Digestive manifestations appear to be common in patients with coronavirus disease 2019 (COVID-19).1 Gastroenterologists will invariably come into contact with infected patients, and the risk of intraprocedural exposure is well established.2 Recommendations have been issued to guide personal protective equipment (PPE) use3 and the triage of procedural urgency,4 among other operational considerations. In response, institutions providing gastroenterology and endoscopy services have taken urgent action to protect patients and staff, but the uptake and extent of these practice changes in North America is unknown. We conducted a survey of gastroenterology and endoscopy practices to assess the response to the COVID-19 pandemic across the continent.
Methods
A Web-based survey was disseminated to gastroenterologists across North America. Distribution was achieved via en masse e-mail, social media promotion, and direct contact by 4 authors (NF, ZLS, SBW, and BJE). The survey consisted of 42 items, stratified into 6 categories: institutional demographics, changes in endoscopy practice, changes in clinical practice, changes in training, periprocedure screening for COVID-19, and changes in PPE practices. Detailed methodology is provided in the Supplementary Material.
Results
Overview
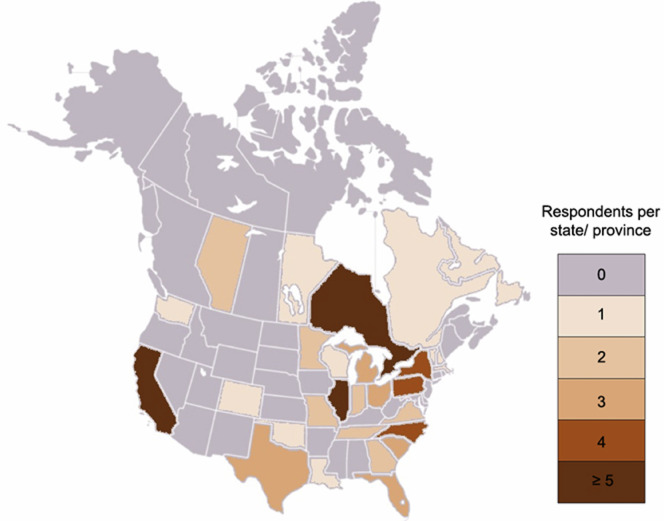
Seventy-three individuals responded on behalf of their institutions, representing 62 US centers across 24 states and the District of Columbia and 11 Canadian centers across 5 provinces (Supplementary Material and Supplementary Figure 1). Most responding centers (63/73) were teaching institutions. Responses were submitted and/or updated from March 21 to April 17, 2020. Aggregate survey responses are presented in the Supplementary Material (Supplementary Figure 2).
Supplementary Figure 1.
Choropleth map demonstrating numbers of survey respondents by state and province.
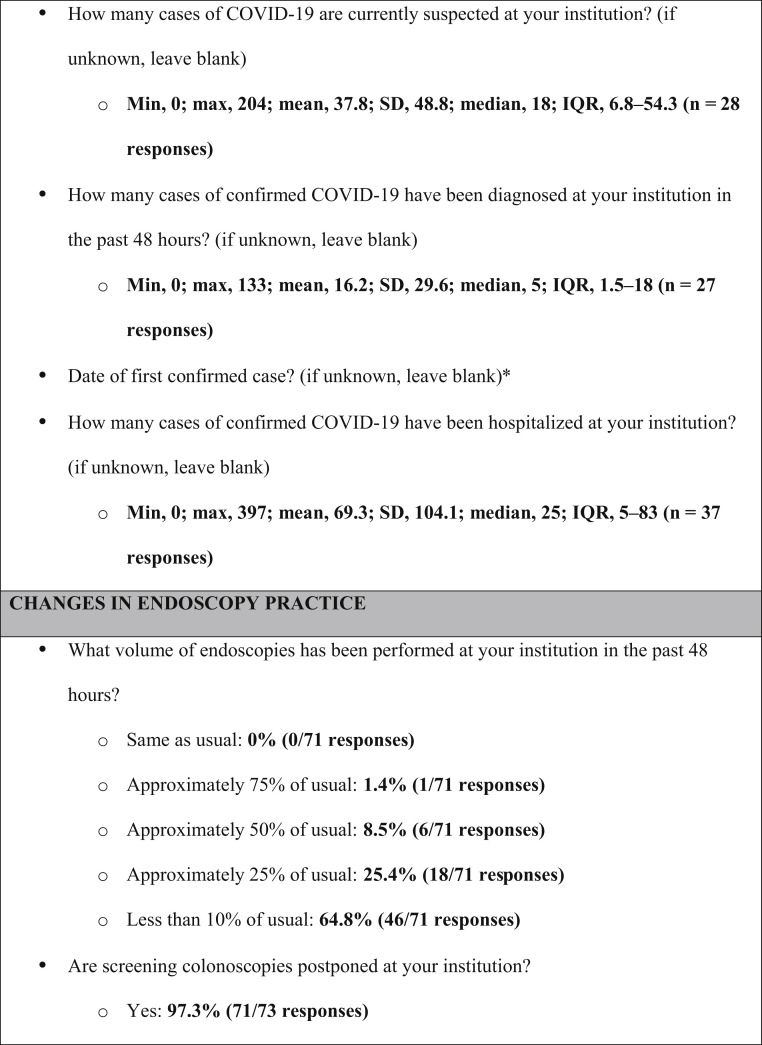
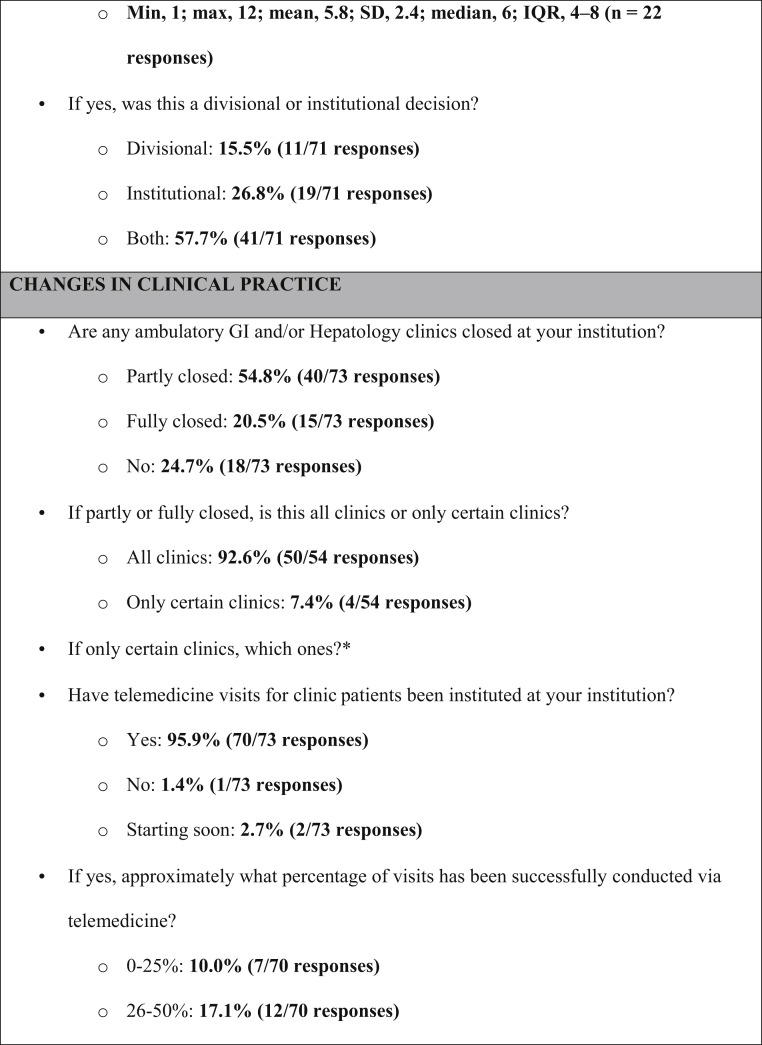
Supplementary Figure 2.
Survey instrument and aggregate responses (in bold). ∗Qualitative responses by free text—no aggregate answers reported. IQR, interquartile range; max, maximum; min, minimum; SD, standard deviation.
Changes in Endoscopy Practice
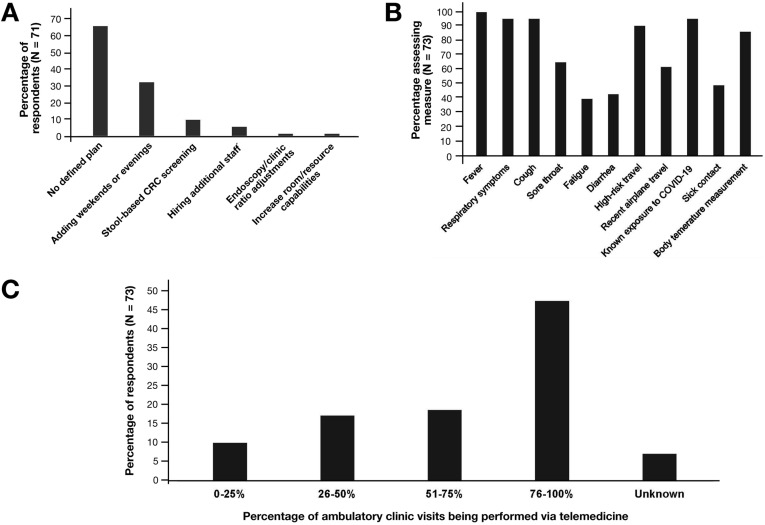
At the time of response, most centers (46/71, 65%) were operating at ≤10% of their normal endoscopy volume, with 18 centers (25%) performing approximately 25% of normal volume. Seventy-one of 73 centers (97%) had postponed screening colonoscopy. Of responding centers, 48 of 71 (68%) stated that they had no defined plan to address the procedural backlog once restrictions are lifted (Figure 1 ). Those that did have a plan favored weekend and after-hours endoscopy or stool-based testing. Twenty of 72 responding centers (27%) had adopted routine endotracheal intubation for upper endoscopic procedures.
Figure 1.
(A) Institutions’ plan(s) to manage the backlog of endoscopic procedures postponed during the COVID-19 pandemic once current restrictions on elective endoscopy are lifted. (B) Proportions of institutions screening for COVID-19 symptoms, exposure history, or performing body temperature measurements. (C) Proportions of institutions’ ambulatory clinic visits currently performed via telemedicine.
Changes in Clinical Gastroenterology Practice
Forty of 73 (55%) and 15 of 73 (21%) institutions reported that clinics were partly or fully closed, respectively. In response, all but 3 had implemented telemedicine visits (70/73, 96%). Approximately half of centers (33/70, 47%) reported conducting >75% of visits via telemedicine (Figure 1). Fifty-four of 73 centers (74%) had adopted modified schedules, limiting the number of attending physicians in the hospital at any given time; a median of 3 staff physicians were expected to be simultaneously present (interquartile range, 2–4).
Changes in Fellowship Training
Fellows continued to evaluate inpatient consults face-to-face in 47 programs (75%) and via electronic-only assessment in 14 programs (22%). Only 3 programs still had fellows seeing clinic patients in person, whereas 39 (62%) had them seeing patients virtually. In contrast, 26 programs (41%) had limited fellow involvement in endoscopy for select cases, whereas 31 (49%) had eliminated their involvement altogether. Many (21/47, 45%) interventional endoscopy training programs had stopped training.
Coronavirus Disease 2019 Screening
The majority of responding centers (63/73, 86%) are screening patients for COVID-19 upon arrival to endoscopy units through symptom and/or exposure assessments (Figure 1). These screening measures had resulted in at least 1 procedure cancellation at 37 of 60 (62%) responding centers. Only 16 of 57 centers (28%) were contacting patients up to 14 days postprocedure to assess for de novo COVID-19 symptoms.
Personal Protective Equipment Processes and Use
Expanded PPE (beyond standard gown, gloves, and goggles) was being used in 63 of 73 (86%) institutions for all endoscopic procedures, whereas 5 of 73 (7%) and 4 of 63 (6%) indicated that expanded PPE use was restricted to patients with suspected or known COVID-19, respectively. A total of 62 of 72 (86%) respondents indicated that they were concerned about their unit’s PPE supply in the event of a case surge. In all comers to endoscopy (COVID-19 negative or unknown status), 69 of 73 (95%) respondents reported using face shields or goggles; 56 of 73 (77%), standard surgical masks; 49 of 73 (67%), hairnets; 43 of 73 (59%), N95 or filtering facepiece particles (FFP) masks; 40 of 73 (55%), double gloves; and 35 of 73 (48%), shoe covers. In high-risk or confirmed COVID-19 cases, 92% of responding centers reported using an N95 or FFP mask. Reuse of N95 or FFP masks was reported by 54 of 71 (76%) respondents.
Stratified Analyses
Over time, the use of N95 masks for all comers increased significantly (χ2 = 22.2; P = .004). A nonsignificant trend was observed in the increased reuse of N95 masks over time (χ2 = 20.7; P = .06). There were no other significant temporal trends. On univariable analyses, US centers (compared to Canadian centers) were significantly more likely to use hairnets (χ2 = 9.1; P = .003), surgical masks (χ2 = 4.1; P = .04), and shoe covers (χ2 = 8.1; P = .004) in all comers and more likely to use hairnets (χ2 = 7.8; P = .005), N95 respirators (χ2 = 13.4; P < .001), and shoe covers (χ2 = 10.9; P = .001) in high-risk/positive patients. US centers were also significantly more likely to reuse N95 masks (χ2 = 19.3; P < .001). There were no significant differences in PPE practices between regions within the United States or between academic and nonacademic centers. Centers in the West were significantly more likely allow fellow participation in general (χ2 = 26.7; P = .02) and interventional endoscopy (χ2 = 15.1; P = .03).
Discussion
Our study shows that institutional responses to COVID-19 have been variable, fluid, multifaceted, and substantial. Although most study centers reported expanded PPE use in all endoscopic procedures, the use of surgical masks vs N95 respirators varied. Increasing trends in N95 mask use were observed over time, and US centers were more likely to use these masks as well and hairnets and shoe covers. Although early evidence suggests that standard surgical masks may be sufficient in non–high-risk scenarios,5 it remains critical to elucidate whether endoscopy produces respiratory aerosols fine enough to penetrate standard masks.
Triaging endoscopic procedures during the pandemic is also crucial, and professional societies have issued guidance on this matter.6 However, the postponement of nonurgent endoscopy will result in a surplus of procedures when clinical operations normalize. Strikingly, nearly two thirds of respondents had no defined plan to address the backlog. Innovative approaches, such as revising CRC surveillance intervals for patients awaiting follow-up colonoscopy according to new multisociety guidelines, should be considered,7 keeping in mind that resumptive strategies may be affected by numerous factors, including new postpandemic infection control precautions and whether a country has a single- or multiple-payer system.
This study provides a current and comprehensive snapshot of the changes that North American centers have implemented in response to the pandemic and—by reporting data on clinical, consultative, and training practices—expands on recent information by our Italian colleagues.8 High-quality research is urgently needed to better inform optimal PPE use and guide the reexpansion of postpandemic clinical and endoscopic services.
Acknowledgments
Members of the North American Alliance for the Study of Digestive Manifestations of COVID-19 who also contributed to the study include Amitabh Chak, MD, Gregory A. Coté, MD, MSc, Don C. Rockey, MD, and Field F. Willingham, MD, MPH.
CRediT Authorship Contributions
Shailendra Singh, MD (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead). Ahmad Khan, MD (Data curation: Lead; Formal analysis: Lead; Methodology: Supporting; Writing – review & editing: Supporting).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.04.071.
Contributor Information
North American Alliance for the Study of Digestive Manifestations of COVID-19:
Amitabh Chak, Gregory A. Coté, Don C. Rockey, and Field F. Willingham
Supplementary Methods
Study Design
This was a multicenter web-based survey study and was exempt from institutional board review. A survey was constructed within the REDCap electronic data capture platform,5 , 6 hosted at the Medical University of South Carolina, and was subsequently disseminated to potential respondents. The survey was accessible via a direct web link (https://gi-covid19.org/survey) without any required login credentials. Respondents were instructed to confer with their institutional leadership prior to completing the survey. In the event surveys were completed by multiple respondents from the same institution, the investigators contacted respondents directly and asked them to reconcile their responses. At the institutional level, this was a cross-sectional analysis; however, because institutions responded at various points in time, this was not a cross-sectional study in aggregate.
Survey Development, Validation and Beta Testing
The survey was developed through a series of electronic communications between the authors. The initial survey was designed by one investigator (BJE) and then iteratively pilot tested by the remainder of the authors to establish face and content validity and to improve factors affecting test-retest reliability, such as the clarity and order of questions, length of the instrument, and ease of administration. The near-final version of the survey was then reviewed and refined by another investigator (SBW) who had not previously commented on the instrument.
Survey Distribution
Due to the rapidly-evolving and critical nature of the COVID-19 pandemic, and the emphasis placed on timely and transparent results as well as widespread survey distribution, the approach to dissemination was unconventional, utilizing several media. A composite list of email addresses representing gastroenterologists at numerous institutions throughout North America was developed by the study team, with an intent to maximize geographic diversity. This list ultimately comprised 187 individuals. Two en masse e-mails were sent (by BJE) to the contact list encouraging participation in the survey and including a direct link. At their discretion, four investigators (NF, ZLS, SBW, BJE) also directly contacted gastroenterologists through email, phone calls and text messages, encouraging survey participation. The survey was also promoted on the social media platform Twitter, and featured in an online editorial regarding gastrointestinal endoscopy amidst the pandemic.7 Finally, respondents that had completed the survey were sent a personalized link 4 days before study closure to encourage them to update their entries.
Survey Items
The survey consisted of 42 items, stratified into 6 categories: institutional demographics, changes in endoscopy practice, changes in GI and hepatology clinical practice, changes in training, peri-procedure screening for COVID-19, and changes in PPE practices. Based on early feedback from respondents after the initial dissemination of the survey, one additional question and three additional response options to existing questions were added. A complete list of survey questions and possible answers is provided in below.
Publication of Intermediate Results During the Study
During the study period, many centers in North America remained in fluid situations regarding practice adaptations during the pandemic. Further, many centers anticipating surges in COVID-19 cases were learning from the experience of centers at which surges were already occurring. Because of the importance of real-time knowledge dissemination during these unprecedented times, the decision was made to publish the survey results, in aggregate, on three separate instances. The intermediate results were published on the survey website (GI-COVID19.org) and promoted on Twitter.
Response Stratification
For subgroup analyses, responses were stratified according to 3 factors: 1) geographic region of origin, including Canada and U.S. regions of the Northeast, Mid-Atlantic, Southeast, Southwest, West, Northwest, and Midwest; 2) whether they represented teaching or non-teaching institutions, as defined by the presence or absence of a gastroenterology fellowship program; and 3) according to the time of completion, in intervals of 7 days after initial survey dissemination.
Statistical Analysis
Individual survey item responses were reported as the proportion of total respondents engaging the question that answered affirmatively to the individual reply. For example, the number of respondents answering “yes” to the cancellation of screening colonoscopies was divided by the total number of responses to that question. For questions containing a free-text response option, attempts were made to codify these replies into de novo categories for analysis purposes. Comparisons of categorical answer responses within questions were performed using Chi-squared tests where appropriate. A Mantel-Haenszel test was used to assess for differences in responses based on the time between survey dissemination and response. A two-sided p-value of 0.05 was considered statistically significant. All analyses were performed using SPSS statistics for Mac version 25.0 (IBM Corp, Armonk, NY).
References
- 1.Pan L. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston E.R. Gastrointest Endosc. 2019;89:818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 3.American Society for Gastrointestinal Endoscopy. https://www.asge.org/home/advanced-education-training/covid-19-asge-updates-for-members/joint-gastroenterology-society-message-covid-19-use-of-personal-protective-equipment-in-gi-endoscopy/ Available at:
- 4.Chiu P.W.Y. Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae S. Ann Intern Med. 2020;173:W22–W23. doi: 10.7326/M20-1342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.American Association of the Study of Liver Disease, American College of Gastroenterology, American Gastroenterological Association, American Society for Gastrointestinal Endoscopy. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf Available at: Published March 31, 2020. Accessed April 5, 2020.
- 7.Gupta S. Gastroenterology. 2020;158:1131–1153. doi: 10.1053/j.gastro.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Repici A. Gastroenterology. 2020;159:363–366. doi: 10.1053/j.gastro.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]