Abstract
Background:
Atherosclerosis of the carotid bifurcation with plaque formation causes asymptomatic carotid artery stenosis (ACAS), which may also be associated with cerebral hypoperfusion. Cerebral hypoperfusion adversely affects multiple aspects of mobility and cognition. This study tests the hypothesis that community-dwelling older adults with a 50% or greater diameter-reducing ACAS will have mobility and cognitive impairments that heighten their risk for falls.
Methods:
Eighty community-dwelling adults completed a mobility assessment (Short Physical Performance Battery, Berg Balance Scale, Four Square Step Test, Dynamic Gait Index, Timed Up and Go, and gait speed), self-reported physical function (Activities-Specific Balance Confidence, SF-12 Physical Function Component), and cognitive tests (Mini-Mental State Examination). Falls were recorded for the past 6 months. Standardized carotid ultrasound examination classified participants into no stenosis (<50% diameter reduction) (n = 54), moderate stenosis (50%−69%) (n = 17), and high-grade stenosis (70%−99%) (n = 9) groups. Linear and logistic regression analyses determined the associations between these measures and the degree of stenosis (three groups).
Results:
Logistic regression analysis showed their degree of stenosis was associated with reductions in mobility (Short Physical Performance Battery [P = .008], Berg Balance Scale [P = .0008], Four Square Step Test [P = .005], DGI [P = .0001], TUG [P = .0004], gait speed [P = .02]), perceived physical function (ABC [P < .0001], SF-12 Physical Function Component [P < .0001]), and cognition (MMSE [P = .003]). Adults with moderate- and high-grade stenosis had a greater incidence of falls compared with those without stenosis (relative risk, 2.86; P = .01). Results remained unchanged after adjustment for age, sex and cardiovascular risk factors.
Conclusions:
ACAS is associated with impaired mobility and cognition that are accompanied with increased fall risk. These impairments increased with worsening severity.
Keywords: Asymptomatic carotid artery stenosis, Cognition, Balance, Physical function, Falls
Asymptomatic carotid artery stenosis (ACAS) with a 50% or greater diameter reduction is a complication of atherosclerotic cardiovascular disease (ASCVD) that occurs in approximately 10% of adults older than 65 years.1 High-grade ACAS (≥70% diameter reduction) identifies individuals at a higher risk of ASCVD events. Although stroke occurs in only 2% of patients with ACAS per year, its prevention has been the traditional focus of treatment.2,3 Recent evidence suggests that 40% to 50% of older adults with ACAS may also have cognitive dysfunction.4,5 This decline in cognitive function may be associated with cerebral hypoperfusion.6 To our knowledge, there is no systematic assessment of mobility function and fall risk in patients with ACAS. Cognitive and mobility dysfunction frequently coexist in older individuals.7–9 This has important clinical and behavioral implications, as because cognition and mobility function are major determinants of health and well-being, independent living, falls, frailty, disability, and survival in the elderly.10–14 This exploratory pilot study tests the hypothesis that community-dwelling, functionally independent older adults with 50% or greater ACAS will have impairments in mobility and cognitive function and will report more falls over the prior 6-month period than participants without stenosis.
METHODS
Ambulatory adults living independently were recruited from the rehabilitation and vascular clinics of the Baltimore Veterans Affairs Medical Center and the University of Maryland. The University of Maryland, Baltimore, Institutional Review Board approved the protocol, and informed consent was obtained from all participants. Carotid artery stenosis was determined by duplex ultrasound in a vascular laboratory accredited by the Intersocietal Accreditation Commission.15 Participants were classified as having no stenosis (<50% diameter reducing), moderate (50%−69% diameter reducing) stenosis, and high grade (70%−99% diameter reducing) stenosis. Doppler velocity thresholds to determine the degree of stenosis were according to consensus criteria used by our group previously (<50% stenosis when the internal carotid artery [ICA] peak systolic velocity [PSV] is <125 cm/second; 50%−69% stenosis when ICA PSV is 125–230 cm/second; and >70% stenosis to near occlusion when ICA PSV is >230 cm/second).16
Exclusions were a disability that limited ambulation or ability to undergo mobility testing, stroke or transient ischemic attack referable to either hemisphere, sedative drugs, prior carotid revascularization procedure, carotid artery occlusion, or known vertebral-basilar or intracranial stenosis or occlusion. Three people were excluded owing to lower extremity amputations and severe knee arthritis. Cardiovascular risk factors, including coronary artery disease, peripheral arterial disease, diabetes mellitus, hypertension, hyperlipidemia, and prior or current smoking, were recorded.17 Coronary artery disease was defined as a positive history of myocardial infarction or angina pectoris.
Five objective, standardized, validated performance tests were used to assess mobility function in all participants.11,18–22 A physical therapist blinded to study participant status (ACAS vs no stenosis) performed all tests in a dedicated space. The order of testing was consistent over time, and scoring occurred within 24 hours. We do not believe that the performance of the measure would induce learning effects. However, a fixed order would ensure that any influence on performance would affect everyone similarly and not influence the results. The total testing time varied from 20 to 30 minutes among the participants because of interindividual variability in the completion time of some tests that had no time limits. The Short Physical Performance Battery (SPPB) assesses gait speed, static balance, and lower extremity strength (time to complete 5 repeated chair rises).11 The items are scored on a scale of 0 to 4, with a total maximum score of 12. The short form Berg Balance Scale (BBS) measures ability to safely balance during a series of predetermined positions that alter the base of support.18,22 The items are scored on a scale of 0 to 4, with a total maximum score of 28. The timed Four Square Step Test (FSST) assesses ability to step quickly over an object forward, sideways and backward.21 The four-item Dynamic Gait Index (DGI) assesses ability to modify balance while walking in the presence of external demands (walking on a level surface, changes in walking speed, walking with horizontal head turns, and walking with vertical head turns).19 The scale ranges from 0 to 3 with a total maximum score of 12. The Timed Up and Go (TUG) requires rising from a chair, walking 3 meters, turning around, walking back to the chair and sitting down.20
We assessed cognitive status, balance confidence, and physical activity as follows: Mini-Mental State Examination (MMSE) for cognitive function; Physical Activity Scale for the Elderly (PASE; range, 0–400) for level of physical activity related to leisure, household and occupational activities23; Activities-Specific Balance Confidence (ABC) for perceived confidence in the ability to maintain balance under different circumstances on a scale of 0% (no confidence) to 100% (total confidence)24; and the Medical Outcome Survey Short Form (SF-12), a 12-item self-report questionnaire for overall health-related quality of life and two subscales related to physical and cognitive health.
At the time of evaluation, participants were asked if they had fallen in the past 6 months. A fall is defined as a sudden, unintentional change in position causing an individual to land at a lower level on an object, the floor, or the ground, other than as a consequence of a sudden onset of paralysis, epileptic seizure, or overwhelming external force.25
Statistical analysis.
Data are reported as means and standard deviations, medians, 25th and 75th percentiles, and minimum and maximum values, and analyzed using SPSS for Windows v. 22.0 (IBM, Chicago, Ill) and SAS version 9.4 (SAS Institute, Cary, NC). The participants were divided into three groups based on carotid ultrasound examination: (1) no stenosis (<50%), (2) moderate stenosis (50%−69%), and (3) high-grade stenosis (70%−99%). Baseline differences in characteristics between the three groups were analyzed using X2 tests and analysis of variance. Linear and logistic regression estimated the association between stenosis and mobility function (SPPB, BBS, FSST, DGI, TUG, and gait speed), cognitive function (MMSE), as well as self-reported measures of physical activity level (PASE), balance confidence (ABC), physical function (SF-12 Physical Function Component) and falls. Models were constructed with stenosis group (one, two, or three) as the independent variable and in a backward elimination selection procedure with a staying P value of .05 with stenosis forced in and cardiovascular risk factors (coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, and prior or current smoking), age, and sex as possible covariates initially. Adjusted and unadjusted P values are presented. Box and whisker plots of measures are presented for each group. Pearson correlation coefficients are used to assess relationships between scores for MMSE, for mobility and balance (SPPB, BBS, FSST, DGI, TUG, gait speed), and for perceived physical activity (PASE), balance confidence (ABC), and physical function (SF-12). The frequency of impairment by the presence of stenosis (yes vs none) was calculated and X2 tests and logistic regression ascertain differences in the frequencies of impairments between groups. For this analysis, impairment is defined as scores that are 1 standard deviation or more below (or above, for the timed tests) the mean of the group without stenosis. Logistic regression modeling with backward selection calculated the relative risk for reported falls in participants with a stenosis compared with those without stenosis. P values of .05 or less indicate statistical significance.
RESULTS
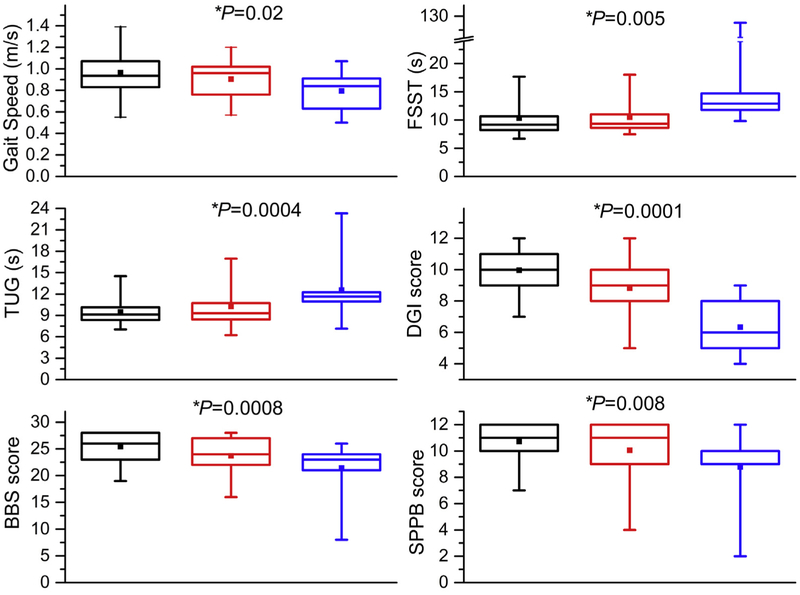
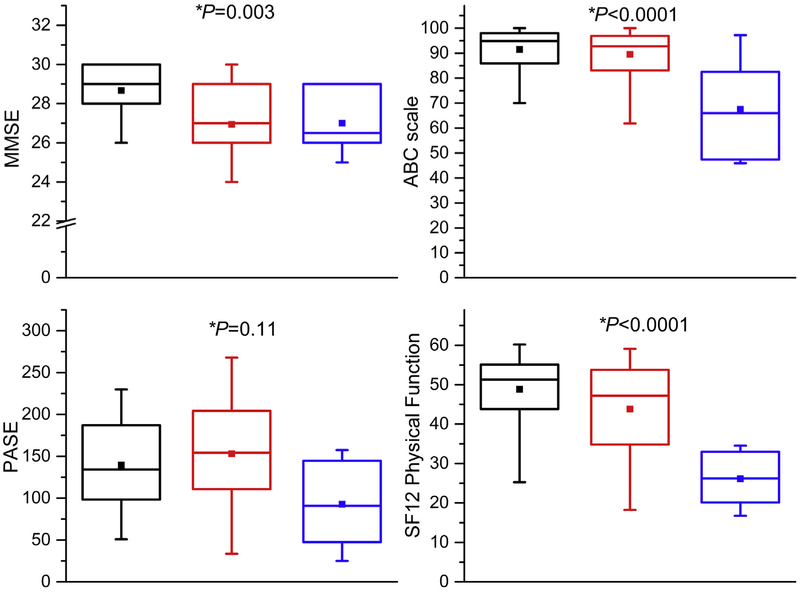
The participants in the three groups were of comparable age, sex, and race (Table I). Consistent with the presence of atherosclerotic disease, rates of coronary artery disease and diabetes are higher in participants with ACAS than in those without a stenosis (Table I). There were 67.5% of participants with no stenosis, 21.3% with moderate stenosis, and 11.2% with high-grade stenosis, of which 12.5% had bilateral disease. The participants with moderate- and high-grade ACAS have worse scores on all measures of mobility function compared with those without a stenosis (Table II; Fig 1), including greater balance deficits (SPPB, BBS, FSST, and DGI) and worse gait measures (TUG, gait speed) with increasing stenosis (Table II). Linear regression shows that scores on all tests of mobility and cognition were worse in participants with moderate or high-grade stenosis than in those without stenosis. Adjusting for clinical characteristics did not change the significance of the association with stenosis. Similarly, the self-reported measures of cognitive function (MMSE), balance confidence (ABC), and SF-12 physical function were lower in participants with stenosis than in those without. The impairments in mobility and cognitive function in the ACAS participants compared with controls persisted after adjustment for the ASCVD risk factors (Table III; Fig 2).
Table I.
Clinical characteristics of participants
| No stenosis (n = 54) | Stenosis 50%–69% (n = 17) | Stenosis 70%–99% (n = 9) | P valuea | |
|---|---|---|---|---|
| Age, years | 74.3 ± 5.9 | 74.9 ± 7.2 | 71.9 ± 7.6 | .51 |
| Female sex | 44.4 | 35.3 | 33.3 | .70 |
| African American race | 53.7 | 52.9 | 77.8 | .38 |
| Index stenosis, right | - | 47.1 | 66.7 | .25 |
| Coronary artery disease | 9.3 | 29.4 | 66.7 | <.001 |
| Diabetes | 25.9 | 52.9 | 55.6 | .049 |
| Hypertension | 63 | 82.4 | 66.7 | .33 |
| Dyslipidemia | 50 | 70.6 | 55.6 | .33 |
| Smoking | 66.7 | 58.8 | 88.9 | .29 |
| Peripheral artery disease | 5.6 | 23.5 | 22.2 | .067 |
Values are presented as mean ± standard deviation or percentages. Percentages are based on total number of individuals for each group.
P value is based on X2 test for categorical variables and analysis of variance for continuous variables.
Table II.
Mobility function (gait and balance) and falls
| Measure | Domain(s) tested | No stenosis (n = 54) | Stenosis 50%–69% (n = 17) | Stenosis 70%–99% (n = 9) | Unadjusted P valuea | Adjusted P valueb |
|---|---|---|---|---|---|---|
| SPPB | Static balance/strength/gait | 10.7 (1.6) | 10.1 (2.3) | 8.8 (3.0) | .006 | .008 |
| BBS | Static/dynamic balance | 25.4 (3.0) | 23.7 (3.6) | 21.4 (5.5) | .001 | .0008 |
| Four Square Test, secondsc | Dynamic balance | 10.3 (3.7) | 10.5 (2.8) | 25.9 (38.7) | .008 | .005 |
| DGI | Gait/dynamic balance | 10.0 (1.6) | 8.8 (2.2) | 6.3 (1.8) | <.001 | <.0001 |
| TUG, seconds | Gait | 9.5 (1.9) | 10.2 (2.8) | 12.6 (4.4) | .001 | .0004 |
| Gait speed | Gait | 0.96 (0.21) | 0.90 (0.17) | 0.79 (0.18) | .015 | .02 |
| Fallers (%) | Falls in last 6 months | 8/54 (14.5%) | 7/17 (41.2%) | 4/9 (44.4%) | .02 | .03 |
BBS, Berg Balance Scale; DGI, Dynamic Gait Index; SPPB, Short Physical Performance Battery; TUG, Timed Up and Go.
All values are presented as mean (standard deviation) except for fallers (percentage).
P value is for linear regression except for Fallers, where logistic regression was used.
P value adjusted for clinical characteristics.
Missing data for one participant in the no stenosis group.
Fig 1.
Boxplots of mobility function measures (gait and balance) for participants without carotid artery stenosis (black boxes), with moderate stenosis (50%−69% diameter reducing) (red boxes) and with high-grade stenosis (70%−99% diameter reducing) (blue boxes). Median, horizontal line in the box; top line of the box, upper quartile (75th percentile); bottom line of the box, lower quartile (25th percentile); Filled square, mean. The ends of the whiskers (vertical lines) indicate the minimum and maximum values. *Adjusted P values based on logistic regression. BBS, Berg Balance Scale; DGI, Dynamic Gait Index; FSST, Four Square Step Test; SPPB, Short Physical Performance Battery; TUG, Timed Up and Go.
Table III.
Cognitive function and self-reported measures of physical function
| Test | Domain | No stenosis (n = 54) | Stenosis 50%–69% (n =16) | Stenosis 70%–99% (n = 6) | Unadjusted P valuea | Adjusted P valueb |
|---|---|---|---|---|---|---|
| MMSE (/30) | Cognitive function | 28.7 (1.3) | 26.9 (3.0) | 27.0 (1.7) | .001 | .003 |
| Physical Activities Scale for the Elderly | Activity level | 139.5 (60.1) | 153.1 (63.8) | 92.7 (52.0) | .36 | .11 |
| Activities Specific Balance Confidence Scale | Balance confidence | 91.4 (9.8) | 89.6 (10.6) | 67.5 (20.6) | <.001 | <.0001 |
| SF12 Physical Function Component | Physical function | 49.4 (9.3) | 42.9 (15.0) | 28.6 (9.5) | <.001 | <.0001 |
MMSE, Mini-Mental State Examination.
All values are presented as mean (standard deviation).
P value for linear regression.
P value adjusted for clinical characteristics.
Fig 2.
Boxplots of cognitive function, and self-reported balance confidence, physical activity, and physical function for participants without carotid artery stenosis (black boxes), with moderate stenosis (50%−69% diameter reducing) (red boxes) and with high-grade stenosis (70%−99% diameter reducing) (blue boxes). Median, central line in the box; top line, upper quartile (75th percentile), lower quartile (25th percentile), mean is central square. The ends of the whiskers (vertical lines) indicate the minimum and maximum values. *Adjusted P values based on logistic regression. ABC, Activities-Specific Balance Confidence Scale; MMSE, Mini-Mental State Examination; PASE, Physical Activity Scale for the Elderly; SF12, Medical Outcome Survey Short Form 12 Physical Function Component.
We next assessed whether mobility function, perceived balance confidence, and physical activity were associated with cognitive function across the three groups. Gait speed measured during complex tasks such as rising from a chair and walking (TUG) or walking while performing additional dual-task maneuvers (DGI) correlated with MMSE (rTUG = −0.30 [P= .008] and rDGI = −0.34 [P = .003]). The performance of other complex physical tasks involving balance (BBS and FSST) also correlated with MMSE (rBBS = 0.27 [P = .02] and rFSST = 0.36 [P = .001]). However, gait speed measured at the participant’s usual self-selected walking pace, correlated weakly with cognitive function (rMMSE = 0.21; P = .07).
A significantly higher proportion of participants with ACAS were cognitively impaired (61% vs 15%; P = .003) and have impaired DGI (62% vs 15%; P = .002) compared with those without stenosis. In addition, a higher proportion of participants with ACAS have a combination of cognitive dysfunction and objective evidence of impaired mobility, compared with those without a stenosis (39% vs 6%; P = .001). These functional impairments in ACAS are associated with a 2.86 times greater (95% confidence interval, 1.31–6.24; P = .01) propensity for falls during the most recent 6 months compared with those without stenosis.
DISCUSSION
In community-dwelling adults living independently without evidence of a prior stroke, the presence of a 50% or greater diameter-reducing asymptomatic carotid stenosis is associated with mobility and cognitive dysfunction that heightens their likelihood of falling. The impaired cognitive and mobility performance, the reduction in perceived physical function, and the reduced confidence in maintaining balance during walking and daily activities worsens with increasing severity of the stenosis. This finding suggests that these functional limitations collectively contribute to the greater incidence of falls in participants with ACAS. Thus, asymptomatic carotid stenosis may not be a benign disease, but rather an insidious one that is accompanied by mobility and cognitive dysfunction and a heightened risk for falls.
There is considerable evidence for an association between cognitive impairment and mobility dysfunction with falls in older adults.8,26–29 The ability to walk at a normal self-selected speed, once considered an automated, spontaneous motor behavior that requires minimal cognitive resources,30–32 depends on more complex neural networks involving executive and visuospatial input.33 Our results show that gait speed and balance are both impaired in participants with ACAS during normal walking, but are impaired to an even greater extent under more cognitively challenging conditions, such as during the DGI, FSST, and TUG. This finding suggests a relationship between impaired cognition with mobility function, congruent with the findings of others where older adults with cognitive dysfunction are limited in their ability to maintain a normal gait rhythm and balance during normal walking.7–9,27,33–36 Furthermore, their mobility impairments are more evident under more challenging conditions, especially in conjunction with acute perturbations, such as turning the head or visual or auditory distractions.27,35,37 The inability to successfully complete these more complex and challenging tasks seem to be related to deficits in the integration of motor signaling across multiple regions of the brain required for the interaction of attention, executive, visuospatial, and motor processing.7,35,36,38 The coexistence of mobility dysfunction with impaired global cognition in adults with ACAS would increase their risk for injurious falls when their cognitive and physical resources are unable to compensate for these impairments, as shown in this study.
Decreased brain perfusion may be related to impaired cognitive function.6 Mobility dysfunction is also found in older adults28 and diabetics39 with impaired cerebrovascular hemodynamics. Carotid stenosis in patients with inadequate cross-collateralization can result in flow restriction, hypoperfusion, and cerebral hemodynamic compromise.5,40 This hemodynamic compromise in patients with ACAS may, therefore, form a potential pathway to explain the mobility and cognitive dysfunction in patients with ACAS observed in our study.5,41 Further studies need to be performed to determine the relationship because we did not perform brain perfusion studies in this population. In addition, atherosclerosis-related inflammation contributes to neurodegeneration, with functional decline and falls in older adults.42,43 Thus, it is likely that a combination of hypoperfusion, cerebrovascular hemodynamic abnormalities, inflammation, silent cerebral infarction, and intracranial atherosclerosis contribute to impaired mobility in carotid stenosis.
Atherosclerosis, a chronic progressive disease with plaque accumulation, has been associated with microembolization and subsequently brain microinfarctions in 15% to 19% of individuals with ACAS.44,45 These microinfarcts could lead to decreases in mobility and cognitive function. However, in our previous study, we did not find a difference in silent brain microinfarct rates between individuals with ACAS who had cognitive impairment and those who were cognitively impaired.5 Further studies would be needed to investigate whether brain microinfarctions are associated with decreases in mobility and cognitive function.
The participants in this study had a high prevalence of coronary artery disease and diabetes, as well as hypertension, dyslipidemia, and cigarette smoking. Cognitive function and mobility worsen with increasing vascular risk factors and with older age,46,47 and these changes are associated with falls.48 Our results indicate that increasing severity of carotid stenosis has a significant and independent effect on mobility and cognition. Low scores on functional activities of daily living have been observed in patients with high-grade ACAS.49,50 Correction of the stenosis by surgery improves quality of life in patients with severe stenosis51; however, a surgical strategy is not applicable for all grades of stenosis. Rehabilitation strategies that emphasize dual task cognitive-motor training and aggressive cardiovascular risk factor management may attenuate these disabilities and decrease fall risk in this population.52,53
The small sample sizes within subgroups classified by the degree of stenosis limits generalizability and the ability to ascertain relative contributions of ACAS and vascular risk factors to cognitive and mobility impairment in this population. This is an exploratory pilot study that lays the groundwork for further studies. More comprehensive vascular risk factor, mobility, and cognitive testing in a larger group of participants with ACAS is needed to clarify the relationship between the severity of stenosis and incident falls. All these participants have multiple comorbidities, and the pathogenesis of their impairments are likely multifactorial. Nevertheless, our analytical model adjusted for clinical risk factors. participants with a stenosis had a 2.8-fold heightened fall rate compared with those without. This fall rate is higher than that reported in participants of a similar age12 and indicates a substantial risk for fracture. Neuroimaging studies and comprehensive cognitive evaluations are required to assess the underlying mechanisms for these functional impairments and aid in the development of effective interventions to slow the progression of ACAS and its impact on cognition, mobility, and risk for fall-related disability.
CONCLUSIONS
This study shows that asymptomatic carotid stenosis is an important risk factor for mobility and cognitive dysfunction and a higher incidence of falls. These disabilities are strongly associated with greater morbidity, disability, nursing home institutionalization, and mortality in older adults. The identification and treatment of risk factors and mechanisms mediating these ACAS-associated disabilities may attenuate declines in cognition and mobility function and reduce the risk of falls in these vulnerable adults. This goal could be accomplished through comprehensive mechanistically driven clinical strategies directed at the reduction of plaque burden, cognitive and mobility rehabilitation, and, in severe stenosis, the addition of surgical revascularization to improve blood flow.
ARTICLE HIGHLIGHTS.
Type of Research: Single-center prospective study
Key Findings: Individuals with asymptomatic carotid stenosis with diameter reducing of more than 50% performed worse on measures of mobility, perceived their physical function as poorer, and scored lower on the Mini Mental State Examination. They also had a greater incidence of falls than those without stenosis (relative risk, 2.86; P = .01).
Take Home Message: Asymptomatic carotid artery stenosis is associated with impaired mobility and cognition and a greater fall risk.
Acknowledgments
The authors acknowledge John Yokemick, RVT, for performing the duplex ultrasound measures in these participants.
Supported by Veteran Affairs Merit (CARA-407-10S and CARA-024-10S to B.K.L.); the National Institute of Neurological Disorders and Stroke (NIH-U01NS080168 to B.K.L.); the National Institute on Aging (R01AG033607 to M.W.R.); the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC); and the National Institute on Aging-Claude D. Pepper Older Americans Independence Center (P30AG028747).
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented at the 2018 Vascular Annual Meeting of the Society for Vascular Surgery, Boston, Mass, June 20–23, 2018.
REFERENCES
- 1.de Weerd M, Greving JP, de Jong AWF, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke 2009;40:1105–13. [DOI] [PubMed] [Google Scholar]
- 2.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg 2011;54:e1–31. [DOI] [PubMed] [Google Scholar]
- 3.Walker M, Marler J, Goldstein M. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–8. [PubMed] [Google Scholar]
- 4.Johnston SC, O’Meara ES, Manolio TA, Lefkowitz D, O’Leary DH, Goldstein S, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med 2004;140:237–47. [DOI] [PubMed] [Google Scholar]
- 5.Lal BK, Dux MC, Sikdar S, Goldstein C, Khan AA, Yokemick J, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg 2017;66:1083–92. [DOI] [PubMed] [Google Scholar]
- 6.Wolters FJ, Zonneveld HI, Hofman A, Van Der Lugt A, Koudstaal PJ, Vernooij MW, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017;136:719–28. [DOI] [PubMed] [Google Scholar]
- 7.Beauchet O, Allali G, Annweiler C, Verghese J. Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT Study. J Gerontol A Biol Sci Med Sci 2016;71:1081–8. [DOI] [PubMed] [Google Scholar]
- 8.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein aging study. Neuropsychology 2006;20:215–23. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013;68:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralnik JM, Simonsick E, Ferrucci L, Glynn R, Berkman L, Blazer D, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 12.Tromp AM, Pluijm SMF, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol 2001;54:837–44. [DOI] [PubMed] [Google Scholar]
- 13.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014;30:421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik J, Ferrucci L, Pieper C, Leveille S, Markides K, Ostir G, et al. Lower extremity function and subsequent disability consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance. J Gerontol A Med Sci 2000;55A:M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IAC standards and guidelines for vascular testing accreditation. Columbia (Md): Intersocietal Accreditation Commission; 2013. [Google Scholar]
- 16.Zierler RE, Jordan WD, Lal BK, Mussa F, Leers S, Fulton J, et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg 2018;68:256–84. [DOI] [PubMed] [Google Scholar]
- 17.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karthikeyan G, Sheikh SG, Chippala P. Test-retest reliability of short form of Berg Balance Scale in elderly people. Glo Adv Res J Med Med Sci 2012;1:139–44. [Google Scholar]
- 19.Marchetti GF, Whitney SL. Construction and validation of the 4-item Dynamic Gait Index. Phys Ther 2006;86:1651–60. [DOI] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 21.Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil 2002;83:1566–71. [DOI] [PubMed] [Google Scholar]
- 22.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Canada 1989;41:304–11. [Google Scholar]
- 23.Washburn R, Ficker J. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sport Med Phys Fit 1999;39:336–40. [PubMed] [Google Scholar]
- 24.Powell L, Myers A. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28–34. [DOI] [PubMed] [Google Scholar]
- 25.Tinetti M, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–7. [DOI] [PubMed] [Google Scholar]
- 26.Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev 2013;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012;60:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology 2010;74:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welmer AK, Rizzuto D, Laukka EJ, Johnell K, Fratiglioni L. Cognitive and physical function in relation to the risk of injurious falls in older adults: a population-based study. J Gerontol A Biol Sci Med Sci 2017;72:669–75. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrijevic M, Gerasimenko Y, Pinter M. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 1998;860:360–76. [DOI] [PubMed] [Google Scholar]
- 31.Grubaugh J, Rhea C. Gait performance is not influenced by working memory when walking at a self-selected pace. Exp Brain Res 2014;232:515–25. [DOI] [PubMed] [Google Scholar]
- 32.Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci 2007;26:275–95. [DOI] [PubMed] [Google Scholar]
- 33.Hausdorff JM, Yogev G, Springer S, Simon E, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res 2005;164:541–8. [DOI] [PubMed] [Google Scholar]
- 34.Hausdorff JM, Hillel I, Shustak S, Del Din S, Bekkers EMJ, Pelosin E, et al. Everyday stepping quantity and quality among older adult fallers with and without mild cognitive impairment: initial evidence for new motor markers of cognitive deficits? J Gerontol A 2017;73:1078–82. [DOI] [PubMed] [Google Scholar]
- 35.Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn 2017;115:41–6. [DOI] [PubMed] [Google Scholar]
- 36.Rosso A, Verghese J, Metti L, Boudreau R, Aizenstein H, Kritchevsky S, et al. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology 2017;89:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh H, Sanders O, McCombe Waller S, Bair WN, Beamer B, Creath RA, et al. Relationship between head-turn gait speed and lateral balance function in community-dwelling older adults. Arch Phys Med Rehabil 2017;98:1955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Best JR, Rosano C, Aizenstein HJ, Tian Q, Boudreau RM, Ayonayon HN, et al. Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiol Aging 2017;57:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jor’dan AJ, Manor B, Novak V. Slow gait speed - an indicator of lower cerebral vasoreactivity in type 2 diabetes mellitus. Front Aging Neurosci 2014;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall R, Festa J, Cheung Y, Chen R, Pavol M, Derdeyn C, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology 2012;78:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology 2013;80:2145–50. [DOI] [PubMed] [Google Scholar]
- 42.Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59:242–8. [DOI] [PubMed] [Google Scholar]
- 43.Poredos P, Spirkoska A, Lezaic L, Mijovski MB, Jezovnik MK. Patients with an inflamed atherosclerotic plaque have increased levels of circulating inflammatory markers. J Atheroscler Thromb 2017;24:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brott T, Tomsick T, Feinberg W, Johnson C, Biller J, Broderick J, et al. Baseline silent cerebral infarction in the Asymptomatic Carotid Atherosclerosis Study. Stroke 1994;25:1122–9. [DOI] [PubMed] [Google Scholar]
- 45.Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 2005;36:2373–8. [DOI] [PubMed] [Google Scholar]
- 46.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62A:844–50. [DOI] [PubMed] [Google Scholar]
- 47.Black S, Rush RD. Cognitive and functional decline in adults aged 75 and older. J Am Geriatr Soc 2002;50:1978–86. [DOI] [PubMed] [Google Scholar]
- 48.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–41. [DOI] [PubMed] [Google Scholar]
- 49.Landgraff NC, Whitney SL, Rubinstein EN, Yonas H. Use of the physical performance test to assess preclinical disability in subjects with asymptomatic carotid artery disease. Phys Ther 2006;86:541–8. [PubMed] [Google Scholar]
- 50.Landgraff NC, Whitney SL, Rubinstein EN, Yonas H. Cognitive and physical performance in patients with asymptomatic carotid artery disease. J Neurol 2010;257:982–91. [DOI] [PubMed] [Google Scholar]
- 51.Cohen DJ, Stolker JM, Wang K, Magnuson EA, Clark WM, Demaerschalk BM, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy versus Stenting Trial). J Am Coll Cardiol 2011;58:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggenberger P, Wolf M, Schumann M, de Bruin ED. Exer-game and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci 2016;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jehu D, Paquet N, Lajoie Y. Gait & Posture Balance and mobility training with or without concurrent cognitive training does not improve posture, but improves reaction time in healthy older adults. Gait Posture 2017;52:227–32. [DOI] [PubMed] [Google Scholar]