Abstract
Background
Colorectal cancer is the third most commonly diagnosed cancer worldwide. A diagnosis of colorectal cancer and subsequent treatment can adversely affect an individuals physical and mental health. Benefits of physical activity interventions in alleviating treatment side effects have been demonstrated in other cancer populations. Given that regular physical activity can decrease the risk of colorectal cancer, and cardiovascular fitness is a strong predictor of all‐cause and cancer mortality risk, physical activity interventions may have a role to play in the colorectal cancer control continuum. Evidence of the efficacy of physical activity interventions in this population remains unclear.
Objectives
To assess the effectiveness and safety of physical activity interventions on the disease‐related physical and mental health of individuals diagnosed with non‐advanced colorectal cancer, staged as T1‐4 N0‐2 M0, treated surgically or with neoadjuvant or adjuvant therapy (i.e. chemotherapy, radiotherapy or chemoradiotherapy), or both.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6), along with OVID MEDLINE, six other databases and four trial registries with no language or date restrictions. We screened reference lists of relevant publications and handsearched meeting abstracts and conference proceedings of relevant organisations for additional relevant studies. All searches were completed between 6 June and 14 June 2019.
Selection criteria
We included randomised control trials (RCTs) and cluster‐RCTs comparing physical activity interventions, to usual care or no physical activity intervention in adults with non‐advanced colorectal cancer.
Data collection and analysis
Two review authors independently selected studies, performed the data extraction, assessed the risk of bias and rated the quality of the studies using GRADE criteria. We pooled data for meta‐analyses by length of follow‐up, reported as mean differences (MDs) or standardised mean differences (SMDs) using random‐effects wherever possible, or the fixed‐effect model, where appropriate. If a meta‐analysis was not possible, we synthesised studies narratively.
Main results
We identified 16 RCTs, involving 992 participants; 524 were allocated to a physical activity intervention group and 468 to a usual care control group. The mean age of participants ranged between 51 and 69 years. Ten studies included participants who had finished active treatment, two studies included participants who were receiving active treatment, two studies included both those receiving and finished active treatment. It was unclear whether participants were receiving or finished treatment in two studies. Type, setting and duration of physical activity intervention varied between trials. Three studies opted for supervised interventions, five for home‐based self‐directed interventions and seven studies opted for a combination of supervised and self‐directed programmes. One study did not report the intervention setting. The most common intervention duration was 12 weeks (7 studies). Type of physical activity included walking, cycling, resistance exercise, yoga and core stabilisation exercise.
Most of the uncertainty in judging study bias came from a lack of clarity around allocation concealment and blinding of outcome assessors. Blinding of participants and personnel was not possible. The quality of the evidence ranged from very low to moderate overall. We did not pool physical function results at immediate‐term follow‐up due to considerable variation in results and inconsistency of direction of effect. We are uncertain whether physical activity interventions improve physical function compared with usual care. We found no evidence of effect of physical activity interventions compared to usual care on disease‐related mental health (anxiety: SMD ‐0.11, 95% confidence interval (CI) ‐0.40 to 0.18; 4 studies, 198 participants; I2 = 0%; and depression: SMD ‐0.21, 95% CI ‐0.50 to 0.08; 4 studies, 198 participants; I2 = 0%; moderate‐quality evidence) at short‐ or medium‐term follow‐up. Seven studies reported on adverse events. We did not pool adverse events due to inconsistency in reporting and measurement. We found no evidence of serious adverse events in the intervention or usual care groups. Minor adverse events, such as neck, back and muscle pain were most commonly reported. No studies reported on overall survival or recurrence‐free survival and no studies assessed outcomes at long‐term follow‐up
We found evidence of positive effects of physical activity interventions on the aerobic fitness component of physical fitness (SMD 0.82, 95% CI 0.34 to 1.29; 7 studies, 295; I2 = 68%; low‐quality evidence), cancer‐related fatigue (MD 2.16, 95% CI 0.18 to 4.15; 6 studies, 230 participants; I2 = 18%; low‐quality evidence) and health‐related quality of life (SMD 0.36, 95% CI 0.10 to 0.62; 6 studies, 230 participants; I2 = 0%; moderate‐quality evidence) at immediate‐term follow‐up. These positive effects were also observed at short‐term follow‐up but not medium‐term follow‐up. Only three studies reported medium‐term follow‐up for cancer‐related fatigue and health‐related quality of life.
Authors' conclusions
The findings of this review should be interpreted with caution due to the low number of studies included and the quality of the evidence. We are uncertain whether physical activity interventions improve physical function. Physical activity interventions may have no effect on disease‐related mental health. Physical activity interventions may be beneficial for aerobic fitness, cancer‐related fatigue and health‐related quality of life up to six months follow‐up. Where reported, adverse events were generally minor. Adequately powered RCTs of high methodological quality with longer‐term follow‐up are required to assess the effect of physical activity interventions on the disease‐related physical and mental health and on survival of people with non‐advanced colorectal cancer. Adverse events should be adequately reported.
Keywords: Aged, Female, Humans, Male, Middle Aged, Anxiety, Anxiety/etiology, Colonic Neoplasms, Colonic Neoplasms/complications, Colonic Neoplasms/psychology, Colonic Neoplasms/therapy, Colorectal Neoplasms, Colorectal Neoplasms/complications, Colorectal Neoplasms/psychology, Colorectal Neoplasms/therapy, Depression, Depression/etiology, Depression/therapy, Exercise, Fatigue, Fatigue/therapy, Mental Health, Physical Fitness, Quality of Life, Randomized Controlled Trials as Topic, Time Factors
Plain language summary
Physical activity interventions for the physical and mental health of people during and after treatment for bowel cancer
Background
Bowel cancer is the third most common cancer diagnosed worldwide. Being diagnosed and receiving treatment for bowel cancer can have a negative impact on a person's physical and mental health. Side effects include reduced fitness levels and increased tiredness. People are also at risk of their cancer returning after treatment and this can cause fear and worry. Research on physical activity programmes in other cancer populations has shown benefits in reducing side effects of treatment. Given that people who are active have a lower chance of developing bowel cancer, physical activity may be beneficial for those with a bowel cancer diagnosis, but the research is not yet clear.
Review question
This review was undertaken to find out whether physical activity programmes are beneficial for the physical and mental health of people with bowel cancer and whether they are safe.
Key results We found 16 studies that included 992 participants, our evidence is current to June 2019. Participants were randomly assigned to receive a physical activity programme or usual care (no physical activity programme). In the included studies, we are unsure whether physical activity programmes improve physical function and we found no effect of physical activity programmes compared to usual care on disease‐related mental health. No serious adverse events occurred in the eight studies that looked at adverse events. There was inconsistency in reporting and measurement of adverse events. We do not know whether physical activity improves survival at any time point as no studies looked at this. The included studies suggest physical activity programmes may increase aerobic fitness, health‐related quality of life (general well‐being) and reduce fatigue (tiredness) in the short term. We are unsure of the long‐term effects of physical activity interventions on physical function, disease‐related mental health, adverse events, physical fitness, fatigue (tiredness), weight, health‐related quality of life (general well‐being) and physical activity levels because no studies assessed this.
Quality of the evidence We rated the quality of the evidence from very low to moderate mainly because of the small number of studies and low number of participants, as well as study limitations. Conclusion The findings of this review should be interpreted with caution due to the low number of studies included and the quality of the evidence. This review shows the need for future high quality research with longer‐term follow‐up to assess the effects of physical activity interventions on the physical and mental health of people with bowel cancer, especially in relation to safety and survival.
Summary of findings
Summary of findings 1. Physical activity compared with usual care in adults with non‐advanced colorectal cancer.
| Physical activity compared with usual care in adults with non‐advanced colorectal cancer | ||||||
|
Population: adults with non‐advanced colorectal cancer treated surgically or with neoadjuvant or adjuvant therapy, or both Settings: all but one study undertaken in high‐income countries. Included home‐based self‐directed and supervised physical activity programmes Intervention: aerobic or resistance training, flexibility or balance training or a combination of these, lasting at least 4 weeks Comparison: control intervention (usual care or no physical activity intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Physical activity | |||||
|
Physical function Assessed with: 30‐Second Chair Stand Test Follow‐up: up to 12 weeks (immediate‐term) |
We did not pool results due to considerable variability and inconsistency in direction of effect. Two studies observed no difference between the physical activity and usual care group for physical function at immediate‐term follow‐up. Two other studies reported significant improvements in physical function in the physical activity group compared with usual care | 185 (4 RCTs) |
⊕⊕⊝⊝a,b Low | We are uncertain whether physical activity interventions improve physical function | ||
|
Disease‐related mental health: depression Assessed with: HADS, CES‐D Follow‐up: more than 12 weeks to 6 months (short term) |
The mean postintervention HADS for depression ranged across control groups from 2.14 to 4.72 | The mean postintervention depression in the intervention group was 0.84 (2 lower to 0.32 higher) points lower than control | 198 (4 RCTs) |
⊕⊕⊕⊝b Moderate | Scores estimated using SMD ‐0.21 (‐0.50 to 0.08)g No evidence of difference in depression in the physical activity group compared with usual care group |
|
|
Disease‐related mental health: anxiety Assessed with: HADS, State‐Trait Anxiety Inventory Follow‐up: more than 12 weeks to 6 months (short term) |
The mean postintervention HADS for anxiety ranged across control groups from 2 to 3 | The mean postintervention anxiety in the intervention groups was 0.40 points (1.2 lower to 0.54 higher) lower than control | 198 (4 RCTs) |
⊕⊕⊕⊝b Moderate | Scores estimated using SMD ‐0.11 (‐0.40 to 0.18)g No evidence of difference in anxiety in the physical activity group compared with usual care group |
|
|
Overall survival (time interval between enrolment in the study and death of the person from any cause) Follow‐up: 12 months |
See comment | See comment | Not estimable | The included studies did not report on overall survival | ||
|
Recurrence‐free survival (time interval between date of enrolment in the study and the date when colorectal cancer recurs or another cancer occurs during the follow‐up) Follow‐up: 12 months |
See comment | See comment | Not estimable | The included studies did not report on recurrence‐free survival | ||
|
Adverse events Follow‐up: range 8 weeks to 11 months |
4 studies reported no adverse events, 3 other studies reported no serious adverse events with 7 participants experiencing minor adverse events in one study, 101 minor adverse events being reported in another study and 39 and 36 minor adverse events being reported in the intervention and control groups, respectively in another study. 1 study did not differentiate between serious and minor adverse events and reported 9 adverse events in the intervention group and one in the control | 305 (8 RCTs) |
⊕⊕⊝⊝c,d Low | |||
|
Physical fitness: aerobic fitness Assessed with: 6‐minute walk test, Bruce Protocol Treadmill Test, estimated V02 peak Follow‐up: up to 12 weeks (immediate term) |
The mean postintervention 6‐minute walk test score ranged across control groups from 293.7 to 588.9 | The mean postintervention physical fitness in the intervention group was 59 metres (24.5 to 93.1) higher than control | 295 (7 RCTs) |
⊕⊕⊝⊝a,e Low | Scores estimated using a SMD 0.82 (0.34 to 1.29)f Evidence suggests an improvement in aerobic fitness in the physical activity group compared with usual care group |
|
|
Cancer‐related fatigue Assessed with: FACIT‐F and FACT‐F (scale 0‐52: higher score indicates lower fatigue) Follow‐up: up to 12 weeks (immediate term) |
The mean postintervention cancer‐related fatigue score ranged across control groups from 37.1 to 44 | The mean postintervention cancer‐related fatigue score in the intervention groups was MD 2.16 higher (0.18 to 4.15 higher) | 230 (6 RCTs) |
⊕⊕⊝⊝a,b Low | Evidence suggests an improvement in cancer‐related fatigue in the physical activity group compared with the usual care group | |
|
Health‐related quality of life (HRQoL) Assessed with: FACT‐C, FACT‐G (higher score indicates better quality of life) Follow‐up: up to 12 weeks (immediate term) |
The mean postintervention FACT‐C scores ranged across control groups from 99.1 to 110.8 | The mean postintervention HRQoL in the intervention group was 6.64 (1.8 to 11.4) points higher than control | 230 (6 RCTs) |
⊕⊕⊕⊝b Moderate | Scores estimated using SMD 0.36 (0.10 to 0.62)h MID 5 to 8 points Evidence suggests an improvement in HRQoL in the physical activity group compared with the usual care group |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CES‐D: Centre for Epidemiological Studies Depression Scale; CI: confidence interval; FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue; FACT‐C: Functional Assessment of Cancer Therapy‐Colorectal; FACT‐F: Functional Assessment of Cancer Therapy‐Fatigue; FACT‐G: Functional Assessment of Cancer Therapy‐General; HADS: Hospital Anxiety and Depression Scale; HRQoL: health‐related quality of life; MID: Minmal important difference, MD: mean difference: RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference (used when studies assess the same outcome but measure it in a variety of ways). | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to indirectness (applicability of results to those undergoing active treatment). bDowngraded one level due to imprecision (small sample size). cDowngraded one levels due to inconsistency in reporting and measuring and numbers of adverse events reported. dDowngraded one level due to indirectness (reporting adverse events and not reporting whether these are 'related' or 'unrelated' to the intervention). eDowngraded one level due to risk of bias (lack of allocation concealment and blinding of outcome assessor). fAnalysed with SMD and back estimated to MD to enable interpretation. SD for performing the calculation was obtained from study by Lee 2017. gAnalysed with SMD and back estimated to MD to enable interpretation. SD for performing the calculation was obtained from study by Van Vulpen 2016. hAnalysed with SMD and back estimated to MD to enable interpretation. SD for performing the calculation was obtained from study by Cramer 2016.
Background
Description of the condition
Colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer death worldwide, accounting for an estimated 881,000 deaths in 2018 (GLOBOCAN 2018). Incidence and mortality rates vary globally, with higher incidence and lower mortality rates in higher‐income countries (Arnold 2017; GLOBOCAN 2018; Stewart 2014). In general, incidence is higher in men than women and is strongly linked with age, with highest incidence among people aged 65 to 74 years (Howlader 2016). Incidence is currently stabilising in high‐income countries, however a two‐fold cumulative increase in incidence is expected by 2025, due to increasing incidence in low‐ to middle‐income countries. With development, comes the adoption of more inactive lifestyles and unhealthy dietary habits; established risk factors for colorectal cancer (Stewart 2014). This is expected to increase the global burden of colorectal cancer, which may be compounded by a lack of health service resources in low‐ and middle‐income countries to deal with the escalation in incidence (Stewart 2014).
Five‐year survival from colon and rectal cancer has reached 60% or more in 22 countries worldwide (Allemani 2015). Between 1989 and 2011, colorectal cancer mortality rates decreased by more than 25% and 30% in men and women, respectively in high‐income countries in Northern and Western Europe, but increased in most Eastern European countries (Ouakrim 2015). Similar trends are evident globally, with decreasing mortality rates in high‐income countries, including Australia, Canada (Coleman 2011), the USA (Ryerson 2016), and Japan (Arnold 2017), and contrasting increasing mortality rates in low‐ and middle‐income regions, such as Latin America and the Phillipines (Arnold 2017). These disparities are not easily explained and are likely due to differences in access to diagnostic and treatment services (Haggar 2009), with advancements in treatment and early detection contributing to decreasing mortality in high‐income countries (Coleman 2011; Stewart 2014).
Although treatments are advancing, anti‐cancer therapies are associated with a range of adverse physiological and psychological side effects, which affect morbidity and mortality (Devin 2016a). Surgical resection is the primary treatment modality for stage I‐III (T1‐4 N0‐2 M0) colorectal cancer, with systemic chemotherapy or radiotherapy (more often in rectal cancer), or both, given either in the adjuvant or neoadjuvant setting in stage III and high risk stage II patients (El‐Shami 2015; Labianca 2010). Major abdominal surgery alone has been associated with declines in physical function (Schroeder 1991), and fatigue (Christensen 1982). Cancer‐related fatigue affects between 60% to 96% of people with cancer during and following chemotherapy, radiotherapy or surgery (Cramp 2012; Thomas 2014; Wagner 2004). It is a distressing symptom defined as a sense of "physical tiredness or exhaustion related to cancer or cancer treatment" (NCCN 2016), which can interfere with one's ability to carry out daily activities (Curt 2000), and negatively affect mood and quality of life (Stone 2008). Cancer‐related fatigue is present in some colorectal cancer survivors at four years following diagnosis (Schneider 2007). Physical inactivity has been identified as both a risk factor for (Bower 2014), and a consequence of (Lynch 2010) cancer‐related fatigue.
Declines in cardiorespiratory fitness can occur following treatment for colorectal cancer (Devin 2016a; West 2014a). Lower levels of cardiorespiratory fitness are linked with higher rates of cancer‐specific morbidity and mortality (Peel 2009; Schmid 2015), and can predict morbidity after colonic and rectal surgery (West 2014b; West 2014c). Furthermore, people with colorectal cancer may be susceptible to sarcopenic obesity (obesity with depleted muscle mass), which is associated with poorer functional status and poorer survival rates (Prado 2008; Wang 2017). These adverse effects, alone or in combination can impact adversely on a patient's quality of life and subsequent physical activity levels (Cramer 2014a). Colorectal cancer survivors are also at an increased risk of developing second colorectal cancers (Green 2002; Markle 2010), non‐colorectal cancers (Birgisson 2005), and other comorbidities (Denlinger 2011).
Concerns surrounding recurrence are common, affecting over half of cancer patients at one year following diagnosis (Baker 2005). Even at five years following surgery for colorectal cancer, survivors have concerns surrounding recurrence (Custers 2016). A significant minority of colorectal cancer patients and longer‐term survivors of colorectal cancer (2 or more years postdiagnosis) experience clinically meaningful levels of psychological distress, including symptoms of anxiety and depression or reduced mental well‐being (Mosher 2016). Colorectal cancer survivors report high quality of life at five years or longer postdiagnosis, but have higher rates of depression than age‐matched populations (Ramsey 2002). Psychological outcomes vary greatly in this population, poorer psychological outcomes have been linked with the presence of existing comorbidities (Lynch 2008; Ramsey 2002), worse general health (Yost 2008), and lower socioeconomic status (Ramsey 2002). Levels of anxiety and depression are reported to be higher in people who undergo surgery with adjuvant chemotherapy or radiotherapy compared with surgery alone (Pereira 2012).
Description of the intervention
Physical activity interventions were the focus of this review. Physical activity is defined as any bodily movement produced by contraction of skeletal muscle that results in energy expenditure above resting energy expenditure (ACSM 2009; Caspersen 1985). For the purpose of this review the term 'physical activity interventions' included 'exercise interventions'. Exercise is a subset of physical activity that is planned, structured and repetitive, done to improve or maintain one, or more of the components of physical fitness (ACSM 2009; Caspersen 1985). Physical activity interventions may be less structured than exercise interventions and often focus on promoting the integration of activities into daily life (e.g. gardening, walking or active travel). Physical activity interventions may be self‐directed or supervised by a healthcare professional. They can involve aerobic or resistance training, flexibility or balance training, or a combination of these, can take place in any setting and can be individual or group based, or both. No restrictions were made regarding frequency, intensity, time or type of physical activity intervention included. Interventions were included if they lasted a minimum of four weeks, this was to exclude studies on the acute effects of physical activity.
Physical activity interventions are not currently delivered as part of standard practice during or following treatment for colorectal cancer. Early postoperative mobilisation is, however, strongly recommended, as part of the Enhanced Recovery After Surgery (ERAS) guidelines following colorectal surgery, encouraging patients to be out of bed for two hours on the day of surgery and six hours per day, thereafter until discharge (Lassen 2009). The American College of Sports Medicine (Schmitz 2010), the American Cancer Society (Rock 2012), and the British Association of Sport and Exercise Science (BASES 2011) guidelines confirm that exercise can be safely performed during and following cancer treatment in the general cancer population. Specific guidance statements on physical activity interventions during and following treatment for colorectal cancer have not yet been published, due to lack of evidence on adverse effects and lack of safety data (Schmitz 2010). Side effects of treatments (cancer‐related fatigue, peripheral neuropathy, immune suppression, digestion issues, bowel dysfunction (including faecal incontinence) and urinary incontinence) may increase the risk of adverse events during physical activity. These side effects may represent barriers to physical activity participation (Denlinger 2009; Denlinger 2011; Rock 2012; Schmitz 2010). Indeed, chronic diarrhoea is a side effect that has been associated with limitations in activity and negative body image (Schneider 2007). The presence of a stoma is also associated with diminished body image (Hong 2014). These side effects have been highlighted as factors to consider when prescribing physical activity. Existing comorbidities (most commonly cardiovascular disease, musculoskeletal problems and lung or breathing problems), particularly in older people with colorectal cancer have been highlighted as other factors requiring consideration, to reduce the risk of injury and adverse events (Denlinger 2009; Rock 2012; Schmitz 2010).
How the intervention might work
Physical activity has been proposed as non‐pharmacologic intervention to attenuate the negative physiologic and psychologic effects of treatment in people with cancer (Courneya 2007; Schmitz 2005). There is a growing body of evidence from Cochrane and non‐Cochrane systematic reviews demonstrating the positive impact of physical activity both during and following cancer treatment (Galvao 2005; Knols 2005; Schmitz 2005; Speck 2010). Exercise training improves cardiorespiratory fitness and muscle strength (Schmitz 2005; Speck 2010), overall health‐related quality of life (HRQoL) (Knols 2005; Mishra 2012a; Mishra 2012b), and cancer‐related fatigue in the general cancer population during and following cancer treatment (Cramp 2012; Furmaniak 2016; Speck 2010), and physical functioning during treatment (Mishra 2012a). Through improved cardiorespiratory fitness and muscle strength, physical activity may help address the physical deconditioning associated with cancer treatments (Schmitz 2005; Speck 2010), and help manage cancer‐related fatigue (Al‐Majid 2009; Cramp 2012). Physical activity may also help the emotional and mental aspects of cancer‐related fatigue (Al‐Majid 2009; Cramp 2012). Benefits of exercise interventions on psychological well‐being (Knols 2005), anxiety and depression show positive trends but the evidence is not consistent (Cramp 2012; Furmaniak 2016; Mishra 2012a).
Cardiorespiratory fitness has been highlighted as an independent predictor of cancer mortality risk. Higher cardiorespiratory fitness is associated with a significant reduction in total cancer mortality (Schmid 2015), and colorectal cancer mortality (Peel 2009). Peel and colleagues report that men with at least a moderate fitness level had a 42% lower risk of colorectal mortality compared with men with a low cardiorespiratory fitness level. Evidence from observational studies suggest that physical activity is associated with overall and disease‐free survival (Haydon 2006; Meyerhardt 2006; Meyerhardt 2009), in both colon and rectal cancer patients.
There is consistent evidence linking physical activity to reduced colon cancer risk (Leitzmann 2015; Wolin 2009). A meta‐analysis of 52 studies found an inverse association between physical activity and colon cancer, with an overall relative risk reduction of 24% (Wolin 2009). This is consistent with findings of an earlier meta‐analysis of 19 cohort studies, which demonstrated a lower risk of colon cancer of 22% and 29% in physically active men and women, respectively (Samad 2005). Conversely, there appears to be no consistent association between physical activity and rectal cancer risk (Robsahm 2013).
The exact biological mechanisms for the observed benefit of physical activity for the prevention and secondary prevention of colorectal cancer are not fully understood. Various mechanisms have been proposed. Physical activity may reduce carcinogen exposure in the mucosa through decreased gastrointestinal transit time (Quadrilatero 2003; Slattery 2003), may alter prostaglandin levels (prostaglandins are unsaturated, free fatty acids that affect colonic function) (Quadrilatero 2003), and may alter the insulin‐like growth factor (IGF) pathway (Denlinger 2011; Fairey 2003). In people with colorectal cancer, moderate‐intensity exercise has resulted in reduced levels of urinary markers of oxidative damage (Allgayer 2008), and decreased interleukin‐1 receptor agonist (Allgayer 2004a), which may enhance immune function. Oxidative DNA damage is thought to be involved in tumour formation and may be associated with malignant transition and recurrence (Allgayer 2008). IGF‐1 is important for cellular proliferation and survival (Hursting 2010), higher levels of which may be associated with increased risk of colorectal cancer (Giovannucci 2000), but this association remains elusive. Decreases in IGF and increases in IGF‐binding proteins have been observed following exercise training in breast cancer survivors, which may be clinically relevant for the colorectal cancer population (Fairey 2003)
Physical activity may therefore be potentially effective in improving overall and recurrence‐free survival. Indeed, given that regular physical activity can decrease the risk of colon cancer and has improved cardiorespiratory fitness, muscle strength, HRQoL and cancer‐related fatigue in other cancer populations, it may be of clinical relevance for the colorectal cancer control continuum.
Why it is important to do this review
Colorectal cancer is a major public health problem. With the projected increasing incidence of colorectal cancer in low‐ and middle‐income regions, increasing mortality rates in low‐ and middle‐income countries and 3.5 million colorectal cancer survivors worldwide (Stewart 2014), there is a need to develop effective interventions that aid physical and psychological recovery, help alleviate treatment side effects and increase overall and recurrence‐free survival. The Lancet Oncology commission has prioritised the reduction in morbidity and mortality associated with cancer, with a focus on "less toxic", "cost‐effective" interventions (Sullivan 2011). There is, therefore, a need for a greater understanding of the effects of physical activity interventions on the disease‐related physical and mental health of individuals with colorectal cancer, for policy, practice and for consumers.
To date, there are currently two published, non‐Cochrane systematic reviews on exercise interventions for people with colorectal cancer (Cramer 2014b; van Rooijen 2018). In the review by Cramer 2014b no recommendations regarding exercise as a routine intervention for people with colorectal cancer were made due to insufficient evidence and lack of safety data. The review undertaken by Cramer and colleagues was limited to individuals who had completed treatment. The second review by van Rooijen and colleagues was undertaken in participants undergoing treatment, and highlighted the limited evidence of exercise training during treatment for colorectal cancer. Six out of seven studies included mixed‐cancer populations, three of these studies were not RCTs. This review is restricted to RCTs only and includes individuals who are receiving adjuvant therapy in addition to those who have finished treatment; no previous review has included such a population. This review will update current evidence and include emerging evidence in relation to physical activity interventions for individuals with colorectal cancer and so identify current evidence gaps.
Objectives
To assess the effectiveness and safety of physical activity interventions on the disease‐related physical and mental health of individuals diagnosed with non‐advanced colorectal cancer, staged as T1‐4 N0‐2 M0, treated surgically or with neoadjuvant or adjuvant therapy (i.e. chemotherapy, radiotherapy or chemoradiotherapy), or both.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised control trials (RCTs) and cluster‐RCTs comparing physical activity interventions to usual care or no physical activity intervention for inclusion in this review.
Types of participants
We included studies that evaluated the effect of physical activity interventions, on adults (aged 18 years or over), regardless of gender, diagnosed with non‐advanced colorectal cancer, staged as T1‐4, N0‐2, M0, treated surgically or with neoadjuvant or adjuvant therapy (i.e. chemotherapy, radiotherapy, chemoradiotherapy), or both. We included studies that examined physical activity interventions delivered during adjuvant therapy, following adjuvant therapy or following surgery alone. We excluded studies that included participants with other cancer types (unless outcomes for colorectal cancer were reported separately), and studies that included participants who were more than five years postdiagnosis.
Types of interventions
We compared physical activity interventions separately to no physical activity intervention or to usual care. Participants in both the control and intervention arms received the same usual care. Physical activity sessions could take place in any setting, be supervised, self‐directed or both, could be individual or group based, or a combination of both. Physical activity modalities could include aerobic or resistance training, flexibility and balance training or a combination of these. No restrictions were made regarding frequency, intensity, time or type of exercise or physical activity intervention. We only included studies with interventions that lasted a minimum of four weeks in duration, to exclude studies on the acute effects of physical activity. We excluded studies with a prehabilitation component. We included studies that provided health education materials or seminars only if the physical activity intervention was the main intervention in the study. We recorded specific details on the intervention according to the FITT‐VP (frequency intensity, time, type, volume, progression) principle (ACSM 2014). We classified physical activity intensity as mild, moderate or vigorous based on the rate of perceived exertion, heart rate or metabolic equivalents report (ACSM 2014), and used the author's classification of mild, moderate, or vigorous when a quantitative measure was unavailable.
Types of outcome measures
We extracted information for the primary and secondary outcomes at all available time points. We sought to analyse overall survival and recurrence‐free survival at 12 months, three years and five years. We analysed the other primary and secondary outcomes according to the length of follow‐up: up to 12 weeks after baseline (immediate); more than 12 weeks but less than or equal to six months after baseline (short term); more than six months but less or equal to 12 months after baseline (medium term) and more than 12 months after baseline (long term).
Primary outcomes
Physical function (e.g. Karnofsky Performance Status Scale; Eastern Cooperative Oncology Group Scale; timed chair rise test; timed 'Up & Go' test) or other valid instruments
Disease‐related mental health (e.g. Hospital Anxiety and Depression Scale (HADS); Beck Depression Index (BDI))
Adverse events (participants experiencing at least 1 adverse event, e.g. injury, death, adverse events resulting in discontinuation of the intervention)
Secondary outcomes
Overall survival (time interval between enrolment in the study and death of the person from any cause)
Recurrence‐free survival (time interval between date of enrolment in the study and the date when colorectal cancer recurs or another cancer occurs during the follow‐up)
Physical fitness (e.g. cardiorespiratory endurance (6‐minute walk test; 10‐metre shuttle walk test; V02 peak or muscle strength (dynamometry; 1 repetition maximum; 5 repetition maximum) or another valid instrument
Cancer‐related fatigue (e.g. Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F); Schwartz Cancer Fatigue Scale (SCFS); Brief Fatigue Inventory (BFI); Piper Fatigue Scale (PFS))
Anthropometric measurements (e.g. weight, body mass index (BMI), body composition, waist measurement, skin‐fold measurement)
HRQoL (e.g. European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30); Medical Outcomes Study Short Form‐36 General Health Survey (SF‐36); Functional Assessment of Cancer Therapy–Colorectal scale (FACT‐C))
Levels of physical activity (e.g. physical activity questionnaires (International Physical Activity Questionnaire (IPAQ), Global Physical Activity Questionnaire (GPAQ) or objective measures of physical activity using pedometers or accelerometers)
Search methods for identification of studies
Electronic searches
We searched the following electronic databases between 6 June 2019 and 14 June 2019 up to the latest issue, with no language or date restrictions to identify relevant RCTs and cluster‐RCTs for this review.
The Cochrane Central Register of Controlled Trials (CENTRAL, in the Cochrane Library) (Appendix 1) (inception to present)
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to Present) (Appendix 2)
Ovid Embase (1974 to present) (Appendix 3)
CINAHL (in EBSCOhost 1982 to present)
Web of Science (1970 to present)
PsycINFO (1806 to present)
Open Grey (formerly SIGLE) (1980 to present)
PEDro (1999 to present)
The searches were conducted by Cochrane Colorectal Cancer's Information Specialist and a review author (MAT).
Searching other resources
We searched clinical trials registries separately on 6 June 2019 for ongoing studies and study protocols of:
Clinical.trials.gov (www.clinicaltrials.gov);
the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/);
the EU Clinical Trials Register (www.clinicaltrialsregister.eu/); and
CenterWatch (www.centerwatch.com).
We screened reference lists of all included studies and any relevant systematic reviews identified. We handsearched conference and meeting abstracts of relevant organisations including:
American Society of Clinical Oncology (ASCO);
European Society for Medical Oncology (ESMO);
American College of Sports Medicine (ACSM);
BIT's Annual World Cancer Congress;
European Multidisciplinary Colorectal Cancer Congress (EMCCC);
European Federation for Colorectal Cancer (EFR); and
European Cancer Congress (ECC).
We contacted individuals or organisations for information on unpublished or ongoing studies.
Data collection and analysis
Selection of studies
We imported all records retrieved from the searches into EndNote and removed duplicates (Endnote 2016). We exported these records to covidence for screening (Covidence 2018). Two review authors (MMG and MAT) independently examined the studies identified in the literature search and screened all studies based on their titles and abstracts, excluding studies that obviously did not meet the eligibility criteria. We did not exclude studies solely on the basis of reporting outcome data. We obtained the full texts of potentially eligible studies and the two review authors (MMG and MAT) independently examined the studies. In covidence, authors coded the studies as 'include', 'exclude' or 'uncertain' based on the outlined criteria. We resolved any disagreements through discussion, where necessary involving a third review author (CC or MMC), and kept a record of decisions made. We recorded reasons for exclusion of full text articles.
Data extraction and management
Two review authors (MMG and MAT) independently extracted data from the studies that met the inclusion criteria. We recorded extracted data on an excel spreadsheet, predeveloped for this purpose. MMG and MAT piloted the data extraction form in a random sample of three studies to ensure it captured the required information. We revised the form as required. We resolved any disagreements through discussion, and where necessary referred to a third review author (CC or MMC). Extracted data were entered into the Cochrane software Review Manager 5 (RevMan 5) (Review Manager 2014) for analyses. We extracted the following data.
Study details; author and year of publication, country of origin, aim, design, funding source, method of randomisation, method of recruitment, trial inclusion and exclusion criteria, duration of participation, conflicts of interest/ethical concerns, risk of bias assessment.
Participant details; total number randomised, age, gender, comorbidities, other relevant sociodemographics, cancer stage, type of cancer treatment, ethnicity, time since diagnosis, time beyond active treatment, baseline imbalances.
Intervention details; exercise type, intensity, frequency, volume, setting, duration of intervention, supervised or self‐directed, details of control/comparison intervention, withdrawals and exclusion and co‐interventions.
Outcomes; primary and secondary relevant to this review, including adverse events, follow‐up time points, measurement tools used for outcomes.
Assessment of risk of bias in included studies
Two review authors (MMG and MAT) independently assessed each included study for risk of bias using the Cochrane 'Risk of bias' tool (Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8.5.d, Higgins 2011a; Higgins 2017; Appendix 4). We assessed random sequence generation, allocation concealment, blinding of personnel and outcome assessment, completeness of outcome data, selective outcome reporting, and any other sources of bias, and judged the risk of bias as 'high', 'low' or 'unclear'. We resolved any disagreements through discussion and where necessary, through involving a third review author (CC or MMC). For each study, we detailed the risk of bias in table form alongside a statement of justification for our judgement. We summarised results in both a 'Risk of bias' summary figure and 'Risk of bias' graph. When interpreting treatment effects, we took into account the risk of bias for studies that contribute to that outcome.
Measures of treatment effect
For continuous outcomes (physical function, disease‐related mental health, physical fitness, cancer‐related fatigue, anthropometric measurements, levels of physical activity and HRQoL) we determined the mean differences (MDs) or standardised mean differences (SMDs) (in cases where different instruments were used to measure the selected outcome), in the intervention group compared with the control with 95% confidence intervals (CIs). We extracted data for final scores and change from baseline scores, if available.
In this version of the review, no outcomes were reported as time‐to‐event and we were unable to report adverse events as a dichotomous outcome. In future versions of this review for time‐to‐event outcomes (overall survival and recurrence‐free survival) we will extract hazard ratios (HRs) with standard errors, assuming that the HR is constant over time to compare the risk of death or recurrence of cancer in the treatment group with that in the control group. Where HRs are not presented, we will estimate them from reported data (e.g. Kaplan‐Meier curves, logrank observed minus expected events and the logrank variance) using methods described by Tierney and colleagues (Tierney 2007). For adverse events, we will calculate the risk ratio (RR) at individual study level by dividing the risk of an event in the intervention group by the risk of the event in the control group. We will define RRs greater than 1.0 as favouring the control group (i.e. fewer adverse events in the control group) and RRs less than 1.0 as favouring the intervention group (Deeks 2017).
Unit of analysis issues
For parallel‐group, individually randomised trials, the colorectal cancer participant was the unit of analysis in each study. No cluster‐RCTs met our inclusion criteria.
For studies reporting multiple follow‐up time points, we conducted separate meta‐analyses to reflect immediate‐, short‐, medium‐ and long‐term periods of follow‐up. For immediate‐term follow‐up we extracted data closest to the 12‐week follow‐up time point. For short‐ and medium‐term follow‐up, we extracted data closest to the six‐ and 12‐month follow‐up time point. For long‐term follow‐up, we extracted the longest time interval.
For studies with multiple arms, we included only relevant intervention arms. We combined all relevant intervention arms into a single group and combined all relevant control arms into a single group, creating a single, pair‐wise comparison.
For future versions of this review, we will extract data from cluster‐RCTs when they report appropriate analyses, adjusting for the sample size in each cluster. Where control of clustering has not been performed we will attempt to correct for the intervention effects of cluster‐RCTs by reducing the size of each study to its 'effective sample size', which is the number of the original sample size divided by the 'design effect'. We will calculate the design effect as 1 + (M‐1)* ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient as described in the Cochrane Handbook for Systematic reviews of interventions, section 16.3.4 (Higgins 2011b). We will use an estimate of the ICC derived from the study (if possible), from a similar study or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered unlikely.
Dealing with missing data
We attempted to contact authors of the included studies to request missing data on outcomes, participants and summary data via email. We documented reasons for missing data (missing at random or missing not at random) and how they were addressed. We assessed the extent to which studies analysed data according to the intention‐to‐treat principle. We assessed the level of missing data for included studies by comparing the number of participants included in the final analysis with the proportion of all participants in each study available in Characteristics of included studies. In future versions of this review, for studies at high risk of attrition bias, we will attempt to perform both the best‐case and worst‐case sensitivity analyses to assess the impact of missing data on the estimates of effect.
Assessment of heterogeneity
We evaluated clinical heterogeneity by examining diversity in participant characteristics, physical activity intervention characteristics, colorectal cancer treatment and outcomes among studies. We evaluated methodological heterogeneity by examining diversity in study designs and risk of bias. We did not pool methodologically heterogeneous studies. We visually inspected forest plots and used the Chi2 test to assess statistical heterogeneity (with P < 0.1). We used the I2 statistic to assess the percentage of variation across studies that is due to heterogeneity and not due to chance (Higgins 2003). We tentatively regarded heterogeneity as 'low' if I2 is less than 49%, 'moderate', if I2 is between 50% and 75% and 'high' if I2 is more than 75% (Deeks 2017). We investigated potential sources of statistical heterogeneity by reassessing diversity in characteristics of studies (participant, intervention, treatment and outcomes) and by means of sensitivity analysis.
Assessment of reporting biases
We attempted to control for time‐lag bias, location bias, citation bias and language bias by using a comprehensive search strategy without language or date restriction, that included searching for unpublished studies and searching trials registers. We controlled for multiple publication bias by identifying duplicate publications of the same study and grouping these together, listing them as one study. For studies published after 1 July 2005, we screened the Clinical Trials Register at the WHO ICTRP for the study protocols (apps.who.int/trialsearch) to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
No analysis in this version of the review included more than 10 studies. For future versions of this review, if there are at least 10 studies included in a meta‐analysis, we will visually inspect funnel plots for asymmetry to investigate potential publication bias or small‐study effects following the recommendations in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions for any statistical testing of funnel plot asymmetry (Sterne 2017).
Data synthesis
We pooled results from comparable groups of studies using both fixed‐effect and random‐effects models, when appropriate. Whenever possible, we used a random‐effects model with inverse variance weighting for meta‐analyses (DerSimonian 1986), due to the nature of physical activity as a highly varied intervention. We used a fixed‐effect model when there were few studies or if the studies were small with few events. Where appropriate, we conducted a sensitivity analysis to investigate the effect of the choice of model (fixed‐effect or random‐effects) on the pooled estimate. MMG and CC conducted statistical analysis using RevMan 5 (Review Manager 2014). We considered a two‐sided P value of less than 0.05 as statistically significant. In cases where measurement tools for outcomes had the opposite direction of effect, we multiplied mean scores of a selected measurement by minus one to ensure all scales had the same direction of effect as discussed in the Cochrane Handbook for Systematic Reviews of Interventions section, 9.2.3.2 (Deeks 2017). Where data aggregation was not possible due to heterogeneity, we provided a narrative synthesis of study results. We summarised the findings of the systematic review alongside an assessment of the quality of evidence for each individual outcome using the GRADE approach (GRADE Working Group 2004).
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were conducted in this version of the review due to insufficient data or inclusion of a range of treatment stages, treatment types, participant ages, gender in each individual study, or both. In future versions of this review where there are sufficient data, we will perform subgroup analyses of the effect of the intervention according to:
exercise and physical activity intervention characteristics (using frequency, intensity, time, type, volume progression (FITT‐VP ) to calculate metabolic equivalents/hours per week);
participant characteristics (gender, age ‐ over 65 years or under 65 years);
cancer stage (T1‐2, N0, M0), (T3‐4, N0, M0), (T1‐4, N1‐2, M0);
cancer type (colon or rectal);
treatment stage (during or post‐treatment);
treatment type (laparoscopic or open surgery, neoadjuvant therapy or no neoadjuvant therapy);
time since diagnosis (zero to one year, two to three years, four to five years).
Sensitivity analysis
We undertook sensitivity analyses to assess the robustness of results. We reanalysed data after excluding studies with high risk of bias, those that had co‐interventions (when appropriate) and studies that had not performed an intention‐to‐treat analysis. We conducted a sensitivity analysis to investigate heterogeneous results with the identification and removal of heterogeneous studies. We conducted a sensitivity analysis to investigate the effect of the choice of model (fixed‐effect or random‐effects) on the pooled estimate. In future versions of this review, for studies at high risk of attrition bias, we will conduct a best‐case/worst‐case sensitivity analysis to assess the impact of missing data on the estimates of effect. If there are any assumptions for ICC value used in cluster‐RCTs, we will perform a sensitivity analysis.
Summary of findings
We assessed the overall quality of evidence of the main review outcomes using the GRADE approach in 'Table 1 (GRADE Working Group 2004). The 'Summary of findings' table highlights the overall quality of the body of evidence for the main review outcomes, using the GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, indirectness, imprecision and publication bias). We used GRADEpro GDT 2015 software to prepare the 'Summary of findings' table. We will also present the results from the prespecified Sensitivity analysis and Subgroup analysis and investigation of heterogeneity when appropriate in 'Summary of findings' tables.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
We conducted the comprehensive database search between 6 June 2019 and 14 June 2019 and found 5061 records. We identified 1092 additional records upon searching clinical trial registries and handsearching references lists, conference and meeting abstracts. After removing duplicates, 3837 potential records remained. We excluded a total of 3640 records based on the title and abstract and retrieved 197 records for more detailed evaluation (Figure 1).
1.
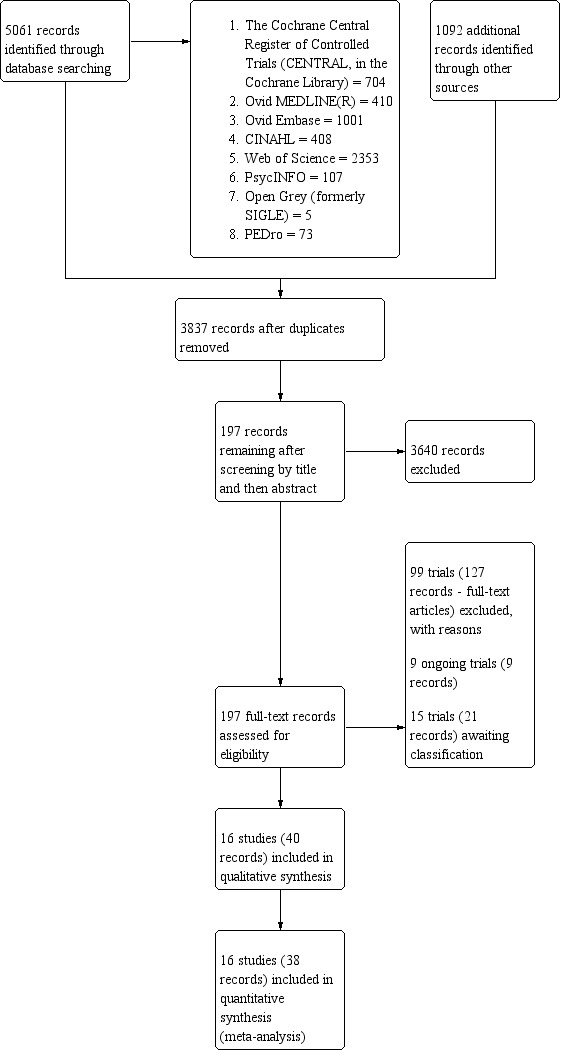
PRISMA flow diagram.
From these, we excluded 99 studies (127 records) as they did not meet the inclusion criteria (see 'Characteristics of excluded studies') and 16 studies (40 records) were appropriate for inclusion in the current review. In addition, nine studies are ongoing and 15 studies (21 records) are awaiting classification; we did not include these studies in the analysis presented below, but will consider them in future updates of this review. We completed all searches by 14 June 2019. Figure 1 illustrates the process of the literature search and study selection for the review based on the PRISMA template (Moher 2009).
Included studies
We included 16 studies in this review (14 journal articles and 2 dissertations) (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Courneya 2016; Cramer 2016; Hubbard 2016; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Pinto 2013; Van Blarigan 2019; Van Vulpen 2016; Waart 2017). We used the main publication as the study reference. We reviewed and included information on study characteristics and outcome related data from an additional 28 publications that were secondary publications of seven of these 16 studies. We contacted 11 study authors for additional information (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Cramer 2016; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Van Vulpen 2016; Waart 2017), eight of these authors replied to information requests (Bourke 2011; Courneya 2003; Cramer 2016; Lewis 2016; McDermott 2017; Nuri 2016; Van Vulpen 2016; Waart 2017). For study characteristics and outcomes, see the Characteristics of included studies table.
Study characteristics
All 16 included studies were randomised controlled trials (RCTs). No cluster‐RCTs met our inclusion criteria. All studies except for two randomised participants to either a physical activity or usual care arm (Brown 2017; Waart 2017). These two studies included an additional study arm, that included variations in exercise volume in Brown 2017 and exercise intensity in Waart 2017. Three studies included co‐interventions (Bourke 2011; Courneya 2016; Hubbard 2016), involving healthy eating seminars and a dietary information pack (Bourke 2011), health education materials for the usual care group (Courneya 2016), and weekly education sessions on topics, including physical activity, diet stress management and cardiac specific issues (Hubbard 2016). In all, investigators allocated 992 participants (mean 62, range 18 to 273) to a physical activity intervention group (n = 524, mean 33, range 9 to 136) or a control group (n = 468, mean 29, range 9 to 137).
Participants
Participants enrolled in the studies had a diagnosis of colon or colorectal cancer, six studies included only participants with colon cancer (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2016; Van Vulpen 2016; Waart 2017), whilst the remaining 10 studies included participants with colorectal cancer (Courneya 2003; Cramer 2016; Hubbard 2016; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Pinto 2013; Van Blarigan 2019). Four studies reported the percentage of rectal cancer participants included (Kim 2018; McDermott 2017; Pinto 2013; Van Blarigan 2019). No studies with exclusively rectal cancer participants met our inclusion criteria. The majority of studies included participants with stage I‐III colorectal cancer, however Courneya 2016, Lee 2017 and Waart 2017 excluded participants with stage I cancer. In addition, Waart 2017, Courneya 2003 and Van Blarigan 2019 included a minority of participants with stage IV cancer; when contacted Waart 2017 confirmed that no stage IV participants were included, Courneya 2003 was unable to provide separate data, excluding the four stage IV participants that were included in the study. Van Blarigan 2019 included one stage IV participant (2% of total participants included). Ten studies included participants who had finished active treatment (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2016; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Pinto 2013; Van Blarigan 2019), the time beyond treatment ranged between two months and five years. Two studies included participants receiving active treatment (Van Vulpen 2016; Waart 2017). Two studies were conducted among participants who were receiving and finished active treatment (Cramer 2016; Hubbard 2016). It was unclear whether participants were receiving or finished treatment in two studies (Courneya 2003; Nuri 2016). The majority of participants had undergone surgery as treatment; chemotherapy was also common across studies with less participants receiving radiotherapy. Mean time since diagnosis was only reported in six studies and ranged between 10 weeks and 2.99 years (Cantarero‐Villanueva 2016; Courneya 2016; Lewis 2016; Pinto 2013; Van Blarigan 2019; Van Vulpen 2016).
The mean age of participants ranged between 51 and 69 years. Fifteen studies included both males and females, with one study including only males (Nuri 2016). Comorbidities and ethnicity were largely unreported in studies. Only two studies reported comorbidities at baseline (Brown 2017; Waart 2017). Two studies reported on ethnicity of participants (Brown 2017; Pinto 2013), in both studies the majority of participants were white. Eight studies reported on education levels of participants (Cantarero‐Villanueva 2016; Courneya 2003; Cramer 2016; Kim 2018; Lee 2017; Van Blarigan 2019; Van Vulpen 2016; Waart 2017) with Kim 2018, Lee 2017, Van Vulpen 2016 and Waart 2017 further reporting on martial status and McDermott 2017, Van Blarigan 2019 and Waart 2017 reporting on employment status. Five studies reported recruiting those who were currently inactive (Bourke 2011; Courneya 2016; Lee 2017; Lewis 2016; McDermott 2017).
Interventions
Type and setting of interventions varied across studies. Three studies opted for exclusively supervised physical activity interventions (Cantarero‐Villanueva 2016; Cramer 2016; Hubbard 2016), likely due to the modes of exercise which included hatha yoga, core stabilisation exercise and cardiac rehabilitation exercise classes. The settings for these interventions were not clearly reported. Five studies opted for exclusively home‐based self‐directed programmes (Brown 2017; Courneya 2003; McDermott 2017; Pinto 2013; Van Blarigan 2019), which consisted of mainly aerobic physical activity (e.g. treadmill walking, cycling). A combination of aerobic and resistance exercise was prescribed in two studies (McDermott 2017 ; Van Blarigan 2019). The Nuri 2016 study involved aerobic physical activity, whether the physical activity was supervised or self‐directed is unclear. Seven studies opted for a combination of supervised and self‐directed physical activity (Bourke 2011; Courneya 2016; Kim 2018; Lee 2017; Lewis 2016; Van Vulpen 2016; Waart 2017), with physical activity logs in Lee 2017 and telephone support in Courneya 2016 and Kim 2018. A combination of aerobic and resistance physical activity was conducted in four of these studies (Bourke 2011; Lee 2017; Van Vulpen 2016; Waart 2017), with Courneya 2016 encouraging activity based on individual preference, with a walking prescription if individuals had no preference.
The intensity of the physical activity varied slightly between studies. Methods used to measure intensity of the physical activity included relatively objective measures, such as percentage of maximum heart rate, heart rate at ventilatory threshold, percentage of predicted maximum workload and ratings of perceived exertion. The majority of studies opted for moderate‐intensity physical activity (Bourke 2011; Brown 2017; Courneya 2003; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Pinto 2013; Waart 2017), with three studies incorporating vigorous physical activity (Lewis 2016; Van Blarigan 2019; Waart 2017). An arm of the Waart 2017 study participated in low‐intensity exercise. Five studies did not report intensity of the physical activity programme (Cantarero‐Villanueva 2016; Courneya 2016; Cramer 2016; Hubbard 2016; Kim 2018).
The most common duration of physical activity intervention was 12 weeks (Bourke 2011; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Pinto 2013; Van Blarigan 2019). In one study, the length of the intervention was determined by duration of chemotherapy, with participants beginning the intervention with the first cycle of chemotherapy and finishing three weeks after the last cycle (Waart 2017). For another study (Hubbard 2016), the length of intervention varied depending on hospital site (6, 10 or 12 weeks). Two studies delivered eight‐week interventions (Cantarero‐Villanueva 2016; Nuri 2016). The duration of the other five studies were 10 weeks (Cramer 2016), 16 weeks (Courneya 2003), 18 weeks (Van Vulpen 2016), six months (Brown 2017), and three years (Courneya 2016). However, the Courneya 2016 study is ongoing and we have extracted one‐year interim data for this review. All studies conducted follow‐up assessments on completion of the exercise intervention. Nine studies conducted a further set of equivalent assessments at a later time point (Cantarero‐Villanueva 2016; Cramer 2016; Hubbard 2016; Lewis 2016; McDermott 2017; Nuri 2016; Pinto 2013; Van Vulpen 2016; Waart 2017). Pinto 2013 was the only study to conduct three postintervention assessments, these took place at three, six and 12 months.
Control groups
The control groups received usual care, were not prescribed physical activity and did not take part in any formal exercise training during the course of the intervention. Five studies provided participants with written information on maintaining a healthy lifestyle (Courneya 2016; Hubbard 2016; Pinto 2013; Van Blarigan 2019), or provided recommendations for a healthy lifestyle (Cantarero‐Villanueva 2016). Three studies reported asking participants in the control arm to maintain their usual daily physical activity levels/lifestyle habits during the intervention (Brown 2017; Lee 2017; Van Vulpen 2016). One study instructed participants not to initiate any structured exercise over the course of the intervention (Courneya 2003). Three studies had a waiting list control group, providing participants the opportunity to take part in the physical activity intervention following completion of the study (Brown 2017; Cramer 2016; Lewis 2016). Van Blarigan 2019 offered a fit bit flex to control group participants following study completion. Pinto 2013 controlled for frequency of contact with participants by having a contact control group.
Outcome measures
The most frequently assessed outcome among the 16 included studies was physical fitness, measured in 12 studies (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Courneya 2016; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Pinto 2013; Van Vulpen 2016; Waart 2017), the most commonly used tools to measure physical fitness were the six‐minute walk test (Brown 2017; Cantarero‐Villanueva 2016; Courneya 2016; Lee 2017; Lewis 2016), and treadmill tests using a variety of protocols (Courneya 2003; Lewis 2016; Pinto 2013; Van Vulpen 2016; Waart 2017). Twelve studies measured levels of physical activity, objectively, using accelerometer data (Brown 2017; Hubbard 2016; Lewis 2016; McDermott 2017; Pinto 2013; Van Blarigan 2019), and subjectively, using a variety of self‐report questionnaires (Bourke 2011; Courneya 2003; Courneya 2016; Kim 2018; Lee 2017; Lewis 2016; Pinto 2013; Waart 2017). Lewis 2016 and Pinto 2013 measured levels of physical activity, both objectively and subjectively. Ten studies assessed anthropometric measurements (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Courneya 2016; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Van Vulpen 2016). All studies except for Courneya 2003 measured weight and only two did not measure body mass index (BMI) (Courneya 2016; Van Vulpen 2016). Fatigue and health‐related quality of life (HRQoL) were assessed in nine studies, out of the 16 included (Bourke 2011; Courneya 2003; Cramer 2016; Kim 2018; Lewis 2016; McDermott 2017; Pinto 2013; Van Vulpen 2016; Waart 2017), most frequently using the Functional Assessment of Cancer Therapy – Fatigue (FACT‐F) scale and Functional Assessment of Cancer Therapy – Colorectal (FACT‐C) scale, respectively. Adverse events were reported in eight studies, of these the absence of any adverse events were reported in four studies (Hubbard 2016; Lewis 2016; Van Vulpen 2016; Waart 2017), whilst four studies recorded the number of adverse events that occurred (Brown 2017; Cantarero‐Villanueva 2016; Cramer 2016; Van Blarigan 2019). Six studies assessed facets of mental health and well‐being (Courneya 2003; Cramer 2016; Kim 2018; McDermott 2017; Van Vulpen 2016; Waart 2017), mainly anxiety and depression, using the Hospital Anxiety and Depression Scale (HADS) score. Other outcomes including overall and recurrence‐free survival were not reported in any of the included studies. Adherence to Enhanced Recovery After Surgery (ERAS) guidelines and length of hospital stay was not reported in any of the included studies. For detailed information on outcome measures, see the Characteristics of included studies table.
Excluded studies
We excluded 99 trials from the review due to the following reasons.
Wrong patient population (n = 19)
Wrong study design (n = 25)
Wrong comparator (n = 18)
Wrong intervention (n = 16)
Intervention too short (n = 10)
Did not analyse colorectal cancer patients separately (n = 8)
Study was not carried out (n = 2)
Outcomes were not relevant (n = 1)
See Characteristics of excluded studies for an overview.
Risk of bias in included studies
We assessed the risk of bias for each included study using the ‘Risk of bias’ assessment tool and recommendations for judging risk of bias provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). See Figure 2 for an overall assessment of risk of bias presented as percentages across all included studies. In addition, Figure 3 provides a 'Risk of bias' summary for each included study. Due to the nature of the intervention, it was expected that blinding of participants and personnel delivering the interventions would not be possible. We therefore judged risk of performance bias as high in all included studies. See Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Twelve studies (75%) were at low risk of selection bias owing to adequate generation of a randomised sequence (Bourke 2011; Cantarero‐Villanueva 2016; Courneya 2003; Cramer 2016; Hubbard 2016; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Van Blarigan 2019; Van Vulpen 2016; Waart 2017). We considered four studies to have an unclear risk of selection bias as they did not describe the generation of a randomised sequence (Brown 2017; Courneya 2016; Nuri 2016; Pinto 2013).
Allocation concealment
Five studies (36%) were at low risk of selection bias owing to adequate concealment of allocation, so that participants and investigators could not foresee assignment to the study groups (Cantarero‐Villanueva 2016; Cramer 2016; Lewis 2016; McDermott 2017; Van Vulpen 2016). The other 11 studies were considered to have an unclear risk of selection bias owing to allocation concealment as they did not describe the method of concealment.
Blinding
Blinding of participants and personnel
Due to the nature of physical activity interventions, blinding of participants and personnel is not possible. Therefore, we judged all included studies at high risk of performance bias.
Blinding of outcome assessors
Six studies were at low risk of detection bias as outcome assessors were blinded to participants group assignment (Bourke 2011; Brown 2017; Courneya 2003; Cantarero‐Villanueva 2016; Pinto 2013; Van Vulpen 2016). We considered seven studies to have unclear risk for detection bias, as blinding of outcome assessors was not described (Courneya 2016; Cramer 2016; Hubbard 2016; Kim 2018; Lee 2017; Nuri 2016; Waart 2017). Three studies were at high risk for detection bias as the outcome assessor was aware of participants' group allocation (Lewis 2016; McDermott 2017; Van Blarigan 2019).
Incomplete outcome data
All included studies reported on adherence. All studies except for Hubbard 2016 were at low risk of attrition bias due to the amount, nature or handling of incomplete outcome data. Hubbard 2016 was at high risk of attrition bias due to the amount of missing data. Adherence to physical activity interventions in other studies varied between 71% and 97%.
Selective reporting
Ten studies were at low risk of reporting bias (Bourke 2011; Courneya 2003; Courneya 2016; Cramer 2016; Kim 2018; Lee 2017; Lewis 2016; McDermott 2017; Pinto 2013; Van Vulpen 2016). Six studies were at high risk of reporting bias, as study protocols or methods sections included outcomes which were not reported in available publications (Brown 2017; Cantarero‐Villanueva 2016; Hubbard 2016; Nuri 2016; Van Blarigan 2019; Waart 2017).
Other potential sources of bias
Baseline imbalances
Eleven studies were at low risk of selection bias owing to the absence of significant imbalances between group at baseline (Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Courneya 2016; Kim 2018; Lee 2017; Lewis 2016; Nuri 2016; Van Vulpen 2016), or in studies were baseline imbalances were present, appropriate allocation concealment was described (Cramer 2016; McDermott 2017). Four studies were at high risk of selection bias because group similarity at baseline was inadequate (Hubbard 2016; Pinto 2013;Van Blarigan 2019; Waart 2017). The risk of selection bias owing to baseline imbalances was unclear in one study as baseline imbalances were not reported (Bourke 2011). All included studies are at risk of participation bias, with the potential for the more motivated participants agreeing to participate.
Effects of interventions
See: Table 1
See: Table 1. For a summary of sensitivity analyses see: Table 2.
1. Summary of sensitivity analysis.
| Outcome | Time point | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical functiond | |||||
| 1.1 Subjective measure of physical function | Short‐term follow‐up | 2 | 114 | SMD (IV, random, 95% CI) | 0.08 (‐0.31 to 0.47)a |
| 2 Disease‐related mental health | |||||
| 2.1 Anxiety | Short‐term follow‐up | 3 | 177 | SMD (IV, random, 95% CI) | ‐0.29 (‐0.60 to 0.01)a,b |
| 2.2. Depression | Short‐term follow‐up | 3 | 177 | SMD (IV, random, 95% CI) | ‐0.18 (‐0.48 to 0.13)a,b |
| 3 Physical fitness | |||||
| 3.1 Aerobic fitness | Immediate‐term follow‐up Short‐term follow‐up |
4 5 |
207 187 |
SMD (IV, random, 95% CI) | 0.38 (0.06 to 0.70)a,b 0.45 (0.15 to 0.75)a,b |
| 4 Cancer‐related fatigued | Immediate‐term follow‐up Short‐term follow‐up |
4 5 |
169 224 |
MD (IV, random, 95% CI) SMD (IV, random, 95% CI) |
2.22 (‐0.34 to 4.79)a,b 0.32 (‐0.04 to 0.67)a,b |
| 5 Anthropometric measuresd | |||||
| 5.1 Weight | Immediate‐term follow‐up Change from baseline to 12 weeks follow‐up |
4 2 |
207 64 |
MD (IV, random, 95% CI) | 0.27 (‐2.87 to 3.42)a,c ‐1.76 [‐4.06 to 0.54]c |
| 5.2 Waist to hip ratio | Immediate‐term follow‐up | 2 | 44 | MD (IV, random, 95% CI) | 0.04 [‐0.01 to 0.10]a,c |
| 5.3 BMI | Immediate‐term follow‐up Change from baseline to 12 weeks follow‐up |
4 2 |
207 64 |
MD (IV, random, 95% CI) | 0.10 [‐0.87 to 1.06]a,c ‐0.42 [‐1.30 to 0.46] |
| 5.4 Body fat % | Immediate‐term follow‐up | 3 | 187 | MD (IV, random, 95% CI) | ‐2.13 [‐4.46 to 0.21]a,d |
| 6 HRQoL | Immediate‐term follow‐up | 4 | 169 | SMD (IV, random, 95% CI) | 0.37 [0.07 to 0.68]a,b,c |
| 7 Levels of physical activity | |||||
| 7.1 Objective measures | Immediate‐term follow‐up | 3 | 80 | MD (IV, random, 95% CI) | ‐2.84 [‐12.40 to 6.73]c |
| 7.2 Subjective measures | Immediate‐term follow‐up | 3 | 138 | SMD (IV, random, 95% CI) | 0.68[0.33 to 1.02]c |
BMI: body mass index; CI: confidence interval; HRQoL: health‐related quality of life; MD: mean difference: SD: standard deviation; SMD: standardised mean difference (used when studies assess the same outcome but measure it in a variety of ways).
a Removal of studies that did not conduct an ITT analysis
b Exclusion of studies at high risk of bias
c Exclusion of studies with an additional intervention component
d Results from choice of model (fixed or random) were consistent
All trial authors reported study results as follow‐up values and six studies also included change in score from baseline to follow‐up (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2016; Hubbard 2016; Lewis 2016). We completed meta‐analyses for both types of outcomes separately and for each follow‐up time period where data were available. We categorised follow‐up as: up to 12 weeks after baseline (immediate); more than 12 weeks but less than or equal to six months after baseline (short term); more than six months but less than or equal to 12 months after baseline (medium term); and more than 12 months after baseline (long term). No included studies reported follow‐up of greater than 12 months after baseline. One study reported follow‐up time points to end of chemotherapy treatment and at six months following completion of chemotherapy treatment (Waart 2017). We calculated follow‐up time points in months, using the reported percentage of chemotherapy treatment received. This was as a proportion of the average total planned duration of chemotherapy. Where studies had two intervention arms (Brown 2017; Waart 2017), or reported gender separately (Van Vulpen 2016), we combined these arms in RevMan to form a single pair‐wise comparison (Review Manager 2014).
Primary outcomes
Physical function
A total of 10 studies reported on physical function, assessed using a variety of measures, including the 30‐Second Chair Stand Test (Bourke 2011; Courneya 2016; Lee 2017; Lewis 2016; McDermott 2017), the Physical Functioning subscale of the Short Form‐36 (SF‐36) (Brown 2017; Pinto 2013), the functional well‐being subscale of the Functional Assessment of Cancer Therapy‐Colorectal (FACT‐C; Courneya 2003), the physical function subscale of the European Organisation for Research and Treatment of Cancer Quality of life Questionnaire‐Core 30 (EORTC QLQ‐C30; Waart 2017), and the Trial Outcome Index‐physical/functional/colorectal (Kim 2018). We conducted separate meta‐analyses for objectively and subjectively measured physical function.
Four studies including 185 participants measured physical function using the 30‐Second Chair Stand Test at immediate‐term follow‐up (Bourke 2011; Lee 2017; Lewis 2016; McDermott 2017), we did not pool these results in a meta‐analysis due to considerable variation in results and inconsistency in direction of effect. Lewis 2016 and McDermott 2017 observed no difference between the physical activity and usual care groups for physical function at immediate‐term follow‐up, whilst, Bourke 2011 and Lee 2017 reported significant improvements in physical function in the physical activity group compared with usual care (P = 0.003 and P = 0.005, respectively). We observed no evidence of difference between groups at short‐term follow‐up when measured objectively (mean difference (MD) 0.76, 95% confidence interval (CI) ‐1.84 to 3.36; 2 studies, 39 participants; I2 = 0%; moderate‐quality evidence; Analysis 1.1; Figure 4), and subjectively at short‐term follow‐up (standardised mean difference (SMD) 0.09, 95% CI ‐0.24 to 0.42; 3 studies, 156 participants; I2 = 0%; low‐quality evidence; Analysis 1.2; Figure 5). There were insufficient data to analyse subjective and objective physical function at medium‐term follow‐up, subjective physical function at immediate‐term follow‐up, and change from baseline results at all four follow‐up time points.
1.1. Analysis.

Comparison 1: Physical activity versus usual care for physical function, Outcome 1: Objective measures more than 12 weeks to 6 months follow‐up (30‐Second Chair Stand Test)
4.

Forest plot of comparison: 1 Physical activity versus usual care for physical function (30‐sec chair sit‐to‐stand test), outcome: 1.2 Objective measures more than 12 weeks to six months follow‐up (short term).
1.2. Analysis.

Comparison 1: Physical activity versus usual care for physical function, Outcome 2: Subjective measures more than 12 weeks to 6 months follow‐up
5.

Forest plot of comparison: 1 Physical activity versus usual care for physical function, outcome: 1.3 Subjective measures more than 12 weeks to six months follow‐up (short term).
Courneya 2016 reported that the structured exercise group (n = 99) improved relative to the health education material group (n = 98) for the 30‐Second Chair Stand Test (mean between group difference (+1.6 repetitions, 95% CI +0.6 to +2.7); P < 0.001) at one year. Pinto 2013 reported no significant group differences at immediate‐ or medium‐term follow‐up for self‐reported physical functioning (n = 23 and n = 19 for physical activity and usual care, respectively). Brown 2017 reported improvements in change from baseline scores for physical function at short‐term follow‐up with higher doses of physical activity resulting in greater improvements. Kim 2018 reported no difference between groups at immediate‐term follow‐up for physical function (P = 0.254). However, change from baseline to immediate‐term follow‐up improved in the intervention group (mean 64.1 (11.2) versus 66.3 (11.8), P = 0.035).
We performed sensitivity analyses to investigate the choice of model on the pooled estimate at all included time points. Sensitivity analyses using a fixed‐effect model were consistent with findings from a random‐effects model. The sensitivity analysis revealed no difference in effect when we removed studies that did not use an intention‐to‐treat (ITT) analysis; this was only possible for subjective measures at short‐term follow‐up (Table 2).
Disease‐related mental health
A total of seven studies reported on disease‐related mental health, assessed by the Hospital Anxiety and Depression Scale (HADS) (Cramer 2016; Van Vulpen 2016; Waart 2017), the Positive and Negative Affect Scale (PANAS) (McDermott 2017), the State‐Trait Anxiety Inventory (Courneya 2003), the Centre for Epidemiological Studies Depression Scale (CES‐D) (Courneya 2003), and the Patient Health Questionnaire (Kim 2018). We obtained postintervention data and separate scores for anxiety and depression through email correspondence with authors Van Vulpen 2016 and Waart 2017, respectively. We conducted meta‐analyses for anxiety and depression separately at 12 weeks to six months after baseline and more than six months to 12 months after baseline.
There was no evidence of difference in depression between the physical activity group and usual care group at short‐term follow‐up (SMD ‐0.21, 95% CI ‐0.50 to 0.08; 4 studies, 198 participants; I2 = 0%; moderate‐quality evidence; Analysis 2.1; Figure 6), or medium‐term follow‐up (assessed using HADS) (MD ‐1.20, 95% CI ‐2.72 to 0.31; 2 studies, 48 participants; I2 = 32%; low‐quality evidence; Analysis 2.2; Figure 7).
2.1. Analysis.

Comparison 2: Physical activity versus usual care for disease‐related mental health, Outcome 1: Depression: more than 12 weeks to 6 months follow‐up
6.

Forest plot of comparison: 2 Physical activity versus usual care for disease‐related mental health, outcome: depression 2.1 More than 12 weeks to six months follow‐up (short term).
2.2. Analysis.

Comparison 2: Physical activity versus usual care for disease‐related mental health, Outcome 2: Depression: more than 6 months to 12 months follow‐up (HADS)
7.

Forest plot of comparison: 2 Physical activity versus usual care for disease‐related mental health (Hospital Anxiety and Depression Scale (HADS)), outcome: depression 2.2 More than six months to 12 months follow‐up (medium term).
There was no evidence of difference in anxiety between the physical activity group and usual care group at short‐term follow‐up (SMD ‐0.11, 95% CI ‐0.40 to 0.18; 4 studies, 198 participants; I2 = 0%; moderate‐quality evidence; Analysis 2.3; Figure 8), or medium‐term follow‐up (assessed using HADS) (anxiety: MD 1.79, 95% CI ‐0.37 to 3.94; 2 studies, 47 participants; I2 = 30%; low‐quality evidence; Analysis 2.4; Figure 9). Data were insufficient for analysis of immediate‐term follow‐up and change scores at all four time points.
2.3. Analysis.

Comparison 2: Physical activity versus usual care for disease‐related mental health, Outcome 3: Anxiety: more than 12 weeks to 6 months follow‐up
8.

Forest plot of comparison: 2 Physical activity versus usual care for disease‐related mental health, outcome: 2.3 Anxiety more than 12 weeks to six months follow‐up.
2.4. Analysis.

Comparison 2: Physical activity versus usual care for disease‐related mental health, Outcome 4: Anxiety: more than 6 months to 12 months follow‐up (HADS)
9.

Forest plot of comparison: 2 Physical activity versus usual care for disease‐related mental health (Hospital Anxiety and Depression Scale (HADS)), outcome: 2.4 Anxiety more than six months to 12 months follow‐up (medium term).
McDermott 2017 (unpublished data) reported no statistically significant differences between intervention (n = 11) and control (n = 9) for PANAS‐positive affect and PANAS‐negative affect at immediate‐ or short‐term follow‐up. There were 10 participants in the control group at 24 weeks follow‐up for PANAS‐negative affect. Kim 2018 reported improvements in change from baseline scores for depression at immediate‐term follow‐up in the intervention group (n = 37) (P = 0.053).
A sensitivity analysis including studies at low risk of bias and those that performed an ITT analysis suggest there may be no effect of physical activity on anxiety (SMD ‐0.29, 95% CI ‐0.60 to 0.01; 3 studies, 177 participants; I2 = 0%) or depression (SMD ‐0.18, CI ‐0.48 to 0.13; 3 studies, 177 participants; I2= 0%) in the physical activity group compared to usual care at short‐term follow‐up (Table 2).
Adverse events
Eight out of the 16 included studies reported on adverse events (Brown 2017; Cantarero‐Villanueva 2016; Cramer 2016; Hubbard 2016; Lewis 2016; Van Blarigan 2019; Van Vulpen 2016; Waart 2017). We did not pool adverse events due to inconsistency in reporting and measurement. Of the eight studies, four studies reported that no adverse events (Hubbard 2016; Lewis 2016; Waart 2017) or serious adverse events (Van Vulpen 2016) occurred during the study period. The method of measurement of adverse events was not described by authors, Van Vulpen 2016 and Waart 2017. Van Vulpen 2016 and Lewis 2016 did not make reference to 'non‐serious' adverse events. Hubbard 2016 recorded adverse events in a participant log and recorded them as "related" or "unrelated" to the study. In the study by Lewis 2016, serious adverse events were recorded by the researcher within 24 hours of becoming aware of the event.
Cramer 2016 recorded all adverse events that occurred during the study period and asked open‐ended questions at weeks 10 and 22 to assess any adverse events not previously mentioned. No serious adverse events occurred and seven participants reported minor adverse events in the intervention group, including transient abdominal pain (n = 1), muscle soreness (n = 3), neck pain (n = 1), minor vertigo (n = 1) and hip pain (n = 1). One patient in the control group experienced a serious adverse event that was reported as "probably not causally related to the study intervention". Brown 2017 assessed adverse events using the Common Terminology Criteria for Assessing Adverse Events (CTCAE). No serious (grade 3) adverse events were reported at six months after baseline. One hundred and one non‐serious (grade 1 and 2) adverse events occurred in the intervention compared to 49 in the usual care group. Common non‐serious adverse events reported in the intervention group were: joint pain, back pain, generalised flu‐like symptoms, foot blisters and myalgia. In the study by Cantarero‐Villanueva 2016 each participant kept a diary to record adverse events. Six participants expressed both neck and abdominal discomfort with some of the exercises in the first sessions. Two participants in the intervention group and one participant in the usual care group experienced postoperative ventral hernias. One participant could not perform the aerobic exercise during one week because he suffered a peripheral neuropathy. The author did not report whether these were recorded as "related" or "unrelated" to the exercise intervention. In the study by Van Blarigan 2019 participants completed an online health check survey which recorded adverse events. Commonly reported non‐serious adverse events included low back, knee, joint, muscle and chest pain, inflammation of joints in both the intervention (n = 39) and control (n = 36) groups.
We graded the evidence of the included studies as low quality (8 studies, 305 participants).
Secondary outcomes
Overall survival
No RCTs reported overall survival.
Recurrence‐free survival
No RCTs reported recurrence‐free survival as an outcome. No studies reported cancer recurrence as reasons for dropout. One study reported lung metastasis in the intervention group as a reason for dropout (Lewis 2016).
Physical fitness
Aerobic fitness
A total of 12 studies reported aerobic fitness, using a variety of different measures including; the six‐minute walk test (Brown 2017; Cantarero‐Villanueva 2016; Courneya 2016; Lee 2017; McDermott 2017), the Bruce Protocol Treadmill Test (Bourke 2011; Lewis 2016), the Modified Balke Treadmill Test (Courneya 2003), V02 peak test (Van Vulpen 2016); Cycle Ergometer Peak Power Output Test (Van Vulpen 2016), Rockport Walk Test (Nuri 2016), treadwalk test (Pinto 2013), and the Steep Ramp Test (Waart 2017). Nuri 2016 and Pinto 2013 estimated V02 peak from submaximal fitness tests; we used this data in our meta‐analysis. No included studies reported change from baseline data at more than 12 months follow‐up.
At immediate‐term follow‐up, we observed an improvement in aerobic fitness in the physical activity group compared with the usual care group (SMD 0.82, 95% CI 0.34 to 1.29; 7 studies, 295 participants; I2 = 68%; low‐quality evidence; Analysis 3.1). This effect was also observed at short‐term follow‐up (SMD 0.56, 95% CI 0.29 to 0.82; 7 studies, 248 participants; I2 = 1%; moderate‐quality evidence; Analysis 3.3), but not at medium‐term follow‐up (SMD 0.44, 95% CI ‐0.04 to 0.92; 4 studies, 272 participants; I2 = 57%; very low‐quality evidence; Analysis 3.5).
3.1. Analysis.

Comparison 3: Physical activity versus usual care for physical fitness (aerobic fitness), Outcome 1: Up to 12 weeks follow‐up
3.3. Analysis.

Comparison 3: Physical activity versus usual care for physical fitness (aerobic fitness), Outcome 3: More than 12 weeks to 6 months follow‐up
3.5. Analysis.

Comparison 3: Physical activity versus usual care for physical fitness (aerobic fitness), Outcome 5: More than 6 months to 12 months follow‐up
Change in aerobic fitness from baseline showed an improvement compared with usual care at immediate‐term follow‐up (SMD 0.89, 95% CI 0.43 to 1.36; 3 studies, 81 participants; I2 = 0%; low‐quality evidence; Analysis 3.2) and short‐term follow‐up (SMD 0.62, 95% CI 0.05 to 1.19; 2 studies, 51 participants; I2 = 0%; moderate‐quality evidence; Analysis 3.4). We were unable to include data from the study by Brown 2017 in the meta‐analysis as the authors reported mean change between groups over time. We emailed to request postintervention and change from baseline data, but no reply was received.
3.2. Analysis.

Comparison 3: Physical activity versus usual care for physical fitness (aerobic fitness), Outcome 2: Change from baseline up to 12 weeks follow‐up
3.4. Analysis.

Comparison 3: Physical activity versus usual care for physical fitness (aerobic fitness), Outcome 4: Change from baseline more than 12 weeks to 6 months follow‐up
We conducted a sensitivity analysis excluding studies with an additional component (dietary advice), studies that did not conduct an ITT analysis and those at high risk of bias; results suggest improvements in aerobic fitness in the physical activity group compared with usual care (SMD 0.38, CI 0.06 to 0.70; 4 studies, 207 participants; I2= 15%; Table 2) at immediate‐ term follow‐up and short‐term follow‐up (SMD 0.45, CI 0.15 to 0.75; 5 studies, 187 participants; I2= 0%; Table 2). We also conducted a sensitivity analysis at medium‐term follow‐up excluding the study in which the physical activity intervention was ongoing. Results of this suggest no improvement in aerobic fitness in the physical activity group compared with usual care (SMD 0.44, CI 0.41 to 1.29; 3 studies, 86 participants).
Upper body strength
A total of five studies reported upper body strength, including arm strength, assessed by hand grip dynamometry (Lee 2017; Lewis 2016; Waart 2017), the 30 second arm‐curl test (Lewis 2016; Courneya 2016), isometric abdominal strength and isometric back strength assessed by the trunk‐curl test and back dynamometry, respectively (Cantarero‐Villanueva 2016), and the push‐up test to assess upper body strength and endurance (Lee 2017). A meta‐analysis was conducted for hand grip strength only, due to the large variability in measurement of upper body strength. Data were insufficient for analysis of more than six months to 12 months follow‐up and for change scores at all four time points.
We observed no difference in hand grip strength, assessed using hand grip dynamometry in the physical activity group compared with usual care at immediate‐term follow‐up (MD 1.92, 95% CI ‐1.17 to 5.00; 2 studies, 147 participants; I2 = 0%, low‐quality evidence; Analysis 4.1) or at short‐term follow‐up (MD 0.94, 95% CI ‐5.98 to 7.87; 2 studies, 39 participants; I2 = 0%; very low‐quality evidence; Analysis 4.2). In the study by Lee 2017, hand grip strength was reported separately for the left and right hand. We contacted Lee 2017 to request combined data, but we received no response. We inputted the data from the right hand only.
4.1. Analysis.

Comparison 4: Physical activity versus usual care for physical fitness (hand grip strength), Outcome 1: Up to 12 weeks follow‐up (hand dynamometry)
4.2. Analysis.

Comparison 4: Physical activity versus usual care for physical fitness (hand grip strength), Outcome 2: More than 12 weeks to 6 months follow‐up (hand dynamometry)
Lee 2017 reported a significantly greater improvement in the push‐up test (P < 0.001) in the intervention group (n = 62) compared with control (n = 61) at 12 weeks. The physical activity group (n = 21) experienced a greater increase in isometric abdominal strength compared with that of the usual care group (n = 19) at eight weeks (P = 0.001) but not at six months follow‐up in the study by Cantarero‐Villanueva 2016. No significant difference was found between groups for isometric back strength. There were no significant group differences in change in arm‐curl repetitions in the study by Lewis 2016 (n = 12 for the physical activity group and usual care group at 12 weeks; n = 9 and n = 11 at 6 months, respectively) or in the study by Courneya 2016 (P = 0.18) (n = 99 and n = 98 for the physical activity and usual care groups, respectively) at one year.
Flexibility
Three studies measured flexibility, assessed by the Sit and Reach Test (Courneya 2003; Courneya 2016), and the Modified Sit and Reach Test (Cantarero‐Villanueva 2016). Cantarero‐Villanueva 2016 reported flexibility of the right and left side separately. We emailed the author, but were unable to obtain combined sit and reach scores. We used data for the right side in our analysis. Mean scores were multiplied by minus one to ensure all scales had the same direction of effect as discussed in the Cochrane Handbook for Systematic Reviews of Interventions section, 9.2.3.2 (Deeks 2017). We observed no difference in flexibility in the physical activity group compared to usual care at short‐term follow‐up (SMD 0.02, 95% CI ‐0.36 to 0.39; 2 studies, 119 participants; I2 = 0%; low‐quality evidence; Analysis 5.1). There were insufficient data for analysis of flexibility at immediate‐ and medium‐term follow‐up and for change scores at all four time points.
5.1. Analysis.

Comparison 5: Physical activity versus usual care for physical fitness (flexibility), Outcome 1: More than 12 weeks to 6 months follow‐up
Courneya 2016 reported that the structured exercise group (n = 99) improved relative to the health education material group (n = 98) for the Sit and Reach Test (mean between group difference of +2.1 cm; 95% CI ‐0.6 to +4.7; P = 0.08) at one year.
Lower limb strength
Two studies measured lower body strength, using dynamometry to assess maximum voluntary torque production of the knee extensors (Bourke 2011; Waart 2017). Meta‐analysis was precluded as studies measured lower limb strength at different time points. In the study by Bourke 2011, no difference in maximum voluntary torque was observed between the intervention (n = 9) and control group (n = 9) over the 12‐week intervention period (P = 0.127). Conversely, Waart 2017 observed improvements in lower body strength in the usual care control group (n = 6), the onco‐move group (low‐intensity home‐based exercise; n = 6) and a reduction in strength in the on‐track intervention group (moderate‐ to high‐intensity supervised exercise; n = 7) at the end of chemotherapy (calculated as 4.8 to 5 months follow‐ up). At six months following chemotherapy (calculated as 10 to 11 months follow‐up), the on‐track group (n = 6) recovered to above baseline values (no P values were reported).
Cancer‐related fatigue
A total of 10 studies reported cancer‐related fatigue, assessed by the Functional Assessment of Chronic Illness Therapy ‐ Fatigue (FACIT‐F) (Cramer 2016; Kim 2018; McDermott 2017), the Functional Assessment of Cancer Therapy ‐ Fatigue (FACT‐F) (Bourke 2011; Courneya 2003; Lewis 2016; Pinto 2013), the Multidimensional Fatigue Inventory (MFI) (Van Vulpen 2016; Waart 2017), the Multidimensional Fatigue Symptom Inventory ‐ Short Form (MFSI‐SF) (McDermott 2017), and the Fatigue Symptom Inventory (FSI) (Brown 2017). Van Vulpen 2016 and Waart 2017 reported results separately for subscales of the MFI. We extracted the MFI general subscale as a comparable measure for inclusion in the meta‐analysis. We chose the general subscale score as it includes general statements about fatigue and decreased functioning, designed to encompass both physical and psychological aspects of fatigue (Lin 2009). We multiplied mean scores from the MFI general subscale by minus one to ensure all scales had the same direction of effect, as discussed in the Cochrane Handbook for Systematic Reviews of Interventions section, 9.2.3.2 (Deeks 2017).
Low‐quality evidence suggests an improvement in cancer‐related fatigue in the physical activity group compared with the usual care group at immediate‐term follow‐up (MD 2.16, 95% CI 0.18 to 4.15; 6 studies, 230 participants; I2= 18%; Analysis 6.1), short‐term follow‐up (SMD 0.34, 95% CI 0.08 to 0.60; 7 studies, 277 participants; I2 = 9%; moderate‐quality evidence; Analysis 6.3), but not medium‐term follow‐up (SMD 0.25, 95% CI ‐0.16 to 0.67; 3 studies, 91 participants; I2 = 0%; low‐quality evidence; Analysis 6.4). Change from baseline to 12 weeks suggests no improvement in fatigue in the physical activity group compared with the usual care group (MD 0.41, 95% CI ‐1.33 to 2.14; 3 studies, 113 participants; I2 = 70%; low‐quality evidence; Analysis 6.2). There were insufficient data to conduct change from baseline analysis at all other time points. Brown 2017 reported improvements in change from baseline scores in the high dose intervention group compared with control at short‐term follow‐up (P trend = 0.045).
6.1. Analysis.

Comparison 6: Physical activity versus usual care for cancer‐related fatigue, Outcome 1: Up to 12 weeks follow‐up (FACT‐F and FACIT‐F)
6.3. Analysis.

Comparison 6: Physical activity versus usual care for cancer‐related fatigue, Outcome 3: More than 12 weeks to 6 months follow‐up
6.4. Analysis.

Comparison 6: Physical activity versus usual care for cancer‐related fatigue, Outcome 4: More than 6 months to 12 months follow‐up
6.2. Analysis.

Comparison 6: Physical activity versus usual care for cancer‐related fatigue, Outcome 2: Change from baseline up to 12 weeks follow‐up (FACT‐F and FACIT‐F)
We conducted a sensitivity analysis with studies at low risk of bias and those that performed an ITT analysis at immediate‐ and short‐term follow‐up. There was no difference in fatigue between groups at immediate‐ or short‐term follow‐up (Table 2). Results from the fixed‐effect sensitivity analysis were consistent with those from the random‐effects model.
Anthropometric measurements
A total of 10 studies reported anthropometric measurements assessed by: weight (Bourke 2011; Cantarero‐Villanueva 2016; Courneya 2016; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016; Van Vulpen 2016), BMI (Bourke 2011; Brown 2017; Cantarero‐Villanueva 2016; Courneya 2003; Lee 2017; Lewis 2016; McDermott 2017; Nuri 2016), waist measurement (Brown 2017; Cantarero‐Villanueva 2016; Lee 2017; McDermott 2017), waist‐to‐hip ratio (Bourke 2011; Brown 2017; Lewis 2016; McDermott 2017), body fat percentage (Cantarero‐Villanueva 2016; Courneya 2003; Lee 2017; Lewis 2016; Nuri 2016), and hip circumference (Brown 2017; Courneya 2016; McDermott 2017). Brown 2017 also assessed visceral fat, subcutaneous fat and fat mass.
We found no evidence of effect of physical activity compared with usual care on weight (Analysis 7.1: moderate‐quality evidence and Analysis 8.3: low‐quality evidence), BMI (Analysis 11.1: low‐quality evidence and Analysis 11.3: low‐quality evidence), waist measurement (Analysis 8.1: very low‐quality evidence and Analysis 8.2: low‐quality evidence), waist‐to ‐hip ratio (Analysis 9.1: very low‐quality evidence and Analysis 9.3: low‐quality evidence), or body fat percentage (Analysis 10.1: low‐quality evidence and Analysis 10.3: very low‐quality evidence) at immediate‐ or short‐term follow‐up time points.
7.1. Analysis.

Comparison 7: Physical activity versus usual care for anthropometric measure of weight (kg), Outcome 1: Up to 12 weeks follow‐up
8.3. Analysis.

Comparison 8: Physical activity versus usual care for anthropometric measure of waist circumference, Outcome 3: Change from baseline more than 12 weeks to 6 months follow‐up
11.1. Analysis.

Comparison 11: Physical activity versus usual care for anthropometric measure of BMI, Outcome 1: Up to 12 weeks follow‐up
11.3. Analysis.

Comparison 11: Physical activity versus usual care for anthropometric measure of BMI, Outcome 3: More than 12 weeks to 6 months follow‐up
8.1. Analysis.

Comparison 8: Physical activity versus usual care for anthropometric measure of waist circumference, Outcome 1: Up to 12 weeks follow‐up
8.2. Analysis.

Comparison 8: Physical activity versus usual care for anthropometric measure of waist circumference, Outcome 2: More than 12 weeks to 6 months follow‐up
9.1. Analysis.

Comparison 9: Physical activity versus usual care anthropometric measure of waist to hip ratio, Outcome 1: Up to 12 weeks follow‐up
9.3. Analysis.

Comparison 9: Physical activity versus usual care anthropometric measure of waist to hip ratio, Outcome 3: More than 12 weeks to 6 months follow‐up
10.1. Analysis.

Comparison 10: Physical activity versus usual care for anthropometric measure of body fat (%), Outcome 1: Up to 12 weeks follow‐up
10.3. Analysis.

Comparison 10: Physical activity versus usual care for anthropometric measure of body fat (%), Outcome 3: More than 12 weeks to 6 months follow‐up
Change from baseline to 12 weeks follow‐up, but not change from baseline from 12 weeks to six months follow‐up suggests a small reduction in weight (MD ‐1.71, 95% CI ‐2.90 to ‐0.51; 3 studies, 82 participants; I2 = 1%; low‐quality evidence; Analysis 7.2; Analysis 7.3) and body fat percentage (MD ‐1.57, 95% CI ‐3.11 to ‐0.04; 2 studies, 60 participants; I2 = 0%; low‐quality evidence; Analysis 10.2 and Analysis 10.4), but not BMI at either time point (Analysis 11.2; Analysis 11.4; low‐ and very low‐quality evidence, respectively, or change from baseline up to 12 weeks for waist‐to‐hip‐ratio (Analysis 9.2; very low‐quality evidence). Low‐quality evidence suggests a small reduction in waist measurement in change from baseline from 12 weeks to six months follow‐up (MD ‐2.79, 95% CI ‐5.21 to ‐0.36; 2 studies, 70 participants; I2 = 38%; Analysis 8.3). When we conducted a sensitivity analysis using a random‐effects model for waist measurement, the difference between the physical activity and usual care group was no longer significant (MD ‐4.36 cm, ‐11.42 to 2.71; 2 studies, 70 participants; I2= 38%).
7.2. Analysis.

Comparison 7: Physical activity versus usual care for anthropometric measure of weight (kg), Outcome 2: Change from baseline up to 12 weeks follow‐up
7.3. Analysis.

Comparison 7: Physical activity versus usual care for anthropometric measure of weight (kg), Outcome 3: Change from baseline more than 12 weeks to 6 months follow‐up
10.2. Analysis.

Comparison 10: Physical activity versus usual care for anthropometric measure of body fat (%), Outcome 2: Change from baseline up to 12 weeks follow‐up
10.4. Analysis.

Comparison 10: Physical activity versus usual care for anthropometric measure of body fat (%), Outcome 4: Change from baseline more than 12 weeks to 6 months follow‐up
11.2. Analysis.

Comparison 11: Physical activity versus usual care for anthropometric measure of BMI, Outcome 2: Change from baseline up to 12 weeks follow‐up
11.4. Analysis.

Comparison 11: Physical activity versus usual care for anthropometric measure of BMI, Outcome 4: Change from baseline more than 12 weeks to 6 months follow‐up
9.2. Analysis.

Comparison 9: Physical activity versus usual care anthropometric measure of waist to hip ratio, Outcome 2: Change from baseline up to 12 weeks follow‐up
There was insufficient postintervention and change from baseline data at six months to 12 months to report on any anthropometric measure. Insufficient data precluded meta‐analysis of change from baseline data to 12 weeks follow‐up for waist measurement and more than 12 weeks to six months follow‐up for waist‐to‐hip‐ratio. We did not pool studies that measured hip circumference from 12 weeks to six months follow‐up (Brown 2017; McDermott 2017), as Brown 2017 reported change from baseline scores whilst McDermott 2017 reported postintervention scores.
Courneya 2016 reported no statistically significant difference between the structured exercise programme (SEP) group and the health education materials (HEM) group for weight (SEP = 115, HEM = 112) (P = 0.38), hip circumference (SEP = 99, HEM = 99) (P = 0.90) or waist circumference (SEP = 99, HEM = 99) (P = 0.31) at one year. Between‐group changes in body weight were not significantly different between intervention (n = 16) and usual care (n =14) for both men and women in the study by Van Vulpen 2016 at 18 and 36 weeks. Changes in waist‐to‐hip ratio were not statistically significance in the study by Lewis 2016 (P = 0.43) or Brown 2017 (P = 0.054). Cantarero‐Villanueva 2016 reported a significant change from baseline scores to eight weeks and six months between the physical activity group (n = 21) and usual care group (n = 19) for waist circumference. Changes in hip circumference up to six months were not significant in the study by Brown 2017 (P = 0.518), whilst McDermott 2017 reported an increase in hip circumference in the control group at week 12 (n = 9) and a decrease at week 24 (n = 9), but not to pre‐intervention levels, whilst the intervention group decreased at week 12 (n = 11) and increased at week 24 (n = 10) but not to pre‐intervention levels (P = 0.012).
We conducted a sensitivity analysis for weight, waist‐to‐hip‐ratio, BMI and body fat percentage at immediate‐term follow‐up, we excluded the study by Bourke 2011 because the intervention contained a dietary advice component and the study by Nuri 2016 as an ITT analysis was not conducted. Results of the sensitivity analysis suggests no difference in effect of physical activity compared with usual care on weight, BMI, waist measurement, waist‐to‐hip ratio or body fat percentage. The choice of model (fixed or random) did not effect the estimates of effect at this time point. A sensitivity analysis for change from baseline to 12 weeks follow‐up suggested no reduction in weight or BMI when the study by Bourke 2011 was removed. A sensitivity analysis with studies at low risk of bias was conducted for weight and BMI for change scores from 12 weeks to six months, results suggest no difference in effect of physical activity compared with usual care on weight or BMI at this time point.
Health‐related quality of life (HRQoL)
A total of 10 studies reported HRQoL; seven of these studies assessed HRQoL using the Functional Assessment of Cancer Therapy‐Colorectal (FACT‐C; Bourke 2011; Brown 2017; Courneya 2003; Cramer 2016; Kim 2018; McDermott 2017; Pinto 2013), the Functional Assessment of Cancer Therapy‐General (FACT‐G; Lewis 2016), the European Organisation for Research and Treatment of Cancer Quality of life Questionnaire‐Core 30 (EORTC QLQ‐C30; Van Vulpen 2016; Waart 2017), the EuroQol‐Visual Analogue Scales (EQ‐VAS; Lewis 2016), and the Satisfaction With Life Scale (SWLS; Courneya 2003).
Moderate‐quality evidence suggests a small positive effect for the physical activity group compared with the usual care group on HRQoL at immediate‐term follow‐up (SMD 0.36, 95% CI 0.10 to 0.62; 6 studies, 230 participants; I2 = 0%; Analysis 12.1) and short‐term follow‐up (SMD 0.45, 95% CI 0.03 to 0.88; 7 studies, 278 participants; I2 = 61%; Analysis 12.3) but no evidence of difference between groups at medium‐term follow‐up (SMD 0.05, 95% CI ‐0.37 to 0.47; 3 studies, 89 participants; I2 = 0%; low‐quality evidence; Analysis 12.5) or change from baseline up to 12 weeks follow‐up (SMD ‐0.10, 95% CI ‐0.47 to 0.28; 3 studies, 113 participants; I2 = 16%; low‐quality evidence; Analysis 12.2). We observed a small positive effect on change from baseline scores at more than 12 weeks to six months follow‐up (SMD 0.70, 95% CI 0.14 to 1.26; 2 studies, 58 participants; I2 = 0%; low‐quality evidence; Analysis 12.4). Data were insufficient to conduct a meta‐analysis of change from baseline scores at more than six months to 12 months follow‐up.
12.1. Analysis.

Comparison 12: Physical activity versus usual care for HRQoL, Outcome 1: Up to 12 weeks follow‐up
12.3. Analysis.

Comparison 12: Physical activity versus usual care for HRQoL, Outcome 3: More than 12 weeks to 6 months follow‐up
12.5. Analysis.

Comparison 12: Physical activity versus usual care for HRQoL, Outcome 5: More than 6 months to 12 months follow‐up
12.2. Analysis.

Comparison 12: Physical activity versus usual care for HRQoL, Outcome 2: Change from baseline up to 12 weeks follow‐up
12.4. Analysis.

Comparison 12: Physical activity versus usual care for HRQoL, Outcome 4: Change from baseline more than 12 weeks to 6 months follow‐up
A sensitivity analysis suggests that physical activity interventions may have a positive effect on HRQoL compared to usual care at immediate‐term follow‐up, (excluding the study by Bourke 2011), and including studies at low risk of bias and those that conducted an ITT analysis (SMD 0.37, 95% CI 0.07 to 0.68; 4 studies, 169 participants; I2 = 0%; Table 2).
Levels of physical activity
A total of 12 studies reported on levels of physical activity, assessed objectively and subjectively. Objective physical activity was assessed by accelerometry (Brown 2017; Hubbard 2016; Lewis 2016; McDermott 2017; Van Blarigan 2019). We pooled total minutes of moderate to vigorous physical activity for the meta‐analysis. Subjective physical activity was assessed by the Godin Leisure Time Exercise Questionnaire (Bourke 2011; Courneya 2003; Kim 2018; Lee 2017), the International Physical Activity Questionnaire (IPAQ; Lewis 2016), the Seven‐Day Physical Activity Recall (7‐day PAR) questionnaire (Courneya 2003; Pinto 2013), the Tartu Physical Activity Questionnaire (TPAQ; Courneya 2016), the Community Healthy Activities Model Programme for Seniors (CHAMPS; Courneya 2016), and the Physical Activity Scale for the Elderly (PASE; Waart 2017).
We found no evidence of difference in levels of physical activity in the physical activity group compared to usual care at immediate‐term follow‐up (MD ‐8.34, 95% CI ‐21.05 to 4.37; 4 studies, 94 participants; I2 = 43%; very low‐quality evidence; Analysis 13.1) or short‐term follow‐up (MD 13.50, 95% CI ‐56.73 to 83.74; 2 studies, 36 participants; I2 = 13%; Analysis 13.4) when measured objectively using accelerometry. We found no evidence of difference in change from baseline scores up to 12 weeks between groups (SMD ‐0.13, 95% CI ‐0.77 to 0.52; 2 studies, 37 participants; I2 = 0%; very low‐quality evidence; Analysis 13.2).
13.1. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 1: Objective measures up to 12 weeks follow‐up (accelerometry moderate to vigorous physical activity mins/per day)
13.4. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 4: Objective measures more than 12 weeks to 6 months follow‐up (accelerometry moderate to vigorous physical activity mins/week)
13.2. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 2: Change from baseline in objective measures up to 12 weeks follow‐up (accelerometry moderate to vigorous physical activity)
Subjective measures of levels of physical activity suggest an increase in levels of physical activity in the physical activity group compared to the usual care group at immediate‐term follow‐up (SMD 0.70, 95% CI 0.38 to 1.03; 4 studies, 156 participants; I2 = 0%; very low‐quality evidence; Analysis 13.3), short‐term follow‐up (SMD 0.39, 95% CI ‐0.05 to 0.82; 4 studies, 176 participants; I2 = 38%; low‐quality evidence; Analysis 13.5) and medium‐term follow‐up (SMD 0.35, 95% CI 0.11 to 0.59; 3 studies, 274 participants; I2 = 21%; very low‐quality evidence; Analysis 13.6).
13.3. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 3: Subjective measures up to 12 weeks follow‐up
13.5. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 5: Subjective measures more than 12 weeks to 6 months follow‐up
13.6. Analysis.

Comparison 13: Physical activity versus usual care for levels of physical activity, Outcome 6: Subjective measures more than 6 months to 12 months follow‐up
We were unable to extract data from studies by Brown 2017 and Lee 2017 because Brown 2017 reported mean change in moderate to vigorous physical activity and Lee 2017 reported moderate and vigorous physical activity separately. We emailed authors but received no response. In the study by Brown 2017, both the low dose (n = 14) and high dose (n = 12) exercise group increased their moderate to vigorous physical activity compared to the usual care group (n = 13) (P trend = < 0.001) over six months. Lee 2017 reported an increase in moderate to vigorous physical activity in the exercise group (n = 62) with no change in the usual care group at three months (group x time interaction, P < 0.01). Data were insufficient to conduct a meta‐analysis for change from baseline from more than 12 weeks to six months follow‐up and more than six months to 12 months follow‐up for objective measures and subjective measures at all time points.
We performed a sensitivity analysis, excluding studies with dietary advice co‐interventions up to 12 weeks follow‐up after baseline. Objectively measured physical activity suggests no difference in levels of physical activity in the physical activity group compared to usual care in immediate‐term follow‐up (MD ‐2.84, 95% CI ‐12.40 to 6.73; 3 studies, 80 participants; I2 = 0%; Table 2). Sensitivity analysis of subjective measures of physical activity suggest an increase in levels of physical activity in the physical activity group compared to the usual care group in immediate‐term follow‐up (SMD 0.68, 95% CI 0.33 to 1.02; 3 studies, 138 participants; I2 = 0%; Table 2). At short‐term follow‐up, we included studies at low risk of bias and studies that conducted an ITT analysis; this sensitivity analysis suggests no difference between levels of physical activity in the physical activity group compared to the usual care group.
Subgroup analysis
Subgroup analyses were precluded as we were unable to extract sufficient compatible data to undertake the predefined subgroup analysis
Discussion
Summary of main results
Summary of main results
This review included 16 randomised controlled trials (RCTs) with a total of 992 participants diagnosed with non‐advanced colorectal cancer. We are uncertain whether physical activity interventions improve physical function. We found no evidence of effect of physical activity interventions on disease‐related mental health, anthropometric measures or levels of physical activity (measured objectively). Interestingy, when levels of physical activity were measured subjectively they were higher in the physical activity group compared to the usual care group at immediate‐, short‐ and medium‐term follow‐up. We found no evidence of serious adverse events in the intervention or usual care groups in the eight studies that reported on adverse events. There was inconsistency in the measurement and reporting of adverse events between studies. Where reported, adverse events were generally minor. We found evidence of positive effects of physical activity interventions on physical fitness (aerobic fitness, but not other components of fitness), cancer‐related fatigue and health‐related quality of life (HRQoL) at immediate‐ and short‐term follow‐up but not medium‐term follow‐up. Only three studies reported medium‐term follow‐up for cancer‐related fatigue and HRQoL. No studies assessed outcomes at long‐term follow‐up. One study that assessed fatigue and levels of physical activity at four years is currently awaiting classification (Van Vulpen 2016).
No studies reported on overall survival or recurrence‐free survival. Our findings should be interpreted with caution as we rated the quality of evidence between very low to moderate overall. Further, higher quality studies are required to assess the effectiveness and safety of physical activity interventions for non‐advanced colorectal cancer patients.
Overall completeness and applicability of evidence
This systematic review includes studies from nine different countries. All studies except for one were undertaken in higher‐income countries (Nuri 2016). This evidence may therefore limit the applicability to low‐middle income countries. All studies in this version of the review except for one, exclusively included individuals with non‐advanced colorectal cancer; Courneya 2003 included four stage IV participants. The majority of studies included only participants that had finished active treatment, this was apparent across all outcomes, especially at immediate‐term follow‐up, limiting applicability to those undergoing active treatment. Ethnicity was only reported in three studies; these studies included mostly white people. Level of education was reported in nine studies and there was some variation in the percentage of participants educated to degree level. The physical activity interventions varied somewhat in their frequency, intensity, time, mode and duration, and because of this we are unable to ascertain optimal mode, frequency, intensity or duration of physical activity interventions for effects on the primary and secondary outcomes assessed in this review, limiting recommendations for clinical practice. We were unable to undertake subgroup analysis according to physical activity intervention or participant characteristics, cancer characteristics, treatment stages and length of time since diagnosis due to lack of comparable data, which limits applicability of findings. The objective measure of physical fitness that was used most commonly amongst included studies was the 6‐minute walk test. This is not the gold standard of measuring physical fitness and has been shown to underestimate VO2 peak in cancer survivors (Schumacher 2017). This may also limit the applicability of the evidence in this population. The highest incidence of colorectal cancer occurs in people aged between 65 and 74, the mean age of participants included in this review ranged between 51 and 69, this may therefore limit the applicability of this evidence to the population with the highest incidence of colorectal cancer.
For primary outcomes, such as physical function and disease‐related mental health, the greatest number of studies included in a meta‐analysis at any given time point was four. These findings should be interpreted with caution due to the limited number of studies and small sample sizes, especially at longer periods of follow‐up, where fewer studies were included. In addition, some of the outcome data included were not normally distributed which may have affected study results. Therefore, findings should be interpreted with caution. We were unable to undertake a meta‐analysis of adverse events due to inconsistencies in measurement and reporting of this data. Less than half of the included studies reported on adverse events and four of these reported that no adverse events occurred. In future, RCTs should systematically record and report adverse events and define whether these events are 'related' or 'unrelated' to the intervention. We could not provide an analysis of effects of physical activity interventions on important outcomes, such as overall survival or recurrence‐free survival because no included studies reported these outcomes. No studies assessed the long‐term effects (more than 12 months follow‐up) of physical activity interventions on any of the primary and secondary outcomes. One study is currently awaiting classification, which reports on four‐year effects of exercise on fatigue and levels of physical activity (Van Vulpen 2016). In addition, across all outcomes, few studies conducted follow‐up measurements at medium‐term follow‐up; these findings should be interpreted with caution
Quality of the evidence
The quality of the findings are reported in Table 1 for the main comparison. We assessed the quality of evidence for each outcome using GRADE methodology (GRADE Working Group 2004). For the immediate‐term follow‐up, GRADE revealed low‐quality evidence for physical function, adverse events, physical fitness (aerobic fitness) and cancer‐related fatigue. Moderate‐quality evidence was revealed for HRQoL (immediate‐term follow‐up) and disease‐related mental health (anxiety and depression) at short‐term follow‐up. The quality of evidence for change from baseline scores and all other time points, ranged between very low to moderate quality. We downgraded the quality of evidence, namely in light of imprecision and indirectness due to the small number of studies/participants included and considering the majority of studies were conducted in participants who had finished active treatment. In addition, three studies included a health education component which may also introduce indirectness, although only one of these studies reported outcomes at immediate‐term follow‐up. Due to the nature of the intervention it was not possible to blind personnel or participants to the intervention, putting all studies at high risk of performance bias, which may be accentuated where participants completed subjective assessments. We found discrepancy in direction of effect of interventions when levels of physical activity were measured subjectively compared to objectively. It has been recently documented that people with cancer self‐report their physical activity levels to be nearly four‐fold higher when compared to objective physical activity monitoring data (Vassbakk‐Brovold 2016). Most of the uncertainty in judging study bias came from a lack of clarity around allocation concealment and blinding of outcome assessors. The 'Risk of bias' table and graph are available in Figure 2 and Figure 3. In addition, some of the data included in the meta‐analysis for measures of mental health, cancer‐related fatigue, aerobic fitness and levels of physical activity were not normally distributed, which may have affected the quality of the evidence.
Potential biases in the review process
This systematic review included comprehensive search strategies of eight electronic databases, and four clinical trial registries. We (MMG and MAT) independently screened reference lists of all included studies and any relevant systematic reviews identified. We handsearched conference and meeting abstracts of relevant organisations and undertook data extraction and 'Risk of bias judgement' independently. We sent emails to seven authors of studies registered in clinical trial registries with recent completion dates and received correspondence from six of these authors. We emailed four authors of unpublished studies, two of these studies are included in this review (Lewis 2016; McDermott 2017). We sent a further 13 emails to study authors that included colorectal cancer participants alongside other cancer cohorts, to request colorectal cancer participant data separately and emailed authors to request missing data,some did not reply and exclusion of these results may also be a source of bias. We were unable to explore publication bias because there were less than 10 studies included in each comparison. The mean age of participants ranged between 51 and 69, which is markedly lower than the incidence of colorectal cancer. The review therefore does not include a cohort of the relevant population, which is a source of selection bias. Furthermore, the mean age range of participants may be indicative of participation bias, where healthier participants are more likely to agree to participate in the physical activity intervention research.
A limitation of this review is that three included studies involved additional components, e.g. healthy eating seminars and a dietary information pack (Bourke 2011), health education materials for the usual care group (Courneya 2016), and weekly education sessions on a range of topics (Hubbard 2016), which could have potential synergistic effects of exercise or physical activity on some outcomes. We investigated their effect on outcomes through exclusion in sensitivity analysis when possible.
Agreements and disagreements with other studies or reviews
To our knowledge only two other systematic reviews have investigated the effectiveness of physical activity interventions in colorectal cancer patients. One if these reviews published in 2014 included 5 RCTs and 238 patients assessing the effect of exercise on HRQoL, fatigue, physical fitness, survival and/or tumour‐associated biomarkers in colorectal cancer patients (Cramer 2014b). The meta‐analysis for physical fitness, fatigue and HRQoL included three studies all of which are included in our review (Bourke 2011; Courneya 2003; Pinto 2013). At short‐term follow‐up (corresponding to our immediate‐term follow‐up) authors observed improvements in physical fitness after aerobic exercise compared with controls which is in agreement with our review. Also in agreement with our review, no studies reported on survival. Conversely, authors found no evidence for short‐term effects on quality of life or cancer‐related fatigue, whereas we found evidence of favouring effects of physical activity interventions on these outcomes at the same time point. We also observed this effect at short‐term follow‐up. Cramer 2014b was unable assess outcomes at this time point due to insufficient data. In addition, authors were unable to report on safety data, whereas we reported adverse events narratively due to the extent of variation in measurement and reporting of this outcome. The differences between these reviews are due to the time of the literature search, indeed all of our other included studies were conducted after 2014. The second review conducted by van Rooijen 2018 highlighted the lack of evidence available on exercise training in those explicitly undergoing active treatment, which was also evident in this review. Six of the seven studies included in the van Rooijen 2018 review included mixed cancer populations, we therefore did not compare results.
Authors' conclusions
Implications for practice.
We are uncertain whether physical activity interventions improve physical function compared to usual care. Physical activity interventions may have no effect on disease‐related mental health. Physical activity interventions may be beneficial for aerobic fitness, cancer‐related fatigue and HRQoL at immediate‐and short‐term follow‐up. There were no serious adverse events in any of the studies that provided safety data. Where reported, adverse events were generally minor. The findings of this review should be interpreted with caution due to the low number of studies included and the quality of the evidence. Due to inconsistency in measuring and reporting of this data, more research is required to inform clinical practice. In addition, the current evidence is based on a small number of studies with few participants. The evidence is graded between low and moderate for the main outcomes, which precludes informed decision making in the clinical setting. Adequately powered RCTs of high methodological quality with longer‐term follow‐up are required to assess the effect of physical activity interventions on disease‐related physical and mental health and on survival of people with non‐advanced colorectal cancer. Adverse events should also be adequately reported. Further, it would be extremely important to understand whether certain exercise components (mode, frequency, duration and intensity) have optimal effects on physical and disease‐related mental health of CRC patients both during and following active treatment.
Implications for research.
This review highlights the need for further large‐scale RCTs to assess the effect of physical activity interventions on the disease‐related physical and mental health of people with non‐advanced colorectal cancer. Future RCTs should be of high methodological quality and adequately powered, and include longer‐term follow‐up to investigate the sustainability of short‐term benefits of physical activity. We identified only two ongoing studies that are investigating disease‐free survival, recurrence‐free survival and overall survival (Piringer 2017; NCT03885817) and only two studies explicitly stating "side effects of the intervention" and "safety" as outcome measures (Ho 2013; Piringer 2017). It is important for future RCTs to investigate the effects of physical activity on overall survival and recurrence‐free survival and report adherence to Enhanced Recovery After Surgery (ERAS) guidelines and length of stay and systematically record and adequately report adverse events, defining whether these events are 'related' or 'unrelated' to the intervention. More research is also required exclusively in those undergoing active cancer treatment for non‐advanced colorectal cancer. Indeed, RCTs undertaken with mixed cancer populations should report data separately for the specific cancers, when appropriate. More robust measures to reduce bias, especially in relation to allocation concealment and blinding of outcome assessors are required. In addition, future research should aim to recruit older participants to increase applicability of results to those with the highest incidence of colorectal cancer. Importantly, a better understanding of the optimal training duration, pattern, intensity, volume, setting and composition of such interventions will be needed to maximise efficacy. This requires a shift in how we record and report these components.
History
Protocol first published: Issue 11, 2017 Review first published: Issue 5, 2020
Acknowledgements
We would like to thank Dr Henning Keinke Andersen and Dr Kristoffer Andersen (Managing Editors), the Cochrane Colorectal Cancer Contact Editor, Dr Nicole Skoetz and Dr Daniel Steffens (peer reviewers) for their help and advice in the development of the Review protocol, Dr Aron Onerup, Dr Rasmus Dahlin Bojesen and Dr Newton Opiyo (peer reviewers) for providing feedback on the review. We would also like to thank Sys Johnsen (Information Specialist) for assisting in the development and adaptation of the search strategy and Dr Lisa A Loughney for her help and advice in the review process. We would like to thank Jill Pearson (consumer referee) and Evelyn (consumer referee) for their assistance and feedback.
MMG has been funded by the Public Health Agency, HSC R & D Division to undertake this review. MAT and MMC are part funded by the Centre of Excellence for Public Health (Northern Ireland), a UKCRC Public Health Research Centre of Excellence. The UKCRC Public Health Research Centres of Excellence are funded by the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, Research and Development Office for the Northern Ireland Health and Social Services and the Wellcome Trust, under the auspices of the UK Clinical Research Collaboration.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL): year, issue number in the Cochrane Library (searched day, month, year)
#1 MeSH descriptor: [Colorectal Neoplasms] explode all trees
#2 ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) near/5 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or malignan*)):ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Exercise] explode all trees
#5 MeSH descriptor: [Exercise Therapy] explode all trees
#6 MeSH descriptor: [Sports] explode all trees
#7 "physical fitness" (Word variations have been searched)
#8 (physical* near/5 (fit* or train* or activ* or endur* or exer*)):ti,ab,kw (Word variations have been searched)
#9 (exercis* near/5 (train* or physical* or activ*)):ti,ab,kw (Word variations have been searched)
#10 sport*:ti,ab,kw (Word variations have been searched)
#11 walk*:ti,ab,kw (Word variations have been searched)
#12 swim*:ti,ab,kw (Word variations have been searched)
#13 pilates*:ti,ab,kw (Word variations have been searched)
#14 tai ji or tai chi or tai‐ji or tai‐chi:ti,ab,kw (Word variations have been searched)
#15 resistance near/3 train*:ti,ab,kw (Word variations have been searched)
#16 #4 and #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 #3 and #16
Appendix 2. MEDLINE search strategy
|
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to present (day, month, year) 1. exp colorectal neoplasms/ |
| 2. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj5 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or malignan*)).mp. |
| 3. 1 or 2 |
| 4. exp exercise/ |
| 5. exp exercise therapy/ |
| 6. exp sports/ |
| 7. Physical Fitness/ |
| 8. (physical* adj5 (fit* or train* or activ* or endur* or exer*)).ti,ab. |
| 9. (exercis* adj5 (train* or physical* or activ*)).ti,ab. |
| 10. sport*.ti,ab. |
| 11. walk*.ti,ab. |
| 12. swim*.ti,ab. |
| 13. pilates.ti,ab. |
| 14. (tai ji or tai chi or tai‐ji or tai‐chi).ti,ab. |
| 15. (resistance adj3 train*).ti,ab. |
| 16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| 17. 3 and 16 |
| 18. randomized controlled trial.pt. |
| 19. controlled clinical trial.pt. |
| 20. randomized.ab. |
| 21. placebo.ab. |
| 22. clinical trials as topic.sh. |
| 23. randomly.ab. |
| 24. trial.ti. |
| 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| 26. exp animals/ not humans.sh. |
| 27. 25 not 26 |
| 28. 17 and 27 |
Appendix 3. Embase search strategy
|
Ovid Embase: 1974 to year week 1. exp large intestine tumor/ |
| 2. ((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel*) adj5 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or malignan*)).mp. |
| 3. 1 or 2 |
| 4. exp exercise/ |
| 5. exp sport/ |
| 6. physical fitness/ |
| 7. exercise therapy/ |
| 8. (physical* adj5 (fit* or train* or activ* or endur* or exer*)).ti,ab. |
| 9. (exercis* adj5 (train* or physical* or activ*)).ti,ab. |
| 10. sport*.ti,ab. |
| 11. walk*.ti,ab. |
| 12. swim*.ti,ab. |
| 13. pilates.ti,ab. |
| 14. (tai ji or tai chi or tai‐ji or tai‐chi).ti,ab. |
| 15. (resistance adj3 train*).ti,ab. |
| 16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| 17. 3 and 16 |
| 18. CROSSOVER PROCEDURE.sh. |
| 19. DOUBLE‐BLIND PROCEDURE.sh. |
| 20. SINGLE‐BLIND PROCEDURE.sh. |
| 21. (crossover* or cross over*).ti,ab. |
| 22. placebo*.ti,ab. |
| 23. (doubl* adj blind*).ti,ab. |
| 24. allocat*.ti,ab. |
| 25. trial.ti. |
| 26. RANDOMIZED CONTROLLED TRIAL.sh. |
| 27. random*.ti,ab. |
| 28. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 |
| 29. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.) |
| 30. 28 not 29 |
| 31. 17 and 30 |
Appendix 4. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘high risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
|
| Criteria for the judgement of ‘unclear risk’ of bias. | Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of ‘low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘high risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
Selective reporting Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘low risk’ of bias | Any of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. It is likely that the majority of studies will fall into this category. |
|
Other bias Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘high risk’ of bias | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgement of ‘unclear risk’ of bias | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Physical activity versus usual care for physical function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Objective measures more than 12 weeks to 6 months follow‐up (30‐Second Chair Stand Test) | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐1.84, 3.36] |
| 1.2 Subjective measures more than 12 weeks to 6 months follow‐up | 3 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.24, 0.42] |
Comparison 2. Physical activity versus usual care for disease‐related mental health.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Depression: more than 12 weeks to 6 months follow‐up | 4 | 198 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.50, 0.08] |
| 2.2 Depression: more than 6 months to 12 months follow‐up (HADS) | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.72, 0.31] |
| 2.3 Anxiety: more than 12 weeks to 6 months follow‐up | 4 | 198 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.40, 0.18] |
| 2.4 Anxiety: more than 6 months to 12 months follow‐up (HADS) | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 1.79 [‐0.37, 3.94] |
Comparison 3. Physical activity versus usual care for physical fitness (aerobic fitness).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Up to 12 weeks follow‐up | 7 | 295 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.34, 1.29] |
| 3.2 Change from baseline up to 12 weeks follow‐up | 3 | 81 | Std. Mean Difference (IV, Random, 95% CI) | 0.89 [0.43, 1.36] |
| 3.3 More than 12 weeks to 6 months follow‐up | 7 | 248 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.29, 0.82] |
| 3.4 Change from baseline more than 12 weeks to 6 months follow‐up | 2 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.05, 1.19] |
| 3.5 More than 6 months to 12 months follow‐up | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.04, 0.92] |
Comparison 4. Physical activity versus usual care for physical fitness (hand grip strength).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Up to 12 weeks follow‐up (hand dynamometry) | 2 | 147 | Mean Difference (IV, Fixed, 95% CI) | 1.92 [‐1.17, 5.00] |
| 4.2 More than 12 weeks to 6 months follow‐up (hand dynamometry) | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐5.98, 7.87] |
Comparison 5. Physical activity versus usual care for physical fitness (flexibility).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 More than 12 weeks to 6 months follow‐up | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.36, 0.39] |
Comparison 6. Physical activity versus usual care for cancer‐related fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Up to 12 weeks follow‐up (FACT‐F and FACIT‐F) | 6 | 230 | Mean Difference (IV, Random, 95% CI) | 2.16 [0.18, 4.15] |
| 6.2 Change from baseline up to 12 weeks follow‐up (FACT‐F and FACIT‐F) | 3 | 113 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐1.33, 2.14] |
| 6.3 More than 12 weeks to 6 months follow‐up | 7 | 277 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.08, 0.60] |
| 6.4 More than 6 months to 12 months follow‐up | 3 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.16, 0.67] |
Comparison 7. Physical activity versus usual care for anthropometric measure of weight (kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Up to 12 weeks follow‐up | 6 | 252 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐2.55, 3.14] |
| 7.2 Change from baseline up to 12 weeks follow‐up | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.90, ‐0.51] |
| 7.3 Change from baseline more than 12 weeks to 6 months follow‐up | 3 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐2.17, 0.72] |
| 7.4 More than 12 weeks to 6 months follow‐up | 3 | 74 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐6.87, 8.04] |
7.4. Analysis.

Comparison 7: Physical activity versus usual care for anthropometric measure of weight (kg), Outcome 4: More than 12 weeks to 6 months follow‐up
Comparison 8. Physical activity versus usual care for anthropometric measure of waist circumference.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Up to 12 weeks follow‐up | 3 | 183 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐2.88, 2.93] |
| 8.2 More than 12 weeks to 6 months follow‐up | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [‐5.58, 8.74] |
| 8.3 Change from baseline more than 12 weeks to 6 months follow‐up | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐5.21, ‐0.36] |
Comparison 9. Physical activity versus usual care anthropometric measure of waist to hip ratio.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Up to 12 weeks follow‐up | 3 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 9.2 Change from baseline up to 12 weeks follow‐up | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 9.3 More than 12 weeks to 6 months follow‐up | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.03, 0.14] |
Comparison 10. Physical activity versus usual care for anthropometric measure of body fat (%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Up to 12 weeks follow‐up | 4 | 214 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐4.04, 0.18] |
| 10.2 Change from baseline up to 12 weeks follow‐up | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐3.11, ‐0.04] |
| 10.3 More than 12 weeks to 6 months follow‐up | 3 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.42, 0.27] |
| 10.4 Change from baseline more than 12 weeks to 6 months follow‐up | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐3.11, 0.59] |
Comparison 11. Physical activity versus usual care for anthropometric measure of BMI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Up to 12 weeks follow‐up | 6 | 252 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.73, 1.02] |
| 11.2 Change from baseline up to 12 weeks follow‐up | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.81, 0.17] |
| 11.3 More than 12 weeks to 6 months follow‐up | 4 | 158 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.32, 0.33] |
| 11.4 Change from baseline more than 12 weeks to 6 months follow‐up | 3 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.17, 0.66] |
Comparison 12. Physical activity versus usual care for HRQoL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Up to 12 weeks follow‐up | 6 | 230 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.10, 0.62] |
| 12.2 Change from baseline up to 12 weeks follow‐up | 3 | 113 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.47, 0.28] |
| 12.3 More than 12 weeks to 6 months follow‐up | 7 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.03, 0.88] |
| 12.4 Change from baseline more than 12 weeks to 6 months follow‐up | 2 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.14, 1.26] |
| 12.5 More than 6 months to 12 months follow‐up | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.37, 0.47] |
Comparison 13. Physical activity versus usual care for levels of physical activity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bourke 2011.
| Study characteristics | ||
| Methods | Study design: RCT Study location: UK Inclusion criteria:
Exclusion criteria:
|
|
| Participants | Number randomised: 18; 9 control; 9 intervention Baseline imbalances: not reported Withdrawals and exclusions: 1 dropout in intervention group (stroke) Age, mean (SD) ‐ total: 69 years (range 52‐80 years); control: 70.3 (8.7) years; intervention: 67.9 (5.7) years Gender ‐ total; 12 male, 6 female; control: 7 male, 2 female; intervention; 5 male, 4 female Ethnicity: not reported Comorbidities: not reported SES: not reported Cancer type: colon Colorectal cancer stage: Dukes A‐C Type of treatment n: surgery: 8 control, 9 intervention; chemotherapy: 2 control, 4 intervention; palliative care: 1 control, 0 intervention Receiving or finished treatment: finished Time beyond treatment (months): 16.7 control; 16.4 intervention Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: 2 group‐based supervised sessions weekly and one home‐based Intensity: 55% to 85% of age predicted maximum heart rate or rate of perceived exertion between 11 and 15 Time: 30 minutes of aerobic exercise per session, 2 and 4 sets of 8 to 12 repetitions of resistance exercises Type: aerobic (treadmills, rowing ergometers, and cycling ergometers) and resistance Volume: not reported Progression: during the final 6 weeks of the intervention, participants attended the university facility once a week and were asked to perform two home‐based sessions a week Length of intervention: 12 weeks Control group: usual care Setting: exercise suite in university and home‐based Supervised or self‐directed: both Co‐interventions: dietary advice information pack, on a fortnightly basis throughout the intervention, engaged in healthy eating seminars in a group format, lasting approximately 15 to 30 minutes |
|
| Outcomes |
Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Adverse events: a stroke occurred in the intervention group |
|
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via code numbers using nQuery statistical software |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An exercise physiologist who was blinded to group allocation assessed outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention as high, attrition (5%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | There appears to be no selective reporting of outcomes. |
| Other bias | Unclear risk | Baseline characteristics are presented, baseline imbalances are not. |
Brown 2017.
| Study characteristics | ||
| Methods | Study design: RCT Study location: USA Inclusion criteria: 1. histologically confirmed stage I‐III colon cancer 2. completed adjuvant therapy 3. age ≥18 years 4. written physician approval 5. the ability to walk unaided for six minutes Exclusion criteria: 1. history of another primary cancer (other than non‐melanoma skin cancer) 2. evidence of metastatic colon cancer 3. planning to receive any additional adjuvant chemotherapy or surgery (i.e. ostomy reversal); pregnant or breast feeding 4. unable to provide baseline blood sample 5. cardiac conditions 6. any other condition which, may impede testing of the study hypothesis or make it unsafe to engage in the exercise programme |
|
| Participants | Number randomised: 39; 13 to usual care, 14 to low‐dose exercise (150 minutes), 12 to high‐dose exercise (300 minutes) Baseline imbalances: no significant differences at baseline Withdrawals and exclusions: 38/39 participants completed study (1 lost to follow‐up in usual care control group) Age, mean (SD): Total; 56.5 (10.0) years Usual care; 57.9 (9.7) Low dose; 58.2 (9.8) High dose; 53.1 (10.5) Gender, n (%): Total; Male and female (57%) Control; male 4(31%), female 9 (69%) Low dose; Male 7 (50%), female 7 (50%) High dose; Male (33%), female 8 (67%) Ethnicity: Majority white (80)% Comorbidities, n (%): hypertension 13 (33%), hyperlipidaemia 6 (15%), T2DM 5 (13%), CVD 4 (10%) SES: not reported Cancer type: colon Cancer stage, n (%): Stage I; 5 (13%) Stage II; 14 (36%) Stage III; 20 (51%) Type of treatment: surgical resection and adjuvant chemotherapy Receiving or finished treatment: finished Time beyond treatment, mean (SD) months: Total; 10.9 (6.1) months (less than or equal to 12 months 25 (64%) greater than 12 months 14 (36%)) Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: not reported Intensity: moderate‐intensity (70.7% of age predicted maximum heart rate) Time: the initial exercise dose prescribed in week one of the study is 60 min Type: home‐based treadmill walking Progression: low‐dose and high‐dose exercise groups increase their exercise to 150 min in week 1 and 300 min in week 1, respectively. Exercise increased by 30 min/week 1 as the participant successfully responds to the exercise dose prescribed in the prior week Length of intervention: 6 months Control group: maintained their pre‐ study levels of physical activity and/or followed the recommendations provided by their physician Setting: home‐based Supervised or self‐directed: self‐directed Co‐interventions: none |
|
| Outcomes | 1. Physical activity (accelerometry)
2. Anthropometric
3. Cardiopulmonary & Functional Status
4. Clinical outcomes
5. Quality of Life
6. Biomarkers including soluble intercellular adhesion molecule‐1 (sICAM‐1) and soluble vascular adhesion molecule‐1 (sVCAM‐1) prognostic biomarkers Time points measured: baseline, six months Time points reported: baseline, six months Adverse events: graded using the Common Terminology Criteria for Adverse Events version 4.0 |
|
| Notes | Funding source: National Cancer Institute and the National Center for Advancing Translational Science | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were obtained by assessors blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant did not provide end point data (97% follow‐up rate) (3% attrition) |
| Selective reporting (reporting bias) | High risk | Study protocol included outcomes (dietary intake, cardiopulmonary and QoL) data that are not presented in available reports |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Cantarero‐Villanueva 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Spain Inclusion criteria: 1. more than 18 years old 2. received curative treatment due to cancer (surgery, chemotherapy and/or radiotherapy) 3. diagnosed with grades I to III A of colorectal cancer 4. completed coadjuvant treatment Exclusion criteria: 1. presented cancer recurrence 2. underwent previous abdominal surgeries 3. were diagnosed with concomitant conditions, such as previous lower‐back pain or musculoskeletal conditions (e.g. osteoarthritis, fibromyalgia or chronic fatigue syndrome) |
|
| Participants | Number randomised:46; 23 to the intervention group and 23 patients to the usual care group Baseline imbalances: no significant differences at baseline Withdrawals and exclusions: six dropouts (2 in intervention group, 4 in usual care group) Age, mean (SD) years: 60 years (usual care: 62.3 (7.9) years, intervention: 57.5 (8.0) years) Gender, n (%): Total: 26 males, 14 females usual care: male 13 (68.4%), female 8 (31.6%) Intervention: male 13 (61.9%) female 6 (38.1%) Ethnicity: not reported Comorbidities: not reported SES: University degree, n (%) Usual care 2 (10.5%) Intervention 6 (28.6%) Cancer type: colon Cancer stage n (%): stage II 14; usual care 7 (36.8%), intervention = 7 (33.3%) stage IIIa 26; usual care 12 (63.2%), intervention 14 (66.7%) Type of treatment: surgery, other treatment not reported Receiving or completed treatment: finished Time beyond treatment, mean (SD): 13.3 months Usual care = 14.6 (10) Months Intervention = 12 (7.4) months Time since diagnosis: mean (SD): 15.4 months Usual care: 17.1 (11.3) months Intervention: 13.8 (7.8) months |
|
| Interventions |
Physical activity description Frequency: 3 times per week Intensity: not reported Time: 90 minutes Type: core stabilisation and stretching exercises Volume, progression =1 set of 8‐10 reps to 2 sets of 10 reps Length of intervention: 8 weeks Control group: usual care, including written general recommendations for a healthy lifestyle Setting: activity centre Supervised or self‐directed: supervised Co‐interventions: none |
|
| Outcomes | 1. Physical fitness/physical function: abdominal isometric endurance (trunk‐curl test), flexibility ("chair sit and reach test"), functional capacity (6‐min walk test with treadmill), static balance (Flamingo Test), fitness perceived (International Fitness Scale), lower back muscle strength (dynamometry)
2. Fatigue (Piper Fatigue Scale)
3. Body composition (bioelectrical impedance analysis)
4. Pain (Brief Pain Inventory)
5. Quality of life
6. Pressure Pain Thresholds
7. Muscle structure Time points measured: baseline, 8 weeks and 6‐month follow‐up Time points reported: baseline 8 weeks and 6‐month follow‐up Adverse events: reported |
|
| Notes | Funding source: Universidad de Granada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sequence created was introduced in numbered, opaque, sealed envelopes by an external researcher who did not participate in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A member of the research group who was blinded to the patient group assessed the variables |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data were described. 13% attrition |
| Selective reporting (reporting bias) | High risk | Study protocol included outcome data that are not presented in available reports |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Courneya 2003.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Canada Inclusion criteria: 1. surgery for colorectal cancer within the past 3 months 2. recovery from surgery as indicated by an attending physician 3. ability to understand and provide written informed consent in English 4. passed the revised Physical Activity Readiness Questionnaire (rPAR‐Q; Thomas et al. 1992) 5. no contraindications to exercise as determined by a submaximal cardiorespiratory fitness test Exclusion criteria: not reported |
|
| Participants | Number randomised: 102; 69 to the exercise group and 33 to a waiting list control group Baseline imbalances: no significant differences at baseline Withdrawals and exclusions: 9 lost to follow‐up in total (7 in intervention group 2 in control group) Age, mean (SD) years: Total; 60.3 years Control; 61.1 (9.93) years Intervention; 59.92 (10.73) years Gender: Control: 64.5% male Intervention: 54.8% male Ethnicity: not reported Comorbidities: not reported SES: Control group: 35% completed university; 53.6% with income > USD 40,000 Intervention group: 46.4% completed university; 65.5% with income > USD 40,000 Cancer type: colorectal cancer Cancer stage: Tumour stage III/IV control; 87.1 % intervention; 77.6 % Node stage 0 control; 61.3% intervention; 58.6% Metastatic control; 0 intervention; 6.9% (4 participants) Type of treatment: 100% had surgery Surgery alone: control 32.3% intervention 34.4% surgery plus RT control 0% intervention 1.6% Surgery plus CT control 51.6% intervention 32.6% surgery plus RT and CT control 16.1% intervention 21.3% Receiving or finished treatment: not reported (most likely a combination of both as surgery had taken place in passed 3 months) Time beyond treatment: surgery within last 3 months Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: 3 to 5 times per week Intensity: to 65% to 75% of heart rate maximum Time: 20 to 30 minutes Type: participants were allowed to choose the mode of exercise they preferred (e.g. swimming, cycling) but if they had no preference they were prescribed walking Length of intervention: 16 weeks Total number of exercise sessions: 48 to 80 Control group: were asked not to initiate any structured exercise over the course of the intervention (wait list) Setting: home based Supervised or self‐directed: self‐directed Co‐interventions: none |
|
| Outcomes | 1. Quality of life (FACT‐C scale, FACT‐G, TOI score‐ sum of physical and FWB subscale and colorectal subscale)
2. Satisfaction with life, measured (Satisfaction with Life scale)
3. Depression and anxiety, (CES‐D scale and STAI, respectively)
4. Cardiovascular fitness (Modified Balke Treadmill Test)
5. Body composition (Harpenden callipers)
6. Flexibility, (sit and reach test)
Time points measured: baseline and 16 weeks Time points reported: baseline and 16 weeks Adverse events: not reported |
|
| Notes | Funding source: National Cancer Institute of Canada, the Alberta Heritage Foundation for Medical Research and Sociobehavioral Cancer Research Network | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Fitness test conducted by person blinded to the experimental group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusions were reported, with reasons. Retention rate was 91.2%. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | There appears to be no selective reporting of outcomes |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Courneya 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Canada and Australia Inclusion criteria: 1. high risk stage II or stage III colon cancer 2. received adjuvant chemotherapy within the past 2–6 months 3. an Eastern Cooperative Oncology Group performance status of 0 or 1 4. are not currently meeting physical activity guidelines (i.e. the equivalent of 150 minutes/week of moderate‐intensity exercise) 5. able to complete at least two stages of a submaximal treadmill test Exclusion criteria: not reported |
|
| Participants | Number randomised: 273; 136 to a structured exercise programme (SEP) and 137 to health education materials (HEM) Baseline imbalances: no significant differences at baseline Withdrawals and exclusions: 62 excluded from primary analysis at year 1 (211 analysed; 105 in the control (HEM) group, 106 in the intervention group) Age, n (%) analysed group: less than 65: Control (HEM): 71 (68%) Intervention: 71 (67%) Gender, n (%) analysed group: Control (HEM) male; 46 (44%), female 59 (56%) Intervention; male 46 (43%), female 60 (57%) Ethnicity: not reported Comorbidities: not reported SES: not reported Cancer type: colon Cancer stage, n (%): High risk stage II: control (HEM): 12 (11%) Intervention: 14 (13%) Stage III: control (HEM): 93 (89%) intervention: 92 (87%) Type of treatment: 100% received chemotherapy Receiving or finished treatment: finished Time beyond treatment: 2‐6 months Time since diagnosis, median (range): Control; 12 (0‐16) months Intervention; 12 (8‐17) months |
|
| Interventions |
Physical activity description: Frequency: biweekly face‐to‐face counselling sessions combined with supervised exercise, with the option of an additional supervised exercise session during alternate weeks Intensity: individual choice Time: individual choice Type: individual choice Volume/progression: increase recreational aerobic physical activity by at least 10 MET‐hours/week from baseline in the first 6 months and sustain this change for 3 years Length of intervention: 3 years altogether (three phases‐ phase 1 adoption, 2 consolidation and 3 maintenance) Control group: health education material (HEM) intervention receive general health education materials promoting physical activity and healthy nutrition as well as standard surveillance follow‐up Setting: not reported Supervised or self‐directed: supervised or telephone support Co‐interventions: HEM |
|
| Outcomes | 1. Disease‐free survival
2. Overall survival
3. Patient reported outcomes (using SF‐36, FACIT‐F, PSQI, and HADS questionnaires)
4. Physical fitness (i.e. body mass index, hip and waist circumference, cardiovascular fitness, and physical function)
5. Physical activity levels (TPAQ)
6. Safety profile according to NCI CTCAE version 3.0
7. Correlative biological markers including biochemical and molecular markers associated with insulin‐related growth factor and cytokines associated with the mechanisms of fatigue
8. Economic evaluations including cost‐effective analysis and cost utility analysis
9. Predictors of physical activity adherence as assessed by Social Cognitive Determinants of Exercise questionnaire Time points measured: baseline and 1 year Time points reported: baseline and 1 year Adverse events: not reported |
|
| Notes | Funding sources: Canada Research Chairs Program Alberta Innovates‐Health Solutions, Alberta Cancer Foundation's Weekend to End Women's Cancers Breast Cancer Chair and Canadian Cancer Society Research Institute National Health and Medical Research Council (Australia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an open‐label study. However, it is not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across intervention groups with reasons. 22% attrition |
| Selective reporting (reporting bias) | Low risk | There appears to be no selective reporting of outcomes |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Cramer 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Germany Inclusion criteria: 1. Colorectal cancer 2. UICC stage I‐III 3. Between 2‐48 months postsurgery 4. aged at least 18 5. physical and cognitive ability to follow the yoga intervention Exclusion criteria: 1. UICC stage IV 2. further active oncological diseases 3. diagnosed and pharmacologically 4. treated psychiatric disorder except for cancer‐related depression or adjustment disorder pregnancy or breastfeeding |
|
| Participants | Number randomised: 54; 27 intervention, 27 usual care control Baseline imbalances: more patients in the yoga group treated with colostomy Withdrawals and exclusions: 6 in the intervention group and 5 from the control group dropped out Age (SD) (age range): Total 68.26 (9.69) years (40–87) control: 67.81 (10.37) years (40–84) intervention: 68.70 (9.13) years (49–87) Gender, n (%): Total; Male, 33 (61.1%) Female, 21 (38.9%) Control; Male, 16 (59.3%) Female, 11 (40.7%) Intervention; Male, 17 (63.0%) Female, 10 (37.0%) Ethnicity: not reported Comorbidities: not reported SES, n (%): university degree 16 (29.6%), employed full time 7 (13%), retired 35 (64.8%) Cancer type: colorectal; colon 24 (44.4%); rectum 29 (53.7%) Cancer stage: Stage I = 20 (37.0%) Stage II = 11 (20.4%) Stage III = 21 (38.9%) Type of treatment, mean (SD): Time since surgery 22.76 (13.09) months (range 3–46 months) current chemotherapy n (%) yes 2 (3.7%) No 52 (96.3%) Receiving or finished treatment: prior chemotherapy n (%) yes 25 (46.3%) No 29 (53.7%) prior radiotherapy yes 12 (22.2%) no 42 (77.8%) Time beyond treatment: 3‐ 46 months postsurgery 3.7% still receiving chemotherapy Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: 1 weekly class Intensity: not reported Time: 90 minutes Type: Hatha yoga Progression: postures in each class built up on the previous ones, and difficulty and intensity levels increased during the course of the programme patients were encouraged to practice at home although no minimum practice time was required Length of intervention: 10 weeks of Hatha yoga Control group: Waiting list control (usual care) Setting: on‐site Supervised or self‐directed: supervised Co‐interventions: none |
|
| Outcomes | 1. Health‐related quality of life (FACT‐C)
2. Fatigue (FACIT‐F)
3. Anxiety, depression (HADS)
4. Sleep quality (PSQI)
5. Safety (number and severity of adverse events)
6. Spiritual well‐being (Functional Assessment of Chronic Illness Therapy ‐ Spirituality)
7. Body awareness/bodily dissociation (Scale of Body Connection (SBC))
8. Body efficacy expectation (Body Efficacy‐Expectation Scale)
9. Qualitative Interviews Time points measured: baseline, week 10, week 22 Time points reported: baseline week 10, week 22 Adverse events: all adverse events occurring during the study period were recorded |
|
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using the random allocation software was used |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label RCT. It is not possible however to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (20%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Clinical trial registry outcomes are consistent with those reported in publication |
| Other bias | Low risk | More patients in yoga group treated with colostomy, however described appropriate allocation concealment |
Hubbard 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: UK Inclusion criteria: 1. ≥18 years 2. diagnosed with primary CRC 3. in recovery period following CRC surgery Exclusion criteria: 1. advanced disease 2. failure of clinical/risk assessment for cardiac rehabilitation 3. deemed unsafe to participate in exercise by cancer nurses during screening 4. severe cognitive impairment 5. unable to communicate in English (no funds available in study for translation services) |
|
| Participants | Number randomised: 41; 21 intervention, 20 usual care Baseline imbalances: Seven (33.3 %) participants allocated to intervention group and four (20%) allocated to the usual care group were classified as T3. More participants allocated to the intervention group had laparoscopic surgery (n = 6) (28.8%) versus n = 3 (15%)). Four (19%) participants allocated to the intervention group had a temporary stoma or permanent stoma, versus nine (45%) in usual care (no rehabilitation) Withdrawals and exclusions: Follow‐up 1; missing 10 participants questionnaire data, and 18 participants accelerometer data Follow‐up 2; missing 16 participants questionnaire data, and 27 participants accelerometer data Age, mean (SD): 66 (11.31) years Control, 64.2 (11.10) years Intervention, 67.9 (11.49) years Gender, n (%): Male, 27 (65.9%) Female, 14 (34.1%) Ethnicity: not reported SES: not reported Comorbidities: not reported Cancer type: colorectal Colorectal cancer stage: T 0‐4, N 0‐1, M0 Type of treatment, n, (%): Colon surgery, yes 25 (61%), no 16 (39%) Rectal surgery, yes 16 (39%), no 25 (61%) Laparoscopic surgery, yes 9 (22%), no 18 (43.9%) Chemotherapy, missing 7 (17.1%), no 27 (65.9%), yes 7 (17.1%) Radiotherapy, missing 7 (17.1%), no 29 (70.7%), yes 5 (12.2%) Other treatment, missing 3 (7.3%), no 35 (85.4%), yes 3 (7.3%) Receiving or finished treatment: both Time beyond treatment: postsurgery (in recovery period), exact time not reported Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: site 1 & 2 once weekly, site 3 twice weekly exercise Intensity: not reported Time: 60–90 min educational sessions Type: cardiac rehabilitation exercise classes of aerobic and strength training Volume: not reported Progression: not reported Length of programme: varied depending on site (site one: 1 hour class over 10 weeks, site two 12 weeks, site 3 twice weekly over 6 weeks) Control group: usual care patients were given a booklet by Bowel Cancer UK (a cancer charity)—Staying healthy after bowel cancer Setting: not reported Supervised or self‐directed: supervised Co‐interventions: weekly education sessions (e.g. diet, physical activity, relaxation/ stress management and cardiac‐specific sessions) |
|
| Outcomes | 1. Feasibility and acceptability 2. Levels of physical activity (accelerometry, Scottish Physical Activity Questionnaire) 3. Quality of life (EQ5D, FACT‐C) 4. Anxiety and depression (HADS) 5. Fatigue (FACIT‐Fatigue) 6. Healthcare resource (seven‐item self‐report questionnaire developed by the research team for the purposes for the study) 7. Physical activity self‐efficacy (12‐ item questionnaire developed by investigators of the Act Well trial 8. Risk perception (operationalisation of the concept in the context of behaviour change research) Time points measured: baseline, weeks 6, 10, 12 (depending on site), second follow‐up assessment was approximately 3 months after the participant had finished cardiac rehabilitation Time points reported: baseline, up to 12 weeks, 3 months after completion of Cardiac rehabilitation Adverse events: no adverse events occurred |
|
| Notes | Funding source: National Institute for Health Research—Health Service and Development Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An automated online randomisation system was used |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to missing data at follow‐up time points. Time point 1: attrition 24% and 43% for subjective and objective measures respectively. Time point 2: attrition 39% and 65% for subjective and objective measures, respectively. |
| Selective reporting (reporting bias) | High risk | Some outcomes described in methods section have not been reported |
| Other bias | High risk | Due to baseline imbalances alongside unclear allocation concealment |
Kim 2018.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Korea Inclusion criteria: 1. between 18 and 75 years of age 2. confirmed stage II to III CRC 3. completed surgery, radiotherapy, and/or chemotherapy within four weeks to two years prior to study enrolment 4. ECOG performance status of 0 or 1 5. ability to understand and provide written informed consent Exclusion criteria: 1. metastatic disease 2. cardiac illness 3. any condition that would make them unsuitable for the study |
|
| Participants | Number randomised: 71 (37 intervention, 34 usual care) Baseline imbalances: no significant difference at baseline Withdrawals and exclusions: 30 and 28 participants completed study in the intervention and control group, respectively Age, mean (SD): control 56.8 (10.2) intervention 55.7 (8.7) Gender n (%): male control 17 (50%) male intervention 18 (48.6%) Ethnicity: not reported Comorbidities: not reported SES (%): Income > USD 3000/month control (38.2%) intervention (54.1%) Married control (79.5%) intervention (75.7%) Completed university control (32.4%) intervention (51.4%) Cancer type, n (%): colon control (55.9%) intervention (73%) rectal control (44.1%) intervention (27%) Colorectal cancer stage: stage II‐III; stage II control (35.3%) intervention (56.8%) stage III control (64.7%) intervention (43.2%) Type of treatment, n (%): chemotherapy 98 (80.3%) chemo+radiotherapy 13 (10.7%) Receiving or finished treatment: finished Time beyond treatment, mean (SD): control 10.6 months (8.4) intervention 10.8 months (5.8) Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Frequency: not reported Intensity: moderate and vigorous intensity Time: not reported Type: aerobic and resistance home‐based exercise e.g. brisk walking, hiking and stationary bike riding were recommended for aerobic exercise 30 min resistance exercise (12‐15 reps) DVD provided Volume: weeks 1‐6 18 MET/hours per week progression: after week 6 increase to 27 MET hours per week Length of programme: 12 weeks Control group: usual care Setting: home‐based with four meetings with exercise trainer, telephone calls and small group sessions Supervised or self‐directed: self‐directed, physical activity logs checked by exercise specialists Co‐interventions: none |
|
| Outcomes | 1. Quality of life
Secondary outcomes:
2. Fatigue
3. Levels of physical activity
4. Anthropometric measurements
5. Levels of physical activity Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Adverse events: not reported |
|
| Notes | Funding source: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number sequence was used |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 18.9% in intervention and 17.6% in control group. Exclusions reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | There appears to be no selective reporting of outcomes |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Lee 2017.
| Study characteristics | ||
| Methods | Study design: RCT Study location: South Korea Inclusion criteria: 1. resident of Seoul or Gyeonggi‐do 2. between 18 and 75 years of age 3. confirmed stage II to III CRC 4. completed surgery, radiotherapy, and/or chemotherapy within four weeks to two years prior to study enrolment 5. ECOG performance status of 0 or 1 6. ability to understand and provide written informed consent 7. not planning extended absences in the three months subsequent to enrolment Exclusion criteria: 1. evidence of recurrent or metastatic disease 2. participation in regular structured physical activity at moderate‐intensity exceeding 200 min/week 3. pregnant or planning to be pregnant within six months 4. we additionally excluded ostomy patients due to lack of evidence in safety of strength training |
|
| Participants | Number randomised: 123; (62 intervention; 61 usual care) Baseline imbalances: no significant difference at baseline Withdrawals and exclusions: 13 lost to follow‐up in control group; 11 lost to follow‐up in intervention group Age, mean (SD): Total 56.3 (9.7) control 56.3 (9.7) intervention 56.3 (9.9) Gender n (%): male 59 (48%) male control 28 (45.9%) male intervention 31 (50%) Ethnicity: not reported Comorbidities: not reported SES (%): Income > USD 3000/month (44.7%) Married (81%) Completed university (48.8%) Cancer type, n (%): colon 84 (68.9%) rectal 38 (31.1%) Colorectal cancer stage: stage II‐III; stage II (46.7%) stage III (53.3%) Type of treatment, n (%): chemotherapy 98 (80.3%) chemo+radiotherapy 13 (10.7%) Receiving or finished treatment: finished Time beyond treatment, mean (SD): 9.8 (6.3) months Time since diagnosis: not reported |
|
| Interventions |
Physical activity description: Frequency: encouraged to do 10,000 steps daily and daily resistance exercise programme Intensity: of these 10,000 steps, participants were asked to complete 3000 steps as exercise that increased their heart rate up to 65% of their age‐predicted maximum heart rate Time: not reported Type: aerobic and resistance home‐based exercise e.g. brisk walking, hiking and stationary bike riding were recommended for aerobic exercise 30 min resistance exercise using their own body weight that could be performed daily at home Volume: weeks 1‐6 18 MET/hours per week progression: after week 6 increase to 27 MET hours per week Length of programme: 12 weeks Control group: usual care Setting: home based with three sessions in hospital based clinic Supervised or self‐directed: self‐directed, physical activity logs checked by exercise specialists Co‐interventions: none |
|
| Outcomes | 1. Fasting insulin
Secondary outcomes:
2. Biomarkers
3. Physical fitness
4. Body composition
5. Levels of physical activity Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Adverse events: not reported |
|
| Notes | Funding source: National R&D programme for cancer control and the national research foundation of Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence was used |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (19%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | There appears to be no selective reporting of outcomes |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Lewis 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: UK Inclusion criteria: 1. a histologically confirmed diagnosis of colorectal cancer with Dukes stages A‐C 2. completed cancer treatment within the last 24 months (later increased to 3 years) 3. be able to understand spoken and written English score of 80 or more on the Karnofsky Performance Status Scale Exclusion criteria: 1. already meeting general physical activity guidelines of 150 min of moderate physical activity or 75 min of vigorous intensity physical activity per week 2. recent myocardial infarction 3. uncontrolled hypertension 4. pacemaker 5. unstable angina |
|
| Participants | Number randomised: 28; 14 control; 14 intervention Baseline imbalances: no significant difference between groups at baseline Withdrawals and exclusions: Control; 3 dropouts (two at three months, one at six months) Intervention: 5 dropouts (two at three months, three at six months) Age, mean (SD): Total mean age; 64.75 Control 65.5 (9.2) Intervention 64.0 (14.8) Gender: Control: 7 male, 7 female Intervention: 8 male, 6 female Ethnicity: not reported Comorbidities: not reported SES: not reported Cancer type: colorectal Colorectal cancer stage: Dukes A‐C Type of treatment, n (%): Surgery; 28 (100%) Chemotherapy; 10 control (71%), 2 intervention (14%) Receiving or finished treatment: finished treatment Time beyond treatment: within three years of completion of treatment Time since diagnosis, months: control 13, intervention 12 months |
|
| Interventions |
Physical activity description Frequency: supervised exercise sessions took place twice per week for the first four weeks. This was tapered off to once per week for the second four weeks. During the last month of the intervention participants continued with the exercise at home Intensity: moderate and vigorous Time: not reported Type: not reported Volume: encouraged to achieve 150 min of moderate to vigorous physical activity per week Progression: not reported Length of programme: supervised exercises for three months Control group: encouraged to continue with their usual lifestyle habits. Supervised exercise sessions were offered after their last appointment Setting: home and supervised Supervised or self‐directed: both Co‐interventions: none |
|
| Outcomes | 1. Moderate and vigorous intensity physical activity (International Physical Activity Questionnaire long version and accelerometry)
2. Behavioural regulation (Behavioural Regulation in Exercise Questionnaire version 2)
3. Psychological needs satisfaction (psychological needs satisfaction in exercise scale)
4. QoL (FACT‐G, FACT‐C)
5. Fatigue (FACT‐F)
6. Intention to exercise (Intention to exercise scale)
7. Barriers to exercise (Barriers to Exercise scale)
8. Physical fitness (treadmill test)
9. Upper body strength (grip strength, arm‐curl test, 30‐Second Chair Stand Test) 10. Body composition (Bioelectrical impedence, waist and waist to hip ratio, height weight, BMI) Time points measured: baseline, 3 and 6 months Time points reported: baseline, 3 and 6 months Adverse events: exercise was deemed safe; there were no adverse events throughout the intervention |
|
| Notes | Funding source: University of East Anglia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised using computer generated list (nQuery software) |
| Allocation concealment (selection bias) | Low risk | A person independent of the research team kept the randomisation sequence. After the completion of the baseline assessments, the research team phoned the independent person to obtain group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collection and analyses were carried out by a researcher not blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (28%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Clinical trial registry outcomes are consistent with those reported in publication |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias. No significant difference between groups at baseline. |
McDermott 2017.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Northern Ireland Inclusion criteria: 1. Dukes A‐C colorectal cancer patients at least 6 weeks post any type anticancer treatment, as identified by the colorectal oncologic and surgical teams. 2. males and females over 18 years of age 3. physically able to undertake the intervention 4. ambulatory and without use of a walking aid Exclusion criteria: 1. still undergoing and/or scheduled for further anti‐cancer treatment 2. any presence of cognitive impairment 3. pregnant or possibility of being pregnant 4. known comorbidities which would severely impact upon physical functioning or nutritional status 5. already meeting the current recommended physical activity guidelines 6. unable to understand and communicate in written and oral English |
|
| Participants | Number randomised: 23; 11 control; 12 intervention Baseline imbalances: mean weight and hip circumference, FACT‐C and FACIT‐F results were significantly higher in the intervention group. Fatigue scores were significantly lower in the intervention group Withdrawals and exclusions: 3 dropouts before week 12 (2 control; 1 intervention), 1 further dropout in intervention group at week 24 Age, mean (SD): total 63 (9) years; control 62.6 (9.1) intervention 63.6 (9.5) Gender n (%): Male; 6 (54.5%) control; 10 (83.3%) intervention Female; 5 (45.5%) control; 2 (16.7%) intervention Ethnicity: not reported Comorbidities: not reported SES (employment): Professional: 5 (45.5%) control; 5 (41.6%) intervention Managerial: 0 control; 3 (25%) intervention Clerical; 1 (9%) control; 2 (16.7%) intervention Manual; 5 (45.5%) control; 2 (16.7%) intervention Cancer type: colorectal Colon; 9 (81.8%) control; 8 (66.7%) intervention Rectal; 2 (18.2%) control; 4 (33.3%) intervention Colorectal cancer stage: 1a: 0 control; 1 (8.3%) intervention 2a/2b: 6 (54.5%) control; 7 (58.3%) intervention 3a/3b/3c: 5 (45.5%) control; 4 (33.3%) intervention Type of treatment: Surgery only: 1 (18.2%) control; 4 (33.3%) intervention Surgery and chemotherapy; 8 (72.7%) control; 6 (50%) intervention Radio/chemo and surgery: 1 (9.1%) control; 2 (16.7%) intervention Receiving or finished treatment: finished (at least 6 weeks post‐treatment) Time beyond treatment: an average of 24 months post‐treatment (25 (17) months in control group; 23 (19) months in the intervention group) Time since diagnosis: not reported |
|
| Interventions | Physical activity description: Frequency: not reported Intensity: moderate‐intensity Time: not reported Type: walking and strengthening intervention Volume: not reported Progression: aim to reach at least 150 minutes a week of moderate‐intensity aerobic activity and strengthening goal of 3 sets of 8‐15 repetitions, 2‐3 days a week Length of programme: 12 weeks Control group: usual care; participants will receive the intervention information on their last visit Setting: home based Supervised or self‐directed: self‐directed with weekly telephone calls Co‐interventions: none |
|
| Outcomes | 1. Feasibility
2. Levels of physical activity (accelerometer)
3. Cardiovascular endurance (6‐minute walk test)
4. Lower limb strength (timed sit to stand)
5. Quality of life (FACT‐C, EQ5D‐3L, PANAS)
6. Demographics
7. Anthropometric tests (BMI, height, weight, hip and waist measurements)
8. Biological markers (venous and capillary blood samples)
9. Fatigue (MFSI‐SF) Time points measured: baseline 12, 24 weeks Time points reported: baseline 12, 24 weeks Adverse events: not reported |
|
| Notes | Funding source: University of Ulster | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used |
| Allocation concealment (selection bias) | Low risk | Numbered concealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researcher carrying out the outcome measures was aware of the participants group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (17%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are consistent with outcomes to be reported in clinical trial register |
| Other bias | Low risk | Baseline imbalances present, however described appropriate allocation concealment |
Nuri 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: Iran Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Participants | Number randomised: 30; 15 control, 15 intervention Baseline imbalances: no significant differences at baseline Withdrawals and exclusions: three subjects in control group who did not participate in the final examinations were excluded Age, mean (SD): 51.56 (11.28) years Gender: 30 (100%) males Ethnicity: not reported Comorbidities: not reported SES: not reported Cancer type: colorectal Colorectal cancer stage: stage I‐III, confirmed via email from author Type of treatment: surgery (no other treatment reported) Receiving or finished treatment: not reported Time beyond treatment: between 2 and 3 months postsurgery Time since diagnosis: not reported |
|
| Interventions | Physical activity description: Frequency: 3 times per week Intensity: 50% to 60% of target heart rate Time: 45 minute (including 5 min warm up cool down and 5 minute rest) Type: aerobic/walking Volume: not reported Progression: Length of programme: 8 weeks Control group: usual care Setting: not reported Supervised or self‐directed: not reported Co‐interventions: participants completed the 3‑day diet recall forms and were instructed to maintain normal dietary habits throughout the study |
|
| Outcomes | 1. Leptin and ghrelin
2. Anthropometric and body composition measures (BMI, waist circumference, waist, hip ratio, fat mass, lean mass)
3. Metabolic characteristics (glucose, insulin, HOMA‐IR)
4. Composition of subjects' diets
5. Physical fitness: Rockport one mile fitness walking Time points measured: baseline, 8 weeks and 9 weeks (i.e. after 1 week of detraining) Time points reported: baseline, 8 weeks and 9 weeks (i.e. after 1 week of detraining) Adverse events: not reported |
|
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information available to permit a judgement but probably 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% attrition |
| Selective reporting (reporting bias) | High risk | Some outcomes described in methods section have not been reported |
| Other bias | Low risk | The study appears to be free of other problems that could put it at a high risk of bias |
Pinto 2013.
| Study characteristics | ||
| Methods | Study design: RCT Study location: USA Inclusion criteria: 1. sedentary adults 2. stage 1‐3 colorectal cancer 3. finished treatment surgery and/or adjuvant therapy 4. less than 5 years postdiagnosis 5. English speaking 6. access to a telephone 7. CVD or diabetes with consent Exclusion criteria: 1. prior history of cancer 2. medical or current psychiatric illness (e.g. orthopaedic problems) that could make compliance with the study protocol difficult or unsafe |
|
| Participants | Number randomised: 46; 20 to physical activity group, 26 to control group Baseline imbalances: more people in employment in the control group Withdrawals and exclusions: 4 withdrew; 1 in physical activity group, 3 in control group Age: 57.3 years (SD 9.7); intervention 55.6 (8.24) control 59.5 (11.2) Gender: male and female (57%) Ethnicity: majority white Comorbidities: not reported SES: majority attended college and had a median household income of USD 60,000 Cancer type: colorectal cancer; 57% colon cancer, 43% rectal cancer Cancer stage: stage I‐III Type of treatment: 100% surgery or surgery with adjuvant therapy 20/46 radiotherapy; 38/46 chemotherapy Receiving or finished treatment: finished Time beyond treatment: < 5 years since completion of treatment Time since diagnosis: mean 2.99 years since diagnosis |
|
| Interventions |
Physical activity description: Frequency: encouraged 10 minutes at least 2 days per week for three weeks, goals were increased over the 12 weeks to 30 minutes a day, 5 days per week Intensity: moderate 64% to 76% estimated heart rate max Type: e.g. brisk walking, biking, home‐based exercise equipment Length of intervention: 12 weeks Control group: contact control group (received weekly calls to match frequency of contact with physical activity group and survivorship tip sheets) Setting: home based Supervised or self‐directed: self‐directed Co‐interventions: none |
|
| Outcomes | 1. Levels of physical activity (7‐day recall, Community Healthy Activities Model Program for Seniors, accelerometry),
2. Movement to action/maintenance stage of motivational
readiness,
3. Submaximal aerobic fitness (Treadwalk test)
4. Psychological outcomes (FACT‐C)
5. Fatigue (FACT‐F)
6. Anthropometric measurements (height, weight, BMI) Time points measured: baseline, 3, 6 and 12 months Time points reported: Adverse events: not reported |
|
| Notes | Funding source: National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments conducted by a staff member who was blinded to the participants' group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data were described (9% attrition) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are consistent with study protocol |
| Other bias | High risk | Due to baseline imbalances alongside unclear allocation concealment |
Van Blarigan 2019.
| Study characteristics | ||
| Methods | Study design: RCT Study location: USA Inclusion criteria: 1. stage II‐III colon or rectal cancer then expanded to include stage I and stage IV 2. previous completion of cancer‐directed therapy ≥ 3 months and < 2years prior to enrolment (time after completion of treatment was dropped) 3. disease free status at enrolment 4. ability to reliably access the Internet and a mobile phone and navigate websites 5. ability to speak and read English Exclusion criteria: 1. any of 19 specific contraindications to moderate‐to‐vigorous physical activity 2. very active at baseline (defined as engaging in exercise for ≥ 30min for ≥ 5days a week) |
|
| Participants | Number randomised: 42; 21 to intervention group, 21 to control group Baseline imbalances: control group more active than intervention group Withdrawals and exclusions: 0 lost to follow‐up in intervention group, 2 in control group Age: 54 years (SD 11); intervention 56 (12); control 54 (11) Gender: male (41%) and female (59%) Ethnicity: white (73%), African American/black (2%), Asian (12%), other (12%) Comorbidities: not reported SES: college degree (93%), employed (63%) Cancer type: colon (56%), rectal (44%) Cancer stage: stage I (20%), II (20%), III (59%), IV (2%) Type of treatment: 100% surgery or surgery with adjuvant therapy 20/46 radiotherapy; 38/46 chemotherapy Receiving or finished treatment: finished Time beyond treatment: not reported Time since diagnosis: mean 1.5 years (SD 1.5) |
|
| Interventions |
Physical activity description Frequency: encouraged to build up to 150 mins per week of moderate activity or 75 min per week of vigorous activity Intensity: moderate or vigorous Type: e.g. brisk walking, biking, jogging, resistance exercise Length of intervention: 12 weeks Control group: received printed educational material about exercise after cancer Setting: home based Supervised or self‐directed: fit bit flex, daily text messages and counselled through print materials Co‐interventions: none |
|
| Outcomes | 1. Levels of physical activity (Actigraph), 2. Fitbit wear time (# days with data / # days of observation), 3. Response to text messages (# of messages responded to / # of messages that asked for a response), Time points measured: baseline and 12 weeks (adverse events measured at baseline 4, 8 and 12 weeks) Time points reported: baseline, 12 weeks Adverse events: reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme was used |
| Allocation concealment (selection bias) | Unclear risk | Whether the treatment assignment was concealed from study personnel and participants was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors state "non‐blinded" trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (5% in intervention arm and 10% in control arm) with reasons reported |
| Selective reporting (reporting bias) | High risk | Some outcomes described in methods section have not been reported |
| Other bias | High risk | Control group more active at baseline |
Van Vulpen 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study location: The Netherlands Inclusion criteria: 1. a histological diagnosis of colon cancer less than 10 wk before study recruitment 2. stage M0 3. scheduled for chemotherapy 4. age 25 to 75 yr 5. able to read and understand the Dutch language 6. Karnofsky Performance Status of 60 or higher 7.able to walk 100m or more Exclusion criteria: 1. the presence of contraindications for physical activity (as assessed through the Revised Physical Activity Readiness Questionnaire) 2. treatment for cancer in the 5 years preceding recruitment (except for basal skin cancer) |
|
| Participants | Number randomised: 33; 17 intervention, 16 control Baseline imbalances: participants in the intervention group were, on average, shorter and heavier Withdrawals and exclusions: 5 lost to follow‐up; 3 in control group, 2 in intervention group Age, mean (SD): Total mean age 58.1; intervention 58.1 (10.3); control 58.1 (9.6) Gender, n (%): Male; 11 (68.8%) control; 10 (58.8%) intervention Female; 5 (31.3%) control; 7 (41.2%) intervention Ethnicity: not reported Comorbidities: SES: High‐income; 9 (56.2%) control; 8 (47.1%) intervention Married; 11 (68.8%) control; 12 (70.6%) intervention Education; low; 3 (18.8%) control; 1 (5.9%) intervention; medium; 4 (25.0%) control; 7 (41.2%) intervention; high; 9 (56.2%) control; 8 (47.1%) intervention; unknown 1 (5.9%) intervention Cancer type: colon Colorectal cancer stage: stage M0 Type of treatment: 100% received surgery, 100% received chemotherapy, 18.4% received radiotherapy Receiving or finished treatment: 81.8% undergoing chemotherapy Time beyond treatment: majority receiving treatment Time since diagnosis: diagnosis of colon cancer less than 10 weeks before study recruitment |
|
| Interventions |
Physical activity description: Frequency: supervised (2 times per week), unsupervised (3 times per week) Intensity: based on heart rate at ventilatory threshold and Borg Time: supervised (1‐hour session) unsupervised (30 mins) Type: supervised ‐ aerobic and muscle strengthening exercise (all large muscle groups) Volume: not reported Progression: the muscle strengthening training started with 2 x 10 repetitions (65% 1‐repetition maximum (RM) and gradually increased to reach 1 x 10 repetitions (75% 1‐RM) and 1 x 20 repetitions (45% 1‐RM)) by the end of the programme Length of programme: 18 weeks Control group: were instructed to maintain their usual physical activity pattern. Setting: not reported Supervised or self‐directed: both Co‐interventions: none |
|
| Outcomes | 1. Fatigue (Multidimensional Fatigue Inventory (MFI) and the Fatigue Quality List)
2. QoL, anxiety, and depression: (EORTC QLQ‐C30), and a general measure of QoL, SF‐36 and (HADS)
3. Physical fitness (peak oxygen uptake (VO2peak)‐Cardiopulmonary exercise test, anthropometry (bodyweight and height)
4. Chemotherapy completion rate: weeks
5. Adherence (attendance percentage of the total offered class)
6. Physical activity level (short questionnaire to assess health‐enhancing physical activity) Time points measured: baseline, 18 and 36 weeks Time points reported: baseline, 18 and 36 weeks Adverse events: no serious adverse events related to exercise were observed during the study period |
|
| Notes | Funding source: The Netherlands Organisation for Health Research and Development, the Dutch Cancer Society and the Dutch Pink Ribbon Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation was used |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by a concealed computer generated randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were assessed by researchers not involved with the exercise interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups, attrition (16%) and exclusions were reported with reasons. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are consistent with those in study protocol |
| Other bias | Low risk | Baseline imbalances present, however described appropriate allocation concealment |
Waart 2017.
| Study characteristics | ||
| Methods | Study design: RCT Study location: The Netherlands Inclusion criteria: 1. there was no upper age limit Exclusion criteria: 1.serious orthopedic, cardiovascular, or cardiopulmonary conditions 2. were suffering from malnutrition 3. had serious psychiatric or cognitive problems 4. did not have basic fluency in Dutch |
|
| Participants | Number randomised: 23; 7 on‐track, 8 onco‐move, 8 usual care Baseline imbalances: participants in onco‐move were more frequently male, had lower education and more often received laparoscopic surgery while participants in on‐track had less comorbidity. Participants in onco‐move tended to have higher physical fitness levels than those in on‐track or usual care Withdrawals and exclusions: on‐track (n = 7) T2: 1 missing onco‐move (n = 8) T1 (1 missing for questionnaires, 2 missing physical performance tests) same at T2 UCC (n = 8) T1 (1 missing for questionnaires, 2 missing physical performance tests) T2 (none missing for questionnaires, 2 missing for physical performance tests) Age, mean (SD): Total 58.2 (10.1) years On‐track; 57.7 (13.2) years Onco‐move; 60.1 (7.3) years usual care; 56.7 (10.6) Gender, n (%): Total 14 (61%) female On‐track; 5 (71%) female Onco‐move; 3 (38%) female Usual care; 6 (75%) female Ethnicity: not reported Comorbidities, n (%): 15 (65%) of total participants SES, n (%): Married 16 (70%) of total College/university education 11 (48%) of total Full or part‐time work 8 (35%) of total Cancer type: colon Colorectal cancer stage: stage II 3 (13%); stage III 18 (78%); stage IV 2 (9%) ‐ author contacted correspondence received confirmed no stage IV patients were included Type of treatment: Larascopic surgery 12 (52%); control 2 (25%); on‐track 4 (57%); onco‐move 6 (75%) Radiotherapy 1 (4%) control Chemotherapy 23 (100%) Receiving or finished treatment: receiving both interventions started with the first cycle of chemotherapy and continued until 3 weeks after the last cycle Time beyond treatment: N/A Time since diagnosis: not reported |
|
| Interventions |
Physical activity description Onco‐move: Frequency: 5 days a week Intensity: low‐intensity 12–14 on the Borg Scale of Perceived Exertion participants Time: at least 30 min Type: not reported On‐track: Frequency: twice weekly Intensity: moderate‐ to high‐intensity Time: six large muscle groups were trained for 20 min per session in two series of eight repetitions at 80% of one‐repetition maximum, 30 mins of aerobic exercises at an intensity of 50% to 80% of the predicted maximal workload of the steep ramp test. Were also encouraged to be physically active 5 days a week for 30 min and to keep an activity diary Type: resistance and aerobic exercise Progression: the intensity was further adjusted using the Borg Length of programme: started with the first cycle of chemotherapy and continued until 3 weeks after the last cycle Control group: usual care Setting: onco‐move was home based; on‐track not specified Supervised or self‐directed: onco‐move was unsupervised; on‐track was partially supervised Co‐interventions: none |
|
| Outcomes | 1. Cardiorespiratory fitness (steep ramp test)
2. Muscle strength (hand‐held dynamometer for elbow flexion and knee extension and the Jamar grip strength dynamometer, and lower‐limb muscle endurance with the 30‐s chair stand test)
3. Fatigue (MFI)
4. HRQoL (EORTC QLQ‐C30)
5. Psychological distress (HADS)
6. Self‐reported physical activity (Physical Activity Scale for the Elderly (PASE))
7. Exercise behaviour and attitudes (questions about exercise stage self‐efficacy, social support benefits barriers, and attitudes toward exercise during chemotherapy) Time points measured: T0 baseline prior to chemotherapy,T1 at completion of chemotherapy, T2 6 months after completion of chemotherapy Time points reported: as above Adverse events: no adverse events occurred |
|
| Notes | Funding source: funding PACES was supported by the Alpe d’HuZes/KWF Fund. The research grant was provided by the Dutch Cancer Society and CZ Fund. The interventions were funded by Zilveren Kruis Achmea and The Comprehensive Cancer Centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to onco‐move, on‐track, or usual care using the minimisation method |
| Allocation concealment (selection bias) | Unclear risk | Whether the treatment assignment was concealed from study personnel and participants was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (low) reported without reasons, however it is balanced across groups |
| Selective reporting (reporting bias) | High risk | Study protocol included outcomes (anthropometric measurements, return to work) data that are not presented in available reports |
| Other bias | High risk | Due to baseline imbalances alongside unclear allocation concealment |
BMI: body mass index; CES‐D ‐ Centre for Epidemiological Studies Depression Scale; CRC: colorectal cancer; CTCAE ‐ Common Terminology Criteria for Assessing Adverse Events; ECOG: Eastern Oncology Cooperative Group; EORTC QLQ‐C30 ‐ European Organisation for Research and Treatment of Cancer Quality of life; EQ‐5D: EuroQol five‐dimension scale; FACIT‐F ‐ Functional Assessment of Chronic Illness Therapy‐Fatigue; FACT‐C ‐ Functional Assessment of Cancer Therapy‐Colorectal; FACT‐F ‐ Functional Assessment of Cancer Therapy‐Fatigue; FACT‐G ‐ Functional Assessment of Cancer Therapy‐General; HADS ‐ Hospital Anxiety and Depression Scale; ITT: intention‐to‐treat; MET: metabolic equivalents; MFSI‐SF: Multidimensional Fatigue Symptom Inventory‐ Short Form; PANAS ‐ Positive and Negative Affect Scale; PSQI: Pittsburgh Sleep Quality Index; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; SES: socioeconomic status; SF‐36 ‐ Medical Outcomes Study Short Form‐36 General Health Survey; STAI: State trait anxiety inventory; TOI: trial outcome index; TPAQ ‐ Tartu Physical Activity Questionnaire; UICC: Union for International Cancer Control; UK: United Kingdom; USA: United States of America
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12618001855213 | Wrong intervention |
| Adamsen 2009 | Wrong patient population (includes advanced disease and a range of cancer diagnoses) |
| Adlard 2016 | Wrong comparator (moderate‐ versus high‐intensity exercise) |
| Ahn 2013 | Intervention was too short (less than 4 weeks in duration) |
| Allgayer 2004b | Intervention was too short (less than 4 weeks in duration) |
| An 2012 | Intervention was too short (less than 4 weeks in duration) |
| Anderson 2010 | Wrong study design (not a randomised controlled trial) |
| Arving 2013 | Wrong intervention (stress management intervention) for people with a range of cancer diagnoses |
| Backman 2014 | Wrong patient population (includes patients with advanced disease) emailed no reply |
| Basen‐Engquist 2015 | Wrong patient population (advanced colorectal cancer) |
| Beck 2015 | Wrong patient population (only one CRC patient included) |
| Beeken 2016 | Wrong intervention (lifestyle advice on a range of behaviours) for people with a range of cancer diagnoses |
| Bennett 2007 | Wrong patient population (majority breast cancer patients, other cancer types not specified) |
| Berntsen 2017 | Wrong comparator (moderate‐ versus high‐intensity exercise) |
| Broderick 2014 | Did not analyse CRC patients separately |
| Buffart 2014 | Did not analyse CRC patients separately |
| Burnham 2002 | Did not analyse CRC patients separately |
| ChiCTROON15005952 | Wrong study design (not a randomised control trial) |
| Demark‐Wahnefried 2012 | Wrong patient population (greater than or equal to 5 years postdiagnosis) |
| DeTroye 2018 | Wrong study design |
| Devin 2016 | Wrong comparator (moderate‐ versus high‐intensity exercise) |
| Dimeo 2004 | Intervention too short (less than 4 weeks in duration) |
| Dodd 2010 | Wrong patient population (only one CRC patient included) |
| DRKS00005793 | wrong comparator (both arms received exercise training) |
| Eakin 2015 | Wrong study design (non‐randomised trial) |
| Forbes 2017 | CRC patients not analysed separately ‐ email confirming this |
| Freitag 2018 | Wrong study design |
| Gabrys 2017 | Wrong study design (non‐randomised trial) |
| Gillis 2014 | Wrong comparator (prehabilitation versus rehabilitation) |
| Gray 2013 | Intervention was too short (less than 4 weeks in duration) |
| Grimmett 2015 | Wrong study design (feasibility study: non‐randomised) |
| Hawkes 2014 | Wrong Intervention (multiple health behaviour change intervention) |
| Hernon 2016 | Wrong patient population (exercise delivered prior to surgery) |
| Hung 2016 | Wrong intervention |
| Ibfelt 2011 | Intervention was too short (6‐day retreat) and focused on multiple health behaviours |
| ISRCTN56928944 | Intervention was too short (less than 4 weeks in duration) |
| ISRCTN62859294 | Wrong patient population (exercise intervention delivered prior to surgery) |
| ISRCTN96374224 | Wrong intervention (multiple components: lifestyle and well‐being support) |
| Kalter 2015 | CRC not analysed separately |
| Kampshoff 2015 | Did not analyse CRC patients separately |
| Kanera 2017 | Wrong intervention (multiple health behaviour change intervention) |
| laStayo 2011 | Wrong patient population (> 5 years postdiagnosis) |
| Lee 2013 | Wrong comparator (both arms received exercise training) |
| Lee 2018a | Dietary co‐intervention included |
| Ligibel 2012 | Did not analyse CRC patients separately "the majority of our participants were breast cancer survivors. We were thus not able to conduct a separate analysis in the colorectal cancer subgroup" |
| Lin 2011 | Wrong comparator (both arms received exercise training) |
| Lin 2014 | Wrong study design (non‐randomised trial) |
| Lin 2016 | Wrong intervention |
| Loughney 2014 | Wrong patient population (exercise delivered prior to surgery) |
| Lyons 2017 | Wrong patient population (CRC patients not included) |
| Macleod 2018 | Wrong intervention |
| Mayer 2017 | Wrong comparator (control group received a pedometer) |
| McCall 2015 | Wrong comparator (all arms received the yoga intervention) |
| Meyerhardt 2017 | Outcomes not relevant (email received "Only 40% of participants were colorectal and the end points were all blood based markers so not sure if this will help your analysis") |
| Midtgaard 2011 | Did not analyse CRC patients separately (email confirmation received) |
| Min 2017 | intervention was too short (less than 4 weeks in duration) |
| Morielli 2018 | Wrong intervention |
| NCT00373022 | Wrong comparator (all arms received physical activity) |
| NCT00977613 | Wrong study design (not a randomised controlled trial) |
| NCT00985400 | Wrong patient population (includes stage IV patients) |
| NCT01032590 | Wrong patient population (includes CRC > 5 years postdiagnosis) |
| NCT01146769 | Wrong intervention |
| NCT01210313 | Wrong study design (observational) |
| NCT01325909 | Wrong study design (non‐randomised trial) |
| NCT01453452 | Wrong study design (non‐randomised trial) |
| NCT01991847 | Wrong study design (non‐randomised trial) |
| NCT02052050 | Wrong study design (non‐randomised trial) |
| NCT02056691 | Wrong study design (non‐randomised trial) |
| NCT02264496 | Wrong patient population (exercise delivered prior to surgery) |
| NCT02442583 | Wrong study design (non‐randomised trial) |
| NCT02512263 | Study was not carried out |
| NCT02522520 | Wrong comparator (both arms received a pedometer intervention) |
| NCT02586701 | Wrong comparator (both arms received exercise training) |
| NCT02647398 | Wrong comparator (both arms received exercise) |
| NCT02677389 | Wrong comparator (both arms received physical activity) |
| NCT02837159 | Study has been suspended due to financing issues |
| NCT02889276 | Wrong comparator (both arms received physical activity) |
| NCT03036436 | Wrong study design (non‐randomised trial) |
| NCT03082495 | Wrong patient population (exercise during neoadjuvant treatment) |
| NCT03120104 | Wrong comparator |
| NCT03232814 | Wrong study design (non‐randomised trial) |
| NCT03630354 | Wrong comparator |
| NTR6383 | Wrong intervention |
| Oh 2008 | Wrong intervention and wrong patient population (mixed cancer diagnoses ‐ three CRC patients included, all in the control arm) |
| Onerup 2017 | Wrong patient population (exercise intervention delivered pre‐ and postsurgery) |
| Phipps 2018 | Wrong study design |
| Piringer 2013 | Wrong study design (non‐randomised trial) |
| Piringer 2018 | Wrong study design |
| Ratjen 2018 | Wrong intervention |
| Ray 2018 | Wrong study design |
| Sandler 2017 | Wrong intervention (intervention group received CBT along with exercise) mixed cancer diagnoses |
| Sellar 2014 | Wrong study design (non‐randomised trial) |
| Sohl 2016 | Intervention was too short (less than 4 weeks in duration) |
| Sprod 2015 | Wrong patient population (less than 3% of patients had gastrointestinal cancers) |
| Ungar 2016 | Wrong comparator |
| van Rooijen 2018 | Wrong study design |
| Wang 2012 | Intervention was too short (less than 4 weeks in duration) |
| West 2015 | Wrong patient population (exercise intervention delivered prior to surgery) |
| Zopf 2018 | Wrong study design |
Characteristics of studies awaiting classification [ordered by study ID]
De Backer 2007.
| Methods | RCT |
| Participants | 57 patients (age 24 to 73 years) who had received chemotherapy for lymphomas, breast, gynaecologic, testicular, or colorectal cancer (8 CRC participants) |
| Interventions | Intervention details: 18‐week high‐intensity strength training programme in cancer survivors Comparator details: standard care |
| Outcomes |
|
| Notes | Emailed to check whether CRC patients analysed separately |
Golsteijn 2017.
| Methods | RCT |
| Participants | 510 prostate and colorectal cancer patients (during treatment or up to 1 year post‐treatment) |
| Interventions | Intervention details: onco‐active (computer‐tailored physical activity intervention participants received tailored advice at 3 time points, a pedometer and access to interactive content on the website) Comparator: usual care waiting list control |
| Outcomes | 1. Physical activity behaviour (self‐report Short Questionnaire to Assess Health Enhancing Physical Activity (SQUASH) and accelerometer data) intention be physically active, 2. HRQoL (EORTC QLQ‐C30) 3. Fatigue (Checklist Individual Strength) |
| Notes | Emailed: data may be shared |
Ho 2013.
| Methods | RCT |
| Participants | 222 colorectal cancer patients who completed curative treatment without evidence of recurrence will be recruited into the study |
| Interventions | intervention: Arm 1: dietary intervention (< 5 servings of red/processed meat weekly, 2 servings of refined grains daily) Arm 2: physical activity intervention (general health target ‐ 30 minutes moderate to vigorous physical activity, 5 days per week (i.e. 10 MET‐hours/week); cancer outcome target ‐ 60 minutes of moderate to vigorous physical activity 5 days per week (i.e. 18‐20 MET‐hours/week)) Arm 3: dietary and physical activity intervention (meeting both physical activity and dietary targets) comparator: usual care |
| Outcomes | 1. Whether physical activity and dietary targets are met 2. Magnitude and mechanism of behavioural change 3. The degree and determinants of compliance 4. The additional health benefits and side effects of the intervention |
| Notes | Estimated study completion date: June 2017 emailed for information, no reply. Trial registration number: NCT01708824 |
Houborg 2006.
| Methods | RCT |
| Participants | 119 patients with colorectal cancer (96), diverticulitis (7) and non‐malignant disease (16) |
| Interventions | Intervention details: demanding exercises covering mobilisation, strength training of upper and lower extremities, and aerobic training comparator: performed relaxation exercises and received hot wrappings and massage |
| Outcomes | 1. Fatigue (visual analogue scale) 2. Muscular strength 3. Walking speed 4. Physical performance test 5. Physical function questions (SF‐36) |
| Notes | Requested data for CRC only, received email ‐ to respond after 1 June |
ISRCTN07465566.
| Methods | RCT |
| Participants | Adults with CRC, Duke stage A‐C2, completed surgery in the past 12 months |
| Interventions | Intervention details: physical activity consultation after baseline (lasting 1‐2 hours) and one at 3 months Comparator: usual care |
| Outcomes | 1. Feasibility (measured by rates of recruitment, completion, adherence), 2. Change in physical activity (Actigraph) 3. Mental well‐being (HADS) 4. QoL (FACT‐G and FACT‐C) 5. Body composition 6. Predictors of change in physical activity 7. Perceptions of cancer risk in partners 8. Fear of cancer recurrence 9. Key components of the revised theory of planner behaviour will be measured in individuals living with colorectal cancer and their partner. 10. The quality of the relationship between the individual living with cancer and their partner will be measured to test if it affects adherence to the physical activity intervention or moderates any of the intervention effects |
| Notes | Emailed ‐ no reply received |
Jacobsen 2013.
| Methods | RCT |
| Participants | 460 cancer patients receiving chemotherapy representing a range of cancer diagnoses (15 colon cancer, 6 rectal/anal cancer) |
| Interventions | Intervention details: arm 1: stress management arm 2: exercise (participants were advised to participate in home‐based exercise 3–5 times per week for 20 to 30 min at approximately 50% to 75% of their estimated heart rate reserve) arm 3: combined stress management and exercise comparator: usual care |
| Outcomes | 1. Mental and physical well‐being 2. Depression and anxiety 3. Exercise 4. Stress reduction activity |
| Notes | Unable to contact via email |
Lee 2018.
| Methods | RCT |
| Participants | 72 colon and rectal cancer patients (38 intervention; 34 usual care) |
| Interventions | Home‐based six‐week exercise programme |
| Outcomes |
Secondary outcomes:
Time points measured: baseline and 12 weeks Time points reported: baseline and 12 weeks Adverse events: not reported |
| Notes | Emailed author to clarify if participants in this study are the same as participants in Kim 2018. If not eligible for inclusion as a separate study. |
Maxwell‐Smith 2019.
| Methods | RCT |
| Participants | Colorectal or endometrial cancer patients at cardiovascular risk |
| Interventions | Intervention details: a wearable tracker, two group sessions and a support phone call Comparator: received printed materials describing physical activity guidelines |
| Outcomes | 1. Moderate to vigorous physical activity 2. Blood pressure 3. BMI 4. Sedentary behaviour |
| Notes | Emailed requesting colorectal cancer patient data separately |
Moller 2015.
| Methods | RCT |
| Participants | breast or colon (33%) cancer patients |
| Interventions | Intervention details arm 1: 12‐week hospital‐based, supervised group exercise intervention of moderate‐ to high‐intensity three times weekly, health promotion counselling and symptom management coaching or arm 2: 12 week home‐based individual pedometer programme, health promotion counselling and symptom management comparator: a control group |
| Outcomes | 1. Maximal oxygen uptake (V02 peak) 2. Psychological well‐being (HADS) 3. QOL (EORTC QLQ‐C30) 4. General well‐being (SF‐36) 5. Perception of pain (Brief Pain Inventory) 6. Leisure time physical activity level (self‐reported) 7. Motivational readiness and ability and barriers to engage in exercise activities ‘stages of motivational readiness’ ‘decisional balance’ and ‘exercise self‐efficacy’ (using validated questionnaires), 8. Social support and network (Multidimensional Scale of Perceived Social Support) |
| Notes | Trial registration number: ISRCTN24901641 Emailed |
NCT02403024.
| Methods | RCT |
| Participants | 42 CRC patients who had completed surgery for local stage disease and patients who had completed surgery and any adjuvant chemotherapy for locally advanced stage disease |
| Interventions | Intervention details: home‐based walking intervention (150 minutes per week) comparator: usual care (waiting list control) |
| Outcomes | 1. Cardiopulmonary fitness (V02 peak during CPET test) 2. Feasibility (inclusion rate, attrition rate, adherence) 3. Body composition (DXA scan) 4. Blood biochemistry (fasting blood samples) 5. Glycaemic control (oral glucose tolerance test) 6. Patient reported outcome measures (health‐related quality of life by FACT‐C, sleep quality by Pittsburgh Sleep Quality Index and exercise motivation by Behavioral Exercise Regulations Questionnaire) |
| Notes | The “Interval Walking in Colorectal Cancer” (I‐WALK‐CRC) study: design, methods and recruitment results of a randomised controlled feasibility trial‐ Publication 2018 |
NCT02564835.
| Methods | RCT |
| Participants | 27 CRC survivors (any stage) |
| Interventions | Intervention details: arm 1: yoga programme ‐ two 90‐minute classes every week tor a total of 12 weeks arm 2: physical activity ‐ two 90‐minute classes every week for a total of 12 weeks, nine resistance exercises (e.g. squats) and 8 flexibility exercises (e.g. shoulder stretch) targeted for the whole body as well as a brief warm up and cool down (walking) comparator: usual care |
| Outcomes | 1. Program evaluation (participant experience and satisfaction with the interventions) 2. Attention Network Test, attention and cognitive control (Digit Span, Digit Symbol Substitution Test, Trail Making Test, Attentional Function Index), 3. Functional Assessment of Cancer Therapy ‐ Cognitive Function 4. Pro‐inflammatory Markers 5. attendance, Adherence and Attrition Log 6. Safety Log 7. Demographic and Medical Information Questionnaires 8. Profile of Mood States‐Brief Form 9. Mindful Attention Awareness Scale, Beliefs about Treatment |
| Notes | NCT02564835 |
Park 2015.
| Methods | RCT |
| Participants | 162 early stage breast (n = 122, 75.3 %) and colorectal cancer survivors (n = 40, 24.7%) who completed primary and adjuvant treatments |
| Interventions | Intervention details: arm 1: oncologists’ exercise recommendation arm 2: oncologists’ exercise recommendation with exercise motivation package (pedometer, exercise education and exercise diary) comparator: control |
| Outcomes | 1. Amount of exercise participation (Godin LeisureTime Exercise Questionnaire) 2. Quality of life 3. Self‐reported physical functioning (EORTC QLQ‐C30) |
| Notes |
Schulz 2002.
| Methods | RCT? There is a control group and exercise group, report does not specify whether participants are randomly allocated |
| Participants | 49 colon cancer patients; 24 control, 25 intervention |
| Interventions | Intervention details: ergometer exercise training performed once a day for approximately 40 minutes, 5 days per week for 5 weeks at a moderate training level Comparator: control group |
| Outcomes | 1. Psychological parameters (EORTC QLQ‐C30, FPI‐R) 2. Immunological parameters |
| Notes | UTC via email |
Schwartz 2009.
| Methods | RCT |
| Participants | 112 females with breast, lymphoma and colon cancer |
| Interventions | Intervention details: arm 1: 12 months, aerobic exercise, moderate‐intensity home‐based exercise 4 days per week. The duration of each specific exercise programme ranged from 20 to 30 minutes arm 2: 12 months, resistance exercise home‐based exercise 4 days per week. The duration of each specific exercise programme ranged from 20 to 30 minutes. comparator: usual care control |
| Outcomes | 1. Body weight 2. Aerobic capacity (12‐minute walk) 3. Muscle strength (1‐repetition maximum tests) 4. Body fat (dual energy x‐ray absorptiometry scans) |
| Notes | UTC via email |
Shun 2016.
| Methods | RCT |
| Participants | 128 postsurgical colorectal cancer patients; 65 control, 63 intervention |
| Interventions | Intervention details: 12‐week home‐based moderate‐intensity exercise programme Comparator: usual care, maintained their normal daily activity |
| Outcomes | 1. Fatigue 2. Psychological distress (depression) |
| Notes | Emailed, no reply received |
BMI: body mass index; CRC: colorectal cancer; EORTC QLQ‐C30 ‐ European Organisation for Research and Treatment of Cancer Quality of life; FACT‐C ‐ Functional Assessment of Cancer Therapy‐Colorectal; FACT‐G ‐ Functional Assessment of Cancer Therapy‐General; FPI‐R‐ Freiburg Personality Inentory; HADS ‐ Hospital Anxiety and Depression Scale; HRQoL‐ health related quality of life; MET: metabolic equivalents; QoL: quality of life; RCT: randomised controlled trial; SF‐36 ‐ Medical Outcomes Study Short Form‐36 General Health Survey; UTC‐ unable to contact
Characteristics of ongoing studies [ordered by study ID]
ChiCTRIOR17012037.
| Study name | The impact of resistance training combined with aerobic exercises on the functional recovery and quality of life of colorectal cancer survivors postsurgeries |
| Methods | Study design: Randomised parallel controlled trial Country where study is being conducted: China |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention details: postoperative cancer survivors in the experimental group would participate in low‐intensity resistance and aerobic exercise for 7 days, supervised by an experienced physiotherapist. And then the patients would follow a 12‐week moderate‐ to high‐intensity home exercise programme Comparator details: will only receive conventional treatment |
| Outcomes |
Time points measured: day 1 preoperation, day 7 postoperation, month 3 postoperation |
| Starting date | 18 May 2016 |
| Contact information | Yuling Wang Tel: +86 13054445587 Email: wangyul@mail.sysu.edu.cn |
| Notes | Trial registration identifier: ChiCTR‐IOR‐17012037 Trial registration link: http://www.chictr.org.cn/showprojen.aspx?proj=20476 Sponsor: Department of Rehabilitation Medicine, The Sixth Affiliated Hospital of Sun Yat‐sen University |
NCT02191969.
| Study name | Physical activity intervention for older patients during chemotherapy for colorectal cancer |
| Methods | Study design: RCT Country where study is being conducted: USA |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention details: participants will take part in the Walk With Ease (WWE) programme during the course of their chemotherapy treatment. They will be requested to initiate the WWE starting on Day 1 of adjuvant chemotherapy. Participants are asked to walk at a safe and comfortable pace, increasing their minutes per day at a rate they can sustain, with the ultimate goal of 30 minutes/day for at least 5 days/week. They are asked to maintain a daily walking log that is provided to them, entering total minutes per day. Participants will be asked to do the walking programme independently (self‐directed, not in a formal group with an instructor) throughout chemotherapy. Comparator details: standard care |
| Outcomes | Primary outcome measures:
Time points measured: baseline, 3 months, and after completion of chemotherapy (24 weeks), some outcomes measured at 1 year |
| Starting date | June 2014 |
| Contact information | Kirsten A Nyrop knyrop@med.unc.edu Amy L Garrrett amy_garrett@med.unc.edu |
| Notes | Trial registration identifier:NCT02191969 Trial registration link: https://clinicaltrials.gov/ct2/show/NCT02191969 Sponsor: UNC Lineberger Comprehensive Cancer Center |
NCT02538913.
| Study name | Exercise training for rectal cancer patients. A randomized controlled trial |
| Methods | Study design: RCT Country where study is being conducted: Norway |
| Participants | 150 people with rectal cancer Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention details: daily pelvic floor muscle training and individualised regular exercise training (aerobic and strength exercise) three days per week. Comparator details: standard care |
| Outcomes | Primary outcome:
Secondary outcomes:
Time points measured: multiple time points measured see above |
| Starting date | September 2015 |
| Contact information | Signe N Stafne, PhD, +47 480 71 766, signe.n.stafne@ntnu.no |
| Notes | Trial registration identifier: NCT02538913 Trial registration link: https://clinicaltrials.gov/ct2/show/NCT02538913 Sponsor: Norwegian University of Science and Technology |
NCT03064308.
| Study name | The Aasessment of the feasibility of a home based exercise programme in the older patient following major surgery (POETold) |
| Methods | Study design: RCT Country where study is being conducted: UK |
| Participants | 30 participants undergoing major body surgery including those with colorectal carcinoma Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention details: participants will complete a High‐Intensity Functional Exercise (HIFE) programme. Comparator details: standard NHS care |
| Outcomes | Primary:
Secondary:
Time points measured: baseline, 3 and 6 months |
| Starting date | 26 June 2017 |
| Contact information | Laura Carrick, BSc MBChB 07429377430 msxlc2@nottingham.ac.uk Bethan Phillips, BSc PhD 01332 724731 |
| Notes | Trial registration number: NCT03064308 Trial registration link: https://clinicaltrials.gov/ct2/show/NCT03064308 Sponsor: University of Nottingham |
NCT03210129.
| Study name | Motivational Interviewing to Increase Physical Activity behaviour in Cancer patients (MIPAClux) |
| Methods | Study design: RCT Country where study is being conducted: Luxembourg |
| Participants | 70 breast, endometrial or colorectal cancer participants Inclusion:
Exclusion:
|
| Interventions | Intervention details: 12 motivational interviewing sessions exploring self‐assessed confidence, ambivalence, and personal values concerning changes in active lifestyle Comparator details: standard care |
| Outcomes | Primary outcome:
Secondary outcomes:
Time points measured: weeks 1, 14 and 26 |
| Starting date | 6 July 2017 |
| Contact information | Alexis Lion, PhD, +35226970849 alexis.lion@lih.lu Eric Besenius, M.Sc., +35226970917 |
| Notes | Trial registration number: Trial registration link: https://clinicaltrials.gov/ct2/show/NCT03210129 Sponsor: Luxembourg Institute of Health |
NCT03212079.
| Study name | Novel individualized intervention for behavioral change among high‐risk group cancer survivors: Physical Activities by Technology Help (PATH) |
| Methods | Study design: RCT Country where study is being conducted: USA |
| Participants | 42 cancer survivors of breast, prostate, lung, colorectal, cervical or oral cancer survivor Inclusion:
Exclusion:
|
| Interventions | Intervention details: Arm 1: Mycoach Smart Text ‐ personalised text messages to cell phone to help participants to increase activity Arm 2: MyCoach via Amazon Alexa‐ will interact with intelligent coach to increase activity Comparator details: control participants will self‐motivate to increase physical activities |
| Outcomes | Primary outcome:
Secondary outcomes:
Time points measured: 5 weeks |
| Starting date | 3 April 2017 |
| Contact information | Ahmed Hassoon, MD, MPH, PMP 443 287 2775 ahassoo1@jhu.edu |
| Notes | Trial registration number: NCT03212079 Trial registration link: https://clinicaltrials.gov/ct2/show/NCT03212079 Sponsor: John Hopins University |
NCT03252821.
| Study name | Effects of high‐intensity training compared to resistance training in cancer patients undergoing radiotherapy |
| Methods | Study design: RCT Country where trial is being conducted: Belgium |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention details Arm 1: high‐intensity aerobic training group will be conducted on cycle ergometers or treadmill with heart rate measured throughout each session Arm 2: resistance training group muscle strengthening will incorporate eight exercises targeting major muscle groups at 60% to 85% of their estimated 1‐repetition maximum (1RM) Comparator details: no intervention, usual care |
| Outcomes | Primary outcome measures:
Secondary outcome measures
Time points measured: baseline, 5 weeks for rectum cancer |
| Starting date | 15 August 2017 |
| Contact information | Gilles Caty gilles.caty@uclouvain.be Elise Piraux elise.piraux@uclouvain.be |
| Notes | Trial registration identifier: NCT03252821 Trial registration link: https://clinicaltrials.gov/ct2/show/NCT03252821 Sponsor: Cliniques universities Saint‐Luc‐ Université Catholique de Louvain |
NCT03885817.
| Study name | Physically active during cancer treatment (FAKT) |
| Methods | Study design: RCT Country where study is being conducted: Norway |
| Participants | 64 participants with CRC radical resection within past 3 months |
| Interventions | Intervention details: exercise training and nutritional guide Comparator details: usual care |
| Outcomes | Primary:
Secondary:
|
| Starting date | Not described ‐ not yet recruiting |
| Contact information | Contact: Ingunn Hatlevoll, +47 90866361
ingunn.hatlevoll@stolav.no Contact: Eva Hofsli, MD PhD eva.hofsli@ntnu.no |
| Notes | NCT03885817 |
Piringer 2017.
| Study name | Phase III randomised trial of endurance exercise following adjuvant chemotherapy for colorectal cancer "aBCSG C08‐II trial" |
| Methods | Study design: RCT Country where study is being conducted: Austria |
| Participants | 788 patients with newly diagnosed, locally advanced colorectal cancer after adjuvant chemotherapy |
| Interventions | Intervention details: one year endurance exercise intervention Comparator details: usual care while maintaining habitual physical activity levels |
| Outcomes | Primary:
Secondary:
Time points measured: not reported |
| Starting date | Not known |
| Contact information | Not known |
| Notes | Supplement in magazine of European Medical Oncology |
BMI: body mass index; ECOG: Eastern Oncology Cooperative Group; EORTC QLQ‐C30 ‐ European Organisation for Research and Treatment of Cancer Quality of life; FACIT‐F ‐ Functional Assessment of Chronic Illness Therapy‐Fatigue; IRB‐approved; institutional review board approved; LAR‐low anterior resection; OEE‐ Outcome Expectations for Exercise Scale; PROMIS‐ patient reported outcome measures information system, PROMIS PF ‐ patient reported outcome measures information system physical function; PROMIS PI‐patient reported outcome measures information system pain interference; PROMS‐ patient reported outcome measures; PSEFSM‐ perceived self‐efficacy for fatigue self‐management TNM‐ classification of malignant tumours; Tx‐ treatment QoL: quality of life; RCT: randomised controlled trial; UK: United Kingdom; USA: United States of America
Differences between protocol and review
We had planned to report adverse events as a dichotomous outcome. As adverse events were inconsistently measured and reported, this precluded meta‐analysis. We therefore provided a narrative description of adverse events. We did not include overall survival and recurrence‐free survival in our 'Summary of findings' table (as indicated in the protocol) because it was not reported in any of the included studies. In measures of treatment effect, we modified the description of our follow‐up periods to further clarify our follow‐up time points, e.g. up to 12 weeks follow‐up became up to or equal to 12 weeks follow‐up. We used the term 'physical activity interventions' throughout the review to encompass exercise interventions for readers ease. We made reference to this in the description of interventions section. We made minor changes to the data extraction form that was proposed in the protocol after testing the pre developed form on a random sample of studies. Subgroup analysis were precluded, and we therefore removed any reference to subgroup analysis from the Assessment of heterogeneity section. We made a minor amendment to the wording under the Types of participants section, as the wording inferred patients that were not treated surgically would not be included in the review; this was not the case and was an oversight by the authors during protocol development. We included three studies that had a health education component in addition to a physical activity intervention, inclusion of these studies was unforeseen and therefore not identified in the protocol. Authors met to discuss inclusion of these studies and agreed to include studies and investigate the effect of inclusion in sensitivity analysis, where appropriate. The effect of including these studies is discussed in Types of interventions, Quality of the evidence and Potential biases in the review process sections.
Contributions of authors
Drafting the protocol and review: MMG, MAT, CC, MMC
Selecting studies: MMG and MAT
Extracting data from studies: MMG and MAT
Entering data into RevMan: MMG
Carrying out the analysis: MMG, MAT and CC
Interpreting the analysis: MMG, MAT and CC
Drafting the final review: MMG, MAT, CC and MMC
Resolving disagreements: MMG, MAT, CC and MMC
Sources of support
Internal sources
Centre for Public Health, Queens University Belfast, UK
External sources
-
Public Health Agency, HSC R & D Division, UK
Provision of funding for the project
Declarations of interest
MMG: none known
CC: none known
MMC: none known
MAT: none known
New
References
References to studies included in this review
Bourke 2011 {published data only}
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation 2011;92(5):749-55. [DOI] [PubMed] [Google Scholar]
Brown 2017 {published data only}
- *.Brown JC, Damjanov N, Courneya KS, Troxel AB, Zemel BS, Rickels MR, et al. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psycho-Oncology 2018;27(4):1221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, et al. A randomized phase II dose-response exercise trial among colon cancer survivors: purpose, study design, methods, and recruitment results. Contemporary Clinical Trials 2016;47:366-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, et al. Dose-response effects of aerobic exercise among colon cancer survivors: a randomized phase II trial. Clinical Colorectal Cancer 2018;17:32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Zemel BS, Troxel AB, Rickels MR, Damjanov N, Ky B, et al. Dose-response effects of aerobic exercise on body composition among colon cancer survivors: a randomised controlled trial. British Journal of Cancer 2017;21:1614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2016 {published data only}
- Cantarero-Villanueva I, Sánchez-Jiménez A, Galiano-Castillo N, Díaz-Rodríguez L, Martín-Martín L, Arroyo-Morales M. Effectiveness of lumbopelvic exercise in colon cancer survivors: a randomized controlled clinical trial. Medicine and Science in Sports and Exercise 2016;48(8):1438-46. [DOI] [PubMed] [Google Scholar]
Courneya 2003 {published data only}
- Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. European Journal of Cancer Care 2003;12(4):347-57. [DOI] [PubMed] [Google Scholar]
Courneya 2016 {published data only}
- Baylock B, Dhillon HM, Courneya K, O'Callaghan C, O'Brien A, Goddard AM, et al. A phase III study of the impact of a physical activity program on disease-free survival in patients with high-risk stage II or stage III colon cancer: a randomised control trial (NCIC CTG CO.21). Asia-Pacific Journal of Clinical Oncology 2014;10(Suppl 8):199-200. [Google Scholar]
- Baylock B, Dhillon HM, Goddard EM, Turner J, Rice H, Kabourakis M, et al. The colon health and life-long exercise change (challenge) trial: Participant adherence to fitness assessments. Asia-Pacific Journal of Clinical Oncology 2014;10(Suppl 8):200. [Google Scholar]
- *.Courneya KS, Booth CM, Gill S, O'Brien P, Vardy J, Friedenreich CM, et al. The colon health and life-long exercise change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Current Oncology 2008;15(6):271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS, Vardy J, Gill S, Jonker D, O'Brien P, Friedenreich CM, et al. Update on the colon health and life-long exercise change trial: a phase III study of the impact of an exercise program on disease-free survival in colon cancer survivors. Current Colorectal Cancer Reports 2014;10(3):321-8. [Google Scholar]
- Courneya KS, Vardy JL, O'Callaghan CJ, Friedenreich CM, Campbell KL, Prapavessis H, et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE Trial. Cancer Epidemiology, Biomarkers & Prevention 2016;25(6):969-77. [DOI] [PubMed] [Google Scholar]
- Vardy J, Dhillon H, Van Der Ploeg H, Goddard E, Clarke S, Spencer L, et al. Challenge: a phase III study of a physical activity program on disease-free survival in patients with high risk stage II/III colon cancer. Asia-Pacific Journal of Clinical Oncology 2010;6:186. [Google Scholar]
- Vardy J, Dhillon HM, Ploeg H, Goddard E, Turner J, Kabourakis M, et al. The challenge trial. A phase III study of the impact of a physical activity program on disease-free survival in patients with high-risk stage II or stage III colon cancer: a randomised controlled trial. Asia-Pacific Journal of Clinical Oncology 2013;9:156. [Google Scholar]
Cramer 2016 {published data only}
- *.Cramer H, Pokhrel B, Fester C, Meier B, Gass F, Lauche R, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psycho-Oncology 2016;25(4):412-20. [DOI] [PubMed] [Google Scholar]
- Cramer H, Pokhrel B, Gass F, Eisenmann C, Lauche R, Meier B, et al. Hatha yoga for patients with colorectal cancer: a randomized controlled mixed-methods study. Journal of Alternative and Complementary Medicine 2014;20:A52-3. [Google Scholar]
Hubbard 2016 {published data only}
- Hubbard G, Adams R, Campbell A, Kidd L, Leslie S, Munro J, et al. Cardiac rehabilitation to increase physical activity among cancer patients: is it feasible and acceptable? Psycho-Oncology 2016;25:5. [Google Scholar]
- *.Hubbard G, Adams R, Campbell A, Kidd L, Leslie SJ, Munro J, et al. Is referral of postsurgical colorectal cancer survivors to cardiac rehabilitation feasible and acceptable? A pragmatic pilot randomised controlled trial with embedded qualitative study. BMJ Open 2016;6:e009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard G, O'Carroll R, Munro J, Mutrie N, Haw S, Mason H, et al. The feasibility and acceptability of trial procedures for a pragmatic randomised controlled trial of a structured physical activity intervention for people diagnosed with colorectal cancer: findings from a pilot trial of cardiac rehabilitation versus usual care (no rehabilitation) with an embedded qualitative study. Pilot & Feasibility Studies 2016;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J, Adams R, Campbell A, Campbell S, Donaldson C, Godwin J, et al. CRIB - The use of cardiac rehabilitation services to aid the recovery of patients with bowel cancer: a pilot randomised controlled trial (RCT) with embedded feasibility study. BMJ Open 2014;4:e004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AJ, Hubbard G, Munro J, Adams R. Is use of cardiac rehabilitation an acceptable and feasible rehabilitation model for patients with colorectal cancer and is a randomised trial of this intervention also acceptable and feasible? Gut 2015;64:A334-5. [Google Scholar]
Kim 2018 {published data only}
- Kim JY, Lee MK, Lee DH, Kang DW, Min JH, Lee JW, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: a randomized controlled trial. Supportive Care in Cancer 2019;27:2933-40. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- *.Lee MK, Kim JY, Kim DI, Kang DW, Park JH, Ahn KY, et al. Effect of home-based exercise intervention on fasting insulin and Adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metabolism 2017;76:23-31. [DOI] [PubMed] [Google Scholar]
- Lee MK, Kim JY, Park JH, Lee J, Kim DI, Kang D, et al. Effect of 12-week home-based exercise program on fasting insulin in stage II-III colorectal cancer survivors: a randomized controlled trial. Journal of Clinical Oncology 2014;32(15 Suppl):3588. [Google Scholar]
Lewis 2016 {published data only}
- Lewis L. A Feasibility Study of an Autonomous Supportive Intervention in People Recovering from CRC: a randomised controlled trial [PhD thesis]. Norwich (UK): University of East Anglia, 2016. [NCT02751892] [Google Scholar]
McDermott 2017 {published data only}
- McDermott L. The Feasibility of a Home-based Walking and Strengthening Intervention on Physiological, Biochemical and Psychological Outcomes in Colorectal Cancer Survivors [PhD thesis]. Belfast (UK): Faculty of Life and Health Sciences of the Ulster University, 2017. [NCT01325909] [Google Scholar]
Nuri 2016 {published data only}
- Nuri R, Moghaddasi M, Darvishi H, Izadpanah A. Effect of aerobic exercise on leptin and ghrelin in patients with colorectal cancer. Journal of Cancer Research & Therapeutics 2016;12:169-74. [DOI] [PubMed] [Google Scholar]
Pinto 2013 {published data only}
- Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N, Pinto BM, et al. Home-based physical activity intervention for colorectal cancer survivors. Psycho-Oncology 2013;22:54-64. [DOI] [PubMed] [Google Scholar]
Van Blarigan 2019 {published data only}
- Chan H, Van Loon K, Kenfield SA, Chan JM, Mitchell E, Zhang L, et al. Effect of physical activity trackers and daily text messages on quality-of-life in colorectal survivors (Smart Pace): a pilot randomized controlled trial. www.researchgate.net/publication/337996933 .
- NCT02966054. Self-monitoring and reminder texts to increase physical activity after cancer: a pilot randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02966054 (first received 17 November 2017).
- *.Van Blarigan E, Chan H, Van Loon K, Kenfield SA, Chan JM, Mitchell E, et al. Self-monitoring and reminder text messages to increase physical activity after colorectal cancer (CRC): a pilot randomized controlled trial. BMC Cancer 2019;19(218):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blarigan E, Van Loon K, Kenfield SA, Chan JM, Mitchell E, Chan H, et al. Self-monitoring and reminder text messages to increase physical activity after colorectal cancer (CRC): a pilot randomized controlled trial. Journal of Clinical Oncology 2018;36(4 Suppl 615):ascopubs.org/doi/abs/10.1200/JCO.2018.36.4_suppl.615. [DOI: 10.1200/JCO.2018.36.4-suppl.615] [DOI] [Google Scholar]
Van Vulpen 2016 {published data only}
- May AM, Bosch MJ, Velthuis MJ, Van Der Wall E, Bisschop CN, Los M, et al. Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and colon cancer undergoing adjuvant chemotherapy: The randomised PACT study. BMJ Open 2017;7:e012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, Van Den Buijs BJ, Backx FJ, et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Medicine and Science in Sports and Exercise 2016;48(5):767-75. [DOI] [PubMed] [Google Scholar]
- Velthuis MJ, May AM, Koppejan-Rensenbrink RA, Gijsen BC, Breda E, Wit GA, et al. Physical Activity during cancer treatment (PACT) study: design of a randomised clinical trial. BMC Cancer 2010;10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox L, Hiensch AE, Velthuis M, Steins C, Bisschop Maartje Los B, Erdkamp FL, et al. Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Medicine 2018;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waart 2017 {published data only}
- *.Waart H, Stuiver M, Harten W, Sonke G, Aaronson N. Design of the Physical exercise during adjuvant chemotherapy effectiveness study (PACES): a randomized controlled trial to evaluate effectiveness and cost-effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer 2010;10:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waart H, Stuiver M, Sonke G, Harten W, Aaronson N. Intensive physical training during adjuvant treatment. Radiotherapy and Oncology 2014;111(Suppl 1):S213. [Google Scholar]
- Waart H, Stuiver MM, Sonke GS, Van Harten WH, Aaronson NK. Effect of low versus high intensity physical exercise during chemotherapy on physical fitness, fatigue and chemotherapy completion rates: results of a randomized, controlled trial. Psycho-Oncology 2014;23:93. [Google Scholar]
- Waart H, Stuiver MM, Harten WH, Geleijn E, Maaker-Berkhof M, Schrama J, et al. Recruitment to and pilot results of the PACES randomized trial of physical exercise during adjuvant chemotherapy for colon cancer. International Journal of Colorectal Disease 2018;33:29-40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12618001855213 {published data only}
- ACTRN12618001855213. A peer support program for the long-term maintenance of physical activity and health outcomes in breast, prostate and colorectal cancer survivors [The PEER Study: Peer support for the maintenance of high intensity interval training and health in cancer survivors]. anzctr.org.au/ACTRN12618001855213.aspx (first received 14 November 2018).
Adamsen 2009 {published data only}
- Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ (Online) 2009;339(7726):895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adlard 2016 {published data only}
- Adlard KN, Devin JL, Jenkins DG, Bolam KA, Aitken JF, Chambers SK, et al. The influence of exercise intensity on fatigue in colorectal cancer survivors: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology 2016;12(Suppl 5):78. [Google Scholar]
Ahn 2013 {published data only}
- Ahn KY, Hur H, Kim DH, Min J, Jeong DH, Chu SH, et al. The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: randomized controlled trial. International Journal of Colorectal Disease 2013;28(5):643-51. [DOI] [PubMed] [Google Scholar]
Allgayer 2004b {published data only}
- Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detection and Prevention 2004;28(3):208-13. [DOI] [PubMed] [Google Scholar]
An 2012 {published data only}
- An KY, Lee MK, Kim DI, Kim DH, Min JH, Kim NK, et al. Randomized controlled trial of colorectal cancer exercise program for colorectal cancer patient after surgery. Medicine and Science in Sports and Exercise 2012;44:276-7. [Google Scholar]
Anderson 2010 {published data only}
- Anderson AS, Caswell S, Wells M, Steele RJ, Macaskill S, Anderson AS, et al. "It makes you feel so full of life" LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Supportive Care in Cancer 2010;18(4):409-15. [DOI] [PubMed] [Google Scholar]
Arving 2013 {published data only}
- Arving C, Thormodsen I, Brekke G, Mella O, Berntsen S, Nordin K. Early rehabilitation of cancer patients - a randomized controlled intervention study. BMC Cancer 2013;13(9):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Backman 2014 {published data only}
- Backman M, Wengström Y, Johansson B, Sköldengen I, Börjesson S, Tärnbro S, et al. A randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer. Acta Oncologica 2014;53(4):510-20. [DOI] [PubMed] [Google Scholar]
- Malin MB, Yvonne YW, Birgitta BJ, Ida IS, Ake AB. Daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer - a randomized pilot study. European Journal of Cancer 2013;49(Suppl 2):397. [Google Scholar]
Basen‐Engquist 2015 {published data only}
- Basen-Engquist K, Parker PA, Eng C, Li YS, Johnson DB, Kee BK, et al. Randomized trial of a home-based exercise intervention for patients with advanced colorectal cancer: effects on physical functioning and activity levels. Journal of Clinical Oncology 2015;33(15 Suppl 9633):ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.9633. [Google Scholar]
Beck 2015 {published data only}
- Beck AC, Garland EL, Hansen PA, Walker D, Reimers C, Thomas EA, et al. Integrated mindfulness-oriented recovery enhancement (MORE) and physical health intervention for cancer survivors with obesity: preliminary results from a pilot randomized controlled trial. Journal of Clinical Oncology 2015;33(29 Suppl 1):237. [Google Scholar]
Beeken 2016 {published data only}
- Beeken RJ, Croker H, Heinrich M, Smith L, Williams K, Hackshaw A, et al. Study protocol for a randomised controlled trial of brief, habit-based, lifestyle advice for cancer survivors: exploring behavioural outcomes for the Advancing Survivorship Cancer Outcomes Trial (ASCOT). BMJ Open 2016;6(11):e011646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2007 {published data only}
- Bennett J, Lyons K, Winters-Stone K, Nail L, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nursing Research 2007;56(1):18-27. [DOI] [PubMed] [Google Scholar]
Berntsen 2017 {published data only}
- Berntsen S, Aaronson NK, Buffart L, Börjeson S, Demmelmaier I, Hellbom M, et al. Design of a randomized controlled trial of physical training and cancer (Phys-Can) - the impact of exercise intensity on cancer related fatigue, quality of life and disease outcome. BMC Cancer 2017;17:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom H, Berntsen S, Hetlelid K, Asenlof P, Demmelmaier I, Henriksson A, et al. Phys-can feasibility study: preparing for physical training and behavioral medicine strategies. Psycho-Oncology 2014;23:95. [Google Scholar]
- Nordin K, Johansson B. Physical training and cancer (phys-can)-design and description of a multicenter clinical trial within a multidisciplinary consortium. US National Library of Medicine 2014;23(Suppl 3):205. [Google Scholar]
Broderick 2014 {published data only}
- Broderick JM, Guinan E, O'Donnell DM, Hussey J, Tyrrell E, Normand C. Calculating the costs of an 8-week, physiotherapy-led exercise intervention in deconditioned cancer survivors in the early survivorship period (the PEACH trial). Physiotherapy 2014;100(2):182-4. [DOI] [PubMed] [Google Scholar]
Buffart 2014 {published data only}
- Buffart L, Ros W, Chinapaw M, Brug J, Knol D, Korstjens I, et al. Mediators of physical exercise for improvement in cancer survivors' quality of life. Psycho-Oncology 2014;23(3):330-8. [DOI] [PubMed] [Google Scholar]
Burnham 2002 {published data only}
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine and Science in Sports and Exercise 2002;34(12):1863-7. [DOI] [PubMed] [Google Scholar]
ChiCTROON15005952 {published data only}
- ChiCTROON15005952. Exercise Prescription for patients with colorectal cancer. www.chictr.org.cn/historyversionpub.aspx?regno=ChiCTR-OON-15005952 (first registered 3 August 2016).
Demark‐Wahnefried 2012 {published data only}
- Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. Journal of Clinical Oncology 2012;30(19):2354-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA 2009;301(18):1883-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00303875. Exercise and dietary counseling in improving physical activity, nutrition, and quality of life in older long-term cancer survivors who are overweight. clinicaltrials.gov/ct2/show/NCT00303875 (first registered 17 March 2006).
- Synder DC, Morey MC, Sloane R, Stull V, Cohen HJ, Peterson B, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psycho-Oncology 2009;18(4):429-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger JG, Mosher CE, Rand KL, Morey MC, Snyder DC, Demark-Wahnefried W. Diet and exercise intervention adherence and health-related outcomes among older long-term breast, prostate, and colorectal cancer survivors. Annals of Behavioral Medicine 2014;48(2):235-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeTroye 2018 {published data only}
- DeTroye A, Christner M, Eganhouse D, Manning B, Sunkin E, Gregory T. The effects of physical activity on survival in patients with colorectal cancer. Journal of the American Academy of Physician Assistants 2018;31(2):21-5. [DOI] [PubMed] [Google Scholar]
Devin 2016 {published data only}
- ACTRN12615000908538. The influence of high-intensity compared with moderate-intensity exercise training on the health of colorectal cancer survivors: a randomised controlled trial. www.anzctr.org.au/TrialSearch.aspx (first registered 1 Sep 2015).
- Devin JL, Jenkins DG, Sax AT, Hughes GI, Aitken JF, Chambers SK, et al. The influence of exercise intensity and frequency on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomized controlled trial. Asia-Pacific Journal of Clinical Oncology 2016;12(Suppl 5):109. [Google Scholar]
- Devin JL, Sax AT, Hughes GI, Jenkins DG, Aitken JF, Chambers SK, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. Journal of Cancer Survivorship 2016;10(3):467-79. [DOI] [PubMed] [Google Scholar]
Dimeo 2004 {published data only}
- Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled trial. Supportive Care in Cancer 2004;12(11):774-9. [DOI] [PubMed] [Google Scholar]
Dodd 2010 {published data only}
- Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, et al. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nursing 2010;33(4):245-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00005793 {published data only}
- DRKS00005793. Effects of supervised physical activity on endurance capacity in colorectal cancer patients undergoing ambulatory Chemotherapy. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005793 (first registered 3 Nov 2014).
Eakin 2015 {published data only}
- Eakin EG, Hayes SC, Haas MR, Reeves MM, Vardy JL, Boyle F, et al. Healthy living after cancer: a dissemination and implementation study evaluating a telephone-delivered healthy lifestyle program for cancer survivors. BMC Cancer 2015;15(992):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forbes 2017 {published data only}
- Forbes CC, Blanchard CM, Mummery WK, Courneya KS. A pilot study on the motivational effects of an internet-delivered physical activity behaviour change programme in Nova Scotian cancer survivors. Psychology and Health 2017;32(2):234-52. [DOI] [PubMed] [Google Scholar]
Freitag 2018 {published data only}
- Freitag N, Weber PD, Sanders TC, Schulz H, Bloch W, Schumann M. High-intensity interval training and hyperoxia during chemotherapy a case report about the feasibility, safety and physical functioning in a colorectal cancer patient. Medicine 2018;97(24):e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabrys 2017 {published data only}
- Gabrys L, Sperzel S, Bernhoerster M, Banzer W, Vogt L. Real-time visual activity feedback for physical activity improvement in breast and colon cancer patients. Research in Sports Medicine 2017;25(1):1-10. [DOI] [PubMed] [Google Scholar]
Gillis 2014 {published data only}
Gray 2013 {published data only}
- Gray NM, Allan JL, Murchie P, Browne S, Hall S, Hubbard G, et al. Developing a community-based intervention to improve quality of life in people with colorectal cancer: a complex intervention development study. BMJ Open 2013;3(4):e002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimmett 2015 {published data only}
- Grimmett C, Simon A, Lawson V, Wardle J. Diet and physical activity intervention in colorectal cancer survivors: a feasibility study. European Journal of Oncology Nursing 2015;19(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN45454522. Feasibility of a personalised, distance-based lifestyle intervention in colorectal cancer patients. www.isrctn.com/ISRCTN45454522 (first registered 2 Nov 2010).
Hawkes 2014 {published data only}
- Gordon LG, Patrao T, Kularatna S, Hawkes AL. A telephone-delivered multiple health behaviour change intervention for colorectal cancer survivors: making the case for cost-effective healthcare. European Journal of Cancer Care 2015;24(6):854-61. [DOI] [PubMed] [Google Scholar]
- Hawkes A, Pakenham K, Courneya K, Peter B, Chambers S. 'Canchange': A trial of a telephone-delivered lifestyle intervention for colorectal cancer (CRC) survivors. Asia-Pacific Journal of Clinical Oncology 2010;6:193. [Google Scholar]
- Hawkes A, Pakenham K, Courneya KS, Patrao T. A randomised controlled trial of the effects of a telephone-delivered program on health behaviours and quality of life for colorectal cancer survivors ('canchange'). Asia-Pacific Journal of Clinical Oncology 2011;7:80-1. [Google Scholar]
- Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. Journal of Clinical Oncology 2013;31(18):2313. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Gollschewski S, Lynch BM, Chambers S. A telephone-delivered lifestyle intervention for colorectal cancer survivors 'CanChange': a pilot study. Psycho-Oncology 2009;18(4):449-55. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Gollschewski S, Lynch BM, Chambers SK. Developing and pilot testing a telephone-delivered lifestyle intervention for colorectal cancer survivors - 'Canchange'. Asia-Pacific Journal of Clinical Oncology 2009;5:A215. [DOI] [PubMed]
- Hawkes AL, Pakenham K, Courneya KS, Patrao TA. A randomised trial of the effects of a multiple health behaviour intervention for colorectal cancer survivors on quality of life and psychosocial outcomes ('CanChange'). Asia-Pacific Journal of Clinical Oncology 2012;8:132. [Google Scholar]
- Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, Courneya KS. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: a randomized controlled trial. Annals of Behavioral Medicine 2014;48(3):359-70. [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Pakenham KI, Courneya KS, Gollschewski S, Baade P, Gordon LG, et al. A randomised controlled trial of a tele-based lifestyle intervention for colorectal cancer survivors ('CanChange'): study protocol. BMC Cancer 2009;9:www.researchgate.net/publication/26750592. [DOI] [PMC free article] [PubMed]
- Lynch B, Courneya K, Sethi P, Patrao T, Hawkes A. A randomized controlled trial of a multiple health behavior change intervention delivered to colorectal cancer survivors: effects on sedentary behavior. Cancer 2014;120(17):2665-72. [DOI] [PubMed] [Google Scholar]
Hernon 2016 {published data only}
- Hernon J. Supportive Exercise Programmes for Accelerating Recovery after Major Abdominal Cancer Surgery (PREPARE-ABC). Norwich (UK): Norfolk and Norwich University Hospital, 2016. [Google Scholar]
- McCulloch JA, Murdoch J, Varley A, Saxton J, Swart A, Sims E, et al. Process evaluation for the Prepare-ABC study: unpicking the standard pre and post-operative care of colorectal cancer patients. Colorectal Disease 2017;19(Suppl 4):3. [Google Scholar]
Hung 2016 {published data only}
- Hung SL, Lin YH, Yang HY, Kao CC, Tung HY, Wei LH. Pelvic floor muscle exercise for fecal incontinence quality of life after coloanal anastomosis. Journal of Clinical Nursing 2016;25(17-18):2658-68. [DOI] [PubMed] [Google Scholar]
Ibfelt 2011 {published data only}
- Ibfelt E, Rottmann N, Kjaer T, Høybye MT, Ross L, Frederiksen K, et al. No change in health behavior, bmi or self-rated health after a psychosocial cancer rehabilitation: results of a randomized trial. Acta Oncologica 2011;50(2):289-98. [DOI] [PubMed] [Google Scholar]
ISRCTN56928944 {published data only}
- ISRCTN56928944. The effect of postsurgery exercise on recovery from colorectal cancer. isrctn.com/ISRCTN56928944 (first registered 17 Feb 2012).
ISRCTN62859294 {published data only}
- ISRCTN62859294. The feasibility of performing a walking programme in patients with rectal cancer undergoing chemo-radiotherapy (the REx trial). isrctn.com/ISRCTN62859294 (first registered 21 Jan 2014).
ISRCTN96374224 {published data only}
- ISRCTN96374224. CLASP Renewed Online Feasibility Study. isrctn.com/ISRCTN96374224 (first registered 31 July 2017).
Kalter 2015 {published data only}
- Kalter J, Buffart L, Korstjens I, Weert E, Brug J, Verdonck-de LI, et al. Moderators of the effects of group-based physical exercise on cancer survivors' quality of life. Supportive Care in Cancer 2015;23(9):2623-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampshoff 2015 {published data only}
- Kampshoff C, Chinapaw M, Brug J, Twisk J, Schep G, Nijziel M, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Medicine 2015;13(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanera 2017 {published data only}
- Kanera IM, Bolman CA, Willems RA, Mesters I, Lechner L. Lifestyle-related effects of the web-based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: a randomized controlled trial. Journal of Cancer Survivorship 2016;10(5):883-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanera IM, Willems RA, Bolman CA, Mesters I, Verboon P, Lechner L. Long-term effects of a web-based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
laStayo 2011 {published data only}
- NCT00335491. Exercise in improving mobility and reducing fatigue and/or weakness in older cancer survivors. clinicaltrials.gov/ct2/show/NCT00335491 (first registered 12 June 2006).
- laStayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer survivors: the impact on muscle and mobility - an exploratory pilot study. BMC Geriatrics 2011;11(5):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee DH, Kim JY, Lee MK, Lee C, Min J-H, Jeong DH, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Supportive Care in Cancer 2013;21(9):2537-45. [DOI] [PubMed] [Google Scholar]
Lee 2018a {published data only}
- Lee CF, Ho J WC, Fong DYT, Macfarlane J, Cerin E, Lee AM, et al. Dietary and physical activity interventions for colorectal cancer survivors: a randomized controlled trial. Scientific Reports 2018;8(5731):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ligibel 2012 {published data only}
- Ligibel J, Meyerhardt J, Najita J, Shockro L, Campbell N, Pierce J. Abstract PD08-09: impact of a Telephone-Based Exercise Intervention on Physical Activity Behaviors and Fitness in a Cooperative Group Setting. Cancer Research 2010;70(Suppl 24):PD 08-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel JA, Meyerhardt J, Pierce JP, Najita J, Shockro L, Campbell N, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Research and Treatment 2012;132(1):205-13. [DOI] [PMC free article] [PubMed]
- NCT00548236. The Active After Cancer Trial (AACT). clinicaltrials.gov/ct2/show/NCT00548236 (first registered 21 October 2007).
Lin 2011 {published data only}
- Lin KY, Tsauo JY. Effects of supervised exercise intervention in patients with colorectal cancer undergoing chemotherapy. Physiotherapy 2011;97:eS693. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
Lin 2016 {published data only}
- Lin YH, Yang HY, Hung SL, Chen HP, Liu KW, Chen TB, et al. Effects of pelvic floor muscle exercise on faecal incontinence in rectal cancer patients after stoma closure. European Journal of Cancer Care 2016;25(3):449-57. [DOI] [PubMed] [Google Scholar]
Loughney 2014 {published data only}
- Loughney L, West M, Kemp GW, Grocott MP, Jack S. Exercise regimen post chemoradiotherapy in patients with operable rectal cancer (empower). Medicine and Science in Sports and Exercise 2014;46(5S):365-6.
Lyons 2017 {published data only}
- Lyons KD, Newman R, Adachi-Mejia AM, Whipple J, Hegel MT. Content analysis of a participant-directed intervention to optimize activity engagement of older adult cancer survivors. OTJR: Occupation, Participation, and Health 2017;38(1):38-45. [DOI] [PMC free article] [PubMed]
Macleod 2018 {published data only}
- Macleod M, Steele RJ, O'Carroll RE, Wells M, Campbell A, Sugden JA, et al. Feasibility study to assess the delivery of a lifestyle intervention (TreatWELL) for patients with colorectal cancer undergoing potentially curative treatment. BMJ Open 2018;8(6):e021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 2017 {published data only}
- Mayer DK, Landucci G, Awoyinka L, Atwood AK, Carmack CL, Demark-Wahnefried W, et al. SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? Journal of Cancer Survivorship 2017;12(1):82-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCall 2015 {published data only}
- McCall M, McDonald M, Thorne S, Ward A, Heneghan C. Yoga for health-related quality of life in adult cancer: a randomized controlled feasibility study. Evidence-based Complementary and Alternative Medicine 2015;2015(816820):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyerhardt 2017 {published data only}
- Meyerhardt JA, Irwin ML, Jones L, Zhang S, Campbell N, Brown JC, et al. Multicenter, randomized phase II trial of physical activity (PA), metformin (Met), or the combination on metabolic biomarkers in stage I-III colorectal (CRC) and breast cancer (BC) survivors. Journal of Clinical Oncology 2017;35(Suppl 15):10059. [Google Scholar]
Midtgaard 2011 {published data only}
- Midtgaard J, Stage M, Møøller T, Andersen C, Quist M, Røørth M, et al. Exercise may reduce depression but not anxiety in self-referred cancer patients undergoing chemotherapy. Post-hoc analysis of data from the ''Body & Cancer'' trial. Acta Oncologica 2011;50(5):660-9. [DOI] [PubMed] [Google Scholar]
Min 2017 {published data only}
- Min J-h, Ki-yong A, Hyuna P, Wonhee C, Hye Jeong J, Nam Kyu K, et al. The effect of post-operative exercise in colorectal cancer patients: a pilot randomized controlled trial (RCT) study. Asian Oncology Nursing 2017;17(1):29-36. [Google Scholar]
Morielli 2018 {published data only}
- Morielli AR, Usmani N, Boule NG, Severin D, Tankel K, Nijjar T, et al. Exercise during and after neoadjuvant rectal cancer treatment (the EXERT trial): study protocol for a randomized controlled trial. Trials 2018;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00373022 {published data only}
- NCT00373022. Moderate physical activity in helping patients recover physically and emotionally from stage II or stage III colorectal cancer. clinicaltrials.gov/ct2/show/NCT00373022 (first registered 7 September 2006).
NCT00977613 {published data only}
- NCT00977613. Adherence to a recommended exercise regimen in colorectal cancer patients. clinicaltrials.gov/ct2/show/NCT00977613 (first received 16 September 2009).
NCT00985400 {published data only}
- NCT00985400. Doctor-recommended home-based exercise program or relaxation training in improving physical function and controlling symptoms in patients with stage iv or recurrent colon cancer that cannot be removed by surgery. clinicaltrials.gov/ct2/show/NCT00985400 (first registered 28 September 2009).
NCT01032590 {published data only}
- NCT01032590. Internet-based weight-loss program for colorectal cancer survivors. clinicaltrials.gov/ct2/show/NCT01032590 (first registered 15 Dec 2009).
NCT01146769 {published data only}
- NCT01146769. Early pelvic floor muscle training improves pelvic floor muscle strength in patient after low anterior resection. clinicaltrials.gov/ct2/show/NCT01146769 (first registered 22 June 2010).
NCT01210313 {published data only}
- NCT01210313. Physical activity for reduction of recurrence rate after adjuvant chemotherapy for localised colorectal carcinoma. clinicaltrials.gov/ct2/show/NCT0121031 (first registered 28 September 2010).
NCT01325909 {published data only}
- NCT01325909. Exercise training in colorectal cancer patients. clinicaltrials.gov/ct2/show/NCT01325909 (first registered 30 March 2011).
NCT01453452 {published data only}
- NCT01453452. S1008: Exercise, diet, & counseling in improving weight loss in overweight female breast or colorectal cancer survivors. clinicaltrials.gov/ct2/show/NCT01453452 (first registered 17 October 2011).
NCT01991847 {published data only}
- NCT01991847. Tertiary prevention by exercise in colorectal cancer therapy. clinicaltrials.gov/ct2/show/NCT01991847 (first registered 25 Nov 2013).
NCT02052050 {published data only}
- NCT02052050. Core stability program in colorectal cancer survivors. clinicaltrials.gov/ct2/show/NCT02052050 (first registered 31 Jan 2014).
NCT02056691 {published data only}
- NCT02056691. EDICT - exercise induced changes in colorectal cancer tissues. clinicaltrials.gov/ct2/show/NCT02056691 (first registered 6 Feb 2014).
NCT02264496 {published data only}
- NCT02264496. Prospective randomised trial of exercise and or antioxidants in colorectal cancer patients undergoing surgery. clinicaltrials.gov/ct2/show/NCT02264496 (first received 15 October 2014).
NCT02442583 {published data only}
- NCT02442583. Reducing Sedentary Behaviors Among Colorectal Cancer Survivors. clinicaltrials.gov/ct2/show/NCT02442583 (first registered 13 May 2015).
NCT02512263 {published data only}
- NCT02512263. Assessment of Adherence and Efficiency of a Home-based Training Program on Muscular Strength, Endurance and Qol for Colon Cancer Patients. ichgcp.net/clinical-trials-registry/NCT02512263 (first registered 7 Jan 2015).
NCT02522520 {published data only}
- NCT02522520. Pedometer intervention and health effects for sedentary colorectal cancer patients during adjuvant chemotherapy. clinicaltrials.gov/ct2/show/NCT02522520 (first registered 13 August 2015).
NCT02586701 {published data only}
- NCT02586701. Supervised versus non-supervised exercise on adherence and functional outcomes in colorectal patients. ichgcp.net/clinical-trials-registry/NCT02586701 (first registered 22 Oct 2015).
NCT02647398 {published data only}
- NCT02647398. Study comparing two strategies of exercise in breast and colon cancer survivors and their impact on fatigue. clinicaltrials.gov/ct2/show/NCT02647398 (first registered 6 Jan 2016).
NCT02677389 {published data only}
- NCT02677389. Survivorship care plan in promoting physical activity in breast or colorectal cancer survivors in Wisconsin. clinicaltrials.gov/ct2/show/NCT02677389 (first registered 9 Feb 2016).
NCT02837159 {published data only}
- NCT02837159. A Mobile Aplication for the Promotion of Healthy Lifestyle Habits in Patients With Colorectal Cancer. clinicaltrials.gov/ct2/show/NCT02837159 (first registered 19 July 2016).
NCT02889276 {published data only}
- NCT02889276. Effects of functional exercise on fitness and QoL in cancer survivors. clinicaltrials.gov/ct2/show/NCT02889276 (first registered 5 September 2016).
NCT03036436 {published data only}
- NCT03036436. The IMPETUS cancer trial. A technology delivered physical activity intervention in cancer. clinicaltrials.gov/ct2/show/NCT03036436 (first registered 30 Jan 2017).
NCT03082495 {published data only}
- NCT03082495. Exercise during and after neoadjuvant rectal cancer treatment. clinicaltrials.gov/ct2/show/NCT03082495 (first registered 17 March 2017).
NCT03120104 {published data only}
- NCT03120104. Physical exercise for colorectal cancer patients after transanal total mesorectal excision. clinicaltrials.gov/ct2/show/NCT03120104 (first registered 19 April 2017).
NCT03232814 {published data only}
- NCT03232814. Walk on: a community-based approach to increase physical activity among men treated for colorectal cancer. clinicaltrials.gov/ct2/show/NCT03232814 (first registered 28 July 2017).
NCT03630354 {published data only}
- NCT03630354. Exercising together for couples coping with cancer. clinicaltrials.gov/ct2/show/NCT03630354 (first registered 14 August 2018).
NTR6383 {published data only}
- NTR6383. The effect of pelvic floor muscle training on bowel symptoms after low anterior resection for rectal cancer. www.trialregister.nl/trial/6227 (first registered 23 Jan 2017).
Oh 2008 {published data only}
- Oh B, Butow P, Mullan B, Clarke S. Medical qigong for cancer patients: Pilot study of impact on quality of life, side effects of treatment and inflammation. American Journal of Chinese Medicine 2008;36(3):459-72. [DOI] [PubMed] [Google Scholar]
Onerup 2017 {published data only}
- Onerup A, Angenete E, Bock D, Börjesson M, Olsén MF, Gillheimer EG, et al. The effect of pre- and post-operative physical activity on recovery after colorectal cancer surgery (PHYSSURG-C): study protocol for a randomised controlled trial. Trials 2017;18:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onerup A, Thorn SE, Angenete E, Bock D, Gryback-Gillheimer E, Haglind E, et al. Physical activity before and after colorectal surgery and its effect on IGF-1, IGFBP-3 and HbA1c: The PHYSSURG-C randomized controlled trial. Colorectal Disease 2017;19(Suppl 2):108.
- Onerup A, Thorn SE, Angenete E, Bock D, Gryback-Gillheimer E, Haglind E, et al. The effect of pre-and postoperative physical activity on recovery after colorectal cancer surgery (PHYSSURG-C): A randomized controlled trial. Colorectal Disease 2017;19(Suppl 2):141. [DOI] [PMC free article] [PubMed]
Phipps 2018 {published data only}
- Phipps AI, Shi Q, Zemla TJ, Dotan E, Gill S, Goldberg RM, et al. Physical activity and outcomes in patients with stage III colon cancer: a correlative analysis of phase III trial NCCTG N0147 (Alliance). Cancer Epidemiology Biomarkers and Prevention 2018;27(6):696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piringer 2013 {published data only}
- Piringer G, Fridrik M, Fridrik A, Zabernigg A, Greil R, Eisterer W, et al. Endurance exercise to reduce the recurrence rate after adjuvant chemotherapy for colorectal cancer. Onkologie 2013;36:69-70. [Google Scholar]
Piringer 2018 {published data only}
- Piringer G, Fridrik M, Fridrik A, Leiherer A, Zabernigg A, Greil R, et al. A prospective, multicenter pilot study to investigate the feasibility and safety of a 1-year controlled exercise training after adjuvant chemotherapy in colorectal cancer patients. Supportive Care in Cancer 2018;26(4):1345-52. [DOI] [PubMed] [Google Scholar]
Ratjen 2018 {published data only}
- Ratjen I, Schafmayer C, Enderle J, di Giuseppe R, Waniek S, Koch M, et al. Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Cancer 2018;18(1156):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2018 {published data only}
- Ray AD, Twarozek AM, Williams BT, Erwin DO, Underwood W, Mahoney MC. Exercise in African American and white colorectal cancer survivors: a mixed-methods approach. Rehabilitation Oncology 2018;36(4):188-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandler 2017 {published data only}
- Sandler C, Goldstein D, Horsfield S, Bennett BK, Friedlander M, Bastick PA, et al. TOPS: a randomised controlled trial of a multidisciplinary intervention for post-cancer fatigue. Journal of Clinical Oncology 2015;33(15 Suppl 1):9571. [Google Scholar]
- Sandler CX, Goldstein D, Horsfield S, Bennett BK, Friedlander M, Bastick PA, et al. Randomized evaluation of cognitive-behavioral therapy and graded exercise therapy for post-cancer fatigue. Journal of Pain and Symptom Management 2017;54(1):74-84. [DOI] [PubMed] [Google Scholar]
Sellar 2014 {published data only}
- Sellar CM, Bell GJ, Haennel RG, Au HJ, Chua N, Courneya KS. Feasibility and efficacy of a 12-week supervised exercise intervention for colorectal cancer survivors. Applied Physiology Nutrition and Metabolism 2014;39(6):715-23. [DOI] [PubMed] [Google Scholar]
Sohl 2016 {published data only}
- Sohl S, Danhauer S, Birdee G, Nicklas B, Yacoub G, Aklilu M, et al. A brief yoga intervention implemented during chemotherapy for colorectal cancer: a randomized controlled pilot study. Psycho-Oncology 2015;24:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl SJ, Danhauer SC, Birdee GS, Nicklas BJ, Yacoub G, Aklilu M, et al. A brief yoga intervention implemented during chemotherapy: a randomized controlled pilot study. Complementary Therapies in Medicine 2016;25:139-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprod 2015 {published data only}
- Sprod LK, Fernandez ID, Janelsins MC, Peppone LJ, Atkins JN, Giguere J, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. Journal of Geriatric Oncology 2015;6(1):8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ungar 2016 {published data only}
- Ungar N, Sieverding M, Weidner G, Ulrich CM, Wiskemann J. A self-regulation-based intervention to increase physical activity in cancer patients. Psychology, Health and Medicine 2016;21(2):163-75. [DOI] [PubMed] [Google Scholar]
van Rooijen 2018 {published data only}
- Rooijen SJ, Engelen MA, Scheede-Bergdahl C, Carli F, Roumen RM, Slooter GD, et al. Systematic review of exercise training in colorectal cancer patients during treatment. Scandinavian Journal of Medicine and Science in Sports 2018;28(2):360-70. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation versus conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Disease 2012;14(8):1009-13. [DOI] [PubMed] [Google Scholar]
West 2015 {published data only}
- West MA, Loughney L, Jack S, Grocott MP, Kemp GJ. Exercise training following neoadjuvant chemoradiotherapy in rectal cancer improves mitochondrial function - a randomized controlled trial. British Journal of Surgery 2015;102:2-2. [Google Scholar]
Zopf 2018 {published data only}
- Zopf EM, Schulz H, Poeschko J, Schroder K, Bloch W, Baumann FT. Effects of supervised aerobic exercise on quality of life and fatigue in colorectal cancer patients undergoing adjuvant chemotherapy. Asia-Pacific Journal of Clinical Oncology 2018;14(Suppl 7):143. [Google Scholar]
References to studies awaiting assessment
De Backer 2007 {published data only}
- De Backer IC, Van Breda E, Vreugdenhil A, Nijziel MR, Kester AD, Schep G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncologica 2007;46(8):1143-51. [DOI] [PubMed] [Google Scholar]
Golsteijn 2017 {published data only}
- Golsteijn RH, Bolman C, Peels DA, De Vries H, Lechner L. Motivating (former) cancer patients to increase their physical activity: the computer tailored oncoactive + project. Psycho-Oncology 2013;22(10):159. [Google Scholar]
- Golsteijn RH, Bolman C, Peels DA, Volders E, Vries H, Lechner L, et al. A web-based and print-based computer-tailored physical activity intervention for prostate and colorectal cancer survivors: a comparison of user characteristics and intervention use. Journal of Medical Internet Research 2017;19(8):1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteijn RH, Bolman C, Volders E, Peels D, Vries H, Lechner L. Development of an e-health physical activity intervention for prostate and colorectal cancer survivors: the oncoactive intervention. Psycho-Oncology 2014;23:258. [Google Scholar]
- Golsteijn RH, Bolman C, Volders E, Peels DA, Vries H, Lechner L. Development of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: OncoActive. BMC Cancer 2017;17(446):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2013 {published data only}
- Ho JWC, Lee AM, Macfarlane DJ, Fong DY, Leung S, Cerin E, et al. Study protocol for "Moving Bright, Eating Smart"- A phase 2 clinical trial on the acceptability and feasibility of a diet and physical activity intervention to prevent recurrence in colorectal cancer survivors. BMC Public Health 2013;13(487):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01708824. Diet and Physical Activity Intervention in CRC Survivors. clinicaltrials.gov/ct2/show/NCT01708824 (first registered 17 October 2012).
Houborg 2006 {published data only}
- Houborg K, Jensen M, Rasmussen P, Gandrup P, Schroll M, Laurberg S. Postoperative physical training following colorectal surgery: a randomised, placebo-controlled study. Scandinavian Journal of Surgery 2006;95(1):17-22. [DOI] [PubMed] [Google Scholar]
- Houborg KB, Jensen MB, Hessov I, Laurberg S. Little effect of physical training on body composition and nutritional intake following colorectal surgery-a randomised placebo-controlled trial. European Journal Clinical Nutrition 2005;59(8):969-77. [DOI] [PubMed] [Google Scholar]
ISRCTN07465566 {published data only}
- ISRCTN07465566. A pilot study to test the effects of physical activity consultations on the physical activity levels and other health outcomes of people living with colorectal cancer and their partners. www.isrctn.com/ISRCTN07465566 (first registered 13 Dec 2011).
Jacobsen 2013 {published data only}
- Jacobsen PB, Phillips KM, Jim HS, Small BJ, Faul LA, Meade CD, et al. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psycho-Oncology 2013;22(6):1229-35. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee MK, Kim NK, Jeon JY. Effect of the 6-week home-based exercise program on physical activity level and physical fitness in colorectal cancer survivors: a randomized controlled pilot study. PloS One 2018;13(4):e0196220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxwell‐Smith 2019 {published data only}
- Maxwell-Smith C, Hince D, Cohen PA, Bulsara MK, Boyle T, Platell C, et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psycho-Oncology 2019;28(7):1420-9. [DOI] [PubMed] [Google Scholar]
Moller 2015 {published data only}
- Moller T, Lillelund C, Andersen C, Bloomquist K, Bang Christensen K, Ejlertsen B, et al. Use of exercise in patients with breast cancer. Supportive Care in Cancer 2015;1(Meeting Abstracts). [Google Scholar]
- Moller T, Lillelund C, Andersen C, Ejlertsen B, Norgaard L, Christensen KB, et al. At cancer diagnosis: a 'window of opportunity' for behavioural change towards physical activity. A randomised feasibility study in patients with colon and breast cancer. BMJ Open 2013;3(11):e003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02403024 {published data only}
- NCT02403024. Feasibility and efficacy of interval walking in patients with colorectal cancer. clinicaltrials.gov/ct2/show/NCT02403024 (first registered 31 March 2015).
NCT02564835 {published data only}
- NCT02564835. Effects of yoga on cognitive and immune function in colorectal cancer. clinicaltrials.gov/ct2/show/NCT02564835 (first registered 1 Oct 2015).
Park 2015 {published data only}
- Park JH, Lee J, Oh M, Park H, Chae J, Kim DI, et al. The effect of oncologists' exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer 2015;121(16):2740-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2002 {published data only}
- Schulz T, Lotzerich H, Niemeier B, Peters C, Michna H. Effects of moderate exercise training on immunological and psychological parameters in the rehabilitation of colon cancer patients. Journal of Cancer Research and Clinical Oncology 2002;128(Suppl 1):158. [Google Scholar]
Schwartz 2009 {published data only}
- Schwartz AL, Winters-Stone K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Physician and Sports Medicine 2009;37(3):62-7. [DOI] [PubMed] [Google Scholar]
Shun 2016 {published data only}
- Shun SC, Lin BR, Jin-Tung Liang JT, Yun-Jen Chou YJ. Effects of individual exercise education on decreasing fatigue and psychological distress in patients in with colorectal cancer after surgery: a clinical randomized control trial. Supportive Care in Cancer 2016;24(1 Suppl 1):S78. [Google Scholar]
References to ongoing studies
ChiCTRIOR17012037 {published data only}
- ChiCTRIOR17012037. Effects of resistance training and aerobic exercise on functional recovery and quality of life of patients with colorectal cancer after surgery. www.chictr.org.cn/showproj.aspx?proj=20476 (first received 18 July 2017).
NCT02191969 {published data only}
- NCT02191969. Physical activity intervention for older patients during chemotherapy for colorectal cancer. clinicaltrials.gov/ct2/show/NCT02191969 (first received 16 July 2014).
NCT02538913 {published data only}
- NCT02538913. Exercise training for rectal cancer patients. A randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02538913 (first received 2 September 2015).
NCT03064308 {published data only}
- NCT03064308. The assessment of the feasibility of a home based exercise programme in the older patient following major surgery. clinicaltrials.gov/ct2/show/NCT03064308 (first received 27 Feb 2017).
NCT03210129 {published data only}
- NCT03210129. Motivational interviewing to increase physical activity behaviour in cancer patients. clinicaltrials.gov/ct2/show/NCT03210129 (first received 6 July 2017).
NCT03212079 {published data only}
- NCT03212079. Physical activities by technology help (PATH). clinicaltrials.gov/ct2/show/NCT03212079 (first received 11 July 2017).
NCT03252821 {published data only}
- NCT03252821. Effects of high-intensity training compared to resistance training in cancer patients undergoing radiotherapy. clinicaltrials.gov/ct2/show/NCT03252821 (first received 17 August 2017).
NCT03885817 {published data only}
- NCT03885817. Physically active during cancer treatment (FAKT). clinicaltrials.gov/ct2/show/NCT03885817 (first received 22 March 2019).
Piringer 2017 {published data only}
- Annual meeting of the Austrian society of haematology and medical oncology. Phase III randomized trial of endurance exercise following adjuvant chemotherapy for colorectal cancer "aBCSG C08-II trial". Annual Meeting of the Austrian Society of Haematology and Medical Oncology; 2017 Memo - Magazine of European Medical Oncology; Austria 2017;10(2 Suppl 1):45. [Google Scholar]
Additional references
ACSM 2009
- American College of Sports Medicine (ACSM). ACSM's Guidelines for Graded Exercise Testing and Prescription. 8th edition. Philadelphia: Lippincott Williams & Wilkins, 2009. [Google Scholar]
ACSM 2014
- American College of Sports Medicine (ACSM). ACSM's Guidelines for Graded Exercise Testing and Prescription. 9th edition. Philadelphia: Lippincott Williams & Wilkins, 2014. [Google Scholar]
Al‐Majid 2009
- Al-Majid S, Gray P. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biological Research for Nursing 2009;10(4):381-91. [DOI: 10.1177/1099800408324431] [DOI] [PubMed] [Google Scholar]
Allemani 2015
- Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population based registries in 67 countries (CONCORD-2). Lancet 2015;385(9972):977-1010. [DOI: 10.1016/S0140-6736(14)62038-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allgayer 2004a
- Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detection and Prevention 2004;28:208-13. [DOI: 10.1016/j.cdp.2004.02.001] [DOI] [PubMed] [Google Scholar]
Allgayer 2008
- Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scandinavian Journal of Gastroenterology 2008;43(8):971-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Arnold 2017
- Arnold M, Sierra MS, Laversanne M, Soerjomateram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [DOI: 10.1136/gutjnl-2015-310912] [DOI] [PubMed] [Google Scholar]
Baker 2005
- Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer 2005;104(S11):2565-76. [DOI: 10.1002/cncr.21488] [DOI] [PubMed] [Google Scholar]
BASES 2011
- British Association of Sport and Exercise Science (BASES). The BASES expert statement on exercise and cancer survivorship. www.bases.org.uk/BASES-Expert-statements (accessed 15 June 2017).
Birgisson 2005
- Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. Journal of Clinical Oncology 2005;23(25):6126-31. [DOI: 10.1200/JCO.2005.02.543] [DOI] [PubMed] [Google Scholar]
Bower 2014
- Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology 2014;11(10):597-609. [DOI: 10.1038/nrclinonc.2014.127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise and physical fitness: definitions and distinctions for health related research. Public Health Reports 1985;100(2):126-31. [DOI: 10.2307/20056429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 1982
- Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. British Journal of Surgery 1982;69(7):417-9. [DOI: 10.1002/bjs.1800690721] [DOI] [PubMed] [Google Scholar]
Coleman 2011
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Courneya 2007
- Courneya KS, Freidenreich CM. Physical activity and cancer control. Seminars in Nursing Oncology 2007;23(4):242-52. [DOI: 10.1016/j.soncn.2007.08.002] [DOI] [PubMed] [Google Scholar]
Covidence 2018 [Computer program]
- Covidence. Version accessed 16 January 2018. Melbourne, Australia: Veritas Health Innovation, 2018.Available at covidence.org.
Cramer 2014a
- Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. Journal of the American College of Cardiology 2014;64(13):1310-19. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cramer 2014b
- Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. European Journal of Cancer Care 2014;23:3-14. [DOI: 10.1111/ecc.12093] [DOI] [PubMed] [Google Scholar]
Cramp 2012
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curt 2000
- Curt GA, Breibart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. The Oncologist 2000;5:353-60. [DOI: 10.1634/theoncologist.5-5-353] [DOI] [PubMed] [Google Scholar]
Custers 2016
- Custers JA, Gielissen MF, Janssen SH, Wilt JH, Prins JB. Fear of cancer recurrence in colorectal cancer survivors. Support Cancer Care 2016;24:555-62. [DOI: 10.1007/s00520-015-2808-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Denlinger 2009
- Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. Journal of the National Comprehensive Cancer Network 2009;7(8):883-94. [DOI: 10.6004/jnccn.2009.0058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Denlinger 2011
- Denlinger C, Engstrom P. Colorectal cancer survivorship: movement matters. Cancer Prevention Research 2011;4(4):502-11. [DOI: 10.1158/1940-6207] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Devin 2016a
- Devin J, Sax A, Hughes G, Jenkins D, Aitken J, Chambers S, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised control trial. Journal of Cancer Survivorship 2016;10(3):467-79. [DOI: 10.1007/s11764-015-0490-7] [DOI] [PubMed] [Google Scholar]
El‐Shami 2015
- El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Champman ML, et al. American Cancer Society colorectal cancer survivorship care guidelines. A Cancer Journal for Clinicians 2015;65(6):427-55. [DOI: 10.3322/caac.21286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote 2016 [Computer program]
- Endnote. Version X8. Philadelphia: Clarivate Analytics, 2016.Available at endnote.com.
Fairey 2003
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiology Biomarkers & Prevention 2003;12(8):721-7. [PubMed] [Google Scholar]
Furmaniak 2016
- Furmaniak A, Menig M, Markes M. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD005001.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galvao 2005
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. Journal of Clinical Oncology 2005;23(4):899-909. [DOI: 10.1200/JCO.2005.06.085] [DOI] [PubMed] [Google Scholar]
Giovannucci 2000
- Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiology, Biomarkers and Prevention 2000;9(4):345-9. [PubMed] [Google Scholar]
GLOBOCAN 2018
- International Agency for Research on Cancer. Estimated age-standardized incidence and mortality rates (World) in 2018, worldwide, both sexes, all ages. gco.iarc.fr (accessed 21 December 2018).
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;7454:1490-4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Green 2002
- Green RJ, Metlay JP, Propert K, Catalano PJ, MacDonald JS, Mayor RJ, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of intergroup 0089. Annals of Internal Medicine 2002;136(4):261-9. [DOI: 10.7326/0003-4819-136-4-200202190-00005] [DOI] [PubMed] [Google Scholar]
Haggar 2009
- Haggar FA, Boushery RP. Colorectal cancer epidemiology: incidence,mortality, survival, and risk factors. Clinics in Colon and Rectal Surgery 2009;22(4):191-7. [DOI: 10.1055/s-0029-1242458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haydon 2006
- Haydon A, MacInnis R, English D, Giles G. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 2006;55:62-7. [DOI: 10.1136/gut.2005.068189] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343(7829):d5928. [DOI: 10.1136/bmj.d5928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hong 2014
- Hong SK, Kim E-J, Chung SS, Kim KH, Lee R-A. Psychological attitude to self-appraisal of stoma patients: prospective observation of stoma duration effect to self appraisal. Annals of Surgical Treatment and Research 2014;86(3):152-60. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howlader 2016
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF. SEER Cancer Statistics Review, 1975-2013. Bethesda (MD): National Cancer Institute 2016.
Hursting 2010
- Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. Journal of Clinical Oncology 2010;28(26):4058-65. [DOI: 10.1200/JCO.2010.27.9935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knols 2005
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology 2005;23(16):3830-42. [DOI: 10.1200/JCO.2005.02.148] [DOI] [PubMed] [Google Scholar]
Labianca 2010
- Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant therapy and follow-up. Annals of Oncology 2010;21(5):70-7. [DOI: 10.1093/annonc/mdq168] [DOI] [PubMed] [Google Scholar]
Lassen 2009
- Lassen K, Soop M, Nygren J, Cox BW, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery. Enhanced recovery after surgery (ERAS) group recommendations. Archives of Surgery 2009;144(10):961-9. [DOI: 10.1001/archsurg.2009.170] [DOI] [PubMed] [Google Scholar]
Leitzmann 2015
- Leitzmann M, Powers H, Anderson A, Scoccianti C, Berrino F, Boutron-Ruault M, et al. European Code against Cancer 4th edition: physical activity and cancer. Cancer Epidemiology 2015;39S:46-55. [DOI: 10.1016/j.canep.2015.03.009] [DOI] [PubMed] [Google Scholar]
Lin 2009
- Lin J, Brimmer D, Maloney E, Nyarko E, Belue R, Reeves WC. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Population Health Metrics 2009;7(18):1-12. [DOI: 10.1186/1478-7954-7-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2008
- Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. Journal of Clinical Oncology 2008;26(27):4480-7. [DOI: 10.1200/JCO.2007.15.7917] [DOI] [PubMed] [Google Scholar]
Lynch 2010
- Lynch BM, Owen N, Hawkes AL, Aitken JF. Perceived barriers to physical activity for colorectal cancer survivors. Support Cancer Care 2010;18:729-34. [DOI: 10.1007/s00520-009-0705-4] [DOI] [PubMed] [Google Scholar]
Markle 2010
- Markle B, May EJ, Majumdar APN. Do nutraceutics play a role in the prevention and treatment of colorectal cancer? Cancer Metastasis Reviews 2010;29:395-404. [DOI: 10.1007/s10555-010-9234-3] [DOI] [PubMed] [Google Scholar]
Meyerhardt 2006
- Meyerhardt J, Heseltine D, Neidzwiechi D, Hollis D, Saltz L, Mayer R, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. Journal of Clinical Oncology 2006;24(22):3535-41. [DOI: 10.1200/JCO.2006.06.0863] [DOI] [PubMed] [Google Scholar]
Meyerhardt 2009
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W. Physical activity and survival in male colorectal cancer survivors. Archives of Internal Medicine 2009;169(22):2102-08. [DOI: 10.1001/archinternmed.2009.412.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012a
- Mishra SI, Scherer RW, Synder C, Geige PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD008465.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012b
- Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007566.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009;339(b2535):1-6. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosher 2016
- Mosher CE, Winger JG, Given BA, Helft PR, O'Neill BH. Mental health outcomes during colorectal cancer survivorship: a review of the literature. Psycho-Oncology 2016;25:1261-70. [DOI: 10.1002/pon.3954] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2016
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: cancer related fatigue. www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (accessed 13 February 2017).
Ouakrim 2015
- Ouakrim DA, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri E, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 2015;351(h4970):1-10. [DOI: 10.1136/bmj.h4970] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peel 2009
- Peel B, Sui X, Matthews C, Adams S, Hebert J, Hardin J, et al. Cardiorespiratory fitness and digestive cancer mortality: findings from the Aerobics Center Longitudinal Study (ACLS). Cancer Epidemiology, Biomarkers and Prevention 2009;18(4):1111-17. [DOI: 10.1158/1055-9965.EPI-08-0846] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pereira 2012
- Pereira MG, Figueiredo AP, Fincham FD. Anxiety, depression, traumatic stress and quality of life in colorectal cancer after different treatments: a study with Portuguese patients and their partners. European Journal of Nursing Oncology 2012;16(3):227-32. [DOI: 10.1016/j.ejon.2011.06.006] [DOI] [PubMed] [Google Scholar]
Prado 2008
- Prado CM, Leiffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncology 2008;9:629-35. [DOI: 10.1016/S1470-2045(08)70153-0] [DOI] [PubMed] [Google Scholar]
Quadrilatero 2003
- Quadrilatero J, Hoffman-Geotz L. Physical activity and colon cancer: a systemic review of potential mechanisms. Journal of Sports Medicine and Physical Fitness 2003;43(2):121-38. [PubMed] [Google Scholar]
Ramsey 2002
- Ramsey SD, Berry K, Moinpour C, Giedzinska A, Anderson MR. Quality of life in long term survivors of colorectal cancer. American Journal of Gastroenterology 2002;97(5):1228-34. [DOI: 10.1111/j.1572-0241.2002.05694.x] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robsahm 2013
- Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical sub sites: a systematic review and meta-analysis of cohort studies. European Journal of Cancer Prevention 2013;22(6):492-505. [DOI: 10.1097/CEJ.0b013e328360f434] [DOI] [PubMed] [Google Scholar]
Rock 2012
- Rock CL, Doyle C, Demrk-Wahnefried W, Meyerhardt J, Courneya K, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians 2012;62:242-74. [DOI: 10.3322/caac.21142] [DOI] [PubMed] [Google Scholar]
Ryerson 2016
- Ryerson AB, Eherman CR, Alterkruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312-37. [DOI: 10.1002/cncr.29936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samad 2005
- Samad AK, Taylor RS, Marshall T, Chapman MAS. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Disease 2005;7(3):204-13. [DOI: 10.1111/j.1463-1318.2005.00747.x] [DOI] [PubMed] [Google Scholar]
Schmid 2015
- Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Annals of Oncology 2015;26(2):272-8. [DOI: 10.1093/annonc/mdu250] [DOI] [PubMed] [Google Scholar]
Schmitz 2005
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention 2005;14(7):1588-95. [DOI: 10.1158/1055-9965.EPI-04-0703] [DOI] [PubMed] [Google Scholar]
Schmitz 2010
- Schmitz KH, Courneya K, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise 2010;42(7):1409-26. [DOI: 10.1249/MSS.0b013e3181e0c112] [DOI] [PubMed] [Google Scholar]
Schneider 2007
- Schneider EC, Malin JL, Kahn KL, Ko CY, Adam J, Epstein AM. Surviving colorectal cancer: patient-reported symptoms 4 years after diagnosis. Cancer 2007;110:2075-82. [DOI: 10.1002/cncr.23021] [DOI] [PubMed] [Google Scholar]
Schroeder 1991
- Schroeder D, Hill DL. Postoperative fatigue: a prospective physiological study of patients undergoing major abdominal surgery. Australian and New Zealand Journal of Surgery 1991;61(10):774-9. [DOI: 10.1111/j.1445-2197.1991.tb00149.x] [DOI] [PubMed] [Google Scholar]
Schumacher 2017
- Schumacher A. Validation of the six-minute walk test for predicting peak oxygen consumption cancer survivors [Master of Science]. Colorado (USA): School of Sport and Exercise Science, 2017. [Google Scholar]
Slattery 2003
- Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R, et al. Physical activity and colorectal cancer. American Journal of Epidemiology 2003;158(3):214-24. [DOI: 10.1093/aje/kwg134] [DOI] [PubMed] [Google Scholar]
Speck 2010
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship 2010;4:87-100. [DOI: 10.1007/s11764-009-0110-5] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Stewart 2014
- Stewart BW, Wild CP, editor(s). World Cancer Report. Vol. 3. Lyon: International Agency for Research on Cancer, 2014. [Google Scholar]
Stone 2008
- Stone PC, Minto O. Cancer-related fatigue. European Journal of Cancer 2008;44(8):1097-104. [DOI: 10.1016/j.ejca.2008.02.037] [DOI] [PubMed] [Google Scholar]
Sullivan 2011
- Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropal NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncology 2011;12(10):933-80. [DOI: 10.1016/S1470-204] [DOI] [PubMed] [Google Scholar]
Thomas 2014
- Thomas RJ, Holm H, Al-Adhami A. Physical activity after cancer: an evidence review of the international literature. British Journal of Medical Practitioners 2014;7(1):16-22. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(16):1-16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vassbakk‐Brovold 2016
- Vassbakk-Brovold K, Kersten C, Fergan L, Mjaland O, Mjaland S, Seiler S, et al. Cancer patients participating in a lifestyle intervention during chemotherapy greatly over-report their physical activity level: a validation study. BMC Sports Science Medicine and Rehabilitation 2016;8(10):1-9. [DOI: 10.1186/s13102-016-0035-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2004
- Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. British Journal of Cancer 2004;91:822-8. [DOI: 10.1038/sj.bjc.6602012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017
- Wang N, Khankari NK, Cai H, Li H, Yang G, Gao Y. Prediagnosis body mass index and waist–hip circumference ratio in association with colorectal cancer survival. International Journal of Cancer 2017;140:292-301. [DOI: 10.1002/ijc.30459] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2014a
- West MA, Loughney L, Barben CP, Sripadam R, Kemp GJ, Grocott MP, et al. The effects of neoadjuvant chemo radiotherapy on physical fitness and morbidity in rectal cancer surgery patients. European Journal of Surgical Oncology 2014;40(11):1421-8. [DOI: 10.1016/j.ejso.2014.03.021] [DOI] [PubMed] [Google Scholar]
West 2014b
- West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S, et al. Cardiopulmonary exercise variables are associated with post operative morbidity after major colonic surgery: a prospective blinded observational study. British Journal of Anaesthesia 2014;112(4):665-71. [DOI: 10.1093/bja/aet408] [DOI] [PubMed] [Google Scholar]
West 2014c
- West MA, Parry MG, Lythgoe D, Barben CP, Kemp GJ, Grocott MP, et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. British Journal of Surgery 2014;101(9):1166-72. [DOI: 10.1002/bjs.9551] [DOI] [PubMed] [Google Scholar]
Wolin 2009
- Wolin KY, Yan Y, Colditz GA, Lee I-M. Physical activity and colon cancer prevention: a meta-analysis. British Journal of Cancer 2009;100:611-16. [DOI: 10.1038/sj.bjc.6604917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yost 2008
- Yost KJ, Hahn EA, Zaslavsky AM, Ayanian JZ, West DW. Predictors of health-related quality of life in patients with colorectal cancer. Health and Quality of Life Outcomes 2008;6(1):66. [DOI: 10.1186/1477-7525-6-66] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
McGettigan 2017
- McGettigan M, Cardwell C, Cantwell MM, Tully MA. Physical activity and exercise interventions for disease‐related physical and mental health during and following treatment in people with non‐advanced colorectal cancer. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012864] [DOI] [PMC free article] [PubMed] [Google Scholar]


