Abstract
The Wnt, Hedgehog, and Notch signaling pathways play a crucial role in early development and the maintenance of adult tissues. When dysregulated, these developmental signaling pathways can drive the formation and progression of cancer by facilitating cell survival, proliferation, and stem-like behavior. While this makes these pathways promising targets for therapeutic intervention, their pharmacological inhibition has been challenging due to the substantial complexity that exists within each pathway and the complicated crosstalk that occurs between the pathways. Recently, several small molecule inhibitors, ribonucleic acid (RNA) molecules, and antagonistic antibodies have been developed that can suppress these signaling pathways in vitro, but many of them face systemic delivery challenges. Nanoparticle-based delivery vehicles can overcome these challenges to enhance the performance and anti-cancer effects of these therapeutic molecules. This review summarizes the mechanisms by which the Wnt, Hedgehog, and Notch signaling pathways contribute to cancer growth, and discusses various nanoparticle formulations that have been developed to deliver small molecules, RNAs, and antibodies to cancer cells to inhibit these signaling pathways and halt tumor progression. This review also outlines some of the challenges that these nanocarriers must overcome to achieve therapeutic efficacy and clinical translation.
Key Terms: small molecule, RNA interference, antibody, multivalency, drug delivery, gene regulation, nanocarrier, nanomedicine
1. Introduction
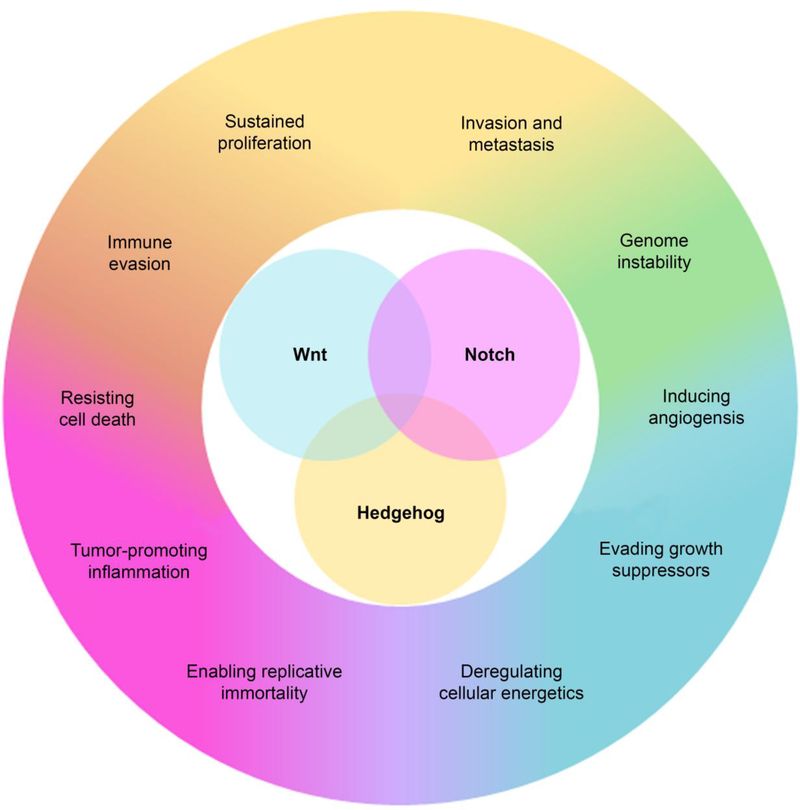
The developmental Wnt, Hedgehog (Hh), and Notch signaling pathways guide early development through precise control of cell proliferation, differentiation, migration, and cell-to-cell communication,6,52 and they also preserve tissue function by regulating adult stem cell populations. 92 Given the important and complex role these pathways play in controlling cellular function, it is unsurprising that their aberrant expression has been associated with the formation and progression of cancer.11,89,105,106 In fact, many of the “hallmarks” of cancer are directly influenced by Wnt, Hh, and Notch signaling (Figure 1), and thus cancer cells have commandeered these developmental pathways to support their ability to grow, resist treatment, metastasize, and recur.33,34,50 Indeed, overactive developmental signaling facilitates cancer cells’ evasion of growth suppressors and the immune system, and supports the formation of new blood vessels to increase nutrient supply, contributing to unchecked disease progression.33,34 Additionally, the Wnt, Hh, and Notch signaling pathways have a significant impact on cancer stem cell populations,12,37,63,82,120 which facilitates disease recurrence if tumors are not fully removed.64,82,131 Overall, the integral role that Wnt, Hh, and Notch signaling play in cancer progression makes them extremely promising targets for new therapeutic strategies. By inhibiting these pathways, cancer progression may be dramatically slowed or even reversed.
Figure 1:
Dysregulation of the developmental Wnt, Notch, and Hedgehog signaling pathways is implicated in all of the “hallmarks” of cancer.
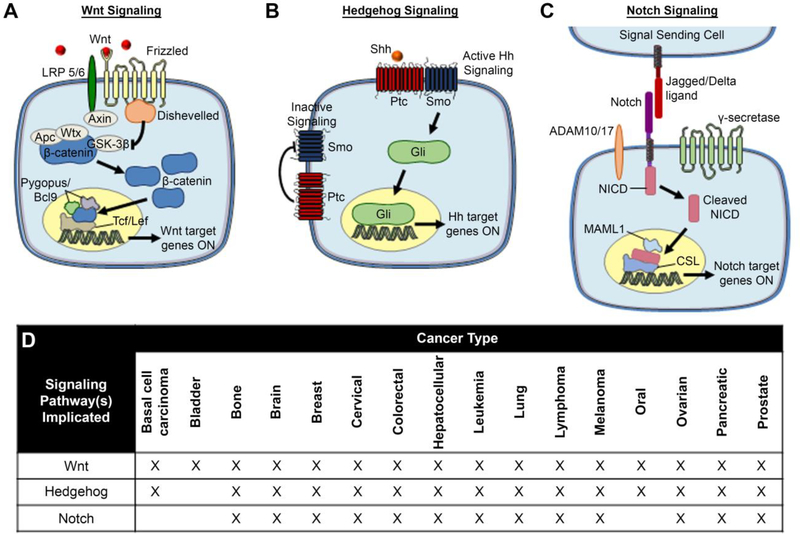
An overview of the Wnt, Hh, and Notch signaling pathways, and the cancers in which they are implicated, is provided in Figure 2. In brief, each pathway is activated when extracellular soluble or cell-bound ligands interact with specific transmembrane receptors on the receiving cell. These ligand-receptor interactions initiate downstream intracellular signaling that leads to the nuclear translocation of specific molecules that subsequently promote the transcription of genes associated with tumorigenesis. Though not depicted in Figure 2, there is substantial crosstalk between the signaling pathways and they often work in concert to support tumor progression. This makes their pharmacological inhibition challenging, as expanded upon in the following paragraph.
Figure 2:
Schematic of activated Wnt, Hh, and Notch signaling pathways and the cancers in which they are implicated. (A) Canonical Wnt signaling is activated in cancer cells when extracellular Wnt ligands bind Frizzled and LRP5/6 co-receptors that are amplified on cancer cell surfaces. This leads to inhibition of the β-catenin destruction complex, allowing β-catenin to accumulate in the cytoplasm and then translocate to the nucleus where it activates the transcription of downstream oncogenes. Reproduced with permission from reference 73, Riley et al. Small. Copyright 2017 Wiley. (B) Simplified scheme of inactive versus active canonical Hedgehog signaling. In inactive Hh signaling, Patched (Ptc) suppresses Smoothened (Smo) to prevent downstream signaling activity. In active Hh signaling, Sonic hedgehog (Shh) ligand binding to Ptc relieves its suppression of Smo, resulting in the activation and translocation of Gli proteins to the nucleus where they promote transcription of target genes. (C) Canonical Notch signaling is activated when Jagged or Delta ligands on a signal-sending cell bind the extracellular domain of the Notch receptor on a signal-receiving cell. The extracellular domain of Notch is subsequently cleaved by ADAM family proteases, then the intracellular domain (NICD) is cleaved by the gamma secretase complex. Released NICD translocates to the nucleus, where it acts as a promoter for the transcription of downstream target genes that support cell survival, proliferation, stemness, and other oncogenic behaviors. (D) List of cancers with known involvement of Wnt, Hh, and Notch signaling.
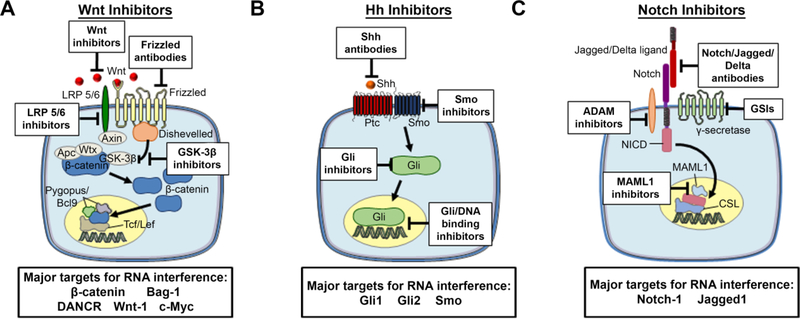
There are many components of the Wnt, Hh, and Notch signaling pathways that are amenable to pharmacological regulation, and a variety of therapeutic moieties have begun to be developed and tested (Figure 3, Table 1). Unfortunately, while inhibiting Wnt, Hh, and Notch signaling is promising in theory, there are many challenges associated with accomplishing this goal. First, given the complexity of each pathway, it is often difficult to determine which receptor(s)/ligand(s)/intracellular molecule(s) to target in different cancer subtypes. Second, resistance is an issue, as cancer cells may activate one pathway to compensate for inhibition of another. Third, since Wnt, Hh, and Notch are active in some adult tissues, side effects are a concern, particularly when using therapeutic moieties that lack specificity. Finally, some of the key mediators of these developmental pathways are “undruggable” because they lack effective binding sites for small molecule therapeutics.47 Overcoming these challenges will revolutionize the treatment of many types of cancer.
Figure 3:
Components of the (A) Wnt, (B) Hh, and (C) Notch signaling pathways that are amenable to inhibition by antibodies, small molecules, and RNA molecules.
Table 1:
Therapeutic moieties available to inhibit Wnt, Hh, and Notch signaling.
| Pathway | Target | Therapeutic Moiety |
|---|---|---|
| Wnt | Extracellular ligands | SFRP1 |
| Frizzled receptor | Wnt5a | |
| Wnt-C59 | ||
| Anti-Frizzled7 antibody | ||
| LRP 5/6 receptor | iWnt peptide | |
| β-catenin | Niclosamide | |
| IWR-1 | ||
| KYA1797K | ||
| FH535 | ||
| siCTNNB1 | ||
| siBag-1 | ||
| iCRT 3/14 | ||
| PNU-74654 | ||
| Gigantol | ||
| DANCR lncRNA | ||
| β-catenin and c-Myc | miR-105 | |
| IWP-2 | ||
| DK419 | ||
| miR-200c | ||
| c-Myc | c-Myc shRNA | |
| GSK-3β | Cromolyn | |
| Pyrvinium pamoate | ||
| Wnt-1 | siWnt-1 | |
| Adavivint | ||
| Wogonin | ||
| Hh | Shh | Robotnikinin |
| Anti-Shh 5E1 antibody | ||
| Anti-Shh MEDI-5304 antibody | ||
| Ptc1 | Ant-Patched-1 antibody | |
| Smo | HhAntag | |
| Cyclopamine | ||
| Vismodegib | ||
| DHCEO | ||
| SMANT | ||
| DY131 | ||
| Itraconazole | ||
| ALLO-1/2 | ||
| Compound 5 | ||
| Jervine | ||
| Cur-61414 | ||
| SANT 1–4 | ||
| Saridegib | ||
| Sonidegib | ||
| TAK-441 | ||
| BMS-833923 | ||
| LEQ506 | ||
| PF-04449913 | ||
| LY2940680 | ||
| Vitamin D3 | ||
| BRD-6851 | ||
| SEN450 | ||
| MRT-83 | ||
| Gli1 | Quinacrine | |
| GANT61 | ||
| GANT58 | ||
| Arcyriaflavin C | ||
| Physalins F | ||
| siGli1 | ||
| Gli1/2 | HPI1–4 | |
| Gli-DNA binding | Anthothecol | |
| Glabrescione B | ||
| Arsenic trioxide | ||
| Notch | ADAM 10/17 | INCB7839 |
| INCB3619 | ||
| INCB8765 | ||
| KP457 | ||
| GM6001 | ||
| GI254023X | ||
| 8C7 antibody | ||
| D1(A12) antibody | ||
| A9(B8) antibody | ||
| MEDI3622 antibody | ||
| ADAM10 prodomain | ||
| Rapamycin | ||
| MAML1 | MAM peptide antagonist | |
| SAHM1 | ||
| Notch receptors | Notch-1 shRNA | |
| Soluble Notch1 | ||
| Soluble DLL1 | ||
| Soluble Jagged1 | ||
| Genistein | ||
| Sulforaphane | ||
| Quercetin | ||
| Curcumin | ||
| Resveratrol | ||
| miR-34a | ||
| Anti-Notch-1–3 antibody | ||
| Jagged/Delta | siJagged1 antibody | |
| Anti-DLL1–4 antibodies | ||
| Nicastrin antibody | ||
| DAPT | ||
| MRK-560 | ||
| MRK-003 | ||
| PF-03084014 | ||
| MK-0752 | ||
| BMS-906024 | ||
| DBZ (LY411575) | ||
| LY450139 | ||
| RO4929097 |
There are three main classes of agents that can be used to suppress developmental signaling pathways in cancer: small molecules, nucleic acids, and antibodies. While small molecule inhibitors have been the most widely explored (Table 1), they often encounter issues with solubility, bioavailability, targeted cellular uptake, and systemic toxicity that hinder their clinical translation. The use of small interfering RNA (siRNA) or microRNA (miRNA) molecules to suppress desired targets through RNA interference (RNAi) is a promising alternative treatment strategy that may enable reduction of cancer cell survival, proliferation, and stemness. However, the clinical translation of RNA therapeutics is limited due to their susceptibility to nuclease degradation, rapid clearance from the bloodstream, and their inability to passively enter cells.30,54,96 For RNA and small molecules to reach their potential, more effective delivery systems must be developed and shown to provide a therapeutic advantage over current standards of care in cancer treatment. Lastly, antagonistic antibodies offer a third way to manipulate developmental signaling pathways. Typically, antagonistic antibodies are designed to bind extracellular ligands or transmembrane receptors to block ligand/receptor interactions, thereby suppressing downstream signaling. Unfortunately, the high required dosages and cost of antagonistic antibodies have limited their translation. As with RNA and small molecule inhibitors, novel delivery vehicles are needed to enhance the efficacy of antibody therapeutics.
Nanoparticles (NPs) offer substantial promise as carriers to enhance the delivery of small molecules, RNAs, and antibodies to cancer cells to manipulate Wnt, Hh, and Notch signaling and halt disease progression. Encapsulating these molecules inside NPs or loading them on NPs’ exterior can improve their stability, pharmacokinetics, biodistribution, and tissue/cell-specific delivery.27,30,65,66 This enables the nanoformulations to be much more effective than their freely delivered counterparts. 7,61,65,88,111 This review summarizes nanocarriers that have been developed to date to deliver small molecules, RNA molecules, and antibodies to cancer cells to suppress the Wnt, Hh, and Notch signaling pathways (Table 2). These new therapeutics offer substantial promise as tools for improved treatment of many types of cancer, and a summary of barriers to address in clinical translation is provided at the end of this review.
Table 2:
Nanoparticle systems designed to inhibit the Wnt, Hh, and Notch signaling pathways.
| NP Name | Pathway (Target) | Molecule Carried | Cancer Targeted | Stage of Development; Key Results | Material | Size; Charge |
|---|---|---|---|---|---|---|
| Chi-Au NC-Alg NPs29 | Wnt (Wnt ligands) | sFRP1 | Cervical | In vitro; 73-fold increase in phosphorylated β-catenin | Gold | 767 nm; −15.8 mV |
| Wnt5a trap56 | Wnt (Wnt5a) | Frizzled7 receptor plasmid DNA | Metastatic melanoma | In vivo; combination with DOX increases median survival from 39 to 65 days | Lipid protamine-DNA | 100 nm; N/A |
| CP-NIC7 | Wnt (β-catenin) | Niclosamide | Colon | In vivo; extended median survival from 13 to 26 days | Chimeric polypeptide | 81.5 nm; N/A |
| CCSNPs73 | Wnt (GSK-3β) | Chromolyn | Colorectal | In vivo; decreased tumor occurrence by 67% | Chitosan | 112.4 nm; +39.9 mV |
| EnCore/CTNNB128 | Wnt (β-catenin) | siCTNNB1 | Colorectal, hepatocellular | In vivo; tumor growth inhibition of 82% | Lipids | 70–100 nm; N/A |
| Nano-plasmid41 | Wnt (β-catenin) | siBag-1 | Colorectal | In vivo; 70% reduction in tumor volume compared to control | Gold | 95.38 nm; +35.03 mV |
| RGD-PEG-ECO/siDANCR110 | Wnt (β-catenin) | siDANCR | Breast | In vivo; 20–40% reduction in tumor volume | Amino lipid ECO, cyclic RGD peptide-PEG | 109.12 nm; +24.67 mV |
| PEG-PEI-Ce6/siRNA58 | Wnt (Wnt-1) | siWnt-1 | Oral | In vitro; 58.55% reduction in cell viability | PEG-PEI-Ce6 | 90nm; +10 mV |
| shRNA + NP107 | Wnt (c-Myc) | c-Myc shRNA | Breast, colorectal | In vivo; more than doubled median survival | PGMA, PEI | 150 nm; +75 mV |
| FZD7-NS88 | Wnt (Frizzled7) | anti-FZD7 antibodies | Breast | In vitro; 25% reduction in cell viability | Gold | 178.3 nm; −27.4 mV |
| iWnt-ATF24-IONPs71 | Wnt (LRP5/6) | iWnt peptide | Breast | In vivo; 94% reduction in tumor volume compared to control | Iron oxide | 25.8 nm; −34 mV |
| CCPM-177Lu126 | Hh (Smo) | Cyclopamine | Breast, pancreatic | In vivo; 70% reduction in tumor weight compared to control | Lecithin | 48.2 nm; N/A |
| HA-SS-PLGA-DOX-CYC39 | Hh (Smo) | Cyclopamine | Breast | In vivo; complete remission in all mice | HA-SS-PLGA | 245.3 nm; N/A |
| P-CYP124 | Hh (Smo) | Cyclopamine | Prostate | In vivo; significantly inhibited tumor growth | mPEG-b-PCC-g-DC | 76.37 nm; N/A |
| M-CPA/PTX130 | Hh (Smo) | Cyclopamine | Pancreatic | In vivo; tumor growth suppressed in 80% of mice | PEG-PMA-PCL | 45.2 nm; N/A |
| CMNP49 | Hh (Smo) | Cyclopamine | Pancreatic | In vivo; 75.3% reduction in tumor volume compared to control | PLGA | 66.1 nm; −26.1 mV |
| SN38 NPs/GDC-0449116 | Hh (Gli1) | GDC-0449 | Pancreatic | In vivo; induced apoptosis in 50% of tumor cells | PEG5K-P(HEMASN38)X | 70 nm; N/A |
| PEG-DB86 | Hh (Gli1) | GDC-0449 | Pancreatic | In vitro efficacy and in vivo accumulation; 70% reduction in cell viability | PEG-b-poly (carbonate) | 149 nm; N/A |
| Antho-NPs114 | Hh (Gli-DNA binding) | Anthothecol | Pancreatic | In vitro; significantly inhibits EMT in stem cells | PLGA | 190.52 nm; 0.02 mV |
| Mang-NPs113 | Hh (Gli-DNA binding) | α-Mangostin | Pancreatic | In vivo; complete elimination of liver metastases | PLGA | 186.3 nm; 0.03 mV |
| NC-GlaB45 | Hh (Gli1) | GlaB | Pancreatic | In vitro efficacy and in vivo biodistribution; 30.2% of cells arrested in G2 phase | PLGA | 159.4 nm; −39.8 mV |
| NQC76 | Hh (Gli1) | Quinacrine | Cervical | In vivo; 10 mm3 reduction in tumor volume compared to control | PLGA | 291.4 nm; +2.38 mV |
| NQC77 | Hh (Gli1) | Quinacrine | Oral | In vitro; 17% increase in cell death | PLGA | 100 nm; +2.38 mV |
| NanoHHI122 | Hh (Gli1) | HPI-1 | Hepatocellular carcinoma | In vivo; 67% reduction in tumor weight compared to control | PLGA-PEG | 60 nm; N/A |
| NanoHHI17 | Hh (Gli1) | HPI-1 | Pancreatic | In vivo; 90% reduction in tumor weight compared to control | PLGA-PEG | 60 nm; N/A |
| GANT61 PLGA NPs9 | Hh (Gli1) | GANT61 | Colorectal, breast | In vitro; significant reduction in tumorsphere formation ability | PLGA | 250 nm; −20 mV |
| DMP-Gli1si132 | Hh (Gli1) | Gli1 siRNA | Brain | In vivo; induced apoptosis in 67.5% of tumor cells | DMP | 35.6 nm; +32.7 mV |
| GSI-MSNPs62 | Notch (γ-secretase) | DAPT | Breast | In vivo; 95% reduction in tumor volume compared to control | Silica | 350 nm; +50 mV |
| MSN-PEI-GAorg-DAPT61 | Notch (γ-secretase) | DAPT | Breast | In vivo; 85% reduction in cancer stem cells | Silica | 216 nm; +27.8 mV |
| CF-NP-EB/DART115 | Notch (γ-secretase) | DAPT | Breast | In vivo; 79% reduction in tumor volume compared to control | PLA | 100 nm; −24.3 mV |
| NPs/PEI-FA/shRNA123 | Notch (Notch-1) | Notch-1 shRNA | Breast | In vitro; 3-fold increase in cellular apoptosis | Iron oxide, silica | 64 nm; +17.5 mV |
| Jagged siRNA-CH100 | Notch (Jagged1) | Jagged1 siRNA | Ovarian | In vivo; 87.5% reduction in tumor weight compared to control | Chitosan | N/A |
| CNPs23 | Notch (multiple) | miR-34a | Breast | In vivo; 80% reduction in tumor weight compared to control | Hyaluronic acid-chitosan | 214 nm; −33 mV |
2. Nanotherapeutics to Suppress Wnt Signaling in Cancer
2.1. Wnt Signaling in Cancer
After the discovery of the wingless gene in a mutagenesis screen for visual phenotypes, many components of the Wnt family of signaling proteins were identified as key mediators of patterning decisions during embryonic development.79 The Wnt pathway was connected to cancer when it was observed that activation of Wnt1 resulted in mammary hyperplasia and tumors.109 Now, Wnt signaling is known to be a key regulator of development and stemness, and its aberrant activity has been implicated in many cancers, including cutaneous melanoma, pancreatic ductal adenocarcinoma, and breast carcinoma, among others.129 Indeed, Wnt signaling has been linked with all stages of cancer development, making its inhibition an exciting strategy to combat tumor progression, drug resistance, metastasis, and recurrence.
The Wnt signaling pathway is divided into β-catenin dependent (canonical) and β-catenin independent (non-canonical) pathways.129 The two non-canonical pathways include the Wnt/Ca2+ and the Wnt/planar cell polarity (Wnt/PCP) pathways.5,20,51,108 Their role in cancer is less established than that of the canonical pathway, and thus the majority of therapeutics under development for Wnt inhibition target the canonical pathway. Accordingly, the canonical Wnt signaling pathway is described in detail below, followed by a discussion of nanotherapeutics designed to suppress this pathway.
In healthy cells with inactive canonical Wnt signaling, the absence of extracellular Wnt ligands to activate the pathway results in the phosphorylation of β-catenin by a destruction complex comprised of Axin, Apc, Wtx, and the kinase GSK-3β. This phosphorylation of β-catenin leads to its proteasomal degradation, and the resulting lack of nuclear β-catenin allows the repressive complex containing Tcf/Lef and Groucho to recruit histone deacetylases to repress Wnt pathway target genes. Conversely, in the cancer microenvironment where there is an abundance of secreted Wnt ligands, the canonical Wnt pathway is activated when the ligands bind to Frizzled (FZD) receptors and LRP co-receptors that are over-expressed on cancer cell surfaces (Figure 2A). This activation initializes a signaling cascade in which LRP receptors are phosphorylated by kinases CK1α and GSK-3β. This is then followed by the recruitment of Dishevelled (Dvl) proteins to the plasma membrane where they polymerize and are activated.16 The Dvl polymer inhibits the destruction complex, which allows β-catenin to stabilize and accumulate in the cytoplasm. Following translocation to the nucleus, β-catenin forms an active complex with Tcf/Lef by displacing Groucho. This new complex activates the transcription of Wnt target genes, including Axin2, Cyclin D1, and c-Myc, to drive disease progression (Figure 2A).57
Therapies designed to suppress canonical Wnt signaling in cancer cells can either attempt to block ligand/receptor interactions to prevent signal cascade activation or attempt to inhibit downstream molecular targets within the pathway, such as β-catenin or its transcriptional targets (Figure 3A). The following sections summarize NPs that have been developed to deliver small molecules, RNA therapeutics, or antibodies to cancer cells to suppress canonical Wnt signaling.
2.2. Nanoparticle Delivery of Small Molecules to Regulate Wnt Signaling
Small molecule inhibitors of Wnt signaling include both naturally occurring compounds 29,56 and synthetic agents7,73. Regarding natural products, researchers have either utilized nanoparticles to deliver Wnt inhibitors that are produced by cells, or they have used nanoparticles to “trap” Wnt ligands and regulate ligand-mediated activation of the signaling pathway.29 One such naturally produced Wnt inhibitor is SFRP1. Healthy cells secrete SFRP1 upon oxidative stress or DNA damage, but its expression is silenced or mutated in cancer cells.25 Upon secretion, SFRP1 blocks Wnt signaling by binding extracellular Wnt ligands so they can no longer bind Wnt receptors18,112, or it directly binds the FZD receptors to form an inhibitory complex.18 By replenishing SFRP1 content in the microenvironment of cervical cancer cells through NP delivery in vitro, Ghosh and team achieved anti-proliferative effects that were mediated through reduction in the expression levels of the Wnt target proteins β-catenin, cyclin D1, and survivin.29 In an alternative approach, Huang and colleagues used NPs to trap Wnt5a molecules that are produced by cancer cells in the tumor microenvironment and demonstrated that this approach could treat metastatic melanoma by preventing signaling feedback.56 Their formulation consisted of cationic lipid-protamine NPs that could deliver plasmid DNA encoding a trimeric trap protein containing the extracellular domain of Frizzled7 receptors to tumors. This caused the tumors to express the trap protein, which bound Wnt5a to reduce its intratumoral expression and limit tumor growth, particularly when combined with doxorubicin chemotherapy.56 Notably, Wnt5a has an innate ability to control the immunosuppressive tumor microenvironment. As a result, Huang and colleagues demonstrated that NP-mediated trapping of Wnt5a could enhance dendritic cell function and T-cell infiltration into tumors, resulting in immunogenic cell death within the tumors.56
In addition to natural Wnt inhibitors, synthetic Wnt inhibitors like Niclosamide (NIC)7 and cromolyn73 have also been incorporated into NP carriers for anti-cancer therapy. NIC is an orally bioavailable drug that has been approved by the Food and Drug Administration (FDA) for the treatment of tapeworms, and it has also recently been identified as a Wnt/β-catenin inhibitor by the National Cancer Institute and other independent researchers.2 Bhattacharrya et al. have exploited this therapeutic discovery by conjugating NIC to polypeptides that self-assemble into NPs, thus overcoming its low solubility and bioavailability and enabling the treatment of colon cancer.7 In in vitro studies, HCT-116 human colon cancer cells treated with NIC-loaded NPs exhibited decreased c-Myc and cyclin D1 expression, indicating these particles effectively inhibit cytosolic β-catenin levels and downstream Wnt targets. In further studies, the team demonstrated that these NPs could slow tumor progression in human colon cancer xenograft models.7 In a different approach, Motawi et al. prepared chitosan NPs incorporating the synthetic Wnt inhibitor, cromolyn, which potently regulates Wnt signaling by inhibiting GSK-3β.73 They used these NPs to deliver cromolyn to rats with dimethylhydrazine-induced colorectal cancer, and demonstrated they could effectively decrease protein levels of the Wnt-related proteins GSK-3β and β-catenin and provide a protective approach to colorectal cancer therapy.73 Taken together, the above studies demonstrate that NP-mediated delivery of small molecule Wnt inhibitors has substantial potential as a cancer treatment strategy.
2.3. Nanoparticle-Mediated RNA Interference of Wnt Signaling
Although the above examples demonstrate that pharmacological inhibition of Wnt signaling is possible, the key mediator of this pathway, β-catenin, is still considered “undruggable” because it lacks an effective binding site for small molecule therapeutics. An attractive alternative strategy to suppress β-catenin and other key targets in the Wnt signaling pathway is the use of siRNA or miRNA to elicit RNAi-mediated gene silencing. In an elegant study, Abrams and colleagues demonstrated that effective inhibition of β-catenin could be achieved using lipid nanoparticles (LNPs) containing Dicer substrate siRNA (DsiRNA) targeting CTNNB1, the gene encoding β-catenin.28 This formulation achieved significant tumor growth inhibition in Wnt-dependent colorectal and hepatocellular carcinoma models in mice, but not in Wnt-independent tumors.28 Excitingly, analysis of histology sections showed the LNPs achieved homogenous DsiRNA delivery throughout tumors, which is impressive given that insufficient NP penetration into tumors is a major barrier that researchers in the field are actively working to overcome.21,22,102,118
Besides targeting β-catenin directly, researchers have also developed NPs to deliver siRNA or miRNA against other genes whose down-regulation can subsequently decrease β-catenin expression. For example, Huang et al. developed magnetic gold NPs to deliver Bag-1 gene-silencing plasmids to colorectal cancer.41 Bag-1 is a positive regulator of the anti-apoptotic gene Bcl-2, so the researchers expected silencing Bag-1 would increase cellular apoptosis. LoVo cells treated with the NPs exhibited reduced Bag-1 mRNA expression, and the team demonstrated a corresponding notable decrease in β-catenin protein expression in LoVo tumors treated with the NPs through immunohistochemistry and Western blotting of excised tumor tissue. Similarly, Vaidya et al. developed polymeric NPs to deliver long noncoding RNA (lncRNA) targeting the gene DANCR to triple negative breast cancer (TNBC).110 These NPs achieved 80–90% knockdown of DANCR expression in MDA-MB-231 and BT549 TNBC cells for up to 7 days, and simultaneously induced a significant decrease in the expression of β-catenin and other proteins involved in epithelial-to-mesenchymal transition and apoptosis regulation.110
Other researchers have also developed platforms to enable RNAi of Wnt signaling by inhibiting Wnt proteins themselves or downstream targets like c-Myc.58,107 For example, Ma et al. developed polyethylene glycol-polyethylenimine-chlorin e6 (PEG-PEI-Ce6) NPs to deliver Wnt-1 siRNA to oral cancer cells and enable simultaneous photodynamic therapy (PDT; Ce6 is an established photosensitizer).58 The rationale for this approach was that PDT is hindered by activation of epithelial-to-mesenchymal transition (EMT), which is regulated by Wnt signaling. Accordingly, the researchers hypothesized that silencing Wnt-1 would enhance the efficacy of PDT by minimizing EMT. Indeed, their in vitro studies confirmed the NPs could inhibit Wnt-1, β-catenin, and vimentin expression in KB oral squamous carcinoma cells, and the combined application of the Wnt-1 siRNA with PDT effectively enhanced cell killing.58 In an alternative approach, Tangudu et al. developed PEG-polyglycidal methacrylate (PEG-PGMA) NPs to deliver short hairpin RNA (shRNA) targeting c-Myc or various types of miRNA to different types of cancer.107 NPs containing c-Myc shRNA, miR-105, or miR-200c silenced the expression of both c-Myc and β-catenin in Jurkat cells in vitro. Further, oral delivery of c-Myc shRNA-containing NPs to a mouse model of colorectal cancer reduced c-Myc mRNA expression in tumors and resulted in β-catenin relocalization from the nucleus to the cytoplasm, indicating inhibition of Wnt signaling.107 Survival was dramatically improved in these mice compared to all other controls.107
Overall, enabling selective inhibition of β-catenin and other Wnt pathway targets could transform cancer management, but is challenging to achieve. As new and improved NP platforms for RNA delivery are developed, the use of RNAi to regulate Wnt signaling will likely yield impressive results against cancer.
2.4. Antibody-Nanoparticle Conjugates for Modulation of Wnt Signaling
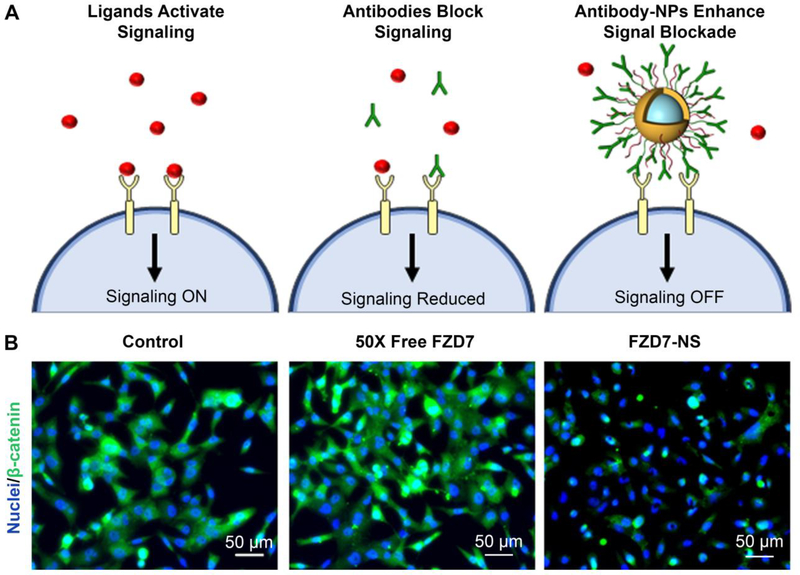
While small molecule therapeutics and RNA molecules must be delivered into cells to elicit their effects, antagonistic antibodies can function from outside the cell, circumventing many delivery barriers. As noted earlier, antagonistic antibodies typically elicit signal cascade interference by binding their targeted receptor on the cell surface to lock it in a ligand-unresponsive state. Recently, researchers have shown that antibody-NP conjugates are drastically more effective than freely delivered antibodies because they can exhibit multivalency by engaging multiple receptors simultaneously, resulting in increased overall binding strength and enhanced signaling inhibition (Figure 4A).88,95 Besides targeting cellular receptors, antibody-NP conjugates can also be designed to bind ligands in the extracellular environment and sequester them from cellular interactions. Additionally, peptide-NP conjugates can be used as an alternative to antibody-NP conjugates.71 Examples of the use of these strategies to suppress Wnt signaling in cancer are described below.
Figure 4:
Comparison of Wnt signaling inhibition mediated by freely delivered antibodies versus antibody-nanoparticle conjugates. (A) In cancer cells, ligand/receptor interactions activate downstream oncogenic signaling. Antibodies can reduce signaling by binding receptors to lock them in a ligand unresponsive state, and antibody-NP conjugates can enhance signaling blockade by engaging multiple receptors simultaneously. (B) Immunofluorescence analysis of β-catenin expression in MDA-MB-231 triple negative breast cancer cells shows that FZD7 antibody-nanoshell (FZD7-NS) conjugates are more effective than freely delivered antibodies at suppressing β-catenin expression and nuclear localization. Reproduced with permission from reference 73, Riley et al. Small. Copyright 2017 Wiley.
Recently, Riley and Day demonstrated that Wnt signaling could be suppressed in TNBC cells using Frizzled7 (FZD7) antibodies conjugated to gold nanoshells (NS).88 As FZD7 overexpression drives hyperactive Wnt signaling and disease progression in TNBC cells, Riley and Day hypothesized that blocking Wnt3a-mediated activation of FZD7 could inhibit cellular viability. In vitro studies showed that FZD7-NS had a lower effective dissociation constant (i.e. greater binding avidity) for TNBC cells than freely delivered FZD7 antibodies, demonstrating the importance of multivalency. Further, MDA-MB-231 cells that were treated with FZD7-NS in the presence of Wnt3a (to competitively bind the receptors) exhibited dramatic reductions in β-catenin expression and nuclear localization, and these effects were not observed when 50X higher doses of free FZD7 antibodies were administered to the cells (Figure 4B). Moreover, only the FZD7-NS-treated cells exhibited decreased expression of the downstream Wnt-target gene Axin2 by qRT-PCR and reduction of cellular metabolic activity by MTT assay.88 Taken together, these findings indicate that FZD7-NS conjugates have substantial potential as a treatment for TNBC and other cancers driven by Wnt signaling.
Notably, peptides offer a cheaper alternative to antibodies that also offer excellent binding strength. Accordingly, using peptide-NP conjugates to block Wnt signaling is also an attractive therapeutic strategy. Miller-Kleinhenz et al. demonstrated this by developing ultra-small magnetic iron oxide nanoparticles (IONPs) coated with peptides that dually target both Wnt/LRP5/6 and uPA receptors.71 This dual-targeted system successfully inhibited the Wnt/β-catenin signaling pathway in breast cancer cells, as indicated by reduced protein expression of β-catenin, Axin, GSK-3β, and Snail.71 Moreover, the systemic administration of the NPs into mice bearing patient derived xenograft (PDX) tumors led to stronger tumor growth inhibition than non-targeted or single-targeted NPs.71 Overall, these findings, coupled with those of Riley and Day, demonstrate the utility of exploiting NP multivalency to achieve and enhance antibody- or peptide-mediated Wnt signaling cascade interference and hinder tumor growth. Future studies should continue to explore the effect of antibody-NP and peptide-NP conjugates against Wnt-dependent tumors, while performing fundamental studies to improve understanding of design characteristics that maximize effect.
3. Nanotherapeutics to Suppress Hedgehog Signaling in Cancer
3.1. Hedgehog Signaling in Cancer
Hh was initially discovered as a “segment polarity” gene that controls Drosophila embryonic cuticle pattern.79 Since then, dysregulated Hh signaling has been associated with birth defects78 and a diverse spectrum of cancers including basal cell carcinoma (BCC), medulloblastoma, pancreatic ductal adenocarcinoma (PDAC), esophageal and stomach cancer, small-cell lung cancer (SCLC), breast cancer, and more.48,60,103 Therefore, pharmacological blockade of the Hh signaling pathway has emerged as a promising anti-cancer therapeutic strategy. In mammals, three Hh homologues with different spatial and temporal distribution patterns have been identified: Sonic (Shh), Indian (Ihh), and Desert (Dhh), of which Sonic is the best studied.46 In the canonical Hh signaling pathway (Figure 2B), activation is most commonly initiated when an extracellular Hh ligand binds to Patched 1 (Ptc), which relieves its repression of Smoothend (Smo) to drive an intracellular signaling cascade that ultimately results in the activation of three Gli transcription factors, with Gli1 and Gli2 functioning primarily as activators and Gli3 as a suppressor. These transcription factors in turn regulate the expression of genes that promote proliferation, apoptosis suppression, migration, invasion, angiogenesis, DNA damage repair, drug efflux, and cancer stemness.48,60,67,91
A wealth of small molecules, RNAs, and antibodies have been developed and studied for their ability to suppress the Hh signaling pathway (Figure 3B, Table 1), with some of them undergoing clinical trials or having been approved by the FDA for cancer treatment.13 Below, recent examples of NPs that have been developed to deliver these molecules to cancer for Hh signaling pathway suppression are summarized, as well as potential opportunities for future therapeutic development.
3.2. Nanoparticle Delivery of Small Molecules to Regulate Hh Signaling
While Hh ligands and Ptc receptors are potential targets for Hh blockade119, the majority of small molecules designed to inhibit Hh signaling target Smo. However, clinical applications of these Smo antagonists are restricted by their poor systemic bioavailability, cellular development of resistance through genetic alterations, and additional Gli activation via non-canonical pathways.37,83 NP delivery systems that seek to overcome these translational issues focus on two classes of Hh inhibitors, similar to those used in Wnt signaling inhibition: naturally occurring inhibitors and synthetic inhibitors.
The most extensively explored natural inhibitors of the Hh signaling pathway are cyclopamine (CPA) and its analog vismodegib. CPA is isolated from corn lily, and although its inhibition of Hh signaling has been well reported15,104, it faces many barriers to clinical translation, such as poor stability and water solubility, and severe adverse effects. Many NP formulations that incorporate CPA have been designed to overcome these clinical application hurdles. For example, You et al. developed a CPA-loaded lipid NP system and found it effectively enhanced radiation therapy in breast and pancreatic cancer cells.126 In addition, Hu et al. developed redox-responsive polymeric NPs to co-deliver CPA and doxorubicin and demonstrated remarkable synergistic anti-tumor effects in an orthotopic breast cancer model.39 In a similar strategy that combines CPA delivery with chemotherapy, researchers have engineered CPA-paclitaxel (PTX) polymer NPs and utilized them to treat prostate cancer124 and pancreatic cancer130 in mice. Finally, Jiang and colleagues recently developed a biomimetic NP delivery system in which CPA was encapsulated in erythrocyte membrane-camouflaged poly(lactic co-glycolic acid) (PLGA) NPs49, which enhances CPA delivery by exploiting the long circulation time of the red blood cell-mimetic NPs.14 Excitingly, combining these NPs with PTX-loaded NPs successfully improved PTX delivery to tumors by disrupting the tumor extracellular matrix, increasing functional vessels, and improving tumor perfusion; consequently, this combination therapy achieved remarkable tumor growth inhibition in vivo.49
In 2012, the FDA approved vismodegib, a functional analogue of cyclopamine, for basal cell carcinoma therapy, making it the first Shh pathway drug approved for treating cancer. Although vismodegib is more water-soluble than cyclopamine, its solubility is still a limiting factor to its systemic bioavailability. To solve this issue, several research groups have developed polymeric NPs for vismodegib delivery. For instance, Wang et al. encapsulated vismodegib in NPs made from SN38 prodrug polymers (SN38 is the active metabolite of irenotecan) to treat pancreatic ductal adenocarcinoma, which is among the most fibrotic and difficult to treat tumors.116 Since Hh signaling plays an important role in the communication between tumor cells and stromal cells, the researchers hypothesized that vismodegib-mediated inhibition of Hh signaling could increase intratumoral drug diffusion and prevent drug resistance. Indeed, they demonstrated that their NPs suppressed Gil1 in the tumor microenvironment in a murine xenograft model, which effectively reversed fibroblast-induced resistance to treatment with SN38.116 Similarly, Ray et al. developed pH-responsive polymeric NPs for vismodegib/gemcitabine co-delivery to pancreatic cancer.86 Both of these studies indicate that NP-mediated delivery of Hh inhibitors has great potential as a strategy to potentiate chemotherapy.
In addition to CPA and vismodegib, several other naturally occurring Hh inhibitors have been reported and incorporated into NPs. Verma et al. found that anthothecol-loaded PLGA NPs could effectively reduce cell proliferation and colony formation and induce apoptosis in pancreatic cancer stem cells (CSCs) by disrupting Gli-DNA binding activity.114 In further studies, the team also found that α-Mangostin-loaded PLGA NPs could inhibit pancreatic CSC growth, development, and metastasis through disruption of Gli-DNA binding activity.113 Similarly, Cinzia et al. found that glabrescione B encapsulated in PLGA NPs had remarkable cell killing activity against Hh-dependent CSCs and some non-stem-like cancer cells.45
NP formulations have also been designed to deliver synthetic Hh inhibitors to cancer. For example, Nayak et al. found that NPs loaded with quinacrine (QC), a drug used to treat malaria, could reduce Gli1 mRNA levels by increasing expression of the Hh components GSK-3β and PTEN, thus inducing apoptosis in CSCs.76,77 Similarly, Anders and colleagues found that PLGA NPs encapsulating the Gli1/2 antagonist HPI-1 could significantly inhibit the growth and metastasis of hepatocellular carcinoma models in mice, and significantly decrease the population of CD133-positive cells, which are implicated as CSCs in liver cancers.122 Chenna et al. explored these NPs further and showed that when they were combined with gemcitabine, they significantly impeded the growth of orthotopic pancreatic cancer xenografts that have a ligand-dependent, paracrine mechanism of Hh activation.17 Finally, researchers have loaded the Gli1 inhibitor GANT61 in PLGA NPs, and demonstrated inhibition of Gli1 nuclear translocation, resulting in increased cancer cell death and reduction of CSCs.9 These observations support the continued development of NPs as vehicles to deliver small molecule Hh inhibitors to cancer.
3.3. RNA Interference of Hedgehog Signaling Mediators
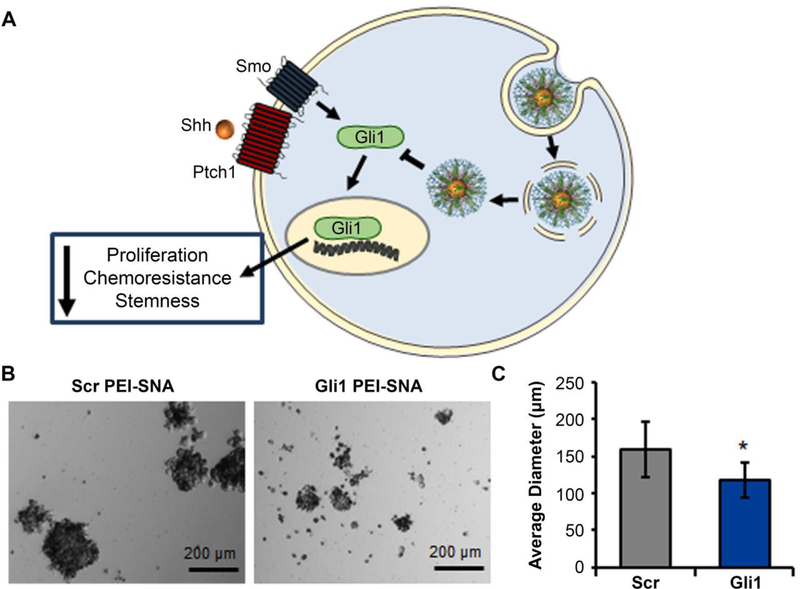
RNAi for Hh signaling inhibition has largely focused on Gli transcription factors, as they are the key oncogenic mediators of this pathway.43 In one study, Melamed et al. developed a robust platform for Gli1 inhibition in which Gli1 siRNA was conjugated to 13 nm diameter gold NPs to produce spherical nucleic acids (SNAs) that were subsequently wrapped with polyethyleneimine (PEI) (Figure 5A).56 The resultant PEI-SNAs efficiently entered glioblastoma (GBM) cells and suppressed the expression of Gli1 and several of its downstream targets (Smo, cyclin D1, c-Myc, Bcl-2, and ABCG2). Through Hh signaling inhibition, these PEI-SNAs slowed cellular proliferation, induced senescence, and increased sensitivity to temozolomide (TMZ), the frontline chemotherapy for GBM.65,67 Additionally, the NPs mitigated the stemness of GBM cells, thereby reducing neurosphere formation (Figure 5B and 5C).56
Figure 5:
(A) Scheme depicting the use of Gli1-targeted PEI-SNAs to suppress Hh signaling in glioblastoma cells and mitigate cellular proliferation, chemoresistance, and stemness. (B) Brightfield images of U87 neurospheres treated with PEI-SNAs carrying Gli1 siRNA or scrambled siRNA. (C) Average diameter of U87 neurospheres treated with PEI-SNAs carrying Gli1 or scrambled siRNA. *p=0.03 by Student’s t-test. Reprinted with permission from reference 65. Copyright 2018 American Chemical Society.
Zhou et al. also demonstrated the ability to deliver siGli1 to cancer cells using NPs formed by self-assembly of 1,2-dioleoyl-3-triethylammonium-propane (DOTAP)-conjugated methoxy-PEG-poly(lactide) copolymer, a non-viral gene delivery vector.132 These particles induced apoptosis and inhibited GBM cell growth in vitro, and slowed the growth of subcutaneous GBM xenografts in vivo.132 These findings corroborate the observations of Melamed et al. that indicate Gli1 inhibition is a promising strategy to combat GBM and other Hh-dependent cancers.
3.4. Future Potential for Antibody Modulation of Hedgehog Signaling
Antibody blockade of Hh signaling has only recently begun to be explored. While several antagonistic antibodies have been developed for Hh suppression, such as the anti-Shh antibodies 5E1 and MEDI-5304,69,75,80,125 they have not yet been incorporated into antibody-NP conjugates. Given that antibody-NP conjugates targeting Wnt signaling have shown greater efficacy than freely delivered antibodies, it is likely that antibody nanocarriers targeting Hh signaling will emerge in the near future as a therapeutic strategy.3,88
4. Nanotherapeutics to Suppress Notch Signaling in Cancer
4.1. Notch Signaling in Cancer
The Notch signaling pathway is a key regulator of cell-to-cell communication that is important for embryonic development and the maintenance and renewal of adult tissues.94 Notch signaling is activated when one of four canonical ligands (Delta1, 3, or 4, or Jagged 1/2) on signal-sending cells bind the extracellular region of Notch receptors 1–4 on signal-receiving cells (Figure 2C).26 This initiates a downstream cascade of cleavages, first by ADAM family proteases that remove the extracellular portion of Notch receptors from their transmembrane domain, and subsequently by the gamma secretase complex that removes the intracellular portion of Notch receptors from the transmembrane domain. Ultimately, this cascade leads to the nuclear translocation of the Notch intracellular domain (NICD) to the nucleus.127 In the nucleus, NICD complexes with MAML1 and CSL to promote the transcription of genes associated with cell survival, proliferation, and stemness (Figure 2C). In addition to this ligand dependent activity, the Notch signaling pathway can also undergo ligand independent activation that is mediated by ADAM17.82
As noted before, Notch signaling is frequently dysregulated in cancer.127 Depending on the type of cancer, different Notch receptors have varying levels of expression and prognostic implications. In certain cases, Notch can even serve as a tumor suppressor 42,44,117, but its overactivation is largely considered oncogenic.1 While overexpression of Notch 2–4 has been implicated in a variety of cancers1,35,36,99, Notch-1 in particular is frequently overexpressed in cancer and serves as a promising target for therapeutic manipulation.26,64,82,90,101 The following sections review the major small molecule, RNA, and antibody-based therapies that utilize nanocarriers to treat cancer through regulation of Notch signaling.
4.2. Small Molecules to Regulate Notch Signaling
Small molecule therapies are being developed to inhibit several different components of the Notch signaling pathway (Figure 3C). ADAM inhibitors block the first of the two cleavages of the Notch receptor that occur after signaling activation. As noted above, ADAM family proteins cleave off the extracellular domain of the Notch receptor. ADAM inhibitors prevent this extracellular cleavage to halt further signaling.26 More specifically, ADAM10 inhibitors prevent ligand-dependent activation of Notch signaling, while ADAM17 inhibitors interfere with ligand-independent receptor activity.82 Further downstream, MAML1 inhibitory peptides block the activity of the NICD-CSL-MAML1 complex, thereby preventing the transcription of oncogenic Notch target genes. 26,68,81 The most widely explored class of small molecule inhibitors of Notch signaling are gamma secretase inhibitors (GSIs), which block cleavage of the NICD and prevent its translocation to the nucleus (Figure 6A).26,127 Neither ADAM inhibitors nor MAML1 inhibitors have been incorporated into NP formulations to date, but GSIs have begun to be explored in nano-formulations.
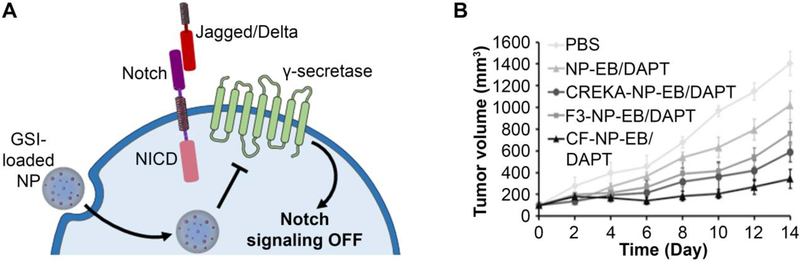
Figure 6:
(A) Scheme depicting the use of nanoparticles carrying a gamma secretase inhibitor (GSI) to suppress Notch signaling in cancer cells. (B) Nanoparticles carrying the GSI DAPT and the drug erlotinib that were coated with both CREKA tumor-homing peptides and F3 cell-penetrating peptides reduced the growth of triple negative breast cancer tumors to a greater extent than NPs coated with either targeting agent individually. Reproduced with permission from reference 115 under the Creative Commons Attribution License. Copyright 2019 Xu Wan, Chaoqian Liu, Yinan Lin, Jie Fu, Guohong Lu, and Zhengmao Lu. Published by Informa UK Limited, trading as Taylor & Francis Group.
While GSIs are potent against Notch-dependent cancers, they commonly exhibit severe gastrointestinal side effects when delivered freely as they are typically delivered orally due to low solubility in aqueous conditions.127 One effective method to minimize these toxic off-target effects is to encapsulate GSIs within NPs. In an intriguing study, Mamaeva et al. developed mesoporous silica NPs to deliver the GSI DAPT to breast cancer.61,62 In testing this NP formulation against MDA-MB-231 breast cancer cells transplanted on chorioallantoic membranes, Mamaeva et al. found that NP-mediated delivery of DAPT successfully inhibited Notch signaling to subsequently reduce the CSC population. Lu and colleagues also delivered DAPT, in combination with the EGFR-inhibitor erlotinib, to triple negative breast cancer (TNBC) using polylactic acid-based NPs.115 When the NPs were coated with both CREKA, a tumor-homing peptide, and F3, a cell-penetrating peptide, they could achieve greater anti-tumor effects in vivo than NPs coated with either external functional agent alone or no coating (Figure 6B).99 Besides DAPT, several other GSIs are in clinical development (e.g., MRK-560, MK-0752, BMS-906024) that may benefit from delivery via NP carriers, as these hydrophobic drugs could be encapsulated in NPs to enhance their systemic tumor delivery and limit the side effects they induce.19,32,72,85
While small molecule inhibitors of Notch like those described above are effective, they also face limitations. For example, GSIs are pan-Notch inhibitors that affect Notch 1–4 simultaneously, which can elevate toxicity versus targeting the receptors individually.121 Further, given that specific receptors play distinct roles in driving the progression of different types of cancer, it would be beneficial to inhibit these receptors individually. Two methods that lend themselves to specific Notch inhibition are RNA interference and antibody-based therapeutics, as discussed below.
4.3. Nanoparticle-Mediated RNA Interference of Notch Signaling
RNAi directed at Notch signaling largely targets the Notch receptors and their ligands, rather than attempting to suppress downstream pathway mediators. Liu and colleagues have developed iron oxide-silica NPs to effectively deliver shRNA targeting Notch-1 to TNBC.123 Through their work, they demonstrated that these NPs effectively reduce Notch-1 expression and cell proliferation, and induce apoptosis in MDA-MB-231 TNBC cells. Similarly, Steg et al. employed chitosan NPs to deliver siJagged1 to treat ovarian cancer. 100 These NPs reduced angiogenic behavior in endothelial cells and inhibited chemoresistance and proliferation of tumor cells in an orthotopic mouse model of ovarian cancer.84 In a less direct approach, Deng et al. examined the effect of delivering the tumor suppressive microRNA miR-34a to TNBC using chitosan-based NPs.23 They showed that miR-34a delivery to MDA-MB-231 TNBC cells via these NPs inhibited cell migration and reduced protein expression of Notch-1. Overall these studies demonstrate that interfering with Notch signaling, particularly at the receptor/ligand level, can significantly reduce the proliferation of cancer cells and halt tumor progression.
4.4. Antibody Nanocarriers to Modulate Notch Signaling
As with the Wnt and Hh signaling pathways, several antibodies have been developed and tested to antagonize Notch signaling, with the majority of research focusing on Notch-1 inhibition.4,26,97,121 However, these antibodies have not yet been incorporated into NP systems, which would likely increase their potency due to multivalency. This is an area with great potential for future development. Notably, Biktasova et al. have demonstrated the potential impact of exploiting multivalency by administering multivalent forms of the Notch receptor ligand DLL-1 to preclinical mouse models of lung cancer.8 They created DLL-1 clusters by biotinylating the Fc portion of IgG antibodies fused with the extracellular domain of DLL-1 and mixing them with NeutrAvidin. These clusters were able to regulate Notch signaling and activate a tumor-specific T-cell response for enhanced anti-tumor effect.8 In the future, antibody nanocarriers designed to modulate Notch signaling could transform the management of Notch-dependent cancers.
5. Challenges and Opportunities
As highlighted in this review, various NP formulations have been designed to deliver small molecule, RNA, and antibody inhibitors of the developmental Wnt, Hh, and Notch signaling pathways to cancer to halt disease progression (Table 2). Although these pathways are not expressed in all cancer types, they are highly implicated in many aggressive cancers, making them valuable targets for NP-mediated inhibition. While the NP formulations discussed in this review can improve the pharmacokinetics and safety profiles of their designated cargo93 and often reduce the administered dose required88, there are still challenges to overcome both in achieving significant levels of therapeutic efficacy and in reaching clinical translation.
One key challenge in targeting Wnt, Notch, or Hh signaling is that cells can develop resistance by exploiting complexity within the pathway. For example, as discussed previously, Smo inhibitors for the Hh signaling pathway can encounter resistance when Gli1 is upregulated through noncanonical pathways.83 A further mechanism of resistance is that there is substantial crosstalk between the Wnt, Notch, and Hh signaling pathways, and a molecule that acts as a tumor-promoter in one pathway may play a tumor-suppressive role in another pathway. GSK-3β, for example, is a key mediator of the Wnt signaling pathway and serves as a popular target for inhibition73, but in the Hh signaling pathway, this protein can serve as a suppressor and its expression is thus desired 76,77. NPs afford the opportunity to counter some of this developed resistance to treatment with the possibility for co-encapsulation or delivery of multiple therapeutic molecules, but this presents additional challenges for future clinical evaluation and implementation. For a more in depth review of the crosstalk between these pathways, readers should refer to the review by Borggrefe et al.10
In addition to the challenges associated with cellular resistance, there are also barriers to effective tumor delivery of NPs that target the Wnt, Notch, and Hh signaling pathways. Recent studies have indicated that only 0.7% of injected NP doses actually reach solid tumors.118 This means off-target toxicity could be a concern, as Wnt, Notch, and Hh signaling play a regulatory role in some adult tissues.38,128 For example, Wnt signaling plays an important role in bone repair and regeneration.38 Inhibiting Wnt signaling can lead to bone loss, but the delivery of bisphosphonates can mitigate these side effects.59 Moving forward, researchers should develop strategies to increase the fraction of NPs that reach tumors versus healthy organs to minimize toxicity, and also explore the possibility of delivering concurrent therapeutics such as bisphosphonates to alleviate any side effects that do occur.
Besides the challenge of reaching tumors versus healthy organs, once in tumors, NPs encounter a variety of cell types and extracellular matrix components that limit their ability to penetrate deeply beyond vessel walls. Chan and colleagues calculated that only 0.0014% of an injected NP dose reaches the desired cancer cells within a tumor.22 Notably, inhibition of Wnt, Notch, and/or Hh signaling may modulate the tumor microenvironment to enhance NP delivery. For example, Jiang et al. demonstrated that cyclopamine-loaded NPs that inhibit Hh signaling could disrupt the tumor extracellular matrix and enhance tumor perfusion. Combining this treatment with PTX-loaded NPs significantly improved the delivery of PTX to the tumor to enhance treatment effect.49 This work provides evidence that modulating the tumor microenvironment through inhibition of developmental signaling pathways may improve the tumor delivery of NPs, but this should be thoroughly investigated through mechanistic studies in the future.
A further consideration when creating NPs to regulate developmental signaling pathways is the location of the targeted protein or molecule within the cancer cell itself (extracellular, cytosolic, or nuclear), as this will dictate the choice of therapeutic agent that should be carried by the NP. For example, large therapeutic molecules such as antibodies can typically only be delivered to extracellular sites, making antibody-NP conjugates best suited for inhibition of soluble ligands or surface-bound receptors. Notably, substantial progress has been made in recent years towards enabling intracellular antibody delivery through clever nano-engineering or chemical modification of NPs with cell-penetrating peptides. 31,55,70,98 With continued optimization, antibody-NP conjugates may be used to suppress both extracellular and intracellular targets in the Wnt, Notch, and Hh signaling pathways.
When designing NPs to attack intracellular targets in developmental pathways, one must also consider that the mechanism of endocytosis can influence the fate of the NP and thus impact the potency of the cargo.53 In particular, the delivery of RNA molecules requires endolysosomal escape so that the released RNA cargo can bind the RNA-induced silencing complex and impact gene expression.24 Studies have shown that cargo internalized by clathrin or caveolae-dependent mechanisms can be routed to lysosomes, but caveolae can also fuse with caveosomes in some cases to avoid lysosome accumulation.74,84,87 Thus, when designing NP carriers, it is important to consider both the mechanism of cellular uptake and of action of the cargo.
Two final hurdles to clinical translation of NPs targeting Wnt, Notch, and Hh signaling include the processing required for large-scale manufacturing and the cost of this production. While NP delivery systems can limit cost of therapeutic agents by reducing the effective dose, the additional cost of the nanocarrier material, the cost of any product lost during NP synthesis, and the scalability of the synthesis are important considerations for these systems as they move toward the clinic. For a more in depth review of the challenges facing NPs and the current standing of clinical translation of these formulations, readers should turn to the review by Hua et al.40
Overall, there are several barriers remaining to clinical translation of NPs targeting Wnt, Notch, and Hh signaling. Biological barriers to translation include complexities within the signaling pathways that can confer resistance on the cancer cells, and continued challenges in delivery not only to tumors, but also to the targeted cells and molecules within tumors. Production barriers including processing constraints and cost also contribute to the challenges of NP therapeutic clinical translation. Successfully overcoming these barriers will result in improved outcomes for patients with a variety of debilitating cancers.
6. Acknowledgements
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award Number R35GM119659 and by the National Cancer Institute of NIH under Award Number R01CA211925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
8. References
- 1.Aburjania Z, Jang S, Whitt J, Jaskula-Stzul R, Chen H, and Rose JB. The Role of Notch3 in Cancer. Oncologist 23:1–12, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend RC, Londoño-Joshi AI, Gangrade A, Katre AA, Kurpad C, Li Y, Samant RS, Li P-K, Landen CN, Yang ES, Hidalgo B, Alvarez RD, Straughn JM, Forero A, and Buchsbaum DJ. Niclosamide and its analogs are potent inhibitors of Wnt/β-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget 7:86803–86815, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruebo M, Valladares M, and Gonzalez-Fernandez A. Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater 1–24, 2009.doi: 10.1155/2009/439389 [DOI] [Google Scholar]
- 4.Aste-Amezaga M et al. Characterization of Notch1 Antibodies That Inhibit Signaling of Both Normal and Mutated Notch1 Receptors. PLoS One 5:e9094, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartscherer K, Pelte N, Ingelfinger D, and Boutros M. Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein. Cell 125:523–533, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Basson MA Signaling in Cell Differentiation and Morphogenesis. Cold Spring Harb. Perspect. Biol 4:a008151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya J, Ren X-R, Mook RA, Wang J, Spasojevic I, Premont RT, Li X, Chilkoti A, and Chen W. Niclosamide-conjugated polypeptide nanoparticles inhibit Wnt signaling and colon cancer growth. Nanoscale 9:12709–12717, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biktasova AK, Dudimah DF, Uzhachenko RV, Park K, Akhter A, Arasada RR, Evans JV, Novitskiy SV, Tchekneva EE, Carbone DP, Shanker A, and Dikov MM. Multivalent Forms of the Notch Ligand DLL-1 Enhance Antitumor T-cell Immunity in Lung Cancer and Improve Efficacy of EGFR-Targeted Therapy. Cancer Res. 75:4728–4742, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borah A, Palaninathan V, Girija AR, Balasubramanian S, Rochani AK, Maekawa T, and Kumar DS. Poly-lactic-co-glycolic acid Nanoformulation of Small Molecule Antagonist GANT61 for Cancer Annihilation by Modulating Hedgehog Pathway. NanoWorld J 3:1–10, 2017. [Google Scholar]
- 10.Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F, and Giaimo BD. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia pathways. Biochim. Biophys. Acta 1863:303–313, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Briscoe J, and Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature 14:416–429, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Burke AR, Singh RN, Carroll DL, Torti FM, and Torti SV. Targeting Cancer Stem Cells with Nanoparticle-Enabled Therapies. J. Mol. Biomarkers Diagnosis 1–8, 2013.doi: 10.4172/2155-9929.S8-003.Targeting [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballo GB, Honorato JR, de Lopes GPF, and de TCL Sampaio e Spohr. A highlight on Sonic hedgehog pathway. Cell Commun. Signal 16:1–15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Che-Ming HJ, Zhang L, Aryal S, Cheung C, Fang RH, and Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci 108:10980–10985, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JK, Taipale J, Cooper MK, and Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16:2743–2748, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen RP, and Blackstock D. Dynamic protein assembly by programmable DNA strand displacement. Nat. Chem. .doi: 10.1038/s41557-018-0016-9 [DOI] [PubMed] [Google Scholar]
- 17.Chenna V, Hu C, Pramanik D, Aftab BT, Karikari C, Campbell NR, Hong S-M, Zhao M, Rudek MA, Khan SR, Rudin CM, and Maitra A. A Polymeric Nanoparticle Encapsulated Small Molecule Inhibitor of Hedgehog Signaling (NanoHHI) Bypasses Secondary Mutational Resistance to Smoothened Antagonists. Mol. Cancer Ther 11:165–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chim CS, Pang R, Fung TK, Choi CL, and Liang R. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 21:2527–2536, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Cook N, Basu B, Smith D-M, Gopinathan A, Evans J, Steward WP, Palmer D, Propper D, Venugopal B, Hategan M, Anthoney DA, Hampson LV, Nebozhyn M, Tuveson D, Farmer-Hall H, Turner H, McLeod R, Halford S, and Jodrell D. A phase I trial of the gamma-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br. J. Cancer 118:793–801, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtois-Cox S, Jones SL, and Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene 27:2801–2809, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Dai Q, Walkey C, and Chan WCW. Polyethylene Glycol Backfilling Mitigates the Negative Impact of the Protein Corona on Nanoparticle Cell Targeting. Angew. Chemie - Int. Ed 53:5093–5096, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, Chen YY, MacMillan P, and Chan WCW. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 12:8423–8435, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Xiao X, Yang Y, Sheng W, Wu Y, and Zeng Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 35:4333–4344, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Dominska M, and Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci 123:1183–1189, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Elzi DJ, Song M, Hakala K, Weintraub ST, and Shiio Y. Wnt Antagonist SFRP1 Functions as a Secreted Mediator of Senescence. Mol. Cell. Biol 32:4388–4399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinoza I, and Miele L. Notch inhibitors for cancer treatment. Pharmacol. Ther 139:95–110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fay BL, Melamed JR, and Day ES. Nanoshell-mediated photothermal therapy can enhance chemotherapy in inflammatory breast cancer cells. Int. J. Nanomedicine 10:6931–6941, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh S, Koser M, Cyr W, Chopda G, Tao J, Shui X, Ying B, Chen D, Pandya P, Chipumuro E, Siddiquee Z, Craig K, Lai C, Dudek H, Monga S, Wang W, Brown BD, and Abrams M. Direct pharmacological inhibition of beta-catenin by RNA interference in tumors of diverse origin. Mol. Cancer Ther 15:2143–2154, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoshal A, Goswami U, Sahoo AK, Chattopadhyay A, and Ghosh SS. Targeting Wnt Canonical Signaling by Recombinant sFRP1 Bound Luminescent Au-Nanocluster Embedded Nanoparticles in Cancer Theranostics. ACS Biomater. Sci. Eng 1:1256–1266, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Goyal R, Kapadia CH, Melamed JR, Riley RS, and Day ES. Layer-by-Layer Assembled Gold Nanoshells for the Intracellular Delivery of miR-34a. Cell. Mol. Bioeng 11:383–396, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta B, Levchenko TS, and Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv. Drug Deliv. Rev 57:637–651, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Habets RA, de Bock CE, Serneels L, Lodewijckx I, Verbeke D, Nittner D, Narlawar R, Demeyer S, Dooley J, Liston A, Taghon T, Cools J, and de Strooper B. Safe targeting of T cell acute lymphoblastic leukemia by pathology-specific NOTCH inhibition. Sci. Transl. Med 11:, 2019. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, and Weinberg RA. The Hallmarks of Cancer. Cell 100:57–70, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, and Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell 144:646–674, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, and Clarke RB. Regulation of Breast Cancer Stem Cell Activity by Signaling through the Notch4 Receptor. Cancer Res. 70:709–719, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Gust KM, Wyatt AW, Goriki A, Jager W, Awrey S, Li N, Oo HZ, Altamirano-Dimas M, Buttyan R, Fazli L, Matsubara A, and Black PC. Not all NOTCH Is Created Equal: The Oncogenic Role of NOTCH2 in Bladder Cancer and Its Implications for Targeted Therapy. Clin. Cancer Res 22:2981–2993, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Hong I-S, Jang G-B, Lee H-Y, and Nam J-S. Targeting cancer stem cells by using the nanoparticles. Int. J. Nanomedicine 10:251–260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houschyar KS, Tapking C, Borrelli MR, Popp D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C, Rein S, Pförringer D, Reumuth G, Grieb G, Mouraret S, Dadras M, Wagner JM, Cha JY, Siemers F, Lehnhardt M, and Behr B. Wnt Pathway in Bone Repair and Regeneration – What Do We Know So Far. Front. Cell Devlopmental Biol 6:1–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu K, Zhou H, Liu Y, Liu Z, Liu J, Tang J, Li J, Zhang J, Sheng W, Zhao Y, Wu Y, and Chen C. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale 7:8607–8618, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Hua S, de Matos MBC, Metselaar JM, and Storm G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol 9:1–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Liu Z, Zhou G, Ling J, Tian A, and Sun N. Silencing Bag-1 gene via magnetic gold nanoparticle-delivered siRNA plasmid for colorectal cancer therapy in vivo and in vitro. Tumor Biol. 37:10365–10374, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Lin L, Shanker A, Malhotra A, Yang L, Dikov MM, and Carbone DP. Resuscitating Cancer Immunosurveillance: Selective Stimulation of DLL1-Notch Signaling in T cells Rescues T-cell Function and Inhibits Tumor Growth. Cancer Res. 71:6122–6132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, Solow-Cordero DE, Jiang J, Rowitch DH, and Chen JK. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. PNAS 106:14132–14137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichimura N, Yamamoto N, Nishikawa M, Furue H, Kondo Y, and Hibi H. Notch3 is frequently downregulated in oral cancer. J. Oral Maxillofac. Surgery, Med. Pathol 29:504–510, 2017. [Google Scholar]
- 45.Ingallina C, Costa PM, Ghirga F, Wang JT, Berardozzi S, Hodgins N, Infante P, Pollard SM, Botta B, and Al-Jamal KT. Polymeric glabrescione B nanocapsules for passive targeting of Hedgehog-dependent tumor therapy in vitro. Nanomedicine 12:711–728, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingham PW, and McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15:3059–3087, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, and Stegh AH. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci. Transl. Med 5:209ra152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J, and Hui C. Hedgehog Signaling in Development and Cancer. Dev. Cell 15:801–812, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang T, Zhang B, Zhang L, Wu X, Li H, Shen S, Luo Z, Liu X, Hu Y, Pang Z, and Jiang X. Biomimetic nanoparticles delivered hedgehog pathway inhibitor to modify tumour microenvironment and improved chemotherapy for pancreatic carcinoma. Artif. Cells, Nanomedicine, Biotechnol 46:S1088–S1101, 2018. [DOI] [PubMed] [Google Scholar]
- 50.Karamboulas C, and Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim. Biophys. Acta 1830:2481–2495, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi A, Yamamoto H, Sato A, and Matsumoto S. New Insights into the Mechanism of Wnt Signaling Pathway Activation. In: International review of cell and molecular biology. 2011, pp. 21–71. [DOI] [PubMed] [Google Scholar]
- 52.Kim M, and Jho E. Cross-talk between Wnt/β-catenin and Hippo signaling pathways: a brief review. BMB Rep. 47:540–545, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kou L, Sun J, Zhai Y, and He Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci 8:1–10, 2013. [Google Scholar]
- 54.Kwok GT, Zhao JT, Weiss J, Mugridge N, Brahmbhatt H, MacDiarmid JA, Robinson BG, and Sidhu SB. Translational applications of microRNAs in cancer, and therapeutic implications. Non-coding RNA Res. 2:143–150, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim SI, Lukianov CI, and Champion JA. Self-assembled protein nanocarrier for intracellular delivery of antibody. J. Control. Release 249:1–10, 2017. [DOI] [PubMed] [Google Scholar]
- 56.Liu Q, Zhu H, Tiruthani K, Shen L, Chen F, Gao K, Zhang X, Hou L, Wang D, Liu R, and Huang L. Nanoparticle-Mediated Trapping of Wnt Family Member 5A in Tumor Microenvironments Enhances Immunotherapy for B-Raf Proto-Oncogene Mutant Melanoma. ACS Nano 12:1250–1261, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, and Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol 22:1184–93, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma C, Shi L, Huang Y, Shen L, Peng H, Zhu X, and Zhou G. Nanoparticle delivery of Wnt-1 siRNA enhances photodynamic therapy by inhibiting epithelial–mesenchymal transition for oral cancer. Biomater. Sci 5:494–501, 2017. [DOI] [PubMed] [Google Scholar]
- 59.Madan B, Mcdonald MJ, Foxa GE, Diegel CR, Williams BO, and Virshup DM. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res 6:, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.di Magliano MP, and Hebrok M. Hedgehog Signalling in Cancer Formation and Maintenance. Nat. Rev 3:903–911, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Mamaeva V, Niemi R, Beck M, Özliseli E, Desai D, Landor S, Gronroos T, Kronqvist P, Pettersen IKN, McCormack E, Rosenholm JM, Linden M, and Sahlgren C. Inhibiting Notch Activity in Breast Cancer Stem Cells by Glucose Functionalized Nanoparticles Carrying γ-secretase Inhibitors. Mol. Ther 24:926–936, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE, Landor S, Toivola DM, Lindén M, and Sahlgren C. Mesoporous Silica Nanoparticles as Drug Delivery Systems for Targeted Inhibition of Notch Signaling in Cancer. Mol. Ther 19:1538–1546, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDermott SP, and Wicha MS. Targeting breast cancer stem cells. Mol. Oncol 4:404–419, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, Allan AL, and Chambers AF. Notch1 Inhibition Alters the CD44hi/CD24lo Population and Reduces the Formation of Brain Metastases from Breast Cancer. Mol. Cancer Res. 9:834–845, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melamed JR, Ioele SA, Hannum AJ, Ullman VM, and Day ES. Polyethylenimine-Spherical Nucleic Acid Nanoparticles against Gli1 Reduce the Chemoresistance and Stemness of Glioblastoma Cells. Mol. Pharmacol 15:5135–5145, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melamed JR, Kreuzberger NL, Goyal R, and Day ES. Spherical Nucleic Acid Architecture Can Improve the Efficacy of Polycation-Mediated siRNA Delivery. Mol. Ther. - Nucleic Acids 12:207–219, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melamed JR, Morgan JT, Ioele SA, Gleghorn JP, Sims-Mourtada J, and Day ES. Investigating the role of Hedgehog/GLI1 signaling in glioblastoma cell response to temozolomide. Oncotarget 9:27000–27015, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendes M, Sousa JJ, Pais A, and Vitorino C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 10:1–47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michaud NR, Wang Y, McEachern KA, Jordan JJ, Mazzola AM, Hernandez A, Jalla S, Chesebrough JW, Hynes MJ, Belmonte MA, Wang L, Kang JS, Jovanovic J, Laing N, Jenkins DW, Hurt E, Liang M, Frantz C, Hollingsworth RE, Simeone DM, Blakey DC, and Bedian V. Novel Neutralizing Hedgehog Antibody MEDI-5304 Exhibits Antitumor Activity by Inhibiting Paracrine Hedgehog Signaling. Mol. Cancer Ther 13:386–398, 2014. [DOI] [PubMed] [Google Scholar]
- 70.Mie M, Takahashi F, Funabashi H, Yanagida Y, Aizawa M, and Kobatake E. Intracellular delivery of antibodies using TAT fusion protein A. Biochem. Biophys. Res. Commun 310:730–734, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Miller-Kleinhenz J, Guo X, Qian W, Zhou H, Bozeman EN, Zhu L, Ji X, Wang YA, Styblo T, O’Regan R, Mao H, and Yang L. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials 152:47–62, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan KM, Fischer BS, Lee FY, Shah JJ, Bertino JR, Rosenfeld J, Singh A, Khiabanian H, and Pine SR. Gamma Secretase Inhibition by BMS-906024 Enhances Efficacy of Paclitaxel in Lung Adenocarcinoma. Mol. Cancer Ther 16:2759–2770, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Motawi TK, El-Maraghy SA, ElMeshad AN, Nady OM, and Hammam OA. Cromolyn chitosan nanoparticles as a novel protective approach for colorectal cancer. Chem. Biol. Interact 275:1–12, 2017. [DOI] [PubMed] [Google Scholar]
- 74.Munsell EV, Ross NL, and Sullivan MO. Journey to the Center of the Cell: Current Nanocarrier Design Strategies Targeting Biopharmaceuticals to the Cytoplasm and Nucleus. Curr. Pharm. Des 22:1227–1244, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura M, Kubo M, Yanai K, Mikami Y, Ikebe M, Nagai S, Yamaguchi K, Tanaka M, and Katano M. Anti-patched-1 Antibodies Suppress Hedgehog Signaling Pathway and Pancreatic Cancer Proliferation. Anticancer Res. 3743–3748, 2007. [PubMed] [Google Scholar]
- 76.Nayak A, Satapathy SR, Das D, Siddharth S, Tripathi N, Bharatam PV, and Kundu CN. Nanoquinacrine induced apoptosis in cervical cancer stem cells through the inhibition of hedgehog-GLI1 cascade: Role of GLI-1. Sci. Rep 1–16, 2016.doi: 10.1038/srep20600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nayak A, Siddharth S, Das S, Nayak D, Sethy C, and Kundu CN. Nanoquinacrine caused apoptosis in oral cancer stem cells by disrupting the interaction between GLI1 and β catenin through activation of GSK3β. Toxicol. Appl. Pharmacol 330:53–64, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Nieuwenhuis E, and Hui C. Hedgehog signaling and congenital malformations. Clin. Genet 67:193–208, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Nusslein-Volhard C, and Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801, 1980. [DOI] [PubMed] [Google Scholar]
- 80.O’Toole SA, Machalek DA, Shearer RF, Millar EKA, Nair R, Schofield P, McLeod D, Cooper CL, McNeil CM, McFarland A, Nguyen A, Ormandy CJ, Qiu MR, Rabinovich B, Martelotto LG, Vu D, Hannigan GE, Musgrove EA, Christ D, Sutherland RL, Watkins DN, and Swarbrick A. Hedgehog Overexpression Is Associated with Stromal Interactions and Predicts for Poor Outcome in Breast Cancer. Cancer Res. 71:4002–4014, 2011. [DOI] [PubMed] [Google Scholar]
- 81.Opačak-Bernardi T, Ryu JS, and Raucher D. Effects of cell penetrating Notch inhibitory peptide conjugated to elastin-like polypeptide on glioblastoma cells. J. Drug Target 25:523–531, 2017. [DOI] [PubMed] [Google Scholar]
- 82.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, and Miele L. Targeting Notch to Target Cancer Stem Cells. Clin. Cancer Res 16:3141–3153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peer E, Tesanovic S, and Aberger F. Next-Generation Hedgehog/GLI Pathway Inhibitors for Cancer Therapy. Cancers (Basel). 11:1–20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pelkmans L, Kartenbeck J, and Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol 3:473–484, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Ran Y, Hossain F, Pannuti A, Lessard CB, Ladd GZ, Jung JI, Minter LM, Osborne BA, Miele L, and Golde TE. Gamma-Secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Mol. Med. 9:950–966, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray P, Confeld M, Borowicz P, Wang T, Mallik S, and Quadir M. PEG-b-poly (carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surfaces B Biointerfaces 174:126–135, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rejman J, Bragonzi A, and Conese M. Role of Clathrin- and Caveolae-Mediated Endocytosis in Gene Transfer Mediated by Lipo- and Polyplexes. Mol. Ther 12:468–474, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Riley RS, and Day ES. Frizzled7 Antibody-Functionalized Nanoshells Enable Multivalent Binding for Wnt Signaling Inhibition in Triple Negative Breast Cancer Cells. Small 13:, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ring A, Kim Y-M, and Kahn M. Wnt/Catenin Signaling in Adult Stem Cell Physiology and Disease. Stem Cell Rev. Reports 10:512–525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, and Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int. J. Cancer 125:1016–1026, 2009. [DOI] [PubMed] [Google Scholar]
- 91.Rubin LL, and de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nature 5:1026–1033, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Sancho R, Cremona CA, and Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. 16:571–581, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanna V, Pala N, and Sechi M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomedicine 9:467–483, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato C, Zhao G, and Ilagen MXG. An Overview of Notch Signaling in Adult Tissue Renewal and Maintenance. Curr. Alzheimer Res. 9:227–240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott AM, Wolchok JD, and Old LJ. Antibody therapy of cancer. Nat. Rev. Cancer 12:278–287, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, and Calin GA. microRNA Therapeutics in Cancer — An Emerging Concept. EBioMedicine 12:34–42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma A, Paranjape AN, Rangarajan A, and Dighe RR. A Monoclonal Antibody against Human Notch1 Ligand–Binding Domain Depletes Subpopulation of Putative Breast Cancer Stem–like Cells. Mol. Cancer Ther 11:77–87, 2012. [DOI] [PubMed] [Google Scholar]
- 98.Slastnikova TA, Ulasov AV, Rosenkranz AA, and Sobolev AS. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol 9:1–21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Speiser J, Foreman K, Drinka E, Godellas C, Perez C, Salhadar A, Ersahin C, and Rajan P. Notch-1 and Notch-4 Biomarker Expression in Triple-Negative Breast Cancer. Int. J. Surg. Pathol 20:139–145, 2012. [DOI] [PubMed] [Google Scholar]
- 100.Steg AD, Katre AA, Goodman B, Han H-D, Nick AM, Stone RL, Coleman RL, Alvarez RD, Lopez-Berestein G, Sood AK, and Landen CN. Targeting the Notch Ligand Jagged1 in Both Tumor Cells and Stroma in Ovarian Cancer. Clin. Cancer Res 17:5674–5686, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stylianou S, Clarke RB, and Brennan K. Aberrant Activation of Notch Signaling in Human Breast Cancer. Cancer Res. 66:1517–1526, 2006. [DOI] [PubMed] [Google Scholar]
- 102.Sykes EA, Chen J, Zheng G, and Chan WCW. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 8:5696–5706, 2014. [DOI] [PubMed] [Google Scholar]
- 103.Taipale J, and Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349–354, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, and Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406:1005–1009, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Takebe N, Harris PJ, Warren RQ, and Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol 8:97–106, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, and Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol 12:445–464, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tangudu NK, Verma VK, Clemons TD, Beevi SS, Hay T, Mahidhara G, Raja M, Nair RA, Alexander LE, Patel AB, Jose J, Smith NM, Zdyrko B, Bourdoncle A, Luzinov I, Iyer KS, Clarke AR, and Kumar LD. RNA Interference Using c-Myc – Conjugated Nanoparticles Suppresses Breast and Colorectal Cancer Models. Mol. Cancer Ther 14:1259–1270, 2015. [DOI] [PubMed] [Google Scholar]
- 108.Tree DRP, Shulman JM, Rousset R, Scott MP, Gubb D, and Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109:371–81, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, and Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619–25, 1988. [DOI] [PubMed] [Google Scholar]
- 110.Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X, Jiang H, Sun D, Scheidt J, Qian V, He S, Gilmore H, Schiemann WP, and Lu Z-R. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjug. Chem. 30:907–919, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valcourt DM, Harris J, Riley RS, Dang M, Wang J, and Day ES. Advances in targeted nanotherapeutics: From bioconjugation to biomimicry. Nano Res. 3:1–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veeck J, Niederacher D, An H, Klopocki E, Wiesmann F, Betz B, Galm O, Camara O, Dürst M, Kristiansen G, Huszka C, Knüchel R, and Dahl E. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene 25:3479–3488, 2006. [DOI] [PubMed] [Google Scholar]
- 113.Verma RK, Yu W, Shrivastava A, Shankar S, and Srivastava RK. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice. Sci. Rep 1–13, 2016.doi: 10.1038/srep32743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verma RK, Yu W, Singh SP, Shankar S, and Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomedicine Nanotechnology, Biol. Med 11:2061–2070, 2015. [DOI] [PubMed] [Google Scholar]
- 115.Wan X, Liu C, Lin Y, Fu J, Lu G, and Lu Z. pH sensitive peptide functionalized nanoparticles for co-delivery of erlotinib and DAPT to restrict the progress of triple negative breast cancer. Drug Deliv. 26:470–480, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang L, Liu X, Zhou Q, Sui M, Lu Z, Zhou Z, Tang J, Miao Y, Zheng M, Wang W, and Shen Y. Terminating the criminal collaboration in pancreatic cancer: Nanoparticle-based synergistic therapy for overcoming fibroblast-induced drug resistance. Biomaterials 144:105–118, 2017. [DOI] [PubMed] [Google Scholar]
- 117.Wen X-F, Chen M, Wu Y, Chen M-N, Glogowska A, Klonisch T, and Zhang G-J. Inhibitor of DNA Binding 2 Inhibits Epithelial-Mesenchymal Transition via Up-Regulation of Notch3 in Breast Cancer. Transl. Oncol 11:1259–1270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, and Chan WCW. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 1:, 2016. [Google Scholar]
- 119.Wu F, Zhang Y, Sun B, McMahon AP, and Wang Y. Hedgehog Signaling: From Basic Biology to Cancer Therapy. Cell Chem. Biol 24:252–280, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu X, Chen H, and Wang X. Can lung cancer stem cells be targeted for therapies? Cancer Treat. Rev 38:580–588, 2012. [DOI] [PubMed] [Google Scholar]
- 121.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, and Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nat. Lett 464:1052–1057, 2010. [DOI] [PubMed] [Google Scholar]
- 122.Xu Y, Chenna V, Hu C, Sun H-X, Khan M, Bai H, Yang X-R, Zhu Q-F, Sun YF, Maitra A, Fan J, and Anders RA . Polymeric Nanoparticle Encapsulated Hedgehog Pathway Inhibitor HPI-1 (“NanoHHI”) Inhibits Systemic Metastases in an Orthotopic Model of Human Hepatocellular Carcinoma. Clin. Cancer Res 18:1291–1302, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang H, Li Y, Li T, Xu M, Chen Y, Wu C, Dang X, and Liu Y. Multifunctional Core/Shell Nanoparticles Cross-linked Polyetherimide-folic Acid as Efficient Notch-1 siRNA Carrier for Targeted Killing of Breast Cancer. Sci. Rep 4:1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang R, Mondal G, Wen D, and Mahato RI. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomedicine Nanotechnology, Biol. Med 13:391–401, 2017. [DOI] [PubMed] [Google Scholar]
- 125.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC Jr, Rubin LL, and de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nat. Lett 455:406–411, 2008. [DOI] [PubMed] [Google Scholar]
- 126.You J, Zhao J, Wen X, Wu C, Huang Q, Guan F, Wu R, Liang D, and Li C. Chemoradiation Therapy using Cyclopamine-Loaded Liquid-Lipid Nanoparticles and Lutetium-177-Labeled Core-Crosslinked Polymeric Micelles. J. Control. Release 202:40–48, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, Wu GS, and Wu K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett 369:20–27, 2015. [DOI] [PubMed] [Google Scholar]
- 128.Yue Z-S, Ruan B, Duan J-L, Han H, and Wang L. The role of the Notch signaling pathway in liver injury and repair. J. Bio-X Res 1:95–104, 2018. [Google Scholar]
- 129.Zhan T, Rindtorff N, and Boutros M. Wnt signaling in cancer. Oncogene 36:1461, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao J, Wang H, Hsiao C-H, Chow DS-L, Koay EJ, Kang Y, Wen X, Huang Q, Ma Y, Bankson JA, Ullrich SE, Overwijk W, Maitra A, Piwnica-Worms D, Fleming JB, and Li C. Simultaneous Inhibition of Hedgehog Signaling and Tumor Proliferation Remodels Stroma and Enhances Pancreatic Cancer Therapy. Biomaterials 159:215–228, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, and Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature 8:806–823, 2009. [DOI] [PubMed] [Google Scholar]
- 132.Zhou P, Cao Y, Liu X, Yu T, Xu Q, You C, Gao X, and Wei Y. Delivery siRNA with a novel gene vector for glioma therapy by targeting Gli1. Int. J. Nanomedicine 13:4781–4793, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]