Abstract
BRAF V600 mutations occur in a wide range of tumor types and RAF inhibition has become standard in several of these cancers. Despite this progress, BRAF V600 mutations have historically been considered a clear demonstration of tumor lineage context-dependent oncogene addiction, based predominantly on the insensitivity of RAF inhibition in colorectal cancer. However, the true broader activity of RAF inhibition pan-cancer remains incompletely understood. To address this, we conducted a multi-cohort ‘basket’ study of the BRAF inhibitor vemurafenib in non-melanoma BRAF V600 mutation-positive solid tumors. In total, 172 patients with 26 unique cancer types were treated, achieving an overall response rate of 33% and median duration of response of 13 months. Responses were observed in 13 unique cancer types, including historically treatment-refractory tumors such as cholangiocarcinoma, sarcoma, glioma, neuroendocrine carcinoma, and salivary gland carcinomas. Collectively, these data demonstrate that single-agent BRAF inhibition has broader clinical activity than previously recognized.
Keywords: vemurafenib, BRAF mutation, solid tumors, basket, pan-cancer
INTRODUCTION
The concept that genomic alterations could be used to guide cancer therapy, regardless of tissue of origin, has been a central objective of genome-driven oncology since its inception. This initial promise was ultimately partially realized with the recent demonstration of tumor-agnostic efficacy and subsequent regulatory approval of PD1 blockade for tumors harboring mismatching repair deficiency (1,2) and TRK inhibition for tumors harboring TRK fusions (3). Despite these significant advancements, experience with the broader array of targeted therapies has demonstrated that their efficacy is often dependent, at least in part, on tumor lineage (4,5).
The efficacy of BRAF V600-mutant selective RAF inhibitors, including vemurafenib, provided an early and prominent example of lineage-dependent response to targeted therapy. Although these agents achieve a high objective response rate and prolong survival in patients with BRAF V600-mutant melanoma (6), they lack meaningful single-agent activity in BRAF V600-mutant colorectal cancer (7). Subsequent biologic studies have demonstrated that the intrinsic resistance to single-agent RAF inhibition in BRAF V600-mutant colorectal cancer is mediated by feedback reactivation of receptor tyrosine kinases (8) and may be overcome with combination targeted therapy targeting these lineage-specific primary resistance mechanisms (9). Despite these findings, the extent to which RAF inhibition efficacy in BRAF V600-mutant tumors is conditioned by lineage beyond colorectal cancer remains largely unknown. Further complicating exploration of this important clinical question is the pattern of BRAF V600 mutations across cancers. Specifically, although BRAF V600 mutations are common, occurring in ~50% of melanomas and papillary thyroid cancers, they occur much with lower frequency (typically <5%) across a variety of other cancer types (10).
To address this important and ongoing knowledge gap, a single-arm, multi-histology, phase 2 study was launched (VE-BASKET; NCT01524978). This study, the first in what became a new wave of ‘basket’ studies, was designed to assess the efficacy of a targeted agent across multiple cancer types characterized by the presence of a single genomic biomarker. Specifically, VE-BASKET was intended to explore the efficacy of vemurafenib in patients with any BRAF V600 mutation-positive cancers other than melanoma, papillary thyroid cancer, and hairy cell leukemia, cancers for which efficacy had been previously defined in traditional tumor-specific studies (6,11,12). The basket study design permitted expansion or discontinuation of enrollment of any specific tumor type based on observed signals of activity following initial enrollment. Previously published preliminary results from the first 95 patients who received vemurafenib monotherapy indicated promising activity in patients with non-small cell lung cancer (NSCLC) as well as histiocytic neoplasms (Erdheim-Chester disease and Langerhans cell histiocytosis) (13). Expanded enrollment of patients with Erdheim-Chester disease ultimately resulted in regulatory approval of vemurafenib in this indication in the United States (14). Subsequently, more mature data in NSCLC (15), primary brain tumors (16), and myeloma (17) have been published.
Despite these tumor-specific descriptions of efficacy, the expanded experience in less commonly represented tumor types, as well as the pan-cancer activity of single-agent vemurafenib, has never been presented or reported. Importantly, during the conduct of this study, only enrollment of patients with colorectal cancer was permanently discontinued. Otherwise, accrual of patients with all other eligible cancer types was permitted throughout the study period, providing a unique opportunity to evaluate the activity of RAF inhibition in a prevalent population of patients with BRAF V600 mutant cancers. We now present the updated and final pan-cancer efficacy data for vemurafenib monotherapy in 172 patients with 26 unique BRAF V600-mutant cancer types. To further increase the value of our findings to the clinical and research community, patient-level demographic and efficacy data are included.
RESULTS
Patient Characteristics
In total, 208 patients with BRAF V600-mutated tumors were enrolled and treated. Of these, 172 had solid tumors and received vemurafenib monotherapy (Table 1, Supplementary Table S1). The remaining 36 patients excluded from this analysis comprised 9 with multiple myeloma and 27 with colorectal cancer who received vemurafenib plus cetuximab.
Table 1.
Patient characteristics
| Characteristic | Vemurafenib Monotherapy (n=172) |
|---|---|
| Median age (range), years | 60 (18–90) |
| Age group, years | |
| 18–64 | 112 (65) |
| 65–84 | 57 (33) |
| ≥85 | 3 (2) |
| Sex, No. (%) | |
| Male | 82 (48) |
| Female | 90 (52) |
| Primary tumor, No. (%) | |
| Non-small cell lung cancer | 63 (37) |
| Histiocytosis | 27 (16) |
| Glioma | 24 (14) |
| Anaplastic thyroid | 12 (7) |
| Colorectal cancer | 10 (6) |
| Cholangiocarcinoma | 9 (5) |
| Sarcoma | 6 (3) |
| Cancer of unknown primary | 5 (3) |
| Ovarian | 4 (2) |
| Neuroendocrine, NOS | 3 (2) |
| Pancreatic | 3 (2) |
| Othersa | 6 (3) |
| BRAF mutation, No. (%) | |
| V600E | 170 (99) |
| V600, other/unknown | 2 (1) |
| ECOG performance status, No. (%) | (n=155) |
| 0 | 47 (30) |
| 1 | 81 (52) |
| 2 | 27 (17) |
| No. of prior systemic therapies, No. (%) | |
| 0 | 28 (16) |
| 1 | 56 (33) |
| 2 | 43 (25) |
| 3+ | 45 (26) |
| Median (range) | 2 (0–10) |
| Median time since diagnosis (range), months | 12.6 (0.9–232.4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NOS, Not Otherwise Specified.
Others were neuroendocrine, head and neck, cervix, squamous cell, and esophageal cancers.
The median age of patients was 60 (range 18–90) years. Patients had received a median of 2 (range 0–10) prior lines of therapy (Table 1). The most common cancer types were NSCLC (37%), histiocytic neoplasms (16%), glioma (14%), anaplastic thyroid cancer (7%), colorectal cancer (6%), and cholangiocarcinoma (5%). A total of 26 unique cancer types were treated.
This analysis was performed after a median follow-up duration across all patients of 10.7 months (range 0.1–46.3 months). As the study was formally closed at the time of this analysis, all patients had discontinued the study. The most common reasons for vemurafenib discontinuation were progressive disease (n=105; 61%), adverse events (n=20; 12%), and withdrawal by the patient (n=13; 8%) (Supplementary Table 2). In addition, 21 patients in an ongoing response or otherwise deriving benefit at the time of study closure were offered the opportunity to continue vemurafenib treatment through an expanded access study (NCT01739764).
Efficacy
Vemurafenib activity is summarized in Table 2 and detailed in Figures 1A and 1B. Data for patients with histiocytosis are shown in Supplementary Figures 1A and 1B; data for patients with other non-histiocytic tumors are shown in Supplementary Figures 2A and 2B. By investigator-assessment, the objective response rate was 32.6% (95% confidence interval: 25.6–40.1%). The best overall response included complete responses in five patients (3%), partial responses in 51 patients (29.7%), stable disease of any duration in 65 patients (37.8%), and progressive disease in 35 patients (20.3%). An additional 16 patients (9.3%) had missing or non-evaluable response assessments and were counted as non-responders per protocol. In total, responses were observed across 13 unique cancer types including NSCLC, histiocytic neoplasms, glioma of various histologies, anaplastic thyroid cancer, ovarian cancer, cholangiocarcinoma, ovarian cancer, sarcoma, salivary duct cancer, and neuroendocrine carcinoma. The clinical benefit rate, defined as a confirmed partial response of any duration or stable disease lasting ≥6 months, was 42% (95% 34–50%).
Table 2.
Treatment efficacy
| Outcome | All Patients (n=172) |
|---|---|
| Objective response rate, % (95% CI) | 32.6 (25.6–40.1) |
| Clinical benefit ratea, % (95% CI) | 41.9 (34.4–49.6) |
| Best overall responseb, No. (%) | |
| Complete response | 5 (2.9) |
| Partial response | 51 (29.7) |
| Stable disease | 65 (37.8) |
| Progressive disease | 35 (20.3) |
| Missing/Not evaluable | 16 (9.3) |
| Median time to event, months (95% CI) | |
| Duration of response | 13.1 (8.0–22.1) |
| Progression-free survival | 5.8 (5.4–7.6) |
| Overall survival | 17.6 (13.0–28.2) |
Abbreviations: CI, confidence interval.
CR + PR (of any duration) + SD (of ≥6 months).
Per investigator-assessment, required confirmation.
Fig 1A.
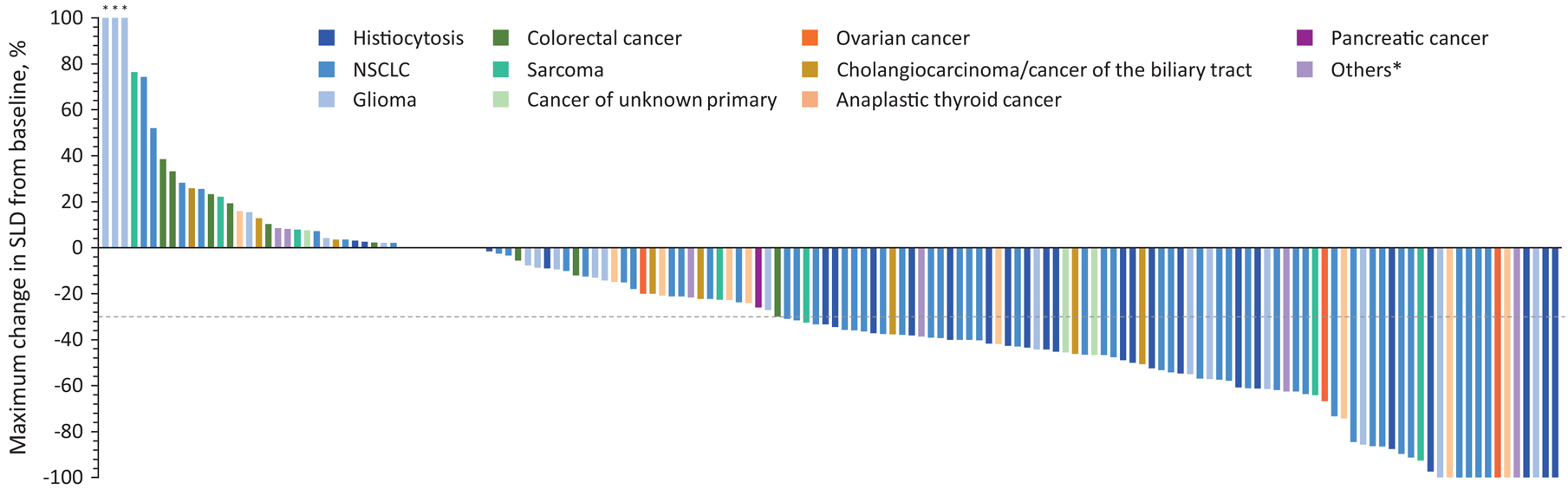

Tumor response to vemurafenib. Plot of time on treatment and time to first response, in individual patients. “Others” includes neuroendocrine, head and neck, cervix, squamous cell, and esophageal cancers. NSCLC, non-small-cell lung cancer. Fig 1B. Waterfall plot of maximum percent decrease from baseline in the sum of diameters of target tumors based on investigator assessment in patients with measurable disease. NSCLC, non-small-cell lung cancer; SLD, sum of the longest diameters. “Others” includes neuroendocrine, head and neck, cervix, squamous cell, and esophageal cancers. The dashed line at −30% represents the cut-off for RECIST response.*>100%.
Among the 56 responding patients, the median duration of response was 13.1 months (95% CI: 8.0–22.1) (Figure 2A). Across the overall population, the median PFS was 5.8 months (95% CI 5.4–7.6) (Figure 2B). The estimated PFS rate at 1 year was 28%. With 83 deaths (48%) at the time of study completion, the median OS was 17.6 months (95% CI 13.0–28.2) (Figure 2C). The estimated OS rates at 1 and 3 years were 60% and 34%, respectively.
Fig 2.
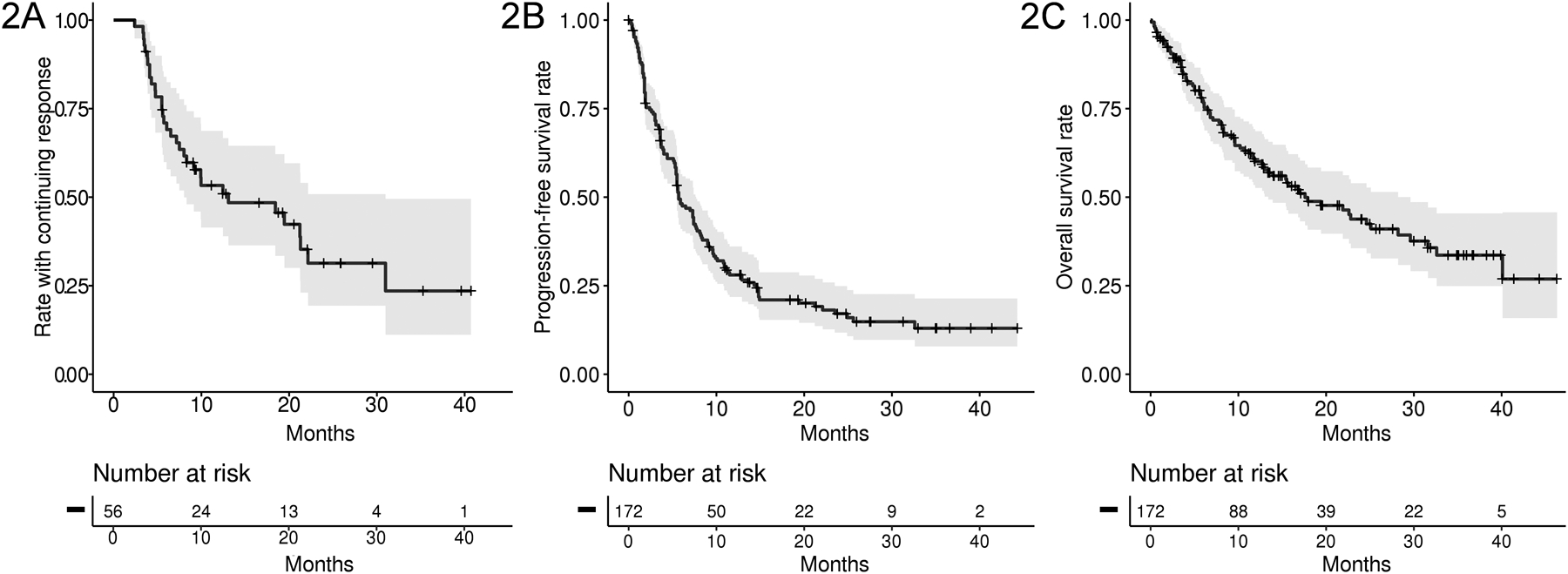
Kaplan Meier plots showing a) duration of response, b) progression-free survival and c) overall survival of 179 patients with BRAF V600 mutation-positive cancers treated with vemurafenib. The 3-, 6-, and 12-month PFS rates were 73.4%, 48.1% and 28.0% respectively. The 3-, 6-, and 12-month survival probabilities were 89.3%, 77.3% and 60.0%, respectively.
Safety
Adverse events occurring in ≥20% of patients, regardless of causality, are shown in Supplemental Table 1. The most common all-grade adverse events were arthralgia (45%), fatigue (34%), and hyperkeratosis (33%). In total, grade ≥3 adverse events occurred in 126 patients (73%), the most common of which were squamous cell carcinoma of the skin (15%), keratoacanthoma (10%), and maculopapular rash (9%). In addition, basal cell carcinoma of the skin occurred in seven patients (4%) and Bowen’s disease in four (2%). Treatment discontinuation due to drug-related adverse events occurred in 13 patients (7.6%). Fatal adverse events occurred in five patients (pulmonary embolism, n=2; respiratory failure and sepsis, n=1, subdural hematoma, n=1; and respiratory failure, n=1). None of these were deemed related to vemurafenib.
DISCUSSION
Here we report the integrated pan-cancer efficacy of BRAF inhibition with single-agent vemurafenib in a large and diverse cohort of 26 unique cancers types harboring BRAF V600 mutations. We found that approximately one-third of patients achieved an objective response and that these responses were generally durable, with a median duration of response exceeding 1 year.
These data are noteworthy for several reasons. Our cohort included many patients with cancer types associated with poor prognosis and treatment refractoriness, including gliomas, pancreatic cancer, colon cancer, sarcomas, and cholangiocarcinomas. Despite this, confirmed objective responses were observed in 13 unique cancer types, including several in which RAF-targeted therapy remains investigational. These efficacy results were achieved despite the fact that patients with melanoma and papillary thyroid cancer were excluded from the outset, as were those with colon cancer following an interim analysis in 11 patients indicated insufficient activity. Interestingly, a separate study of vemurafenib in BRAF V600-mutant papillary thyroid cancer study reported a response rate of 38%, similar to the response rate achieved pan-cancer here, suggesting that the exclusion of this tumor type did not bias our results (11). Beyond these limited enrollment restrictions, the cohort of patients accrued here appears to be broadly reflective of the BRAF V600-mutant prevalent pan-cancer population. As such, these data further contribute to our understanding of the therapeutic relevance of BRAF V600 across multiple cancer types.
When initially launching this early basket study, our primary goal was to screen for potential efficacy of RAF inhibition beyond melanoma, where this approach had already been shown to improve survival. Demonstrating the value of this approach, data from this study were used to support approval of vemurafenib in the histiocytic neoplasm Erdheim-Chester disease in the United States (14,18) as well as inclusion of vemurafenib in the National Comprehensive Cancer Network (NCCN) guidelines for the management of patients with non-small cell lung cancer. In parallel, alternative studies evaluating combined RAF and MEK inhibition have led to regulatory approval of such combinations in NSCLC (19) and anaplastic thyroid cancer (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-anaplastic-thyroid-cancer-braf-v600e-mutation) (20). Moreover, since conceptualizing of this study, subsequent iterations of basket studies have been used to evaluate a broader range of hypotheses, including initial clinical validation of investigational genomic targets and even tumor-agnostic regulatory approval of novel molecular entities (3,21).
This study has some important limitations. Most notably, it was launched before multiple studies demonstrated that the combination of BRAF and MEK inhibition is frequently superior to BRAF inhibition alone (22,23). As such, the efficacy reported here may actually represent an estimate of the lower limit of what might have been achieved if this same population had been treated with a RAF/MEK combination, although this remains unproven. Similarly, in patients with colorectal cancer, subsequent clinical data have shown that sensitivity can be induced through use of RAF inhibitor combinations that incorporate anti-EGFR monoclonal antibodies (9,24). Moreover, best response is reported as the primary efficacy endpoint, whereas in studies with a primary endpoint of response, RECIST advises that confirmed response be reported. There may therefore be a modest over-reporting of efficacy as a result of this approach. The inclusion of histiocytosis in the pan-cancer analysis may also increase the reported efficacy. In addition, this study was conducted before tumor next-generation sequencing became widely available and prior to use of blood-based circulating tumor DNA sequencing. This, as well as the lack of central collection of archival tumor or plasma in the majority of patients, significantly limits our ability to interrogate the broader genomic landscape of patients treated here and our understanding of how this may contribute to the likelihood of treatment responsiveness.
In conclusion, the results of this analysis demonstrate that although tumor lineage can sometimes play an important role in conditioning response to single-agent RAF inhibition, few cancer types exhibit complete insensitivity to vemurafenib, and overall pan-cancer efficacy appears clinically meaningful. In patients with BRAF V600-mutant tumor types for which RAF targeted therapy, alone or in combination, is not currently approved, these data can be used to guide further discussion and decision making.
METHODS
Study Design
VE-BASKET was a multicenter, single-arm, phase II study of vemurafenib in patients with a variety of non-melanoma cancers harboring BRAF V600 mutations identified through testing as routinely practiced by each participating site. All patients received single-agent vemurafenib (960 mg orally twice daily). This study was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by institutional review boards or human research ethics committees at the participating centers. All patients provided written informed consent.
Patients
Eligible patients were aged ≥16 years, with histologically confirmed, measurable (Response Evaluation Criteria in Solid Tumors [RECIST]; version 1.1) disease, BRAF V600 mutation-positive cancer that was refractory to standard therapy or for which standard or curative therapy did not exist or was not considered appropriate by the investigator. Patients had adequate hematologic, renal, and liver function. Prior treatment with a BRAF or MEK inhibitor was not allowed. Patients with active or untreated CNS metastases were excluded, as were those with melanoma, papillary thyroid cancer, and leukemia. Detailed inclusion and exclusion criteria are available in the Protocol Appendix.
Assessments
Assessments were performed using computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis at study entry and every 8 weeks thereafter until disease progression, death, or study withdrawal. Response was investigator assessed using RECIST version 1.1. Adverse events were graded by the investigators using National Cancer Institute Common Terminology Criteria version 4 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf) from consent until 28 days after discontinuation of study treatment.
Outcomes
The primary objective of the analysis was to evaluate the efficacy of vemurafenib (best overall response [BOR]). Key secondary objectives included duration of response (DOR), progression-free survival (PFS), clinical benefit rate (CBR, defined as response or stable disease of ≥6 months), and overall survival (OS), and safety.
Statistical Analysis
This study was primarily intended to analyze efficacy in a tumor-specific context. As such, the original statistical design utilized a modified, two-stage Simon design study for each pre-specified tumor cohort (NSCLC, ovarian cancer, colorectal cancer, cholangiocarcinoma, breast cancer, and multiple myeloma). During Stage 1, seven patients with measurable disease were enrolled into each cohort; this stage was considered complete when all patients had completed a minimum of 8 weeks’ treatment, developed progressive disease, prematurely withdrawn, or died. A further six or 12 patients could be enrolled into Stage 2, depending on the results for Stage 1: if two to four of the seven patients had a response, an additional 12 patients could be enrolled; if five or more of the seven responded, six additional patients were recruited. Recruitment into any cohort/indication could be further expanded up to 70 patients if a response rate was demonstrated in Stage 2 of that cohort according to protocol-defined stopping rules or a clear clinical benefit was observed, as determined by the steering committee.
In the current analysis, efficacy data were pooled across all solid tumor patients treated with single-agent vemurafenib with the goal of defining pan-cancer efficacy. DOR, PFS, and OS were calculated using Kaplan–Meier methodology. Confidence intervals (CIs) for proportions were calculated using the Clopper–Pearson method. All analyses were performed using SAS (versions 9.2 and 9.4; SAS Institute Inc., Cary, NC, USA).
Supplementary Material
SIGNIFICANCE.
These data suggest that BRAF V600 mutations lead to oncogene addiction and are clinically actionable in a broad range of non-melanoma cancers, including tumor types in which RAF inhibition is not currently considered standard of care.
ACKNOWLEDGMENTS
J-YB is funded in part by grants (NETSARC+ and LYRICAN [INCA-DGOS-INSERM 12563])
Editorial support was provided by Lee Miller and Deirdre Carman PhD of Miller Medical Communications Ltd., funded by F. Hoffmann-La Roche, Ltd.
Research support: This study was supported by F. Hoffmann-La Roche Ltd (Basel, Switzerland). Editorial support, provided by Miller Medical Communications Ltd (Brindle, UK), was funded by F. Hoffmann-La Roche Ltd. Additional support from the National Institutes of Health (P30 CA008748).
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Vivek Subbiah: Research funding/grant support for clinical trials: Roche/Genentech, Novartis, Bayer, GlaxoSmithKline, NanoCarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharma, InhibRx, Exelixis, Blueprint Medicines, Loxo Oncology, MedImmune, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals. Travel: Novartis, Pharmamar, ASCO, ESMO, Helsinn Therapeutics, Incyte. Consultancy/advisory board: Helsinn, Loxo Oncology/Eli Lilly, R-Pharm US, Incyte, QED Pharma, MedImmune, Novartis. Other: Medscape.
Igor Puzanov: Consulting or Advisory Role: Roche, Amgen, Bristol-Myers Squibb
Jean-Yves Blay: research support and honoraria from Roche Genentech, GSK, and Novartis
Ian Chau: Research Funding: Sanofi Oncology, Janssen-Cilag, Merck Serono, Novartis; Personal Fees: Sanofi Oncology, Eli Lilly, Bristol Meyers Squibb, MSD, Bayer, Roche, Five Prime Therapeutics, Taiho, Pfizer, Amgen, Gilead Science
A. Craig Lockhart: None declared.
Noopur S. Raje: Consulting or Advisory Role: Celgene, Takeda, Amgen, Novartis; Research Funding: Eli Lilly, AstraZeneca
Juergen Wolf: Research grants from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Ignyta, Lilly, MSD, Novartis, Pfizer, Roche, BMS, MSD, Novartis, Pfizer, and advisory boards and lecture fees from Roche
Jose Baselga: Employed by AstraZeneca. On the board of directors of Foghorn and past board member of Varian Medical Systems, Bristol‐Myers Squibb, Grail, Aura Biosciences and Infinity Pharmaceuticals. Performed consulting and/or advisory work for Grail, PMV Pharma, ApoGen, Juno, Lilly, Seragon, Novartis, and Northern Biologics. Stock or other ownership interests in PMV Pharma, Grail, Juno, Varian, Foghorn, Aura, Infinity Pharmaceuticals, ApoGen, as well as Tango and Venthera, of which JB is a co‐founder. Previously received honoraria or travel expenses from Roche, Novartis, and Eli Lilly
Funda Meric-Bernstam: Commercial research grants from Novartis, AstraZeneca, Calithera, Aileron Therapeutics, Bayer, Debiopharm, Jounce Therapeutics, CytomX, eFFECTOR, Zymeworks, Puma Biotechnology, Taiho, Seattle Genetics, Curis, Millennium, Daiichi Sankyo, AbbVie, Pfizer, Guardant Health, Takeda, and GlaxoSmithKline. Institutional funding and technology support from Royal Philips. Travel related fees from Taiho and Seattle Genetics. Speaker fees from Chugai Biopharmaceuticals. Consultant fees from Pieris Pharmaceuticals, Pfizer, Debiopharm, Dialectica, Sumitomo Dainippon, Samsung Bioepis, Aduro, OrigiMed, Xencor, Jackson Laboratory, Zymeworks, Kolon Life Science, Parexel International, F. Hoffmann-La Roche. Advisor to Inflection Biosciences, GRAIL, Darwin Health, Spectrum, Mersana, Seattle Genetics, Immunomedics, and IBM Watson Health
Jason Roszik: None declared
Eli L. Diamond: has an advisory board relationship with Third Rock Ventures
Gregory Riely: Research funding from Novartis, Genentech, Millennium, GlaxoSmithKline, Pfizer, Infinity Pharmaceuticals, Ariad, Mirati Therapeutics, Merck. Travel, accommodation, expenses from Merck Sharp & Dohme. Other relationships with Pfizer, Genentech, and Takeda
Eric Sherman: is an advisory board member at Loxo, Regeneron, Novartis, and Eisai
Todd Riehl: Employed by Genentech, Inc.
Bethany Pitcher: Employed by F. Hoffmann-La Roche, Ltd.
David M. Hyman: Personal Fees from Chugai Pharma, CytomX Therapeutics, Boehringer Ingelheim, Genentech/Roche and AstraZeneca, and research funding from Puma Biotechnology, AstraZeneca, Loxo Oncology and Bayer AG
REFERENCES
- 1.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–13 doi 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 2019;25(13): 3753–3758 doi 10.1158/1078-0432. [DOI] [PubMed] [Google Scholar]
- 3.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378(8):731–9 doi 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554(7691):189–94 doi 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman DM, Taylor BS, Baselga J. Implementing Genome-Driven Oncology. Cell 2017;168(4):584–99 doi 10.1016/j.cell.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364(26):2507–16 doi 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol 2015;33(34):4032–8 doi 10.1200/jco.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2(3):227–35 doi 10.1158/2159-8290.Cd-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Huijberts S, Grothey A, Yaeger R, Cuyle PJ, Elez E, et al. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study. J Clin Oncol 2019;37(17):1460–9 doi 10.1200/jco.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23(6):703–13 doi 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17(9):1272–82 doi 10.1016/s1470-2045(16)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiacci E, Park JH, De Carolis L, Chung SS, Broccoli A, Scott S, et al. Targeting Mutant BRAF in Relapsed or Refractory Hairy-Cell Leukemia. N Engl J Med 2015;373(18):1733–47 doi 10.1056/NEJMoa1506583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373(8):726–36 doi 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, et al. Vemurafenib for BRAF V600-Mutant Erdheim-Chester Disease and Langerhans Cell Histiocytosis: Analysis of Data From the Histology-Independent, Phase 2, Open-label VE-BASKET Study. JAMA oncology 2018;4(3):384–8 doi 10.1001/jamaoncol.2017.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbiah V, Gervais R, Riely G, Hollebecque A, Blay J-Y, Felip E, et al. Efficacy of vemurafenibv in patients with non-small cell lung cancer with BRAF V600 mutation: an open-label, single-arm cohort of the histology-independent VE-BASKET study. JCO Precision Oncology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF Inhibition in BRAFV600-Mutant Gliomas: Results From the VE-BASKET Study. J Clin Oncol 2018;36(35):3477–84 doi 10.1200/jco.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raje N, Chau I, Hyman DM, Ribrag V, Blay J-Y, Tabernero J, et al. Vemurafenib in Patients With Relapsed Refractory Multiple Myeloma Harboring BRAFV600 Mutations: A Cohort of the Histology-Independent VE-BASKET Study. JCO Precision Oncology 2018(2):1–9 doi 10.1200/po.18.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oneal PA, Kwitkowski V, Luo L, Shen YL, Subramaniam S, Shord S, et al. FDA Approval Summary: Vemurafenib for the Treatment of Patients with Erdheim-Chester Disease with the BRAFV600 Mutation. Oncologist 2018;23(12):1520–4 doi 10.1634/theoncologist.2018-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist 2018;23(6):740–5 doi 10.1634/theoncologist.2017-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600- Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36(1):7–13 doi 10.1200/jco.2017.73.6785.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao JJ, Schram AM, Hyman DM. Basket Studies: Redefining Clinical Trials in the Era of Genome-Driven Oncology. Annu Rev Med 2018;69:319–31 doi 10.1146/annurev-med-062016-050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016;17(9):1248–60 doi 10.1016/s1470-2045(16)30122-x. [DOI] [PubMed] [Google Scholar]
- 23.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017;28(7):1631–9 doi 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, et al. Phase IB Study of Vemurafenib in Combination with Irinotecan and Cetuximab in Patients with Metastatic Colorectal Cancer with BRAFV600E Mutation. Cancer Discov 2016;6(12):1352–65 doi 10.1158/2159-8290.Cd-16-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


