Abstract
Objectives:
People after stroke demonstrate alterations in vascular endothelial function measured by flow-mediated dilation. Limited information is available in the literature to possible protective factors following stroke. The aims of the secondary analysis were: 1) characterize the time course of vascular endothelial function using flow-mediated dilation at 72 hours post-stroke and one week later during inpatient stroke rehabilitation and 2) whether flow-mediated dilation was related to vascular endothelial growth factor, brain-derived neurotrophic factor or estimated pre-stroke peak oxygen uptake.
Methods:
Flow-mediated dilation using Doppler ultrasound was assessed in bilateral brachial arteries at the defined time points. Flow-mediated dilation and blood draws occurred on the same day between 7:30 am and 9:00 am following an overnight fast. ELISA was used to quantify plasma vascular endothelial growth factor and brain-derived neurotrophic factor values. A non-exercise estimate was used to calculate pre-stroke peak oxygen uptake.
Results:
We have shown that between limb differences are evident within 72 hours post-stroke and remain one week later during inpatient rehabilitation. Higher values for vascular endothelial growth factor were associated with increased flow-mediated dilation at both time points. Higher estimated pre-stroke peak oxygen uptake was related to flow-mediated dilation. Brain-derived neurotrophic factor was not related to any outcome measures.
Conclusions:
Unique vascular adaptations start early after stroke in the stroke-affected limb and remain through inpatient stroke rehabilitation. Vascular endothelial growth factor and pre-stroke physical activity may have a protective role in vascular function following stroke. Future work should focus on mechanistic pathways for preservation of vascular health.
Introduction
A healthy vascular endothelium is essential for regulating the blood vessel’s function and delivery of blood to tissues. Negative physiological adaptations can occur as a result of chronic disease1-3 including stroke.3-7 Our previous work found that vascular endothelial function using flow-mediated dilation (FMD) was reduced in the stroke affected arm.4 Unilateral adaptations are not typically observed in healthy young and older adults8,9 and can have the potential to affect blood flow delivery during exercise or activities of daily living.6
Reduced blood flow and arterial diameter could not only have implications for physical activity in the community but may influence rehabilitation where optimal blood flow regulation is needed for exercise, standing activities and walking. Therefore, we have been interested in better understanding the timeline of when these unilateral vascular adaptations begin post-stroke and the relationship of any protective factors that may be a target for future interventions. We have previously shown that people with acute stroke have reduced FMD when compared to age- and gender-matched controls.5 A recent publication reported similar findings within the first 10 days of stroke.3 The authors reported patients with stroke had reduced FMD even when compared to patients with similar cardiovascular risk factors. This highlights that there may be factors uniquely related to stroke.
The purpose of this secondary analysis was to first characterize the time course of vascular endothelial function using FMD early after stroke and then one week later during inpatient rehabilitation. Second, we examined whether vascular endothelial growth factor (VEGF) and pre-stroke estimated peak oxygen uptake (peak VO2) was related to FMD. We have previously reported that within 72 hours post-stroke between limb differences exist for vascular endothelial function.5 Therefore, we hypothesized we would detect between limb differences in FMD at 1 week post-stroke. We wanted to explore whether VEGF and pre-stroke estimated peak VO2 were related to FMD measures. We hypothesized that 1) VEGF and 2) pre-stroke estimated peak VO2 would be moderately related to FMD. We also measured brain-derived neurotrophic factor (BDNF) from our blood samples to explore relationships to our selected outcome measures.
Materials and Methods
Participants
Participants admitted to KU Hospital between March 2013 and April 2015 with acute stroke were approached for enrollment into the study. Since this is a secondary analysis, these study participants have been described elsewhere.5 Briefly, patients were admitted to the acute stroke unit and screened for the following inclusion criteria: 1) diagnosis of unilateral stroke from neuroimaging, 2) the ability to consent within 72 hours of admission to the stroke unit, and 3) between 30-80 years of age. Individuals were not enrolled if the following exclusion criteria were present: 1) acute renal failure, 2) ischemic cardiovascular event or coronary artery bypass surgery less than 3 months prior to stroke, 3) severe peripheral artery disease, 4) diagnosis of congestive heart failure, and 5) unable to maintain position of the upper extremity to access the brachial artery during ultrasound scanning.
The study was approved by the Human Subjects Committee at the University of Kansas Medical Center, which complies with the Declaration of Helsinki [approval number 12490]. Institutionally approved written consent was obtained prior to enrollment from all participants or the surrogate decision maker.
Questionnaires and medical information
We have previously published data on the use of a non-exercise estimate for pre-stroke peak VO2 in acute stroke.10, 11 The medical record was used to gather information on heart function using ejection fraction (%) from the ultrasound report since low cardiac output could affect vascular endothelial function.
Flow-mediated dilation
FMD Time 1 was performed within 72 hours of admission to the acute stroke unit5 and Time 2 was performed within the inpatient rehabilitation setting one week later. FMD was conducted using Doppler ultrasound on bilateral brachial arteries in the upper extremities using previously published methodology.4, 5 Participants refrained from food or caffeine for 12 hours prior to the ultrasound scan and no medications were given from midnight until after the procedure. Vasoactive medications were withheld overnight.5, 12 All ultrasound scans occurred within a 2-hour window in the morning between 7:30 am and 9:00 am.
We have previously reported on our FMD methodology4, 5 and follow published recommendations for conducting FMD13 using Doppler ultrasound. All images were stored on a computer and analyzed off-line using specialized software (Brachial Analyzer, Medical Imaging Applications, Coralville, Iowa).
Blood analysis
Approximately 10 mLs of venous blood were obtained from each participant on the same day as the Time 1 FMD procedure. Blood was immediately stored on ice to be transported to the laboratory for processing and storage at −80°C within one hour of collection from the participant.5 After all samples were collected from participants, the KU Biobehavioral Measurement Core (P30 HD002528) performed the assays to quantify VEGF and BDNF.
Statistical analysis
The data used in this secondary analysis was based on a previously determined sample size for FMD.5
Data analysis was performed with SPSS Version 22 (IBM, Armonk, NY) for Windows. Values are expressed as mean ± SD. Paired t-test was used to assess whether between limb differences existed between variables. Pearson correlation was used for correlation analyses. We used criteria defined by Portney and Watkins:14 Pearson’s coefficient (r) = 0.00 – 0.25, little to no relationship; r = 0.25 – 0.50, fair relationship; r = 0.50 – 0.75, moderate to good relationship; and r >0.75, good to excellent relationship. A significant p-value was ≤ 0.05.
Results
Participant demographics are reported in Table 1. According to the medical record, our participants had values that were considered normal cardiac ejection fraction (62% ± 4%). The pre-stroke estimated peak VO2 was 27.3 ± 8.8 ml/kg/min, which is considered poor for males and females in their respective age group.15
Table 1.
Participant Demographics
| Characteristics; n = 12 | |
|---|---|
| Male/Female | 7/5 |
| Age, years | 59.6 (10.8) |
| Body Mass Index | 31.1 (5.6) |
| Type of Stroke | |
| Hemorrhagic | 3 |
| Ischemic | 9 |
| Stroke Affected Side | |
| Left | 6 |
| Right | 6 |
| Race/Ethnicity | |
| White/Caucasian | 10 |
| African American | 2 |
| Asian | 0 |
| Hispanic | 0 |
| Diabetes | |
| None | 10 |
| Type II | 2 |
| Medications | |
| Blood Thinner | 2 |
| Anti-hypertensive | 8 |
| Cholesterol | 6 |
Data are expressed as mean (SD) unless stated otherwise stated. SD= standard deviation
Flow-mediated dilation, VEGF and estimated pre-stroke peak VO2
Time 1:
We previously reported that FMD in the stroke affected limb was lower when compared to the non-affected limb early after stroke.5 We found a good to excellent, positive relationship between FMD for the stroke-affected limb and VEGF (r = 0.85, p < 0.01) shown in Figure 1. A good to moderate relationship, but not statistically significant was found for the non-affected limb (r = 0.52, p = 0.08). We also found a fair to moderate, non-significant relationship between FMD for the stroke-affected side and estimated pre-stroke peak VO2 (r = 0.46, p = 0.13) while a moderate to good, significant relationship was found between FMD for the non-affected limb and estimated pre-stroke peak VO2 (r = 0.62, p = 0.03). Peak shear and blood flow velocity were not different between limbs (p> 0.05). Values for BDNF were not related to any of our outcome measures (p > 0.20).
Figure 1.
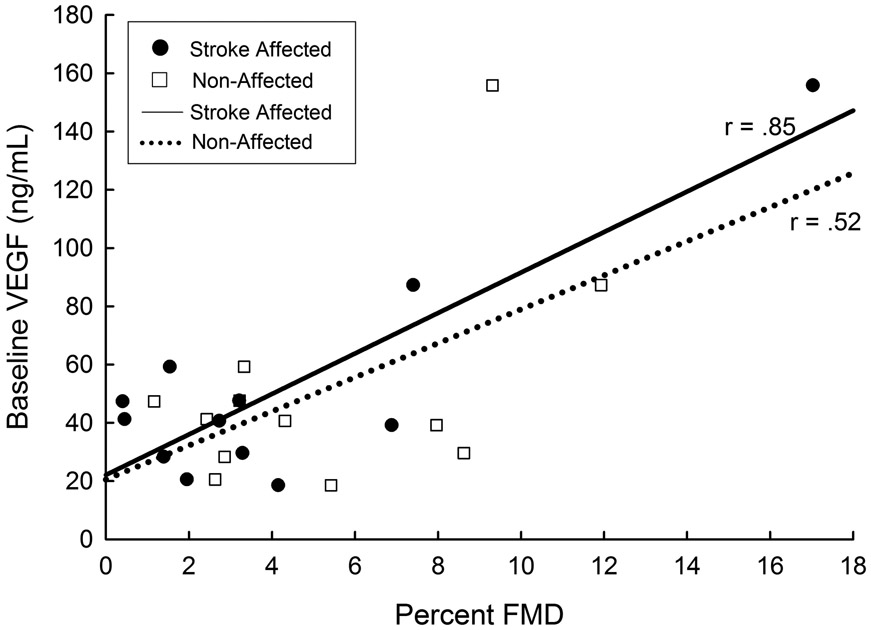
Baseline VEGF Concentration and Limb Percent FMD Time 1 after Stroke
Time 2:
One individual was discharged home and lost to follow up. One individual experienced adverse medical complications (not study related) and was not allowed to participate in study procedures per the patient’s physician. Therefore, ten individuals had complete data sets and were included in the Time 2 analysis. We report significant differences for FMD remain at one week post-stroke for the stroke-affected limb (FMD = 3.3% ± 2.3) and the non-affected limb (FMD = 5.7% ± 2.0, p < 0.01). No significant differences were found for peak shear and velocity (p> 0.20). We found higher VEGF values at Time 1 were significantly related to FMD values at Time 2 for the stroke-affected limb (r = 0.72, p = 0.02) and the non-affected limb (r = 0.65, p = 0.04) shown in Figure 2. Estimated pre-stroke peak VO2 and FMD on the stroke affected side (r = 0.95, p < 0.01) and the non-affected side (r = 0.70, p = 0.02) showed a significant good to excellent relationship.
Figure 2.
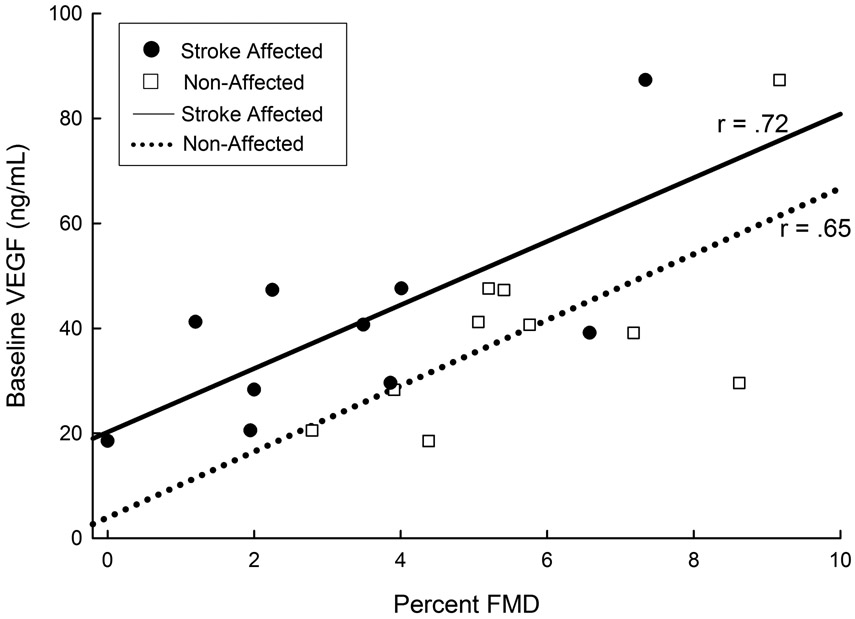
Baseline VEGF Concentration and Limb Percent FMD Time 2 after Stroke
Discussion
There is a limited but growing body of evidence regarding vascular endothelial function in stroke. Our study extends the literature by providing information on vascular endothelial function using FMD in the acute stroke period and then within the first week of inpatient rehabilitation. We also examined the relationship between vascular endothelial function using FMD and 1) VEGF and 2) estimated pre-stroke peak VO2 at both time points. The major novel findings generated from this study were: 1) FMD was reduced in the stroke-affected limb within the first week of inpatient stroke rehabilitation which is similar to our findings within the first 72 hours post-stroke,5 2) circulating VEGF was strongly associated with FMD in the stroke-affected limb at both time points. The non-affected side was moderately associated at both time points but only Time 2 was significant, and 3) estimated pre-stroke peak VO2 was strongly associated with the non-stroke affected side at both time points and only the stroke-affected side at Time 2. BNDF was not related to any of our outcome measures.
Time course of FMD after acute stroke
Our findings support the current literature suggesting FMD is reduced in acute stroke.3, 5 A strength of the present study was that we examined between-limb differences across the early stage of stroke recovery at 2 time points. Our data suggest that within 72 hours post-stroke between limb differences are observed and remain from the acute hospital stay to inpatient rehabilitation. A recent study reported acute stroke patients had a lower FMD when compared to those hospitalized with no stroke but similar cardiovascular risk factor profiles.3 The authors suggested that the reductions in FMD could not be solely attributed to cardiovascular risk but may be the result of the acute stroke event.
FMD and VEGF
It is important to examine potential protective factors of vascular health following stroke. Nitric oxide has been shown to be a regulator of vascular endothelial function16 and interact with VEGF to increase vascular dilation.17 Although the presence of pro-inflammatory markers in circulation may impair vascular function,2, 18, 19 the presence of increased circulating VEGF may be beneficial for stroke recovery.17, 20, 21 One report suggests that the increase in VEGF levels during acute stroke were proportional to stroke recovery (NIH Stroke Scale) at 3 months.22 This was an important finding to show those with higher VEGF levels in acute stroke had a “favorable prognosis.” Our work extends the literature by assessing a measure of vascular health that may contribute to stroke recovery. To our knowledge, we are the first to report on the relationship between VEGF and FMD following an acute stroke. In the present study, individuals within 72 hours of stroke with increased VEGF values presented with higher FMD values. The relationship between VEGF and FMD was stronger with the stroke-affected limb (r = 0.85, p < 0.01) than the non-affected limb (r = 0.52, p = 0.08). We also report higher VEGF values taken at Time 1 were associated with higher FMD at Time 2. These findings suggest VEGF helps maintain vascular integrity within the first few weeks of stroke recovery. However, our study did not include any follow up assessments of stroke recovery. One limitation of the study was that we did not collect minutes of activity in therapy during the hospital stay to determine whether those who were more active had higher VEGF levels and FMD. Future work should examine whether pharmacologic or therapeutic interventions such as physical activity during rehabilitation maximize vascular function following stroke.
FMD and estimated pre-stroke peak VO2
Lack of physical activity and low aerobic capacity has been well-documented after stroke.23-29 Aerobic capacity4, 30-32 and vascular health4, 33 can be improved after exercise training in people with stroke. Therefore, examining pre-stroke peak VO2 and vascular health may support the protective relationship of exercise to vascular health. Previous work has shown higher estimated metabolic equivalents per hour per week were positively and significantly related to a higher blood flow response in the paretic limb in chronic stroke survivors.6 In our study we used a non-exercise estimate based on physical activity to estimate peak VO2, which is a measure of aerobic fitness. We have previously reported on the use of an non-exercise estimate for pre-stroke peak VO2 on the acute stroke unit.10 Using this screening tool for physical activity levels and estimated aerobic fitness provides important information when formal exercise testing is not available within the first few days post-stroke.10
Similar to previous literature,6 we observed those with higher estimated peak VO2 was related to greater vascular responsiveness. However, our hypothesis was partially supported. We found a stronger relationship between FMD and estimated peak VO2 for the non-stroke affected side while only a fair to moderate relationship with the stroke-affected side at Time 1. The relationship between FMD and estimated peak VO2 strengthened at Time 2. We found these results between FMD and non-exercise estimate for peak VO2 intriguing. The possibility exists that circulating endothelial factors may have a greater contributory role on FMD in the stroke-affected limb than pre-stroke activity or fitness within the first 72 hours post-stroke. We acknowledge that the Time 2 results could be influenced by the drop-out of two participants. Therefore, we believe future investigation that is focused on vascular function early post-stroke is warranted. We acknowledge the small sample size limits our ability to interpret the potential magnitude of this effect and that the non-exercise estimate for peak VO2 may not reflect measured peak VO2, which could affect the results.
The strengths of this study were the examination of FMD across two time points of stroke recovery and using a well-defined protocol that followed published recommendations,13 which is important for obtaining reproducible measurements.34 We examined shear stress, which is known to influence FMD response where a greater peak shear results in a greater FMD. In our study, no significant differences for shear stress were found, which provides some evidence that other circulating factors such as VEGF may play a role in vascular health. We also examined whether BDNF would be related to any of our measures and found no significance. Since we were focused on vascular health, and not measures of neurogenesis or neuroplasticity,35 this would likely support our findings. Lastly, cardiac ejection fraction was reported as normal for all participants and did not influence results related to blood flow measures or FMD. This study did not capture endothelial independent function in this early post-stroke recovery. This information would be valuable to determining sympathetic nervous system input in addition to circulating peripheral factors. We believe future research is warranted to better understand vascular function and blood flow delivery following stroke especially for secondary prevention of future events.
Acknowledgements
We want to thank the participants for their time and effort on the study. SAB was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [grant number K01HD067318]. JFS and AW were supported in part by NICHD [grant number T32HD057850]. This project was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS [grant number UL1TR000001] and in part by the Kansas Intellectual and Developmental Disabilities Research Center [grant number P30 HD002528]. The content is solely the responsibility of the authors and does not necessarily represent the official views of NICHD or the National Institutes of Health. REACH laboratory space is supported by the Georgia Holland Endowment Fund.
Footnotes
Declaration of conflicting interests
SAB, JFS, and AW declare no conflict of interest. MA reports personal fees from Stryker Neurovascular and from Boehringer Ingelheim that is outside the submitted work. The authors alone are responsible for the content and writing of the paper.
References
- 1.Gerrits HL, de Haan A, Sargeant AJ, van Langen H and Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001; 82: 832–9. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari R, Bachetti T, Agnoletti L, Comini L and Curello S. Endothelial function and dysfunction in heart failure. Eur Heart J. 1998; 19 Suppl G: G41–7. [PubMed] [Google Scholar]
- 3.Omisore AD, Ayoola OO, Ibitoye BO, Fawale MB and Adetiloye VA. Sonographic Evaluation of Endothelial Function in Brachial Arteries of Adult Stroke Patients. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2017; 36: 345–51. [DOI] [PubMed] [Google Scholar]
- 4.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G and Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. 2012; 36: 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billinger SA, Sisante JV, Mattlage AE, et al. The relationship of pro-inflammatory markers to vascular endothelial function after acute stroke. Int J Neurosci. 2016: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durand MJ, Murphy SA, Schaefer KK, et al. Impaired Hyperemic Response to Exercise Post Stroke. PLoS One. 2015; 10: e0144023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivey FM, Gardner AW, Dobrovolny CL and Macko RF. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc Dis. 2004; 18: 283–9. [DOI] [PubMed] [Google Scholar]
- 8.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P and Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. American journal of physiology Heart and circulatory physiology. 2005; 288: H1747–55. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker MW, De Groot PC, Rongen GA, et al. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005; 99: 1293–300. [DOI] [PubMed] [Google Scholar]
- 10.Mattlage AE, Redlin SA, Rosterman LR, et al. Use of a Nonexercise Estimate for Prestroke Peak Vo2 During the Acute Stroke Hospital Stay. Cardiopulm Phys Ther J. 2016; 27: 96–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Mattlage AE, Rippee MA, Abraham MG, Sandt J and Billinger SA. Estimated Prestroke Peak VO2 Is Related to Circulating IGF-1 Levels During Acute Stroke. Neurorehabil Neural Repair. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen PL, Wang PY, Sheu WH, et al. Changes of brachial flow-mediated vasodilation in different ischemic stroke subtypes. Neurology. 2006; 67: 1056–8. [DOI] [PubMed] [Google Scholar]
- 13.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology Heart and circulatory physiology. 2011; 300: H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portney L and Watkins M. Foundations of clinical research: applications to practice. East Norwalk: Appleton & Lange, 1993. [Google Scholar]
- 15.ACSM. ACSM's guidelines for exercise testing and prescription. Philadephia, PA: Lippincott Williams & Wilkins, 2014, p.456. [Google Scholar]
- 16.Lerman A and Burnett JC Jr. Intact and altered endothelium in regulation of vasomotion. Circulation. 1992; 86: III12–9. [PubMed] [Google Scholar]
- 17.Talwar T and Srivastava MV. Role of vascular endothelial growth factor and other growth factors in post-stroke recovery. Annals of Indian Academy of Neurology. 2014; 17: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum A and Miller H. Role of cytokines in heart failure. Am Heart J. 1998; 135: 181–6. [DOI] [PubMed] [Google Scholar]
- 19.Aukrust P, Ueland T, Lien E, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999; 83: 376–82. [DOI] [PubMed] [Google Scholar]
- 20.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A and Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000; 31: 1863–70. [DOI] [PubMed] [Google Scholar]
- 21.Medvedkova S and Berezin A. Vascular Endothelial Growth Factor-1 Level and Functional Neurologic Recovery after Ischemic Hemispheric Stroke. Neurochemistry and Neuropharmacology. 2015; 1. [Google Scholar]
- 22.Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH and Lee YJ. Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur J Neurol. 2010; 17: 45–51. [DOI] [PubMed] [Google Scholar]
- 23.Billinger SA, Arena R, Bernhardt J, et al. Physical Activity and Exercise Recommendations for Stroke Survivors: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014; 45: 2532–53. [DOI] [PubMed] [Google Scholar]
- 24.Billinger SA, Coughenour E, Mackay-Lyons MJ and Ivey FM. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat. 2012; 2012: 959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billinger SA, Taylor JM and Quaney BM. Cardiopulmonary response to exercise testing in people with chronic stroke: a retrospective study. Stroke Res Treat. 2012; 2012: 987637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay-Lyons MJ and Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002; 83: 1697–702. [DOI] [PubMed] [Google Scholar]
- 27.Ivey FM, Macko RF, Ryan AS and Hafer-Macko CE. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 28.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005; 36: 2206–11. [DOI] [PubMed] [Google Scholar]
- 29.Macko RF, Katzel LI, Yataco A, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke. 1997; 28: 988–92. [DOI] [PubMed] [Google Scholar]
- 30.Tang A, Sibley KM, Thomas SG, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabil Neural Repair. 2009; 23: 398–406. [DOI] [PubMed] [Google Scholar]
- 31.MacKay-Lyons M, Makrides L and Speth S. Effect of 15% body weight support on exercise capacity of adults without impairments. Phys Ther. 2001; 81: 1790–800. [PubMed] [Google Scholar]
- 32.Mackay-Lyons M, McDonald A, Matheson J, Eskes G and Klus MA. Dual Effects of Body-Weight Supported Treadmill Training on Cardiovascular Fitness and Walking Ability Early After Stroke: A Randomized Controlled Trial. Neurorehabil Neural Repair. 2013. [DOI] [PubMed] [Google Scholar]
- 33.Billinger SA, Gajewski BJ, Guo LX and Kluding PM. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke. 2009; 40: 3086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greyling A, van Mil AC, Zock PL, et al. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis. 2016; 248: 196–202. [DOI] [PubMed] [Google Scholar]
- 35.Mang CS, Campbell KL, Ross CJ and Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013; 93: 1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


