Dear Editor
During the current nascent pandemic, anosmia has been increasingly reported among patients with coronavirus disease-2019 (COVID-19) (1). While postviral olfactory loss secondary to nasal congestion or conductive pathway alteration is a known sequela of sinonasal viral infections (2), anosmia of COVID-19 is less commonly associated with rhinorrhea or nasal congestion (3). This may indicate sensory neural loss as the underlying cause of the olfactory dysfunction rather than the conductive mechanism in most cases of postviral olfactory loss.
We have recently reported normal morphology of the olfactory bulb on magnetic resonance imaging in anosmia of COVID-19 (4). Whether there is decreased neural activity in olfactory pathways despite normal morphology is unknown. We sought to assess the neural metabolic activity in anosmia of COVID-19 by 18fluoro-2-deoxy-d-glucose (18FDG) positron emission tomography–computed tomography (PET-CT).
We included a 27-year-old healthy, right-handed woman, diagnosed with COVID-19 by polymerase chain reaction assay. The patient had persistent isolated anosmia for 6 weeks. She had no history of alcohol intake or tobacco smoking and no background of psychiatric problems. The patient was asked to fast for 6 hours prior to imaging. We performed 18FDG-PET/CT in a neutral environment using 5 ml of aerosolized 0.9% NaCl delivered with O2 at 3.5 ml/min via facial mask for 9 minutes. The patient was instructed to breath normally without sniffing. After 3 minutes, the patient received intravenous 18FDG (4.6 Megabecquerel/kg, Masih Daneshvari Hospital, Tehran), and the neutral olfactory condition continued for another 6 minutes. The patient laid down in a semi-darkened, noiseless, and odorless room, with her eyes closed. After an uptake time of 60 minutes, the brain and whole-body PET/CT scan was performed with sequential TOF-PET/CT (Discovery 690 PET/CT with 64 slices, GE Healthcare, USA). The PET tomography consisted of LYSO (Lu1.8Y0.2 SiO5 (Ce)) crystals with dimensions of 4.2 × 6.3 × 25 mm3. Data was acquired in 3D mode with scan duration of 10 minutes for the brain scan and 2 minutes per bed position for the whole-body scan. The images were reviewed by a nuclear medicine physician and a radiologist with substantial experience in PET/CT reporting in consensus.
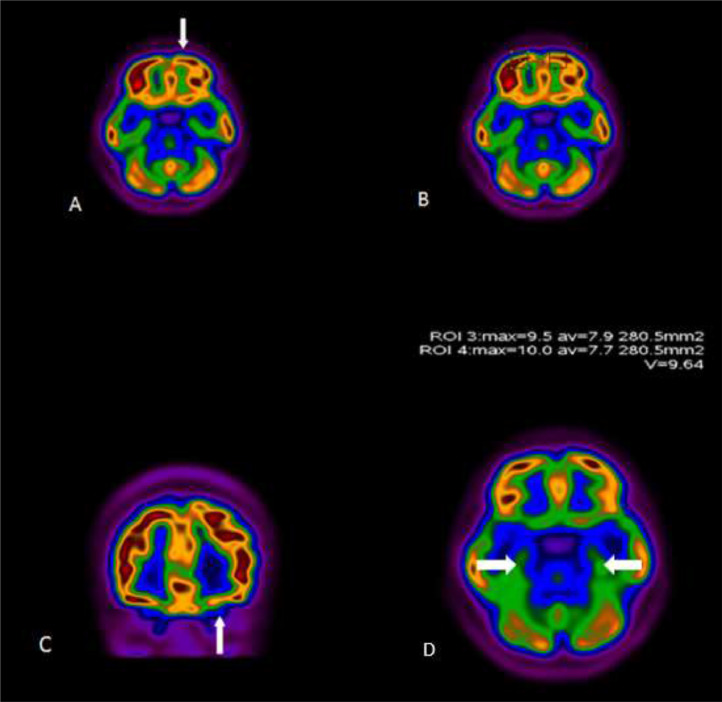
The PET-CT images suggested hypometabolism of the left orbitofrontal cortex (Fig 1 ). The standardized uptake value (SUV) of 9.5 on the left side was reduced compared with the SUV of 10 on the right side. Our finding is in contrast to higher metabolic activity of the left orbitofrontal cortex compared with the right side in right-handed subjects with normal olfaction (5). The FDG uptake in the left inferior temporal cortex was normal (Fig 1).
Figure. 1.
18FDG-PET/CT scan of a 27-year-old woman with persistent anosmia and positive PCR for SARS-CoV-2. Representative axial (A, B, D) and coronal (C) images are shown. There was decreased uptake in the left orbitofrontal cortex (arrows). The uptake of temporal lobes were symmetric and normal (arrows, D).
Olfactory perception is a sophisticated chemosensory process involving different parts of the cerebral cortices, mainly orbitofrontal and limbic system. Peripherally located olfactory dendrites within receptor cells that connect to the central nervous system (ie, olfactory bulb) may provide a route for neuro-invasion in SARS-CoV-2-induced anosmia (6).
There are two separate subsystems for smelling and sniffing resulting in stimulation of different cerebral cortices, and therefore, asymmetric cortical activity. Under olfactory condition (using a pure odor), Cuneus, lingual gyrus, and parahipocampal gyrus have higher glucose metabolic activity mainly in the left hemisphere in right-handed subjects (7, 8, 9). In contrast, under neutral condition (normal saline used in our study), superior, middle, inferior, medial frontal, and orbital gyrus (ie, orbitofrontal cortex) as well as anterior cingulate gyrus have higher metabolic activity on the left-side compared to the right-side in right-handed subjects.
Orbitofrontal cortex is responsible for detection of common odors and on FDG-PET-CT has a lower uptake under olfactory condition than under neutral condition (5). The present study examined the FDG uptake patterns under neutral condition. To elucidate the full spectrum of olfactory abnormalities in anosmia of COVID-19, it is necessary to perform 18FDG-PET-CT under olfactory condition in future studies.
In conclusion, to the best of our knowledge, this is the first report of reduced metabolic activity in the orbitofrontal cortex in anosmia of COVID-19, suggesting impaired neural function in this region as an underlying cause of anosmia, likely due to direct neurotropism of SARS-CoV-2.
Footnotes
Prior Presentations: No
Author contributions: M.K. and A.Y have provided the case and images and M.K, N.R, M.B and S.H. have written the article.
References
- 1.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagheri S.H., Asghari A.M., Farhadi M. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. medRxiv. 2020 doi: 10.34171/mjiri.34.62. https://www.medrxiv.org/content/10.1101/2020.03.23.20041889v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020:1. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galougahi M.K., Ghorbani J., Bakhshayeshkaram M. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micarelli A., Pagani M., Chiaravalloti A. Cortical metabolic arrangement during olfactory processing: Proposal for a 18F FDG PET/CT methodological approach. Medicine. 2014;93:e103. doi: 10.1097/MD.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brann J.H., Firestein S.J. A lifetime of neurogenesis in the olfactory system. Front Neurosci. 2014;8:182. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessandrini M., Micarelli A., Chiaravalloti A. Cortico-subcortical metabolic correlates of olfactory processing in healthy resting subjects. Scientific reports. 2014;4:5146. doi: 10.1038/srep05146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolding K.A., Franks K.M. Recurrent cortical circuits implement concentration-invariant odor coding. Science. 2018;361 doi: 10.1126/science.aat6904. eaat6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litaudon P., Bouillot C., Zimmer L. Activity in the rat olfactory cortex is correlated with behavioral response to odor: a microPET study. Brain Struct Funct. 2017;222:577–586. doi: 10.1007/s00429-016-1235-8. [DOI] [PubMed] [Google Scholar]