The coronavirus disease 2019 (COVID-19) pandemic is, undoubtedly, posing unprecedented challenges on patient care including those without COVID-19 [1]. Given the complexity of cancer care, which is often multidisciplinary requiring immunosuppressive therapy, surgery and radiation, oncologists have to be judicious more than ever to protect patients, caregivers and all healthcare workers [2]. COVID-19 has challenged operations of all hospitals, travel industry, food industry, and closure of academic institutions. It has inflicted a major impact on international economy. Without a specific therapy or vaccine which are still in early clinical trials, quarantine, social distancing, hand washing, testing, contact tracing and isolation seem to be few of the mitigating measures in our battle against COVID-19. In this context, how do we manage patients with cancer? Recently, emirates became the first in the airline industry to offer testing to passengers to open up its flights. In a similar manner, we propose a way forward to manage cancer care in the COVID-19 era with tiered testing of patients with cancer for COVID-19.
Patients with cancer could present several scenarios that include presentation with active COVID-19 infection, as symptomatic or asymptomatic carriers, history of COVID-19, exposed to COVID-19, or unexposed to COVID-19. We need to manage all these situations in the context of cancer therapy.
First, in the absence of an effective vaccine or anti-viral therapy, we should try to prevent patients with cancer from contracting infection. Second, how do we adjust cancer therapy in patients with COVID-19? Evidence is lacking to support changing or withholding chemotherapy or immunotherapy in cancer patients. However, the general expectation is to continue treatments if lifesaving. For non-curative treatments, like maintenance therapy in follicular lymphoma (FL), delaying drug dosing is reasonable. Alongside chemotherapy, steroids are frequently used in cancer management and have been linked to adverse outcomes of COVID-19 [3] arguing for routine testing in patients receiving them. As to immunotherapy, it is not known if their use puts patients at a higher risk for pneumonitis or cytokine storm. Managing COVID-19 and its complications should be prioritized over management of cancer. However, clinical decisions should be individualized to consider factors such as the risk of cancer relapse if treatment is modified, the number of cycles already completed, and the patient's tolerance of therapy. Third, in asymptomatic patients who are carriers of COVID-19 planning ahead could help minimize complication rate of chemotherapy. Cancer directed therapy (e.g. anti-CD20 monoclonal antibody) has been known to reactivate certain viral infections. In these circumstances, delaying or de-intensifying semi urgent chemotherapy should be considered.
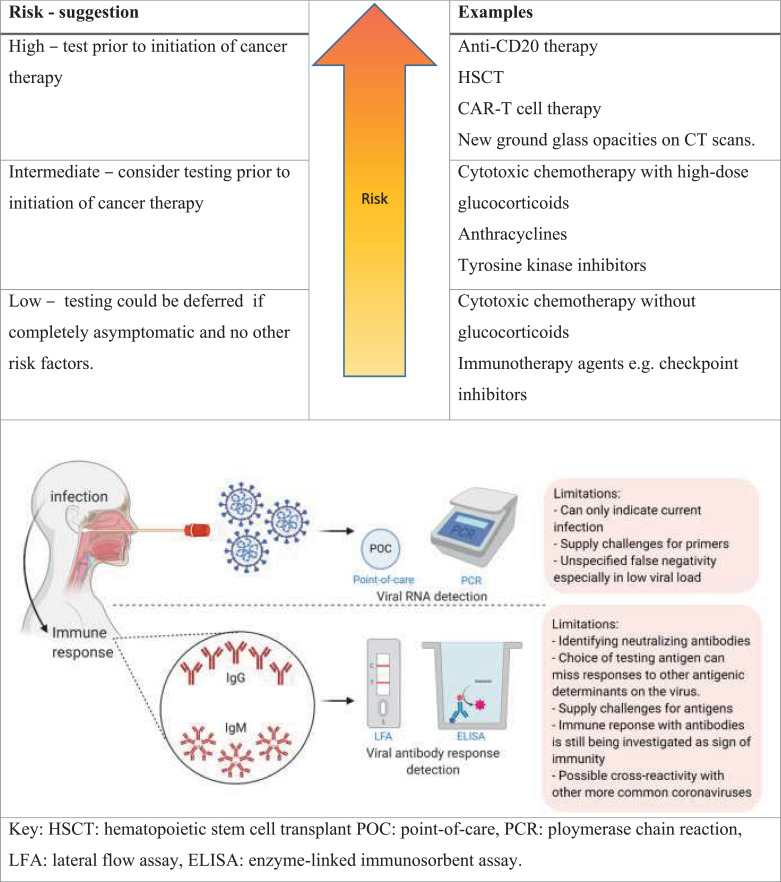
Antibody testing could aid decision of when to proceed with immune compromising chemotherapy especially those with curative intent. A return-to-chemotherapy criteria could be entertained once reliable antibody testing and viral titers are developed (Fig. 1). After development of an effective vaccine, antibody testing may aid in vaccinating those with low titer of protective antibodies. Patients with B cell malignancies such as lymphoma, chronic lymphocytic leukemia, or multiple myeloma will likely have distinct antibody responses and may require a specialized pathway. Finally, patients with a known exposure need to be tested for COVID-19, quarantined and closely followed up.
Fig. 1.
Delaying may not be clinically feasible due to urgency and need for therapy and unknown cycle of the COVID-19 pandemic. Testing all patients with lung cancer for COVID-19 has been suggested [4]. Herein, we offer a tiered model (Fig. 1) based on the presumed risk of immunosuppression with different chemotherapy drug classes to prioritize testing COVID-19 carriers. Early evidence showing activity of convalescent plasma against COVID-19 [5] suggests the role of humoral immunity against COVID-19. Therefore, patients who are carriers and are going to receive anti-CD20 therapy or undergo hematopoietic cell transplantation or CAR-T cell might be at a high risk of progression to fulminant infection. Similarly, regimens containing high-dose glucocorticoids (e.g. lymphoma regimens) may increase risk of viral spread. Another consideration would be to test preoperative patients (e.g. cystectomy patients who have completed neoadjuvant chemotherapy) as precautionary measure intended to reduce risk to both the patient and to the multiple team members who are in close proximity during the surgery.
Several limitations exist facing current panels that include availability, under-estimating rate of false-positivity or false-negativity (Fig. 1). However, technology is evolving at a rapid pace and, hopefully, viral RNA and antibody testing will mature over time and become more available. In summary, cancer patients represent a special situation during this pandemic and a tiered approach to testing could help provide them with life-saving chemotherapy without jeopardizing their chances of benefit.
Declaration of Competing Interest
VS reports Research funding/ Grant support for clinical trials: Roche/ Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint medicines, Loxo oncology, Medimmune, Altum, Dragonfly therapeutics, Takeda and, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning point therapeutics, Boston Pharmaceuticals Travel: Novartis, Pharmamar, ASCO, ESMO, Helsinn, Incyte, Consultancy/ Advisory board: Helsinn, LOXO Oncology/ Eli Lilly, R-Pharma US, INCYTE, QED pharma, Medimmune, Novartis. Other: Medscape. Others report no conflict of interest.
References
- 1.Rosenbaum L. The Untold Toll — The Pandemic's Effects on Patients without Covid-19. Engl J Med. 2020 doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 2.Masumi U., Renato M., Paul C.H. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J Natl Comprehens Cancer Netw J Natl Compr Canc Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Int `Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passaro A., Peters S., Mok T.S.K., Attili I., Mitsudomi T., de Marinis F. Testing for COVID-19 in lung cancer patients. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Diseases. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]