Abstract
A 49-year-old man presented with worsening high-grade fevers, dry cough, and shortness of breath. He tested positive for severe acute respiratory syndrome-coronavirus-2 and was noted to have bradycardia with intermittent high-degree atrioventricular block. However, cardiac biomarkers and echocardiographic findings were normal, thus making this an unusual and interesting manifestation of myocardial involvement of this novel coronavirus. (Level of Difficulty: Beginner.)
Key Words: bradycardia, cardiomyopathy, complication, COVID-19
Abbreviations and Acronyms: ACE 2, angiotensin-converting enzyme 2; AV, atrioventricular; COVID-19, coronavirus disease-2019; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Graphical abstract
A 49-year-old man presented with worsening high-grade fevers, dry cough, and shortness of breath. He tested positive for severe acute respiratory…
History of Presentation
A 49-year-old man presented to the emergency department with acute-onset high-grade fevers accompanied by dry cough and shortness of breath that had been ongoing for a week before presentation. He denied any associated nausea or vomiting, diarrhea, sore throat, congestion, or skin rash. Of note, he had recently returned from a high-prevalence area for coronavirus disease-2019 (COVID-19) within the United States and was in self-quarantine. He was monitoring his symptoms; however, when his shortness of breath was not improving with his asthma medications (albuterol inhaler and cetirizine), he presented to the emergency department. On arrival, he was noted to be febrile at 102.5°F, he was tachypneic to 22 breaths/min, he was normotensive at 125/75 mm Hg, his heart rate was 75 beats/min, and he was saturating 98% oxygen on room air. Physical examination was remarkable for decreased breath sounds bilaterally.
Learning Objectives
-
•
To anticipate and diagnose conduction disturbances associated with the novel coronavirus.
-
•
To understand the mechanism responsible for high-degree AV block associated with COVID-19 without evidence of overt myocarditis.
Past Medical History
His past medical history was significant for mild intermittent asthma.
Differential Diagnosis
Our patient’s clinical presentation was concerning for viral or bacterial lower respiratory tract infection.
Investigations
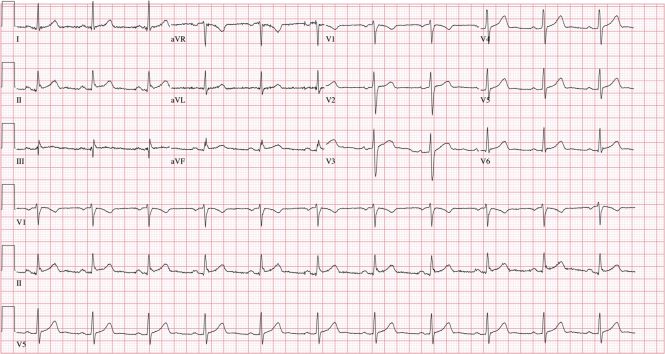
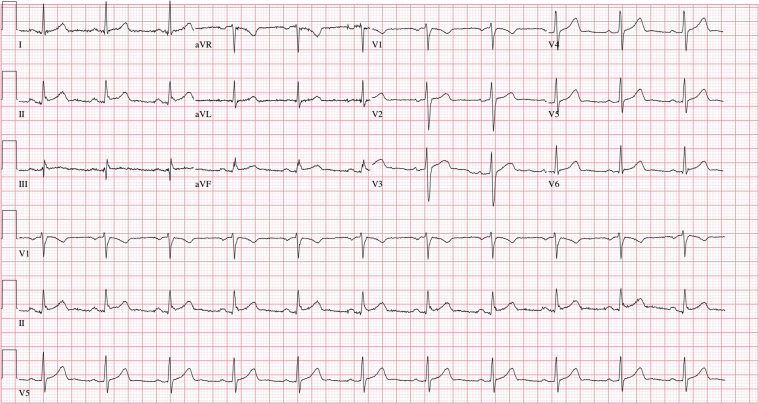
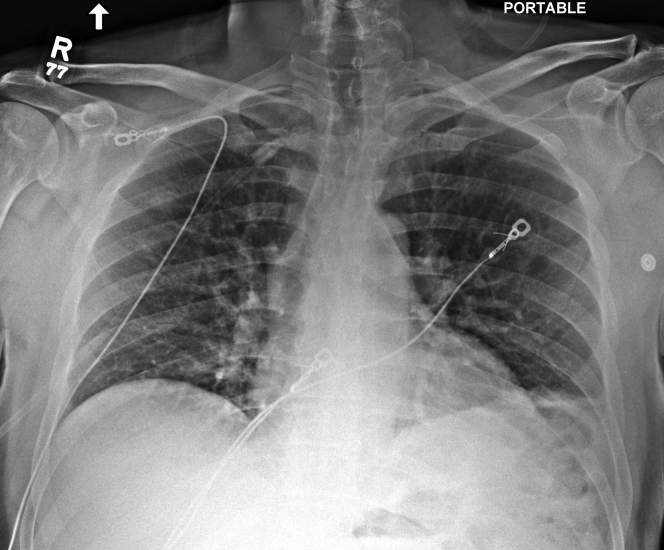
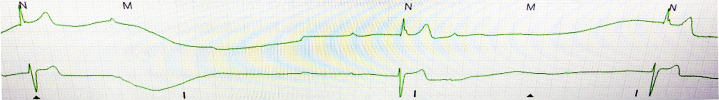
An electrocardiogram revealed normal sinus rhythm with normal PR (172 ms) and QRS (94 ms) intervals (Figure 1). No acute ST-T wave changes were noted. A single-view chest radiograph showed blunted costophrenic angles bilaterally with concern for right middle lobe opacity (Figure 2). Laboratory test results were remarkable for leukopenia to 3,900 cells/μl with lymphopenia (absolute lymphocyte count was reduced at 900 cells/μl). Serum chemistry results, including liver function tests, were normal. Arterial blood gas revealed acute respiratory alkalosis, with pH of 7.55, partial pressure of carbon dioxide of 25 mm Hg, partial pressure of oxygen of 94 mm Hg, and bicarbonate of 22 mmol/l on room air. The results of a nasopharyngeal respiratory panel (adenovirus, non−COVID-19 coronavirus, influenza A and B, human metapneumovirus, parainfluenza virus 1 to 4, respiratory syncytial virus, Bordetella pertussis, Mycoplasma pneumoniae, and Chlamydophila pneumoniae) were negative. Given the high pre-test probability for COVID-19, a nasopharyngeal swab for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) was collected, and the patient was admitted to the medical floor, given the concern for viral or bacterial pneumonia. During his hospital course, he was noted to have evidence of bradycardia with intermittent high-degree AV block and AV dissociation; the ventricular rate was <20 beats/min (telemetry strips in Figures 3 and 4). He was symptomatic with diaphoresis during these episodes but remained hemodynamically stable. Pacemaker pads were placed; however, he did not require transcutaneous pacing. Cardiac biomarker levels, including troponin I (<0.012 ng/ml; normal range 0 to 0.034 ng/ml) and N-terminal pro–B-type natriuretic peptide (38.3 pg/ml; normal range 0 to 125 pg/ml), were normal. A transthoracic echocardiogram showed a normal ejection fraction, normal diastolic function, no wall motion abnormalities, and no significant valvular disease (Videos 1, 2, and 3). Inflammatory markers were mildly elevated; the ferritin level was 571 mg/ml (normal range 0 to 400 ng/ml), and C-reactive protein was elevated at 1.2 mg/dl (normal range 0 to 0.9 mg/dl). The procalcitonin level was negative at 0.039 ng/ml (normal range 0 to 0.080 ng/ml), and thyroid hormone levels were within normal limits. His nasopharyngeal swab tested positive for SARS-CoV-2 ribonucleic acid.
Figure 1.
Initial Electrocardiogram Findings
A 12-lead electrocardiogram on presentation showing normal QRS complex and PR interval.
Figure 2.
Initial Chest Radiograph
Anteroposterior single-view chest radiograph on presentation showing blunted costophrenic angles bilaterally and a right middle lobe opacity.
Figure 3.
Telemetry Strip Showing Bradycardia With High-Degree Atrioventricular Block
Figure 4.
Telemetry Strip Showing High-Degree Atrioventricular Block With Slow Escape Ventricular Rates <20 Beats/Min
Online Video 1.
Echocardiographic Findings
Echocardiogram (parasternal long axis view) showing normal left ventricular size and function.
Online Video 2.
Echocardiographic Findings
Echocardiogram (apical 4-chamber view) showing normal biatrial size and biventricular function.
Online Video 3.
Echocardiographic Findings
Echocardiogram showing preserved longitudinal left ventricular strain at −18.6%.
Management
Given the patient’s underlying asthma, which predisposed him to an increased risk for pulmonary complications, he was started on a course of azithromycin and hydroxychloroquine after consultation with infectious disease doctors.
Follow-Up
He was monitored as an inpatient because of worsening hypoxemia for 5 days. The rest of his hospital course remained uneventful. No further evidence of conduction disease was noted on telemetry, and the QTc interval remained normal while he was receiving hydroxychloroquine. He continued to improve clinically. He was discharged home with a plan to continue self-quarantine, and outpatient follow-up was arranged.
Discussion
SARS-CoV-2 invades cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is abundant in the heart (1). Among 138 patients hospitalized with COVID-19 in Wuhan, China, acute cardiac injury (defined if the serum levels of cardiac biomarkers were above the 99th percentile upper reference limit or new abnormalities were shown on electrocardiography and echocardiography) was diagnosed in 7% patients, and approximately 16.7% patients were noted to have cardiac arrhythmias (2). COVID-19 involvement of the heart has ranged from asymptomatic myocardial injury to acute coronary syndrome, mild to fulminant myocarditis, stress cardiomyopathy, and cardiogenic shock; however, the mechanism of cardiac involvement is not exactly clear (3). Furthermore, underlying cardiovascular disease or risk factors and myocardial injury have been shown to portend poor prognosis in these patients (4). In this case, we present a patient with moderate COVID-19 infection who showed evidence of transient conduction disturbances with high-degree atrioventricular (AV) block. High-degree AV block is known to be an uncommon presentation of acute myocarditis in adults, more commonly seen in cardiac sarcoidosis and giant cell myocarditis (5). However, because our patient did not have any other overt evidence of myocardial involvement, with normal cardiac biomarkers and a normal echocardiogram, his presentation is unusual and interesting. It is possible that COVID-19 may have caused subclinical myocarditis leading to high-degree AV block in this case. ACE2 receptors are abundant in the heart and are present in multiple cell types, including macrophages, endothelial cells, smooth muscle cells, and cardiomyocytes (6). Further, animal models have shown the presence of ACE2 receptors in sinoatrial nodal cells in rats (7), and conduction disturbances and ventricular fibrillation have been noted with overexpression of the ACE2 receptor in experimental mice models (8). Hence, another possibility is that isolated involvement of the AV node and infra-Hisian conduction system by SARS-CoV-2 may have caused transient high-grade AV block. Whether this block is secondary to direct viral involvement or is an autoimmune response is unknown at this time. Our patient did not have a recurrence of these conduction disturbances after he was started on supportive treatment with azithromycin and hydroxychloroquine. This outcome may have reflected recovery from the viral infection or the anti-inflammatory effects of the medications, or both.
Conclusions
We reported an unusual presentation of a young patient without any significant cardiac comorbidities or underlying conduction disease who presented with transient high-degree AV block that was associated with a diagnosis of moderate COVID-19. Conduction disturbances in our patient were likely the result of subclinical myocarditis or isolated involvement of the AV node and infra-Hisian block secondary to infection with this novel coronavirus. We need to maintain a high index of suspicion for cardiovascular complications in these patients. Conduction disturbances were transient in the case presented here. However, with increasing severity of disease, persistent conduction disturbances could require pacing.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkin K.J., Fried J.A., Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 4.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Cooper L.T., Jr., Blauwet L.A. When should high-grade heart block trigger a search for a treatable cardiomyopathy? Circ Arrhythm Electrophysiol. 2011;4:260–261. doi: 10.1161/CIRCEP.111.963249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zisman L.S. ACE and ACE2: a tale of two enzymes. Eur Heart J. 2005;26:322–324. doi: 10.1093/eurheartj/ehi043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira A.J., Moraes P.L., Foureaux G., Andrade A.B., Santos R.A., Almeida A.P. The angiotensin-(1-7)/Mas receptor axis is expressed in sinoatrial node cells of rats. J Histochem Cytochem. 2011;59:761–768. doi: 10.1369/0022155411411712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]