Abstract
Exposure to gestational stress is implicated in increased risk for neuropsychiatric disorders in offspring. We assessed association between prenatal exposure to a one month period of repeated rocket attacks during the 2006 Second Lebanon War in Northern Israel and emergence of childhood neuropsychiatric disorders from birth through nine years of age. Children born to women who were pregnant during the war (N=6,999) were identified and compared to children in the same district born a year later (N=7,054), whose mothers were not exposed to rocket attacks during pregnancy. Multivariable regression models assessed risk for attention deficit hyperactivity disorder, autism, epilepsy, depression and/or anxiety, or any of these disorders (composite outcome) in offspring. Models controlled for multiple confounders including parents’ demographics, parity, maternal use of psychotropic medications during pregnancy, post-partum depression and parental psychiatric history. Results show that exposed and comparison groups did not differ with respect to demographics, parity or psychiatric history. Exposed and comparison groups were similar with regards to gestational age and weight at birth. Multivariable models did not demonstrate an association between exposure to rocket attacks during pregnancy and neuropsychiatric outcomes by age nine. No interactions were found between exposure and gestational trimester at exposure or child’s sex. Our findings suggest that in utero exposure to isolated, one month repeated rocket attacks on a civilian population was not associated with major neuropsychiatric outcomes in children by age nine. Future studies should evaluate whether this exposure is associated with psychiatric and/or other health-related outcomes later in life.
Keywords: Gestational stress, Neurodevelopment, ADHD, Autism spectrum disorder, Depression, Resilience
INTRODUCTION
The prenatal environment is known to influence health outcomes throughout the lifecycle [1]. While this hypothesis was previously suggested in the context of limited supply of nutrients during pregnancy as a predictor of later life medical conditions [2], recent studies suggest that other stress-imposing exposures during pregnancy could induce in utero programming to disease susceptibility [3]. Multiple mechanisms have been proposed to explain the prenatal reprogramming effect of stress, those include the placenta, gene–environment interactions, epigenetics, and neuro-endocrine-immune systems, including the hypothalamic–pituitary–adrenal axis and cytokines (for review see Glover et al.) [4]. Studies show that psychological stress of the pregnant mother is associated with various early conditions in the offspring, including length of gestation and birth related abnormalities [5–7], along with immune related conditions in childhood, such as asthma and allergies [8, 9]. Few studies have suggested that prenatal exposure to gestational stress is associated with neurodevelopmental disorders like attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and other mental health outcomes [10]. Ongoing conflict in the Middle East often exposes civilians to repeated rocket attacks [11]. Several studies on pregnancy exposure to these long-lasting attacks have reported an increased rate of spontaneous abortions [12], low birth weight [13] and other pregnancy complications [14] compared to non-exposed controls. In the summer of 2006, from July 13th to August 13th, during the Second Lebanon War, the population of Northern Israel endured roughly 4,000 rocket attacks, with more than 40 civilian fatalities [15].
To date, no study has evaluated the association of exposure to an isolated period of repeated rocket attacks during pregnancy and offspring development. In the current study, we evaluated the association of exposure to repeated rocket attacks during pregnancy with neuropsychiatric outcomes in childhood using the integrated longitudinal electronic health record data of members from Israel’s largest integrated payer-provider health care organization. We hypothesized that children born to exposed mothers would be at increased risk for a variety of neuropsychiatric conditions, such as ADHD, epilepsy, ASD and depression and/or anxiety compared to a comparison group of children whose mothers were not exposed to the rocket attacks.
METHODS
Study Design
This retrospective cohort study followed an exposed and comparison group from birth (index date) over a nine year period. The exposed group was composed of children born to women who, while pregnant, lived in the Haifa district in Israel during repeated rocket attacks over a one month period during the Second Lebanon War (July 14 – August 14, 2006). The comparison group was composed of children born to women who, while pregnant, lived in the same district one year later (July 14, 2007 – August 14, 2007) during a time of no rocket attacks. Following the ceasefire on August 14, 2006 and extending throughout the study period, there were no additional periods of rocket attacks in proximity to the Haifa district. Children in both groups were followed from birth until a mean age of nine years (April 2016 for the exposed group and April 2017 for the comparison group).
Data Source
Data were extracted from the comprehensive electronic health record (EHR) database of Clalit Health Services (CHS), the largest integrated payer-provider health care organization in Israel, serving over four million members and over half of Israel’s population. CHS provides inpatient and outpatient services in 14 hospitals and over 1,500 clinics. The database includes claim-based inpatient and outpatient clinical and procedural data, socio-demographic, laboratory data, information on all births, pharmacy medication prescription and dispensing records, as well as details regarding health behavior.
Access to the data warehouse and the analyses were approved for this study by the CHS Institutional Review Board.
Study population
We included CHS members whose mothers lived in the Haifa district and were pregnant during the full duration of the Second Lebanon War (exposed group), and CHS members whose mothers were pregnant and lived in the same district 12 months later (non-exposed comparison group). We excluded children whose mothers did not have continuous CHS membership for at least two years before the index birth and through the nine year follow-up period. Additionally, children whose mothers took systemic steroids during pregnancy were excluded, as it was assumed that systemic steroid exposure overrides the physiologic stress response (since it interferes with the physiologic stress response hypothalamus-pituitary-adrenal axis). Lastly, children whose parent (either mother or father) died during the entire study period were excluded in order to avoid the impact of the stress of losing a parent in childhood, that has been shown to be associated with stress response physiology [16], and hence may override the gestational stress possibly associated with the exposure.
Variables
Primary outcomes: Four neuropsychiatric diagnoses outcomes were considered over the nine year follow-up period: ADHD, ASD, epilepsy and depression and/or anxiety disorder (diagnosis of anxiety or depressive disorders or any purchase of an antidepressant medication). In addition, a binary composite outcome was created that indicates whether or not any of the defined neuropsychiatric outcomes occurred (a case was considered if a participant had at least one of the following diagnoses: ADHD, ASD, epilepsy, depression or anxiety). A complete list of International Classification of Diseases (ICD) codes and medications are described in the Supplemental data (Supplemental tables S1 and S2). Clinical diagnoses and ICD codes are commonly added by CHS physicians as part of routine medical care. The diagnoses are based on standard medical practice in Israel and are added to the EHR either by the primary care pediatrician, or alternatively the consulting physician (child and adolescent psychiatrist, child neurologist or developmental pediatrician). Purchase of antidepressant medications was considered for participants with at least one dispensed prescription of any antidepressant (ATC N06A). According to the Israeli Health Ministry instructions, mandatory for all physicians, antidepressant treatment in children is prescribed only following a comprehensive evaluation by a certified child psychiatrist.
The neuropsychiatric outcomes among the children were followed up for nine years due to data constraints (data were extracted on April 2017, allowing a nine year follow-up for the study population).
Covariates
We tested potential confounders at the start of the war (July 14, 2006) for mothers in the exposed group and again on the same date a year later (July 14, 2007) for mothers in the comparison group, including maternal population ethnicity (Arab or Jewish), maternal socio-economic status (low, medium, high), maternal and paternal age at index birth (in years), maternal and paternal smoking status (never, past, current), singleton vs. multiple birth, parity of index birth (ordinal), gestational age at birth (in weeks), psychiatric medication treatment during pregnancy (defined as a purchase of antidepressants or antipsychotics; or at least three purchases of benzodiazepines), maternal post-partum depression (yes, no), trimester of pregnancy at exposure (first, second, third), and the following maternal or paternal diagnoses as documented in the EHR at the time of exposure (i.e. in the prenatal/pregnancy period): ADHD (yes, no), ASD (yes, no), epilepsy (yes, no) and depression and/or anxiety (yes, no).
Statistical Analysis
The distributions of socio-demographic and clinical variables of the exposed and comparison groups were compared using chi-square test for categorical variables, Student’s t-test for continuous variables and Mann-Whitney U test for aparametric cases. Univariate associations between the exposed and comparison groups and the five neuropsychiatric developmental outcomes (ADHD, ASD, epilepsy, depression and/or anxiety, and the composite outcome) were assessed using chi-square tests. Univariate analyses were further stratified by trimester to determine whether there was a differential association between gestational stress and neuropsychiatric developmental outcomes based on the timing (trimester) of the exposure during the mother’s pregnancy.
Five hierarchical logistic regression models assessed the association between exposure and neuropsychiatric developmental outcomes among children controlling for child’s sex, parents’ population sector, socioeconomic status (SES), age, smoking status at index- birth, parity, gestational age and weight at birth, maternal psychiatric medications treatment during index-pregnancy, maternal post-partum depression, trimester of exposure and parental neuropsychiatric diagnoses. Interaction terms were introduced to determine the modifying effect of trimester, child’s sex, maternal ethnic background and maternal post-partum depression on the association between exposure and children’s neuropsychiatric outcomes throughout the nine year study period. Odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Analyses were conducted using R (R Foundation for Statistical Computing) version 3.4.0.
RESULTS
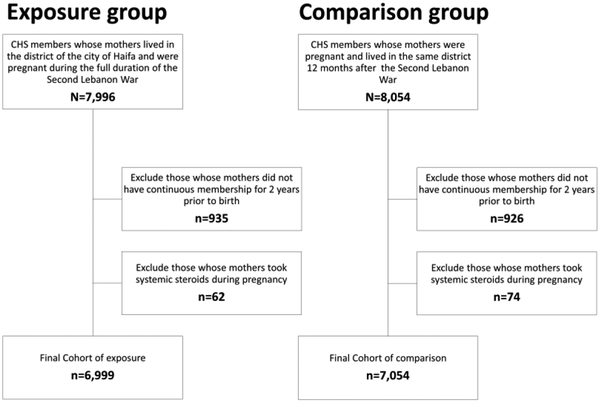
Exposed and comparison groups had similar rates of participants who met exclusion criteria for not continuing membership in CHS during study period (11.7% vs. 11.5%, respectively) and similar rates of prenatal exposure to systemic steroids (0.7% vs. 0.5%, respectively). See Figure 1 for the population flow chart. Characteristics of the included exposed (N=6,999) and comparison (N=7,054) groups are presented in Table 1. Among the entire sample, 49% of mothers were Arab and 51% were Jewish, mean maternal age at birth was 28.3 years, mean parity was 2.5 children and 1% was treated with psychiatric medications during pregnancy (0.9% in the exposed group). Women exposed to the rocket attacks during pregnancy had a slightly greater gestational age at delivery (38.8 vs. 38.7 weeks, p=0.04), and their rate of postpartum depression was slightly lower than the comparison group (2.0% vs. 2.5%, p=0.03). Children in both the exposed and comparison group were born with a mean weight of 3.2 kilograms.
Figure 1. Population flowchart.
CHS= Clalit Health Services
Table 1.
Cohort characteristics for pregnant women exposed to gestational stress, 14 July 2006 – 4 June 2007, and the unexposed comparison group, 14 July 2007 – 4 June, 2008, Haifa, Israel
| Total N= 14,053 |
EXPOSED N= 6,999 |
COMPARISON N=7,054 |
P-Value | |
|---|---|---|---|---|
| Offspring sex, No. (%) | ||||
| Female | 6,926 (49.3) | 3,457 (49.4) | 3,469 (49.2) | |
| Male | 7,127 (50.7) | 3,542 (50.6) | 3,585 (50.8) | 0.8 |
| Maternal population sector, No. (%) | ||||
| Arab | 6,814 (48.5) | 3,444 (49.2) | 3,370 (47.8) | |
| Jewish | 7,239 (51.5) | 3,555 (50.8) | 3,684 (52.2) | 0.09 |
| Maternal SES, No. (%) | ||||
| Low | 6,883 (49.3) | 3,489 (50.1) | 3,394 (48.4) | |
| Medium | 3,935 (28.2) | 1,946 (28.0) | 1,989 (28.4) | 0.1 |
| High | 3,151 (22.6) | 1,527 (21.9) | 1,624 (23.2) | |
| Maternal age at birth, yrs. | ||||
| Mean (SD) | 28.3 (5.3) | 28.3 (5.3) | 28.3 (5.3) | 0.5 |
| Maternal smoking status, No. (%) | ||||
| Never | 10,131 (88.5) | 4,391 (88.9) | 5,740 (88.2) | |
| Past | 416 (3.6) | 132 (2.7) | 284 (4.4) | <0.001 |
| Current | 905 (7.9) | 418 (8.5) | 487 (7.5) | |
| Paternal age at birth# | ||||
| Mean (SD) | 32.4 (5.8) | 32.4 (5.9) | 32.4 (5.8) | 0.8 |
| Paternal smoking status, No. (%)# | ||||
| Never | 4,998 (59.0) | 2,038 (59.0) | 2,960 (59.0) | |
| Past | 525 (6.2) | 189 (5.5) | 336 (6.7) | 0.05 |
| Current | 2,949 (34.8) | 1,228 (35.5) | 1,721 (34.3) | |
| Singleton birth, No. (%) | ||||
| Yes | 13,427 (95.5) | 6,706 (95.8) | 6,721 (95.3) | |
| No | 626 (4.5) | 293 (4.2) | 333 (4.7) | 0.1 |
| Parity | ||||
| Mean (SD) | 2.5 (1.5) | 2.5 (1.5) | 2.5 (1.6) | 0.2 |
| Gestational age at birth, wks. | ||||
| Mean (SD) | 38.8 (1.8) | 38.8 (1.7) | 38.7 (1.9) | 0.04 |
| Birth weight, grams. | ||||
| Mean (SD) | 3,189.0 (560.6) | 3,205.5 (525.6) | 3,171.9 (594.6) | 0.158 |
| Psychiatric medication treatment during pregnancy, No. (%) | ||||
| Yes | 132 (0.9) | 64 (0.9) | 68 (1.0) | |
| No | 13,921 (99.1) | 6,935 (99.1) | 6,986 (99.0) | 0.8 |
| Post-partum depression, No. (%) | ||||
| Yes | 314 (2.2) | 137 (2.0) | 177 (2.5) | |
| No | 13,739 (97.8) | 6,862 (98.0) | 6,877 (97.5) | 0.03 |
| Trimester of pregnancy at exposure, No. (%) | ||||
| First | 5,396 (38.4) | 2,667 (38.1) | 2,729 (38.7) | |
| Second | 5,734 (40.8) | 2,865 (40.9) | 2,869 (40.7) | 0.8 |
| Third | 2,923 (20.8) | 1,467 (21.0) | 1,456 (20.6) | |
| Maternal ADHD | ||||
| Yes | 28 (0.2) | 12 (0.2) | 16 (0.2) | |
| No | 14,025 (99.8) | 6,897 (99.8) | 7,038 (99.8) | 0.6 |
| Paternal ADHD | ||||
| Yes | 27 (0.2) | 13 (0.2) | 14 (0.2) | |
| No | 14,026 (99.8) | 6,986 (99.8) | 7,040 (99.8) | 1.0 |
| Maternal ASD | ||||
| Yes | 6 (0.04) | 4 (0.1) | 2 (0.03) | |
| No | 14,047 (99.96) | 6,995 (99.9) | 7,052 (99.97) | 0.4 |
| Paternal ASD | ||||
| Yes | 6 (0.04) | 4 (0.1) | 2 (0.03) | |
| No | 14,047 (99.96) | 6,995 (99.9) | 7,052 (99.97) | 0.4 |
| Maternal Epilepsy | ||||
| Yes | 69 (0.5) | 39 (0.6) | 30 (0.4) | |
| No | 13,984 (99.5) | 6,960 (99.4) | 7,024 (99.6) | 0.3 |
| Paternal Epilepsy | ||||
| Yes | 71 (0.5) | (36 (0.5) | 35 (0.5) | |
| No | 13,982 (99.5) | 6,963 (99.5) | 7,019 (99.5) | 0.97 |
| Maternal Depression and/or Anxiety | ||||
| Yes | 1,213 (8.6) | 578 (8.3) | 635 (9.0) | |
| No | 12,840 (91.4) | 6,421 (91.7) | 6,419 (91.0) | 0.1 |
| Paternal Depression and/or Anxiety | ||||
| Yes | 892 (6.3) | 426 (6.1) | 466 (6.6) | |
| No | 13,161 (93.7) | 6,573 (93.9) | 6,588 (93.4) | 0.2 |
There were similar rates of fathers that were not CHS members from 2 years prior to index birth, hence were not included in the analyses. In the exposed group, 1,996 (28.5%) of fathers; in the control group, 1,922 (27.2%) fathers.
Abbreviations: SES, socio-economic status; SD, standard deviation; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder.
The five multivariable logistic models examining associations between gestational stress and developmental neuropsychiatric outcomes are presented in Table 2. There was no observed association between gestational stress and neuropsychiatric outcomes, including ADHD (OR:1.02; 95% CI: 0.92–1.14), ASD (OR: 1.03; 95% CI: 0.62–1.69), epilepsy (OR: 1.14; 95% CI: 0.78–1.68), depression and/or anxiety (OR: 1.02; 95% CI: 0.82–1.27) or the composite outcome of any of these diagnoses (OR: 1.06; 95% CI: 0.95–1.17) over the nine year course of the children’s follow-up. The following variables emerged as positively associated with the composite outcome: male sex (OR: 2.48; 95% CI: 2.23–2.76); being from the Jewish sector (OR: 2.66; 95% CI: 2.38–2.98); maternal and paternal smoking (OR: 1.40; 95% CI: 1.18–1.66 and OR: 1.18; 95% CI: 1.05–1.32, respectively) and post-partum depression up to one year following birth (OR: 1.75; 95% CI: 1.29–2.36); gestational age at delivery was negatively associated with ADHD and the composite outcome (OR: 0.93; 95% CI: 0.91–0.96). The four interaction terms were examined in all models (gestational stress*trimester, gestational stress*sex, gestational stress*maternal population ethnicity and gestational stress*maternal post-partum depression); none were statistically significant.
Table 2.
Associationsa between exposure to gestational stress and neuropsychiatric developmental outcomes among offspring until nine years of age.
| Gestational Stress (Y/N) | ADHD (n=1905) | ASD (n=77) | Epilepsy (n=177) | Depression/Anxiety (n=398) | Composite outcome (n=2225) |
|---|---|---|---|---|---|
| β | 0.02 | 0.03 | 0.13 | 0.01 | 0.01 |
| SE β | 0.06 | 0.25 | 0.2 | 0.11 | 0.11 |
| OR | 1.02 | 1.03 | 1.14 | 1.02 | 1.06 |
| Lower 95% CI | 0.92 | 0.62 | 0.78 | 0.82 | 0.95 |
| Upper 95% CI | 1.14 | 1.69 | 1.68 | 1.27 | 1.17 |
| P-value | 0.66 | 0.92 | 0.49 | 0.84 | 0.3 |
| Nagelkerke R2 | 0.31 | 0.26 | 0.15 | 0.21 | 0.21 |
Odds ratios were calculated via logistic multivariable regression models and adjusted for offspring sex, parents′ population sector, age at child’s birth and smoking status, parity, child’s gestational age and weight at birth, maternal psychiatric medication use during pregnancy, post-partum depression and parental history of ADHD, ASD, epilepsy or depression/anxiety.
Abbreviations: OR, odds ratio; CI, confidence interval; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder.
A sensitivity analysis was conducted among the study population that included children who lost their parents during study period (that were excluded in the main analyses) and found similar results for all models.
DISCUSSION
In the current study, we found no association between prenatal exposure to an isolated period of repeated rocket attacks and neuropsychiatric outcomes on children by age nine. This finding is inconsistent with a group of previous studies that observed an association of gestational stress due to stressful life events [17–23] or natural disasters [24, 25] with neuropsychiatric outcomes in children. Our results do concur with two studies that found no association between gestational stress and ASD in offspring [26, 27].
There are several potential explanations for our null findings. First, it is possible that there is a resilience effect of gestational exposure to stress on select children outcomes. Research has suggested that there may be an “inoculation effect” of stress up to a certain degree, before it reaches a “toxic” level [28, 29], along with the notion of community resilience at times of extreme hardship, such as disasters or other adverse events [30]. It may be that the one month exposure to rocket attacks resulted in the development of tolerance to the stress rather than imposing toxic levels of stress on the child’s development in utero. Indeed, the lower post-partum depression rate among exposed mothers in this study may support this notion of resilience. Second, it is possible that the outcomes we evaluated are not sensitive enough to detect the signal of the risk imposed by the exposure. For example, previous studies show associations of prenatal stress exposure with increased autistic traits or ADHD symptoms [18]; outcomes in the current analysis were defined by diagnoses (ASD, ADHD), rather than symptom-level outcomes, which may be more sensitive to smaller effect size of exposure. Additionally, the adverse neuropsychiatric effects of exposure may present at ages older than nine years (end of the follow-up period), as suggested by studies describing adolescence and early adulthood depression in youth exposed to prenatal stress [31–33], and by a recent study linking prenatal exposure to terror attacks with increased risk for offspring schizophrenia in adulthood [34]. Future follow-up of this cohort through adulthood may reveal long-term consequences of the exposure.
Strengths and Limitations
The major strength of the current study was the large sample that we were able to study using comprehensive EHR data from Israel’s largest health care organization. This permitted the longitudinal examination of the effect of multiple mother and child covariates along with interactions such as offspring sex and timing of exposure by trimester on the neuropsychiatric outcomes. An additional strength was the ability to create a control group composed of a population of women who were pregnant one year following the exposure who closely resembled the exposed group in their socio-demographic and clinical covariates.
The current study had several limitations. Primarily, we did not have any measure of the subjective stress that the pregnant mothers experienced during the exposure period. The rationale for the study hypothesis was that the exposed mothers would be stressed during pregnancy in a manner that would trigger biological processes that would contribute to an increase in the identified outcomes compared to control mothers. However, others have suggested that the subjective experience of distress following exposure, such as in the case of a natural disaster like Project Ice Storm [24], is the dominant factor that drives associations with offspring adverse outcomes beyond the exposure itself. In our study, if exposed pregnant mothers were indeed resilient to the traumatic stress and did not respond with a marked subjective distress, we would not have captured the expected associations. Another limitation is that we were unable to track pregnant mothers who left the rocket-targeted area during the exposure period, which may serve to dilute the expected association. Another key limitation was that we did not have dimensional outcome measures (i.e. scales for neuropsychiatric conditions) that might have been more sensitive to subtle changes in brain and behavior, rather we used dichotomous outcomes composed of threshold clinical disorders (i.e. ICD diagnoses), and the diagnoses were not established in a systematic procedure by child psychiatrists, rather were added to the EHR as part of standard clinical practice. This limitation is augmented by the fact that interrater reliability for child psychiatric disorders is hard to be established among practitioners. In addition, we cannot rule out the possibility that some participants received consultations outside of the CHR system and therefore were not included in the EHR. It is also possible that some multivariable models included too many predictor variables as co-variates, given that for some outcomes the number of cases was rather small. Lastly, while we selected an exposed and comparison group that were similar on most observable clinical and socio-demographic variables (with the exception of gestational age and smoking status that were both controlled for in the multivariable analyses), there is always the possibility in observational studies that there are unobservable differences, which are not considered, that could substantially bias the detected associations.
To conclude, we did not find an association between prenatal exposure to an isolated one month rocket attack on a civilian population and neuropsychiatric outcomes in children nine years old. Despite the inherent limitations of the study design, our findings suggest that our study population may be resilient to the impact of the exposure to repeated short bursts of stress. Presumably, if the effect was immense, we would have still seen non-null results despite the methodological limitations that bias the findings to the null. We therefore postulate that a huge pathogenic effect of rocket attacks in pregnancy is not obvious. Our findings highlight the need for future studies on prenatal reprogramming effect of gestational stress to focus on subjective perception of the stress, examining cohorts prospectively, evaluating child’s behavior with established and reliable instruments and a long-term follow-up including directly observed traits, behaviors and diagnoses.
Supplementary Material
FUNDING
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH120437.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
DECLARATION OF CONFLICT OF INTEREST
Dr. Barzilay serves on the scientific board and reports stock ownership in ‘Taliaz Health’, with no conflict of interest relevant to this work. All other authors declare no potential conflict of interest.
REFERENCES
- 1.Barker DJP (1998) In utero programming of chronic disease. Clin. Sci 95: [PubMed] [Google Scholar]
- 2.Roseboom T, de Rooij S, Painter R (2006) The Dutch famine and its long-term consequences for adult health. Early Hum Dev 82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 3.Mulder EJH, Robles de Medina PG, Huizink AC, et al. (2002) Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev 70:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Glover V, O’Donnell KJ, O’Connor TG, Fisher J (2018) Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—A global perspective. Dev Psychopathol 30:843–854. doi: 10.1017/S095457941800038X [DOI] [PubMed] [Google Scholar]
- 5.Tegethoff M, Greene N, Olsen J, et al. (2010) Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: evidence from a population-based cohort study. Psychosom Med 72:419–26. doi: 10.1097/PSY.0b013e3181d2f0b0 [DOI] [PubMed] [Google Scholar]
- 6.Zhu P, Tao F, Hao J, et al. (2010) Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol 203:34e1–34.e8. doi: 10.1016/j.ajog.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Class QA, Lichtenstein P, Långström N, D’Onofrio BM (2011) Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: A population study of 2.6 million pregnancies. Psychosom Med 73:234–241. doi: 10.1097/PSY.0b013e31820a62ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandoli G, von Ehrenstein O, Ghosh JKC, et al. (2016) Prenatal maternal stress and the risk of lifetime wheeze in young offspring: An examination by stressor and maternal ethnicity. J Immigr Minor Heal 18:987–95. doi: 10.1007/s10903-015-0269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartwig IR V, Sly PD, Schmidt LA, et al. (2014) Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol 134:160–9. doi: 10.1016/j.jaci.2014.01.033 [DOI] [PubMed] [Google Scholar]
- 10.Van den Bergh BRH, van den Heuvel MI, Lahti M, et al. (2017) Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Henrich CC, Shahar G (2013) Effects of exposure to rocket attacks on adolescent distress and violence: A 4-year longitudinal study. J Am Acad Child Adolesc Psychiatry 52:619–627. doi: 10.1016/j.jaac.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 12.Wainstock T, Lerner-Geva L, Glasser S, et al. (2013) Prenatal stress and risk of spontaneous abortion. Psychosom Med 75:228–235. doi: 10.1097/PSY.0b013e318280f5f3 [DOI] [PubMed] [Google Scholar]
- 13.Wainstock T, Anteby E, Glasser S, et al. (2013) The association between prenatal maternal objective stress, perceived stress, preterm birth and low birthweight. J Matern Neonatal Med 26:973–977. doi: 10.3109/14767058.2013.766696 [DOI] [PubMed] [Google Scholar]
- 14.Keren M, Keren N, Eden A, et al. (2015) The complex impact of five years of stress related to life-threatening events on pregnancy outcomes: A preliminary retrospective study. Eur Psychiatry 30:317–321. doi: 10.1016/j.eurpsy.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Rubin U (2007) The rocket campaign against Israel during the 2006 Lebanon War. Begin-Sadat Center for Strategic Studies. Retrieved from http://www.jstor.org/stable/resrep04280 [Google Scholar]
- 16.Tyrka AR, Wier L, Price LH, et al. (2008) Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry 63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okano L, Ji Y, Riley AW, Wang X (2018) Maternal psychosocial stress and children’s ADHD diagnosis: a prospective birth cohort study. J Psychosom Obstet Gynecol 1–9. doi: 10.1080/0167482X.2018.1468434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronald A, Pennell CE, Whitehouse AJO (2010) Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol 1:223. doi: 10.3389/fpsyg.2010.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson M, Mattes E, Oddy WH, et al. (2011) Prenatal stress and risk of behavioral morbidity from age 2 to 14 years: the influence of the number, type, and timing of stressful life events. Dev Psychopathol 23:507–20. doi: 10.1017/S0954579411000241 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Olsen J, Vestergaard M, Obel C (2010) Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry 19:747–53. doi: 10.1007/s00787-010-0113-9 [DOI] [PubMed] [Google Scholar]
- 21.Zhu P, Hao J-H, Tao R-X, et al. (2015) Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry 24:1139–47. doi: 10.1007/s00787-015-0701-9 [DOI] [PubMed] [Google Scholar]
- 22.Rijlaarsdam J, van IJzendoorn MH, Verhulst FC, et al. (2017) Prenatal stress exposure, oxytocin receptor gene (OXTR) methylation, and child autistic traits: The moderating role of OXTR rs53576 genotype. Autism Res 10:430–438. doi: 10.1002/aur.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Class QA, Abel KM, Khashan AS, et al. (2014) Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med 44:71–84. doi: 10.1017/S0033291713000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King S, Dancause K, Turcotte-Tremblay A-M, et al. (2012) Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today 96:273–88. doi: 10.1002/bdrc.21026 [DOI] [PubMed] [Google Scholar]
- 25.Walder DJ, Laplante DP, Sousa-Pires A, et al. (2014) Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res 219:353–360. doi: 10.1016/j.psychres.2014.04.034 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Vestergaard M, Obel C, et al. (2009) A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics 123:1102–1107. doi: 10.1542/peds.2008-1734 [DOI] [PubMed] [Google Scholar]
- 27.Rai D, Golding J, Magnusson C, et al. (2012) Prenatal and early life exposure to stressful life events and risk of autism spectrum disorders: population-based studies in Sweden and England. PLoS One 7:e38893. doi: 10.1371/journal.pone.0038893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seery MD (2011) Resilience: A silver lining to experiencing adverse life events? Curr Dir Psychol Sci 20:390–394. doi: 10.1177/0963721411424740 [DOI] [Google Scholar]
- 29.Lyons DM, Parker KJ (2007) Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress 20:423–433. doi: 10.1002/jts.20265 [DOI] [PubMed] [Google Scholar]
- 30.Norris FH, Stevens SP, Pfefferbaum B, et al. (2008) Community resilience as a metaphor, theory, set of capacities, and strategy for disaster readiness. Am J Community Psychol 41:127–150. doi: 10.1007/s10464-007-9156-6 [DOI] [PubMed] [Google Scholar]
- 31.Grizenko N, Fortier M-È, Gaudreau-Simard M, et al. (2015) The effect of maternal stress during pregnancy on IQ and ADHD symptomatology. J Can Acad Child Adolesc Psychiatry 24:92–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Kingsbury M, Weeks M, MacKinnon N, et al. (2016) Stressful life events during pregnancy and offspring depression: Evidence from a prospective cohort study. J Am Acad Child Adolesc Psychiatry 55:709–716.e2. doi: 10.1016/j.jaac.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 33.Betts KS, Williams GM, Najman JM, Alati R (2015) The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety 32:82–90. doi: 10.1002/da.22272 [DOI] [PubMed] [Google Scholar]
- 34.Weinstein Y, Levav I, Gelkopf M, et al. (2018) Association of maternal exposure to terror attacks during pregnancy and the risk of schizophrenia in the offspring: A population-based study. Schizophr Res 199:163–167. doi: 10.1016/j.schres.2018.04.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.