Highlights
-
•
COVID-19 is a new respiratory and systemic disease which needs quick identification of potential critical patients.
-
•
There is a significant reduction of lymphocyte count in the severe COVID-19 group compared to the non-severe group.
-
•
Those with lymphopenia have a 3-fold higher risk of developing severe COVID-19.
-
•
Lymphopenia is a prominent feature of COVID-19 and lymphocyte counts may be a useful, easily available biomarker in predicting the severity and clinical outcomes.
Keywords: COVID-2019, lymphocyte count, lymphopenia
Abstract
Objectives
Coronavirus Disease 2019 (COVID-19) is a new respiratory and systemic disease which needs quick identification of potential critical patients. This meta-analysis aimed to explore the relationship between lymphocyte count and the severity of COVID-19.
Methods
A comprehensive systematic literature search was carried out to find studies published from December 2019 to 22 March 2020 from five databases. The language of literatures included English and Chinese. Mean difference (MD) of lymphocyte count in COVID-19 patients with or without severe disease and odds ratio (OR) of lymphopenia for severe form of COVID-19 was evaluated with this meta-analysis.
Results
Overall 13 case-series with a total of 2282 cases were included in the study. The pooled analysis showed that lymphocyte count was significantly lower in severe COVID-19 patients (MD -0.31 × 109/L; 95%CI: -0.42 to -0.19 × 109/L). The presence of lymphopenia was associated with nearly threefold increased risk of severe COVID-19 (Random effects model, OR = 2.99, 95% CI: 1.31-6.82).
Conclusions
Lymphopenia is a prominent part of severe COVID-19 and a lymphocyte count of less than 1.5 × 109/L may be useful in predicting the severity clinical outcomes.
1. Background
Coronavirus Disease 2019 (COVID-19) is a new form of respiratory disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(El Zowalaty and Järhult, 2020). Since its first appearance and reporting in December 2019, it has infected over 462,684 cases and caused 20,834 deaths as of 26th March 2020. (WHO Coronavirus disease situation reports). Patients with COVID-19 may develop acute respiratory distress syndrome and occasionally may progress to multiorgan failure(Hui et al., 2020). Latest reports suggest that for COVID-19 the hospitalization rate is 20.7-31.4%, ICU admission rate is 4.9-11.5%, and case-fatality rate is 1.8-3.4%(SOAPCD, 2020). It is expected that COVID-19 will continue to increase and there will be a growing demand for intensive care(Wu et al., 2020a). Quick identification of potential critical patients is important in the management of this disease to prioritize health-care resources, which are under strain in practically every part of the world(Zhao et al., 2020, He et al., 2020).
Cytotoxic T lymphocytes and natural killer cells are necessary for the control of viral infection. A functional exhaustion of antiviral lymphocytes is reported in COVID-19 patients(Zheng et al., 2020a, Alves da Silva et al., 2018). However, there is still limited evidence for the predictive role of lymphocyte count in predicting the severity of COVID-19. Herein, we reviewed all the literature on COVID-19 from December 2019 to 22 March to explore the possible role of lymphocyte counts in differentiating between severe and non-severe COVID-19 patients, so as to find a simple tool for quick identification of potential critical patients that will help with the clinical management of this new disease.
2. Methods
2.1. Literature Search and Study Selection
We carried out a comprehensive systematic literature search of online databases, including PubMed, Web of Science, Cochrane, WanFang and CNKI databases from December 2019 to 22 March 2020 to identify all reported case studies in Chinese and English languages. The WanFang and CNKI databases are Chinese databases available online which can be used to find full-text articles. The following terms and their relative variants were used for literature search: “COVID-19” OR “2019 novel coronavirus infection” OR “coronavirus disease 2019” OR “2019 novel coronavirus disease” OR “coronavirus 2019” OR “2019-nCoV” OR “SARS-CoV-2” OR “COVID19” OR “coronavirus disease-19”.
The title, abstract and full text of all documents identified according to this search strategy were then screened by two investigators (M.M and QW.Z). The reference list of each review and original article was reviewed for identifying other eligible reports. The inclusion criteria for the studies to be included in the meta-analysis were as follows: studies presenting the data of lymphocyte counts or information on lymphopenia in COVID-19 cases with or without severe presentation. The definition of severe COVID-19 varied in different literature. In this study we defined the “severe presentation” as a requirement for intensive care, mechanical ventilation or death which is consistent with most articles. All the search results were evaluated according to the Methodological Index Non-Randomized Studies (MINORS) statement.
2.2. Data Extraction and Quality Assessment
Data extraction and the evaluation of literature quality were conducted independently by 2 investigators (M.M and QW.Z). A Microsoft Excel database was created to record all available information, including baseline details, and rate of development of primary end point in patients with different respiratory conditions. Any disagreement was resolved by another investigator (ZY.W).
2.3. Statistical Analysis of Data
Microsoft Excel was used to analyze the clinical symptoms and the laboratory results. A meta-analysis was carried out using R software (version 3.6.3, available on https://www.r-project.org). Heterogeneity among studies was tested using the Cochran Chi-square test and I 2, When I 2 < 50%, a fixed-effects model was used, while when I 2 > 50%, a random-effects model was selected. If statistical heterogeneity was found among the results, a further sensitivity analysis was conducted to determine the source of heterogeneity. After the significant clinical heterogeneity was excluded, the randomized effects model was used for meta-analysis. Funnel plots were used to detect publication bias. P < 0.05 was considered as statistical significance.
3. Results
3.1. Research Selection and Quality Assessment
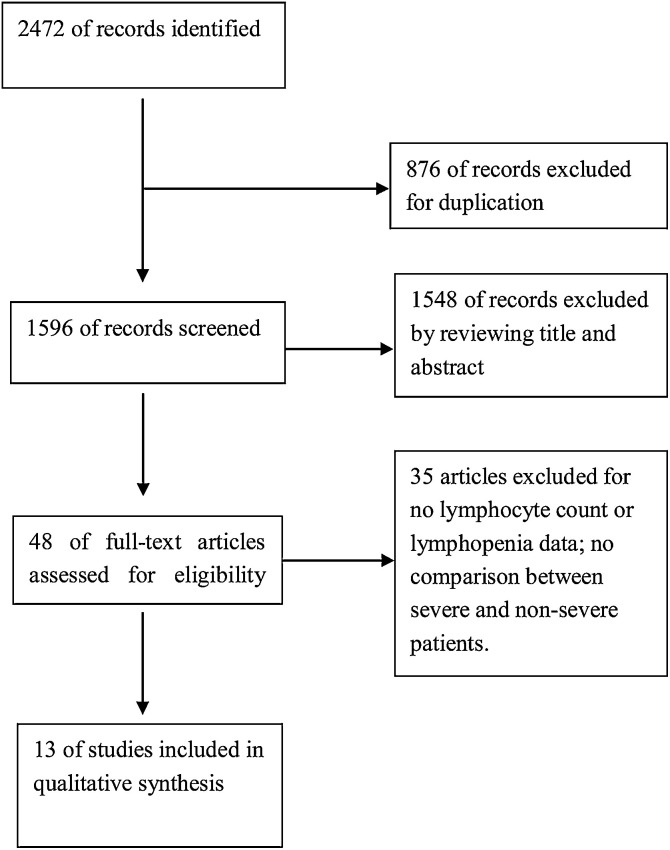
Based on the described search strategy, a total of 2472 studies were found in the five online databases as described above. After removing the duplicate records, 1596 studies were retained. Furthermore, 1548 studies were excluded as they were not relevant to current meta-analysis. Full text of the remaining 48 articles was assessed for eligibility, and 35 of these were removed for various reasons. Eventually 13 studies (11 in English and 2 in Chinese) [9-20]were included in the analysis (Fig. 1 ). The characteristics and demographic data of the included studies are shown in Table 1 . As all the studies included in this meta-analysis were retrospective case-series, the calculation of sample size was not reported. Most studies reported the follow-up period inadequately. As a result, the overall quality of literature included in this study was not high, with MINORS scores from 10-13.
Fig. 1.
A flow diagram of the inclusion criteria of studies eligible for meta-analysis.
Table 1.
Characteristics and demographic data of the included studies
| author | year | region | district | language | outcome | mean age | male n(%) | Non-Severe Lymphocyte Count: Mean(SD) × 10^9/L | Severe Lymphocyte Count: Mean(SD) × 10^9/L | Severe cases/total cases —no./total no. (%) | severe cases in lymphopenia/total cases of lymphopenia —no./total no. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| X. Chen | 2020 | China | Chongqing | Chinese | Composite End Point | 45.33 | 76(54.68%) | 1.18(0.49) | 0.87(0.38) | 31/139 (22.30%) | not reported |
| X. Fang | 2020 | China | Anhui | Chinese | Composite End Point | 45.1 | 9(20.93%) | 1.3(0.61) | 0.73(0.55) | 24/79 (30.38%) | not reported |
| Y. Gao | 2020 | China | Anhui | English | Composite End Point | 44.08 | 26(60.47%) | 1.07(0.4) | 1.2(0.42) | 15/43 (34.88%) | not reported |
| W. Guan | 2020 | China | 30 provinces | English | Composite End Point | 46.67 | 459(58.12) | not report | not report | 67/1099 (6.10%) | 50/731 (6.84%) |
| C. Huang | 2020 | China | Wuhan | English | ICU | 49.33 | 30(73.17%) | 0.93(0.31) | 0.47(0.5) | 13/41 (31.71%) | 11/26 (42.31%) |
| W. Liu | 2020 | China | Wuhan | English | Composite End Point | 42.67 | 39(50.00%) | 1.02(0.54) | 0.66(0.72) | 11/78 (14.10%) | not reported |
| Y. Liu | 2020 | China | Shenzhen | English | Composite End Point | 53.67 | 8(66.67%) | 1.56(0.99) | 0.63(0.42) | 3/12 (25.00%) | 3/6 (50.00%) |
| C. Wu | 2020 | China | Wuhan | English | Composite End Point | 51.33 | 128(63.68) | 1.08(0.55) | 0.72(0.38) | 84/201 (41.79%) | not reported |
| D. Wang | 2020 | China | Wuhan | English | ICU | 55.33 | 75(54.35%) | 0.9(0.45) | 0.73(0.31) | 36/138 (26.09%) | not reported |
| Z. Wang | 2020 | China | Wuhan | English | Composite End Point | 46.33 | 32(46.38%) | 1.2(0.42) | 0.66(0.48) | 14/69 (20.29%) | not reported |
| X. Yang | 2020 | China | Wuhan | English | Death | 59.7 | 35(66.67%) | 0·74(0.4) | 0·62(0.37) | 32/52 (61.54) | 11/28 (39.29%) |
| F. Zhou | 2020 | China | Wuhan | English | Death | 56.33 | 119(62.30) | 1.13(0.52) | 0.63(0.23) | 54/191 (28.27%) | not reported |
| J. Zhang | 2020 | China | Wuhan | English | Composite End Point | 56.33 | 71(50.71%) | 0.87(0.45) | 0.73(0.38) | 58/140 (41.43) | 46/104 (44.23%) |
* Composite end point in original studies was defined as SpO2 < 90% or requirement of an intensive care or the use of mechanical ventilation, or death.
3.2. Clinical Data
The characteristics of the included studies are presented in Table 1. The pooled clinical data of 13 studies involving 2282 cases showed that a total of 442 patients required intensive care or ventilatory support, or died. The clinical severity was defined as the composite of ICU admission, use of mechanical ventilation or death as reported in nine studies(Xiaowei et al., 2020, Gao et al., 2020, Liu et al., 2020a, Liu et al., 2020b, Wu et al., 2020b, Wang et al., 2020b, Zhang et al., 2020, Guan W-j et al., 2020, Chen et al., 2020), death only as reported in two studies(Yang et al., 2020, Zhou et al., 2020) and ICU admission as reported in two studies(Wang et al., 2020a, Huang et al., 2020). The blood tests were measured at the time of hospitalization in all studies. Eight studies reported the lymphocyte count(Xiaowei et al., 2020, Gao et al., 2020, Liu et al., 2020a, Wu et al., 2020b, Wang et al., 2020a, Wang et al., 2020b, Zhou et al., 2020, Chen et al., 2020) and one study only reported information of lymphopenia(Guan W-j et al., 2020). The original data was presented as the prevalence of lymphopenia in severe or non-severe COVID-19 patients. To better visualize the risk, we converted the dataset and present the data as the incidence of severe pneumonia in lymphopenia population.
3.3. Lymphocyte count and the severity of COVID-19
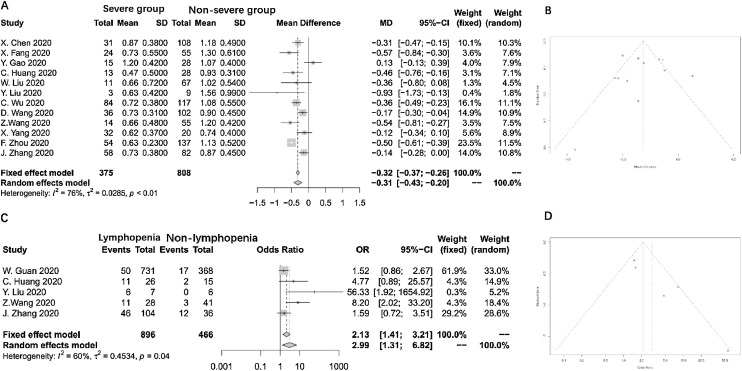
Twelve studies were included in this meta-analysis(Xiaowei et al., 2020, Gao et al., 2020, Liu et al., 2020a, Liu et al., 2020b, Wu et al., 2020b, Wang et al., 2020a, Wang et al., 2020b, Yang et al., 2020, Zhou et al., 2020, Zhang et al., 2020, Chen et al., 2020). The meta-analysis for the mean difference in lymphocyte count showed an overall significant reduction of 0.31 × 109/L in the severe COVID-19 group compared to the non-severe group (random effects model, CI: -0.42 to -0.19 × 109/L). The overall heterogeneity in this meta-analysis was high (I 2 = 76%; p < 0.01, Fig. 2A). Sensitivity analysis revealed that this heterogeneity was not a result from an individual study. Funnel plot showed no publication bias in this analysis (Fig. 2B).
Fig. 2.
Forest plot and funnel plot of all outcomes.
3.4. Lymphopenia and the severity of COVID-19
Five studies reported the relationship between lymphopenia and the severity of COVID-19(Liu et al., 2020a, Liu et al., 2020b, Wang et al., 2020b, Zhang et al., 2020, Guan W-j et al., 2020). Lymphopenia was defined as a lymphocyte count of less than 1.1 × 109/L in four studies(Liu et al., 2020a, Liu et al., 2020b, Yang et al., 2020, Zhang et al., 2020), and as less than 1.5 × 109/L in one(Guan W-j et al., 2020). The pooled OR as summarized in Fig. 2C shows that the presence of lymphopenia results in an approximately 3-fold increased risk of severe COVID-19 (Random effects model, OR = 2.99, 95% CI: 1.31-6.82). The heterogeneity among the different studies was high (I 2 = 60%, p = 0.04). A sensitivity analysis by excluding each study was performed, showing the study from Z. Wang was the major source of heterogeneity. After excluding this study, the I 2 of heterogeneity reduced to 48%, the OR of lymphopenia was 2.17 (95% CI: 1.0126-4.6812). The funnel plot indicated no publication bias inside this study (Fig. 2D).
4. Discussion
This meta-analysis included the latest studies of COVID-19 from December 2019 to 22 March 2020 published in the English and Chinese language, showing that patients with severe COVID-19 displayed a lymphocyte count reduction compared with the non-severe COVID-19 group. Lymphopenia, defined as a lymphocyte count of less than 1.5 × 109/L, is associated with a 3-fold increased risk of severe COVID-19 infection.
The lack of awareness of severity of disease in the early stages of COVID-19 coupled with the high infectivity of the virus has led to a dramatic increase in the number of patients and relatively high fatality rates worldwide. A cheap, easily acquired biomarker is needed to identify severe disease among hospitalized patients at early stages. According to our results, lymphocyte count and lymphopenia may serve as a rapid tool that can quickly identify COVID-19 patients with more severe clinical presentation. Previous studies observed that lymphopenia is a common observation in patients with severe acute respiratory syndrome (SARS) caused by SARS virus with reported prevalence of 69.6%-54%(Lee et al., 2003, Booth et al., 2003). Lymphopenia is quite notable in SARS infection(Yang et al., 2004). SARS infection may either directly suppress bone marrow or induce an immune-mediated destruction of lymphocytes resulting in lymphopenia (He et al., 2005). SARS-COV-2 might share a similar inner mechanism with SARS virus, including direct infection and destruction of lymphocytes (Zheng et al., 2020b) and cytokine-mediated lymphocyte destruction (Zheng et al., 2020c, Sarzi-Puttini et al., 2020, Xie and Chen, 2020).
The main drawback of this meta-analysis is the heterogeneity of included cases. All the included studies were retrospective case series as no data from either prospective observation study or randomized trials are available. The patients in different studies might be at different stages of disease. Furthermore, the different definitions of severity of COVID-19 and the discrepancy in the cut-offs for lymphopenia, which can be partially mitigated by considering lymphocyte count of less than 1.5 × 109/L as lymphopenia, might be one of the reasons for the heterogeneity.
In addition, another drawback of our study is that there is no data relating to fluctuations of lymphocyte counts on the disease course, which is significant to the detection of COVID-19 clinical course. So, it is too early to draw the conclusion that lymphopenia is related to deterioration of COVID-19. A recent research study with small sample size showed the dynamic changes of lymphocyte counts could predict the deterioration of COVID-19, yet studies with large sample size are still lacking in this field(Tan et al., 2020).
Due to the extremely rapid spread of COVID-19 worldwide, waiting for the results from prospective studies will delay the understanding of this novel disease, as a result of which there may be a delay in the clinical management of patients. Even in light of these limitations, the result of this meta-analysis show that the presence of lymphopenia in the evolution of COVID-19 may help to rapidly identify patients at risk of severe pneumonia and worse outcomes. Further studies should focus on the time-line of lymphopenia development, severity of COVID-19 and ARDS, which may confirm our findings about the relationship between lymphopenia and severity of COVID-19.
5. Conclusion
Lymphopenia is a prominent part of severe COVID-19 and a lymphocyte count of less than 1.5 × 109/L may be useful in predicting the severity of clinical outcomes. Further studies are needed to focus on lymphocyte changes in COVID-19 to confirm the predictive ability of lymphopenia in COVID-19.
Declarations
Ethics: the study does not require ethical approval because the meta-analysis is based on published research and the original data are anonymous.
Consent for publication: Not applicable.
Availability of data and materials: All data are fully available on line without restriction.
Competing interests
The authors declare that they have no competing interests.
Funding Source
Scientific research “Pei Yu” project of Beijing Municipal Hospital Administration (PZ2018011)
Author Contributions
Guarantor of the article: Li Yang and Zhiyuan Weng; Designed the study: Li Yang and Zhiyuan Weng; Interpreted data and wrote the manuscript: Qianwen Zhao and Rahul Kumar; Screened and extracted data: Qianwen Zhao and Meng Meng; Statistical analyses: Zhiyuan Weng; Reviewed the results and made critical comments on the manuscript: Ningfang Lian, Rahul Kumar, Yunlei Deng, Jiaofeng Huang and Yinlian Wu; All authors approved the final version of the manuscript.
Acknowledgments
Not applicable.
References
- El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans - Call for a One Health approach. One health (Amsterdam, Netherlands) 2020;9:100124. doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar I.E., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:343-6. [DOI] [PMC free article] [PubMed]
- Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Meng M., Kumar R. The impact of COPD and smoking history on the severity of Covid-19: A systemic review and meta-analysis. Journal of medical virology. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Deng Y., Li W. Coronavirus disease 2019: What we know? Journal of medical virology. 2020 doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves da Silva R., de Souza Todão J., Kamitani F.L. Molecular characterization of hepatitis C virus in end-stage renal disease patients under hemodialysis. Journal of medical virology. 2018;90:537–544. doi: 10.1002/jmv.24976. [DOI] [PubMed] [Google Scholar]
- Xiaowei F., Qing M., Lei Z. Clinical features and treatment analysis of 79 Coronovirus infected pneumonia. Chinese Pharmacological Bulletin. 2020;36:12–18. [Google Scholar]
- Gao Y., Li T., Han M. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tao Z.W., Lei W. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory medicine. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Guan W-j, Ni Z-y, Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Xi, Tong Jin, Xiang Jianhua, Hu Jingjing. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of Severity. Chongqing Medicine. 2020:1–9. [Google Scholar]
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Yang M., Li C.K., Li K. Hematological findings in SARS patients and possible mechanisms (review) Int J Mol Med. 2004;14:311–315. [PubMed] [Google Scholar]
- He Z., Zhao C., Dong Q. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.Y., Zhang M., Yang C.X. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P., Giorgi V., Sirotti S. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020 [PubMed] [Google Scholar]
- Xie M., Chen Q. Insight into 2019 novel coronavirus - An updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]