Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a global public health crisis. Liver injury has been reported during the COVID-19 disease progression.1 However, previous studies did not examine in detail the interaction of preexisting liver disease and COVID-19.1 Therefore, we aimed to study the impact of preexisting liver disease on outcomes in a large cohort of patients with COVID-19 in the United States.
Methods
A real-time search and analysis were performed for patients (≥10 years age) diagnosed with COVID-19 by using the TriNetX (Cambridge, MA) Research Network with COVID-19–specific diagnosis and terminology recommended by the World Health Organization and Centers for Disease Control and Prevention. TriNetX provided real-time access to the electronic medical records of more than 49 million patients from 37 health care organizations.
Identified patients with COVID-19 were then stratified into 2 groups based on the presence (LD) or absence (non-LD) of preexisting liver disease. The LD group consisted of patients with a diagnosis of chronic liver disease, cirrhosis, or related complications either at the time of diagnosis of COVID-19 or any time before that.
The outcomes studied were mortality, hospitalization, and laboratory findings in the time window up to 30 days from the diagnosis of COVID-19. All statistical analyses were performed with TriNetX. TriNetX obfuscates patient counts to safeguard protected health information by rounding patient counts in analyses up to the nearest 10.
Details of the data source, search criteria, diagnosis, study variables, statistical analysis, and limitations of the methodology are available in the Supplementary Methods.
Results
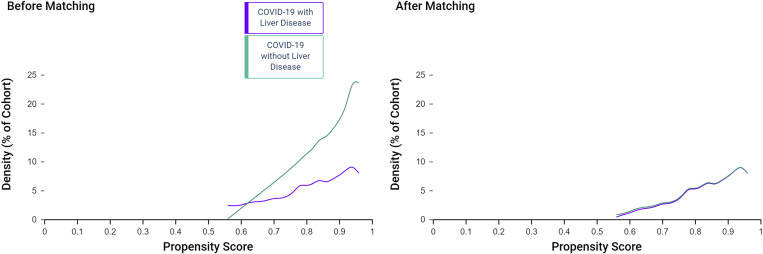
We identified a total of 2780 patients with COVID-19 across 34 health care organizations in the United States. There were 250 (9%) patients with preexisting liver disease included in the LD group, and the remaining 2530 were included in the non-LD group. Among the LD group patients, 50 (1.8%) were also diagnosed with cirrhosis. LD group patients were older than those in the non-LD group (55.2 ± 14.6 vs 51.6 ± 17.8 years; P < .01). LD group patients had substantially higher comorbidities, and a large proportion had hypertension (68%) or diabetes (48%). Fatty liver disease or nonalcoholic steatohepatitis (42%) was the most common among LD group patients. Therefore, we performed (1:1) propensity score matching for body mass index, hypertension, and diabetes in addition to age, race, and nicotine use. The groups were relatively balanced after propensity matching (n = 250 in each group) (Table 1 and Supplementary Figure 1).
Table 1.
Outcomes and Baseline Characteristics Among Patients With COVID-19 Stratified Into Those With Preexisting Liver Disease and Without Liver Disease
| Before propensity matching |
After propensity matching |
|||||
|---|---|---|---|---|---|---|
| COVID-19 with liver disease (n = 250) | COVID-19 without liver disease (n = 2530) | RR, RD, or P value | COVID-19 with liver disease (n = 250) | COVID-19 without liver disease (n = 250) | RR, RD, or P value | |
| Outcomes | ||||||
| Mortality, %, (n/total) | 12.0 (30/250) | 4.3 (110/2530) | RR: 2.8 (1.9, 4.0) RD: 7.7% (3.5%, 11.75%) P < .001 |
12.0 (30/250) | 4.0 (10/250) | RR: 3.0 (1.5, 6.0) RD: 8.0% (3.3%, 12.7%) P = .001 |
| Hospitalization rate | 52.0 (130/250) | 30.0 (760/2530) | RR: 1.7 (1.2, 2.0) RD: 22.0% (15.5%, 28.4%) P < .001 |
48.0 (120/250) | 36.0 (90/250) | RR: 1.3 (1.1, 1.6) RD: 12.0% (3.4%, 20.6%) P = .006 |
| Characteristics | ||||||
| Age, y, mean ± SD | 55.2 ± 14.6 | 51.6 ± 17.8 | <.01 | 55.4 ± 14.4 | 56.7 ± 15.3 | .36 |
| Female, n (%) | 140 (56) | 1570 (62) | .06 | 140 (56) | 140 (56) | 1.00 |
| Race: white, n (%) | 130 (52) | 1220 (48.2) | .25 | 130 (52) | 130 (52) | 1.00 |
| Nicotine dependence, n (%) | 60 (24) | 190 (7.5) | <.01 | 50 (20) | 50 (20) | 1.00 |
| Body mass index >30.0 to 30.9 kg/m2, n (%) | 60 (24) | 310 (12.5) | <.01 | 50 (20) | 50 (20) | 1.00 |
| Race: black or African American, n (%) | 100 (40) | 1000 (39.5) | .88 | 100 (40) | 110 (44) | .36 |
| Hypertension, n (%) | 170 (68) | 1020 (40.3) | <.01 | 170 (68) | 170 (68) | 1.00 |
| Diabetes mellitus, n (%) | 120 (48) | 520 (14.8) | <.01 | 110 (44) | 110 (44) | 1.00 |
| Chronic lower respiratory diseases, n (%) | 100 (40) | 280 (11.0) | <.01 | 100 (40) | 70 (28) | .01 |
| Chronic kidney disease, n (%) | 80 (32) | 305 (7.2) | <.01 | 80 (32) | 50 (20) | .01 |
| Heart failure, n (%) | 60 (24) | 220 (8.7) | <.01 | 60 (24) | 50 (20) | .28 |
NOTE. Outcomes and baseline characteristics are compared before and after propensity score matching of groups.
RD, risk difference; RR, risk ratio; SD, standard deviation.
Supplementary Figure 1.
Propensity score density graph before and after matching of the LD and non-LD groups.
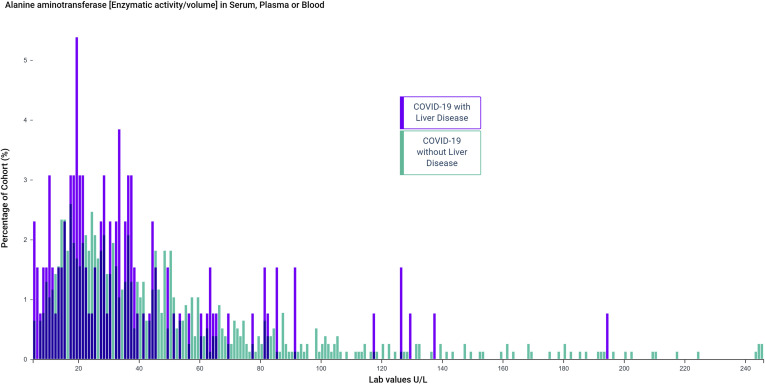
The mean values of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were elevated from baseline after COVID-19 in both the LD (ALT, 100 ± 444 U/L; AST, 221 ± 1799 U/L) and the non-LD group (ALT, 80 ± 227 U/L; AST, 133 ± 678U/L). ALT elevations (>50 U/L) were seen in 46.1% in the LD group and 50.6% in the non-LD group (Table 2 and Supplementary Figure 2). Similarly, elevations in mean values of gamma-glutamyl transferase, alkaline phosphatase, and total bilirubin were also seen. Concentrations of ferritin, C-reactive protein, lactate dehydrogenase, interleukin 6, creatine kinase, and D-dimer were also elevated in both groups (Table 2).
Table 2.
Laboratory Findings Among Patients With COVID-19 Stratified Into Those With Preexisting Liver Disease and Without Liver Disease
| Laboratory findings | COVID-19 with liver disease (n = 250) |
COVID-19 without liver disease (n = 2530) |
||
|---|---|---|---|---|
| Baseline | After COVID-19 | Baseline | After COVID-19 | |
| Liver chemistries (serum, plasma, or blood) | ||||
| ALT, U/L, mean ± SD (n) | 30 ± 24 (220) | 100 ± 444 (130) | 21 ± 17 (1520) | 80 ± 227 (70) |
| ALT > 50 U/L, % (n/total) | 46.1 (60/130) | 50.6 (390/770) | ||
| ALT >150 U/L, % (n/total) | 15.4 (20/130) | 11.7 (90/770) | ||
| AST, U/L, mean ± SD (n) | 34 ± 26 (220) | 221 ± 1799 (130) | 22 ± 20 (1520) | 133 ± 678 (770) |
| AST elevation > 50 U/L, % (n/total) | 61.5 (80/130) | 67.5 (520/770) | ||
| AST elevation > 150 U/L, % (n/total) | 15.4 (20/130) | 16.9 (130/770) | ||
| GGT, U/L, mean ± SD (n) | 219 ± 485 (70) | 278 ± 521 (10) | 44 ± 54 (70) | 99 ± 146 (30) |
| GGT > 50 U/L, % (n/total) | 100 (10/10) | 66.6 (20/30) | ||
| Total bilirubin, mg/dL, mean ± SD (n) | 0.8 ± 1.3 (220) | 1.2 ± 2.9 (120) | 0.5 ± 0.3 (1510) | 0.8 ± 1.2 (770) |
| Total bilirubin > 2 mg/dL, % (n/total) | 25.0 (30/120) | 9.1 (70/770) | ||
| Alkaline phosphatase, U/L, mean ± SD (n) | 112 ± 111 (220) | 153 ± 175 (120) | 778 ± 29 (1520) | 93 ± 62 (770) |
| Alkaline phosphatase > 150 U/L, % (n/total) | 41.6 (50/120) | 15.6 (120/770) | ||
| Serum albumin, g/dL, mean ± SD (n) | 3.9 ± 0.8 (220) | 2.6 ± 0.8 (120) | 4.1 ± 0.6 (1510) | 2.5 ± 0.7 (770) |
| Metabolic panel (serum, plasma, or blood) | ||||
| Sodium, mEq/L, mean ± SD (n) | 139 ± 3 (230) | 137 ± 5 (130) | 140 ± 3 (1660) | 137 ± 5 (880) |
| Potassium, mEq/L, mean ± SD (n) | 4.0 ± 0.4 (230) | 4.1 ± 0.4 (130) | 4.3 ± 0.8 (1660) | 4.1 ± 1.0 (880) |
| Creatinine, mg/dL, mean ± SD (n) | 1.2 ± 1.0 (230) | 1.8 ± 2.1 (130) | 1.0 ± 1.0 (1680) | 1.8 ± 2.1 (880) |
| Complete blood count, mean ± SD (n) | ||||
| Hemoglobin, g/dL | 12.2 ± 2.4 (220) | 10.4 ± 2.1 (130) | 12.9 ± 1.9 (1660) | 11.08 ± 2.2 (860) |
| Leukocytes/μL | 7.6 ± 3.6 (220) | 8.8 ± 5.9 (130) | 7.4 ± 2.9 (1660) | 9.7 ± 6.3 (860) |
| Platelets/μL | 229 ± 92 (220) | 237 ± 122 (130) | 253 ± 78 (1660) | 276 ± 123 (860) |
| Lymphocytes/μL | 2.4 ± 4.4 (130) | 1.9 ± 3.7 (50) | 2.2 ± 2.9 (800) | 2.5 ± 5.8 (220) |
| Neutrophils/100 leukocytes | 63 ± 12 (110) | 70 ± 16 (50) | 61 ± 12 (800) | 69.2 ± 13 (230) |
| Coagulation profile, mean ± SD (n) | ||||
| Prothrombin time, s | 12.5 ± 3.45 (170) | 14.94 ± 4.81 (50) | 12 ± 3.62 (650) | 14.03 ± 7.36 (250) |
| Activated partial thromboplastin time, s | 31.0 ± 10.9 (140) | 39.0 ± 16.4 (30) | 29.1 ± 8.2 (540) | 38.9 ± 15.7 (220) |
| Inflammatory, infectious and other markers (serum, plasma, or blood), mean ± SD (n) | ||||
| Ferritin, ng/mL | 371 ± 94 (130) | 1925 ± 2567 (30) | 234 ± 735 (380) | 2225 ± 6179 (270) |
| C-reactive protein, mg/L | 25 ± 53 (110) | 131 ± 103 (70) | 17 ± 40 (370) | 130 ± 113 (570) |
| Erythrocyte sedimentation rate, mm/h | 36 ± 29 (110) | 56 ± 42 (10) | 24 ± 24 (440) | 71 ± 35 (70) |
| Lactate dehydrogenase, U/L | 270 ± 159 (80) | 440 ± 568 (30) | 260 ± 460 (190) | 555 ± 747 (270) |
| Interleukin 6, pg/mL | 86 ± 111 (10) | 99 ± 188 (50) | ||
| D-dimer, μg/mL | 1.0 ± 0.3 (10) | 2.9 ±3.5 (30) | ||
| Creatinine kinase, U/L | 1068 ± 2797 (20) | 1032 ± 3358 (170) | ||
| Procalcitonin, ng/mL | 3.4 ± 5.3 (20) | 2.3 ± 8.3 (220) | ||
NOTE. Laboratory findings are compared before and after COVID-19 diagnosis.
GGT, gamma glutamyl transferase.
Supplementary Figure 2.
Distribution of the values of ALT after COVID-19 in patients with preexisting liver disease and without liver disease.
Patients in the LD group had a significantly higher risk of mortality (risk ratio [RR], 2.8; 95% confidence interval [CI], 1.9–4.0; P < .001), and the risk remained high even after the propensity matching of the 2 groups (RR, 3.0; 95% CI, 1.5–6.0; P = .001). In the subgroup analysis of the LD group, patients with cirrhosis had an even higher relative risk of mortality compared to patients in the non-LD group (RR, 4.6; 95% CI, 2.6–8.3; P < .001). Similarly, the risk of hospitalization was higher in the LD group before and after the matching of cohorts (Table 1).
Discussion
We compared the outcomes of patients with preexisting liver disease and without liver disease in a large and diverse cohort of 2780 patients with COVID-19 in the United States. Elevation in liver chemistry test levels was seen in the vast majority of patients, suggesting possible liver injury in COVID-19. We found that patients with preexisting liver disease were at increased risk for mortality (RR, 2.8; 95% CI, 1.9–4.0; P <.001) compared to patients without liver disease, and the relative risk was markedly higher in patients with cirrhosis (RR, 4.6; 95% CI, 2.6–8.3; P < .001).
Our findings are similar to those reported for other comorbidities, such as hypertension, diabetes, or cardiovascular disease, yielding poor outcomes.2 Many comorbidities overlap in patients or have a similar profile; a large proportion of patients in the preexisting liver disease group had fatty liver disease along with diabetes and hypertension. Therefore, we performed propensity matching of the groups and still found a higher risk for mortality and hospitalization in patients with preexisting liver disease.
The possible reasons for poor outcomes among COVID-19 with preexisting liver disease need further investigation; however, it appears to be an interplay of local liver injury and systemic disturbances. SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor to gain entry and damage the target organ.3 The expression of angiotensin-converting enzyme 2 receptors has been suggested in both liver and bile duct cells.4, 5 Previous studies have reported abnormalities in transaminases in 14%–53% of patients with COVID-19.1 We also noticed elevations in liver chemistry test results from baseline values, suggesting possible liver injury from SARS-CoV-2. However, we cannot rule out medications or other possible etiologies for these abnormalities and are unable to specify a pattern of liver injury. Hypoxia often seen in COVID-19 can induce diminished cellular activity and high-level oxygen free radicals, resulting in liver injury and organ failure.1 , 6 Patients with COVID-19 with preexisting liver dysfunction, especially with cirrhosis, are theoretically more susceptible to poor outcomes from these direct injuries to liver. Moreover, the immune deficiency and accompanying persistent systemic inflammation reflected by the activated circulating immune cells and increased serum levels of proinflammatory cytokines that occurs in patients with advanced liver disease can predispose them to uncontrollable proinflammatory cytokine production.6 , 7 Many patients with cirrhosis can also have underlying hepatopulmonary syndrome, portopulmonary hypertension, or hepatic hydrothorax, which can increase the risk of respiratory failure in itself.8
In conclusion, liver injury can be seen in the majority of patients with COVID-19. However, patients with preexisting liver disease, notably cirrhosis, are at higher risk for hospitalizations and mortality. Early isolation, intensive surveillance, and timely diagnosis are essential in these patients. Further research identifying interventions to reduce poor outcomes in high-risk patients with COVID-19 is needed.
Acknowledgments
We acknowledge the West Virginia Clinical and Translational Science Institute, which provided us with access to and training in the TriNetX global health care network. We also acknowledge the TriNetX (Cambridge, MA) health care network for design assistance in completing this project.
CRediT Authorship Contributions
Shailendra Singh, MD (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead). Ahmad Khan, MD (Data curation: Lead; Formal analysis: Lead; Methodology: Supporting; Writing – review & editing: Supporting).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.04.064.
Supplementary Methods
Description of the Data Source
The TriNetX (Cambridge, MA) COVID-19 Research Network for health care organizations (HCOs) actively seeking to participate in coronavirus research was recently created. TriNetX fast-tracked data inflow to incorporate COVID-19–specific diagnosis and terminology following the World Health Organization and Centers for Disease Control and Prevention (CDC) COVID-19 criteria. As a federated network, TriNetX received a waiver from Western IRB because only aggregated counts and statistical summaries of deidentified information, but no protected health information, is received, and no study-specific activities are performed in retrospective analyses.
TriNetX cloud-based features allow real-time access to the deidentified longitudinal clinical data, along with the analytics to analyze research questions. The deidentified clinical data are aggregated directly from the electronic medical records of the participating HCOs continuously. Both the patients and HCOs as data sources stay anonymous. Participating HCOs include a mix of inpatient, outpatient, and specialty care services and provide care to a diverse patient population from different geographic regions.
Selection of Patients With Coronavirus Disease 2019
The search was conducted (April 12, 2020) following the criteria provided by TriNetX to identify potential patients with COVID-19 as per the CDC COVID-19 coding guidelines. These codes included International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-10-CM) codes U07.1 (COVID-19, virus identified), B34.2 (coronavirus infection, unspecified), B97.29 (other coronavirus as the cause of diseases classified elsewhere), and J12.81 (Pneumonia due to SARS-associated coronavirus). Patients identified with diagnosis code 079.89 (other specified viral infection) were excluded. Only patients diagnosed with the abovementioned codes after January 20, 2020, (the first confirmed case in the United States) were included. The B97.29 code was specifically included based on the recommendation from the general guidance of the ICD-10-CM Official Coding Guidelines released by the CDC on February 20, 2020. Similarly, U07.1 is the new specific code for a confirmed diagnosis of the COVID-19 with a positive COVID-19 test result starting April 1, 2020, as per the new CDC guidelines. The codes B34.2 and J12.81 were used more often before the CDC guidelines. Patients with ICD-9 code 079.89 (mapped to ICD-10 code B34.2 and B97.2) were excluded to reduce any patients with false positive COVID-19 diagnoses, because this ICD-9 code can still be used occasionally as catch-all code for more than 50 viral infections.
Selection of the Preexisting Liver Disease Patient Cohort
Queries for patients with liver disease were made using the ICD-10-CM codes alone or in combination: K70, alcoholic liver disease; K74, fibrosis and cirrhosis of liver; K83.2, primary sclerosing cholangitis; K76.89, other specified diseases of liver; K76.9, liver disease, unspecified; K72.9, hepatic failure, unspecified; K71, toxic liver disease; K75.81, nonalcoholic steatohepatitis; K76.0, fatty (change of) liver, not elsewhere classified; B18, chronic viral hepatitis; B19.1, unspecified viral hepatitis B; K73, chronic hepatitis, not elsewhere classified; K75.3, granulomatous hepatitis, not elsewhere classified; K75.4, autoimmune hepatitis; K75.89, other specified inflammatory liver diseases; K75.9, inflammatory liver disease, unspecified; K76.6, portal hypertension; K65.2, spontaneous bacterial peritonitis; K76.81, hepatopulmonary syndrome; K76.7, hepatorenal syndrome; I85.0, esophageal varices.
Diagnosis Associated With Preexisting Liver Disease
Based on the criteria as mentioned for liver disease, the following diagnoses with the total numbers of patients (%) identified were as follows: fatty liver disease or nonalcoholic steatohepatitis, 100 (42%); alcoholic liver disease, 20 (8%); chronic viral hepatitis B, 10 (4%); chronic viral hepatitis C, 40 (17%); inflammatory liver disease, unspecified, 40 (17%); primary sclerosing cholangitis, 10 (4%); primary biliary cirrhosis, 10 (4%); toxic liver disease, 20 (8%); liver disease, unspecified, 50 (21%); hepatic fibrosis, 20 (8%); chronic hepatic failure, 10 (4%); chronic hepatitis, not elsewhere classified, 10 (4%); granulomatous hepatitis, not elsewhere classified, 10 (4%); autoimmune hepatitis, 10 (4%); cirrhosis, 50 (20%); secondary biliary cirrhosis, 10 (4%); portal hypertension, 30 (13%); hepatorenal syndrome, 10 (4%); esophageal varices, 20 (8%); gastric varices, 10 (4%); ascites, 40 (17%); spontaneous bacterial peritonitis, 10 (4%); hepatocellular carcinoma, 10 (4%); portal vein thrombosis, 10 (4%).
Definition of Study Variables
The diagnosis of COVID-19 was defined as the index event. Baseline comorbidities were estimated based on diagnosis any time before or at the time of the index event. The timing of a patient’s presenting signs and symptoms was estimated from the index event to 14 days before the index event. Baseline laboratory values were the most recent findings from any time 14 days before the index event. The time window to estimate all outcomes was from the day of diagnosis of COVID-19 up to 30 days after that.
Statistical Analysis
All statistical analyses were performed in real time using TriNetX. TriNetX obfuscates patient counts to safeguard protected health information by rounding patient counts in analyses up to the nearest 10. Rounding may influence measures of association results for small cohorts and infrequent outcomes.
The means, standard deviations, and proportions of clinical facts were used to describe and compare patient characteristics. We performed 1:1 propensity score matching using a greedy nearest-neighbor matching algorithm with a caliper of 0.1 pooled standard deviations to account for confounding variables. For each outcome, the risk difference and risk ratio were calculated to compare the association of the liver disease with the outcome. An a priori defined 2-sided alpha value of <.05 was used for statistical significance.
Limitations of Methodology
The data derived from an electronic medical records–based database is susceptible to errors in coding or data entry when patient information is translated into ICD-10 code. However, the ability of TriNetX to aggregate the data directly from the EMRs in a real-time fashion minimizes the risk of data collection errors at the investigator’s end. Patients with mild disease who remained undiagnosed or were treated at home and did not present to any HCOs were missed; therefore, our cohort may represent the more severe spectrum of the disease. Data on the exposure history, incubation time, specific radiographic findings, and dynamic changes in patients’ clinical condition could not be estimated. The laboratory findings were not performed for all patients, and hence, their role might be undervalued. Patient counts were rounded up to the nearest 10 in our analysis to safeguard protected health information. Rounding may influence measures of association results for small cohorts and infrequent outcomes.
References
- 1.Zhang C. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wang D. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai X. bioRχiv. https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1 Published February 4, 2020.
- 5.Xu L. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L. Respir Res. 2018;19:242. doi: 10.1186/s12931-018-0934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albillos A. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Karcz M. Acute respiratory failure complicating advanced liver disease. Semin Respir Crit Care Med. 2012;33:96–110. doi: 10.1055/s-0032-1301738. [DOI] [PubMed] [Google Scholar]