Dear Editor, The clinical presentation and pathology of discoid lupus erythematosus (DLE) varies by stage. Erythematous scaly papules and plaques characterize the early lesions. They progress to dyspigmented patches and plaques with central scarring and atrophy. Under histology, early lesions demonstrate a perivascular and perifollicular inflammatory infiltrate, liquefactive degeneration and keratinocyte apoptosis. Increased collagen deposition within the dermis and reduced inflammatory infiltrate is seen in later stages [1].
The cellular and molecular underpinnings associated with the progression of the lesions from inflammatory to scarring stages in DLE is not well understood. We previously identified higher CD8+ T cells in early-stage DLE skin and elevated CD20+ B cells in later-stage DLE skin [2]. To further explore the immune cell microenvironment, we sought to compare the T-helper 1 (Th1) and T-helper 2 (Th2) subpopulations in early-, mid- and late-stage DLE skin using immunohistochemistry. Th1 and Th2 cells have distinct effector functions where Th1 cells potentiate a cell-mediated inflammatory response and Th2 cells promote a humoral and fibrotic response [3]. We hypothesized that there would be a Th1 cell predominance in early-stage DLE skin, where recruitment and activation of macrophages and CD8+ T cells can occur. We postulated that late-stage DLE skin would show Th2 cell predominance, leading to activation of fibrotic pathways and plasma cell differentiation.
We collected lesional skin biopsies from the face (6), scalp (10) and arms (5) of 21 participants with DLE. Subjects on immunosuppressive medications (e.g. prednisone, methotrexate) and antimalarials were excluded. A dermatopathologist (GH) blinded to the clinical data examined H&E slides of formalin-fixed, paraffin-embedded biopsy specimens, and divided them into three DLE stages: inflammatory (DLE-I; N=9), inflammatory with scarring (DLE-I/S; N=7), or scarring (DLE-S; N=5). DLE-I lesions featured robust perifollicular, interface, and/or perivascular inflammatory infiltrates with no increased dermal collagen deposition. DLE-S lesions had sparse infiltrates and substantially increased collagen deposition. DLE-I/S lesions had moderate inflammation and increased dermal collagen [1]. Following published immunohistochemistry protocols [4], specimens were stained with chromogen-linked monoclonal antibodies against T-bet (Abcam) (Th1 cells), GATA-3 (Biocare) (Th2 cells) and CD4 (Abcam) (T cells). Double and single positive cells were manually counted by independent readers (BFC, GAH) in representative high-power fields in three microanatomic regions: epidermal-dermal junction (interface), perifollicular and perivascular. Cell counts and percent ratios of CD4+T-bet+ (Th1) over percent CD4+GATA-3+ (Th2) cells were calculated for each specimen in each region. Non-parametric comparisons were performed with Kruskal-Wallis tests and Dunn pairwise comparison tests using GraphPad Prism (v. 8.1).
For all regions, the median percent ratio of Th1 to Th2 cells was highest in DLE-I skin (1.70 (IQR:1.04-1.97)) compared to DLE-I/S (0.57 (0.44-0.89)), and DLE-S skin (0.27 (0.17-0.92)) (p=0.002, Fig. 1a). Pairwise comparison revealed a significant difference between DLE-I and DLE-I/S skin (p=0.03), and DLE-I and DLE-S skin (p=0.02). Within the perivascular region, the median percent ratio of Th1 to Th2 cells for DLE-I, DLE-I/S and DLE-S skin in the perivascular areas were 1.22 (0.96-2.97), 0.47 (0.32-0.99) and 0.45 (0.32-0.58), respectively (p=0.01, Fig. 1b). Pairwise comparisons found a significant difference between DLE-I and DLE-S skin (p=0.03). In the interface region, the median percent Th1 to Th2 ratios trended higher in DLE-I skin (2.40 (1.16-3.41)) versus DLE I/S (0.88 (0.57-1.17)) and DLE-S (0.29 (0.0-0.99)) skin (p=0.06). Low cell counts and missing follicles in the perifollicular region of DLE-S skin precluded further sub-analyses. Examples of immunostainings in DLE-I and DLE-I/S skin are shown in Fig. 1c–1f.
Fig. 1.
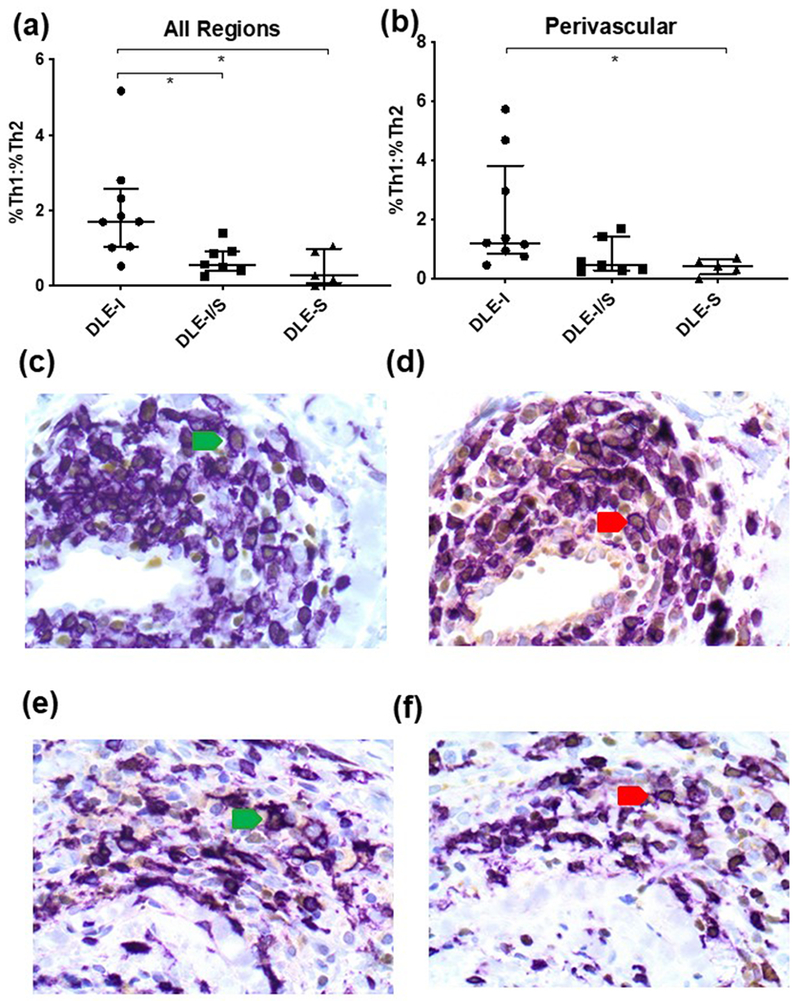
The percent ratios of Th1 to Th2 cells are different among the three stages of DLE skin. (a) The three combined microanatomic regions demonstrated significantly higher percent ratios of Th1 to Th2 cells in DLE-I skin vs. DLE-I/S and DLE-S skin (p=0.002). Pairwise comparison p-values are represented by astericks (*: p<0.05) between groups. Each data point represents the mean percent ratio of Th1 to Th2 cells for all three regions for each biopsy specimen. The error bars represent the median and IQR. (b) In the perivascular region, there were significantly higher percent ratios of Th1 to Th2 cells in DLE-I skin vs. DLE-I/S and DLE-S skin (p=0.014). (c-f) Representative double immunostaining photos of perivascular DLE-I (c, d) and DLE-I/S (e, f) skin. DLE-I and DLE-I/S skin were stained with CD4 (purple) and T-bet (brown, c,e) or GATA-3 (brown, d,f). The green and red arrows highlight examples of CD4+T-bet+ Th1 cells and CD4+GATA-3+ Th2 cells, respectively. Magnification: 600X. Additional results for the other microanatomic regions and cell counts are available upon request.
Our data supports a predominance of Th1 cells in early DLE lesions. Th1 cells secrete pro-inflammatory cytokines including IL-2, and IFN-γ. IL-2 promotes proliferation of CD8+ T cells [3], which are elevated in early DLE [2]. IFN-γ can activate macrophages and other immunocytes [5]. We found elevations in Th2 cells in late DLE skin. Th2 cells promote fibrosis through upregulation of TGF-β and secretion of IL-13, which signals fibroblast proliferation and collagen production [3, 6]. Additionally, Th2 cells inhibit pro-inflammatory M1 macrophages, suppress the secretion of pro-inflammatory cytokines, and upregulate production of anti-inflammatory mediators (IL-4, IL-10, TGF-β) [5, 7]. Finally, through IL-4 and IL-13, Th2 cells promote B-cell proliferation and plasma cell differentiation, which were previously observed in latter stages of DLE [2, 8].
Limitations include small sample size and CD4+ cells being expressed in other cells such as macrophages. Nonetheless, we have identified differences in T cell subsets that may drive histologic and clinical changes in DLE. Further elucidation of immune changes in different stages of DLE skin will potentially lead to development of therapies that reduce the chronic skin sequelae of scarring and dyspigmentation in DLE.
Funding Acknowledgements:
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR061441, and by the National Center for Advancing Translation Sciences of the National Institutes of Health under Award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, and the National Institutes of Health.
Abbreviations:
- CLE
Cutaneous lupus erythematosus
- DLE
Discoid lupus erythematosus
- Th1
T-helper 1 cell
- Th2
T-helper 2 cell
Footnotes
Conflict of Interest: Dr. Chong is an investigator for Daavlin Corporation, Biogen Incorporated, and Pfizer Incorporated. He is a consultant for Viela Bio.
References
- 1.Baltaci M, Fritsch P. Histologic features of cutaneous lupus erythematosus. Autoimmun Rev. 2009;8(6):467–73. doi: 10.1016/j.autrev.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien JC, Hosler GA, Chong BF. Changes in T cell and B cell composition in discoid lupus erythematosus skin at different stages. J Dermatol Sci. 2017;85(3):247–9. doi: 10.1016/j.jdermsci.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florez-Pollack S, Tseng LC, Kobayashi M, Hosler GA, Ariizumi K, Chong BF. Expansion of Myeloid-Derived Suppressor Cells in the Peripheral Blood and Lesional Skin of Cutaneous Lupus Patients. J Invest Dermatol. 2019;139(2):478–81. doi: 10.1016/j.jid.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci. 2018;19(6). doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Meyer C, Müller A, Herweck F, Li Q, Müllenbach R et al. IL-13 Induces Connective Tissue Growth Factor in Rat Hepatic Stellate Cells via TGF-β–Independent Smad Signaling. The Journal of Immunology. 2011;187(5):2814–23. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Timares L, Elmets CA. 19 - Host Defenses in Skin In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology (Fifth Edition). London: Content Repository Only; 2019. p. 273–83.e1. [Google Scholar]
- 8.Romagnani S Th1/Th2 cells. Inflamm Bowel Dis. 1999;5(4):285–94. [DOI] [PubMed] [Google Scholar]


