See Nephrology Rounds on Page 940
Coronavirus disease 2019 (COVID-19) is an emerging human infectious disease caused by a novel β coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical manifestations range from asymptomatic infection, self-limited flu-like symptoms, to severe acute pneumonia with high mortality. Whereas the lung is the primary target in this pandemic disease, other organs can be affected, including the gastrointestinal tract, liver, heart, blood, and kidneys.1 Acute respiratory distress syndrome and multiorgan damage, which are responsible for most mortalities, are thought to be mediated by an acute release of inflammatory cytokines, representing cytokine release syndrome.2
Reports from China have highlighted frequent kidney involvement in COVID-19, and this was associated with increased mortality.3,4 In a prospective cohort of 401 patients with COVID-19, 44% of patients had proteinuria, 27% had hematuria, 14% had elevated serum creatinine on admission, and 5.1% had acute kidney injury (AKI) during admission; all of these renal abnormalities were independent risk factors for in-hospital death.3 In another study, 59% had proteinuria, 44% hematuria, and 10% elevated serum creatinine on admission.4 Proposed mechanisms for AKI include hypoperfusion-induced tubular injury associated with sepsis and cytokine storm, and direct tubular cell toxicity by the virus.5 The latter is supported by the findings of a recent postmortem study that analyzed the renal pathologic abnormalities in 26 patients with COVID-19.6 A third of patients had clinical evidence of elevated serum creatinine and/or new-onset proteinuria. Prominent acute tubular injury was seen by light microscopy, viral particles were detected within tubular epithelial cells and podocytes by electron microscopy, and immunofluorescence staining for SARS-CoV-2 nucleoprotein was positive in tubular cells. Collectively, these pathologic findings indicate that SARS-CoV-2 infects kidney parenchymal cells, similar to a closely related β coronavirus, Middle East respiratory syndrome coronavirus.7
Collapsing glomerulopathy (CG) is an aggressive and distinct histologic variant of focal segmental glomerulosclerosis characterized by segmental or global glomerular tuft collapse with hypertrophy and hyperplasia of the overlying podocytes.8 Mouse model data have been variously interpreted to suggest that the extraglomerular cells characteristic of CG may include dedifferentiated podocytes9 or parietal epithelial cells. Because segmental glomerular scars are not always seen, the term CG is preferred to collapsing focal segmental glomerulosclerosis. Accompanying acute tubular injury, tubular dilation with microcyst formation and interstitial inflammation are common.
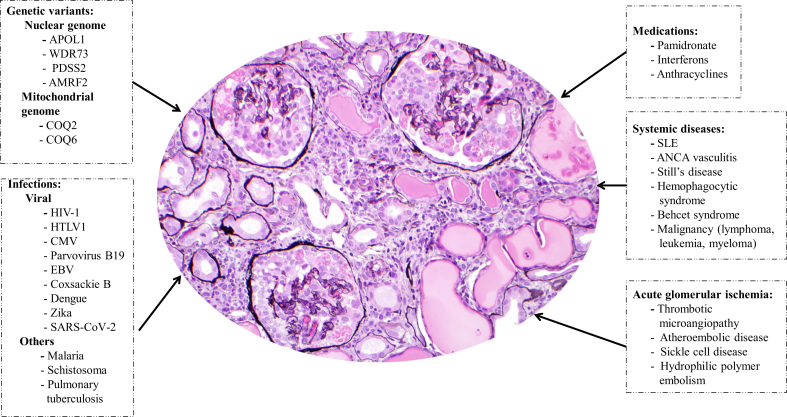
CG can be primary or associated with a wide variety of infectious agents, inflammatory conditions (such as systemic lupus erythematosus and hemophagocytic syndrome), malignancies, glomerular ischemic insult (associated with thrombotic microangiopathy, cholesterol embolization, or sickle cell disease), genetic mutations, and drugs (such as pamidronate and interferon) (Figure 1).8,S1–S3 A causal association between HIV-1 infection and CG is well-established, based in part from work on HIV-transgenic mice. Other viruses, including cytomegalovirus, parvovirus B19, and Epstein-Barr virus, also have been linked to CG.S4
Figure 1.
Conditions associated with collapsing glomerulopathy. Collapsing glomerulopathy is characterized histologically by glomerular tuft collapse with hypertrophy and hyperplasia of the overlying podocytes and podocyte intracytoplasmic protein resorption droplets. It is frequently accompanied by acute tubular injury, tubular dilation with microcyst formation, and interstitial inflammation. There are 5 broad categories of disorders associated with collapsing glomerulopathy: genetic conditions, infections (particularly viral infections including the recently reported association with severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), systemic conditions (including autoimmune, inflammatory, and malignant conditions), medications, and conditions associated with acute glomerular ischemia. AMRF2, action-myoclonus-renal failure syndrome; ANCA, anti-neutrophil cytoplasmic antibodies; APOL1, apolipoprotein L1; CMV, cytomegalovirus; COQ2, Coenzyme Q2; COQ6, Coenzyme Q6; EBV, Epstein-Barr virus; HTLV1, human T-cell lymphotropic virus type 1; PDSS2, decaprenyl diphosphate synthase subunit 2; SLE, systemic lupus erythematosus; WDR73, WD repeat domain 73 (Galloway-Mowat syndrome).
A major genetic contributor to risk for glomerulosclerosis and particularly to CG, regardless of etiology, among patients of African ancestry, is the presence of APOL1 high-risk genotype (carriage of G1/G1, G1/G2, or G2/G2 genotypes). How these APOL1 risk alleles alter podocyte biology, phenotype, and function is not fully understood. Various mechanisms have been proposed from kidney biopsy studies, transgenic mouse studies, and cell culture studies. These include opening of plasma membrane cation channels, impaired mitochondrial function, altered endolysosomal trafficking, inflammasome activation, protein kinase R activation, and most recently, through interference with APOL3 control of actomyosin in podocytes.S5
In this issue of Kidney International Reports, Larsen et al.S6 and Peleg et al.S7 independently report 2 patients with CG associated with COVID-19. Simultaneously, a third case of CG associated with COVID-19 is being reported by Kissling et al.S8 in Kidney International. One of the authors (SHN) is also aware of more than a dozen additional cases that have not been reported yet. The 3 reported patients all presented with severe AKI, heavy proteinuria, and hypoalbuminemia. In 2 of them, the AKI coincided with moderate respiratory symptoms (without associated sepsis or acute respiratory distress syndrome),S6,S8 but interestingly in the third patient, AKI occurred 1 week after recovery from mild respiratory symptoms.S7 Despite improvement of pulmonary symptoms, AKI did not recover in 2 patients, who needed dialysis at discharge. These observations suggest that kidney involvement is independent of lung involvement. Histologically, severe CG, prominent acute tubular injury, diffuse podocyte foot process effacement, and endothelial tubuloreticular inclusions were present.
The pathogenesis of COVID-19–associated CG is likely multifactorial. Coronavirus particles were observed by electron microscopy within the cytoplasm of podocytes in the patient with CG reported by Kissling et al.S8 and by postmortem examination of patients with COVID-19 who had AKI and proteinuria,6 supporting direct viral infection of podocyte. This is not surprising because podocytes (and tubular cells) express membrane-bound angiotensin-converting enzyme 2,S9 the receptor for SARS-CoV-2. Thus, direct toxic viral effect on podocytes, as occurs in HIV-associated nephropathy, is possible in some cases. However, in the 2 other reported cases of COVID-19–associated CG, in situ hybridization studies for SARS-CoV-2 RNA failed to show viral RNA in the kidney and no viral inclusions were seen in renal tissue by electron microscopy,S6,S7 arguing against viral infection. The authors postulated that CG could be a consequence of the cytokine release syndrome characteristic of patients with COVID-19.S6,S7 Indeed, plasma inflammatory markers (C-reactive protein, interleukin-6, and interleukin-2 receptor) were elevated in the patient described by Peleg et al.S7 Importantly, all 3 patients were of sub-Saharan African descent, and the 2 tested had APOL1 high-risk genotype, suggesting that this genotype is an important risk factor, similar to CG associated with HIV and other viruses.S4 It has been previously shown that APOL1 expression is upregulated by viral infections and other inflammatory diseases that activate the Toll-like receptor-3.S10 Viral infections stimulate host interferon production, and interferon is a potent stimulus to APOL1 gene expression.S10 Thus, it appears likely that, in African American individuals, SARS-CoV-2 infection acts as a “second hit” that leads to podocyte dysregulation and injury leading to CG.
In summary, although reports from China indicate that COVID-19 manifestations can include renal tubular injury, there are emerging reports highlighting CG as another renal manifestation of COVID-19. The 2 plausible mechanisms by which SARS-CoV-2 causes CG are direct toxic viral effect on podocytes and/or virus-induced cytokine injury to podocytes. Genetic susceptibility, particularly the presence of high-risk APOL1 genotypes, likely play a crucial role in the pathogenesis of this entity among individuals of African descent. Further work is needed to define the clinico-pathologic characteristics, outcome, and the role of antiviral therapy and corticosteroids for treatment of this lesion, and to understand the molecular mechanisms of podocyte injury by SARS-CoV-2.
Disclosure
All the authors declared no competing interests.
Acknowledgment
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (ZO1-DK043308).
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu B., Li M., Zhou Z. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z., Wu M., Yao J. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 2020.2002.2008.20021212. [Google Scholar]
- 5.Naicker S., Yang C.-W., Hwang S.-J. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China [e-pub ahead of print]. Kidney Int.https://doi.org/10.1016/j.kint.2020.04.003. Accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 7.Alsaad K.O., Hajeer A.H., Al Balwi M. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection—clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 9.Shkreli M., Sarin K.Y., Pech M.F. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2011;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.