Abstract
Rationale:
Some mood disorders, such as major depressive disorder, are more prevalent in women than in men. However, historically preclinical studies in rodents have a lower inclusion rate of females than males, possibly due to the fact that behavior can be affected by the estrous cycle. Several studies have demonstrated that chronic antidepressant treatment can decrease anxiety-associated behaviors and increase adult hippocampal neurogenesis in male rodents.
Objective:
Very few studies have looked at the effects of antidepressants on behavior and neurogenesis across the estrous cycle in naturally cycling female rodents.
Methods:
Here we analyze the effects of chronic treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac) on behavior and adult hippocampal neurogenesis in naturally cycling C57BL/6J females across all four phases of the estrous cycle.
Results:
In naturally cycling C57BL/6J females, fluoxetine decreases negative valence behaviors associated with anxiety in the elevated plus maze and novelty suppressed feeding task, reduces immobility time in forced swim test, and increases adult hippocampal neurogenesis. Interestingly, the effects of fluoxetine on several negative valence behavior and adult hippocampal neurogenesis measures were mainly found within the estrus and diestrus phases of the estrous cycle.
Conclusions:
Taken together these data are the first to illustrate the effects of fluoxetine on behavior and adult hippocampal neurogenesis across all four phases of the murine estrous cycle.
Keywords: Estrous Cycle, Adult Neurogenesis, Anxiety, Depression, Antidepressants, Females
Introduction
Although several mood disorders, including major depressive disorder, are more prevalent in women than men (Kessler 2003; Sloan and Kornstein 2003), historically females are excluded from rodent preclinical studies because fluctuations in ovarian steroid hormones (Arakawa et al. 2014; Lovick 2012) across the estrous cycle may confound experimental results. Many preclinical studies have used rodents to demonstrate behavioral effects of treatment with various antidepressants, including selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine (FLX), on negative valence behaviors associated with anxiety such as elevated plus maze and novelty suppressed feeding and on forced swim test (Bodnoff et al. 1988; Cryan et al. 2002; David et al. 2009; Dulawa et al. 2004; Lucki et al. 2001; Samuels et al. 2015; Santarelli et al. 2003). However, nearly all of these studies only studied the effects of antidepressants in male rodents. Similarly, although treatment with antidepressants such as FLX was shown nearly two decades ago to increase adult hippocampal neurogenesis in male rodents (Malberg et al. 2000), and follow-up work suggested this effect on neurogenesis is required for the behavioral effects of antidepressants (Santarelli et al. 2003), surprisingly little is known about how FLX affects adult hippocampal neurogenesis in naturally cycling females.
In humans, women can experience depression and anxiety due to premenstrual syndrome, where variations in mood states are correlated with different secretion patterns of estrogen and progesterone across the menstrual cycle (Shors and Leuner 2003). Rodents display similar fluctuations in behavior, with diestrus female rodents showing increased stress responses, immobility time in the forced swim test (FST), and anxiety-associated behaviors within the elevated plus maze (EPM) relative to proestrus females (D’Souza and Sadananda 2017; Lovick 2012; Marcondes et al. 2001; Sayin et al. 2014). Administration of estradiol to ovariectomized (OVX) rats reduces immobility time in the forced swim test (Bekku and Yoshimura 2005), an effect that is similar to antidepressant treatment (Green and Galea 2008; Mahmoud et al. 2016). Finally, in comparing two different mouse strains, Meziane and colleagues observed that unlike BALB/cByJ females, C57BL/6J female mice have less behavioral variation across the estrous cycle in the open field (OF), tail flick, and tail suspension tests (Meziane et al. 2007).
In addition to effects on behavior, variations in gonadal steroid hormone secretion patterns, as seen in cycling females, contribute to alterations in adult hippocampal neurogenesis (Barha et al. 2009; Tanapat et al. 2005) within the subgranular zone of the dentate gyrus (DG) (Tanapat et al. 1999). For instance, proestrus rats have higher DG cell proliferation than estrus or diestrus rats possibly due to higher estradiol levels during the proestrus phase (Pawluski et al. 2009; Sadrollahi et al. 2014; Tanapat et al. 1999). Whether the estrous cycle impacts other phases of adult hippocampal neurogenesis, such as differentiation into and maturation of young neurons (Plumpe et al. 2006) remains unknown. Furthermore, while many studies document that estrogen administration to ovariectomized (OVX) rats affects DG cell proliferation, these effects may be species specific since Lagace and colleagues observed no significant differences in adult hippocampal cell proliferation in ovariectomized C57BL/6J mice (Lagace et al. 2007). While Sayin and colleagues illustrate that citalopram, a selective serotonin reuptake inhibitor (SSRI), alleviates differences in anxiety-associated behaviors between proestrus and non-proestrus rats (Sayin et al. 2014), no studies have examined the impact of SSRIs on behavior and neurogenesis in intact, cycling females across all four phases of estrous.
Therefore, given the gaps in knowledge in how FLX affects behavior and adult hippocampal neurogenesis in naturally cycling female rodents, coupled with the fact that the estrous cycle has clear effects, this study aims to resolve the behavioral and adult neurogenesis effects of FLX across all four phases of the estrous cycle.
Methods
Subjects
Adult 8-week-old female C57BL/6J strain mice were purchased from Jackson laboratories. All mice were grouped housed (up to 5 per cage) in standard cages with corncob bedding, maintained on a 12L:12D (6am:6pm) schedule with food (Purina 5002 LabDiet) and autoclaved water provided ad libitum. For the duration of the project FLX (n= 25; 18 mg/kg/day; BioTrend, BG0197) or vehicle (n = 20; deionized water) was administered within the first 3 hours of the light phase via oral gavage with volume administered contingent on bodyweight. Specifically, mice were individually weighed with oral gavage volume calculated via ((18mg/kg (dose of FLX)*mouse weight kg)/concentration mg/ml). The final volume delivered by gavage each day ranged from 0.15ml to 0.23 ml. After 3 weeks of FLX treatment behavioral testing began and was run during the morning hours (8:00–12:00), with two days separating each behavioral test. On behavioral testing days FLX or vehicle was administered after mice completed the behavioral test to avoid acute effects. All testing was conducted in compliance with the NIH laboratory animal care guidelines and approved by Rutgers University Institutional Animal Care and Use Committee.
Vaginal Lavage
To examine estrous cycle state vaginal lavages were performed throughout FLX/vehicle treatment, after completing each behavioral test, and prior euthanasia. In order to collect the samples, a pipet was filled with ddH2O, placed at the opening of the mouse’s vaginal canal (without penetration) with ddH2O gently expelled and suctioned back (Byers et al. 2012; McLean et al. 2012). Samples were then placed on a slide warmer to dry for approximately 5 minutes and imaged under a EVOS FL Auto 2.0 microscope (Thermofisher Scientific) at 10× magnification. Estrous phase was identified by the presence or absence of nucleated epithelial cells, cornified epithelial cells, and leukocytes (Byers et al. 2012; Felicio et al. 1984). Mice in proestrus displayed mostly nucleated and some cornified cells (Figure 1B). Estrus was recorded as the presence of mostly cornified epithelial cells, with the presence of a few nucleated cells in early estrus (Figure 1B). Metestrus was determined by the presence of cornified epithelial cells and polymorphonuclear leukocytes (Byers et al. 2012), while mice in diestrus contained mainly polymorphonuclear leukocytes with few epithelial cells being present (Figure 1B).
Figure 1. Behavioral differences between FLX- and vehicle-treated female mice are also mediated by estrous phase.
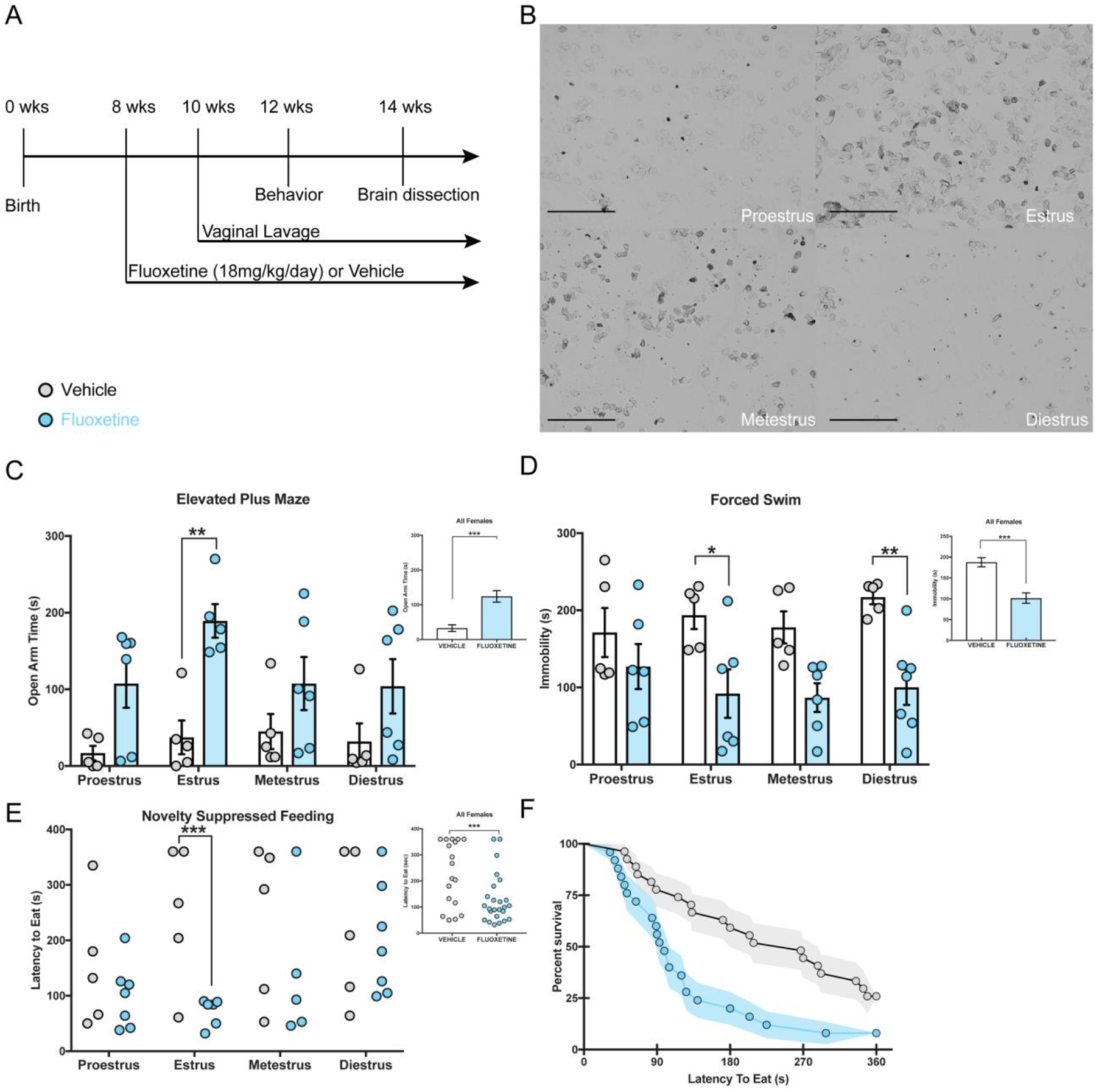
A timeline depicting the experimental study is depicted (A) with representative images of the four phases of the estrous cycle (10× magnification; scale = 500 μm; B). (C) Separate 2×4 ANOVAs were run and revealed that overall females treated with FLX (smaller inset panel) spend less time in the open arms than vehicle mice (F(1,35)=22.8, p<0.001, η2 = 0.373), with this group difference being evident within the estrus phase of the estrous cycle (p = 0.003, Bonferroni corrected). (D) In the FST, FLX appeared to reduce overall immobility time (treatment F(1,35) = 25.77, p < 0.001, η2 = 0.356), with FLX-treated females in the estrus (p = 0.025) and diestrus (p = 0.006) phases having significantly lower immobility scores than vehicle-treated females. (E) Small scatter plot represents Kaplan Meier survival analysis (F) with FLX-treated females have shorter latencies to eat in the NSF (x2(1)=8.95, p=0.0028) task than vehicle-treated females. Larger graph (E) represents Kaplan Meier survival analysis within group differences being evident within the estrus phase (*** x2(1)=10.5, p = 0.008).
Behavioral Testing
Open Field (OF)
Motor activity was quantified in five Plexiglass open field boxes 43 × 43 cm (Kinder Scientific). The recording of x-y ambulatory movements was recorded by two sets of 16 pulse-modulated infrared photobeams placed on opposite walls 2.5 cm apart to. As previously described, activity chambers were computer interfaced for data sampling at 100ms resolution (David et al. 2009). The computer software predefines grid lines that divide each open field chamber into center and periphery regions, with the center being a square 11cm from the wall. The number of entries, distance traveled, and total time spent in the center were recorded, as well as percent of distance traveled in the center defined as center distance divided by total distance traveled (Supplemental Figure 1A). To measure overall motor activity total distance (cm) was quantified.
Light Dark (LD)
The light/dark test was conducted in an open field chamber measuring 43 × 43 cm (Kinder Scientific, USA), with a clear floor and walls. To divide the open field into separate light and dark compartments, a dark plastic box that covered one third of the chamber was inserted. The dark box was opaque to visible light, but transparent to infrared light, and contained an opening that allowed passage between the light and dark compartments (David et al. 2009). The light compartment was brightly illuminated at 1000 lux. At the beginning of each 5-minute test, mice were placed in the dark compartment. An observer blind to treatment groups recorded the latency to emerge into the light (Supplemental Figure 1C). Using Activity Monitor (Kinder Scientific, USA) software, total time in the light and ambulatory distance in both compartments was analyzed. To calculate percent distance traveled in the light, distance traveled in the light was divided by total distance traveled (Supplemental Figure 1B).
Elevated Plus Maze (EPM)
The EPM test consisted of a plus-shaped apparatus with two open and two closed arms (side walls), elevated 2 feet from the floor. During the five-minute test, the mouse’s behavior was recorded from a video camera mounted above each EPM arena. EthoVision (Noldus) software was then used to score time spent in the open arms, entries, and distance traveled in both open and closed arms. By dividing total open arm distance traveled by total distance traveled, we were able to analyze percent distance traveled in open arms.
Novelty Suppressed Feeding (NSF)
After undergoing 18 hours of food deprivation within their home cage, mice were placed in the corner of a testing apparatus (50×50×20 cm) filled with approximately 2 cm of corncob bedding and a single pellet of food attached via a rubberband to a white platform in the center of the box (Samuels and Hen 2011). The center of the box was illuminated at 1500 lux. The NSF test lasted 6 minutes, where the latency to eat (defined as the mouse sitting on its haunches and biting the pellet with the use of forepaws) was timed/recorded. If a mouse did not consume food during the NSF a latency of 360secs was recorded. Immediately afterwards, the mice were transferred to their home cage to assess home cage feeding behavior for 5 minutes (Supplemental Figure 1D–F). During this task latency to eat and amount of food consumed was measured as a control for feeding behavior observed in the NSF task. Each mouse was weighed before food deprivation and after home cage feeding to assess the percentage of body weight loss. Following home cage feeding, mice were placed in a new home cage with cage mates and returned to the colony room.
Forced Swim Test (FST)
A modified FST procedure suitable for mice was used (David et al. 2009). Individual cylinders (46 cm tall × 32 cm in diameter × 30 cm deep) were filled with room-temperature water (25–26°C) before placing each mouse into the cylinder. Two sets of photobeams were mounted on opposite sides of the cylinder (Kinder Scientific, USA) to allow for the recording of swimming behavior during the 6-minute test. Immobility times (measured by beam breaks over 5-second intervals) were assessed during the last 4-minutes of the test since mice are habituating to the task during the initial 2-minutes of the test.
Brain Collection, Sectioning and Immunohistochemistry
Brain Collection and Sectioning
48 hours after completion of behavioral testing and 24 hours after the last FLX dosage, brains were collected from all experimental mice. To ensure mice were euthanized in each estrous cycle phase, vaginal lavages were collected prior to anesthesia to ensure that enough mice were sacrificed during different phases. Mice were anesthetized with ketamine (80mg/kg) and perfused transcardially with PBS followed by 4% paraformaldehyde. Brains were collected and stored in 4% paraformaldehyde overnight at 4°C. Next, brains were switched to a 30% sucrose 0.1% sodium azide (NaN3) in PBS solution and stored at 4°C until they were sectioned. Using a cryostat, every sixth section (40μm) of the hippocampus (Bregma −1.22 to −3.88) was collected onto Superfrost Plus slides (Thermofisher Scientific) and stored at −20°C until staining and further analysis.
Ki67 labeling
The effects of FLX treatment on cell proliferation were assessed across a total of 12 sections (every sixth section) of the hippocampus. Slides were washed in 1% Triton X-100 PBS for 5 minutes before undergoing three PBS washes. Next, slides were incubated in warm citrate buffer for 30 minutes and then washed three times in PBS. Slides were then transferred to an opaque moisture chamber (TedPella) for the blocking and overnight incubation step. Slides were blocked for 1 hour in 10% normal goat serum (NGS) diluted in PBS before being incubated overnight at 4°C in anti-rabbit Ki67 (1:250; polyclonal abCam, ab15580) diluted in 2% NGS PBS. Following 18 hours of incubation slides were washed 3 times in PBS before being incubated at room temperature for 2 hours in CY-5 goat anti-rabbit (1:1000, Invitrogen, Thermo Fisher Scientific, A10523) diluted in 2% NGS PBS. Next slides were washed with PBS then counterstained with DAPI (1:15000) for 15 minutes. Finally, slides were washed with PBS and cover slipped using the mounting medium Prolong Diamond (Thermofisher Scientific). Fluorescent images were taken using a EVOS FL Auto 2.0 microscope (Thermofisher Scientific) at 10× or 40× magnification, where Ki67+ cells overlayed with DAPI (Thermofisher Scientific) across the 12 sections of hippocampus were collected and counted via an observer blind to treatment (Supplemental Figure 2A–E).
Doublecortin (DCX) labeling
12 hippocampal sections (every sixth section) for doublecortin (DCX) were stained using the primary antibody doublecortin anti-goat (1:500; Life technologies; 481200) and secondary antibody CY-5 goat anti-rabbit (1:1000, Invitrogen, Thermo Fisher Scientific, A10523). Fluorescent images were taken using using a EVOS FL Auto 2.0 microscope (Thermofisher Scientific) at 10× or 40× magnification, where DCX+ cells across the 12 sections of hippocampus were collected and counted by an observer blind to treatment (Supplemental Figure 2F–O). Following imaging, DCX+ cells were counted and subcategorized according to their dendritic morphology: DCX+ cells with no tertiary dendritic processes and DCX+ cells with complex, tertiary dendrites. The maturation index was defined as the ratio of DCX+ cells possessing tertiary dendrites over the total DCX+ cells.
Statistical Analyses
To analyze both behavioral and molecular differences between treatment groups and estrous cycle phases separate 2×4 analyses of variance (ANOVA) were conducted. Each ANOVA was followed by subsequent Bonferroni post-hoc analyses to further assess between and within group differences across the estrous cycle. Since we imposed a cutoff time during the NSF, we ran a Kaplan Meier survival analysis (nonparametric test) that permits censoring of these data points to analyze differences in feeding latencies (Samuels and Hen 2011). GraphPad Prism 7 was used for all statistical analyses.
Results
FLX and vehicle behavioral differences across estrous cycle
We treated adult C57BL/6J female mice (n= 45) with 18mg/kg FLX for three weeks (Figure 1A) and then exposed these mice to the Open Field (OF), Light Dark Test (LD), Elevated Plus Maze (EPM), Novelty Suppressed Feeding (NSF), and Forced Swim Test (FST). To assess estrous cycle phase (Figure 1B), vaginal lavages were performed daily beginning two weeks prior to behavior until the end of the experiment. On behavioral days lavages were performed upon completion of testing. We found significant treatment effects in the EPM and FST, such that FLX mice spent more time in the open arms (Two-Way ANOVA cycle × treatment F(1,35)=22.8, p<0.001, η2 = 0.373; Figure 1C) and less time immobile (Two-Way ANOVA cycle × treatment F(1,35) = 25.77, p < 0.001, η2 = 0.356; Figure 1D) than vehicle mice. In the EPM, planned Bonferroni post-hoc comparisons revealed treatment differences in open arm time within the estrus phase, such that estrus FLX-treated mice spent more time in the open arms than estrus vehicle-treated mice, p = 0.003 (Figure 1C). Additionally, planned Bonferroni post-hoc comparisons revealed immobility time in the FST was significantly different between treatment groups within the estrus and diestrus phases, such that vehicle mice spent significantly more time immobile than FLX mice within both the estrus (Bonferroni post-hoc comparison, p = 0.025) and diestrus (Bonferroni post-hoc comparison, p = 0.006) phases (Figure 1D). We found no significant effects of treatment or estrous phase nor interaction effect on behaviors within the OF and LD tests (Supplemental Figure 1A–C).
In the NSF, a Kaplan Meier survival analysis log rank test revealed that FLX significantly reduces latency to feed, p = 0.0038 (Figure 1E, 1F), as compared to vehicle mice. Additionally, we observed an effect of FLX treatment in the estrus phase of the estrous cycle, with FLX females in estrus having lower latencies to feed than estrus vehicle females, p = 0.008. There was no treatment effect nor estrous cycle effect on home cage latency to eat, amount of food consumed in home cage, or percent weight change (Supplemental Figure 1D–F).
Taken together, these data suggest that behavior across the estrous cycle is consistent across estrous phases, and that FLX treatment reduces anxiety-associated behaviors in the estrus phase and decreases FST immobility in both the estrus and diestrus phases.
Fluoxetine treatment and estrous cycle state impact adult neurogenesis
We next performed immunostaining to determine the effects of FLX on the distinct stages of adult hippocampal neurogenesis across the different phases of the estrous cycle. We first stained for the cell proliferation marker Ki67 and found that FLX-treated mice had more Ki67+ cells than vehicle-treated mice (Two-Way ANOVA cycle × treatment, F(1, 32) = 11.79, p = 0.002, η2 = 0.234) (Figure 2A–B, Supplemental Figure 2A–E). We observed no estrous cycle effects nor interaction on number of Ki67+ cells (p > 0.05 for all phases). However, planned Bonferroni post-hoc comparisons showed that FLX-treated mice had more Ki67+ cells than vehicle mice within the estrus (p = 0.007) and there was a trend in diestrus (p = 0.053). Additionally, within the vehicle group we found no differences in the number of Ki67+ cells within the DG subgranular zone (SGZ) across all phases of the estrous cycle (Figure 3A).
Figure 2. FLX increases all stages of neurogenesis with differences most pronounced in the estrus and diestrus phases.
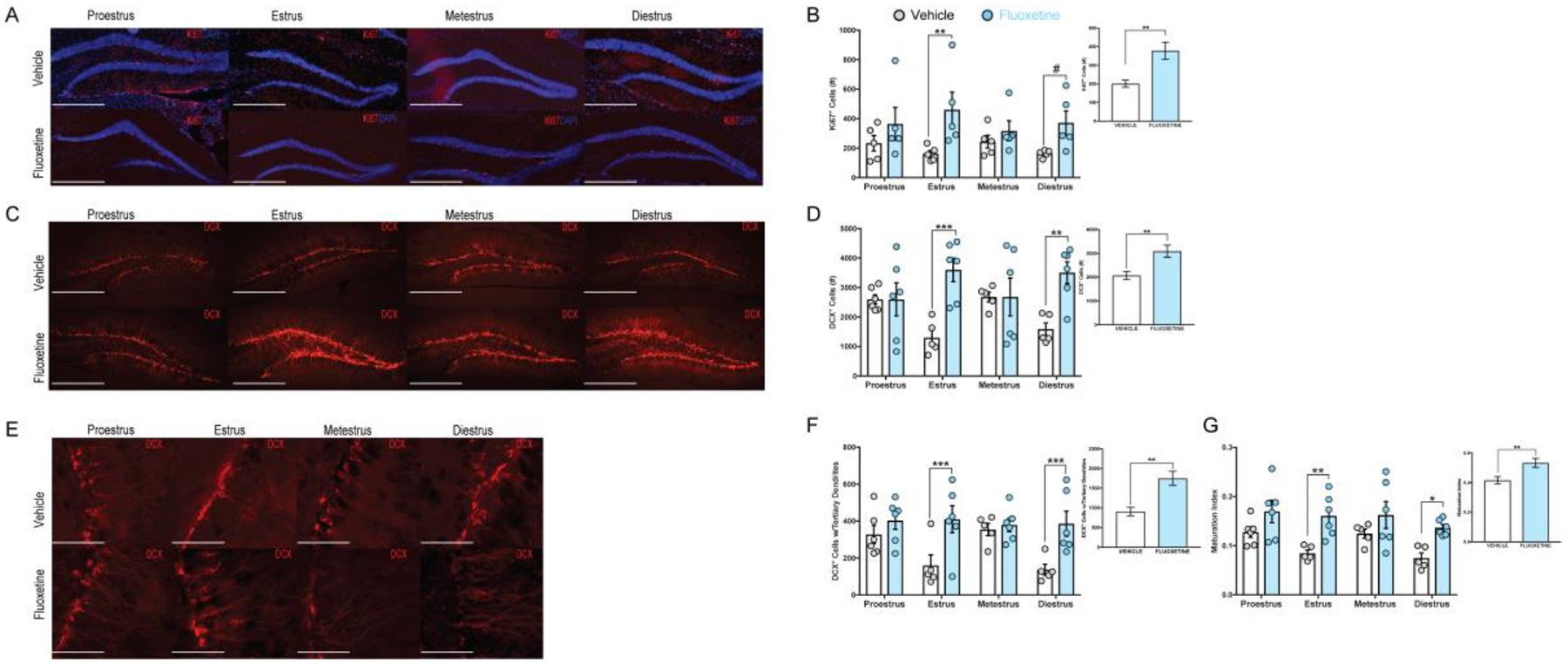
Visual representation of Ki67 and DCX immunostaining is depicted above for each treatment group across the estrous cycle phases. Ki67 (A) and DCX (C) images were taken at 10× magnification (scale = 500 μm) with mature neurons (E) taken at 40× magnification (scale = 125 μm). Smaller inset panels (B, D, F, G) represent 2×4 ANOVAs (cycle × treatment FLX-treated females had higher adult cell proliferation (Ki67+, B, ** F(1, 32) = 11.79, p = 0.002, η2 = 0.234) more immature (D, *** F(1, 37) = 13.44, p < 0.001, η2 = 0.280) and mature (F, ***F(1, 37) = 15.85, p < 0.001, η2 = 0.401) neurons as well as a higher maturation index (G, ***F(1, 37) = 21.71, p < 0.001) compared to vehicle animals. Larger panels represent the estrous effect from 2×4 ANOVAs (cycle × treatment) with FLX-treated females having more cell proliferation (B **estrus p = 0.002 and #diestrus p = 0.053), more immature neurons (D F(3, 37) = 4.57, p = 0.008, η2 = 0.275, estrus ***p < 0.001, diestrus ** p=0.009) and mature (F, F(3, 37) = 3.26, p = 0.032, η2 = 0.126; **estrus p = 0.007 and *diestrus p = 0.012) neurons as well as a higher maturation index (G, **estrus p = 0.002 and *diestrus p = 0.013) compared to vehicle-treated females.
Figure 3. Estrous cycle impacts adult hippocampal neurogenesis in intact, cycling vehicle-treated female mice.
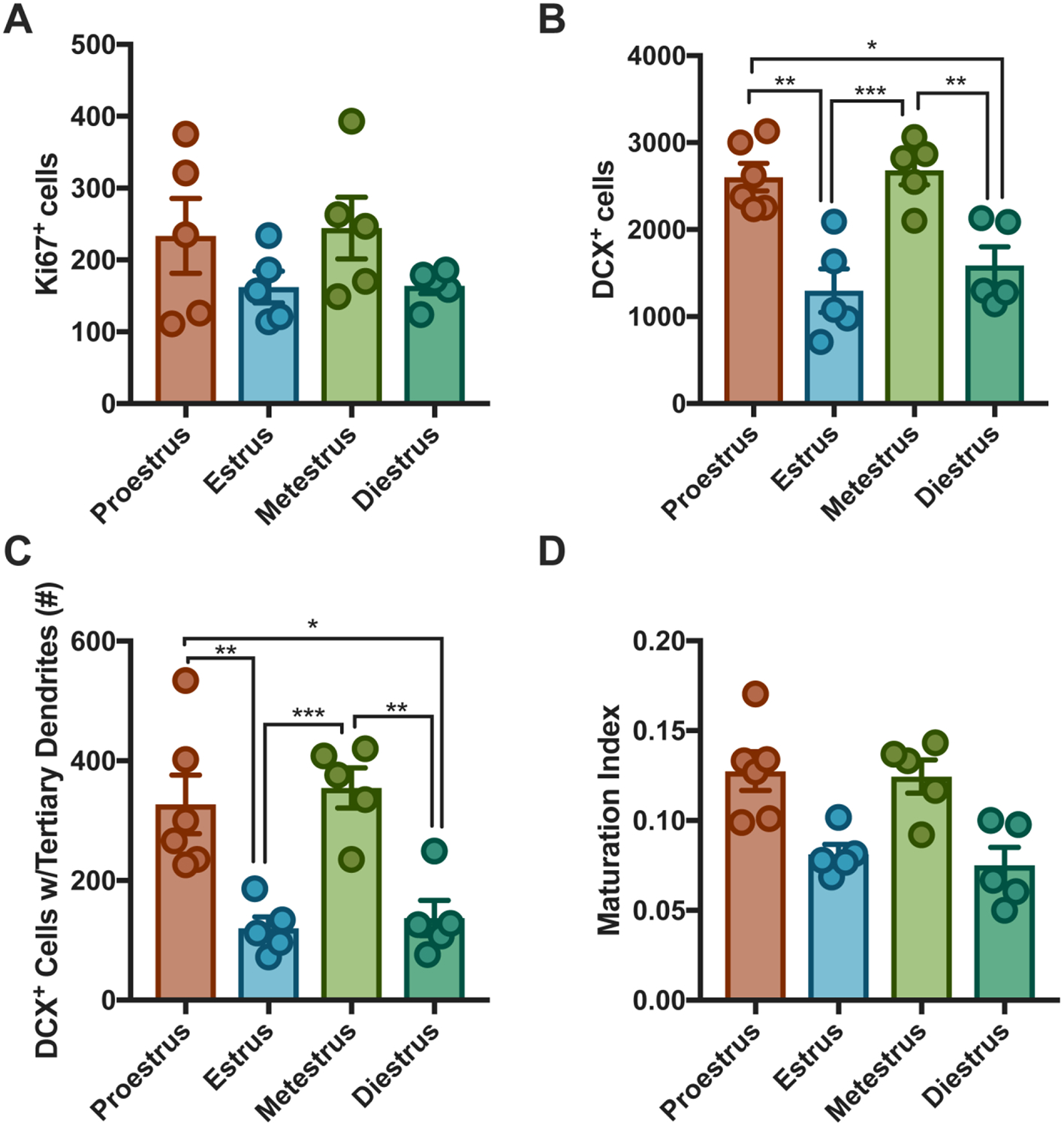
Planned post-hoc analyses within the vehicle group was conducted to assess impact of estrous cycle on adult hippocampal neurogenesis. No differences in adult hippocampal cell proliferation was noted across the estrous cycle in vehicle females (A). Proestrus females had higher expression of immature (B **estrus p=0.001, *diestrus p=0.01) and mature (C, **estrus p=0.007; *diestrus p=0.012) neurons than estrus and diestrus females. Metestrus females had more immature (***estrus p < 0.001; **diestrus p = 0.008) and (C) mature (***estrus p=0.004; **diestrus p=0.006) neurons estrus and diestrus. There was no impact of estrous cycle on maturation index (D).
We next performed immunostaining with the young neuron marker DCX, and observed that FLX-treated mice had more DCX+ cells within the DG than vehicle-treated mice (Two-Way ANOVA cycle × treatment, F(1, 37) = 13.44, p < 0.001, η2 = 0.280) (Figure 2C–D, Supplemental Figure 2F–J). We found a significant interaction effect between treatment and estrous phase (Two-Way ANOVA cycle × treatment, F(3, 37) = 4.57, p = 0.008, η2 = 0.275). Bonferroni post-hoc comparisons revealed that FLX-treated mice had more DCX+ cells than vehicle mice within the estrus (p < 0.001) and diestrus (p = 0.009; Figure 2D) phases. Within the vehicle group, Bonferroni post-hoc comparisons revealed that proestrus females had significantly more DCX+ cells than both estrus (p = 0.001) and diestrus (p = 0.01) female mice (Figure 3B). Additionally, metestrus vehicle-treated mice had significantly more DCX+ cells than both estrus (p < 0.001) and diestrus (p = 0.008) vehicle-treated mice (Figure 3B). Within the FLX group no differences in DCX+ cell expression was observed across the estrous cycle.
To assess maturation of the young neurons, we counted the DCX+ neurons that displayed tertiary dendrites. As expected, FLX-treated mice had more mature neurons than vehicle mice as indicated by the number of DCX+ cells with tertiary dendrites (Two-Way ANOVA cycle × treatment, F(1, 37) = 15.85, p < 0.001, η2 = 0.401; Figure 2E–F, Supplemental Figure 2K–O). Planned Bonferroni post-hoc comparisons revealed that FLX-treated mice had more DCX+ cells with tertiary dendrites than vehicle mice within the estrus (p < 0.001) and diestrus (p = 0.001; Figure 2F) phases. We also observed a significant interaction effect between treatment group and estrous cycle phase (Two-Way ANOVA cycle × treatment, F(3, 37) = 3.26, p = 0.032, η2 = 0.126). Within the vehicle group, planned post-hoc comparisons revealed that proestrus females have more DCX+ cells with tertiary dendrites than estrus (p = 0.007) and diestrus (p = 0.012) females (Figure 3C). Planned comparisons also revealed that metestrus vehicle-treated females had more mature neurons than estrus (p = 0.004) and diestrus (p = 0.006) vehicle-treated females (Figure 3C). Within the FLX group, we observed no differences in expression of DCX+ cell with tertiary dendrites across the estrous cycle.
Lastly, we observed that FLX mice have a higher maturation index (Two-Way ANOVA cycle × treatment, F(1, 37) = 21.71, p < 0.001; Figure 2G) than vehicle mice, with FLX females having a significantly higher maturation index than vehicle females in both the estrus (Bonferroni post-hoc comparison, p = 0.003) and diestrus (p = 0.013) phases (Figure 2G). Within both the vehicle (Figure 3D) and FLX group we observed no differences in the maturation index across the estrous cycle. Taken together, these data suggest that the effects of fluoxetine on adult hippocampal neurogenesis are most pronounced during both the estrus and diestrus phases.
Discussion
FLX behavioral effects across estrous cycle
We used negative valence behavioral tests to evaluate the impact of antidepressant treatment on behavior across the estrous cycle. Prior to behavioral testing we tracked the females estrous cycle for two weeks to assess whether females were cycling together within the same housing room. Although Meziane and colleagues observed that females within the same room cycle together (Meziane et al. 2007), we found that females in our vivarium had out of sync cycles, which allowed us to investigate the different estrous cycle phases during each behavioral test. Similar to previous studies in males (David et al. 2009), our data illustrate that FLX treatment in females reduces anxiety-associated behaviors within the EPM and NSF, and decreases immobility in the FST, with no effects seen on behavior within the OF and LD. However, our data demonstrate that estrous cycle significantly impacts the effects of FLX on behavior. FLX-treated females in the estrus phase display a reduction in anxiety-associated behavior in the EPM and NSF, and reduced immobility in the FST relative to vehicle-treated estrus females. Furthermore, diestrus FLX-treated females had lower immobility times in the FST than diestrus vehicle females, but FLX did not affect the anxiety-associated EPM and NSF tasks in this phase. The behavioral effects of FLX were not significant in any of these tasks during metestrus and proestrus. Sayin and colleagues observed that proestrus rats display decreased anxiety-associated behaviors relative to non-proestrus rats in the EPM, with estrous cycle differences attenuated following citalopram administration (Sayin et al. 2014). Compared to our data, these findings suggest that species differences may exist in anxiety-associated behaviors across the estrous cycle. Although we did not observe an effect of the estrous cycle on behavior within treatment, a previous study observed that C57BL/6J females have less variation in anxiety-associated behaviors across the estrous cycle than BALB/cByJ females (Meziane et al. 2007). Despite this, our data illustrate that FLX treatment has significant effects on behavior. However, our detailed analyses suggest that the behavioral effects of FLX in the EPM and NSF are mainly driven by changes during the estrus phase, while the effects of FLX in the FST are driven by changes during the estrus and diestrus phases.
Differences in behavior between treatment groups within estrus and diestrus may be related to fluctuations in estradiol and progesterone levels (Lovick 2012; Pawluski et al. 2009). Specifically, estradiol levels are lowest during estrus and diestrus (Pawluski et al. 2009; Wood et al. 2007). Exogenous estradiol treatment to mimic diestrus in OVX rats results in decreases in anxiety-associated behavior in the EPM compared to non-estrogen treated freely cycling diestrus rats (Marcondes et al. 2001). In mice, females in the estrus and diestrus phases are more susceptible to individual housing stress and spend less time in the center of the open field arena than proestrus mice (Palanza et al. 2001). The impact of FLX administration on ovarian steroid hormones across the estrous cycle is understudied in rodents, and future studies will need to assess the relationship between antidepressant and endogenous ovarian hormone levels.
One potential caveat of this study is that behavioral testing is performed during the first few hours of the light phase. Some studies report that testing during the light vs dark phase can influence anxiety-associated behaviors in rodents. For example, wild-type males show more rears and time in the light side of the light/dark test than males with a dysfunctional androgen receptor during the dark phase but not during the light phase (Chen et al. 2014). Therefore, it will be informative for future studies to also assess behavioral testing during the dark phase.
Fluoxetine treatment and estrous cycle state impact adult neurogenesis
Similar to several other studies (Lagace et al. 2007; Pawluski et al. 2014), we observed that chronic FLX administration increased adult hippocampal neurogenesis levels relative to vehicle-treated females. Within the DG, females treated with FLX had increased numbers of proliferating cells and young neurons, and increased maturation of young neurons relative to vehicle-treated females. However, similar to the effects on behavior, our study is the first to illustrate that the effects of FLX on adult hippocampal neurogenesis are most pronounced during the estrus and diestrus phases. This is in part due to the relatively lower levels of neurogenesis in the vehicle-treated mice during these phases, which may be attributable to natural low levels of estradiol in these phases. Estrogens, such as estradiol, impact both cell proliferation and cell survival in the DG (Ormerod et al. 2003) and more proliferating cells are found in the proestrus phase than the non-proestrus phases (Pawluski et al. 2009; Sadrollahi et al. 2014; Tanapat et al. 1999). Discrepancies in proliferating cell numbers across the estrous cycle can be attributed to endogenous estrogen levels naturally peaking during the proestrus phase and decreasing during the estrus and diestrus phase (Butcher et al. 1974; Pawluski et al. 2009; Tanapat et al. 1999). However, Lagace and colleagues show that endogenous levels of estradiol do not appear to impact adult hippocampal cell proliferation in C57BL/6J mice, since OVX female mice have similar number of proliferating cells (BrdU+) and immature neurons (DCX+) in the hippocampus as intact female mice (Lagace et al. 2007). Furthermore, in assessing cell proliferation across three phases of the estrous cycle (proestrus, estrus, and diestrus), Lagace and colleagues observed no differences in cell proliferation in the different phases. Similar to Lagace and colleagues, we show that estrous cycle phase does not significantly impact DG cell proliferation levels within vehicle-treated mice (Lagace et al. 2007).
We found that proestrus and metestrus vehicle-treated female mice have higher numbers of young neurons (DCX+) within the DG than estrus and diestrus vehicle-treated female mice. Additionally, we show that estrus and diestrus vehicle treated female mice have less maturation of young neurons in the DG than proestrus and metestrus vehicle-treated female mice. Interestingly, FLX specifically increased the number of young neurons and maturation of the young neurons in the DG during estrus and diestrus relative to vehicle-treated mice. This finding is surprising, since individual phases of the estrous cycle only last about twenty-four hours and the processes of fate specification and maturation can take several days (Ming and Song 2005). However, chronic FLX accelerates maturation of immature neurons (Wang et al. 2008) and our data indicate that these effects are primarily observed during estrus and diestrus. One possible explanation is that the effects of FLX on DCX expression are in part due to dematuration of mature granule cells within the DG (Kobayashi et al. 2010), which could happen on a fast timescale. A second possible explanation is that DCX as a neurogenesis marker has been widely described in male rodents, where it is only transiently expressed during an intermediate phase in which the cells are young neurons. However, DCX expression in these cells may be more dynamic than previously appreciated in females. Ultimately, our data suggest that the effects of FLX on adult hippocampal neurogenesis are mainly driven by changes in the estrus and diestrus phases of the estrous cycle. Interestingly, estrogen treatment of OVX or castrated rats has antidepressant-like effects (Martinez-Mota et al. 2008). We found that adult hippocampal neurogenesis levels were relatively lower (although not significantly different from other phases) in the estrus phase when estrogen levels are lowest. It is therefore possible that FLX acts as a substitute for estrogen during estrus but that the naturally higher levels of estrogen during proestrus mask or prevent any effects of FLX on adult hippocampal neurogenesis.
Interestingly, the effects of FLX on DCX expression appear to be more pronounced in the ventral dentate gyrus during estrus and diestrus (Supplemental Figure 2). A few lines of evidence suggest that dorsal (posterior in primates) and ventral (anterior in primates) hippocampus play different roles in mediating behavior (Fanselow and Dong 2010). First, dorsal and ventral hippocampus have distinct anatomical connections (Fanselow and Dong 2010). Second, relatively specific manipulations suggest that dorsal performs primarily cognitive functions while ventral hippocampus performs functions related to stress and affective state (Kheirbek et al. 2013). Therefore, more profound effects of FLX on DCX in the ventral DG may permit a larger effect on affect-related behaviors.
Overall our study illustrates that different estrous phases show significant differences in behavior and adult hippocampal neurogenesis. Furthermore, the effects of FLX treatment on behavior and adult hippocampal neurogenesis are mainly driven by changes during the estrus and diestrus phases. Future studies should assess whether antidepressants influence endogenous levels of ovarian hormones, such as estrogen and progesterone, since fluctuations in these hormones across the estrous cycle would also affect behavior and adult hippocampal neurogenesis. Given that sex differences in the etiologies of mood disorders and symptomologies exist, preclinical studies that determine differences across the estrous cycle are critical for developing a better understanding of how these disorders develop and should be treated in females.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health [Grant Number R01 MH112861 to BAS]. The authors would like to thank Mark M. Gergues, Kylee Shivok, and Debbie Ma for their assistance with this project.
Abbreviations
- DG
Dentate Gyrus
- FLX
Fluoxetine
- OVX
Ovariectomized
- EPM
Elevated Plus Maze
- NSF
Novelty Suppressed Feeding
- FST
Forced Swim Test
- LD
Light Dark
- OF
Open Field
- SSRI
Selective Serotonin Reuptake Inhibitor
- DCX
Doublecortin
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
References
- Arakawa K, Arakawa H, Hueston CM, Deak T (2014) Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology 100: 162–77. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA (2009) Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol 21: 155–66. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H (2005) Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 183: 300–7. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ (1988) The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 95: 298–302. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW (1974) Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94: 1704–8. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA (2012) Mouse estrous cycle identification tool and images. PLoS One 7: e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CV, Brummet JL, Lonstein JS, Jordan CL, Breedlove SM (2014) New knockout model confirms a role for androgen receptors in regulating anxiety-like behaviors and HPA response in mice. Horm Behav 65: 211–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23: 238–45. [DOI] [PubMed] [Google Scholar]
- D’Souza D, Sadananda M (2017) Estrous Cycle Phase-Dependent Changes in Anxiety- and Depression-Like Profiles in the Late Adolescent Wistar-Kyoto Rat. Ann Neurosci 24: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29: 1321–30. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Finch CE (1984) Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod 31: 446–53. [DOI] [PubMed] [Google Scholar]
- Green AD, Galea LA (2008) Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav 54: 203–11. [DOI] [PubMed] [Google Scholar]
- Kessler RC (2003) Epidemiology of women and depression. J Affect Disord 74: 5–13. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R (2013) Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77: 955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H (2010) Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci U S A 107: 8434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ (2007) Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17: 175–80. [DOI] [PubMed] [Google Scholar]
- Lovick TA (2012) Estrous cycle and stress: influence of progesterone on the female brain. Braz J Med Biol Res 45: 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ (2001) Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 155: 315–22. [DOI] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Chaiton JA, Lieblich SE, Galea LAM (2016) Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology 107: 278–293. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC (2001) Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74: 435–40. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Cruz-Martinez JJ, Marquez-Baltazar S, Fernandez-Guasti A (2008) Estrogens participate in the antidepressant-like effect of desipramine and fluoxetine in male rats. Pharmacol Biochem Behav 88: 332–40. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp: e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W (2007) Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6: 192–200. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223–50. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA (2003) Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol 55: 247–60. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, Parmigiani S (2001) Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav 73: 411–20. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA (2009) Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 30: 343–57. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, van Donkelaar E, Abrams Z, Houbart V, Fillet M, Steinbusch HW, Charlier TD (2014) Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats. Neural Plast 2014: 123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G (2006) Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrollahi M, Ghorbanian MT, Zavareh S (2014) Hippocampal neurogenesis in mice at different phases of the estrous cycle. Feyz 18: 336–44. [Google Scholar]
- Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madronal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R (2015) 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci 18: 1606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R (2011) Novelty-suppressed feeding in the mouse In: Gould TD (ed) Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Volume II. Humana Press, New York, NY, pp 107–21 [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–9. [DOI] [PubMed] [Google Scholar]
- Sayin A, Derinoz O, Yuksel N, Sahin S, Bolay H (2014) The effects of the estrus cycle and citalopram on anxiety-like behaviors and c-fos expression in rats. Pharmacol Biochem Behav 124: 180–7. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B (2003) Estrogen-mediated effects on depression and memory formation in females. J Affect Disord 74: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Kornstein SG (2003) Gender differences in depression and response to antidepressant treatment. Psychiatr Clin North Am 26: 581–94. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E (2005) Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol 481: 252–65. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19: 5792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28: 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KL, Khokha R (2007) Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 133: 1035–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


