Abstract
Background
Relaxin/relaxin family peptide receptor 1 (RXFP1) signaling is important for both normal physiology and disease. Strong preclinical evidence supports relaxin as a potent antifibrotic molecule. However, relaxin‐based therapy failed in clinical trial in patients with systemic sclerosis. We and others have discovered that aberrant expression of RXFP1 may contribute to the abnormal relaxin/RXFP1 signaling in different diseases. Reduced RXFP1 expression and alternative splicing transcripts with potential functional consequences have been observed in fibrotic tissues. A relative decrease in RXFP1 expression in fibrotic tissues—specifically lung and skin—may explain a potential insensitivity to relaxin. In addition, receptor dimerization also plays important roles in relaxin/RXFP1 signaling.
Methods
This review describes the tissue specific expression, characteristics of the splicing variants, and homo/heterodimerization of RXFP1 in both normal physiological function and human diseases. We discuss the potential implications of these molecular features for developing therapeutics to restore relaxin/RXFP1 signaling and to harness relaxin's potential antifibrotic effects.
Results
Relaxin/RXFP1 signaling is important in both normal physiology and in human diseases. Reduced expression of RXFP1 in fibrotic lung and skin tissues surrenders both relaxin/RXFP1 signaling and their responsiveness to exogenous relaxin treatments. Alternative splicing and receptor dimerization are also important in regulating relaxin/RXFP1 signaling.
Conclusions
Understanding the molecular mechanisms that drive aberrant expression of RXFP1 in disease and the functional roles of alternative splicing and receptor dimerization will provide insight into therapeutic targets that may restore the relaxin responsiveness of fibrotic tissues.
Keywords: alternative splicing, fibrosis, relaxin, RXFP1
The failure of antifibrotic effects in systemic sclerosis patients by relaxin may be the result of reduced expression of its receptor relaxin family peptide receptor 1 (RXFP1) in the fibrotic tissues. Alternative splicing and RXFP1 dimerization with other receptors also play roles in regulating relaxin/RXFP1 signaling. Molecular mechanisms underline the reduced RXFP1 expression and alternative splicing are potential therapeutic targets for restoring relaxin sensitivity in fibrotic diseases.
1. INTRODUCTION
The Relaxin/relaxin family peptide receptor 1 (RXFP1) axis is an “old” pathway (Bennett, 2009; Chihal & Espey, 1973) and the idea that relaxin's actions could be harnessed as an antifibrotic emerged from the seminal work in 1926 identifying relaxin as a hormone that could relax pelvic ligaments (Fevold, Hisaw, & Meyer, 1930; Hisaw, 1926). More recent studies suggest that aberrant expression of RXFP1—with its potentially negative consequences on relaxin signaling—is an important contributor to several diseases (Bahudhanapati et al., 2019; Corallo et al., 2019; Fallowfield et al., 2014; Feng & Agoulnik, 2011; Feng et al., 2010; Giordano et al., 2012; Nagorniewicz et al., 2019; Tan et al., 2016; Thanasupawat et al., 2019). RXFP1 expression can be modulated, and alternative mRNA splicing transcripts may have potential functional consequences in disease tissues (Bahudhanapati et al., 2019; Chow et al., 2014; Chow et al., 2019; Fagerberg et al., 2014; Hsu et al., 2000; Kern & Bryant‐Greenwood, 2009; Kern, Hubbard, Amano, & Bryant‐Greenwood, 2008; Muda et al., 2005; Sasser, 2014; Scott et al., 2006; Scott, Tregear, & Bathgate, 2005; Tan et al., 2016). Therefore, this review will focus on the aberrant expression, functional alterations associated with mRNA splicing variants, and posttranslational heterodimerization of RXFP1 in human physiology and disease. We will discuss the potential implications of the abnormal RXFP1 changes in developing therapeutics to restore relaxin/RXFP1 signaling.
The relaxin family peptide receptor 1 (RXFP1) mediates relaxin‐2 (relaxin) signaling (Hsu et al., 2002). A total of four relaxin receptors, RXFP1 to RXFP4, have been identified. All four are members of the class A seven‐transmembrane G‐protein‐coupled receptor (7TM GPCRs) superfamily based on sequence homology and functional similarity (Banerjee & Mahale, 2015; Kleinlogel, 2016; Yegorov, Bogerd, & Good, 2014). RXFP3 and RXFP4 are classical peptide receptors with a short N‐terminus extracellular domain, while RXFP1 and RXFP2 contain a leucine‐rich repeat (LRR) domain and a low‐density lipoprotein class A (LDLa) module in their extracellular region and belong to the LRR‐containing G protein‐coupled receptor (LGR) subfamily (Bathgate et al., 2013; Yegorov et al., 2014). The extracellular domain of RXFP2 mediates the effects of insulin‐like peptide 3 (INSL3) (Halls et al., 2005; Wilkinson, Speed, Tregear, & Bathgate, 2005). Although relaxin and INSL3 both activate RXFP1 and RXFP2 in vitro, there is no evidence that RXFP2 is activated by relaxin in vivo (Hsu et al., 2002; Kumagai et al., 2002; Scott, Fu, et al., 2005). Moreover, the linker in RXFP2 lacks the proposed binding region for relaxin and thus has a lower affinity for relaxin than RXFP1 (Hoare et al., 2019). The relaxin/RXFP1 system has a much wider range of tissue distribution and function than INSL3/RXFP2 (Halls, Bathgate, & Summers, 2006; Halls, Bathgate, Sutton, Dschietzig, & Summers, 2015).
2. RELAXIN/RXFP1 SIGNALING
Relaxin is a heterodimeric peptide hormone with a two‐chain structure (Wilkinson et al., 2005). It was first identified by Frederick Hisaw in a guinea pig model of pregnancy and parturition (Fevold, Hisaw, & Meyer, 1930; Hisaw, 1926). Relaxin was observed to loosen pelvic ligaments to facilitate parturition by reducing the density of collagen bundles and relaxing the collagen fibers (Chihal & Espey, 1973; Hisaw, 1926; Wilkinson et al., 2005). Additional roles of relaxin/RXFP1 signaling axis were identified in many physiological processes including development of mammary nipples and vaginal epithelium in mice (Kaftanovskaya et al., 2017), cervix growth during pregnancy in rats and pigs (Burger & Sherwood, 1998; Huang, Li, & Anderson, 1997), growth of vagina and uterus in pregnant pigs (Min, Hartzog, Jennings, Winn, & Sherwood, 1997), new blood vessel formation and endometrial connective tissue maintenance in early pregnancy of rhesus monkeys (Goldsmith et al., 2004), and improvement of spermatozoan motility (Lessing et al., 1986).
The relaxin/RXFP1 system has been associated with cAMP, PI3K/Akt, NO/cGMP, MAPK and ERK1/2 signaling (reviewed in Valkovic, Bathgate, Samuel, & Kocan, 2019) (Valkovic et al., 2019). Binding of relaxins to their receptors recruits G‐proteins with subsequent activation of adenylyl cyclase and elevation of cAMP (Bathgate et al., 2013). Activation of NF‐κB by a cAMP/protein kinase A‐dependent mechanism may promote NOS2 (iNOS) expression and nitric oxide (NO) (Bani et al., 1998; Failli et al., 2002). NO has been shown to inhibit profibrotic TGFβ signaling by blocking phosphorylation of Smad2 (Heeg et al., 2005). PI3K/Akt‐associated signaling pathways can be activated by relaxin/RXFP1 to provide vasodilation in the cardiovascular system and regulate cell differentiation (Boccalini, Sassoli, Bani, & Nistri, 2018).
3. PROTEIN STRUCTURE AND FUNCTIONAL CHARACTERISTICS OF RXFP1
While much is known about the cell signaling pathways activated by relaxin, it is clear that ligand–receptor interactions are multidimensional and represent a potential site for cell signaling regulation. In experimental binding assays, relaxin dose, treatment length, and assay temperature contributed to the efficiency of relaxin binding to its receptor (Svendsen et al., 2009; Svendsen, Zalesko, et al., 2008). Cellular pH levels control ligand/receptor complex stability and play an important role in modulating the downstream signaling pathway (Svendsen, Zalesko, et al., 2008). Heterodimerization of WT RXFP1 with its splicing variants or other receptors exhibit negative cooperativity in relaxin/RXFP1 binding (Kern et al., 2008; Svendsen, Zalesko, et al., 2008). Furthermore, overexpression of RXFP1 in HEK‐293T cells resulted in its intracellular accumulation and inhibition of relaxin/RXFP1 signaling (Hoare et al., 2019). Crosstalk between RXFP1 and other receptors is also a current focus in RXFP1 research (Valkovic et al., 2019).
Like other GPCRs, RXFP1 protein consists of three major regions: the extracellular (EC), the transmembrane (TM), and the intracellular (IC) regions (Venkatakrishnan et al., 2013).
3.1. EC region
The EC region of RXFP1 consists of an N‐terminus and three extracellular loops (ECL1–ECL3) (Venkatakrishnan et al., 2013). ECLs link TM segments and contribute to ligand binding, TM positioning, and activation of GPCRs (Palczewski et al., 2000; Wheatley et al., 2012). Three protein domains are identified in the EC region: an LDLa module, a linker domain, and a LRR domain (Hoare et al., 2019).
The LDLa was first described and characterized in LDL receptor and was subsequently identified in other proteins with diverse biological functions (Brown & Goldstein, 1986; Hopkins, Bathgate, & Gooley, 2005). It contains three disulfide bonds and requires a bound calcium ion for its correct folding and stabilization (Hopkins et al., 2005). Although relaxin does not bind to LDLa directly, the binding of relaxin to RXFP1 stabilizes the LDLa/linker structure that leads to the direct contact between the EC and TM region (Diepenhorst et al., 2014; Hoare et al., 2019; Sethi et al., 2016). Removing LDLa module from RXFP1 abolished the ligand‐activated receptor signaling (Scott et al., 2006). Mutagenesis introduced in the LDLa module altered its native three‐dimensional structure (Hopkins et al., 2005; Koduri & Blacklow, 2001; Varret et al., 1997) and fully disrupted receptor activity (Hopkins, Layfield, Ferraro, Bathgate, & Gooley, 2007).
There is a 32‐residue linker hitching the N‐terminus LDLa module and the LRR domain together in RXFP1 (Sethi et al., 2016). This helically shaped linker provides a binding site essential for the steady binding of the relaxin A‐chain (Scott et al., 2006; Sethi et al., 2016). Although mutations in the linker residues have not been shown to affect receptor trafficking or G‐protein coupling, profound effects on reducing relaxin binding and decreasing cAMP response were observed (Sethi et al., 2016).
Different from most of the class A GPCR ligands that bind to the TM region directly, relaxin binds to the RXFP1 through the primary ligand binding site in the LRR domain of the EC region (Hoare et al., 2019). A shallow curvature structure formed by the 10 LRRs is predicted to serve as the primary high‐affinity relaxin binding site (Petrie, Lagaida, Sethi, Bathgate, & Gooley, 2015). The LRR domain potentially interacts with the linker after relaxin binding (Petrie et al., 2015; Scott et al., 2006). When relaxin binds to the LRR, it induces a conformational change of the receptor to position the LDLa module for interacting with the TM region (Hopkins et al., 2007).
3.2. TM region
In addition to the high‐affinity binding site in the EC region, there is a low‐affinity relaxin binding site in the TM region of RXFP1 (Halls et al., 2005). The TM regions of GPCRs form the main structural core of the receptor with seven α‐helices (TM1–TM7) folded together. Conformational changes of different TMs are important for transducing the ligand/receptor interaction to the IC region (Venkatakrishnan et al., 2013). Mutations in the TM region affected receptor conformational selectivity and ligand‐binding affinity in vitro (Dore et al., 2011; Heitz et al., 1999). Two single amino acid changes in the TM6 resulted in dose‐dependent increases of cAMP production (Hsu et al., 2000).
3.3. IC region
The IC region of GPCR interfaces with cytosolic signaling proteins. It includes three intracellular loops (ICL1–ICL3), an intracellular amphipathic helix, and a unique C‐terminal tail containing a phosphorylation site (Hsu et al., 2000; Scheerer et al., 2008). The C‐terminal half of ICL3 plays an important role in linking the relaxin‐activated RXFP1 receptor with G protein (Shpakov et al., 2007).
In summary, activation of RXFP1 by relaxin is a complex multistep process. Relaxin binding initiates RXFP1 signaling. However, the completion of RXFP1‐dependent signal transduction requires the interactions between different receptor regions and correct conformation of the ligand/receptor complex to initiate downstream IC signaling (Sethi et al., 2016).
4. ALTERNATIVE SPLICING VARIANTS AND RXFP1 FUNCTION
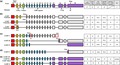
The RXFP1 gene is localized on chromosome 4 with 18 exons and encodes a protein with 757 amino acids (Figure 1a) (Hsu et al., 2000). RXFP1 mRNA is detectable in testis, ovary, adrenal gland, uterus, small intestine, colon, kidney, brain, endometrium, lung, heart, and placenta (https://www.ncbi.nlm.nih.gov/gene/59350) (Fagerberg et al., 2014; Hsu et al., 2000). Interestingly, multiple smaller RXFP1 transcripts were detected in different tissues suggesting the presence of alternative splicing (Hsu et al., 2000). As many as 29 alternative splicing variants have been identified, and 9 of them have been characterized in detail (Hsu et al., 2000; Kern & Bryant‐Greenwood, 2009; Kern et al., 2008; Muda et al., 2005; Scott, Fu, et al., 2005; Scott et al., 2006). Figure 1 summarizes the skipped exons, corresponding protein regions, and known functional consequences for these characterized RXFP1 variants.
Figure 1.

Alternative splicing variants of RXFP1. The genomic structures are shown on the left. The functions of each splicing variant in relaxin binding, signaling, and interfering of wild‐type RXFP1 function are shown on the right. The designations from original reports for each alternative splicing variant are shown. Coding exons are shown in colors and noncoding exons are shown as gray boxes. The locations of novel premature stop codons are shown. (a) Wild‐type RXFP1 gene. Only exons were drawn based on their relative size. The coding exons for each protein domains are shown. (b) Genomic structures of three truncated N‐terminus RXFP1 splicing variants that retain the LDLa module. (c) Genomic structures of truncated N‐terminus RXFP1 that retain both LDLa module and linker domain. For the novel exons 6A and 15A in LGR7 are shown in red‐framed boxes. (d) Genomic structure of a truncated N‐terminus RXFP1 splicing variant, LGR7‐C, retains LDLa module, linker domain and majority of LRRs. (e) Genomic structures of two splicing variants resulted from in‐frame deletion. For the summary table, positive function is labeled as (✓), lack of function is labeled as (X), inconclusive findings in the literature is labeled as (??), and not analyzed is labeled as (N/A). Ref, cited references are: 1. Kern et al. (2008); 2. Scott et al. (2006); 3. Muda et al. (2005); 4. Kern and Bryant‐Greenwood (2009); 5. Hsu et al. (2000); 6. Scott et al. (2006). Abbreviations: LDLa, low‐density lipoprotein class A; LRR, leucine‐rich repeat
4.1. Truncated N‐terminus RXFP1 retaining the LDLa module
Three splicing variants that encode truncated RXFP1 proteins retaining the N‐terminus LDLa module are identified in human uterus tissue (Figure 1b) (Scott et al., 2006). Since RXFP1 was initially named as LGR7, alternative splicing variants for RXFP1 were all designated based on the old nomenclature. These include one exon 4 skipping (designated as LGR7‐Truncate), one exon 3 and exon 4 skipping (LGR7‐Truncate 2) and one exon 3 and exon 4 skipping with extended intronic sequences attached to the end of exon 2 (LGR7‐Truncate 3) (Scott et al., 2006; Scott, Tregear, et al., 2005). All three splicing variants result in open reading frameshifts and a premature stop codon in exon 5 (LGR7‐Truncate and LGR7‐Truncate 2) and a new extended exon 2 (LGR7‐Truncate 3). Additional amino acids, ranging from 1 to 7, are attached to the C‐terminus of these truncated RXFP1 proteins (Scott et al., 2006). Functional analysis of the LGR7‐Truncate showed no interference with surface expression of the wild‐type (WT) RXFP1 co‐expressed in HEK‐293T cells (Scott et al., 2006). In contrast, LGR7‐Truncate inhibited cAMP accumulation induced by WT RXFP1 dose dependently (Scott et al., 2006; Scott, Tregear, et al., 2005). Interestingly, the naturally occurring splicing variant partially lacking the LDLa module (LGR7.10 in Figure 1e) results in normal relaxin binding but abolished its ability of inducing cAMP accumulation (Scott et al., 2006). In mouse, the LGR7‐Truncate is expressed in pregnant uterus and not in brain (Scott et al., 2006). These studies suggest that the LDLa module may act as an antagonist affecting cAMP accumulation and may differentially regulate relaxin/RXFP1 signaling during pregnancy and delivery.
4.2. Truncated N‐terminus RXFP1 retaining both LDLa module and linker domain
In contrast to the splicing variants that retain the LDLa module but lack the linker domain, three of the known splicing variants encoded truncated RXFP1 proteins retain both the LDLa module and the linker domains (Figure 1c) (Kern et al., 2008; Muda et al., 2005). These variants were identified initially in the human fetal membrane and placental tissues and encode RXFP1 proteins lacking the majority of the LRR domain, the TM, and the IC regions (Kern et al., 2008; Muda et al., 2005). One of them (LGR7.1) has two novel exons after exon 6 (exon 6A) and exon 15 (15A) (Muda et al., 2005). Exon 15A contains an alternative poly‐A signal while 6A has a premature stop codon (Muda et al., 2005). LGR7.1 is translated into a RXFP1 protein containing the N‐terminus region with only two LRRs and 10 nonhomologous amino acids at the C‐terminus of the truncated protein (Muda et al., 2005). Kern and colleagues have also identified and characterized two splicing variants (LGR7‐D and LGR7‐F) that encode similarly truncated RXFP1 proteins as LGR7.1 (Kern & Bryant‐Greenwood, 2009; Kern et al., 2008). The LGR7‐D protein is encoded by a splicing variant lacking exon 6 through exon 15 generated by cryptic splice sites and contains the LDLa module, one LRR, and 25 nonhomologous amino acids (Kern et al., 2008). The LGR7‐F is a result of an alternative splicing of exon 6 to exon 18 with cryptic splicing sequences (Kern et al., 2008). It encodes a RXFP1 protein containing the N‐terminus part up to and including the first two LRRs and 10 nonhomologous amino acids at the end (Kern et al., 2008). The LGR7.1 is expressed in different tissues (Muda et al., 2005). Direct comparison of expression levels in the placenta demonstrated much lower levels of the LGR7‐D and LGR7‐F compared to the WT RXFP1 (Kern et al., 2008). Functional analysis revealed that the LGR7.1 and LGR7‐F are predominantly retained within cells (Kern et al., 2008; Muda et al., 2005), while the LGR7‐D is expressed intracellularly and on the cell surface (Kern et al., 2008). When co‐expressed with WT in HEK‐293 cells, both LGR7‐D and LGR7‐F colocalized with WT RXFP1 within the cells and reduce RXFP1‐mediated cAMP accumulation (Kern et al., 2008). In addition, these two truncated variants have dominantly negative effects in WT RXFP1 maturation, homodimerization in the endoplasmic reticulum, and cell surface expression (Kern et al., 2008).
4.3. Truncated N‐terminus RXFP1 retaining LDLa module, linker domain, and majority of LRRs
Another splicing variant (LRP7‐C) misses the TM and IC regions but retains 8 of the 10 LRRs (Figure 1d) (Kern et al., 2008). The LGR7‐C is a result of alternative splicing between exon 12 and exon 18 that creates a novel stop codon at the beginning of exon 18 (Kern et al., 2008). Although it contains 8 LRRs, the LGR7‐C is mainly retained inside the cells and has a similar function as the three truncated N‐terminus RXFP1 retaining only 1 or 2 LRRs (Figure 1c).
4.4. RXFP1 variants with in‐frame deletions
Two splicing variants of RXFP1 result from in‐frame deletions and have been characterized (Figure 1e) (Hsu et al., 2000; Muda et al., 2005). One variant skips exon 3 [LGR7.10 based on Muda et al. (2005), LGR7(2) based on Hsu et al. (2000) or LGR7‐Short based on Scott et al. (2006), Scott, Tregear, et al. (2005) and will be referred as LGR7.10 in this review] and the other skips both exon 12 and 13 (LGR7.2) which is different from the LGR7(2) mentioned above (Hsu et al., 2000) (Muda et al., 2005). The LGR7.10 is detected in the ovary, pituitary, placental, prostate, and uterus tissues and encodes a RXFP1 with an in‐frame deletion of the linker region (Hsu et al., 2000; Muda et al., 2005). When LGR7.10 or LGR7.2 are overexpressed in HEK‐293T cells, only the LGR7.10 was detected on the cell surface but at a very lower level compared to the WT (Muda et al., 2005). The LGR7.2 lost its responsiveness to relaxin and relaxin binding (Muda et al., 2005). Specific binding of relaxin to the LGR7.10 was not detected in a study reported by Muda et al., however, a later study demonstrated specific relaxin binding to this RXFP1 variant in HEK‐293T cells (Muda et al., 2005; Scott et al., 2006).
In summary, all characterized RXFP1 splicing variants have been shown to lose their ability to activate relaxin‐dependent cAMP accumulation. Seven of the nine splicing variants have been shown to interfere with cAMP accumulation mediated by WT RXFP1 signaling. Given the large size of the RXFP1 gene and the numbers of coding exons, we speculate that tissue‐specific splicing variants will be discovered in the future. The differential tissue expression and antagonistic (dominant‐negative) function of these splicing variant receptors suggest that complex posttranscriptional regulation of RXFP1 gene may play important roles in spatial and temporal expression and signaling of relaxin/RXFP1 (Halls, van der Westhuizen, Bathgate, & Summers, 2007).
5. RXFP1 AND CANCER
Studies related to relaxin/RXFP1 and human diseases have been centered on cancer and fibrotic diseases. Relaxin/RXFP1‐mediated cancer growth and invasion have been reported in breast, thyroid, prostate, and other cancer models (Bigazzi, Brandi, Bani, & Sacchi, 1992; Binder, Hagemann, Husen, Schulz, & Einspanier, 2002; Feng et al., 2007; Hombach‐Klonisch et al., 2006; Hombach‐Klonisch, Buchmann, Sarun, Fischer, & Klonisch, 2000; Tashima, Mazoujian, & Bryant‐Greenwood, 1994; Vinall et al., 2011). In most of these cancers, relaxin and relaxin‐like peptides are overexpressed and exert their effects by activating different signaling cascades (Bigazzi et al., 1992) (Hombach‐Klonisch et al., 2000; Tashima et al., 1994) (Vinall et al., 2011). Although the role of RXFP1 in cancer has not been fully understood, it has emerged as a therapeutic target to reverse the procancer effects of increased relaxin (recently reviewed by Thanasupawat et al., 2019) (Thanasupawat et al., 2019). Downregulation of RXFP1 in prostate cancer cells decreased tumor formation induced by these cells in nude mice (Feng et al., 2010).
Are splice variants associated with disease states? Overexpression of LDLa module of RXFP1 in prostate cancer cells resulted in a decrease in proliferation, soft agar colony formation, adhesion and invasion in vitro, and tumor growth in mouse model (Feng & Agoulnik, 2011). These findings suggest that alternative splicing variants retaining different domains of the RXFP1 protein may modulate relaxin function in cancer. In addition, the formation of both GPCR homodimer and heterodimer contributes to the complexity of GPCR signaling (Angers, Salahpour, & Bouvier, 2002). RXFP1 forms a homodimer when it is transported from the ER to the cell membrane and negative cooperativity occurs when it forms a heterodimer with RXFP2 (Svendsen, Vrecl, et al., 2008; Svendsen et al., 2009; Svendsen, Zalesko, et al., 2008). Therefore, dimerization with other receptors or RXFP1 splicing variants may play important roles in normal RXFP1 function and in diseases.
6. RXFP1 AND FIBROTIC DISEASE
Strong preclinical studies support relaxin as a potent antifibrotic molecule (Lam, Royce, Samuel, & Bourke, 2018; McVicker & Bennett, 2017; Ng, Leo, Parry, & Ritchie, 2018; Pini et al., 2010; Samuel, 2005; Samuel et al., 2017; Sasser, 2013). However, relaxin‐based clinical trials failed to show any therapeutic effects in patients with systemic sclerosis (SSc or scleroderma) (Casten & Boucek, 1958; Jefferis & Dixon, 1962; Khanna et al., 2009). Emerging studies demonstrate that the unresponsiveness to relaxin‐based therapy is due to the downregulation of RXFP1 expression in fibrotic tissues, such as lung and skin (Bahudhanapati et al., 2019; Corallo et al., 2019; Giordano et al., 2012; Tan et al., 2016). Therefore, RXFP1 becomes a therapeutic target to restore the responsiveness of fibrotic tissues (Bathgate et al., 2018). Table 1 summarized studies on relaxin and RXFP1 expressions in different fibrotic diseases.
Table 1.
Tissue‐specific expression of relaxin and RXFP1 in fibrotic diseases
| Disease | Tissue/cell | Relaxin | RXFP1 | Reference | ||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | |||
| SSc | Skin | ↓ | Giordano et al. (2012) | |||
| Skin fibroblast | ↓ | Giordano et al. (2012) | ||||
| Skin fibroblast | ↑ | Corallo et al. (2019) | ||||
| Lung | ↓ | Tan et al. (2016) | ||||
| Lung fibroblast | ↓ | Tan et al. (2016) | ||||
| Blood | ↑ | Giordano et al. (2005) | ||||
| IPF | Lung tissue | ↑ | ↓ | ↓ | Tan et al. (2016) | |
| Lung fibroblast | ↓ | ↓ | Tan et al. (2016) | |||
| Lung fibroblast | ↓ | ↓ | Bahudhanapati et al. (2019) | |||
| ESRD | Blood | ↑ with death | Hocher et al. (2004) | |||
| CHF | Blood | ↑ | Han et al. (2017) | |||
| AMI | Blood | ↑ | Zhang et al. (2015) | |||
| AHF | Blood | ↑ with severity | Pintalhao et al. (2017) | |||
| HF | Blood | ↓ | Kupari et al. (2005) | |||
| AF | Blood | ↑ in patients with recurrence | Qu et al. (2019) | |||
| Blood | ↑ in HF | Zhou et al. (2016) | ||||
| HT | Blood | ↓ | Gedikli et al. (2009) | |||
| Liver Cirrhosis | Liver | ↑ | Nagorniewicz et al. (2019) | |||
| Liver | ↑ | Fallowfield et al. (2014) | ||||
The changes in RXFP1 expression are indicated by up or down arrows.
Abbreviations: AF, atrial fibrillation; AHF, acute heart failure; AMI, acute myocardial infarction; CHF, congestive heart failure; ESRD, end‐stage renal disease; HF, heart failure; HT, hypertension; IPF, idiopathic pulmonary fibrosis; RXFP1, relaxin family peptide receptor 1; SSc, systemic sclerosis.
6.1. Lung and skin fibrosis
SSc is a group of heterogeneous disorders characterized by varying degrees of fibrosis of the skin and internal organs (Haustein, 2002; Silman, 1997). Lung fibrosis is one of the most common manifestations and is a major cause of SSc‐related mortality (Denton, Wells, & Coghlan, 2018). The protective role of relaxin signaling in lung fibrosis has been demonstrated in relaxin knockout mice (Samuel et al., 2005; Unemori et al., 1996). Similarly, the RXFP1‐null mice develop early onset peribronchiolar and perivascular fibrosis compared to the relaxin‐null mice (Kamat et al., 2004). Relaxin has been tested in SSc patients as early as 1958 with no beneficial effects (Casten & Boucek, 1958; Jefferis & Dixon, 1962). A smaller study with relaxin showed some efficacy in reducing skin fibrosis (Seibold et al., 2000) which was not validated in a large clinical trial with SSc patients (Khanna et al., 2009).
RXFP1 protein expression in fibrotic lung and skin of SSc patients is dramatically reduced. RXFP1 is similarly downregulated in SSc lung and skin fibroblasts (Corallo et al., 2019; Giordano et al., 2012; Tan et al., 2016). Increased relaxin in peripheral blood was also reported in SSc patients (Giordano et al., 2005). However, the relative reduction of RXFP1 expression in fibrotic tissues may potentially render these tissues insensitive to relaxin. Interestingly, bulk RNA sequencing of SSc skin fibroblasts detected upregulation of 13 different mRNA isoforms without detectable expression of RXFP1 protein in these cells (Corallo et al., 2019). This study supports that the splicing variants of RXFP1 may be important regulators of RXFP1 expression in different fibrotic diseases.
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with an average survival of 2.5 years (King, Pardo, & Selman, 2011). Patients with IPF or other forms of interstitial lung disease may have better pulmonary function if their lung‐specific RXFP1 expression is higher (Tan et al., 2016). In the bleomycin lung fibrosis mouse model, treating with a relaxin‐like agonist reduced bleomycin‐induced collagen deposition in vivo (Pini et al., 2010; Tan et al., 2016). Most notable, RXFP1 expression is dramatically decreased in both lung tissues and lung fibroblasts of IPF patients (Tan et al., 2016). In vitro, silencing of RXFP1 expression was associated with insensitivity to exogenous relaxin, which could be reversed by enhancement of RXFP1 expression in IPF lung fibroblasts (Tan et al., 2016). The findings in both SSc and IPF support that the lack of or reduced expression of RXFP1 in fibrotic tissues of IPF and SSc contributes to the failed responses to relaxin for IPF lung fibroblasts in vitro and relaxin‐based therapies in SSc clinical trials (Casten & Boucek, 1958; Jefferis & Dixon, 1962; Khanna et al., 2009; Tan et al., 2016).
What drives downregulation of RXFP1? Reduction of RXFP1 mRNA suggests that transcriptional mechanisms may account for this. TGFβ decreases expression of RXFP1 at the level of mRNA (Bahudhanapati et al., 2019; Corallo et al., 2019; Tan et al., 2016). Our group recently reported that microRNA‐144‐3p (miR‐144‐3p) regulates RXFP1 in fibrotic lung fibroblasts (Bahudhanapati et al., 2019). MiR‐144‐3p is upregulated in IPF fibroblasts compared with control donor lung fibroblasts. Overexpression of a miR144‐3p mimic and anti‐miR144‐3p in the donor lung fibroblasts resulted in the down‐ and upregulation RXFP1, respectively. Interestingly, Yong and colleagues have also demonstrated that knocking down RXFP1 gene by a synthetic microRNA resulted in a loss of relaxin responsiveness of human dermal fibroblasts (Yong, Callander, Bergin, Samuel, & Bathgate, 2013). In addition to the micro‐RNA regulation, RXFP1 may be regulated by transcription factors important in fibrotic diseases. Therefore, abnormal regulation of RXFP1 expression in fibrotic lung and skin tissues is a therapeutic target for reversing tissue fibrosis.
6.2. Kidney fibrosis
Relaxin has been reported as a natural protective agent against induced or age‐related renal fibrosis (Samuel et al., 2004). Relaxin treatment in an animal model of kidney disease decreases serum creatinine, proteinuria, and interstitial fibrosis (McDonald et al., 2003). Mice lacking relaxin experienced more kidney interstitial fibrosis (Hewitson et al., 2007). Interestingly, in patients with end‐stage renal disease, higher levels of circulating relaxin are associated with mortality (Hocher et al., 2004) although the status of renal‐specific RXFP1 expression in these patients is not known. Short time infusion of serelaxin in patients with alcohol‐related liver cirrhosis increases renal blood flow and decreases renal vascular resistance (Snowdon et al., 2017). Relaxin/RXFP1 signals through pERK1/2 and a neuronal nitric oxide (nNOS)‐NO‐sGC‐cGMP‐dependent pathway to inhibit TGF‐β1/Smad2 pathway and to reduce renal myofibroblast differentiation (Chow et al., 2012; Heeg et al., 2005; Mookerjee et al., 2009). Both angiotensin II type 1 receptor (AT1R) and type 2 receptor (AT2R) can form a heterodimer with RXFP1 (Chow et al., 2014; Chow et al., 2019; Sasser, 2013, 2014). Antagonists for either AT1R or AT2R block relaxin/RXFP1 signaling, supporting comprehensive crosstalk between RXFP1 homodimer and RXFP1‐AT1R and RXFP1‐AT2R heterodimers (Chow et al., 2019). In addition, AT2R activation reduces TGF‐β1 stimulation of profibrotic pathways (Jones, Vinh, McCarthy, Gaspari, & Widdop, 2008; Peluso, Santos, Unger, & Steckelings, 2017; Wang et al., 2017). The complexity of RXFP1 homo and heterodimerization suggests that RXFP1 is at the center of renal‐specific relaxin/RXFP1 antifibrotic signaling.
6.3. Cardiac fibrosis
Relaxin was also introduced as a cardioprotective factor in ischemic heart diseases, atrial fibrillation, and cardiac remodeling in aged heart (Henry et al., 2016; Martin, Romero, & Salama, 2019; Parikh et al., 2013; Zhang et al., 2005). Treatment with relaxin specifically reverses cardiac fibrosis in the cardiomyopathy or hypertrophy fibrosis animal models (Bathgate et al., 2008; Parikh et al., 2013; Sun et al., 2019). Several reviews focusing on the relaxin and their protective roles in cardiac fibrosis have published in recent years (Barker, Tan, & Clevers, 2013; Devarakonda & Salloum, 2018; Du, Bathgate, Samuel, Dart, & Summers, 2010; MacLean & Pasumarthi, 2014; Martin et al., 2019; Ng et al., 2018; Sarwar, Du, Dschietzig, & Summers, 2017; van der Westhuizen et al., 2008). Lower levels of circulating relaxin were reported in patients with heart failure and hypertension (Gedikli et al., 2009; Kupari, Mikkola, Turto, & Lommi, 2005). However, there are multiple studies that reported increases of peripheral blood relaxin and positive correlation of relaxin levels with disease severity in cardiovascular diseases (Han et al., 2017; Pintalhao et al., 2017; Qu et al., 2019; Zhang et al., 2015; Zhou et al., 2016). It will be important to determine the tissue‐specific relaxin and RXFP1 expression in the affected cardiac tissues for understanding the complexity of cardiac‐specific relaxin/RXFP1 signaling. The RXFP1‐AT1R and RXFP1‐AT2R heterodimers described in kidney fibrosis also play important roles in the cardiac system (Chow et al., 2014; Chow et al., 2019; Sasser, 2014). A nonpeptide‐based small molecule relaxin mimetic, ML290, exerts its antifibrotic effects by inhibiting TGF‐β1‐induced Smad2 and Smad3 phosphorylation and increasing matrix metalloprotease 2 expression (Kocan et al., 2017). In addition, a relaxin B‐chain‐only analog, B7‐33, has strong antifibrotic effects in multiple rodent cardiac fibrosis models by activating RXFP1‐AT1R and RXFP1‐AT2R heterodimers and downstream signaling (Barker et al., 2013; Chow et al., 2019; Hossain et al., 2016).
6.4. Liver fibrosis
The major cause of liver fibrosis is the over activation of hepatic stellate cells and their transformation into myofibroblast‐like cells after liver damage (Williams et al., 2001). In the rat carbon tetrachloride model, relaxin increases intrahepatic NO level and reduces hepatic expression of profibrotic markers and portal pressure (Fallowfield et al., 2014). A phase II randomized open‐label clinical study of serelaxin in patients with alcohol‐related liver cirrhosis and portal hypertension was reported (Snowdon et al., 2017). The small molecule relaxin agonist, ML290, also shows antifibrotic effects in an in vitro liver organoid model and an in vivo liver fibrosis mouse model (Kaftanovskaya et al., 2019). Interestingly, unlike skin and lung fibrosis, dramatically increased hepatic expression of RXFP1 has been observed in a rat model of liver cirrhosis and—in contrast to lung and skin‐‐higher expression of RXFP1 is correlated with increased liver fibrosis in human (Fallowfield et al., 2014; McBride et al., 2017; Nagorniewicz et al., 2019). However, whether the upregulation RXFP1 is related to the alternative splicing transcripts or protein variants is not known. The regulation of RXFP1 in liver fibrosis may be fundamentally different from that in lung, skin, and other fibrotic organs.
7. CONCLUSIONS
Relaxin/RXFP1 signaling is important in both normal physiology and in human diseases. Reduced expression of RXFP1 in fibrotic lung and skin tissues surrenders both relaxin/RXFP1 signaling and their responsiveness to exogenous relaxin treatments. Several questions remain. These include how splice variants of RXFP1 regulate expression and relaxin sensitivity. Understanding the molecular mechanisms that drive aberrant expression of RXFP1 in disease will provide insight into therapeutic targets that may restore the relaxin responsiveness of fibrotic tissues.
Chen T‐Y, Li X, Hung C‐H, et al. The relaxin family peptide receptor 1 (RXFP1): An emerging player in human health and disease. Mol Genet Genomic Med. 2020;8:e1194 10.1002/mgg3.1194
Funding information
This project was supported in part by the Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH 1R21AR076024‐01 (YZ), R01 HL126990 (DJK) and a pilot grant from the 5P50AR060780‐08 (YZ).
REFERENCES
- Angers, S. , Salahpour, A. , & Bouvier, M. (2002). Dimerization: An emerging concept for G protein‐coupled receptor ontogeny and function. Annual Review of Pharmacology and Toxicology, 42, 409–435. 10.1146/annurev.pharmtox.42.091701.082314 [DOI] [PubMed] [Google Scholar]
- Bahudhanapati, H. , Tan, J. , Dutta, J. A. , Strock, S. B. , Sembrat, J. , Àlvarez, D. , … Kass, D. J. (2019). MicroRNA‐144‐3p targets relaxin/insulin‐like family peptide receptor 1 (RXFP1) expression in lung fibroblasts from patients with idiopathic pulmonary fibrosis. Journal of Biological Chemistry, 294(13), 5008–5022. 10.1074/jbc.RA118.004910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, A. A. , & Mahale, S. D. (2015). Role of the extracellular and intracellular loops of follicle‐stimulating hormone receptor in its function. Frontiers in Endocrinology, 6, 110 10.3389/fendo.2015.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani, D. , Failli, P. , Bello, M. G. , Thiemermann, C. , Bani Sacchi, T. , Bigazzi, M. , & Masini, E. (1998). Relaxin activates the L‐arginine‐nitric oxide pathway in vascular smooth muscle cells in culture. Hypertension, 31(6), 1240–1247. 10.1161/01.hyp.31.6.1240 [DOI] [PubMed] [Google Scholar]
- Barker, N. , Tan, S. , & Clevers, H. (2013). Lgr proteins in epithelial stem cell biology. Development, 140(12), 2484–2494. 10.1242/dev.083113 [DOI] [PubMed] [Google Scholar]
- Bathgate, R. A. , Halls, M. L. , van der Westhuizen, E. T. , Callander, G. E. , Kocan, M. , & Summers, R. J. (2013). Relaxin family peptides and their receptors. Physiological Reviews, 93(1), 405–480. 10.1152/physrev.00001.2012 [DOI] [PubMed] [Google Scholar]
- Bathgate, R. A. D. , Kocan, M. , Scott, D. J. , Hossain, M. A. , Good, S. V. , Yegorov, S. , … Gooley, P. R. (2018). The relaxin receptor as a therapeutic target ‐ Perspectives from evolution and drug targeting. Pharmacology & Therapeutics, 187, 114–132. 10.1016/j.pharmthera.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Bathgate, R. , Lekgabe, E. D. , McGuane, J. T. , Su, Y. , Pham, T. , Ferraro, T. , … Du, X.‐J. (2008). Adenovirus‐mediated delivery of relaxin reverses cardiac fibrosis. Molecular and Cellular Endocrinology, 280(1–2), 30–38. 10.1016/j.mce.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Bennett, R. G. (2009). Relaxin and its role in the development and treatment of fibrosis. Translational Research, 154(1), 1–6. 10.1016/j.trsl.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigazzi, M. , Brandi, M. L. , Bani, G. , & Sacchi, T. B. (1992). Relaxin influences the growth of MCF‐7 breast cancer cells. Mitogenic and antimitogenic action depends on peptide concentration. Cancer, 70(3), 639–643. https://doi.org/10.1002/1097-0142(19920801)70:3<C639:aid-cncr282070031=E3.0.co;2-v"> [DOI] [PubMed] [Google Scholar]
- Binder, C. , Hagemann, T. , Husen, B. , Schulz, M. , & Einspanier, A. (2002). Relaxin enhances in‐vitro invasiveness of breast cancer cell lines by up‐regulation of matrix metalloproteases. Molecular Human Reproduction, 8(9), 789–796. 10.1093/molehr/8.9.789 [DOI] [PubMed] [Google Scholar]
- Boccalini, G. , Sassoli, C. , Bani, D. , & Nistri, S. (2018). Relaxin induces up‐regulation of ADAM10 metalloprotease in RXFP1‐expressing cells by PI3K/AKT signaling. Molecular and Cellular Endocrinology, 472, 80–86. 10.1016/j.mce.2017.11.021 [DOI] [PubMed] [Google Scholar]
- Brown, M. S. , & Goldstein, J. L. (1986). A receptor‐mediated pathway for cholesterol homeostasis. Science, 232(4746), 34–47. 10.1126/science.3513311 [DOI] [PubMed] [Google Scholar]
- Burger, L. L. , & Sherwood, O. D. (1998). Relaxin increases the accumulation of new epithelial and stromal cells in the rat cervix during the second half of pregnancy. Endocrinology, 139(9), 3984–3995. 10.1210/endo.139.9.6210 [DOI] [PubMed] [Google Scholar]
- Casten, G. G. , & Boucek, R. J. (1958). Use of relaxin in the treatment of scleroderma. Journal of the American Medical Association, 166(4), 319–324. 10.1001/jama.1958.02990040005002 [DOI] [PubMed] [Google Scholar]
- Chihal, H. J. , & Espey, L. L. (1973). Utilization of the relaxed symphysis pubis of guinea pigs for clues to the mechanism of ovulation. Endocrinology, 93(6), 1441–1445. 10.1210/endo-93-6-1441 [DOI] [PubMed] [Google Scholar]
- Chow, B. S. , Chew, E. G. , Zhao, C. , Bathgate, R. A. , Hewitson, T. D. , & Samuel, C. S. (2012). Relaxin signals through a RXFP1‐pERK‐nNOS‐NO‐cGMP‐dependent pathway to up‐regulate matrix metalloproteinases: The additional involvement of iNOS. PLoS ONE, 7(8), e42714 10.1371/journal.pone.0042714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, B. S. M. , Kocan, M. , Bosnyak, S. , Sarwar, M. , Wigg, B. , Jones, E. S. , … Samuel, C. S. (2014). Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney International, 86(1), 75–85. 10.1038/ki.2013.518 [DOI] [PubMed] [Google Scholar]
- Chow, B. S. M. , Kocan, M. , Shen, M. , Wang, Y. , Han, L. , Chew, J. Y. , … Samuel, C. S. (2019). AT1R‐AT2R‐RXFP1 functional crosstalk in myofibroblasts: Impact on the therapeutic targeting of renal and cardiac fibrosis. Journal of the American Society of Nephrology, 30(11), 2191–2207. 10.1681/asn.2019060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corallo, C. , Pinto, A. M. , Renieri, A. , Cheleschi, S. , Fioravanti, A. , Cutolo, M. , … Giordano, N. (2019). Altered expression of RXFP1 receptor contributes to the inefficacy of relaxin‐based anti‐fibrotic treatments in systemic sclerosis. Clinical and Experimental Rheumatology, 37 Suppl, 119(4), 69–75. [PubMed] [Google Scholar]
- Denton, C. P. , Wells, A. U. , & Coghlan, J. G. (2018). Major lung complications of systemic sclerosis. Nature Reviews Rheumatology, 14(9), 511–527. 10.1038/s41584-018-0062-0 [DOI] [PubMed] [Google Scholar]
- Devarakonda, T. , & Salloum, F. N. (2018). Heart disease and relaxin: New actions for an old hormone. Trends in Endocrinology and Metabolism, 29(5), 338–348. 10.1016/j.tem.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenhorst, N. A. , Petrie, E. J. , Chen, C. Z. , Wang, A. , Hossain, M. A. , Bathgate, R. A. , & Gooley, P. R. (2014). Investigation of interactions at the extracellular loops of the relaxin family peptide receptor 1 (RXFP1). Journal of Biological Chemistry, 289(50), 34938–34952. 10.1074/jbc.M114.600882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré, A. S. , Robertson, N. , Errey, J. C. , Ng, I. , Hollenstein, K. , Tehan, B. , … Marshall, F. H. (2011). Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure, 19(9), 1283–1293. 10.1016/j.str.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. J. , Bathgate, R. A. , Samuel, C. S. , Dart, A. M. , & Summers, R. J. (2010). Cardiovascular effects of relaxin: From basic science to clinical therapy. Nature Reviews Cardiology, 7(1), 48–58. 10.1038/nrcardio.2009.198 [DOI] [PubMed] [Google Scholar]
- Fagerberg, L. , Hallström, B. M. , Oksvold, P. , Kampf, C. , Djureinovic, D. , Odeberg, J. , … Uhlén, M. (2014). Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Molecular & Cellular Proteomics: MCP, 13(2), 397–406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli, P. , Nistri, S. , Quattrone, S. , Mazzetti, L. , Bigazzi, M. , Sacchi, T. B. , & Bani, D. (2002). Relaxin up‐regulates inducible nitric oxide synthase expression and nitric oxide generation in rat coronary endothelial cells. The FASEB Journal, 16(2), 252–254. 10.1096/fj.01-0569fje [DOI] [PubMed] [Google Scholar]
- Fallowfield, J. A. , Hayden, A. L. , Snowdon, V. K. , Aucott, R. L. , Stutchfield, B. M. , Mole, D. J. , … Iredale, J. P. (2014). Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology, 59(4), 1492–1504. 10.1002/hep.26627 [DOI] [PubMed] [Google Scholar]
- Feng, S. , & Agoulnik, A. I. (2011). Expression of LDL‐A module of relaxin receptor in prostate cancer cells inhibits tumorigenesis. International Journal of Oncology, 39(6), 1559–1565. 10.3892/ijo.2011.1159 [DOI] [PubMed] [Google Scholar]
- Feng, S. , Agoulnik, I. U. , Bogatcheva, N. V. , Kamat, A. A. , Kwabi‐Addo, B. , Li, R. , … Agoulnik, A. I. (2007). Relaxin promotes prostate cancer progression. Clinical Cancer Research, 13(6), 1695–1702. 10.1158/1078-0432.ccr-06-2492 [DOI] [PubMed] [Google Scholar]
- Feng, S. , Agoulnik, I. U. , Truong, A. , Li, Z. , Creighton, C. J. , Kaftanovskaya, E. M. , … Agoulnik, A. I. (2010). Suppression of relaxin receptor RXFP1 decreases prostate cancer growth and metastasis. Endocrine‐Related Cancer, 17(4), 1021–1033. 10.1677/erc-10-0073 [DOI] [PubMed] [Google Scholar]
- Fevold, H. L. , Hisaw, & F. L. , Meyer, R. K. (1930). The relaxative hormone of the corpus luteum. Its purification and concentration. Journal of the American Chemical Society, 52(8), 3340–3348. 10.1021/ja01371a051 [DOI] [Google Scholar]
- Gedikli, O. , Yilmaz, H. , Kiris, A. , Karaman, K. , Ozturk, S. , Baykan, M. , … Celik, S. (2009). Circulating levels of relaxin and its relation to cardiovascular function in patients with hypertension. Blood Pressure, 18(1–2), 68–73. 10.1080/08037050902864086 [DOI] [PubMed] [Google Scholar]
- Giordano, N. , Papakostas, P. , Lucani, B. , Amendola, A. , Cipolli, F. , Agate, V. M. , … Nuti, R. (2005). Serum relaxin in systemic sclerosis. Journal of Rheumatology, 32(11), 2164–2166. [PubMed] [Google Scholar]
- Giordano, N. , Volpi, N. , Franci, D. , Corallo, C. , Fioravanti, A. , Papakostas, P. , … Nuti, R. (2012). Expression of RXFP1 in skin of scleroderma patients and control subjects. Scandinavian Journal of Rheumatology, 41(5), 391–395. 10.3109/03009742.2012.669496 [DOI] [PubMed] [Google Scholar]
- Goldsmith, L. T. , Weiss, G. , Palejwala, S. , Plant, T. M. , Wojtczuk, A. , Lambert, W. C. , … Cole, D. M. (2004). Relaxin regulation of endometrial structure and function in the rhesus monkey. Proceedings of the National academy of Sciences of the United States of America, 101(13), 4685–4689. 10.1073/pnas.0400776101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls, M. L. , Bathgate, R. A. , & Summers, R. J. (2006). Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Molecular Pharmacology, 70(1), 214–226. 10.1124/mol.105.021691 [DOI] [PubMed] [Google Scholar]
- Halls, M. L. , Bathgate, R. A. D. , Sutton, S. W. , Dschietzig, T. B. , & Summers, R. J. (2015). International union of basic and clinical pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1–4, the receptors for relaxin family peptides. Pharmacological Reviews, 67, 389–440. 10.1124/pr.114.009472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halls, M. L. , Bond, C. P. , Sudo, S. , Kumagai, J. , Ferraro, T. , Layfield, S. , … Summers, R. J. (2005). Multiple binding sites revealed by interaction of relaxin family peptides with native and chimeric relaxin family peptide receptors 1 and 2 (LGR7 and LGR8). Journal of Pharmacology and Experimental Therapeutics, 313(2), 677–687. 10.1124/jpet.104.080655 [DOI] [PubMed] [Google Scholar]
- Halls, M. L. , van der Westhuizen, E. T. , Bathgate, R. A. D. , & Summers, R. J. (2007). Relaxin Family Peptide Receptors – Former orphans reunite with their parent ligands to activate multiple signalling pathways. Br J Pharmacol (Vol., 150, 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. , Luo, J. , Bai, S. , Jia, Y. E. , Chen, X. , Zhao, Y. , … Qi, Y. (2017). Combined assessment of relaxin and B‐Type natriuretic peptide improves diagnostic value in patients with congestive heart failure. American Journal of the Medical Sciences, 354(5), 480–485. 10.1016/j.amjms.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Haustein, U. F. (2002). Systemic sclerosis‐scleroderma. Dermatology Online Journal, 8(1), 3. [PubMed] [Google Scholar]
- Heeg, M. H. , Koziolek, M. J. , Vasko, R. , Schaefer, L. , Sharma, K. , Muller, G. A. , & Strutz, F. (2005). The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney International, 68(1), 96–109. 10.1111/j.1523-1755.2005.00384.x [DOI] [PubMed] [Google Scholar]
- Heitz, F. , Holzwarth, J. A. , Gies, J. P. , Pruss, R. M. , Trumpp‐Kallmeyer, S. , Hibert, M. F. , & Guenet, C. (1999). Site‐directed mutagenesis of the putative human muscarinic M2 receptor binding site. European Journal of Pharmacology, 380(2–3), 183–195. 10.1016/s0014-2999(99)00439-2 [DOI] [PubMed] [Google Scholar]
- Henry, B. L. , Gabris, B. , Li, Q. , Martin, B. , Giannini, M. , Parikh, A. , … Salama, G. (2016). Relaxin suppresses atrial fibrillation in aged rats by reversing fibrosis and upregulating Na+ channels. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 13(4), 983–991. 10.1016/j.hrthm.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson, T. D. , Mookerjee, I. , Masterson, R. , Zhao, C. , Tregear, G. W. , Becker, G. J. , & Samuel, C. S. (2007). Endogenous relaxin is a naturally occurring modulator of experimental renal tubulointerstitial fibrosis. Endocrinology, 148(2), 660–669. 10.1210/en.2006-0814 [DOI] [PubMed] [Google Scholar]
- Hisaw, F. L. (1926). Experimental relaxation of the pubic ligament of the guinea pig. Proceedings of the Society for Experimental Biology and Medicine, 23, 661–663. https://doi.org/10.3181_00379727-23-3107 [Google Scholar]
- Hoare, B. L. , Bruell, S. , Sethi, A. , Gooley, P. R. , Lew, M. J. , Hossain, M. A. , … Bathgate, R. A. D. (2019). Multi‐component mechanism of H2 relaxin binding to RXFP1 through nanoBRET kinetic analysis. iScience, 11, 93–113. 10.1016/j.isci.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher, B. , Ziebig, R. , Krause, R. , Asmus, G. , Neumayer, H. H. , Liefeldt, L. , & Stasch, J. P. (2004). Relaxin is an independent risk factor predicting death in male patients with end‐stage kidney disease. Circulation, 109(19), 2266–2268. 10.1161/01.CIR.0000128598.72920.B5 [DOI] [PubMed] [Google Scholar]
- Hombach‐Klonisch, S. , Bialek, J. , Trojanowicz, B. , Weber, E. , Holzhausen, H.‐J. , Silvertown, J. D. , … Klonisch, T. (2006). Relaxin enhances the oncogenic potential of human thyroid carcinoma cells. American Journal of Pathology, 169(2), 617–632. 10.2353/ajpath.2006.050876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach‐Klonisch, S. , Buchmann, J. , Sarun, S. , Fischer, B. , & Klonisch, T. (2000). Relaxin‐like factor (RLF) is differentially expressed in the normal and neoplastic human mammary gland. Cancer, 89(11), 2161–2168. [DOI] [PubMed] [Google Scholar]
- Hopkins, E. J. , Bathgate, R. A. , & Gooley, P. R. (2005). The human LGR7 low‐density lipoprotein class A module requires calcium for structure. Annals of the New York Academy of Sciences, 1041, 27–34. 10.1196/annals.1282.006 [DOI] [PubMed] [Google Scholar]
- Hopkins, E. J. , Layfield, S. , Ferraro, T. , Bathgate, R. A. , & Gooley, P. R. (2007). The NMR solution structure of the relaxin (RXFP1) receptor lipoprotein receptor class A module and identification of key residues in the N‐terminus region of the module that mediate receptor activation. Journal of Biological Chemistry, 282(6), 4172–4184. 10.1074/jbc.M609526200 [DOI] [PubMed] [Google Scholar]
- Hossain, M. A. , Kocan, M. , Yao, S. T. , Royce, S. G. , Nair, V. B. , Siwek, C. , … Samuel, C. S. (2016). A single‐chain derivative of the relaxin hormone is a functionally selective agonist of the G protein‐coupled receptor, RXFP1. Chemical Science, 7(6), 3805–3819. 10.1039/c5sc04754d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, S. Y. , Kudo, M. , Chen, T. , Nakabayashi, K. , Bhalla, A. , van der Spek, P. J. , … Hsueh, A. J. W. (2000). The three subfamilies of leucine‐rich repeat‐containing G protein‐coupled receptors (LGR): Identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Molecular Endocrinology, 14(8), 1257–1271. 10.1210/mend.14.8.0510 [DOI] [PubMed] [Google Scholar]
- Hsu, S. Y. , Nakabayashi, K. , Nishi, S. , Kumagai, J. , Kudo, M. , Sherwood, O. D. , & Hsueh, A. J. (2002). Activation of orphan receptors by the hormone relaxin. Science, 295(5555), 671–674. 10.1126/science.1065654. 295/5555/671 [pii] [DOI] [PubMed] [Google Scholar]
- Huang, C. J. , Li, Y. , & Anderson, L. L. (1997). Relaxin and estrogen synergistically accelerate growth and development in the uterine cervix of prepubertal pigs. Animal Reproduction Science, 46(1–2), 149–158. 10.1016/S0378-4320(96)01593-X [DOI] [PubMed] [Google Scholar]
- Jefferis, J. E. , & Dixon, A. S. (1962). Failure of relaxin in the treatment of scleroderma. Annals of the Rheumatic Diseases, 21, 295–297. 10.1136/ard.21.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. S. , Vinh, A. , McCarthy, C. A. , Gaspari, T. A. , & Widdop, R. E. (2008). AT2 receptors: Functional relevance in cardiovascular disease. Pharmacology & Therapeutics, 120(3), 292–316. 10.1016/j.pharmthera.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftanovskaya, E. M. , Ng, H. H. , Soula, M. , Rivas, B. , Myhr, C. , Ho, B. A. , … Agoulnik, A. I. (2019). Therapeutic effects of a small molecule agonist of the relaxin receptor ML290 in liver fibrosis. The FASEB Journal, 33(11), 12435–12446. 10.1096/fj.201901046R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftanovskaya, E. M. , Soula, M. , Myhr, C. , Ho, B. A. , Moore, S. N. , Yoo, C. , … Agoulnik, A. I. (2017). Human relaxin receptor is fully functional in humanized mice and is activated by small molecule agonist ML290. Journal of the Endocrine Society, 1, 712–725. 10.1210/js.2017-00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat, A. A. , Feng, S. , Bogatcheva, N. V. , Truong, A. , Bishop, C. E. , & Agoulnik, A. I. (2004). Genetic targeting of relaxin and insulin‐like factor 3 receptors in mice. Endocrinology, 145(10), 4712–4720. 10.1210/en.2004-0515 [DOI] [PubMed] [Google Scholar]
- Kern, A. , & Bryant‐Greenwood, G. D. (2009). Mechanisms of relaxin receptor (LGR7/RXFP1) expression and function. Annals of the New York Academy of Sciences, 1160, 60–66. 10.1111/j.1749-6632.2008.03826.x [DOI] [PubMed] [Google Scholar]
- Kern, A. , Hubbard, D. , Amano, A. , & Bryant‐Greenwood, G. D. (2008). Cloning, expression, and functional characterization of relaxin receptor (leucine‐rich repeat‐containing G protein‐coupled receptor 7) splice variants from human fetal membranes. Endocrinology, 149, 1277–1294. 10.1210/en.2007-1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, D. , Clements, P. J. , Furst, D. E. , Korn, J. H. , Ellman, M. , Rothfield, N. , … Seibold, J. R. (2009). Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: A randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism, 60(4), 1102–1111. 10.1002/art.24380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, T. E. Jr , Pardo, A. , & Selman, M. (2011). Idiopathic pulmonary fibrosis. Lancet, 378(9807), 1949–1961. 10.1016/S0140-6736(11)60052-4 [DOI] [PubMed] [Google Scholar]
- Kleinlogel, S. (2016). Optogenetic user's guide to Opto‐GPCRs. Frontiers in Bioscience, 21, 794–805. 10.2741/4421 [DOI] [PubMed] [Google Scholar]
- Kocan, M. , Sarwar, M. , Ang, S. Y. , Xiao, J. , Marugan, J. J. , Hossain, M. A. , … Summers, R. J. (2017). ML290 is a biased allosteric agonist at the relaxin receptor RXFP1. Scientific Reports, 7(1), 2968 10.1038/s41598-017-02916-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri, V. , & Blacklow, S. C. (2001). Folding determinants of LDL receptor type A modules. Biochemistry, 40(43), 12801–12807. 10.1021/bi011344k [DOI] [PubMed] [Google Scholar]
- Kumagai, J. , Hsu, S. Y. , Matsumi, H. , Roh, J.‐S. , Fu, P. , Wade, J. D. , … Hsueh, A. J. W. (2002). INSL3/Leydig insulin‐like peptide activates the LGR8 receptor important in testis descent. Journal of Biological Chemistry, 277(35), 31283–31286. 10.1074/jbc.C200398200 [DOI] [PubMed] [Google Scholar]
- Kupari, M. , Mikkola, T. S. , Turto, H. , & Lommi, J. (2005). Is the pregnancy hormone relaxin an important player in human heart failure? European Journal of Heart Failure, 7(2), 195–198. 10.1016/j.ejheart.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Lam, M. , Royce, S. G. , Samuel, C. S. , & Bourke, J. E. (2018). Serelaxin as a novel therapeutic opposing fibrosis and contraction in lung diseases. Pharmacology & Therapeutics, 187, 61–70. 10.1016/j.pharmthera.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Lessing, J. B. , Brenner, S. H. , Colon, J. M. , Ginsburg, F. W. , Schoenfeld, C. , Goldsmith, L. T. , … Weiss, G. (1986). Effect of relaxin on human spermatozoa. Journal of Reproductive Medicine, 31(5), 304–309. [PubMed] [Google Scholar]
- MacLean, J. , & Pasumarthi, K. B. (2014). Signaling mechanisms regulating fibroblast activation, phenoconversion and fibrosis in the heart. Indian Journal of Biochemistry & Biophysics, 51(6), 476–482. [PubMed] [Google Scholar]
- Martin, B. , Romero, G. , & Salama, G. (2019). Cardioprotective actions of relaxin. Molecular and Cellular Endocrinology, 487, 45–53. 10.1016/j.mce.2018.12.016 [DOI] [PubMed] [Google Scholar]
- McBride, A. , Hoy, A. M. , Bamford, M. J. , Mossakowska, D. E. , Ruediger, M. P. , Griggs, J. , … Fallowfield, J. A. (2017). In search of a small molecule agonist of the relaxin receptor RXFP1 for the treatment of liver fibrosis. Scientific Reports, 7(1), 10806 10.1038/s41598-017-10521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, G. A. , Sarkar, P. , Rennke, H. , Unemori, E. , Kalluri, R. , & Sukhatme, V. P. (2003). Relaxin increases ubiquitin‐dependent degradation of fibronectin in vitro and ameliorates renal fibrosis in vivo. American Journal of Physiology‐Renal Physiology, 285(1), F59–F67. 10.1152/ajprenal.00157.2002 [DOI] [PubMed] [Google Scholar]
- McVicker, B. L. , & Bennett, R. G. (2017). Novel anti‐fibrotic therapies. Frontiers in Pharmacology, 8, 318 10.3389/fphar.2017.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, G. , Hartzog, M. G. , Jennings, R. L. , Winn, R. J. , & Sherwood, O. D. (1997). Evidence that endogenous relaxin promotes growth of the vagina and uterus during pregnancy in gilts. Endocrinology, 138(2), 560–565. 10.1210/endo.138.2.4909 [DOI] [PubMed] [Google Scholar]
- Mookerjee, I. , Hewitson, T. D. , Halls, M. L. , Summers, R. J. , Mathai, M. L. , Bathgate, R. A. , … Samuel, C. S. (2009). Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. The FASEB Journal, 23(4), 1219–1229. 10.1096/fj.08-120857 [DOI] [PubMed] [Google Scholar]
- Muda, M. , He, C. , Martini, P. G. V. , Ferraro, T. , Layfield, S. , Taylor, D. , … Bathgate, R. A. D. (2005). Splice variants of the relaxin and INSL3 receptors reveal unanticipated molecular complexity. Molecular Human Reproduction, 11(8), 591–600. 10.1093/molehr/gah205 [DOI] [PubMed] [Google Scholar]
- Nagorniewicz, B. , Mardhian, D. F. , Booijink, R. , Storm, G. , Prakash, J. , & Bansal, R. (2019). Engineered relaxin as theranostic nanomedicine to diagnose and ameliorate liver cirrhosis. Nanomedicine, 17, 106–118. 10.1016/j.nano.2018.12.008 [DOI] [PubMed] [Google Scholar]
- Ng, H. H. , Leo, C. H. , Parry, L. J. , & Ritchie, R. H. (2018). Relaxin as a therapeutic target for the cardiovascular complications of diabetes. Frontiers in Pharmacology, 9, 501 10.3389/fphar.2018.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski, K. , Kumasaka, T. , Hori, T. , Behnke, C. A. , Motoshima, H. , Fox, B. A. , … Miyano, M. (2000). Crystal structure of rhodopsin: A G protein‐coupled receptor. Science, 289(5480), 739–745. 10.1126/science.289.5480.739 [DOI] [PubMed] [Google Scholar]
- Parikh, A. , Patel, D. , McTiernan, C. F. , Xiang, W. , Haney, J. , Yang, L. , … Salama, G. (2013). Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circulation Research, 113(3), 313–321. 10.1161/CIRCRESAHA.113.301646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso, A. A. , Santos, R. A. , Unger, T. , & Steckelings, U. M. (2017). The angiotensin type 2 receptor and the kidney. Current Opinion in Nephrology and Hypertension, 26(1), 36–42. 10.1097/MNH.0000000000000289 [DOI] [PubMed] [Google Scholar]
- Petrie, E. J. , Lagaida, S. , Sethi, A. , Bathgate, R. A. D. , & Gooley, P. R. (2015). In a class of their own – RXFP1 and RXFP2 are unique members of the LGR family. Frontiers in Endocrinology, 6, 10.3389/fendo.2015.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini, A. , Shemesh, R. , Samuel, C. S. , Bathgate, R. A. D. , Zauberman, A. , Hermesh, C. , … Rotman, G. (2010). Prevention of bleomycin‐induced pulmonary fibrosis by a novel antifibrotic peptide with relaxin‐like activity. Journal of Pharmacology and Experimental Therapeutics, 335(3), 589–599. 10.1124/jpet.110.170977 [DOI] [PubMed] [Google Scholar]
- Pintalhao, M. , Castro‐Chaves, P. , Vasques‐Novoa, F. , Gonçalves, F. , Mendonça, L. , Fontes‐Carvalho, R. , … Bettencourt, P. (2017). Relaxin serum levels in acute heart failure are associated with pulmonary hypertension and right heart overload. European Journal of Heart Failure, 19(2), 218–225. 10.1002/ejhf.611 [DOI] [PubMed] [Google Scholar]
- Qu, X. , Chen, L. , Sun, L. , Chen, C. , Gao, Z. , Huang, W. , & Zhou, H. (2019). Serum relaxin level predicts recurrence of atrial fibrillation after radiofrequency catheter ablation. Heart and Vessels, 34(9), 1543–1551. 10.1007/s00380-019-01386-1 [DOI] [PubMed] [Google Scholar]
- Samuel, C. S. (2005). Relaxin: Antifibrotic properties and effects in models of disease. Clinical Medicine & Research, 3(4), 241–249. 10.3121/cmr.3.4.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, C. S. , Royce, S. G. , Hewitson, T. D. , Denton, K. M. , Cooney, T. E. , & Bennett, R. G. (2017). Anti‐fibrotic actions of relaxin. British Journal of Pharmacology, 174(10), 962–976. 10.1111/bph.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, C. S. , Zhao, C. , Bond, C. P. , Hewitson, T. D. , Amento, E. P. , & Summers, R. J. (2004). Relaxin‐1‐deficient mice develop an age‐related progression of renal fibrosis. Kidney International, 65(6), 2054–2064. 10.1111/j.1523-1755.2004.00628.x [DOI] [PubMed] [Google Scholar]
- Samuel, C. S. , Zhao, C. , Yang, Q. , Wang, H. , Tian, H. , Tregear, G. W. , & Amento, E. P. (2005). The relaxin gene knockout mouse: A model of progressive scleroderma. The Journal of Investigative Dermatology, 125(4), 692–699. 10.1111/j.0022-202X.2005.23880.x [DOI] [PubMed] [Google Scholar]
- Sarwar, M. , Du, X. J. , Dschietzig, T. B. , & Summers, R. J. (2017). The actions of relaxin on the human cardiovascular system. British Journal of Pharmacology, 174(10), 933–949. 10.1111/bph.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser, J. M. (2013). The emerging role of relaxin as a novel therapeutic pathway in the treatment of chronic kidney disease. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 305(6), R559–565. 10.1152/ajpregu.00528.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser, J. M. (2014). New targets for renal interstitial fibrosis: Relaxin family peptide receptor 1‐angiotensin type 2 receptor heterodimers. Kidney International, 86(1), 9–10. 10.1038/ki.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer, P. , Park, J. H. , Hildebrand, P. W. , Kim, Y. J. , Krauß, N. , Choe, H.‐W. , … Ernst, O. P. (2008). Crystal structure of opsin in its G‐protein‐interacting conformation. Nature, 455(7212), 497–502. 10.1038/nature07330 [DOI] [PubMed] [Google Scholar]
- Scott, D. J. , Fu, P. , Shen, P.‐J. , Gundlach, A. , Layfield, S. , Riesewijk, A. , … Bathgate, R. A. D. (2005). Characterization of the rat INSL3 receptor. Annals of the New York Academy of Sciences, 1041, 13–16. 10.1196/annals.1282.003 [DOI] [PubMed] [Google Scholar]
- Scott, D. J. , Layfield, S. , Yan, Y. , Sudo, S. , Hsueh, A. J. , Tregear, G. W. , & Bathgate, R. A. (2006). Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. Journal of Biological Chemistry, 281(46), 34942–34954. 10.1074/jbc.M602728200 [DOI] [PubMed] [Google Scholar]
- Scott, D. J. , Tregear, G. W. , & Bathgate, R. A. (2005). LGR7‐truncate is a splice variant of the relaxin receptor LGR7 and is a relaxin antagonist in vitro. Annals of the New York Academy of Sciences, 1041, 22–26. 10.1196/annals.1282.005 [DOI] [PubMed] [Google Scholar]
- Seibold, J. R. , Korn, J. H. , Simms, R. , Clements, P. J. , Moreland, L. W. , Mayes, M. D. , … Sanders, M. E. (2000). Recombinant human relaxin in the treatment of scleroderma. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine, 132(11), 871–879. 10.7326/0003-4819-132-11-200006060-00004 [DOI] [PubMed] [Google Scholar]
- Sethi, A. , Bruell, S. , Patil, N. , Hossain, M. A. , Scott, D. J. , Petrie, E. J. , … Gooley, P. R. (2016). The complex binding mode of the peptide hormone H2 relaxin to its receptor RXFP1. Nature Communications, 7, 10.1038/ncomms11344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpakov, A. O. , Gur'yanov, I. A. , Kuznetsova, L. A. , Plesneva, S. A. , Shpakova, E. A. , Vlasov, G. P. , & Pertseva, M. N. (2007). Studies of the molecular mechanisms of action of relaxin on the adenylyl cyclase signaling system using synthetic peptides derived from the LGR7 relaxin receptor. Neuroscience and Behavioral Physiology, 37(7), 705–714. 10.1007/s11055-007-0071-y [DOI] [PubMed] [Google Scholar]
- Silman, A. J. (1997). Scleroderma–demographics and survival. Journal of Rheumatology. Supplement, 48, 58–61. [PubMed] [Google Scholar]
- Snowdon, V. K. , Lachlan, N. J. , Hoy, A. M. , Hadoke, P. W. F. , Semple, S. I. , Patel, D. , … Fallowfield, J. A. (2017). Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Medicine, 14(2), e1002248 10.1371/journal.pmed.1002248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Hao, W. , Fillmore, N. , Ma, H. , Springer, D. , Yu, Z.‐X. , … Murphy, E. (2019). Human relaxin‐2 fusion protein treatment prevents and reverses isoproterenol‐induced hypertrophy and fibrosis in mouse heart. Journal of the American Heart Association, 8(24), e013465 10.1161/jaha.119.013465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen, A. M. , Vrecl, M. , Ellis, T. M. , Heding, A. , Kristensen, J. B. , Wade, J. D. , … Nøhr, J. (2008). Cooperative binding of insulin‐like peptide 3 to a dimeric relaxin family peptide receptor 2. Endocrinology, 149(3), 1113–1120. 10.1210/en.2007-0412 [DOI] [PubMed] [Google Scholar]
- Svendsen, A. M. , Vrecl, M. , Knudsen, L. , Heding, A. , Wade, J. D. , Bathgate, R. A. D. , … Nøhr, J. (2009). Dimerization and negative cooperativity in the relaxin family peptide receptors. Annals of the New York Academy of Sciences, 1160, 54–59. 10.1111/j.1749-6632.2009.03835.x [DOI] [PubMed] [Google Scholar]
- Svendsen, A. M. , Zalesko, A. , Kønig, J. , Vrecl, M. , Heding, A. , Kristensen, J. B. , … Nøhr, J. (2008). Negative cooperativity in H2 relaxin binding to a dimeric relaxin family peptide receptor 1. Molecular and Cellular Endocrinology, 296(1–2), 10–17. 10.1016/j.mce.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Tan, J. , Tedrow, J. R. , Dutta, J. A. , Juan‐Guardela, B. , Nouraie, M. , Chu, Y. , … Kass, D. J. (2016). Expression of RXFP1 is decreased in idiopathic pulmonary fibrosis. Implications for relaxin‐based therapies. American Journal of Respiratory and Critical Care Medicine, 194(11), 1392–1402. 10.1164/rccm.201509-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima, L. S. , Mazoujian, G. , & Bryant‐Greenwood, G. D. (1994). Human relaxins in normal, benign and neoplastic breast tissue. Journal of Molecular Endocrinology, 12(3), 351–364. 10.1677/jme.0.0120351 [DOI] [PubMed] [Google Scholar]
- Thanasupawat, T. , Glogowska, A. , Nivedita‐Krishnan, S. , Wilson, B. , Klonisch, T. , & Hombach‐Klonisch, S. (2019). Emerging roles for the relaxin/RXFP1 system in cancer therapy. Molecular and Cellular Endocrinology, 487, 85–93. 10.1016/j.mce.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Unemori, E. N. , Pickford, L. B. , Salles, A. L. , Piercy, C. E. , Grove, B. H. , Erikson, M. E. , & Amento, E. P. (1996). Relaxin induces an extracellular matrix‐degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. Journal of Clinical Investigation, 98(12), 2739–2745. 10.1172/JCI119099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkovic, A. L. , Bathgate, R. A. , Samuel, C. S. , & Kocan, M. (2019). Understanding relaxin signalling at the cellular level. Molecular and Cellular Endocrinology, 487, 24–33. 10.1016/j.mce.2018.12.017 [DOI] [PubMed] [Google Scholar]
- van der Westhuizen, E. T. , Halls, M. L. , Samuel, C. S. , Bathgate, R. A. , Unemori, E. N. , Sutton, S. W. , & Summers, R. J. (2008). Relaxin family peptide receptors–From orphans to therapeutic targets. Drug Discovery Today, 13(15–16), 640–651. 10.1016/j.drudis.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Varret, M. , Rabes, J. P. , Collod‐Beroud, G. , Junien, C. , Boileau, C. , & Beroud, C. (1997). Software and database for the analysis of mutations in the human LDL receptor gene. Nucleic Acids Research, 25(1), 172–180. 10.1093/nar/25.1.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan, A. J. , Deupi, X. , Lebon, G. , Tate, C. G. , Schertler, G. F. , & Babu, M. M. (2013). Molecular signatures of G‐protein‐coupled receptors. Nature, 494(7436), 185–194. 10.1038/nature11896 [DOI] [PubMed] [Google Scholar]
- Vinall, R. L. , Mahaffey, C. M. , Davis, R. R. , Luo, Z. , Gandour‐Edwards, R. , Ghosh, P. M. , … de Vere White, R. W. (2011). Dual blockade of PKA and NF‐kappaB inhibits H2 relaxin‐mediated castrate‐resistant growth of prostate cancer sublines and induces apoptosis. Horm Cancer, 2(4), 224–238. 10.1007/s12672-011-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Del Borgo, M. , Lee, H. W. , Baraldi, D. , Hirmiz, B. , Gaspari, T. A. , … Widdop, R. E. (2017). Anti‐fibrotic potential of AT2 receptor agonists. Frontiers in Pharmacology, 8, 564 10.3389/fphar.2017.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley, M. , Wootten, D. , Conner, M. T. , Simms, J. , Kendrick, R. , Logan, R. T. , … Barwell, J. (2012). Lifting the lid on GPCRs: The role of extracellular loops. British Journal of Pharmacology, 165(6), 1688–1703. 10.1111/j.1476-5381.2011.01629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, T. N. , Speed, T. P. , Tregear, G. W. , & Bathgate, R. A. (2005). Evolution of the relaxin‐like peptide family. BMC Evolutionary Biology, 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. J. , Benyon, R. C. , Trim, N. , Hadwin, R. , Grove, B. H. , Arthur, M. J. , … Iredale, J. P. (2001). Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut, 49(4), 577–583. 10.1136/gut.49.4.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegorov, S. , Bogerd, J. , & Good, S. V. (2014). The relaxin family peptide receptors and their ligands: New developments and paradigms in the evolution from jawless fish to mammals. General and Comparative Endocrinology, 209, 93–105. 10.1016/j.ygcen.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Yong, K. L. , Callander, G. E. , Bergin, R. , Samuel, C. S. , & Bathgate, R. A. (2013). Development of human cells with RXFP1 knockdown using retroviral delivery of microRNA against human RXFP1. Italian Journal of Anatomy and Embryology, 118(1 Suppl), 10–12. [PubMed] [Google Scholar]
- Zhang, D. , Wang, Y. , Yu, S. , Niu, H. , Gong, X. , & Miao, X. (2015). Serum relaxin levels as a novel biomarker for detection of acute myocardial infarction. International Journal of Clinical and Experimental Medicine, 8(9), 16937–16940. [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Qi, Y.‐F. , Geng, B. , Pan, C.‐S. , Zhao, J. , Chen, L. I. , … Tang, C.‐S. (2005). Effect of relaxin on myocardial ischemia injury induced by isoproterenol. Peptides, 26(9), 1632–1639. 10.1016/j.peptides.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Qu, X. , Gao, Z. , Zheng, G. , Lin, J. , Su, L. , … Huang, W. (2016). Relaxin level in patients with atrial fibrillation and association with heart failure occurrence: A STROBE compliant article. Medicine, 95(21), e3664 10.1097/MD.0000000000003664 [DOI] [PMC free article] [PubMed] [Google Scholar]


