Abstract
Newcastle disease (ND) is prevalent among the domesticated and the wild birds and is caused by the avian paramyxovirus serotype-I (APMV-I). It is commonly known to affect chicken, pheasant, ostrich, pigeon and waterfowl. Depending on the virulence, the velogenic NDV strains cause severe respiratory and nervous disorders with a high mortality rate. The live and killed vaccines are available for the prevention of infection in the market, but the drug for the treatment is not available. Nitazoxanide (NTZ), a member of thiazolides, is an antiparasitic drug. In the present study, the effect of NTZ on the NDV replication was explored. The experiments were conducted in chicken fibroblast cells (DF-1), PBMC, embryonated chicken eggs, and two-week old chickens. The inhibition of the NDV was observed upon post-treatment of NTZ at a concentration of ~12.5 μM. Cytokine profiling of the DF-1, PBMC, and chicken embryonic tissue treated with NTZ revealed significant upregulation in all the cytokines studied except for IL-1β in DF-1 cells. It is plausible that NTZ is involved in causing immune-modulatory effects in poultry. NTZ treatment in two weeks old chicken showed significant reduction in NDV replication in trachea, and lungs, respectively, at 72 h post-infection. Encouraging results from the present study warrants repurposing NTZ as a drug for the treatment of viral infection in poultry. It will also pave the way towards understanding of similar effect against other animal pathogens.
Keywords: In vitro, Nitazoxanide, Avian paramyxovirus, Cytokines, Repurposing
1. Introduction
Newcastle Disease is a panzootic disease which affects the avian species. It is caused by Newcastle disease virus (NDV) belonging to the Avulavirus genus of the family Paramyxoviridae. It is an enveloped virus having linear, non-segmented single-stranded RNA of negative polarity [1]. Based on the genome size, strains of NDV are classified into 3 groups having 15186, 15192, and 15,198 nucleotides [2], [3], [4], [5]. The genome of NDV has six transcriptional units, comprising nucleocapsid protein (N), matrix protein (M), phosphoprotein (P), fusion protein (F), haemagglutinin-neuraminidase protein (HN), and large polymerase protein (L) from 3′ to 5′ direction. The N and P, along with L, forms an RNA polymerase complex for the viral genome replication [6].
Depending on the severity of the infection, NDV strains are classified into lentogenic (less virulent), mesogenic (moderate virulent), and velogenic (high virulent) [7]. Furthermore, based on the tissue tropism, it can be further classified as viscerotropic velogenic, which produces lethal haemorrhagic lesions in the viscera and neurotropic velogenic that cause respiratory and neurological disorders [8]. The clinical signs of the infection vary depending on the virus, host species, the age of the host, infection with other organisms, environmental stress, and immune status. The average incubation time of the virus is 15–21 days and can be transmitted easily, causing significant loss to the poultry industry [9]. It can cause a substantial economic loss by reduced egg production and the death of the ailing birds. There are live, inactivated, and vectored vaccines that are available for the prevention of the disease in poultry [10], [11]. Among these, the traditional live vaccines such as LaSota, B1, and VG/GA strains are commonly used in the endemic areas [12], [13]. However, vaccine failures have been frequently reported due to the emergence of its variant strains [14]. There is no available drug against NDV; the reason why the development of the antiviral drug is of prime importance.
Nitazoxanide (NTZ), also known as 2-(acetyloxy)-N-(5-nitro-2-thiazolyl) benzamide is a commercially available antiprotozoal drug which was initially developed for the cure of cryptosporidiosis and giardiasis [15]. NTZ has also been classified as a broad-spectrum first-class antiviral compound against the respiratory syncytial virus, parainfluenza virus, Sendai virus, coronavirus, rotavirus, and norovirus infection [16], [17], [18], [19], [20]. Additionally, NTZ has been shown to inhibit hepatitis viruses [21], [22], flaviviruses [23], [24], [25], chikungunya virus [26], human immunodeficiency virus [27] and vaccinia virus [28]. Clinical trials are under progress for the use of NTZ against influenza and its subtypes [29], [30]. Several mechanisms of action have been suggested for NTZ mediated viral inhibition. It can curtail influenza virus replication by inhibiting the maturation of hemagglutinin protein [29]. In the Sendai virus, the active metabolite of NTZ acts as a non-competitive inhibitor of ER-resident proteins to misfold the F protein and halts its trafficking towards the plasma membrane [17]. The reduction in the size of viroplasm was observed against rotavirus, which led to a decrease in the formation of dsRNA [31]. As an anti-hepatitis C compound, NTZ was shown to activate protein kinase R (PKR), resulting in the phosphorylation of eukaryotic initiation factor 2α (eIF2-α) [32]. NTZ blocks the replication of bovine viral diarrhoea virus by causing stress in the endoplasmic reticulum leading to depletion of ATP sensitive intracellular Ca2+ stores [33]. However, the effect of NTZ on NDV biology has not been addressed to date. The present study reports the inhibition of NDV upon NTZ treatment on chicken fibroblast cells (DF-1), embryonated chicken eggs, and two-week old chickens.
2. Materials and methods
2.1. Cell culture and compound
The chicken embryo fibroblast cells (DF-1) cells used in the experiments were obtained from ATCC (Manassas, VA, USA). The DF-1 cells were maintained in Dulbecco’s minimum essential medium (DMEM) with 10% fetal bovine serum (FBS) (HiMedia Laboratories, India) at 37 °C with 5% CO2. The stock solution (10 mg/mL) of NTZ (Sigma Aldrich, USA) was made by dissolving it in dimethyl sulfoxide (DMSO). Further dilutions of NTZ for treatment were made in DMEM with 2% FBS, unless differently specified.
2.2. Cytotoxicity assay
The cytotoxicity of the compound was found by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide formazan conversion assay, as described previously [34]. DF-1 cells were seeded in a 96 well plate with a seeding density of 105 cells/well prior to the experiment. Further, the cells were incubated with NTZ (0.3 μM to 100 μM) for 24 h and 48 h. The absorbance was taken at 570 nm using the microplate reader, and the percentage cell viability was calculated. DMSO was used as vehicle control.
2.3. Virus titration and infection
The NDV strain of Bareilly (virulent), previously sequenced in our lab [35], was propagated in 9-day-old specific pathogen free (SPF) embryonated chicken eggs. Recombinant NDV expressing the green fluorescent protein (rNDV-GFP) available in the lab was used to visualize the infection in the cells [36]. The virus titer was calculated by hemagglutination assay (HA) with 1% chicken RBC and by plaque assay. Plaque assay was performed by infecting the DF-1 cells with NDV and incubating it at 37 °C for an hour [37]. The virus containing media was discarded, and an overlay of DMEM containing 0.8% methylcellulose supplemented with 2% FBS was given. It was further incubated for nearly 72 h for the development of plaques. The cells were then fixed and stained with 1% crystal violet solution. The plaque forming units were counted, and the viral titer was calculated. For all the experiments, the virus infection was given at 0.01 MOI.
2.4. Time of addition assay
DF-1 cells were incubated with NTZ concentration of 12.5 μM prior to (pre-treatment), during (co-treatment), or after (post-treatment) virus infection. The NTZ treatment (~12.5 µM) was given 12 h before infection in case of pre-treatment, whereas in co-treatment, the drug was given during the NDV infection for approximately 1 h. In post-treatment, NTZ was given after NDV infection for 12 h, and the media was replaced with DMEM supplemented with 2% FBS. Infection of DF-1 by rNDV-GFP was visualized using a fluorescence microscope at 24 h, 36 h, and 48 h. The infected cell culture supernatant of all the conditions mentioned above was collected every 8 h till 96 h for analyzing the growth kinetics of NDV using TCID50 assay. TCID50 was performed according to the Reed and Muench algorithm [38]. Additionally, the virus was also quantified by plaque assay and compared with the controls.
2.5. Dose-dependent response of NTZ
Depending on the cytotoxicity, varying concentrations of NTZ were used to check the antiviral effect in DF-1 cells (3.125 μM, 6.25 μM, 12.5 μM, and 25 μM). The infection by rNDV-GFP was visualized by fluorescence microscopy at 24 h, 36 h, and 48 h post infection. The total cell lysates were collected using lysis buffer 48 h post infection along with the mock infected and NTZ controls. Protein expression was analyzed with the lysates collected using HN and N specific monoclonal antibodies (a kind gift from Dr. R Iorio, University of Massachusetts, Worcester, MA, USA). The NDV titer post NTZ treatment was quantified using a plaque assay. Additionally, TCID50 assay was performed with the cell culture supernatant collected every 8 h till 96 h to analyze the growth kinetics of the virus in the presence of NTZ.
The total RNA was isolated from the DF-1 cells infected with NDV using RNAiso plus reagent (Takara Bio, India) with and without NTZ treatment. cDNA was prepared using high-capacity cDNA reverse transcription kit (Thermo Fischer Scientific, USA). SYBR Green master mix (Thermo Fischer Scientific, USA) was used to quantify the gene expression levels with GAPDH as an internal control. The relative gene expression values were determined using the 2-ΔΔCt method relative to mock, and each experiment was performed in triplicates. Protein lysates were collected at 24 h and 48 h post virus infection along with the mock infected and NTZ controls. The media was then discarded, and the cell lysate was collected with RIPA lysis buffer containing 1x ProteoGuard EDTA free protease inhibitor cocktail (Clontech, USA). Cell lysates were stored at −80 °C till use. The NDV protein expression was analyzed by western blotting using NDV gene specific monoclonal antibodies.
2.6. In ovo studies
The 9-day-old SPF embryonated chicken eggs were inoculated with NDV (MOI of 0.01) and NTZ with a concentration of 12.5 μM. Four groups of eggs were used in the experiment: uninfected control, NDV control, NTZ control, and NTZ treated along with NDV inoculation. The eggs were incubated at 37 °C with regular candling until 48 h post inoculation after which the eggs were kept at 4 °C to harvest the embryo and the allantoic fluid. The titration of the virus from the allantoic fluid was done using a plaque assay, as described previously. Further, the embryos were examined for pathological lesions, which are indicative of NDV infection, and the tissue samples were collected from the embryos, which was used for virus quantification and gene expression studies.
2.7. Cytokine profiling
Differential gene expression of host cytokines viz., IL18, TLR7, TNF-α, IL-1β, NLRP3, IFN-α, and IFN-β on NTZ treatment was evaluated on DF-1 cells, PBMC, and chicken embryo tissue samples. 5x 105 DF-1 cells were seeded in 6 well plates, and total RNA lysates were collected at 12 and 24 h post NTZ treatment at a final concentration of 12.5 µM. PBMC was collected by bleeding SPF chickens into tubes with EDTA; blood was carefully layered on Histopaque-1077 (Sigma-Aldrich) and centrifuged at 400 × g for 30 min. The white layer containing PBMC was collected and washed in serum free DMEM three times, and 5 × 107 cells were seeded in 6 well plate. After overnight incubation, PBMC was treated with NTZ at a final concentration of 12.5 µM, and RNA lysates were collected at 12 and 24 h post-treatment. 500 mg of chicken embryo tissue was collected from the NTZ treated and mock treated groups from in ovo experiment 48 h post-treatment. Total RNA from all three experiments were isolated using RNAiso plus reagent (Takara Bio, India). cDNA was prepared using high capacity cDNA reverse transcription kit (Thermo Fischer Scientific, USA), and qPCR was performed using chicken specific primers to check the expression of host cytokines (Table 1 ). qPCR results are an average of three independent experiments. Normalization was done with the mock-treated controls. GAPDH was used as an endogenous gene expression control.
Table 1.
Real-time primers used in the host gene expression post-NDV and/or nitazoxanide treatment.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| NDV N | TACATCCTCYACCCCGTATG | GAAGAATGCAGTAAGCCCG |
| NDV P | GGCAGAGCCAAGACAATAC | TCATRGGGATGGAGGATGTC |
| NDV L | ATCAGGTCAGACACATTCTTC | AATGTTGGCACARGACATTAC |
| IFN-α | CTTCTGAAAGCTCTCGCC | TCGTTGAAGGAGCAAGAC |
| IFN- β | CTGCCAGCTCTCACCACCAC | ATGCGGAGGGTGGTGATGG |
| IL18 | CTGGCAGTGGAATGTACTTCG | TTCACCAGGAATGTCTTTGGG |
| TLR7 | TTGTGGAGATTGACTTCAGG | CTCAGCAGACGTAAAGTAGC |
| TNF-α | GTCTGCTCCTAGTGGCTTTC | CTACGGGTTGCTGCACATAC |
| IL1β | GCTGACCCGCTTCATCTTCTA | CGCCCACTTAGCTTGCAGGT |
| NLRP3 | GCTGCAAGGGAGGAGTATTT | CATCTGTGTGGTGGTCTTAGAG |
2.8. Animal study
Two weeks old specific pathogen free (SPF) chickens were used for animal studies. The chickens were randomly divided into four groups, namely untreated-uninfected control, NTZ treated, virus-infected, and NTZ treated-virus infected groups. Each group consisted of three birds. The animal studies were performed at biosafety level-2 facility in Centre for Medical Biotechnology, Maharshi Dayanand University, Rohtak, India. The study was conducted following the guidelines of institutional animal ethics and biosafety committee. On day 0, the NTZ alone and with virus groups were given an intramuscular injection of 12.5 µM NTZ. On day 1, virus and virus with NTZ groups were infected with 50 µl of 1:500 dilution of 5 × 108 PFU/ml NDV through the oral-nasal route. On day 4, all the birds were sacrificed, followed by the collection of trachea, lungs, and spleen for viral estimation by tissue titration. Briefly, 200 mg of each organ was triturated with an equal volume of DMEM with 10x antibiotic solution. The mixture was centrifuged at 4000 rpm for 5 min to settle down the tissue material, and the supernatant was transferred in fresh tubes. The supernatant was subjected to 10 fold serial dilutions in DMEM without serum and was applied to DF1 cells to determine TCID50 titer.
2.9. Statistical analysis
Experimental results were analyzed using the ANOVA for multiple comparisons (GraphPad Prism 8). All data were obtained from at least three independent experiments, and a p-value of less than 0.05 was considered significant.
3. Results
3.1. Cytotoxicity study
The MTT assay determined the cytotoxicity of NTZ in DF-1 cells. A decrease in cell viability was observed in the concentrations higher than 50 μM (Fig. 1 a). The cell toxicity was linearly related to the NTZ when treated from 25 μM to 100 μM concentrations. The IC50 (50% inhibitory concentration) value of NTZ was calculated as ~71.6 μM (Fig. 1b), whereas it decreased to ~63.3 μM at 48 h (Fig. 1c and d). Its toxicity was temporal and dose-dependent, as evident in the cell images visualized under bright field microscopy (Fig. 1e).
Fig. 1.
Cytotoxicity of NTZ in DF-1 cells using MTT assay. Graph depicting the percentage cell viability after 24 h treatment with increasing concentrations of NTZ (0.3–100 µM) was plotted (a). Linear regression graph was plotted to determine the IC50 value of NTZ (~71.6 μM) after 24 h (b). Graph showing the percentage cell viability after 48 h treatment with increasing concentrations of NTZ (0.3–100 µM) was plotted (c). Linear regression graph was plotted to determine the IC50 value of NTZ (~63.3 μM) after 48 h (d). DF-1 cell images were taken in an inverted microscope after 24 h and 48 h treatment with NTZ (12.5 μM, 25 μM and 50 μM) (e).
3.2. Antiviral effect of NTZ
In the time of addition assay, the DF-1 cells were infected with NDV based on the schematic representation shown in Fig. 2 a. To directly visualize the effect of NTZ, the rNDV-GFP was used, and a significant viral reduction in post NTZ treatment was observed as compared to the pre- and co-treatment (Fig. 2b). The antiviral effect of NTZ sustained until 48 h post NTZ treatment, whereas it was not visualized in the pre and co-treatment conditions. Growth kinetics was performed to monitor the release of NDV progeny, which showed complete inhibition of NDV post NTZ treatment until 96 h (Fig. 2c). It was observed that in other conditions of pre and co-treatment, the NDV replication in the initial time points was less but later became equal to the NDV control. The results obtained in the growth kinetics of NDV were further corroborated by plaque assay. In pre-treatment of NTZ, approximately 30% of NDV reduction was observed. The reduction of NDV in the post-treatment of NTZ was found to be 65% (Fig. 2d and e), whereas it was not significant in the co-treatment conditions. From the results obtained, it was concluded that NTZ was effective in reducing NDV when it is post-treated, and therefore, for further experiments post-treatment of NTZ was preferred.
Fig. 2.
Time of addition assay in DF-1 cells to determine the anti-NDV effect of NTZ. Schematic representation of the time of addition assay performed (a). DF-1 cell images were taken in an inverted fluorescence microscope after infection with rNDV/GFP and pre, co and post-treatment of NTZ (b). Mock-infected DF-1 cells were used as a control. Growth kinetics of NDV was determined after pre, co and post-treatment of NTZ using TCID50 assay (c). NDV reduction was observed when treated with NTZ (pre, co and post-treatment) using plaque assay (d). The graph was plotted from the number of plaques observed after NDV infection and pre, co, and post-treatment of NTZ (e). Statistical significance difference was determined by using ordinary one way ANOVA (Dunnett’s multiple comparisons test, GraphPad Prism 8). A p-value less than 0.01 is flagged with one star (**), and less than 0.001 is flagged with three stars (***).
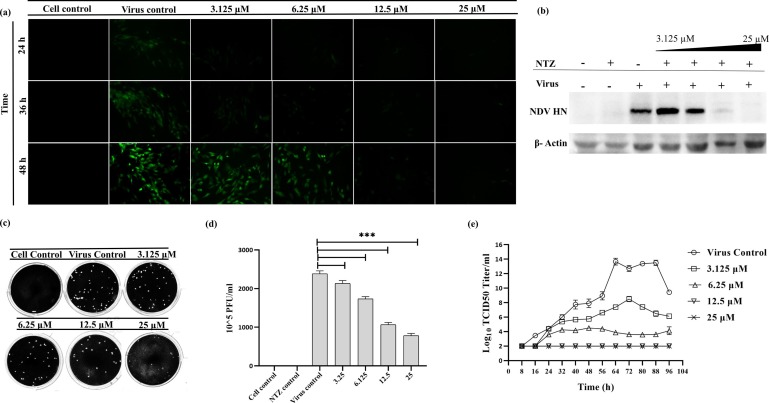
The infection of DF-1 cells with the rNDV-GFP showed a reduction in a dose and time-dependent manner (Fig. 3 a). A significant reduction was observed in the NTZ treated DF-1 cells at a concentration of 12.5 μM as compared to the untreated and mock infected controls. The NDV reduction, when treated with NTZ, was further confirmed by analyzing protein expression using western blotting (Fig. 3b). Furthermore, the NDV quantification by plaque assay showed a reduction of 50% as compared to the virus control upon 12.5 μM treatment of NTZ (Fig. 3c and d). A dose-dependent NTZ treatment showed a reduction of virus titer in the growth kinetics (Fig. 3e). No viral load was observed in the cells treated with NTZ at a concentration of 12.5 μM. Further experiments were carried out using EC50 ~12.5 μM of NTZ.
Fig. 3.
Dose-dependent anti-NDV effect of NTZ. DF-1 cell images were taken in an inverted fluorescence microscope after infection with rNDV/GFP and post-treatment of NTZ with varying concentrations (3.125 μM, 6.25 μM, 12.5 μM and 25 μM) (a). Mock-infected DF-1 cells were used as a control. Reduction in NDV protein HN after dose-dependent treatment of NTZ was observed using western blotting (b). β-Actin was used as a loading control. NDV reduction was observed after dose-dependent treatment with NTZ using plaque assay (c). The graph was plotted from the number of plaques observed after NDV infection and dose-dependent treatment of NTZ. Statistical significance difference was determined by using ordinary one way ANOVA (Dunnett’s multiple comparisons test, GraphPad Prism 8). A p-value less than 0.001 is flagged with three stars (***) (d). Growth kinetics of NDV was determined after treatment with varying concentrations of NTZ using TCID50 assay (e).
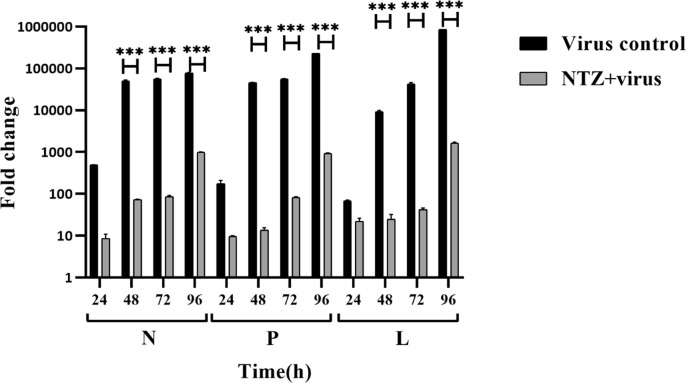
3.3. Expression analysis of NDV genes upon NTZ treatment
The treatment of NTZ showed ~700-fold downregulation of N gene of NDV as compared to the untreated control at 48 h post-treatment (Fig. 4 ). The analysis of P and L genes of NDV post-NTZ treatment at different time intervals showed a significant reduction as compared to untreated DF-1 cells (Fig. 4). The mock infected DF-1 cells were used as a baseline control for all the experiments.
Fig. 4.
Reduction of NDV gene expression after treatment with NTZ in vitro. A comparison of the expression of NDV non-structural genes (N, P, and L) was done using real-time PCR. Graph depicting the fold change in NDV gene expression in the presence and absence of NTZ at different time points (24, 48, 72, and 96 h) was plotted. Normalization was done with mock-infected cells. GAPDH was used as an endogenous gene expression control. Statistical significance difference was determined by using two way ANOVA (Sidak’s multiple comparisons test, GraphPad Prism 8). A p-value less than 0.001 is flagged with three stars (***). Bars that are not flagged are not significant.
3.4. In ovo experiment
The 9-day-old embryo showed lesions after infection with NDV; however, the treatment of NTZ after virus infection did not show any visible lesion of infection (Fig. 5 a). NDV quantification of the allantoic fluid and tissue sample by plaque assay showed a 1.5-fold reduction in its titer upon NTZ treatment (Fig. 5b and c). Similarly, an about 2-fold reduction in the kinetics of NDV was observed in the tissue isolated from the NTZ treated embryo (Fig. 5c).
Fig. 5.
In ovo experiment showing the anti-NDV effect of NTZ. Nine-day-old embryos after 48 h of NDV infection in the presence and absence of NTZ (~12.5 μM) was observed (a). Visible gross lesions indicated NDV infection. Quantification of NDV from the allantoic fluid and the tissue collected from the embryos using plaque assay (b). The graph was plotted from the number of plaques observed from the allantoic fluid and the tissue collected from the embryos (c).
3.5. Cytokine profiling
NTZ treated DF-1 cells showed upregulation in mRNA levels of all the cytokines studied (Fig. 6 a). Except for IL-1β, a significant increase in mRNA level of cytokines studied was observed upto 24 h post-NTZ treatment. In chicken PBMC, it was observed that the expression level of all the cytokines studied was significantly upregulated until 24 h post-NTZ treatment (Fig. 6b). IFN-α, IFN-β, NLRP3, and IL-1β showed maximum upregulation upon NTZ treatment as compared to others. The tissue sample collected from chicken embryo treated with NTZ was analyzed for various cellular cytokines 48 h post-treatment (Fig. 6c). The cytokine gene expression analysis showed upregulation of IL18, TLR7, TNF-α, IL-1β NLRP3, IFN-α, and IFN-β, upon NTZ treatment, as compared to the control. Maximum regulation was observed in TLR7 and TNF-α, while the minimum was observed in IL-1β and NLRP3. All the observed changes were found to be significant.
Fig. 6.
Cytokine expression profiling (IL18, TLR7, TNF-α, IL-1β, NLRP3, IFN-α, and IFN-β) of DF-1, PBMC and chicken embryo tissue treated with NTZ was performed using qPCR (a, b, c). Normalization was done with the mock-treated controls. GAPDH was used as an endogenous gene expression control. Statistical significance difference was determined by using two way ANOVA (Tukey’s multiple comparisons test, GraphPad Prism 8). A p-value less than 0.05 is flagged with one star (*), less than 0.01 is flagged with two stars (**), and less than 0.001 is flagged with three stars (***). Bars that are not flagged are not significant.
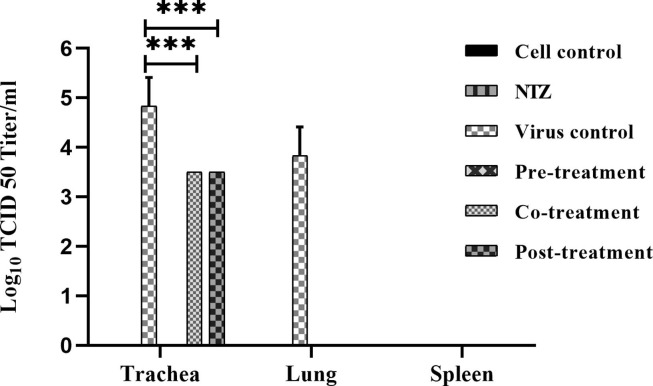
3.6. Animal study
Replication of the virus was observed in the trachea and lungs, whereas no virus was found in the spleen for virus control group ( Fig. 7 ). The pre-treatment group showed a complete reduction of the virus in all the three organs studied. Co and post-treatment groups showed no virus in the lungs and 1-log reduction of the virus in the trachea as compared to virus control. NTZ treated groups had no gross pathological lesions on organs.
Fig. 7.
In vivo experiment showing the anti-NDV effect of NTZ. NDV viral titer in trachea, lung, and spleen in six different groups of chicken, namely, mock treated control group, NTZ only treated group, virus alone infected group, Pre-treatment NTZ with virus infected group, Co-treatment NTZ with virus infected group, and Post-treatment NTZ with virus infected group. All the birds were harvested 72 h post virus infection, and various tissues were collected, triturated and subjected to TCID50 assay. Statistical significance difference was determined by using two way ANOVA (Tukey’s multiple comparisons test, GraphPad Prism 8). A p-value less than 0.001 is flagged with three star (***).
4. Discussion
Newcastle disease is an economically important disease, causing substantial loss to the poultry industry. The recommended strategy for the prevention of the disease is by using killed or live attenuated vaccines. Vaccination failure is a common problem and has been reported from places where the velogenic strains of NDV are endemic [10], [39], [40]. An alternative to this problem is the use of antiviral drugs against NDV, which is not commercially available. NTZ is a licensed drug for the treatment of diarrhoea induced by Cryptosporidium parvum and Giardia intestinalis [15], [41]. NTZ has also been used as a broad-spectrum antiviral drug [16], [42]. The present study is an attempt to repurpose NTZ as an antiviral against NDV infection. The IC50 value of NTZ in the chicken fibroblast cells was found to be ~60 μM in 48 h. The cytotoxic concentration that reduced the exponential growth in baby hamster kidney (BHK-21) cells was earlier reported as 18.59 μg/ml (60.4 μM) [24], which is as per the IC50 value reported in our study.
We performed the time of addition assay to identify the stage of NDV replication affected by NTZ. The minimal reduction was observed in the pre-treatment of NTZ, as observed in the rNDV-GFP infected cells. No reduction was observed in the co-treatment, while the most significant reduction was visible in the post-treatment of NTZ. Our results were similar to the results reported earlier [17], [29], [43]. It was concluded that the drug does not directly affect the virus binding or entry into target cells.
In previous reports, concentration ranging from 3 to 25 μM was used to show the antiviral activity of NTZ [26], [33]. A peak concentration of up to 200 μM has been detected in the blood plasma of humans after the oral dosage of NTZ (4 g) and is safe [44]. In order to minimize the cytotoxic effect of NTZ, concentrations ranging from 3.125 to 25 μM was chosen for checking it’s effect against NDV. Our study showed a reduction of NDV by two-fold at NTZ concentration of 12.5 μM, suggesting that the drug is significantly effective far below the calculated IC50 value, which further suggests the efficacy of NTZ as anti-NDV.
The expression of NDV non-structural genes (N, P, and L), which make up the polymerase complex, was analyzed in our study. The significant reduction in the viral genes further confirmed the blocking of viral replication. Effect of NTZ against rubella virus replication has been previously reported showing the reduction of RNA transcription of viral genes [43]. We found a reduction in the expression of viral genes in accordance with the earlier report; however, further experimentation is required to understand whether NTZ blocks the transcription of mRNA or whether the reduced gene expression is due to lower infectivity. Our data suggested a decrease in viral structural and non-structural proteins post-NTZ treatment, which could perhaps have perturbed the effective assembly of the NDV virion in the cytoplasm. Earlier reports suggested inhibition of enveloped viruses by downregulating the structural and non-structural proteins [24], [42]. However, we did not get an appreciated protein level post 24 h of NDV infection, which could be due to a lesser viral protein that falls below the detection threshold.
We also wanted to explore other possible mechanisms. NTZ has been reported to stimulate innate immunity and reduce HIV replication [45]. Our results showed significant up-regulation in TLR7 and type I interferons, along with few other cytokines such as IL18 and IL-1β. Out of all reported TLRs in birds, TLR7 has been reported to reduce the avian influenza replication [46]. TLR7, which induces the type I interferon production, might play a role in reducing the NDV infection [47]. Type I interferons and pro-inflammatory cytokines are shown to modulate virus replication [48]. Our results showed significant upregulation of pro-inflammatory genes such as IL-1β, NLRP3, and IL18 at the transcription level. Furthermore, NTZ treatment upregulated type I interferons suggesting that the innate immune modulation could help effectively to clear the NDV from the host. However, the effect could be appreciated only at the level of transcription due to the unavailability of chicken specific antibodies. A significant reduction of the virus in NTZ treated groups was observed as compared to non-treated infected birds. The pre-treatment group was found to be the most effective NTZ treated group. Decreased viral replication in the trachea and lungs indicates that NTZ is more effective by enhancing the immune system for the rapid clearance of the virus. Spleen did not show any virus even in the virus control group suggesting that the virus spreads only in trachea and lungs.
Repurposing of the drug as an antiviral has been less explored and neglected to a large extent. The present study demonstrates that NTZ can be repurposed as an antiviral against NDV infection. The possible mechanisms in which the drug acts might be through the stimulation of cytokines. The results presented in the current work is a step towards understanding the drug induced modulation of host immune response, which could be explored to design effective therapeutics against NDV infection in chicken. Although, there might be several other ways by which NTZ could modulate the host system to curtail viral infectivity. Perhaps in a limited way, the study will benefit to use NTZ as an antiviral for other poultry pathogens.
CRediT authorship contribution statement
Ferrin Antony: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing - original draft. Yoya Vashi: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing - original draft. Sudhir Morla: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing - original draft. Vandna: Data curation, Formal analysis, Methodology. Hari Mohan Saini: Formal analysis, Methodology, Resources. Sachin Kumar: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to all lab members for their constant support and help. The NDV research work is supported by grants from the department of biotechnology BT/PR16147/NER/95/83/2015 and BT/562/NE/U-Excel/2016.
References
- 1.R.A. Lamb, G.D. Parks, Paramyxoviridae: The Viruses and their Replication, 5th ed., Lippincott, Williams, and Wilkins, 2007.
- 2.Czegledi A., Ujvari D., Somogyi E., Wehmann E., Werner O., Lomniczi B. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 2006;120(1–2):36–48. doi: 10.1016/j.virusres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 3.de Leeuw O., Peeters B. Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J. Gen. Virol. 1999;80(Pt 1):131–136. doi: 10.1099/0022-1317-80-1-131. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y., Wan H.Q., Liu H.Q., Wu Y.T., Liu X.F. Genomic sequence of an isolate of Newcastle disease virus isolated from an outbreak in geese: a novel six nucleotide insertion in the non-coding region of the nucleoprotein gene. Brief Rep. Arch. Virol. 2004;149(7):1445–1457. doi: 10.1007/s00705-004-0297-8. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurthy S., Samal S.K. Nucleotide sequences of the trailer, nucleocapsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequence. J. Gen. Virol. 1998;79(Pt 10):2419–2424. doi: 10.1099/0022-1317-79-10-2419. [DOI] [PubMed] [Google Scholar]
- 6.Errington W., Emmerson P.T. Assembly of recombinant Newcastle disease virus nucleocapsid protein into nucleocapsid-like structures is inhibited by the phosphoprotein. J. Gen. Virol. 1997;78(Pt 9):2335–2339. doi: 10.1099/0022-1317-78-9-2335. [DOI] [PubMed] [Google Scholar]
- 7.Alexander D.J. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 2000;19(2):443–462. doi: 10.20506/rst.19.2.1231. [DOI] [PubMed] [Google Scholar]
- 8.Allan W.H. Newcastle disease virus pathotypes AU – Alexander, D.J. Avian Pathology. 1974;3(4):269–278. doi: 10.1080/03079457409353840. [DOI] [PubMed] [Google Scholar]
- 9.Capua I., Alexander D.J. Springer Science & Business Media; 2009. Avian Influenza and Newcastle Disease: A Field and Laboratory Manual. [Google Scholar]
- 10.Dimitrov K.M., Afonso C.L., Yu Q., Miller P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mebatsion T., Koolen M.J., de Vaan L.T., de Haas N., Braber M., Romer-Oberdorfer A., van den Elzen P., van der Marel P. Newcastle disease virus (NDV) marker vaccine: an immunodominant epitope on the nucleoprotein gene of NDV can be deleted or replaced by a foreign epitope. J. Virol. 2002;76(20):10138–10146. doi: 10.1128/JVI.76.20.10138-10146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson R.P., Brandly C.A. Identification of vaccine strains of Newcastle disease virus. Science. 1955;122(3160):156–157. [PubMed] [Google Scholar]
- 13.Hanson R.P., Spalatin J., Estupinan J., Schloer G. Identification of lentogenic strains of Newcastle disease virus. Avian Dis. 1967;11(1):49–53. [PubMed] [Google Scholar]
- 14.Perozo F., Marcano R., Afonso C.L. Biological and phylogenetic characterization of a genotype VII Newcastle disease virus from Venezuela: efficacy of field vaccination. J. Clin. Microbiol. 2012;50(4):1204–1208. doi: 10.1128/JCM.06506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox L.M., Saravolatz L.D. Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 2005;40(8):1173–1180. doi: 10.1086/428839. [DOI] [PubMed] [Google Scholar]
- 16.Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piacentini S., La Frazia S., Riccio A., Pedersen J.Z., Topai A., Nicolotti O., Rossignol J.F., Santoro M.G. Nitazoxanide inhibits paramyxovirus replication by targeting the Fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57. Sci. Rep. 2018;8(1):10425. doi: 10.1038/s41598-018-28172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossignol J.F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Publ. Health. 2016;9(3):227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahapatro S., Mahilary N., Satapathy A.K., Das R.R. Nitazoxanide in acute rotavirus diarrhea: a randomized control trial from a developing country. J. Trop. Med. 2017;2017:7942515. doi: 10.1155/2017/7942515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiq D.M., Koo H.L., Adachi J.A., Viola G.M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63(5):394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachulski A.V., Pidathala C., Row E.C., Sharma R., Berry N.G., Iqbal M., Bentley J., Allman S.A., Edwards G., Helm A., Hellier J., Korba B.E., Semple J.E., Rossignol J.F. Thiazolides as novel antiviral agents. 1. Inhibition of hepatitis B virus replication. J. Med. Chem. 2011;54(12):4119–4132. doi: 10.1021/jm200153p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolova K., Gluud C., Grevstad B., Jakobsen J.C. Nitazoxanide for chronic hepatitis C. Cochrane Database Syst. Rev. 2014;4:CD009182. doi: 10.1002/14651858.CD009182.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao R.Y., Xu Y.F., Zhang T.H., Yang J.J., Yuan Y., Hao P., Shi Y., Zhong J., Zhong W. Pediatric drug nitazoxanide: a potential choice for control of zika. Open Forum Infect. Dis. 2017;4(1):ofx009. doi: 10.1093/ofid/ofx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Z., Wei J., Deng X., Li S., Qiu Y., Shao D., Li B., Zhang K., Xue F., Wang X., Ma Z. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014;11:10. doi: 10.1186/1743-422X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botta L., Rivara M., Zuliani V., Radi M. Drug repurposing approaches to fight Dengue virus infection and related diseases. Front Biosci. (Landmark Ed.) 2018;23:997–1019. doi: 10.2741/4630. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y.M., Lu J.W., Lin C.C., Chin Y.F., Wu T.Y., Lin L.I., Lai Z.Z., Kuo S.C., Ho Y.J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antiviral Res. 2016;135:81–90. doi: 10.1016/j.antiviral.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan X., Hu L., Luquette L.J., 3rd, Gao G., Liu Y., Qu H., Xi R., Lu Z.J., Park P.J., Elledge S.J. Systematic identification of synergistic drug pairs targeting HIV. Nat. Biotechnol. 2012;30(11):1125–1130. doi: 10.1038/nbt.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickson S.E., Margineantu D., Hockenbery D.M., Simon J.A., Geballe A.P. Inhibition of vaccinia virus replication by nitazoxanide. Virology. 2018;518:398–405. doi: 10.1016/j.virol.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossignol J.F., La Frazia S., Chiappa L., Ciucci A., Santoro M.G. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J. Biol. Chem. 2009;284(43):29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koszalka P., Tilmanis D., Hurt A.C. Influenza antivirals currently in late-phase clinical trial. Influenza Other Respir. Viruses. 2017;11(3):240–246. doi: 10.1111/irv.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Frazia S., Ciucci A., Arnoldi F., Coira M., Gianferretti P., Angelini M., Belardo G., Burrone O.R., Rossignol J.F., Santoro M.G. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J. Virol. 2013;87(20):11096–11106. doi: 10.1128/JVI.01213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elazar M., Liu M., McKenna S.A., Liu P., Gehrig E.A., Puglisi J.D., Rossignol J.F., Glenn J.S. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137(5):1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 33.Ashiru O., Howe J.D., Butters T.D. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores. Virology. 2014;462–463:135–148. doi: 10.1016/j.virol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.Morla S., Kumar Tiwari A., Joshi V., Kumar S. Complete genome sequence of a newcastle disease virus isolate from an outbreak in northern India. Genome Announc. 2014;2(2):00342–414. doi: 10.1128/genomeA.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das M., Baro S., Kumar S. Evaluation of imidazole and its derivative against Newcastle disease virus infection in chicken: a drug repurposing approach. Virus Res. 2019;260:114–122. doi: 10.1016/j.virusres.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Lomniczi B. Plaque assay for avirulent (lentogenic) strains of Newcastle disease virus. Appl. Microbiol. 1974;27(6):1162–1163. doi: 10.1128/am.27.6.1162-1163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27(3):493–497. [Google Scholar]
- 39.Ezema W.S., Okoye J.O., Nwanta J.A. LaSota vaccination may not protect against the lesions of velogenic Newcastle disease in chickens. Trop. Anim. Health Prod. 2009;41(4):477–484. doi: 10.1007/s11250-008-9210-x. [DOI] [PubMed] [Google Scholar]
- 40.Khorajiya J.H., Pandey S., Ghodasara P.D., Joshi B.P., Prajapati K.S., Ghodasara D.J., Mathakiya R.A. Patho-epidemiological study on Genotype-XIII Newcastle disease virus infection in commercial vaccinated layer farms. Vet World. 2015;8(3):372–381. doi: 10.14202/vetworld.2015.372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doumbo O., Rossignol J.F., Pichard E., Traore H.A., Dembele T.M., Diakite M., Traore F., Diallo D.A. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am. J. Trop. Med. Hyg. 1997;56(6):637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol J.F. Thiazolides: a new class of antiviral drugs. Expert Opin. Drug Metab. Toxicol. 2009;5(6):667–674. doi: 10.1517/17425250902988487. [DOI] [PubMed] [Google Scholar]
- 43.Perelygina L., Hautala T., Seppanen M., Adebayo A., Sullivan K.E., Icenogle J. Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antiviral Res. 2017;147:58–66. doi: 10.1016/j.antiviral.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockis A., Allemon A.M., De Bruyn S., Gengler C. Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. Int. J. Clin. Pharmacol. Ther. 2002;40(5):213–220. doi: 10.5414/cpp40213. [DOI] [PubMed] [Google Scholar]
- 45.Trabattoni D., Gnudi F., Ibba S.V., Saulle I., Agostini S., Masetti M., Biasin M., Rossignol J.F., Clerici M. Thiazolides Elicit Anti-Viral Innate Immunity and Reduce HIV Replication. Sci. Rep. 2016;6:27148. doi: 10.1038/srep27148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barjesteh N., Behboudi S., Brisbin J.T., Villanueva A.I., Nagy E., Sharif S. TLR ligands induce antiviral responses in chicken macrophages. PLoS One. 2014;9(8):e105713. doi: 10.1371/journal.pone.0105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St Paul M., Brisbin J.T., Abdul-Careem M.F., Sharif S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 2013;152(3–4):191–199. doi: 10.1016/j.vetimm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Mogensen T.H., Paludan S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001;65(1):131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]