Abstract
The apparent predicament of the representative chemotherapy for managing respiratory distress calls for an obligatory deliberation for identifying the pharmaceuticals that effectively counter the contemporary intricacies associated with target disease. Multiple, complex regulatory pathways manifest chronic pulmonary disorders, which require chemotherapeutics that produce composite inhibitory effect. The cost effective natural product based molecules hold a high fervor to meet the prospects posed by current respiratory-distress therapy by sparing the tedious drug design and development archetypes, present a robust standing for the possible replacement of the fading practice of poly-pharmacology, and ensure the subversion of a potential disease relapse. This study summarizes the experimental evidences on natural products moieties and their components that illustrates therapeutic efficacy on respiratory disorders.
Keywords: COPD, Natural products, Respiratory disorders, Alkaloids, Flavonoids
Highlights
-
•
Plant derived therapeutics for managing chronic respiratory disorders.
-
•
Activity of natural product based molecules on key regulatory pathways of COPD.
-
•
Preclinical evidence for the efficacy of natural product moieties.
-
•
Clinical significance of plant derived molecules in pulmonary distress.
1. Introduction
The chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD), pulmonary sarcoidosis, asthma, pneumoconiosis, and lung cancer pose major healthcare and economic strain across the globe. As per the annual report by World Health Organization (WHO) on ‘The Global Impact of Respiratory Disease’, COPD alone claims a global morbidity of 65 million, including 3 million annual mortalities. The figures for asthma further raise the alarm with 334 million people suffering from the disease, which includes 14% of children [1]. Notably, pneumonia; caused by bacteria streptococcus pneumonia raises the mortality rate among children below 5 years of age. Tuberculosis, a chronic respiratory ailment caused by bacteria mycobacterium tuberculosis affects 10 million people annually, out of which 1.4 million lose lives. In addition, lung cancer represents a fatal, yet most common neoplasm claiming roughly 2 million lives annually [2].
The pathophysiology of COPD manifests chronic inflammation in the lung parenchyma mediated by macrophages, neutrophils, and cytotoxic (CD8+) T lymphocytes. The subsequent fibrosis results in narrowing of small airways and obliteration of parenchyma by proteases. Reportedly, the development of COPD focused precision medicine faces considerable challenges due to dearth of animal models for preliminary drug testing, and due to a lack of information about the surrogate markers for monitoring the efficacy of rationally designed drugs [3]. Bronchial asthma, an inflammatory condition develops due to the abnormal activity of enzymes and prostanoids [4], coupled with oxidative stress in the airways resulting in hypertrophy and hyperplasia of bronchial smooth muscles, hyper-responsiveness and hypersecretion of mucins in the airway passages [5]. Besides the anti-asthma drugs targeting cysteinyl leukotrienes, immunoglobulin E, anticholinergics, and β-AR agonists, the contemporary chemotherapy development efforts against asthma could not yield clinically efficacious results in the last 3 decades [6]. The emergence of multi-drug resistance microbial strains tainted the drug development efforts directed at pneumonia and tuberculosis [7], which are emerging as leading cause of excessive and unregulated antibiotic consumption [8]. Similarly, adenocarcinoma presents the recurring and most prevalent cancer type among the various lung cancer forms [9].
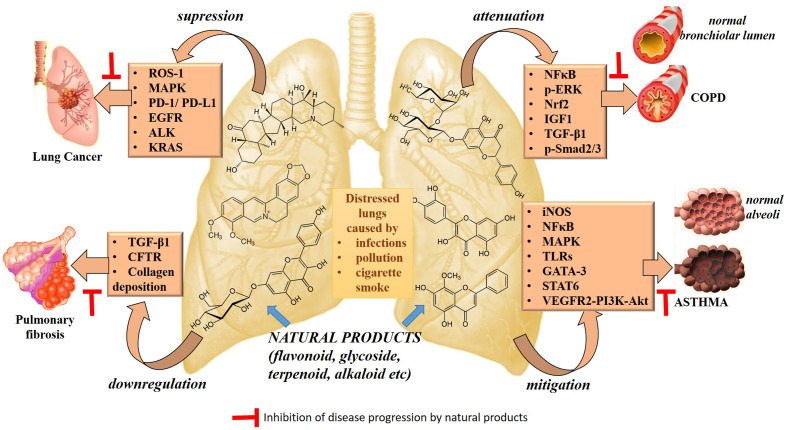
Various cell signaling pathway are involved in inflammatory and oxidative response, remodeling of extracellular matrix leading to asthma, COPD and pulmonary fibrosis whereas cell migration and proliferation pathway leading to lung cancer progression [[10], [11], [12], [13]]. In asthma and COPD, oxidative stress leads to inflammation in airway by through redox sensitive transcription factor, nuclear factor (NF)-kappaB (NF-kB) pathway [14]. The activation NF-κB in cytoplasm and subsequent translocation to nucleus is induced by inflammatory cytokines such as interleukin (IL)-1β and tumour necrosis factor (TNF)-α whereas via activation of toll like receptors (TLRs) during pathogenic infections (bacterial or viral) [15]. Similarly, increase in transforming growth factor (TGF)-b by airway epithelial cells and inflammatory cells are involved in the pathogenesis of pulmonary fibrosis. High level of TGF-b results in activation, migration, and proliferation of resident fibroblasts. These fibroblasts can differentiation into activated myofibroblasts promoting abundant extracellular matrix (ECM) deposition and abnormal collagen build-up [16]. Likewise, activation of pathway such as epidermal growth factor receptor-tyrosine kinase, anaplastic lymphoma kinase (ALK), c-ros oncogene-1 (ROS1), programmed cell-death-1/program cell death ligand −1 (PD-1/PD-L1), mitogen activated protein kinases (MAPK), phosphoinositide 3-kinases (PI3Ks) are involved in migration and proliferation pathway leading to lung cancer progression [[17], [18], [19], [20], [21]].
Various natural compound are able to target the cell-signaling pathway showing beneficial activity against respiratory disease (Fig. 1 ). The natural products containing alkaloids, flavonoids and terpenes serve as storehouse of essential chemotherapeutics [[22], [23], [24]], which produce desirable effects against chronic respiratory ailments (Table 1 ). These also prompt the development of novel drug systems by providing suitable pharmacophores for producing optimum effect against the target pathways associated with the manifestation of respiratory disorders [25]. This review presents a succinct discussion on the potential of natural product derived drugs based on alkaloids, flavones and terpenes for capping the conventional and emerging respiratory disorders.
Fig. 1.
Natural products targeting different cell signaling pathway.
Table 1.
Natural products showing effect in respiratory disorders.
| S.No. | Natural product | Nature | Chemical structure | Pharmacological/Mechanism of action | Ref. |
|---|---|---|---|---|---|
| 1. | Apigenin | Flavonoid |  |
|
[26] |
| 2. | Asperuloside | Iridoid glycoside | 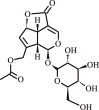 |
|
[27] |
| 3. | Eugenol | Terpenoid | 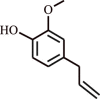 |
|
[28] |
| 4. | Curcumin | Alkaloid | 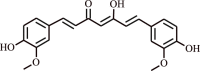 |
|
[29] |
| 5. | Berberine | Alkaloid | 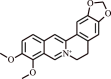 |
|
[30] |
| 6. | Naringin | Flavanone glycoside | 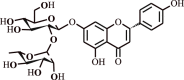 |
|
[31] |
| 7. | Naringenin | Flavonoid | 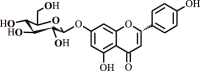 |
|
[32] |
| 8. | Luteolin | Flavone | 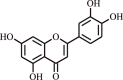 |
|
[33] |
| 9. | Quercetin | Flavonoid | 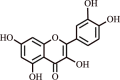 |
|
[34] |
| 10. | Kaempferol glycoside | Flavonoid | 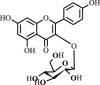 |
|
[35] |
| 11. | Epicatechin | Flavonol | 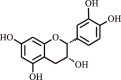 |
|
[36] |
| 12. | Picroside II | Iridoid glycoside | 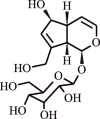 |
|
[37] |
| 13. | Lactiflorin | Monoterpene glycoside | 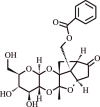 |
|
[38] |
| 14. | Resveratrol | Polyphenolic compound | 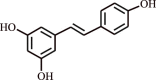 |
|
[39] |
1.1. Alkaloids
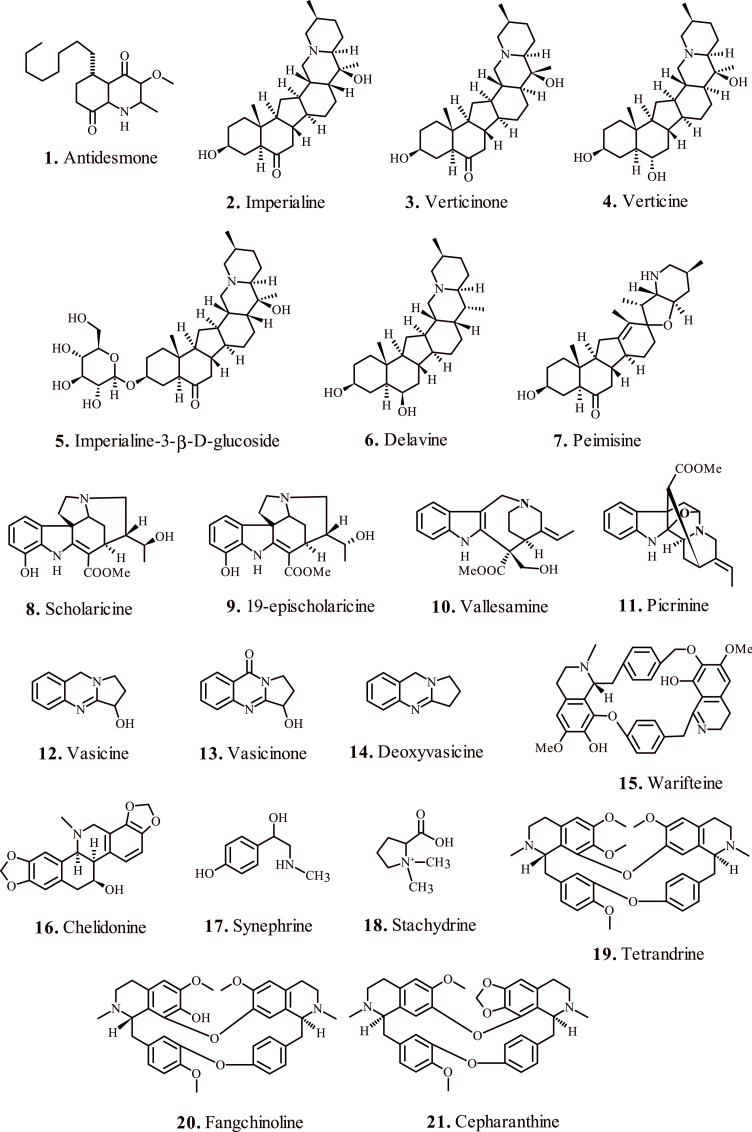
Lu et al., 2007; reported the medicinal properties of tetrahydroquinoline alkaloid ‘Antidesmone’ (1, Fig. 2 ) for the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), which represent prolonged inflammatory disorders instigated by membrane lipopolysaccharide (LPS) present on gram-negative bacteria, characterized by lung parenchymal injury and interstitial edema [26]. The microbial LPS instigate neutrophil infiltration, and trigger the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), mitogen-activated protein kinase (MAPK) [27], cyclooxygenase-2 (COX-2) [28], interleukin-1β (IL-1β) [29], inducible nitric oxide synthase (iNOS) [30], nuclear factor-kappa B (NF-κβ) [31] and interleukin-6 (IL-6) [32] at the target site, thereby inducing acute lung injury.
Fig. 2.
Alkaloid based medicinal compounds for the treatment of respiratory disorders.
Exposure with antidesmone significantly down-regulated MAPK and TNF-α signaling pathway and apparently lowered the expression of NF-κβ by offsetting the nuclear translocation of REL-associated protein p65, responsible for its activation [33]. Interestingly, the antidesmone exposure demonstrated significant inhibition of pulmonary myeloperoxidase (MPO), the biomarker for accumulation of neutrophils in the morbid lungs [34], further validating the therapeutic privilege of the alkaloid.
Reportedly, cigarette smoke (CS) potentiates the redox imbalance by activating lung epithelial cells and macrophages, thereby resulting in chronic respiratory ailments [35]. The heightened oxidative stress caused by CS dissociates transcription factor Nrf2 from kelch-like ECH-associated protein 1 (Keap1) [36], eventually translocating into nucleus followed by binding to antioxidant response elements (ARE). These events regulate downstream gene expression for phase II detoxification enzymes such as glutamate cysteine ligase (GCL), glutathione S-transferase (GST), and heme oxygenase 1 (HO-1) [37], that promote in maintaining cellular redox balance [38]. Therefore, Nrf2 signaling pathways present potential therapeutic target against the physiological redox imbalance [39].
Liu et al., 2020; investigated the therapeutic potential of six isosteroid alkaloids (2–7, Fig. 2) obtained from F. cirrhosa bulbus against the CS induced oxidative stress in RAW264.7 macrophages. Reportedly, the identified six test alkaloids significantly attenuated the production of reactive oxygen species (ROS), upregulated the level of antioxidant molecule glutathione (GSH), and promoted the Nrf2 induced expression of HO-1 protein. Notably, the presence of glucoside moiety at C-3 position in alkaloid 5, and absence of β-OH at C-17 position in alkaloid 6 resulted in higher GSH/GSSG ratio. Similarly, the presence of β-CH3 substituent at C-20 position in alkaloid 3 favored its efficacy and tolerable cytotoxicity. The presence of –OH substitution at C-3 position in all the test alkaloids demonstrated a paramount importance for inducing HO-1 expression, which diminished in the presence of C O group at the same position [40].
Zhao et al., 2017; investigated the curative effects and pharmacokinetics of alkaloids 8–11, Fig. 2, obtained from A. scholaris on ovalbumin induced airways allergic inflammatory model [41]. Exposure to the test alkaloids lowered the levels of leukocytes and eosinophils, further confirmed by histopathological analysis of lungs. Notably, the test alkaloids downregulated the secretion of proinflammatory cytokine IL-4, a key mediator of allergic responses eventually resulting in a significant reduction of pulmonary eosinophils, and balancing the rise of immunoglobulin E (IgE) in serum.
Importantly, the animal models administered with the test alkaloids 3 times a day demonstrated lower levels of eotaxin in the serum. Eotaxin overexpressed in the serum and airways of asthmatics serves as a biomarker for the pathogenesis of chronic asthma [42]. Therefore, its elimination from the systemic circulation in the morbid groups further validates the anti-asthma efficacy of test alkaloids. The administration of total alkaloids produced marked effect as compared to the single test alkaloid, hence confirming a synergistic effect. Moreover, these alkaloids proved useful for the treatment of post-infectious symptoms in animal models by mitigating the levels of inflammatory cytokines followed by down-regulation of the expression of IL-6. Histopathological examination of the morbid lungs further confirmed these observations [43]. Liu et al., 2015; isolated quinazoline alkaloids 12–14, Fig. 2, from the aerial parts of Peganum harmala L [44], and tested their antitussive, expectorant, and bronchodilating effects in animal models.
The alkaloids effectively lowered the symptoms associated with capsaicin induced acute pulmonary inflammation at doses 5, 15, and 45 mg/kg, compared to codeine phosphate administered at 30 mg/kg. The test alkaloids appreciably promoted expectorant activity as indicated by phenol red secretion in mice trachea, compared to the standard drug ammonium chloride. Further, the bronchodilating test verified that the test alkaloids considerably prolonged the pre-convulsive times in animal models, superior to the standard drug aminophylline, thereby validating the potency of these alkaloids for treating bronchial asthma.
Alkaloids from Cissampelos sympodialis ‘warifteine’ (15, Fig. 2) display notable efficacy in airway hyperreactivity in animal model of asthma [45]. Oral pretreatment of animal models with the test alkaloid warifteine significantly reduced the allergen induced airway hyperreactivity (AHR) to inhaled methacholine. Moreover, it also reduced the IL-13 levels in bronchoalveolar lavage, which serves as the key regulator of AHR [46]. The test alkaloid checked the ovalbumin (OVA)-induced eosinophil intrusion in the tissues, and downregulated the mucus production and subepithelial fibrosis. Further analysis of airway mucins by periodic acid-Schiff (PAS) staining revealed OVA induced metaplasia and mucus accumulation in animal models, which reduced significantly after warifteine administration.
Kim et al., 2015; reported attenuation of IL-4 and eotaxin-2 mediated eosinophilic airway inflammation in asthmatic animal models by the alkaloid ‘chelidonine’ (16, Fig. 2) isolated from Chelidonium majus [47]. The test alkaloid significantly suppressed the level of eosinophils in the airways, in addition to downregulation of eotaxin-2, interleukins, and cytokines in the bronchoalveolar lavage fluid. Notably, chelidonine treated animal models exhibited considerable decrease of CD4+ and CD8+ T cells, Gr-1+/CD11b+ and 351 CD3/CCR3+ positive cells, which otherwise intensify inflammation process by secreting Th2 cytokines and degranulation of eosinophils [48]. Histological analysis suggested marked attenuation of OVA-induced eosinophil subversion, goblet cell hyperplasia, and accumulation of collagen in ling tissues in the presence of chelidonine. These findings validated the therapeutic potency of this alkaloid in the treatment of pathologic inflammatory disorders associated with respiratory airways.
Alkaloids 17 and 18 (Fig. 2) obtained from Pericarpium Citri Reticulatae reportedly exhibit appreciable anti-asthmatic activity. The administration of alkaloids in animal models with histamine induced asthma, downregulated eosinophils expression in bronchoalveolar lavage fluid and serum along with notable attenuation of IgE, IL-4 and IL-5. The alkaloid demonstrated spasmolytic effects on acetylcholine chloride-induced contractions in the animal trachea [49]. Kim et al., 2019; identified natural bis-benzylisoquinoline alkaloids 19, 20 and 21 (Fig. 2) form stephania tetrandra, which inhibited human coronavirus OC43 infection of MRC-5 human lung cells at its early stages.
Coronaviruses infect the respiratory tract thereby manifesting severe conditions such as bronchiolitis, and pneumonia [50]. The test alkaloids reportedly restricted the replication of human coronavirus OC43, restrained the expression of viral protein and virus-induced response of the host MRC-5 cells. Notably, the alkaloid 19 (Fig. 2) activated the p38 Mitogen-Activated Protein Kinase (MAPK) pathway in the virus infected cells, which eventually improved their viability with minimal signs of cytotoxicity. The MRC-5 cells exposed to the test alkaloids showed negligible expression of proinflammatory cytokines, which are otherwise upregulated by the virus infection [51].
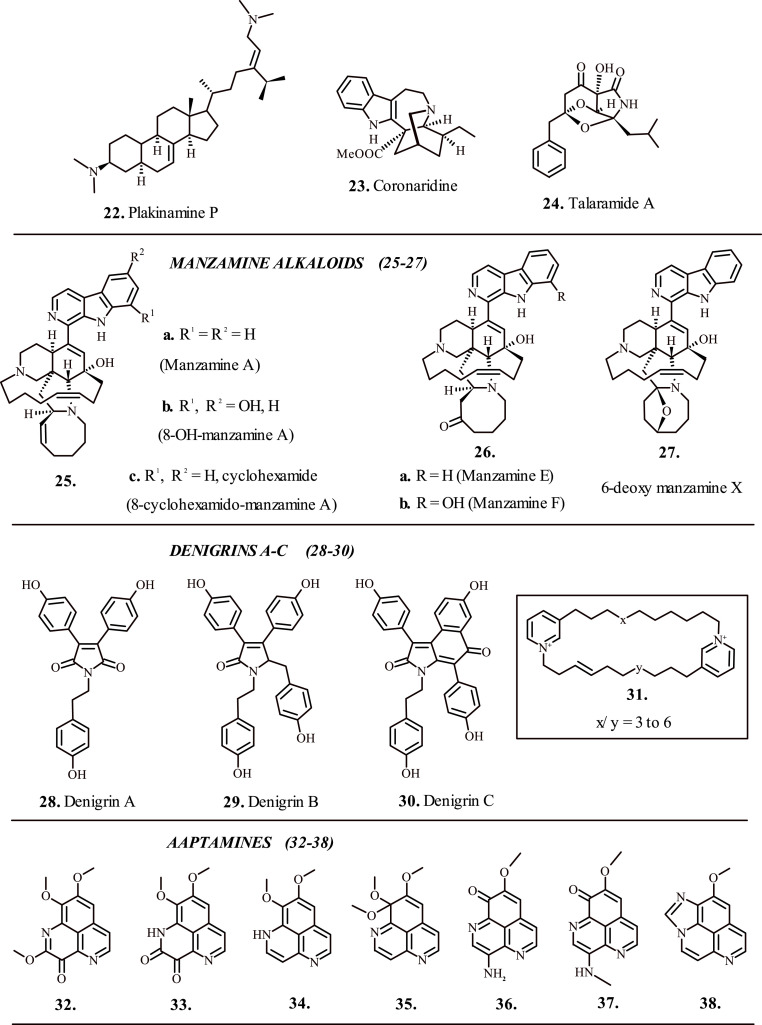
Deep-water sponges of the genus Pakina yield therapeutic alkaloid 22 (Fig. 3 ), which displayed considerable bactericidal efficacy against Mycobacterium tuberculosis strain CDC1551. The test alkaloid Plakinamine P reportedly inhibited actively growing bacterial strain by 98%, and dormant non-replicating bacteria by 67%. The test alkaloid, being structurally similar to cholesterol and bearing undegradable side chains allegedly inhibits the cholesterol catabolism pathway in the host microbe, which is essential for its survival and for the persistence of ensuing infection. In addition, the metabolic breakdown of test alkaloid by cholesterol degradation pathway yields toxic products fatal to the host microbe [52].
Fig. 3.
Alkaloid based medicinal compounds for the treatment of tuberculosis.
Coronaridine alkaloid 23 (Fig. 3) isolated from Tabernaemontana ternifolia displayed marked antibacterial potency against Mycobacterium tuberculosis strain H37Rv with IC50 = 82.64 μg/ml. The activity, however was lower than the commercial drug rifampicin (IC50 = 0.05 μg/ml) [53]. Chen et al., 2017; isolated the alkaloid ‘Talaramide A’ (24, Fig. 3) from Talaromyces sp. (strain HZ-YX1) and investigated its antimycobacterial potency by targeting mycobacterial protein kinase G (PknG), which plays a key role in the persistent localization of mycobacteria in macrophages. The alkaloid displayed significant inhibition of PknG with IC50 = 55 μM, thereby validating its potential for the treatment of chronic tuberculosis [54].
Besides several contemporary targets for capping tuberculosis, shikimate pathway represents the most recently identified means for the survival of mycobacterium. MtSK, one of the key enzymes associated with the shikimate pathway mediates the phosphorylation of shikimate substrate by abstraction of phosphate group from adenosine triphosphate (ATP) thereby producing shikimate-3-phosphate and adenosine diphosphate products. Manzamine alkaloids 25, 26 and 27 (Fig. 3) isolated form Indo-Pacific sponge, Acanthostrongylophora sp. present mixed noncompetitive inhibition of MtSK enzyme. The kinetic profiling suggested that the inhibition of target enzyme occurred via two-step mechanism where the isomerization of preliminary EI complex results in highly unstable EI* intermediate. Notably, the alkaloid 25c (Fig. 3) due to 6-cyclohexamide substitution displayed superior potency compared to the peers with inhibition constant Ki = 0.06 μM [55]. Further research on marine sponge Dendrilla nigra for the identification of MtSK inhibitors led to the discovery of 3,4-diarylpyrrole alkaloids: Denigrin A, B and C (28, 29 and 30; Fig. 3) [56].
Maarisit et al., 2017; identified the cyclic 3-alkyl pyridinium dimer alkaloids 31 (Fig. 3) with antimycobacterial properties from the marine sponge Haliclona sp. [56]. Also referred to as cyclostellettamines when isolated from sponge Pachychalina sp., the test alkaloids with longer alkyl chains connecting the pyridinium moieties exhibited excellent activity against Mycobacterium Tuberculosis strain H37Rv [57]. The precise mechanism of action for the test alkaloids 31 (Fig. 3) is still under investigation. Besides the active mycobacterium, the non-replicating persistent Mycobacterium tuberculosis pathogen requires uninterrupted treatment for several months to prevent the relapse of disease [58].
The dormant mycobacterium requires hypoxic conditions for survival and displays considerable resistance against the representative drugs [59]. The marine sponge Aaptos sp. served as an excellent source for the isolation of aaptamine alkaloids 32 to 38 (Fig. 3). Oxygen depletion instigated dormancy response of the mycobacterium in the form of resistance towards the antibiotic isoniazid served as the model for evaluating the anti-dormant mycobacterial efficacy of the test alkaloids. The test alkaloids displayed highly effective anti-dormant mycobacterial activity with IC50 in the range 1.5–6.25 μg/ml. Further investigations suggested that the presence of carbonyl groups at C-3 and/or C-9 position of the candidate alkaloids played a critical role in the expression of bioactivity against non-replicating dormant Mycobacterium tuberculosis [60]. These alkaloids formed the basis of identification of suitable pharmacophores for rationally designing the pharmaceuticals against dormant mycobacterium.
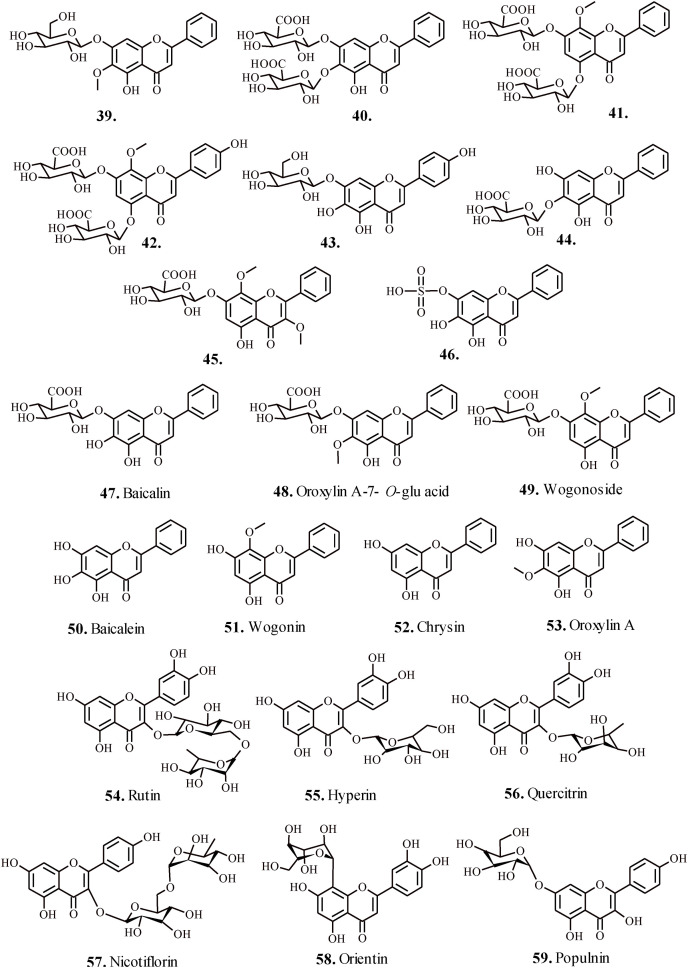
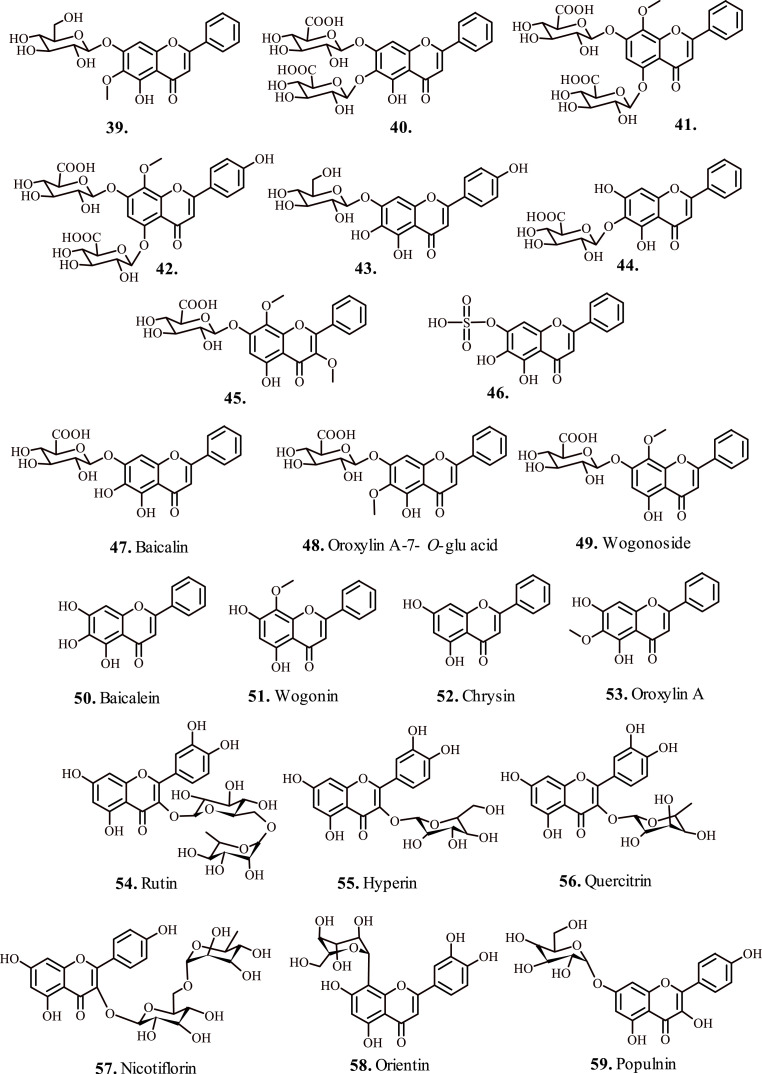
2. Flavonoids
Zhi et al., 2020; investigated the efficacy of flavonoids 39–46 (Fig. 4 ) isolated form Scutellaria baicalensis against influenza A virus (IAV) induced acute lung injury (ALI). The inactive form of test flavonoids metabolized in vivo to anti-complimentary active metabolite aglycones 47–53 (Fig. 4) by the enzyme β-glucuronidase secreted by the epithelial cells, which demonstrated superior absorption into IAV-induced ALI animal models [61]. The infected animal models demonstrated a better metabolic transformation of the test flavonoids to active metabolites, with elevated levels in lungs and intestine, with higher content in the latter suggesting that the test flavonoids reach intestine first and then transformed to active metabolites before finally becoming a part of systemic circulation. The active metabolites 47–53 after reaching the lungs reportedly downregulate the levels of TNF-α, IL-6, monocyte chemotactic protein (MCP-1), while raising the levels of IL-10 and interferon-γ (IFN-γ), and considerably lowered the production of nitric oxide (NO) from lipopolysaccharide (LPS)-stimulated RAW264.7 cells thereby attenuating the activity of target virus [62].
Fig. 4.
Flavonoids for the treatment of respiratory disorders (a).
Flavonoids 54–56 (Fig. 4) extracted from Houttuynia cordata appreciably mitigated acute lung injury caused by H1N1 virus [63]. The animal models exposed to test flavonoids maintained the morphology of diseased lung microstructures, attenuated the penetration of proinflammatory factors MCP-1, IL-8, TNF-α, and malondialdehyde that play crucial role in the recruitment of macrophages and neutrophils at the site of stimulus [64]. However, the levels of interferon-β elevated, which played a key role in restricting the replication of virus [65]. In addition, the expression of TLR3/4/7 and level of NF-κ β p65 phosphorylation lowered in the lung tissues, which are associated with heightened activation and secretion of interferon-β [66]. Hence, the test flavonoids directed anti-inflammatory therapy served deliberated approach for countering IAV induced pathological processed manifesting acute lung injury. Reportedly, the flavonoids 54 and 56 (Fig. 4) displayed enhanced biocidal potential against Mycobacterium tuberculosis H37Rv strain with IC50 = 6.25 and 25 μg/ml respectively [67]. The biocidal potential of the test flavonoids proved better than the standard anti-TB drugs isoniazid and rifampicin.
Besides numerous efforts entailing anti-TB drug development, the emergence of drug-resistant mycobacterial strains necessitated the identification of novel drug targets by highly efficacious pharmacophores. MtPKnG, a protein kinase G produced by Mycobacterium tuberculosis represents serine/threonine kinase enzyme, which prolongs the survival rate of the bacterium in host macrophages by preventing the phagosome-lysosome fusion [68]. The enzyme mediates phosphorylation of proteins associated with essential signal transduction pathways in bacteria and allegedly sustains the tuberculosis infection in the host [69]. Notably, the MtPKnG enzyme prompts anti-TB drug resistance in mycobacteria and serves as a key component for mycobacterial biofilms production. Hence, it forms a desirable target for anti-TB drug discovery against resistant mycobacteria.
Qasaymeh et al., 2019; reported bioactive flavonoids 57–59 (Fig. 4) isolated from Pelargonium sp. with excellent binding affinity towards MtPKnG [70]. The in silico docking analysis confirmed the interactions of flavonoid 57 (Fig. 4) with target enzyme MtPknG via H-bonding (<2.5 Å) with essential residues E233, E280, S239 and Q238. Similarly, the –OH groups present on B-ring of the test flavonoid 58 (Fig. 4) showed H-bonding interaction with the residues K181, D293, and its flavone moiety interacted with Ala158 and Ile157 residues of the target enzyme. Nevertheless, the test flavonoid 59 (Fig. 4) exhibited significant H-bonding interactions with residues K181 (2.498 Å), D293 (2.213 Å) and Q238 (2.278 Å) and hydrophobic interactions with the active site residues A158, I157, I165, I292 and M283 of the target MtPknG enzyme. These naturally occurring flavonoids hold tremendous potential for rationally designing pharmaceuticals and chemical therapeutics, essentially for targeting multidrug-resistant TB.
The drug resistance in microbes arises due to over-activity of the membrane bound efflux pumps that prevent the drug internalization in cells. The lack of clinically approved efflux pump inhibitors further aggravate the disease pathogenesis. Polymethoxylated flavonoids 60–64 (Fig. 5 ) reportedly reduced the rifampicin resistance and adversely affected the survival of Mycobacterium tuberculosis. Importantly, the bioactivity of anti-TB drug isoniazid enhanced in the presence of test flavonoids [71]. However, further investigations could not quantify the insight mechanism for the behavior of test flavonoids towards mycobacterium efflux pumps. Reportedly, the flavonoid 60 (Fig. 5) considerably attenuated AHR, and reduced the expression of airway eosinophils and Th2 cytokines, whereas it amplified the levels of transforming growth factor-β1 (TGF-β1) in the bronchoalveolarlavage (BAL) fluids in OVA-sensitized animal models.
Fig. 5.
Flavonoids for the treatment of respiratory disorders (b).
The administration of flavonoid 60 (Fig. 5) also mitigated the collagen deposition in sub-epithelium, and suppressed goblet cell hyperplasia. Hence, the test flavonoid served as bradykinin antagonist and diminished the principal pathophysiological characteristics associated with allergic asthma [72]. Jeon et al., 2017; isolated flavonoid 65 (Fig. 5), which exerted bactericidal effect on drug-resistant mycobacterium tuberculosis H37Rv strain. The test flavonoid reportedly inhibited the release of proinflammatory interleukins and attenuated the expression of TNF-α. The test flavonoid 65 (Fig. 5) exhibits high affinity binding to M. tuberculosis β-ketoacyl acyl carrier protein synthase III (mtKASIII) enzyme via interactions of A-ring 2-OH and B-ring 4-OH groups of the flavonoid with N261 and C122 residues [73]. Importantly the open chain structure of phloretin permits membrane permeability against the mycobacterial cell wall, which protects the microbe from antibiotics and allows its persistence and proliferation in macrophages.
The flavonoids 66 and 67 (Fig. 5) isolated from Mosla chinensis Maxim exerted inhibitory potential against H1N1 influenza virus mainly by downregulating the expression of the host TLR signaling pathways [74]. In addition, the upregulation of critical factors (IL-6, TNF-α, IFNγ, and NO) and suppression of IL-2, SOD and cytokine GSH caused significant reduction of virus instigated inflammatory damage to lung tissues. Pawar et al., 2020, investigated the anti-tubercular activity of flavonoids 68 and 69 (Fig. 5) by targeting glutamate racemase enzyme of the microbe associated with the synthesis of membrane peptidoglycans by transforming l-glutamate to d-glutamate.
The enzyme also plays a key role in DNA gyrase sequestration. The test flavonoids introduced deformations in the secondary and tertiary structure of the target enzyme by attenuating the helical contents, which manifested morphological changes in the microbial membrane. The interaction of test flavonoids with active site residues D12, C75, C185, and H187; present at the substrate binding site of glutamate racemase enzyme prompted its inhibition [75]. The flavonoids 70, 71 and 72 (Fig. 5) proved highly efficacious for capping severe acute respiratory syndrome (SARS) caused by coronaviruses by inhibition of 3C-like proteases (3CLpro), responsible for autocleavage of viral polyproteins essential for viral propagation. The test flavonoids significantly blocked the enzyme activity by interacting effectively with the substrate-binding site via hydrophobic aromatic rings and hydrophilic –OH groups [76].
Mulberry root bark served as principal component for the isolation of prenylated flavonoid class ‘sanggenols’ 73–77 (Fig. 5) bearing dual activity against influenza virus and Streptococcus pneumoniae. The prenylated flavonoids displayed high physiological tolerance while having an inhibitory potential superior to the standard drug olestamivir against the target neuraminidase (NA) enzyme, which sustains lethal synergy between the influenza virus and S. pneumoniae assisting the sustenance and longevity of the both [77].
In virus, the NA enzyme promotes reproduction and spread, whereas in the bacterium, NA enzyme facilitates the cleavage of sialic acid from glycoproteins present on the cell surface thereby affording receptors for attaching of microbe hence providing the host cell nutrients for further growth and colonization [78]. Hence, it makes a desirable target for attenuating the pathogenesis caused by Influenza virus and S. pneumoniae. These events allow the release of virus from infected cells and provides defense against representative drugs. The prenylated flavonoids effectively break this synergism thereby preventing the bacterial coinfection in the subjects with influenza [79].
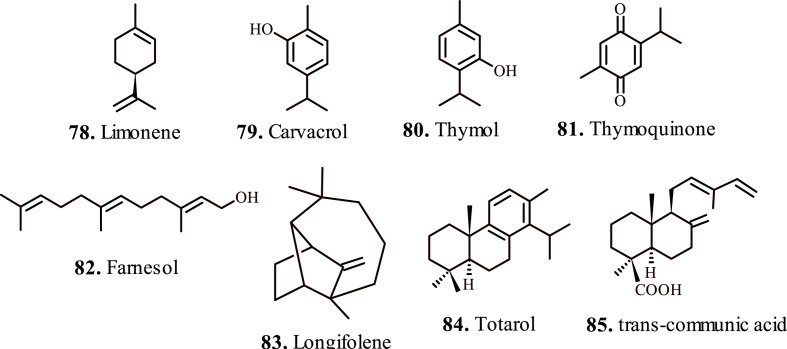
3. Terpenes
Hirota et al., 2012, evaluated the potential of monoterpene limonene (78, Fig. 6 ) in treating airway inflammation. Reportedly, limonene inhibits attenuates the allergic airway inflammation in Dermatophagoides farina-treated animal models, mainly by mitigating reactive oxygen species. The exposure to limonene significantly reduced the levels of interleukins-3/5, eotaxin, MCP-1, TGF-β in the bronchoalveolar lavages. In addition, the limonene administration abrogated gblet cell metaplasia, airway fibrosis, and thickness of the airway smooth muscles. These finding validated the candidature of limonene as prophylactic agent in asthma treatment [80].
Fig. 6.
Important terpenes for the treatment of respiratory disorders (a).
Alavinezhad et al., 2017; investigated the therapeutic effect of carvacrol (79, Fig. 6) on asthma subjects. The carvacrol exposure induced relaxant effect on the tracheal smooth muscles by reducing the total WBC, neutrophil, monocyte and eosinophil count in the blood and bronchoalveolar lavage fluid of the sensitized animal models. The relaxant effect of carvacrol occurs due to the significant attenuation of pro-inflammatory biometabolites in systemic circulation [81]. Boskabady et al., 2013; validated the preventive effect of carvacrol on tracheal responsiveness in ovalbumin treated animal models. Interestingly, the treatment of animal models with carvacrol considerably lowered the levels of nitric oxide in serum, produced by endothelial nitric oxide synthase, which reportedly triggers plasma extravasation and lung edema [82]. Similarly, the higher levels of iNOS-derived nitric oxide raises vascular permeability, instigates mucus hypersecretion, and worsenes the inflammatory cell permeation, and epithelial cell damage that contribute to asthma pathology [83].
Wan et al., 2017; investigated the therapeutic effects of thymol (80, Fig. 6) in animal models with LPS-induced acute lung injury. The LPS-challenged animals showed improved pathological changes in lung tissues on thymol exposure. Reportedly, the LPS-induced influx of the inflammatory cells and metabolites, interlukins and TNF-α attenuated in bronchoalveolar lavage fluid on thymol administration. In addition, thymol also restrained the LPS-mediated upsurge of MDO and MPS levels, and considerably lowered the activity of SOD thereby pausing the activation of NF-κB in the lungs [84]. These neutrophil localized enzymes primarily influence the adhesion and margination of neutrophils in the lung thereby causing severe lung injury.
Mohammadi et al., 2018; evaluated immunomodulatory effects of thymol in mitigating oxidative stress associated with asthma immunopathogenesis. Thymol-exposure attenuated the levels of 8-OHdG, an oxidative marker in asthma and carbonyl protein released by peroxidases from eosinophils in the airway passages, which elevates musine secretion, overexpresses cytokines and elevates apoptosis of epithelial cells lining airway passages. Reportedly, thymol affords considerable cytoprotective effect against the oxidative stress and moderately reinstates the defective trace element levels in asthma [85].
Gabri et al., 2019; studied the ameliorative effects of thymoquinone (81, Fig. 6) on the LPS-induced pulmonary vascular damage. The protective effect appears because of the downregulation of proinflammatory cytokines and interleukins in the presence of thymoquinone. In addition, thymoquinone administration attenuated the expression of NF-κB, TNFα, and IL-1β in the respiratory airways produced due to the LPS sensitization [86]. Thymoquinone also protects against lung damage caused by cigarette smoke by mitigating the expression of proinflammatory leukotrienes, prostaglandins, thromboxanes and importantly IL-1β, the principal biomarker cytokine in cigarette smoker's lung [87]. Su et al., 2016; evaluated the protective effects of thymoquinone in asthma animal models. Thymoquinone reportedly attenuated the expression of IL-4/5 and improved the expression of platelet endothelial cell adhesion molecule1 (CD31) and α-smooth muscle actinalpha (α-SMA) in ovalbumin-sensitized animals. In addition, thymoquinone deactivated VEGFR2-PI3K-Akt pathway and upregulated the expression of Slit glycoprotein-2 (Slit-2), which validates its anti-neoangiogenesis effect in asthma amelioration [88].
Qamar et al., 2008; investigated farnesol (82, Fig. 6) for the effective amelioration of lung injury caused by cigarette smoke, known to cause pulmonary emphysema, COPD, and pulmonary fibrosis. The isoprenoids such as farnesol possesses excellent anti-nociceptive and chemopreventive potency and demonstrates protection against chronic lung inflammation, oxidative stress and lung injury caused by cigarette smoke intoxicants. The prophylactic treatment with farnesol showed lung-protective symptoms by lowering LDH levels, and lowered activity of reduced glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx) and catalase enzymes. The lowered H2O2 content in lung tissues further validated the cytoprotective effects of lung tissues against cigarette smoke [89]. Farnesol proved efficacious in the alleviation of benzopyrene-induced respiratory stress in animal models. Farnesol also sustained optimal levels of phospholipids and altered the catalytic activity of benzopyrene enzymes NADPH–cytochrome P450 reductase, glutathione S-transferase (GST) and microsomal epoxide hydrolase (mEH) in the lung tissues of animal models. These findings suggested the protective role of farnesol against benzopyrene induced lung inflammation, edema, and epithelial damages in subject animals [90].
Gordien et al., 2009; reported the antimycobacterial activity of terpenes 83, 84 and 85 (Fig. 6) isolated from Juniperus communis L. Reportedly, the terpene 84 (Fig. 6) displayed enhanced activity against H37v strain of Mycobacterium tuberculosis with IC50 = 73.7 μM, and against the isoniazid-, streptomycin- and moxifloxacin-resistant strains with IC50 in the range 38.4–83.4 μM. The terpenes 83, and 84 (Fig. 6) demonstrated excellent activity against rifampicin-resistant variants with IC50 = 24 μM and 20.2 μM respectively. The terpene 85 (Fig. 6) displayed noticeable inhibitory activity against Mycobacterium aurum with IC50 = 13.2 μM; however with a low selectivity index which indicated physiological toxicity [91].
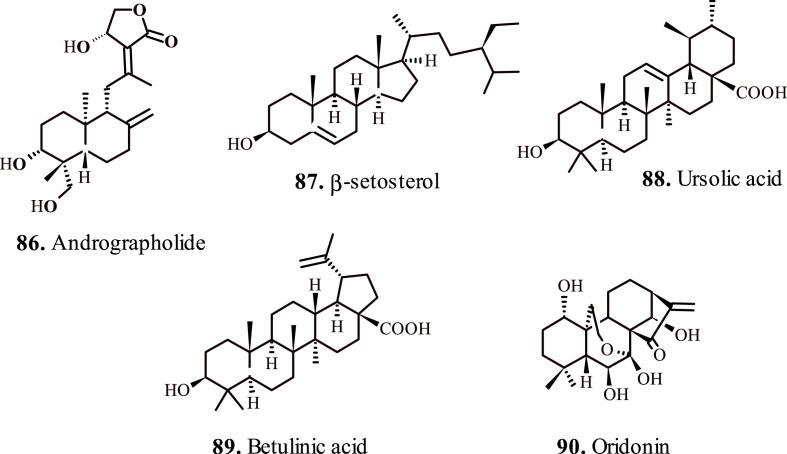
Andrographolide (86, Fig. 7 ) reportedly ameliorates LPS-induced acute lung injury by an effective downregulation of MAPK and NF-κB pathways, which regulate the production of inflammatory cytokines such as TNF-α and IL-6 in bronchoalveolar lavage fluid [92]. The anti-oxidative potency of andrographolide effectively restores the steroid sensitivity for blocking LPS-induced production of IL-27 and airway hyper-responsiveness. Moreover, andrographolide considerably restored the levels of nuclear HDAC2 protein and their total activity, whereas it contracted the total activity of histone acetyltransferase/HDAC in the animal lungs exposed to LPS/IFN-γ. These events occurred due to the suppression of phosphorylation of PI3K/Akt/HDAC2, and the upregulation of antioxidant transcription factor NF erythroid-2–related factor 2 level [93].
Fig. 7.
Important terpenes for the treatment of respiratory disorders (b).
Sulaiman et al., 2018; presented the beneficial effects of andrographolide in offsetting toluene diisocyanate (TDI)-induced occupational asthma and aberrant distribution of E-cadherin in the airways. TDI represents the major cause of occupational asthma culminating 15% of the global asthma deaths. The exposure of the respiratory airways to oxidants results in epithelial cell necrosis and functional impairment due to defective expression of adherens junction proteins such as E-cadherin and β-catenin. Hence, E-cadherin maintains epithelial barrier stability of the airway mucosa and allergic sensitization, which lowers in asthma condition thereby promoting the infiltration of oxidants via defected airway epithelium [94]. Andrographolide exposure maintained airway integrity by reversing the distribution of aberrant airway epithelial E-cadherin and β-catenin. In addition, it led to the attenuation of TNF-α induced production of oxidants and ROS via upregulation of Nrf2 through Akt phosphorylation [95].
Lampronti et al., 2017; reported therapeutic potential of β-sitosterol (87, Fig. 7) extracted from Nigella arvensis L., in mitigating the expression of cytokine genes in cystic fibrosis epithelial cells lining bronchi. The test terpene significantly reduced the expression of neutrophilic chemokines IL-8, GRO-α, and GRO-β in LPS-sensitized human bronchial epithelial cells. These chemokines play a critical role in the recruitment of neutrophils in the lungs inflamed with cystic fibrosis. The investigations suggested that β-sitosterol partially inhibited LPS-triggered activation of Protein Kinase C isoform alpha, associated with transmembrane signaling for the activation of the expression of IL-8 gene in bronchial epithelial cells. Hence, β-sitosterol effectively mitigates the chronic lung inflammation in the patients with cystic fibrosis [96]. Further investigations suggested the attenuation of pulmonary fibrosis by β-sitosterol, by preventing the abnormal accumulation of extracellular matrix (ECM), manifesting deleterious effects to the alveolar epithelium resulting in epithelial–mesenchymal transition (EMT). β-sitosterol, reportedly displayed anti-fibrotic effect while inhibiting EMT by downregulating the expression of transforming growth factor-β1 (TGF-β1). Notably, treatment with β-sitosterol convincingly blocked TGF-β1-induced protein expression of EMT markers, N-cadherin, vimentin, and E-cadherin [97].
Kim et al., 2012; studied effect of Ursolic acid (88, Fig. 7) on ovalbumin-induced airway inflammation and airway hyper-responsiveness in animal asthma models. Ursolic acid displayed significant inhibition of airway inflammation via suppression of Th2 cytokines such as IL-5, IgE, and CCR3 expression [98]. Ursolic acid reportedly displays chemotherapeutic effect on COPD, and provides protection against cigarette smoke induced cell injury by suppressing the lung tumor metastasis [99]. The therapeutic effect of allergic asthma arises due to increase in the expression of PPARg and attenuated GATA-3 and STAT6 expression, resulting in a decrease of antigen-induced Penh, eosinophilia, lung inflammation, and the production of cytokines and antigen-specific IgE that play vital role in coordinating and intensifying the allergic inflammation in asthma [100]. Reportedly, the treatment with ursolic acid alleviates emphysema instigated by cigarette smoke by PERK pathway, as well as Nrf2 pathway [101]. The activation of PERK discourages protein synthesis via phosphorylation of eukaryotic translation initiator factor, thereby causing a selective translation of ATF4, which controls the expression of critical apoptotic association genes.
Nuclear erythroid-related factor 2 (Nrf2) represents a transcription factor regulating several antioxidant and detoxification genes [102]. Nrf2 signaling pathway plays a critical role in cigarette smoke-induced emphysema. Reportedly, ursolic acid demonstrates protective effects against cigarette smoke-induced emphysema through PERK andNrf2 signaling pathway. Further investigations on beneficial pulmonary effects of ursolic acid suggested that the terpene effectively alleviates cigarette smoke induced emphysema and airway remodeling involving unfolded protein response (UPR) pathways by suppression of ERS-associated apoptosis, and downregulation of IGF1, TGF-β1, and p-Smad2/3 expression [103,104]. These factors mediate bronchial epithelial and muscle cell regeneration in COPD patients through effects on airway vessel remodeling and muscle atrophy.
Lee et al., 2010; appraised theanti-asthma potency of Betulinic acid (89, Fig. 7) isolated form Forsythia viridissima. The terpene successfully paused the activity of histamine and phospholipase A2 in bronchoalveolar lavage fluid at a 12.5 mg/kg dose. The activity of eosinophil peroxide (EPO) and eosinophil recruitment lowered at a dose of 25 mg/kg. These metabolites act as the precursors of chronic inflammation in the airway passages [105]. Ekuadzi et al., 2017; further validated the potency of betulinic acid for the treatment of respiratory ailments, chiefly the carrageenan induced lung inflammation in animal models by successful inhibition of the production of proinflammatory chemokines and cytokines [106].
Jiang et al., 2017; reported the beneficial effects of Oridonin (90, Fig. 7) for capping acute respiratory distress syndrome (ARDS) in animal models. The test terpene notably mitigates the expression of inflammatory metabolites and factors such as TNF-α, IL-6, and NF-κB p56. Conversely, the expression of Iκ-B α augmented in lung tissues and bronchoalveolar lavage fluid [107]. Wang et al., 2016; further verified the protective effects of oridonin by studying its anti-asthma potential in ovalbumin-sensitized animal models. Reportedly, oridonin sustains Th1/Th2 cytokines balance in sensitized animal models and demonstrated anti-asthmatic effects in acute asthma model [108].
4. Clinical significance
Structurally diverse, plant derived natural products bear a privileged status in rational designing of commercially successful medicines. The identification of plant based natural molecules with therapeutic properties prompted systemic efforts to explore and commercially exploit the nature-derived drugs for countering clinical intricacies associated with the chronic diseases [109]. The natural product based drugs present considerable commercial representation under various brand names, as well as a significant number of candidate molecules in clinical trials [110]. However, the nature-derived molecules in chronic respiratory diseases present only a limited clinical profile, with even fewer molecules in commercial scaling [111].
A randomized controlled trial conducted in 60 patients with moderate to severe asthma suggested the safety and efficacy of Squill oxymel (a popular Iranian medicine obtained from Drimia maritima). Squill oxymel was able to significantly increases the force expiratory volume (FEV) 1 L, FEV1%, FEV1/force vital capacity (FVC%), and maximal mid expiratory flow (MEF) as compared to placebo. There was a remarkable improvement in symptoms, activity, and total score in the patients administered Squill Oxymel but not in the placebo group. Moreover, there was no serious adverse observed except minor nausea and vomiting reported in five patients in Squill oxymel group. This trail showed a strong potential of Squill Oxymel in terms of safety and efficacy for asthma patient [112]. Another double-blind randomized controlled clinical trial involving 85 asthma patients investigated whether plant stanol ester improves immune function in those patients. The effect of plant stanol added in soy-based yogurts in half of the patient on comparison with remaining half receiving control yogurt (placebo group) suggested considerable therapeutic results. It was observed that patients receiving plant based stanol ester group showed increased antibody titres against hepatitis A virus after 3 week [19% (P = 0.037)] and 4 wk [22% (P = 0.030)] of vaccination. Likewise, there was a marked depletion in plasma total IgE, TNF-α, IL-1β in plant-stanol ester group compared to placebo control. Furthermore, a correlation appeared between the increase in serum plant-stanol concentrations and decrease in IL-13 concentrations as well as Th1 switch in the Th1/Th2 balance [113]. The alkaloids such as theophylline, lobeline, and narceine have been commercialized as adenovasin, citotal, and peneraj respectively for relieving chronic symptoms associated with asthma, however several molecules fail to clear the clinical trials prior to successful commercialization [114,115].
Sulforaphane is a derivative of broccoli and commonly found in cruciferous vegetables. A phase 2 randomized trail conducted in 89 COPD patients were given either placebo or 25 μmoles or 150 μmoles of sulforaphane orally for 4 wks. The changes in nuclear factor erythroid-2 related factor 2 (Nrf2) target gene expression in bronchial epithelial cells and alveolar macrophages as well as measurement of oxidative damage, airway inflammation, and pulmonary function tests were tested. However, sulforaphane did not induce the Nrf2 target gene expression and no significant anti-oxidant and anti-inflammatory activity appeared as compared to placebo control [116]. Thyme herb, ivy leaves and primose roots exhibit beneficial effects against acute bronchitis and productive cough [117].
Gruenwald et al., 2005 and Kemmerich B. 2007 separately conducted a double-blind randomized control trial to study the efficacy and tolerability of thyme herb and primose root combination therapy In Gruenwald et al., 2005 study, the combination therapy was administered as 1 ml orally in 75 outpatient suffering with bronchitis while remaining 75 patients were given placebo. The combination therapy was effective in significantly reducing the bronchitis severity score leading to more patients with symptom free in combination therapy as compared to placebo group at the end of study. Both groups tolerate the treatment very well as there was no serious adverse events observed [118]. Similarly in Kemmerich B study, 183 patients with acute bronchitis were given with 1 tablet of thyme-primose combination thrice a day for 11 days and the efficacy was compared with placebo (N = 178 patients). There was a mean reduction of 67.1% of coughing fits in combination therapy group compared to 51.3% in placebo group on day 7–9. The combination therapy reduces the coughing fits to 50% two days earlier than placebo group. Further, no severe adverse events appeared in both groups, which concludes the well tolerability of thyme-primose combination therapy [119].
Kemmerich B et al., 2006 further reported in another trial investigating the efficacy and tolerability of thyme herb and ivy leaves in acute bronchitis patient. Similarly, the safety and efficacy of alcoholic extract of Echinacea purpurea herb (95%) and root (5%) in a large population (755 healthy volunteer) over 4 month's duration demonstrated significant therapeutic effects. The efficacy of extract to prevent the common cold episode when compared with placebo group showed beneficial activity against common cold by reducing the total number of cold episode as well as cumulated episode days. Furthermore, the extract was able to prevent enveloped virus infection. Regarding safety issue, 9% of participants in Echinacea extract treated group and 10% in placebo group showed adverse effect concluding the safety of Echinacea as non-inferior to placebo. The clinical trials evaluating the safety and efficacy of various natural product compounds in chronic respiratory disease are scarce. The limited clinical trials with few promising natural moieties need further validation with multiple evidence [120].
In Table 2 , we present the clinical studies on natural product-based molecules for countering the chronic respiratory disorders in human subjects.
Table 2.
Clinically significant natural product based molecules in attenuating respiratory disorders.
| Alkaloids | ||||||
|---|---|---|---|---|---|---|
| Sr. No | Compound | Source | Structure | Application | Mechanism | Ref. |
| 1. | Theophylline | Camellia sinensis and Theobroma cacao | 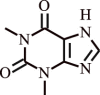 |
Inhibits the Exacerbations in human subjects with COPD, bronchodilation | Suppression of inflammatory genes by enhanced HDAC activation | [121,122] |
| 2. | Ligustrazine | Ligusticum wallichii Rhizome, Curcuma aromatica Salisb, Jatropha podagrica Hook | 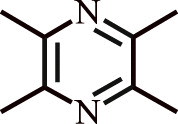 |
Treatment of pulmonary arterial hypertension (PAH) in human subjects | Upregulates levels of NO and downregulates ET-1; lowers mPAP levels. |
[123,124] |
| 3. | Atropine | Family Solanaceae | 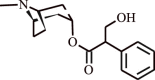 |
Relieves asthma chronic bronchitis | Reduction in sputum volume | [125] |
| Flavonoids | ||||||
| 4. | Puerarin | Pueraria montana var. lobata | 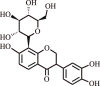 |
Treatment of pulmonary arterial hypertension (PAH) in human subjects | Restrains pulmonary vascular remodeling | [126] |
| Procyanidin | Cocoa | 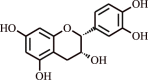 |
Relieves oxidant stress in human subjects | Trigger Nrf2 activity | [127] | |
| Terpenoids | ||||||
| 5. | Carvacrol | Origanum vulgare | 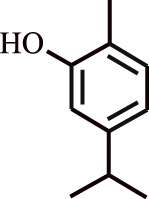 |
Therapeutic effect on asthma in human subjects | Downregulation of inflammatory cells, and high-sensitivity C-reactive protein (hs-CRP) | [128] |
| 6. | Eukalyptol | Eucalyptus sp. | 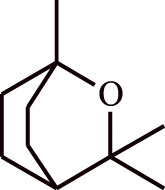 |
Relieves bronchial asthma and rhinosinusitis in human subjects | Mucolytic agent in upper and lower airway passages | [129,130] |
| 7. | Boswellic acid | Boswellia serrata | 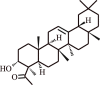 |
Alleviates symptoms of asthma in human subjects | Deactivation of lipoxygenase pathway | [131] |
| Miscellaneous | ||||||
| 8. | Sulforaphane | cruciferous vegetables |  |
Attenuates inflammatory effects of oxidative stress in respiratory passages in human subjects | Induces the expression of mucosal Phase II enzymes in the upper airway passage | [132] |
| 9 | Curcumin | Zingiberaceaefamily | 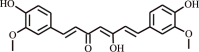 |
Prevention of deleterious cardiovascular events in COPD human subjects | Decreased the atherosclerotic AT-LDL levels, resulting in prevention of possible cardiovascular disorders in COPD subjects | [133] |
| 10 | Pycnogenol | Pinus pinaster | Complex antioxidant compounds | Relieves asthma inflammation in human subjects | Inactivation of NF-κB and attenuation of MMP-9 secretion | [134] |
| 11 | Linoleic acid | Vegetable oils | Polyunsaturated fatty acid | Improves the airway hyper‐reactivity in asthma human subjects | Downregulation of stimulated TNF-α | [[135], [136], [137]] |
| 12 | Caffeic acid | Eucalyptus globulus | 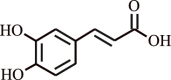 |
Significant inhibition in the incidence and severity of nocturnal attacks, improvement of ventilatory functions in asthma human subjects | Reduction in pro‐inflammatory factors (TNF)‐α, ICAM‐1, IL‐6, IL‐8, prostaglandins E2 and F2α and leukotriene D4; upsurge in IL‐10. The levels of | [138] |
5. Conclusion and future perspectives
Chronic respiratory disorders mainly arise due to the bronchial and pulmonary inflammation arising due to the heightened innate response causing an upsurge of proinflammatory metabolites, and over activity of enzyme such as cyclooxygenases, and lipoxygenases. The inhalation of toxicants manifests the recruitment of inflammatory cells in the bronchial and alveolar mucosa. The perseverance of nonspecific macrophages and adaptive T-lymphocytes even after the prolonged stimuli cassation inappropriately instigates the memory cells of adaptive immunity, an event further maintained by dendritic cells, which also induce remodeling of lung tissue. In addition, the oxidative stress and disturbance in protease/antiprotease balance deter the beneficial effects of corticosteroids in managing airway inflammation.
The ensuing effects include increased thickness of the bronchial wall leading to narrowing of the lumen, increased tone of bronchial smooth muscle leading to loss of elasticity and mucus oversecretion, proliferation of fibroblasts, detrimental extracellular matrix, and apoptosis in the endothelial cells. The involvement of multiple regulatory pathways in mediating respiratory disorders necessitates the development of multi-targeting medications. The practice of polypharmacy holds considerable potential for effectively managing the composite pulmonary maladies, considering the patient compliance. Mucoactive drugs such as ‘erdosteine’ combat broncho-obstructive symptoms by reinstating mucociliary clearance.
Notably, erdosteine displays significant antibiotic effect thereby attenuating the bacterial load during the respiratory distress. Similarly, the pulmonary disorders caused by multidrug-resistant microbes such as mycobacterium tuberculosis, and streptococcus pneumoniae requires innovative pharmaceuticals with multifarious targeting potential. Reportedly, the isoniazid monotherapy or rifampicin or pyrazinamide bitherapy proved inadequate for the disease management and resulted in its recurrent cycles. Hence, the recommended anti-TB regimen suggests polypharmacy as preliminary combination therapy of rifampin, isoniazid, pyrazinamide, ethambutol, and pyridoxine to prevent the recurrence of disease. Nevertheless, the amenability of the subjects to the combination of drugs, and the probability of microbial susceptibility to the administered doses raises compulsion for the identification of physiologically tolerable, novel pharmacophores for which the natural products present an ideal profile.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Meenu is supported by the Graduate School of Health and University of Technology Sydney (International Research Training Program Scholarship (IRTP)), Australia.
Kamal Dua is supported by a project grant from Rebecca L. Cooper Medical Research Foundation and Sydney Partnership for Health, Education, Research and Enterprise (SPHERE) for the TRIPLE I CAG Secondment/Exchange grant.
Philip M. Hansbro is supported by a fellowship from the National Health and Medical Research Council of Australia (NHMRC #1079187).
Keshav Raj Paudel is supported by a fellowship from Prevent Cancer Foundation (PCF) and the International Association for the Study of Lung Cancer (IASLC) foundation, USA.
References
- 1.Kyu H.H., Pinho C., Wagner J.A., Brown J.C., Bertozzi-Villa A., Charlson F.J., Coffeng L.E., Dandona L., Erskine H.E., Ferrari A.J., Fitzmaurice C., Fleming T.D., Forouzanfar M.H., Graetz N., Guinovart C., Haagsma J., Higashi H., Kassebaum N.J., Larson H.J., Lim S.S., Mokdad A.H., Moradi-Lakeh M., Odell S.V., Roth G.A., Serina P.T., Stanaway J.D., Misganaw A., Whiteford H.A., Wolock T.M., Wulf Hanson S., Abd-Allah F., Abera S.F., Abu-Raddad L.J., AlBuhairan F.S., Amare A.T., Antonio C.A., Artaman A., Barker-Collo S.L., Barrero L.H., Benjet C., Bensenor I.M., hutta Z.A., Bikbov B., Brazinova A., Nonato Campos, Castaneda-Orjuela C.A., Catala-Lopez F., Chowdhury R., Cooper C., Crump J.A., Dandona R., Degenhardt L., Dellavalle R.P., Dharmaratne S.D., Faraon E.J., Feigin V.L., Furst T., Geleijnse J.M., Gessner B.D., Gibney K.B., Goto A., Gunnell D., Hankey G.J., Hay R.J., Hornberger J.C., Hosgood H.D., Hu G., Jacobsen K.H., Jayaraman S.P., Jeemon P., Jonas J.B., Karch A., Kim D., Kim S., Kokubo Y., Kuate Defo B., Kucuk Bicer B., Kumar G.A., Larsson A., Leasher J.L., Leung R., Li Y., Lipshultz S.E., Lopez A.D., Lotufo P.A., Lunevicius R., Lyons R.A., Majdan M., Malekzadeh R., Mashal T., Mason-Jones A.J., Melaku Y.A., Memish Z.A., Mendoza W., Miller T.R., Mock C.N., Murray J., Nolte S., Oh I.H., Olusanya B.O., Ortblad K.F., Park E.K., Paternina Caicedo A.J., Patten S.B., Patton G.C., Pereira D.M., Perico N., Piel F.B., Polinder S., Popova S., Pourmalek F., Quistberg D.A., Remuzzi G., Rodriguez A., Rojas-Rueda D., Rothenbacher D., Rothstein D.H., Sanabria J., Santos I.S., Schwebel D.C., Sepanlou S.G., Shaheen A., Shiri R., Shiue I., Skirbekk V., Sliwa K., Sreeramareddy C.T., Stein D.J., Steiner T.J., Stovner L.J., Sykes B.L., Tabb K.M., Terkawi A.S., Thomson A.J., Thorne-Lyman A.L., Towbin J.A., Ukwaja K.N., Vasankari T., Venketasubramanian N., Vlassov V.V., Vollset S.E., Weiderpass E., Weintraub R.G., Werdecker A., Wilkinson J.D., Woldeyohannes S.M., Wolfe C.D., Yano Y., Yip P., Yonemoto N., Yoon S.J., Younis M.Z., Yu C., Zaki M. El Sayed, Naghavi M., Murray C.J., Vos T. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. 2016;170:267–287. doi: 10.1001/jamapediatrics.2015.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabe K.F., Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 3.Mehta M., Deeksha, Tewari D., Gupta G., Awasthi R., Singh H., Pandey P., Chellappan D.K., Wadhwa R., Collet T., Hansbro P.M., Kumar S.R., Thangavelu L., Negi P., Dua K., Satija S. Oligonucleotide therapy: an emerging focus area for drug delivery in chronic inflammatory respiratory diseases. Chem. Biol. Interact. 2019;308:206–215. doi: 10.1016/j.cbi.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta M., Deeksha, Sharma N., Vyas M., Khurana N., Maurya P.K., Singh H., Andreoli de Jesus T.P., Dureja H., Chellappan D.K., Gupta G., Wadhwa R., Collet T., Hansbro P.M., Dua K., Satija S. Interactions with the macrophages: an emerging targeted approach using novel drug delivery systems in respiratory diseases. Chem. Biol. Interact. 2019;304:10–19. doi: 10.1016/j.cbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Dua K., Malyla V., Singhvi G., Wadhwa R., Krishna R.V., Shukla S.D., Shastri M.D., Chellappan D.K., Maurya P.K., Satija S., Mehta M., Gulati M., Hansbro N., Collet T., Awasthi R., Gupta G., Hsu A., Hansbro P.M. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: an emerging need for novel drug delivery systems. Chem. Biol. Interact. 2019;299:168–178. doi: 10.1016/j.cbi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Mullane K. The increasing challenge of discovering asthma drugs. Biochem. Pharmacol. 2011;82:586–599. doi: 10.1016/j.bcp.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Dua K., Rapalli V.K., Shukla S.D., Singhvi G., Shastri M.D., Chellappan D.K., Satija S., Mehta M., Gulati M., Pinto T.J.A., Gupta G., Hansbro P.M. Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: emerging need for novel drug delivery approaches. Biomed. Pharmacother. 2018;107:1218–1229. doi: 10.1016/j.biopha.2018.08.101. [DOI] [PubMed] [Google Scholar]
- 8.Kaziani K., Sotiriou A., Dimopoulos G. Duration of pneumonia therapy and the role of biomarkers. Curr. Opin. Infect. Dis. 2017;30:221–225. doi: 10.1097/QCO.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P., Mehta M., Dhanjal D.S., Kaur S., Gupta G., Singh H., Thangavelu L., Rajeshkumar S., Tambuwala M., Bakshi H.A., Chellappan D.K., Dua K., Satija S. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Biol. Interact. 2019;309 doi: 10.1016/j.cbi.2019.06.033. 108720. [DOI] [PubMed] [Google Scholar]
- 10.Chellappan D.K., Yee L.W., Xuan K.Y., Kunalan K., Rou L.C., Jean L.S., Ying L.Y., Wie L.X., Chellian J., Mehta M., Satija S., Singh S.K., Gulati M., Dureja H., Da Silva M.W., Tambuwala M.M., Gupta G., Paudel K.R., Wadhwa R., Hansbro P.M., Dua K. Targeting neutrophils using novel drug delivery systems in chronic respiratory diseases. Drug Dev. Res. 2020 doi: 10.1002/ddr.21648. [DOI] [PubMed] [Google Scholar]
- 11.Malyla V., Paudel K.R., Shukla S.D., Donovan C., Wadhwa R., Pickles S., Chimankar V., Sahu P., Bielefeldt-Ohmann H., Bebawy M., Hansbro P.M., Dua K. Recent advances in experimental animal models of lung cancer. Future Med. Chem. 2020 doi: 10.4155/fmc-2019-0338. [DOI] [PubMed] [Google Scholar]
- 12.Kim T.M., Paudel K.R., Kim D.W. Eriobotrya japonica leaf extract attenuates airway inflammation in ovalbumin-induced mice model of asthma. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2019.112082. 112082. [DOI] [PubMed] [Google Scholar]
- 13.Mehta M., Dhanjal D.S., Paudel K.R., Singh B., Gupta G., Rajeshkumar S., Thangavelu L., Tambuwala M.M., Bakshi H.A., Chellappan D.K., Pandey P., Dureja H., Charbe N.B., Singh S.K., Shukla S.D., Nammi S., Aljabali A.A., Wich P.R., Hansbro P.M., Satija S., Dua K. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: an update. Inflammopharmacology. 2020 doi: 10.1007/s10787-020-00698-3. [DOI] [PubMed] [Google Scholar]
- 14.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards M.R., Bartlett N.W., Clarke D., Birrell M., Belvisi M., Johnston S.L. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez I.E., Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 17.Liu T.C., Jin X., Wang Y., Wang K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Canc. Res. 2017;7:187–202. [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golding B., Luu A., Jones R., Viloria-Petit A.M. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC) Mol. Canc. 2018;17:52. doi: 10.1186/s12943-018-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk P.P., Selinger C.I., Mahar A., Cooper W.A. Biomarkers for ALK and ROS1 in lung cancer: immunohistochemistry and fluorescent in situ hybridization. Arch. Pathol. Lab Med. 2018;142:922–928. doi: 10.5858/arpa.2017-0502-RA. [DOI] [PubMed] [Google Scholar]
- 21.Ciuffreda L., Incani U.C., Steelman L.S., Abrams S.L., Falcone I., Curatolo A.D., Chappell W.H., Franklin R.A., Vari S., Cognetti F., McCubrey J.A., Milella M. Signaling intermediates (MAPK and PI3K) as therapeutic targets in NSCLC. Curr. Pharmaceut. Des. 2014;20:3944–3957. doi: 10.2174/13816128113196660763. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P., Mehta M., Satija S., Garg M. Enzymatic in vitro anti-diabetic activity of few traditional Indian medicinal plants. J. Biol. Sci. 2013;13:540–544. [Google Scholar]
- 23.Garg M., Lata K., Satija S. Cytotoxic potential of few Indian fruit peels through 3-(4, 5-dimethylthiazol-yl)-2, 5-diphenyltetrazolium bromide assay on HepG2 cells. Indian J. Pharmacol. 2016;48:64. doi: 10.4103/0253-7613.174552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta M., Satija S., Kalsi V. Invitro Antioxidant evaluation of Psidium guajava strem extracts. Int. J. Drug Dev. Res. 2011;3:213–216. [Google Scholar]
- 25.Mannhold R., Kubinyi H., Folkers G. John Wiley & Sons; 2013. Natural Products in Medicinal Chemistry. [Google Scholar]
- 26.Lu X., Pu Y., Kong W., Tang X., Zhou J., Gou H., Song X., Zhou H., Gao N., Shen J. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-κB activities. Int. Immunopharm. 2017;45:34–42. doi: 10.1016/j.intimp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Arya S.B., Kumar G., Kaur H., Kaur A., Tuli A. ARL11 regulates lipopolysaccharide-stimulated macrophage activation by promoting mitogen-activated protein kinase (MAPK) signaling. J. Biol. Chem. 2018;293:9892–9909. doi: 10.1074/jbc.RA117.000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacob S.P., Lakshmikanth C.L., Chaithra V.H., Kumari T.R., Chen C.H., McIntyre T.M., Marathe G.K. Lipopolysaccharide cross-tolerance delays platelet-activating factor-induced sudden death in Swiss albino mice: involvement of cyclooxygenase in cross-tolerance. PloS One. 2016;11 doi: 10.1371/journal.pone.0153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choe S.H., Choi E.Y., Hyeon J.Y., Keum B.R., Choi I.S., Kim S.J. Telmisartan, an angiotensin II receptor blocker, attenuates Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1beta in murine macrophages. Int. Immunopharm. 2019;75 doi: 10.1016/j.intimp.2019.105750. 105750. [DOI] [PubMed] [Google Scholar]
- 30.Choudhury M.G., Saha N. Induction of inducible nitric oxide synthase by lipopolysaccharide and the influences of cell volume changes, stress hormones and oxidative stress on nitric oxide efflux from the perfused liver of air-breathing catfish, heteropneustes fossilis. PloS One. 2016;11 doi: 10.1371/journal.pone.0150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azuma Y., Taniguchi F., Nakamura K., Nagira K., Khine Y.M., Kiyama T., Uegaki T., Izawa M., Harada T. Lipopolysaccharide promotes the development of murine endometriosis-like lesions via the nuclear factor-kappa B pathway. Am. J. Reprod. Immunol. 2017;77 doi: 10.1111/aji.12631. [DOI] [PubMed] [Google Scholar]
- 32.Winkler C., Ferdous F., Dimmick M., Scott T. Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFkappaB in chicken thrombocytes. Dev. Comp. Immunol. 2017;73:124–130. doi: 10.1016/j.dci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Giridharan S., Srinivasan M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018;11:407–419. doi: 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickerhof N., Huang J., Min E., Michaelsson E., Lindstedt E.L., Pearson J.F., Kettle A.J., Day B.J. Myeloperoxidase inhibition decreases morbidity and oxidative stress in mice with cystic fibrosis-like lung inflammation. Free Radic. Biol. Med. 2020;152:91–99. doi: 10.1016/j.freeradbiomed.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Aridgides D.S., Mellinger D.L., Armstrong D.A., Hazlett H.F., Dessaint J.A., Hampton T.H., Atkins G.T., Carroll J.L., Ashare A. Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci. Rep. 2019;9:9624. doi: 10.1038/s41598-019-46045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian W., Rojo de la Vega M., Schmidlin C.J., Ooi A., Zhang D.D. Kelch-like ECH-associated protein 1 (KEAP1) differentially regulates nuclear factor erythroid-2-related factors 1 and 2 (NRF1 and NRF2) J. Biol. Chem. 2018;293:2029–2040. doi: 10.1074/jbc.RA117.000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki T., Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshmukh P., Unni S., Krishnappa G., Padmanabhan B. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9:41–56. doi: 10.1007/s12551-016-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Yang T., Ming T.W., Gaun T.K.W., Zhou T., Wang S., Ye B. Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia. 2020;140 doi: 10.1016/j.fitote.2019.104434. 104434. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y.L., Cao J., Shang J.H., Liu Y.P., Khan A., Wang H.S., Qian Y., Liu L., Ye M., Luo X.D. Airways antiallergic effect and pharmacokinetics of alkaloids from Alstonia scholaris. Phytomedicine. 2017;27:63–72. doi: 10.1016/j.phymed.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Corrigan C.J. Eotaxin and asthma: some answers, more questions. Clin. Exp. Immunol. 1999;116:1–3. doi: 10.1046/j.1365-2249.1999.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y.L., Yang Z.F., Shang J.H., Huang W.Y., Wang B., Wei X., Khan A., Yuan Z.W., Liu Y.P., Wang Y.F., Wang X.H., Luo X.D. Effects of indole alkaloids from leaf of Alstonia scholaris on post-infectious cough in mice. J. Ethnopharmacol. 2018;218:69–75. doi: 10.1016/j.jep.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W., Wang Y., He D.D., Li S.P., Zhu Y.D., Jiang B., Cheng X.M., Wang Z., Wang C.H. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (+/-)-vasicine, deoxyvasicine, and (+/-)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine. 2015;22:1088–1095. doi: 10.1016/j.phymed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Bezerra-Santos C.R., Vieira-de-Abreu A., Vieira G.C., Filho J.R., Barbosa-Filho J.M., Pires A.L., Martins M.A., Souza H.S., Bandeira-Melo C., Bozza P.T., Piuvezam M.R. Effectiveness of Cissampelos sympodialis and its isolated alkaloid warifteine in airway hyperreactivity and lung remodeling in a mouse model of asthma. Int. Immunopharm. 2012;13:148–155. doi: 10.1016/j.intimp.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Corren J. Role of interleukin-13 in asthma. Curr. Allergy Asthma Rep. 2013;13:415–420. doi: 10.1007/s11882-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 47.Kim S.H., Hong J.H., Lee Y.C. Chelidonine, a principal isoquinoline alkaloid of Chelidonium majus, attenuates eosinophilic airway inflammation by suppressing IL-4 and eotaxin-2 expression in asthmatic mice. Pharmacol. Rep. 2015;67:1168–1177. doi: 10.1016/j.pharep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Lourenco O., Fonseca A.M., Taborda-Barata L. Human CD8+ T cells in asthma: possible pathways and roles for NK-like subtypes. Front. Immunol. 2016;7:638. doi: 10.3389/fimmu.2016.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu M., Zou B., An K., Yu Y., Tang D., Wu J., Xu Y., Ti H. Anti-asthmatic activity of alkaloid compounds from Pericarpium Citri Reticulatae (citrus reticulata 'chachi') Food Funct. 2019;10:903–911. doi: 10.1039/c8fo01753k. [DOI] [PubMed] [Google Scholar]
- 50.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., Kim H.R., Kim S., Jin Y.H., Kwon S. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9 doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues Felix C., Roberts J.C., Winder P.L., Gupta R., Diaz M.C., Pomponi S.A., Wright A.E., Rohde K.H., Plakinamine P. A steroidal alkaloid with bactericidal activity against Mycobacterium tuberculosis. Mar. Drugs. 2019;17 doi: 10.3390/md17120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcellano R.C., Cort J.R., Moinuddin S.G.A., Franzblau S.G., Ma R., Aguinaldo A.M. An iboga alkaloid chemotaxonomic marker from endemic Tabernaemontana ternifolia with antitubercular activity. Nat. Prod. Res. 2019:1–5. doi: 10.1080/14786419.2018.1550759. [DOI] [PubMed] [Google Scholar]
- 54.Chen S., He L., Chen D., Cai R., Long Y., Lu Y., She Z. Talaramide A, an unusual alkaloid from the mangrove endophytic fungus Talaromyces sp.(HZ-YX1) as an inhibitor of mycobacterial PknG. New J. Chem. 2017;41:4273–4276. [Google Scholar]
- 55.Simithy J., Fuanta N.R., Alturki M., Hobrath J.V., Wahba A.E., Pina I., Rath J., Hamann M.T., DeRuiter J., Goodwin D.C., Calderon A.I. Slow-binding inhibition of Mycobacterium tuberculosis shikimate kinase by manzamine alkaloids. Biochemistry. 2018;57:4923–4933. doi: 10.1021/acs.biochem.8b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murali Krishna Kumar M., Devilal Naik J., Satyavathi K., Ramana H., Raghuveer Varma P., Purna Nagasree K., Smitha D., Venkata Rao D., Denigrins A.-C. New antitubercular 3,4-diarylpyrrole alkaloids from Dendrilla nigra. Nat. Prod. Res. 2014;28:888–894. doi: 10.1080/14786419.2014.891112. [DOI] [PubMed] [Google Scholar]
- 57.De Oliveira J.H., Seleghim M.H., Timm C., Grube A., Köck M., Nascimento G.G., Martins A.C.T., Silva E.G., De Souza A.O., Minarini P.R. Antimicrobial and antimycobacterial activity of cyclostellettamine alkaloids from sponge Pachychalina sp. Mar. Drugs. 2006;4:1–8. [Google Scholar]
- 58.Jakkala K., Ajitkumar P. Hypoxic non-replicating persistent Mycobacterium tuberculosis develops thickened outer layer that helps in restricting rifampicin entry. Front. Microbiol. 2019;10:2339. doi: 10.3389/fmicb.2019.02339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rustad T.R., Harrell M.I., Liao R., Sherman D.R. The enduring hypoxic response of Mycobacterium tuberculosis. PloS One. 2008;3 doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arai M., Han C., Yamano Y., Setiawan A., Kobayashi M. Aaptamines, marine spongean alkaloids, as anti-dormant mycobacterial substances. J. Nat. Med. 2014;68:372–376. doi: 10.1007/s11418-013-0811-y. [DOI] [PubMed] [Google Scholar]
- 61.Zhi H., Jin X., Zhu H., Li H., Zhang Y., Lu Y., Chen D. Exploring the effective materials of flavonoids-enriched extract from Scutellaria baicalensis roots based on the metabolic activation in influenza A virus induced acute lung injury. J. Pharmaceut. Biomed. Anal. 2020;177 doi: 10.1016/j.jpba.2019.112876. 112876. [DOI] [PubMed] [Google Scholar]
- 62.Zhi H.J., Zhu H.Y., Zhang Y.Y., Lu Y., Li H., Chen D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine. 2019;57:105–116. doi: 10.1016/j.phymed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Ling L.-j., Lu Y., Zhang Y.-y., Zhu H.-y., Tu P., Li H., Chen D.-f. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153150. 153150. [DOI] [PubMed] [Google Scholar]
- 64.Galan A., Mayer I., Rafaj R.B., Bendelja K., Susic V., Ceron J.J., Mrljak V. MCP-1, KC-like and IL-8 as critical mediators of pathogenesis caused by Babesia canis. PloS One. 2018;13 doi: 10.1371/journal.pone.0190474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart C.E., Randall R.E., Adamson C.S. Inhibitors of the interferon response enhance virus replication in vitro. PloS One. 2014;9 doi: 10.1371/journal.pone.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barton G.M., Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 67.Sasikumar K., Ghosh A.R., Dusthackeer A. Antimycobacterial potentials of quercetin and rutin against Mycobacterium tuberculosis H37Rv. 3 Biotech. 2018;8:427. doi: 10.1007/s13205-018-1450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brust B., Lecoufle M., Tuaillon E., Dedieu L., Canaan S., Valverde V., Kremer L. Mycobacterium tuberculosis lipolytic enzymes as potential biomarkers for the diagnosis of active tuberculosis. PloS One. 2011;6 doi: 10.1371/journal.pone.0025078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arora G., Chaudhary D., Kidwai S., Sharma D., Singh R. CitE enzymes are essential for Mycobacterium tuberculosis to establish infection in macrophages and Guinea pigs. Front. Cell. Infect. Microbiol. 2018;8:385. doi: 10.3389/fcimb.2018.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qasaymeh R.M., Rotondo D., Oosthuizen C.B., Lall N., Seidel V. Predictive binding affinity of plant-derived natural products towards the protein kinase G enzyme of Mycobacterium tuberculosis (MtPknG) Plants. 2019;8:477. doi: 10.3390/plants8110477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solnier J., Martin L., Bhakta S., Bucar F. Flavonoids as novel efflux pump inhibitors and antimicrobials against both environmental and pathogenic intracellular mycobacterial species. Molecules. 2020;25:734. doi: 10.3390/molecules25030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang H.-Y., Ahn K.-S., Park M.-J., Kwon O.-K., Lee H.-K., Oh S.-R. Skullcapflavone II inhibits ovalbumin-induced airway inflammation in a mouse model of asthma. Int. Immunopharm. 2012;12:666–674. doi: 10.1016/j.intimp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Jeon D., Jeong M.C., Jnawali H.N., Kwak C., Ryoo S., Jung I.D., Kim Y. Phloretin exerts anti-tuberculosis activity and suppresses lung inflammation. Molecules. 2017:22. doi: 10.3390/molecules22010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X.X., Wu Q.F., Yan Y.L., Zhang F.L. Inhibitory effects and related molecular mechanisms of total flavonoids in Mosla chinensis Maxim against H1N1 influenza virus. Inflamm. Res. 2018;67:179–189. doi: 10.1007/s00011-017-1109-4. [DOI] [PubMed] [Google Scholar]
- 75.Pawar A., Jha P., Chopra M., Chaudhry U., Saluja D. Screening of natural compounds that targets glutamate racemase of Mycobacterium tuberculosis reveals the anti-tubercular potential of flavonoids. Sci. Rep. 2020;10:949. doi: 10.1038/s41598-020-57658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McAuley J.L., Gilbertson B.P., Trifkovic S., Brown L.E., McKimm-Breschkin J.L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019;10:39. doi: 10.3389/fmicb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Syed S., Hakala P., Singh A.K., Lapatto H.A.K., King S.J., Meri S., Jokiranta T.S., Haapasalo K. Role of pneumococcal NanA neuraminidase activity in peripheral blood. Front. Cell. Infect. Microbiol. 2019;9:218. doi: 10.3389/fcimb.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grienke U., Richter M., Walther E., Hoffmann A., Kirchmair J., Makarov V., Nietzsche S., Schmidtke M., Rollinger J.M. Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumoniae. Sci. Rep. 2016;6 doi: 10.1038/srep27156. 27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirota R., Nakamura H., Bhatti S.A., Ngatu N.R., Muzembo B.A., Dumavibhat N., Eitoku M., Sawamura M., Suganuma N. Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal. Toxicol. 2012;24:373–381. doi: 10.3109/08958378.2012.675528. [DOI] [PubMed] [Google Scholar]
- 81.Alavinezhad A., Khazdair M.R., Boskabady M.H. Possible therapeutic effect of carvacrol on asthmatic patients: a randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother Res. 2018;32:151–159. doi: 10.1002/ptr.5967. [DOI] [PubMed] [Google Scholar]
- 82.Boskabady M.H., Jalali S. Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized Guinea pigs. Exp. Biol. Med. 2013;238:200–208. doi: 10.1177/1535370212474604. [DOI] [PubMed] [Google Scholar]
- 83.Ricciardolo F.L., Sterk P.J., Gaston B., Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 84.Wan L., Meng D., Wang H., Wan S., Jiang S., Huang S., Wei L., Yu P. Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation. 2018;41:183–192. doi: 10.1007/s10753-017-0676-4. [DOI] [PubMed] [Google Scholar]
- 85.Mohammadi A., Mahjoub S., Ghafarzadegan K., Nouri H.R. Immunomodulatory effects of Thymol through modulation of redox status and trace element content in experimental model of asthma. Biomed. Pharmacother. 2018;105:856–861. doi: 10.1016/j.biopha.2018.05.154. [DOI] [PubMed] [Google Scholar]
- 86.Al-Gabri N.A., Qaid M.M., El-Shaer N.H., Ali M.H., Abudabos A.M. Thymoquinone ameliorates pulmonary vascular damage induced byEscherichia coli-derived lipopolysaccharide via cytokine downregulation in rats. Environ. Sci. Pollut. Res. Int. 2019;26:18465–18469. doi: 10.1007/s11356-019-05229-4. [DOI] [PubMed] [Google Scholar]
- 87.Yetkin N.A., Buyukoglan H., Sonmez M.F., Tutar N., Gulmez I., Yilmaz I. The protective effects of thymoquinone on lung damage caused by cigarette smoke. Biotech. Histochem. 2019:1–8. doi: 10.1080/10520295.2019.1681511. [DOI] [PubMed] [Google Scholar]
- 88.Su X., Ren Y., Yu N., Kong L., Kang J. Thymoquinone inhibits inflammation, neoangiogenesis and vascular remodeling in asthma mice. Int. Immunopharm. 2016;38:70–80. doi: 10.1016/j.intimp.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 89.Qamar W., Sultana S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: an initial step in lung chemoprevention. Chem. Biol. Interact. 2008;176:79–87. doi: 10.1016/j.cbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Qamar W., Khan A.Q., Khan R., Lateef A., Tahir M., Rehman M.U., Ali F., Sultana S. Benzo(a)pyrene-induced pulmonary inflammation, edema, surfactant dysfunction, and injuries in rats: alleviation by farnesol. Exp. Lung Res. 2012;38:19–27. doi: 10.3109/01902148.2011.632064. [DOI] [PubMed] [Google Scholar]
- 91.Gordien A.Y., Gray A.I., Franzblau S.G., Seidel V. Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae) J. Ethnopharmacol. 2009;126:500–505. doi: 10.1016/j.jep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X., Xu Q., Sun Y. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-kappaB pathways. Acta Pharm. Sin. B. 2016;6:205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao W., Tan W.S., Wong W.S. Andrographolide restores steroid sensitivity to block lipopolysaccharide/IFN-gamma-Induced IL-27 and airway hyperresponsiveness in mice. J. Immunol. 2016;196:4706–4712. doi: 10.4049/jimmunol.1502114. [DOI] [PubMed] [Google Scholar]
- 94.Van Roy F., Berx G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sulaiman I., Tan K., Mohtarrudin N., Lim J.C.W., Stanslas J. Andrographolide prevented toluene diisocyanate-induced occupational asthma and aberrant airway E-cadherin distribution via p38 MAPK-dependent Nrf2 induction. Pulm. Pharmacol. Therapeut. 2018;53:39–51. doi: 10.1016/j.pupt.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Lampronti I., Dechecchi M.C., Rimessi A., Bezzerri V., Nicolis E., Guerrini A., Tacchini M., Tamanini A., Munari S., D'Aversa E., Santangelo A., Lippi G., Sacchetti G., Pinton P., Gambari R., Agostini M., Cabrini G. Beta-sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front. Pharmacol. 2017;8:236. doi: 10.3389/fphar.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park Y.J., Bang I.J., Jeong M.H., Kim H.R., Lee D.E., Kwak J.H., Chung K.H. Effects of beta-sitosterol from corn silk on TGF-beta1-induced epithelial-mesenchymal transition in lung alveolar epithelial cells. J. Agric. Food Chem. 2019;67:9789–9795. doi: 10.1021/acs.jafc.9b02730. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.H., Kim B.K., Lee Y.C. Effects of Corni fructus on ovalbumin-induced airway inflammation and airway hyper-responsiveness in a mouse model of allergic asthma. J. Inflamm. 2012;9:9. doi: 10.1186/1476-9255-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu W., Tan X., Shu L., Sun H., Song J., Jin P., Yu S., Sun M., Jia X. Ursolic acid inhibits cigarette smoke extract-induced human bronchial epithelial cell injury and prevents development of lung cancer. Molecules. 2012;17:9104–9115. doi: 10.3390/molecules17089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim S.H., Hong J.H., Lee Y.C. Ursolic acid, a potential PPARgamma agonist, suppresses ovalbumin-induced airway inflammation and Penh by down-regulating IL-5, IL-13, and IL-17 in a mouse model of allergic asthma. Eur. J. Pharmacol. 2013;701:131–143. doi: 10.1016/j.ejphar.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 101.Lin L., Yin Y., Hou G., Han D., Kang J., Wang Q. Ursolic acid attenuates cigarette smoke-induced emphysema in rats by regulating PERK and Nrf2 pathways. Pulm. Pharmacol. Therapeut. 2017;44:111–121. doi: 10.1016/j.pupt.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 102.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 103.Lin L., Hou G., Han D., Kang J., Wang Q. Ursolic acid protected lung of rats from damage induced by cigarette smoke extract. Front. Pharmacol. 2019;10:700. doi: 10.3389/fphar.2019.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin L., Hou G., Han D., Yin Y., Kang J., Wang Q. Ursolic acid alleviates airway-vessel remodeling and muscle consumption in cigarette smoke-induced emphysema rats. BMC Pulm. Med. 2019;19:103. doi: 10.1186/s12890-019-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J.Y., Moon H., Kim C.J. Effects of hydroxy pentacyclic triterpene acids from Forsythia viridissima on asthmatic responses to ovalbumin challenge in conscious Guinea pigs. Biol. Pharm. Bull. 2010;33:230–237. doi: 10.1248/bpb.33.230. [DOI] [PubMed] [Google Scholar]
- 106.Ekuadzi E., Biney R.P., Benneh C.K., Osei Amankwaa B., Jato J. Antiinflammatory properties of betulinic acid and xylopic acid in the carrageenan-induced pleurisy model of lung inflammation in mice. Phytother Res. 2018;32:480–487. doi: 10.1002/ptr.5993. [DOI] [PubMed] [Google Scholar]
- 107.Jiang J., Shan X., Zhu L. Effects and mechanisms of oridonin in the treatment of acute respiratory distress syndrome mice. Int. J. Clin. Exp. Med. 2017;10:6191–6197. [Google Scholar]
- 108.Wang J., Li F., Ding J., Tian G., Jiang M., Gao Z., Tuyghun E. Investigation of the anti-asthmatic activity of Oridonin on a mouse model of asthma. Mol. Med. Rep. 2016;14:2000–2006. doi: 10.3892/mmr.2016.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atanasov A.G., Waltenberger B., et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yao J., Weng Y., Dickey A., Wang K.Y. Plants as factories for human pharmaceuticals: applications and challenges. Int. J. Mol. Sci. 2015;16:28549–28565. doi: 10.3390/ijms161226122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Nejatbakhsh F., Borzi H.K., Amin G., Eslaminejad A., Hosseini M., Bozorgi M., Gharabaghi M.A. Quill Oxymel, a traditional formulation from Drimia Maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: a pilot, triple-blind, randomized clinical trial. J. Ethanopharmacol. 2017;196:186–192. doi: 10.1016/j.jep.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 113.Brull F., De Smet E., Mensink R.P., Vreugdenhil A., Kerksiek A., Lutjohann D., Plat J. Dietary plant stanol ester consumption improves immune function in asthma patients: results of a randomized, double-blind clinical trial. Am. J. Clin. Nutr. 2016;103:444–453. doi: 10.3945/ajcn.115.117531. [DOI] [PubMed] [Google Scholar]
- 114.May C.D. History of the introduction of theophylline into the treatment of asthma. Clin. Exp. Allergy. 1974;4:211–217. doi: 10.1111/j.1365-2222.1974.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 115.Amirkia V., Heinrich M. Alkaloids as drug leads - a predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014;10 xlviii-liii. [Google Scholar]
- 116.Wise R.A., Holbrook J.T., et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PloS One. 2017;12 doi: 10.1371/journal.pone.0175077. Article e0175077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gruenwald J., Graubaum H.J., Busch R. Efficacy and tolerability of a fixed combination of thyme and primrose root in patients with acute bronchitis. A double-blind, randomized, placebo-controlled clinical trial. Arzneimittelforschung. 2005;55:669–676. doi: 10.1055/s-0031-1296916. [DOI] [PubMed] [Google Scholar]
- 118.Kemmerich B., Eberhardt R., Stammer H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled clinical trial. Arzneimittelforschung. 2006;56:652–660. doi: 10.1055/s-0031-1296767. [DOI] [PubMed] [Google Scholar]
- 119.Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneimittelforschung. 2006;56:607–615. doi: 10.1055/s-0031-1296656. [DOI] [PubMed] [Google Scholar]
- 120.Jawad M., Schoop R., Suter A., Klein P., Eccles R. Safety and efficacy profile of Echinacea purpurea to prevent common cold episodes: a randomized, double-blind, placebo-controlled trial. Evid based Complement Alternat. Med. 2012 doi: 10.1155/2012/841315. (2012) Article 841315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Devereux G., Cotton S., Fielding S., et al. Effect of theophylline as adjunct to inhaled corticosteroids on exacerbations in patients with COPDA randomized clinical trial. J. Am. Med. Assoc. 2018;320:1548–1559. doi: 10.1001/jama.2018.14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ito K., Lim S., Caramori G., Cosio B., Chung K.F., Adcock I.M., Barnes P.J. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99:8921–8926. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M., Handa S., Ikeda Y., et al. Specific inhibiting characteristics of tetramethylpyrazine, one of the active ingredients of the Chinese herbal medicine ‘Chuanxiong’, on platelet thrombus formation under high shear rates. Thromb. Res. 2001;104:15–28. doi: 10.1016/s0049-3848(01)00343-7. [DOI] [PubMed] [Google Scholar]
- 124.Feng E.Z., Yang S.Y., Huang N.X., et al. Plasma endothelin–1 and nitric oxide correlate with ligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation (in Chinese) Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014;30:532–537. [PubMed] [Google Scholar]
- 125.Vidriero M.T.L., Costello J., Clark T.J.H., Das I., Keal E.E., Reid L. Effect of atropine on sputum production. Thorax. 1975;30:543–547. doi: 10.1136/thx.30.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen C., Chen C., Wang Z.Y., et al. Puerarin induces mitochondria–dependent apoptosis in hypoxic human pulmonary arterial smooth muscle cells. PloS One. 2012;7 doi: 10.1371/journal.pone.0034181. Article e34181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Izquierdo T.R., Nemzer B., Argumedo R., Cervantes M., Pietrzkowski Z. Stimulatory effect OF acute single dose OF dried whole coffee cherry powder ON NRF2 activity IN freshly isolated blood cells. A single-blind, placebo controlled cross-over pilot clinical study. J. Ageing Res. Clin. Pract. 2016;5:120–127. [Google Scholar]
- 128.Alavinezhad A., Khazdair M.R., Boskabady M.H. Possible therapeutic effect of carvacrol on asthmatic patients: a randomized, double blind, placebo‐controlled, Phase II clinical trial. Phytother Res. 2017;32:151–159. doi: 10.1002/ptr.5967. [DOI] [PubMed] [Google Scholar]
- 129.Geurgens U.R., Dethlefsen U., Steinkamp G., Repges A.G.R., Vetter H. Anti-inflammatory activity of1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Med. 2003;97:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- 130.Kehri W., Sonnemann U., Dethlefsen U. Therapy for acute nonpurulent rhinosinusitis with cineole: results of a double‐blind, randomized, placebo‐controlled trial. Laryngoscope. 2004;114:738–742. doi: 10.1097/00005537-200404000-00027. [DOI] [PubMed] [Google Scholar]
- 131.Yogandhar P., Rao K.M., Sengupta K. A novel herbal composition containing extracts of Boswellia serrata gum resin and Aegle marmelos fruit alleviates symptoms of asthma in a placebo controlled double‐blind clinical study. Phytother Res. 2018;32:140–150. doi: 10.1002/ptr.5963. [DOI] [PubMed] [Google Scholar]
- 132.Reidl M.A., Saxon A., Sanchez D.D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Funamoto M., Sunagawa Y., Katanasaka Y., et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:2029–2034. doi: 10.2147/COPD.S104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grimm T., Chovanova Z., Muchova J., et al. Inhibition of NF-κB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol) J. Inflamm. 2006;3 doi: 10.1186/1476-9255-3-1. Article 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hodge L., Salome C.M., Hughes J.M., Brennan D.L., Rimmer J., Allman M., Pang D., Armour C., Woolcock A.J. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur. Respir. J. 1998;11:361–365. doi: 10.1183/09031936.98.11020361. [DOI] [PubMed] [Google Scholar]
- 136.Redmond R.M., Singhera G., Attridge S., Bahzad M., Fava C., Lai Y., Hallstrand T.S., Dorscheid D.R. Conjugated linoleic acid improves airway hyper‐reactivity in overweight mild asthmatics. Clin. Exp. Allergy. 2010;40:1071–1078. doi: 10.1111/j.1365-2222.2010.03531.x. [DOI] [PubMed] [Google Scholar]
- 137.Jaudszus A., Mainz J.G., Pittag S., Dornaus S., Dopfer C., Roth A., Jahreis G. Effects of a dietary intervention with conjugated linoleic acid on immunological and metabolic parameters in children and adolescents with allergic asthma – a placebo-controlled pilot trial. Lipids Health Dis. 2016;15 doi: 10.1186/s12944-016-0187-6. Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khayyal M.T., El-Ghazaly M.A., El-Khatib A.S., hatem A.M., De Vries P.J.F., El-Shafei S., Khattab M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fund. Clin. Pharmacol. 2003;17:93–102. doi: 10.1046/j.1472-8206.2003.00117.x. [DOI] [PubMed] [Google Scholar]