CD41+LSK from 5-FU-treated GFP mice were transplanted and the recipients’ peripheral blood was analyzed for the frequency of GFP+ platelets (Fig. 6E; Supporting Information Fig. S6C). On day 7, GFP+ cells were detectable only in the platelet fraction (Fig. 6E, left panel). The frequency of GFP+ platelets on day 14 was also statistically higher than GFP+ cell frequencies of the other lineages.
These results together indicate that megakaryopoiesis during emergency hematopoiesis is positively regulated at the proximity of or within the repopulating cell level, through increasing the number of megakaryopoiesis-primed CD41+LSK, along with upregulation of CD42b.
TPO/cMpl Signaling Plays a Key Role in the Platelet Production During Hematopoietic Recovery Phase
To investigate the in vivo TPO dependence in various progenitors, we analyzed cMpl-deficient mice. As reported previously, these mice showed severe thrombocytopenia but not anemia (Supporting Information Fig. S7A) [14, 31]. The frequencies of total LSK and long-term HSC identified as CD150+CD48−LSK in bone marrow were significantly reduced compared with the littermate control (Supporting Information Fig. S7B), as previously reported [16].
CD41+LSK and MKP were also markedly reduced in frequencies (Fig. 7A). In contrast, the frequencies of MEP (Fig. 7A) and Flt3+LSK (Supporting Information Fig. S7B) were not reduced at all, and that of PreMegE was only slightly reduced (Fig. 7A). These observations highlighted the TPO/cMpl-dependent pathway constituting HSC, CD41+LSK, and MKP in a continuous manner.
Figure 7.
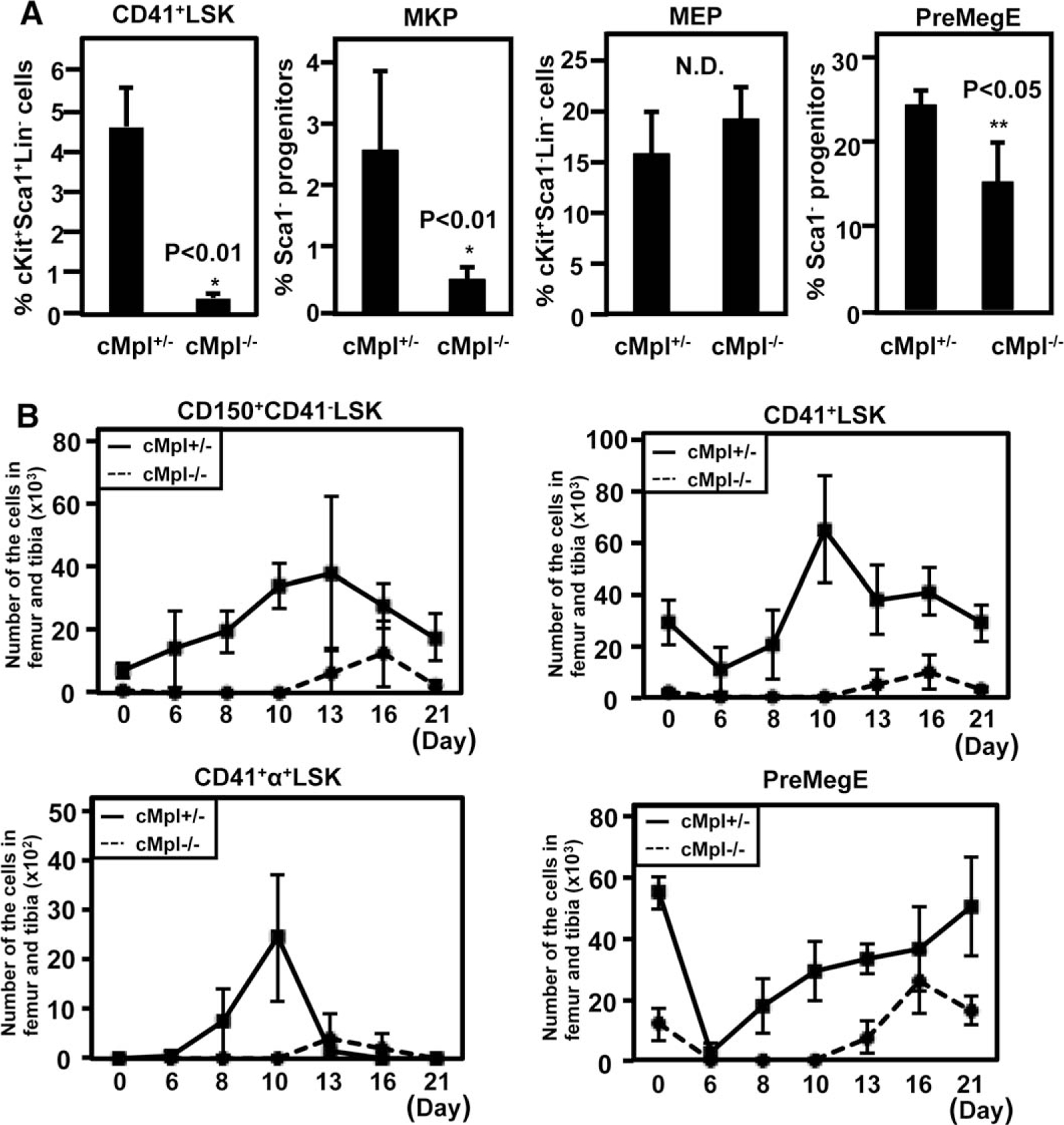
Distinct megakaryocyte progenitors at the steady and platelet-demanding state show variable degrees of dependency on thrombopoietin/cMpl signaling. (A): Frequencies of CD41+LSK, megakaryocytic progenitor (MKP), megakaryocyte–erythroid progenitor (MEP), and PreMegE in cMpl-deficient (cMpl−/−) and heterozygous (cMpl+/−) mice. The means of quadlicates are shown. (B): The total number of each progenitor cell population in cMpl−/− and cMpl+/− mice at several time points after 5-FU treatment (n 5 4, each group). Abbreviations: LSK, lineage−Sca1+cKit+; MEP, megakaryocyte–erythroid progenitor; MKP, megakaryocytic progenitor; PreMegE, pre-megakaryocyte/erythrocyte progenitors.
We next analyzed 5-FU-treated cMpl-deficient mice to evaluate whether emergency megakaryopoiesis-specific CD41+α+LSK is affected. The total number of CD41+α+LSK in bone marrow was significantly decreased in cMpl-deficient mice compared with the littermate control, similar to the decreases in CD150+CD41−LSK and whole CD41+LSK, while the frequency of PreMegE was mildly decreased in cMpl-deficient mice (Fig. 7B). These observations imply that TPO/cMpl signaling plays an important role for megakaryocyte/platelet production during the hematopoietic recovery phase, including the emergence of CD41+α+LSK.
DISCUSSION
Here, we have described a unipotent megakaryocyte progenitor-differentiating pathway branching within the LSK compartment in steady state and emergency hematopoiesis. This unipotent megakaryocytes-producing pathway, separately from the biEMP pathway, is continuously dependent on TPO-cMpl signaling, and thus, might be mapped at the right downstream to the part of the recently identified PbHSC/MkRP [7, 8].
Heterogeneity in the CD34−LSK compartment, in conjunction with the presence of megakaryocytes-biased repopulating cells, has been repeatedly demonstrated [7, 8]. In the current work, we identified highly biased megakaryocyte progenitors in the same CD34−LSK compartment (>90% of CD41+a+LSK) during bone marrow recovery phase. Because MkRP are identified only by single cell transplantation, some multipotent HSCs acquire CD42b expression while being biased to be megakaryocytic lineage when transplanted.
The degree of contributions of biEMP and unipotent pathway to megakaryopoiesis in physiologic conditions could be only virtually estimated. The megakaryocyte-producing potential of MEP, PreMegE, MKP, and CD41+LSK (through MKP) on an average per-cell basis could be calculated as 0.1, 0.9, 1.5, and 7.6 (calculation from data shown in Figs. 1B, 2C; Supporting Information Fig. S2B), providing a possibility for the subCD41+LSK/MKP pathway to contribute to physiologic megakaryopoiesis. Moreover, during emergency megakaryopoiesis, it is likely that this pathway plays a more dominant role.
Among the major differences between biEMP and subCD41+LSK/MKP is the expression of cMpl and CD41, in addition to CD42b. cMpl, CD41, and CD42b may be once repressed in biEMP, and likely to be re-expressed during differentiation to mature megakaryocytes. Accordingly, TPO signaling may be once reduced in biEMP and then upregulated. On the other hand, TPO signaling is continuously used in HSC maintenance through the subCD41+LSK/MKP pathway, as indicated by a single cell signaling analysis (Supporting Information Fig. S8).
CONCLUSIONS
In conclusion, our current data imply that platelet-biased population in the HSC compartment can directly differentiate into the megakaryocyte lineage and bypass the BiMEP pathway. CD150+CD41+CD42b+LSK, which are recognizable during the bone marrow recovery phase, showed restricted unipotent megakaryocyte differentiation potential and likely to represent intermediate progenitors in this direct differentiation pathway, strictly depending on TPO signaling. These findings should facilitate further understanding of the cell fate decision mechanism in the normal and emergency hematopoiesis, such as hematopoietic reconstitution after bone marrow transplantation or chemotherapy, and may provide a clue to solve diverse medical questions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. K. Akashi of Kyushu University for critical reading of the manuscript. This work was supported by the Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23118503 and 22390191 to S.C.; 22790896 and 24790959 to H.N.) and by the Uehara Memorial Foundation, Yasuda Medical Foundation, and SENSHIN Medical Research Foundation (to H.N.).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Akashi K, Traver D, Miyamoto T et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000;404:193–197. [DOI] [PubMed] [Google Scholar]
- 2.Pronk CJ, Rossi DJ, Mansson R et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 2007; 1:428–442. [DOI] [PubMed] [Google Scholar]
- 3.Cornejo MG, Mabialah V, Sykes SM et al. Crosstalk between NOTCH and AKT signaling during murine megakaryocyte lineage specification. Blood 2012;118:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercher T, Cornejo MG, Sears C et al. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell 2008;3:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez M, Weissman IL, Pallavicini M et al. Differential amplification of murine bipotent megakaryocytic/erythroid progenitor and precursor cells during recovery from acute and chronic erythroid stress. STEM CELLS 2006;24:337–348. [DOI] [PubMed] [Google Scholar]
- 6.Klimchenko O, Mori M, Distefano A et al. A common bipotent progenitor generates the erythroid and megakaryocyte line-ages in embryonic stem cell-derived primitive hematopoiesis. Blood 2009;114:1506–1517. [DOI] [PubMed] [Google Scholar]
- 7.Sanjuan-Pla A, Macaulay IC, Jensen CT et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 2013;502:232–236. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto R, Morita Y, Ooehara J et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013; 154:1112–1126. [DOI] [PubMed] [Google Scholar]
- 9.Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci USA 2003;100:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng AP, Kauppi M, Metcalf D et al. Characterization of thrombopoietin (TPO)-responsive progenitor cells in adult mouse bone marrow with in vivo megakaryocyte and erythroid potential. Proc Natl Acad Sci USA 2012;109:2364–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferkowicz MJ, Starr M, Xie X et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 2003;130:4393–4403. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch VR, Tomer A. Advances in megakaryocytopoiesis and thrombopoiesis: From bench to bedside. Br J Haematol 2013;161: 778–793. [DOI] [PubMed] [Google Scholar]
- 13.Kaushansky K, Lok S, Holly RD et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 1994;369:568–571. [DOI] [PubMed] [Google Scholar]
- 14.Kimura S, Roberts AW, Metcalf D et al. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA 1998;95:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bersenev A, Wu C, Balcerek J et al. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest 2008;118: 2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshihara H, Arai F, Hosokawa K et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007;1:685–697. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol 2014;165:259–268. [DOI] [PubMed] [Google Scholar]
- 18.Ghosn EE, Yamamoto R, Hamanaka S et al. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci USA 2012;109: 5394–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canobbio I, Balduini C, Torti M. Signalling through the platelet glycoprotein Ib-V-IX complex. Cell Signal 2004;16:1329–1344. [DOI] [PubMed] [Google Scholar]
- 20.Nishikii H, Eto K, Tamura N et al. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med 2008;205:1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson A, Laurenti E, Oser G et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 2008;135:1118–1129. [DOI] [PubMed] [Google Scholar]
- 22.Osawa M, Hanada K, Hamada H et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996;273:242–245. [DOI] [PubMed] [Google Scholar]
- 23.Gekas C, Graf T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood 2013;121: 4463–4472. [DOI] [PubMed] [Google Scholar]
- 24.Kiel MJ, Yilmaz OH, Iwashita T et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121:1109–1121. [DOI] [PubMed] [Google Scholar]
- 25.Adolfsson J, Mansson R, Buza-Vidas N et al. Identification of Flt31 lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005;121:295–306. [DOI] [PubMed] [Google Scholar]
- 26.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med.207:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyawaki K, Arinobu Y, Iwasaki H et al. CD41 marks the initial myelo-erythroid line-age specification in adult mouse hematopoiesis: Redefinition of murine common myeloid progenitor. STEM CELLS 2014;33:976–987. [DOI] [PubMed] [Google Scholar]
- 28.Seita J, Ema H, Ooehara J et al. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci USA 2007;104:2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozuma Y, Ninomiya H, Murata S et al. The pro-apoptotic BH3-only protein Bim regulates cell cycle progression of hematopoietic progenitors during megakaryopoiesis. J Thromb Haemost 2010;8:1088–1097. [DOI] [PubMed] [Google Scholar]
- 30.Sudo T, Yokota T, Oritani K et al. The endothelial antigen ESAM monitors hematopoietic stem cell status between quiescence and self-renewal. J Immunol 2012;189:200–210. [DOI] [PubMed] [Google Scholar]
- 31.Gurney AL, Carver-Moore K, de Sauvage FJ et al. Thrombocytopenia in c-mpl-deficient mice. Science 1994;265:1445–1447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


