Abstract
Radiologically isolated syndrome (RIS), in which asymptomatic demyelinating-appearing lesions are detected incidentally on MRI, can be a pre-clinical form of multiple sclerosis (MS). In this study, we measured cerebellar volumes on 3D T1-weighted 3T MR images in 21 individuals with RIS and 38 age and sex-matched healthy controls (HC). Normalized cerebellar white matter volume and the anterior cerebellar grey matter volume were significantly decreased in RIS compared to HC (p = 0.003 and p = 0.005, respectively). Our findings support reports of regional brain atrophy in RIS prior to the development of a seminal attack related to inflammatory demyelination.
Keywords: Radiologically isolated syndrome, multiple sclerosis, cerebellum, brainstem, cerebellar atrophy, white matter lesions
Introduction
Radiologically Isolated Syndrome (RIS), or the incidental identification of lesions consistent with multiple sclerosis (MS) on MRI in otherwise asymptomatic individuals, is likely the earliest detectable manifestation of MS [1]. It is estimated that approximately one third of RIS subjects will develop clinical symptoms related to an acute attack within five years and that a meaningful group may experience a primary progressive phenotype [2]. Even at this pre-symptomatic stage, studies have demonstrated brain volume loss in deep gray matter structures, particularly the thalamus [3]. As the same inflammatory and degenerative processes are at work in the posterior fossa, our objective was to assess whether cerebellar volume loss is also detectable at the RIS stage.
Material and methods
Participants
Twenty-one individuals fulfilling 2009 RIS criteria [1] who were retrospectively identified at the University of California, San Francisco (UCSF) MS Center were included in this analysis. These subjects have been described previously [3]. Thirty-eight age- and sex-matched healthy controls (HC) with imaging covering the cerebellum were selected from an available dataset that was collected at UCSF and has also been described previously [3]. At the time of study enrollment, the protocol was approved by the Committee on Human Research at UCSF, and informed consent was obtained from all participants.
MRI data acquisition and processing
All subjects were scanned on a 3T GE Signa scanner with a standardized imaging protocol which included an inversion recovery spoiled gradient echo 3D T1-weighted isotropic volumetric sequence (3D-IRSPGR, 1 × 1 × 1 mm3 resolution, 180 slices, TE/TE/TI = 2/7/400 msec, flip angle = 15°, 256 × 256 × 180 matrix, 240 × 240 × 180 mm3 field of view, number of excitations = 1).
White matter (WM) lesions in RIS subjects were segmented on the 3D T1-weighted images using a semiautomatic segmentation technique (Jim 7, Xinapse Systems, Northants, UK). Lesion-filled 3D T1-weighted images were segmented into GM, WM and CSF using the “Segment” module implemented in the Statistical Parametric Mapping (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm) [4]. Cerebellar nuclei were excluded from WM segmentation. Cerebellar GM (anterior and posterior), cerebellar WM, and brainstem volumes were calculated on the lesion-filled 3D T1-weighted images using the Spatially Unbiased Infratentorial for enhanced resolution (SUITer) method [5]. Cerebral and cerebellar volumes were normalized for head size using the intracranial volume.
Binary T1 lesion masks were transformed to the Montreal Neurological Institute (MNI) standard space by using FSL FLIRT [6]. Normalized masks were then averaged, which yielded a T1 lesion probability map (LPM), where voxel value from 0 to 1 is the probability for the voxel to be a lesion [7].
Statistical Analysis
A general linear model was used to assess the difference in infratentorial structures normalized volumes between RIS and HC. The threshold for significance was 5% (α=0.05, uncorrected p-values for multiple comparisons). Spearman correlation coefficient was used to assess the correlations between T1 lesions volumes (T1LV) and total GM, total WM, supratentoral and infratentorial normalized volumes. All statistical analyses were carried out using Statistical Package for Social Science (SPSS Inc, v. 19.0, Chicago, III).
Results
Group differences
Demographic and MRI characteristics of RIS and HC subjects are summarized in Table 1. Figure 1 shows an example of segmentation of infratentorial structures in an RIS subject obtained by our customized version of SUIT method. RIS subjects showed a significant decrease in cerebellar WM (95% CI 0.0131–0.0139 vs 0.0141–0.015, p = 0.003) and in the anterior cerebellar GM (95% CI 0.0093–0.0101 vs 0.0101–0.0107, p = 0.005) compared to HC. Trends toward lower but not significant total GM (95% CI 0.4673–0.5065 vs 0.4952–0.5157, p = 0.062), supratentorial GM (95% CI 0.3897–0.4231 vs 0.4127–0.43, p = 0.077) and cerebellar GM (95% CI 0.077–0.0839 vs 0.0818–0.0864, p = 0.071) volumes were found in RIS subjects compared to HC. There were no significant differences between RIS subjects and HC for total WM (95% CI 0.2814–0.2999 vs 0.2862–0.2968, p = 0.850), supratentorial WM (95% CI 0.2494–0.2667 vs 0.2526–0.2621, p = 0.870), posterior cerebellar GM (95% CI 0.0676–0.0739 vs 0.0716–0.0757, p = 0.107) and brainstem (95% CI 0.0184–0.0198 vs 0.0192–0.0202, p = 0.136) volumes.
Table 1.
Demographic and MRI characteristics of radiologically isolated syndrome and healthy control subjects.
| RIS subjects (n=21) | HC (n=38) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age at scan date, y | 41.9 ± 12.7 | 41.3 ± 11.2 | - |
| Age at RIS diagnosis, y | 39.9 ± 12.8 | - | - |
| Gender, F:M | 13:8 | 27:11 | - |
| Normalized volumes | |||
| Total GM | 0.4869 ± 0.00938 | 0.5054 ± 0.00507 | 0.062 |
| [0.4673–0.5065] | [0.4952–0.515] | ||
| Total WM | 0.2906 ± 0.00443 | 0.2915 ± 0.00262 | 0.850 |
| [0.2814–0.2999] | [0.2862–0.2968] | ||
| Supratentorial GM | 0.4064 ± 0.008 | 0.4213 ± 0.00428 | 0.077 |
| [0.3897–0.4231] | [0.4127–0.43] | ||
| Supratentorial WM | 0.258 ± 0.00416 | 0.2573 ± 0.00234 | 0.870 |
| [0.2494–0.2667] | [0.2526–0.2621] | ||
| Cerebellar GM | 0.0805 ± 0.0076 | 0.0841 ± 0.0071 | 0.071 |
| [0.077–0.0839] | 0.0818–0.0864 | ||
| Anterior cerebellar GM | 0.0097 ± 0.0009 | 0.0104 ± 0.0009 | 0.005* |
| [0.0093–0.0101] | [0.0101–0.0107] | ||
| Posterior cerebellar GM | 0.0708 ± 0.0069 | 0.0737 ± 0.0063 | 0.107 |
| [0.0676–0.0739] | [0.0716–0.0757] | ||
| Cerebellar WM | 0.0135 ± 0.0009 | 0.0145 ± 0.0014 | 0.003* |
| [0.0131–0.0139] | [0.0141–0.015] | ||
| Brainstem | 0.0191 ± 0.0016 | 0.0197 ± 0.0014 | 0.136 |
| [0.0184–0.0198] | [0.0192–0.0202] | ||
| T1LV, ml | 3.8259 ± 2.8477 | - | - |
| [2.52962–5.12212] |
Statistically significant.
Values are expressed as mean ± SD [95% IC min-max], unless otherwise indicated. F, female; GM, grey matter; HC, healthy controls; M, male; ml, milliliter; RIS, radiologically isolated syndrome; T1LV, T1 lesions volume; WM, white matter; y, year.
Figure 1.
Example of anterior (blue) and posterior (green) cerebellum, cerebellar WM (yellow) and brainstem (red) segmentations in an RIS subject.
Correlation with T1 lesions volumes
Significant correlations were detected between T1LV and total GM (r = −0.529, p = 0.014), supratentorial GM (r = −0.556, p = 0.009) normalized volumes. No correlations were detected between T1LV and total WM (r = −0.181, p = 0.434), supratentorial WM (r = −0.129, p = 0.579), cerebellar GM (r = −0.294, p = 0.197), anterior cerebellar GM (r = −0.279, p = 0.220), posterior cerebellar GM (r = −0.275, p = 0.227), cerebellar WM (r = −0.330, p = 0.144) and brainstem (r = - 0.303, p = 0.182) normalized volumes.
Lesion Probability Map
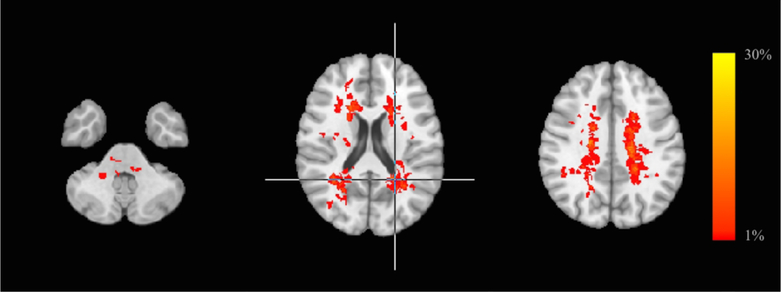
Figure 2 shows LPM in RIS subjects. Lesions were mainly located in the periventricular WM and the bilateral corona radiata. The peak of WM lesion frequency was in the left periventricular WM (28 %). Only 7 RIS subjects had lesions in the brainstem and/or cerebellar WM (pons n = 3; pons and medulla oblongata n = 1; pons and middle cerebellar peduncle n = 1; middle cerebellar peduncle n = 2). T1 lesion volume was 3.8259 ± 2.8477 ml (95% CI 2.52962–5.12212 ml) in RIS subjects.
Figure 2.
White matter lesion probability map in RIS subjects, overlaid on the MNI standard brain template, showing the probability of each voxel containing a lesion. The peak of WM lesion frequency is identified by a crosshair. The color scale denotes the probability range. RIS, radiologically isolated syndrome; MNI, Montreal Neurological Institute.
Discussion
Our results demonstrate that cerebellar volume loss is detectable in RIS individuals in grey and white matter. Previous studies of CIS patients have demonstrated cerebellar atrophy is present even at this early stage of disease [8,9]. We have previously demonstrated that anterior cerebellar volume is associated with sensorimotor deficits and posterior cerebellar atrophy linked to cognitive impairment [4] and it is possible that our current findings are in line with these results, but we are limited by the single time point included in this study.
The etiology of these changes is likely multifactorial. The cerebellum has complex interconnections within incoming and outgoing tracts throughout the brain and spinal cord, and is subject to injury via focal demyelinating lesions within the cerebellum, as well as subsequent neurodegeneration [10]. In our study, most lesions were located in the periventricular white matter, suggesting this might be a prevailing etiology of volume loss in this cohort (Figure 2). Meningeal inflammatory activity in the posterior fossa likely also affects volume loss, particularly in RRMS [11]. Finally, microstructural changes in the cerebellar white matter in early MS have been demonstrated in the absence of lesions and may later link to volume loss [12].
There are limitations to this study. A relatively small sample size was used and the study was underpowered to draw conclusions on cerebellar volume loss and an eventual diagnosis or specific clinical subtype of MS. A larger cohort would help clarify the significance of this pattern of atrophy as well as the cause of preferential atrophy of the anterior cerebellum. Similar to findings involving the thalamus in RIS [3], our new data also support volume loss in the cerebellum in this pre-symptomatic population.
Funding Statement
This research was supported by the NIH (National Institute of Neurological Disorders and Stroke R01NS062885 to D.P.), the National Multiple Sclerosis Society (Sylvia Lawry Physician Fellowship Award to I.C.G.), and the Race to Erase MS Foundation (14–003399 to C.J.A.).
Footnotes
Disclosures
The authors have no conflicts of interest.
References
- 1.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800–5. [DOI] [PubMed] [Google Scholar]
- 2.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS ONE. 2014;9(3):e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocozza S, Petracca M, Mormina E, et al. Cerebellar lobule atrophy and disability in progressive MS. J Neurol Neurosurg Psychiatry 2017;88:1065–1072. [DOI] [PubMed] [Google Scholar]
- 5.El Mendili MM, Petracca M, Podranski K, Fleysher L, Cocozza S, Inglese M. SUITer: an automated method for improving segmentation of infratentorial structures at ultra-high field MRI. J Neuroimaging. In press. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002. October;17(2):825–41. [DOI] [PubMed] [Google Scholar]
- 7.Rossi F, Giorgio A, Battaglini M, Stromillo ML, Portaccio E, Goretti B, Federico A, Hakiki B, Amato MP, De Stefano N. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS One. 2012;7(11):e44826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese M, Mattisi I, Rinaldi F, et al. Magnetic resonance evidence of cerebellar cortical pathology in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81(4):401–4. [DOI] [PubMed] [Google Scholar]
- 9.Rocca MA, Preziosa P, Mesaros S, et al. Clinically Isolated Syndrome Suggestive of Multiple Sclerosis: Dynamic Patterns of Gray and White Matter Changes-A 2-year MR Imaging Study. Radiology. 2016;278(3):841–53. [DOI] [PubMed] [Google Scholar]
- 10.Inglese M, Petracca M, Mormina E, et al. Cerebellar volume as imaging outcome in progressive multiple sclerosis. PLoS ONE. 2017;12(4):e0176519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain. 2018;141(6):1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deppe M, Tabelow K, Krämer J, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler. 2016;22(1):73–84. [DOI] [PubMed] [Google Scholar]