Abstract
The coronavirus disease-19 pandemic (COVID-19), which appeared in China in December 2019 and rapidly spread throughout the world, has forced clinicians and scientists to take up extraordinary challenges. This unprecedented situation led to the inception of numerous fundamental research protocols and many clinical trials. It quickly became apparent that although COVID-19, in the vast majority of cases, was a benign disease, it could also develop a severe form with sometimes fatal outcomes. Cytokines are central to the pathophysiology of COVID-19; while some of them are beneficial (type-I interferon, interleukin-7), others appear detrimental (interleukin-1β, -6, and TNF-α) particularly in the context of the so-called cytokine storm. Yet another characteristic of the disease has emerged: concomitant immunodeficiency, notably involving impaired type-I interferon response, and lymphopenia. This review provides an overview of current knowledge on COVID-19 immunopathology. We discuss the defective type-I IFN response, the theoretical role of IL-7 to restore lymphocyte repertoire, as well as we mention the two patterns observed in severe COVID-19 (i.e. interleukin-1β-driven macrophage activation syndrome vs. interleukin-6-driven immune dysregulation). Next, reviewing current evidence drawn from clinical trials, we examine a number of cytokine and anti-cytokine therapies, including interleukin-1, -6, and TNF inhibitors, as well as less targeted therapies, such as corticosteroids, chloroquine, or JAK inhibitors.
Keywords: Sars-CoV2, COVID-19, Cytokine storm, Tocilizumab, Anakinra, Type-I interferon, IL-6, IL-1
Highlights
-
•
COVID-19 induces both impairment and hyperactivation of the immune system.
-
•
Early viral clearance by type-I IFN is a key to preventing further viral replication, T cell exhaustion, and cytokine storm.
-
•
Timely administration of treatments may reduce viral load and avoid hyperinflammation.
1. Introduction
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is a new β-coronavirus responsible for the pandemic viral pneumonia known as COVID-19. The disease started in Wuhan, China in December 2019 and has rapidly spread throughout the world [1]. As of April 24, 2020, the overall outbreak had caused over 191,000 deaths and approximately 2,700,000 infections. SARS-CoV-2 shares genomic similarities with two human coronaviruses, SARS-CoV and MERS-CoV (about 80% and 50%, respectively), which have caused fatal infections in the past 20 years [2]. COVID-19 causes a wide spectrum of clinical manifestations, ranging from asymptomatic or paucisymptomatic forms (with cough, fever, myalgia, and malaise) to full-blown viral pneumonia, which can lead to acute respiratory distress syndrome (ARDS) [3,4]. Currently, three phenotypes are observed in COVID-19 patients, indicating three stages of the infection’s progression and extent: (i) “mild” (benign infection: 80%) in patients with minor and nonspecific symptoms who will not progress to a more severe disease; (ii) “moderate” (overt pneumonia with or without hypoxia and localized inflammation: 15%) in patients requiring hospitalization; and (iii) “severe” (systemic hyperinflammation and ARDS: 5%) in patients who require critical care management and at risk of fatal outcome (1-2%) [5,6]. Despite multiple targeted and non-targeted interventions, no treatment has yet proven effective in treating COVID-19. Numerous clinical trials are ongoing.
As the pandemic progresses, the pathophysiology of COVID-19 is becoming clearer and the potential of multiple cytokine and/or anti-cytokine interventions is raised. Indeed, it has been reported that COVID-19 associates states of both immunodeficiency and hyperinflammation, with the latter being manifested by a cytokine storm.
In this paper, we summarize today’s principal findings and attempt to establish a more definitive view of COVID-19 pathophysiology, focusing on one key question: “How can antiviral immunity be reinforced and hyperinflammatory damages be avoided?” In the second half of this paper, we will describe the early results of clinical investigations which have assessed targeted and non-targeted therapies.
In this review, we will not discuss antiviral strategies used to treat COVID-19, nor will we discuss how to manage the wide spectrum of SARS-CoV2 infection-related complications, such as thromboembolism, vasculitis (chilblains), acute kidney injury or neurologic involvements.
2. Overview of COVID-19 immunopathology
2.1. Act 1: the virus entry
SARS-CoV-2 is a zoonotic β-coronavirus, which infects human airways and enters cells by binding its S (spike) protein envelope to the human angiotensin-converting enzyme 2 (hACE2) after S protein priming by host serine protease TMPRSS2 [7]. hACE2 is present in type II alveolar cells (representing about 80% of all hACE2-expressing cells), nasal mucosa, upper respiratory tract, endothelium, heart, kidney, and intestine cells [8]. It has been reported that patients with diabetes, hypertension, or patients treated with ibuprofen are at higher risk of contracting severe COVID-19 [[9], [10], [11]]. In these patients, because there is increased expression of hACE2 on lung epithelial cells, some authors have postulated a connection between these points [12]. Of note, another receptor, CD147, has been implicated in mediating host cell invasion by SARS-CoV-2 [13]. After binding to its receptor, the virus enters the cells through endocytosis, viral RNA is released into the cytosol, the virus exploits the cell machinery to replicate, and it is further excreted from the cell by exocytosis [14].
Lung injury directly induced by the virus remains poorly explained. Patients with high viral loads and long virus-shedding periods are at higher risk of severe COVID-19 [15]. The early onset of rapid viral replication may cause massive epithelial and endothelial cell apoptosis, vascular leakage, as well as pro-inflammatory mediator release [16]. Furthermore, hACE2 downregulation and shedding by viral S protein can cause dysfunction of the renin-angiotensin system and exacerbate inflammation and vascular permeability leading to acute lung injury [17,18]. Finally, recent data have suggested that SARS-CoV-2 can directly infect T cells through receptor-dependent, S protein-mediated membrane fusion [19]. Nevertheless, T cells have a very low expression level of hACE2, suggesting either an alternative receptor or high S protein affinity for hACE2. T cell infection is abortive, meaning that SARS-CoV-2 cannot replicate within T cells but rather induces cell death [19]. In SARS-CoV infection, the modulation of TNF-α-converting enzyme (TACE or ADAM17) by the spike protein of SARS-CoV and hACE2 induces TNF-α production which may accentuate T cell apoptosis [20,21]. In this vein, Xiong et al. reported upregulation of apoptosis, autophagy, and p53 pathways in PBMCs from COVID-19 patients, when compared to healthy controls [22].
2.2. Act 2: the innate immune response, first cytokine wave
Epidemiological studies have demonstrated an elevation of acute phase reactants in patients with COVID-19, including ESR, C-reactive protein (CRP), serum amyloid A, and ferritin, suggesting a rapid activation of the innate immune response [3,[23], [24], [25]]. Accordingly, COVID-19 patients have high levels of circulating TNF-α, IL-1β, IL-1Ra, sIL-2Rα, IL-6, IL-10, IL-17, IL-18, IFN-γ, MCP-3, M-CSF, MIP-1a, G-CSF, IP-10 and MCP-1 [23,26].
These results are suggestive of hypercytokinemia, which is a hallmark of COVID-19. Nevertheless, only the serum concentrations of certain of these cytokines make it possible to discriminate between mild, moderate, and severe cases (mainly IL-1 β, IL-1Ra, IL-6, IL-7, IL-10, IP-10, and TNF-α) [26]. In addition, the levels of these cytokines in mild/moderate cases are generally below levels observed in usual macrophage activation syndrome/reactive hemophagocytic lymphohistiocytosis (MAS/reHLH) or in severe cytokine release syndrome (CRS) [27,28]. Thus, hypercytokinemia should be regarded as a general marker of SARS-CoV-2, while the term ‘cytokine storm’ should be kept for those situations of overly exuberant inflammation leading to critical conditions, such as ARDS, disseminated intravascular coagulation or multiple organ failure.
Within cells, RNA viruses are sensed by the innate immune system through three major classes of pattern recognition receptors (PRRs): toll-like receptors (i.e. TLR-3, -7, -8), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs) [29]. Among TLRs, only TLR-7 and -8 recognize single stranded viral genomic RNA. Nonetheless, other cytosolic sensors such as TLR-3 and RIG-I/MDA5 may detect the virus intermediates during intracellular replication, including double stranded RNA [30]. These recognitions then activate downstream signaling effectors, i.e. NF-ĸB and IRF3/7. NF-ĸB promotes the transcription of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, further triggering Th1 and Th17 inflammatory responses with subsequent secretion of IFN-γ and IL-17. IRF3/7 promote type-I IFN production, which in turn activates the JAK1/TYK2-STAT1/2 pathway. STAT1 and STAT2 form a complex with IRF9 that translocates into the nucleus where it initiates the transcription of hundreds of genes called IFN-stimulated genes (ISGs). An early and robust type-I IFN response is required as the very first line of defense to suppress viral replication and spread.
Early NLR engagement has also been postulated during the innate immune response against coronaviruses [16]. Evidence of NLRs directly recognizing viral RNA is scant [31], however, NLRs are more possibly involved in detecting intracellular homeostasis perturbations or danger signals, such as ROS production [32,33]. Evidence of their involvement can be found in the observation of high levels of circulating IL-1β, IL1-Ra, and IL-18 [23,26] and synergistic upregulation of IL1 and IL1RN genes in the lung [34]. Indeed, some NLRs are able to assemble a multimolecular platform termed inflammasome (via recruiting the adaptor ASC, apoptosis-associated speck-like protein containing a CARD), which in turn activates the proinflammatory caspase-1. Caspase-1 cleaves and activates IL-1β and IL-18, and induces a hyperinflammatory form of cell death, termed pyroptosis, through the cleavage of the pore-forming gasdermin-D [32,35]. IL-1 family cytokines include IL-1α, IL-1β, IL-18 and their bioavailability is regulated by soluble antagonists (IL-1Ra, IL-18BP) of either endogenous origin or resulting from therapeutic intervention [36,37]. The most frequently studied inflammasome sensor, NLRP3, drives inflammation during SARS-CoV infection through several activating pathways [[38], [39], [40], [41]]. Given the similarities between SARS-CoV and SARS-CoV-2, comparable mechanisms likely intervene during the acute phase of COVID-19.
Of note, the cytosolic sensor RIG-I can also associate with ASC to form a non-canonical inflammasome inducing caspase-1 activation and subsequent IL1-β/IL-18 secretion and pyroptosis [42]. IL-1β secretion may itself contribute to hypercytokinemia since IL-1β further induces the expression of other proinflammatory cytokines such as TNF-α and IL-6.
Lastly, high levels of chemokines and their coupled receptors have been observed in COVID-19 patients [22,23,26]. Accordingly, analysis of the molecular signature within the BALFs of patients revealed a plethora of upregulated chemokine transcripts, including neutrophil-recruiting mediators (CXCL8, CXCL1, CXCL2, CXCL10, CCL2, CCL7) and other attractants of monocytes and immune cells (CXCL6, CXCL11, CCL2, CCL3, CCL4, CCL7, CCL8, CCL20) [34]. These results are consistent with pathological findings attesting to lung infiltration by monocytes, macrophages and neutrophils, in contrast with lower amounts of lymphocytes [43]. This hyperactivated chemotaxis may play a major role in developing a pulmonary-centric disease by driving accumulated immune cells into the lungs and restricting the immune response to this particular organ.
2.3. Act 3: the immunodeficient state
As stated above, type-I IFN is critical for protecting against viral infections, since it promotes intracellular RNA degradation and virus clearance, induces tissue repair, and triggers a prolonged adaptive immune response [[44], [45], [46]]. Type-I IFN is mainly produced by plasmacytoid dendritic cells (pDCs), which are less sensitive to productive viral infection and/or virus-mediated cytotoxicity and can produce other inflammatory cytokines, such as TNF-α and IL-6 or control T cell response [47,48]. pDCs are circulating immune cells, which act as sentinels and are activated after physical contact with virally-infected cells and transfer of PAMPs to the TLR7 sensors in pDCs, part of a process termed interferogenic synapse [49]. This synapse enables robust type-I IFN production at the infected site, thereby possibly limiting viral replication and systemic deleterious response. Data derived from the SARS-CoV and MERS-CoV outbreaks have revealed that coronaviruses suppress type-I IFN response by interfering with PRRs or type-I IFN receptor-signaling pathways. For instance, SARS-CoV induces the degradation of RNA sensor adaptor molecules, MAVS and TRAF3/6, thus inhibiting IRF3 translocation into the nucleus. MERS-CoV employs additional strategies, such as repression of histone modifications. Both viruses inhibit JAK/STAT signaling by decreasing STAT1 phosphorylation.
Considering the 80% overlap between SARS-CoV and SARS-CoV-2, it has been speculated that the latter uses similar strategies to dampen innate immune response. Nevertheless, SARS-CoV2 appears to have lower pathogenicity than SARS-CoV (with about a 10%-fatality rate) suggesting a lesser IFN antagonism [50]. On the other hand, exceptional genetic defects or immunosenescence may account for a reduced type-I IFN response and greater severity of COVID-19. Accordingly increased age is associated with a poorer outcome [3,6,23,51].
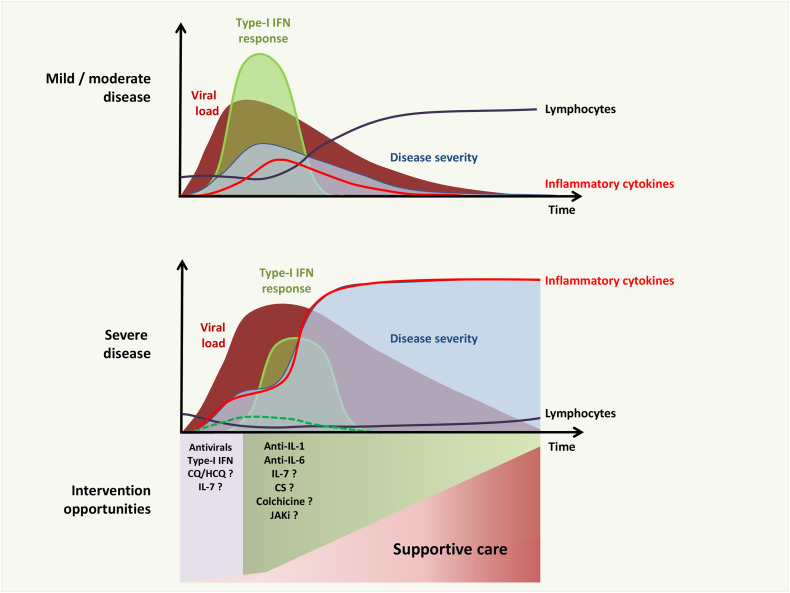
In mouse models of SARS-CoV and MERS-CoV infections, a delayed type-I IFN response may explain more severe disease, with compromised virus control and paradoxical hyperinflammation induced by type-I IFN itself [52]. This leads to an influx of neutrophils and monocytes-macrophages (the major sources of pro-inflammatory cytokines) and further apoptosis of T cells, epithelial and endothelial cells [[52], [53], [54], [55]]. These acute inflammatory mechanisms damage the pulmonary microvascular and alveolar barrier and cause vascular leakage and alveolar edema, converging to ARDS. Therefore, not only the strength of the response but also its timing would appear to play a critical role in coronavirus infection (Fig. 1 ).
Fig. 1.
Kinetics and intensity of the antiviral response are decisive in COVID-19 outcome. In mild to moderate COVID-19, the early antiviral response, mostly type-I interferon (IFN), allows the rapid reduction of viral load and prevents T-cell depletion and hypercytokinemia. In severe COVID-19, delayed (solid green line) or low (dotted green line) antiviral response results in elevated lung cytokine/chemokine levels, impaired virus-specific T-cell responses, and acute clinical deterioration. Optimal times for therapeutic interventions are proposed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In vitro, SARS-COV-2 displays a greater sensitivity to type-I IFN than SARS-COV[56]. Furthermore, SARS-CoV-2 does not impair STAT1 phosphorylation. Interestingly, mild/moderate infection by SARS-CoV-2 is associated with a potent type-I IFN response, characterized by the robust expression of ISGs in patients’ BALFs [34]. Moreover, preliminary results have revealed early IFN production in peripheral blood from patients with mild/moderate to severe forms during the first week of COVID-19 ([203]). In contrast, about 20% displayed no IFN production. Another study found the type-I IFN response to be high (between days 8-12) in mild-to-moderate patients while reduced in more severe patients who had a striking downregulation of IFN-stimulated genes [57]. These results are in line with previous conclusions and would indicate that the characteristics of type-I IFN response - both in terms of kinetics and intensity - could be linked to clinical outcome [52]. How exactly SARS-CoV-2 escapes type-I IFN in some patients remains to be elucidated.
Lymphopenia is one of the most prominent markers of COVID-19 and has been observed in over 80% of patients [23,58,59]. Analyses have shown that all subsets of lymphocytes were decreased, including CD4+ and CD8+ cytotoxic T cells [57,60], natural killer (NK) cells, memory and regulatory T cells [61] along with B cells [62]. Lower lymphocyte counts are closely linked to severe disease [61,62]. In addition to being quantitatively decreased, T cells exhibit elevated exhaustion levels and reduced functional diversity [60,63]. T cell numbers are negatively correlated with serum IL-6, IL-10 and TNF-α and with higher levels of exhaustion markers, such as PD-1 or Tim-3 [57,60,64]. Patients with severe form of COVID-19 have less multifunctional and more non-functional CD4+ T cells, as well as fewer non-exhausted CD8+ T cells than patients with mild COVID-19 [63].
Several explanations are posited to explain this SARS-CoV-2-induced lymphopenia.
First, the virus can directly infect T cells but cannot replicate within the cells [19]. T cell infection may thus result in cell death by apoptosis, necrosis, or pyroptosis [19,65,66].
Second, a number of inhibitory cytokines are released by infected lung macrophages or epithelial cells (first wave of hypercytokinemia), including TNF-α which causes T cell apoptosis [21], IL-10 which is known to prevent T cell proliferation [67], and type-I IFN which regulates lymphocyte recirculation [68]. T cell exhaustion, characterizing severe COVID-19, may stem from increased PD-1 or Tim-3 expression, from elevated levels of inhibitory IL-10 [60] or from increased IL-6-induced expression of SOCS3 [[69], [70], [71]]. Interestingly, IL-2 and IL-7, the cytokines responsible for expansion and differentiation of various T cell subsets are increased in the sera of patients having either mild/moderate or severe forms of COVID-19 [23]. Nevertheless, these increased levels most likely represent attempts by the immune system to reverse lymphopenia and T cell exhaustion.
Lastly, lymphopenia has been thought to be the result of immune cells redistribution, with accumulation of lymphocytes in the lungs or lymphoid organs [72]. However, though pathological results are scarce and contradictory, it would seem that alveolar or interstitial tissues are not invaded by lymphocytes but rather by monocytes, macrophages and moderate numbers of multinucleated giant cells [43]. More intriguingly, autopsies have revealed that secondary lymphoid tissues had been destroyed with atrophied spleen and decreased lymphocyte numbers, accompanied by significant cell degeneration, focal hemorrhagic necrosis, plus macrophage proliferation and phagocytosis. Lymph nodes were atrophied and their number decreased, with signs of necrosis. Immunohistochemical staining demonstrated decreased rates of CD4+ and CD8+ T cells in the spleen and lymph nodes [61].
2.4. Act 4: the cytokine storm, a lethal second wave
Sudden and rapidly progressing clinical deterioration has been widely mentioned in late stages of COVID-19 (around 7-10 days). This often manifests as an unexpected aggravation of symptoms (fever, dyspnea) and is correlated with increased levels of acute phase reactants (ESR, CRP, ferritin), coagulopathy (elevated titers of d-dimers, disseminated intravascular coagulation), and cell lysis (CK, LDH) [3,4,11,23,59]. In the most severe patients, clinical and laboratory parameters correlated with increased levels of proinflammatory cytokines (IL-1β, IL-1Ra, IL-6, TNF-α, and sIL2-Rα), evocative of a cytokine storm [3,[73], [74], [75]]. Interestingly, ARDS occurs in SARS-CoV patients despite a diminishing viral load, suggesting that exuberant host immune response may be responsible for this outcome rather than viral virulence. Such a cytokine profile is strongly reminiscent of both Cytokine Release Syndrome (CRS, seen in CAR T cell therapy) and hemophagocytic lymphohistiocytosis (HLH) [76,77]. Numerous authors have paralleled the COVID-19 cytokine storm to either primary or reactive HLH (reHLH) because of its close resemblance, including high fever, cytopenia, hyperferritinemia, abnormal liver tests, coagulopathy, and pulmonary involvement (including ARDS), occurring in approximately 50% of patients with reHLH [73,74,78]. In adults, reHLH is most often triggered by viral infections and is observed in 3 - 4% of sepsis cases [79]. Herpesviridae (e.g. Epstein-Barr virus) and influenza are major triggers of such cytokine storms [77]. Systemic diseases, like systemic lupus erythematosus, or the autoinflammatory adult-onset Still’s disease and its pediatric counterpart, can also be complicated by cytokine storm, known as the macrophage activation syndrome (identical to reHLH) [[80], [81], [82]]. In all these conditions, IL-1β, IL-18, IFN-γ, and IL-6 are key mediators of hyperinflammation.
Similar to what is observed in SARS-CoV-2 infection, the immunodeficiency linked to abnormal T cell number or function (genetically determined in primary HLH) appears to be the primum mobile of most cytokine storms [[83], [84], [85], [86], [87]]. Although an alternative pathway of primary hyperactivated innate immunity is possible, it is more likely that this cytokine storm occurs due to the combination of a defective (or delayed) first line of defense, followed by persistent hypercytokinemia (IL-6, IL-1β, and TNF-α), and dysfunctional T cell response (generally cytotoxicity). This results in an impaired clearance of apoptic cells or infected/activated macrophages, an increase in viral replication and dissemination, followed by an IL-18/IFN-γ feedforward loop activating macrophages, culminating in multiple cytokine release, hemophagocytosis, coagulopathy, and ARDS (Fig. 2 ) [85,88,89]. Some of these mediators may further fuel this vicious cycle, including NK cell function impairment by IL-6 [71] or macrophage activation by the H-chain of ferritin [[90], [91], [92]]. Quite importantly, hemophagocytosis has been reported in lung tissues from patients who succumbed to SARS-CoV infection [93].
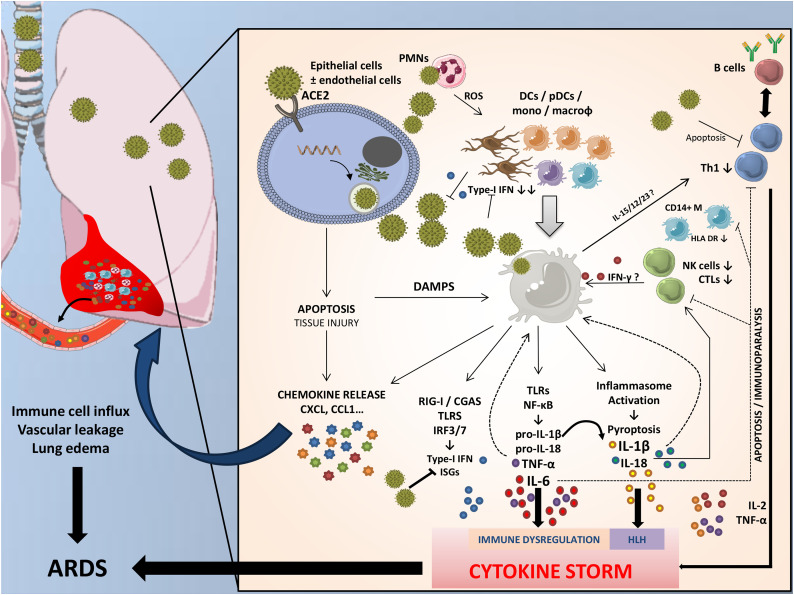
Fig. 2.
The immunopathology of COVID-19.
The entry of SARS-CoV-2 in epithelial/endothelial cells, via binding to ACE2 (and CD147), induces apoptotic and necroptotic pathways resulting in lung injury and release of numerous chemokines driving the recruitment of large amounts of immune cells within the lungs. Dendritic cells (DCs) and plasmacytoid DCs (pDCs, the main source of type-I interferon (IFN)), along with alveolar macrophages and neutrophils, promote the innate immune response by secreting alarmins and antiviral or proinflammatory cytokines, plus presenting the antigen to adaptive immune cells. SARS-CoV-2 may have evolved strategies to downregulate the type-I IFN response and induce T cell apoptosis. The recognition of molecular patterns (viral RNA, particles, or danger signals) by various Toll-like receptors (TLRs), NOD-like receptors (NLRs) or RIG-I like receptors (RLRs) activates the transcription and release of proinflammatory mediators, such as interleukin (IL)-1β, -6, -18, and tumor necrosis factor (TNF)-α. These mediators further skew naïve T-cells to Th1 or cytotoxic lymphocytes (CTLs or CD8+), which in turn secrete amounts of cytokines. A pro-inflammatory feedforward loop of cytokines on innate immune cells results in cytokine storm, coagulopathy, and acute respiratory distress syndrome (ARDS). COVID-19 cytokine storm may intertwine two mechanisms; one highly suggestive of macrophage activation syndrome (hemophagocytic lymphohistiocytosis, HLH) driven by IL-1β, and another pattern characterized by immune dysregulation driven by IL-6, which triggers immunoparalysis (decreased HLA-DR on CD14 monocytes) and global lymphopenia.
Thus, in COVID-19, the onset of ARDS may be the consequence of a HLH-like syndrome, after a first period of lung damage, mainly localized within the lungs due to a prior accumulation of large amounts of innate immune cells. Systemic features of HLH are lacking (such as extremely high titers of ferritinemia, organomegaly, and severe end-organ disease) but this is most probably the consequence of lymphoid organ immune cell depopulation of [43].
Analysing more precisely the immunopathology of SARS-CoV2-related ARDS, Giamarellos-Bourboulis et al. have come to the conclusion that two patterns of immune dysfunction exist in worsening COVID-19: (i) one pattern highly suggestive of macrophage activation syndrome (hyperferritinemia and elevated H score : 25% of patients), which is driven by IL-1β; and (ii) one pattern with immune dysregulation driven by IL-6 [71]. The latter was characterized by a combination of hypercytokinemia, immunoparalysis (as indicated by decreased HLA-DR molecules on CD14 monocytes), and global lymphopenia (including CD4+ and NK cells). Interestingly, IL-6 blockade with tocilizumab partially restored HLA-DR expression on CD14 monocytes and increased the circulating lymphocyte count.
A number of key points stand out in this overview of the immunopathology of SARS-CoV-2 infection:
-
1)
While the virus induces both impairment and hyperactivation of the immune system, these are sequential, and the latter seems to ensue from the former, at least partially;
-
2)
An early viral clearance by type-I IFN is a key to preventing further viral replication, T cell exhaustion, and subsequent cytokine storm;
-
3)
Therapies (when applicable) should be administered with the right timing; i.e. antivirals and immune boosters should be initiated promptly after symptom onset, whereas immunosuppressants should be administered at the very start of the cytokine storm (Fig. 1);
-
4)
Given the great frequency of non-severe presentations, therapeutic interventions should be kept for selected patients (i.e. those with risk factors or worsening diseases). Nonetheless, except for advanced age, elevated body mass index, and comorbid conditions, there is still a lack of robust prognostic factors [3,23,24,75].
Routine and non-routine markers can be monitored to help evaluate the risk of developing cytokine storm. However, at present, no cut-off value has yet been proposed for any of these markers. It would be of great benefit to analyze their trends in depth, along with daily clinical evaluation.
3. Cytokine-based interventions
3.1. Type-I interferon
Since type-I IFN’s key role in antiviral response and low or delayed IFN response has been associated with poor outcome, IFN-α and IFN-β have emerged as potentially effective drugs against SARS-CoV-2 [52,94]. Evidence is mainly derived from experience with SARS-CoV and MERS-CoV infections [95]. In vivo, type-I is most often used in combination with other antiviral drugs, such as lopinavir/ritonavir [[96], [97], [98], [99]] or ribavirin [[100], [101], [102]]. In vitro, IFN-α and -β have very systematically demonstrated robust efficacy against coronaviruses, but proved disappointing when transposed to human diseases [95,96,100,103]. Multiple biases in these trials have prevented drawing conclusions from them. These biases include: coronavirus escape strategies to type-I IFN, limited sample sizes, heterogeneous experimental designs/clinical conditions, the nature of IFN isoform, administration route, plus difficulties in assessing whether disease outcome was linked to type-I IFN or to the drugs used in combination. Key points which have emerged from these studies are: (i) IFN-β1b and IFN-β1a are the most potent subtypes for SARS-CoV inhibition (probably even more for SARS-CoV-2) [56,104,105]; (ii) type-I IFN must be administered as soon as possible after infection (ideally before symptom onset) although not in the late phase, because of possible tissue damage [5,52,106,107]; and (iii) although IFN-α inhalation can lower SARS-CoV-2 infection rate and serve in prophylaxis or treatment (Chinese guidelines) [108], the preferred solutions remain the better-evaluated, safer intravenous and subcutaneous routes [94,109].
Even though type-I IFN is being evaluated in several clinical trials, either alone (NCT04293887, NCT04320238, ChiCTR2000029989) or in combination (NCT04254874, NCT04276688, NCT04273763, NCT04315948, NCT04350684, NCT04350281, NCT04343768, NCT04350671), only one single retrospective study is currently available [110]. Zhou et al. compared 77 adults with laboratory-confirmed COVID-19 who were treated with either nebulized IFN-α2b (5 mU b.i.d.), oral umifenovir (200 mg t.i.d.), or a combination of both [110]. The authors found that treatment with IFN-α2b - with or without umifenovir - significantly reduced the duration of detectable virus in the upper respiratory tract and reduced the duration of elevated inflammatory markers (IL-6 and CRP). Unfortunately, this study has major flaws: (i) the study did not include a control group; (ii) only moderate forms of COVID-19 were included, with a median time from symptom onset to treatment of 11 days (range, 5 -22); (iii) patients in the IFN-α-treated group were significantly younger and had fewer comorbidities; (iv) the only patients treated with IFN-α2b alone were women; and (v) treatment regimens were quite heterogeneous since, in the combination group, 34% of patients received IFN-α2b as add-on to umifenovir and 52% started IFN-α2b after umifenovir had been stopped. None of the patients developed end organ dysfunction, nor respiratory failure requiring oxygen or critical care management. Interestingly, another study prospectively evaluated IFN-α1b nasal drops to prevent medical staff SARS-CoV-2 infection [111]. 2,944 medical staff members were allocated to either a low-risk group (n=2,415, 4-6 drops q.i.d. for 28 days) or a high-risk group (nasal drops + subcutaneous thymosin-α1 every week) depending on their exposure to the coronavirus. The control group consisted of medical staff in the same areas of Hubei Province, China. The 28-day incidence of COVID-19 amounted to zero for both groups, whereas over 2,000 new COVID-19 cases were diagnosed among medical personnel over the same period. Despite problems of major bias, this study suggests that IFN-α1b could serve as an efficient prophylactic against COVID-19.
Additional well-designed studies involving timely administration of IFN-α are thus eagerly awaited.
3.2. Interleukin-7
Lymphopenia and lymphocyte exhaustion are hallmarks of COVID-19 and are possibly responsible for the cytokine storm due to defective clearance of both virus and infected cells. It is thus tempting to speculate that IL-7 - the major cytokine promoting lymphocyte expansion and possibly reversal of T cell exhaustion - may be useful in restoring immune system homeostasis. IL-7 exerts anti-apoptotic properties and induces potent proliferation of naive and memory T cells leading to replenishment of the circulating pool (CD4+ and CD8+) [112,113]. IL-7 also increases T cell receptor repertoire diversity [114] and promote the expression of cell adhesion molecules, improving the capacity of T cells to traffic to infection sites [115]. More importantly, the administration of recombinant IL-7 (rIL-7) does not induce hyperinflammatory response through stimulating adaptive immunity. Indeed, rIL-7 has been successfully used to treat T cell exhaustion following septic shock and restore CD4+ T cells in HIV patients, with no evidence of clinical deterioration nor proinflammatory marker exacerbation (TNF-α, IL-6, CRP) [116,117]. Surprisingly, at the time of writing this review, there is no trial registered yet to evaluate this strategy. If such a study were to be launched, the perfect timing for lymphocyte restoration would need to be strictly determined.
4. Anti-cytokine interventions
4.1. Interleukin-6 inhibition
Not only is IL-6 a major mediator within the cytokine storm, its levels have been closely correlated with ARDS severity and outcome, along with blood SARS-CoV-2 viral load [71,74,118]. A recent meta-analysis reported 2.9-fold higher serum levels in patients with complicated COVID-19 compared to patients with non-complicated disease [119]. Inhibitors of IL-6 or its receptor have been successful in treating other cytokine storm syndromes, such as reHLH associated with adult-onset Still’s disease [120], or CRS secondary to CAR T cell therapies [28,121]. Several drugs are available, including IL-6 receptor inhibitors (tocilizumab, sarilumab) and IL-6 inhibitors (siltuximab, clazakizumab, sirukumab) [122].
To date, only four studies and case reports are available [[123], [124], [125], [126], [127]].
Xu et al. have reported on 21 severe or critical patients treated with tocilizumab although there was no control group [123]. This first study demonstrated decreased oxygen requirements (75%), resolution of CT-scan abnormalities (90.5%), and clinical improvement (100%). No adverse events or deaths were reported. To estimate the results of a potential control group not administered tocilizumab, Xu et al. referred to the results of a previous study which found a baseline mortality rate of 60% in critical patients and 11% in severe patients admitted to one ICU [9].
The second study, from China, retrospectively analyzed 15 patients (median age, 73 years) with moderate (n=2), severe (n=6), and critical (n=7) forms of COVID-19 [124]. Eight patients received concurrent methylprednisolone. Three patients died (all from the critical group), 1 patient improved, 9 were clinically stabilized and 2 experienced disease worsening. A persistent increase of serum IL-6 was observed in 4 (out of 5) patients resulting in either aggravation or fatal outcome.
The third study, performed in Italy, analyzed 21 patients with COVID-19 who developed ARDS and were enrolled into a compassionate-use program [125]. All patients received siltuximab at doses ranging from 700 to 1,200 mg. Clinical improvement was observed in 33% of patients, 43% stabilized as evidenced by absence of clinically-relevant change in condition, and 24% experienced a worsened condition. One patient died, and one patient suffered a cerebrovascular event. The absence of control group precludes final conclusions, basically because the mortality rate for patients undergoing standard of care was not mentioned.
Finally, Roumier et al. reported their experience regarding tocilizumab in 30 selected patients (23% in ICU) [127]. Patients were aged <80, with >5-days disease duration, and with severe pneumonia (i.e. requiring > 6L/min of oxygen) and rapidly deteriorating (i.e. increase by more than 3L/min of oxygen flow within the previous 12 hours). The control group (standard of care) included patients matched for age, gender, and disease severity. Patients were followed-up for a median of 8 days. Tocilizumab significantly reduced mechanical ventilation requirement (OR: 0.42; p=0.025) and risk of subsequent ICU admission (OR: 0.17; p=0.001). There was a trend, though no statistical difference, toward lower mortality. Tocilizumab was well-tolerated, yet the author reported two mild liver test disturbances, and one ventilator-acquired pneumonia.
These mixed results must be further clarified. Most notably, since experimental models have shown that IL-6 can either suppress or facilitate viral replication [128], one crucial remaining question to answer will be the optimal timing for anti-IL6 administration. If too early, the drugs may adversely affect viral clearance. If too late, the drugs may not be effective enough. The perfect timing of this must be assessed in trials. At present, several clinical trials are under way to evaluate the safety and efficacy of IL-6 inhibitors, with various protocols and comparators (clinicaltrials.gov identifiers: NCT04332913, NCT04322773, NCT04317092, NCT04320615, NCT04306705, NCT04324073, NCT04315298, NCT04315480, NCT04321993, NCT04335071, NCT04348500, NCT04329650, NCT04330638, NCT04345289, NCT04327388, NCT04341870). In China, tocilizumab is also being evaluated either alone or in combination (identifiers: ChiCTR2000029765, ChiCTR2000030796, ChiCTR2000030442, ChiCTR2000030894).
It is noteworthy that since March 2020 tocilizumab has been formally included in the National Health Commission of China's COVID-19 diagnosis and treatment program (7th edition): “Tocilizumab can be used in patients with extensive bilateral lung lesions opacity or in severe or critical patients, who have elevated laboratory detected IL-6 levels”. More recently, the Infectious Diseases Society of America (IDSA) has published guidelines and recommends that, for patients who have been admitted to the hospital with COVID-19, tocilizumab should be used only in the context of a clinical trial, due to “knowledge gap” [129].
4.2. Interleukin-1 family: IL-1 and IL-18 blockade
IL-1β and its natural antagonist (i.e. IL-1Ra) have been observed in the peripheral blood and BALF of patients with COVID-19-induced pneumonia [22,23,26,71]. This is a striking result since IL-1β has a short half-life in serum and is rarely isolated in peripheral blood. This finding suggests high levels of IL-1β production, reinforced by reports indicating IL1B and IL1R1 gene upregulations [57].
IL-1β is a proinflammatory cytokine that is activated and secreted upon activation of the inflammasome [32]. NLRP3, the most-frequently studied inflammasome, is activated by danger signals, which have been suggested to be viroporin A, E protein, or ORF3 proteins from SARS-CoV and MERS-CoV [38,40,130]. Pyroptosis has also been associated with coronavirus infection [16,131]. Elevated IL-1β is central to ARDS [132] and HLH [89,133]. Indeed, HLH is a well-known and frequent complication in about 10% of juvenile and adult-onset Still’s disease, whith pathophysiology marked by very high levels of IL-1β [81,134]. Anakinra, a recombinant IL-1Ra, has proved effective in treating HLH (via continuous intravenous infusion) [135,136], and it is currently used in IL-1-induced autoinflammatory diseases, including cryopyrinopathies (dominant NLRP3 gain-of-function) [137], familial Mediterranean fever (pyrin inflammasome deregulation) [138], PFAPA [139], and Still’s disease [140]. In a reanalysis of data from a phase-III randomized trial, anakinra was associated with significant survival improvement in patients with severe sepsis and features of reHLH, without triggering any serious adverse events [141]. Today, anakinra is being evaluated in this context in the PROVIDE study (NCT03332225). Often efficacious within hours, anakinra has a short half-life and is considered safe. It may thus constitute a drug of choice for certain selected COVID-19 patients presenting signs of imminent cytokine storm. So far, no clinical data are available but clinical trials are ongoing to test anakinra in COVID-19 (NCT04330638, NCT04341584, NCT04339712, NCT04324021).
Canakinumab, a monoclonal antibody targeting IL-1β, is also being investigated in a single-arm observational study (NCT04348448); however its longer half-life (26 days) may be problematic for infected patients.
IL-18 is mainly produced by macrophages as an inactive precursor which is processed by inflammasome-activated caspase-1, like IL-1β [32,142]. By binding to IL-18R, IL-18 (in combination with IL-12/-15) acts on CD4+, CD8+ T cells, and NK cells to induce IFN-γ, the major driver of macrophage activation syndrome (i.e. HLH) [85,86,89,143]. IL-18 is one of the upregulated cytokines in sera and BALF from COVID-19 patients [26,144]. In addition, IL-18 has been reported as a biomarker of Still’s disease, its levels being correlated with disease activity [145]. High levels of serum IL-18 have also been reported in patients with a newly-described autoinflammatory disease associating pulmonary alveolar proteinosis and recurrent macrophage activation syndrome (IL18PAP-MAS) [146]. Tadekinig alfa is a recombinant IL-18 binding protein (IL-18BP, a naturally-occurring inhibitor of IL-18) which has shown encouraging results in Still’s disease [135,136]. Interestingly, rIL-18BP was uused to successfully treat a 6-week old girl with life-threatening NLRC4-associated hyperinflammation (HLH), which was refractory to corticosteroids, IL-1 blockade, anti-TNF, cyclosporine, and vedolizumab [147]. Although blocking IL-18 could be of interest in COVID-19, there is no clinical evidence, nor any registered RCT assessing the safety and efficacy of rIL-18BP in this framework.
4.3. Interferon-γ inhibition
Intriguingly, while being central to cytokine storm occurrence in SARS-CoV infection [148], IFN-γ was not found to be elevated in the sera or BALFs of patients with severe forms of COVID-19 [22,23,26,149]. Actually, there are even conflicting results showing a mild elevation in the sera of non-severe patients [23,26,75,150]. However, one meta-analysis has shown a link between high IL-6/IFN-γ ratio and disease severity, thereby indicating that lower levels of IFN-γ should rather predict poorer outcomes [151]. Emapalumab, a monoclonal antibody directed against IFN-γ is approved for treating pediatric and adult primary HLH patients with refractory, recurrent, or progressive disease or intolerance to conventional therapy (despite a lack of clinical trial data in adults) [152,153]. However, in the single RCT on 34 patients, serious adverse reactions occurred in 53% of the recipients, including (bacterial, viral, and opportunistic) infections and multiple organ dysfunction syndrome. In addition, data from mouse models suggest that simultaneously inhibiting multiple cytokine-signaling pathways (such as with JAK inhibitors) may be more effective than targeting IFN-γ alone [154,155]. RCTs are now ongoing to evaluate emapalumab in HLH (either primary or reactive: NCT01818492, NCT0331275, NCT03985423, NCT03311854) and in COVID-19 (NCT04324021).
4.4. Tumor necrosis factor-α inhibition
TNF is present in COVID-19 patients’ blood and diseased tissues and its levels are even higher in patients with severe disease [23,61,75,156]. Since TNF inhibitors are readily available and well-evaluated drugs, some authors postulate that there is sufficient evidence to conduct clinical trials in COVID-19 [157]. Nevertheless, the literature remains very scanty when exploring possible treatments for cytokine storm or HLH. Indeed, while a few case reports described the benefits of etanercept [[158], [159], [160]], other studies have shown that it may trigger or worsen disease progression [[161], [162], [163]]. However, some cases argue for TNF inhibitors use in COVID-19: (i) TNF neutralization provides protection against SARS-CoV infection in animal models [164]; (ii) anti-TNF induce a rapid decrease of IL-6 and IL-1 concentrations in patients with active rheumatoid arthritis [165]; (iii) anti-TNF trigger a reduction of adhesion molecules and vascular endothelial growth factor, which is partly responsible for capillary leak [166,167]; and (iv) anti-TNF lead to less leucocyte traffick to inflamed tissues due to reduced adhesion molecules and chemokines with subsequent reduction of cell content and exudate [168]. It is suggested that initial assessments of TNF inhibitors should be done in patients with moderate disease, as soon as possible after their admission to hospital [157]. To date, only a single RCT evaluating adalimumab in COVID-19 has been registered (ChiCTR2000030089).
4.5. Non-targeted therapies
4.5.1. Corticosteroids
Corticosteroids are potent cytokine inhibitors working through several mechanisms but mainly by inhibiting the NF-κB transcription factor. They are the cornerstone of treatments for cytokine storms and HLH associated with autoimmune/autoinflammatory diseases. However, their use in managing SARS-CoV-2 patients is currently highly contested [[169], [170], [171]]. Indeed, a systematic review of observational studies on corticosteroids administered to SARS-CoV infected patients reported no survival benefit, plus potential harm (although mostly inconclusive) [95]. Similarly, a systematic review of observational studies in influenza found a higher risk of mortality and secondary infections with (high-dose) corticosteroids [172]. Further, a study of patients given corticosteroids for MERS-CoV infection revealed delayed clearance of the virus and zero effect of corticosteroids on mortality [173]. Finally, while most previous guidelines did not recommend corticosteroid use for treating sepsis in the absence of refractory shock, a recent statement (triggered by the analysis of two RCTs) made a weak recommendation to use corticosteroids in sepsis patients [174]. Altogether, it has become apparent that corticosteroids may be more detrimental than beneficial in COVID-19 and the WHO (as well as IDSA) recommends that they not be used outside of clinical trials [175]. However, given that these data are derived from previous infections, corticosteroids have been recommended as adjuvant therapy by China's National Health Commission. In particular, one study of 201 patients has found that, among patients who developed ARDS (n=84, 41.8%), treatment with methylprednisolone decreased the risk of death (HR, 0.38; 95% CI, 0.20-0.72, p=0.003) [24]. Nevertheless, in a recent meta-analysis of 15 studies on COVID-19, corticosteroids were associated with significantly higher mortality (RR=2.11, p=0.019), longer length-of-stay (P<0.001), and higher rates of bacterial infection (RR=2.08, P<0.001) [176].
Such conflicting findings are most likely linked to the heterogeneity of protocols and clinical conditions and, above all, to different timings of in corticosteroid administration. Thus, there is no doubt that the main challenge of up-coming RCTs will be to define the precise moment and best dosages for preventing hyperinflammation, while lessening the risks of prolonged viral shedding and secondary bacterial infections.
4.5.2. Chloroquine and hydroxychloroquine
There has been tremendous debate about chloroquine or its derivative, hydroxychloroquine, as potentially effective treatments in COVID-19. Although these drugs have in vitro antiviral properties [[177], [178], [179]], including on SARS-CoV-2 [180], studies have systematically failed to demonstrate positive effects in human viral diseases. Both drugs can prevent the virus’ binding to hACE2 in vitro, or inhibit viral cycle [181]. In addition, they are used in autoimmune and inflammatory diseases (mainly systemic lupus erythematosus) for their immunomodulating properties. It is hypothesized that their action is tied to their ability to accumulate in lysosomes where they raise pH and interfere with antigen degradation and presentation to CD4+ T cells [182,183]. Data also indicate that they reduce proinflammatory IL-6, IL-18, and TNF-α [184,185]. Lastly, they inhibit endosomal TLRs and have anti-inflammatory effects, such inhibiting prostaglandin synthesis or lipid peroxidation [[186], [187], [188]].
Altogether, chloroquine and hydroxychloroquine are thought to be able to reduce viral load and prevent cytokine storm. At the time of writing this review, seven articles are reporting clinical data (observational studies or clinical trials), with either positive (n=4) [[189], [190], [191], [192]] or negative (n=5) results [[193], [194], [195], [196]], but no definitive conclusions can currently be drawn because of major biases in all of the studies [197]. Several RCTs are also ongoing to evaluate these drugs in the context of COVID-19 (n=32 registered with www.clinicaltrials.gov; n=16 with the Chinese clinical trial registry).
In conclusion, it is noteworthy that several national guidelines already include chloroquine or hydroxychloroquine whereas the IDSA recommends reserving its use (in combination with azithromycin or not) for clinical trials.
4.5.3. JAK inhibitors and colchicine
Other drugs are currently being investigated, such as the JAK inhibitors (i.e. tofacitinib, NCT04332042; baricitinib, NCT04321993, NCT04345289, NCT04320277, NCT04346147, NCT04340232; ruxolitinib, NCT04348695, NCT04331665, NCT04337359, NCT04338958, NCT04334044, NCT04348071) or colchicine. The JAK/STAT pathway lies downstream of several cytokines which are increased in HLH and thus it is an attractive target to abrogate the signaling of multiple cytokine pathways [198]. However, caution should be applied in using JAK inhibitors because: (i) COVID-19 can be complicated by coagulopathy with increased frequency of thromboembolic events, and the FDA has recently warned clinicians about increased thromboembolism risk with some JAK inhibitors [199]; ii) treatment with JAK inhibitors is associated with increased frequency of herpes zoster virus reactivation; and (iii) pan-JAK inhibitors may repress some cytokines required for antiviral defense (type-I IFN) or for immune restoration (IL-2, IL-7) [200,201].
Colchicine is a long-established drug with anti-inflammatory properties used to treat patients with Behçet’s disease or familial Mediterranean fever. Colchicine inhibits IL-1β and its subsequent inflammatory cascade principally by blocking pyrin and (to a lesser extent) NLRP3 inflammasome activation. Until now, there has been no data indicating that pyrin is activated upon SARS-CoV-2 infection and this would appear rather unlikely. On the other hand, NLRP3 is likely to be activated following virus entry into the cell [[38], [39], [40],65,130]. Nevertheless, the inhibition of NLRP3 inflammasome by colchicine has not been firmly demonstrated since in vitro it does not prevent IL-1β secretion induced by typical NLRP3 stimuli (i.e. ATP, nigericin) [202]. Five RCTs are currently under way to test the efficacy of colchicine in COVID-19 patients (NCT04322682, NCT04322565, NCT04328480, NCT04326790, NCT04350320).
5. Conclusion
The rapid diffusion of COVID-19 drives an urgent search for effective treatments, mainly for its severe forms. The disease is polyphasic in nature, with secondary cytokine storm and ARDS resulting in poor outcomes, plus overwhelmed intensive care units and hospitals. In the very early stages of the disease, treatment should focus on reducing viral load either by specific antivirals or by stimulating type-I IFN. Later on, in selected patients, therapies targeting proinflammatory cytokines could suppress hyperinflammation, while some cytokines (IL-7) could theoretically trigger immune restoration. In clinical practice, unfortunately, patients are unlikely to be detected early enough (i.e. before/at symptom onset) to benefit from antiviral strategies. Therefore, factors to predict progression toward severe forms of the disease are, at present, the most urgently needed and awaited determinants. A highly-structured approach, which includes immune monitoring, would thus be of utmost importance.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet Lond Engl. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivellese F., Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev. 2020;102536 doi: 10.1016/j.autrev.2020.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 12.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv. 2020;2020(3):14.988345. doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 14.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M. Social Science Research Network; Rochester, NY: 2020. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. [DOI] [Google Scholar]
- 17.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020:1–3. doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta A.K., Gracias D.T., Croft M. TNF activity and T cells. Cytokine. 2018;101:14–18. doi: 10.1016/j.cyto.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X. SAA is a biomarker to distinguish the severity and prognosis of Coronavirus Disease 2019 (COVID-19) J Infect. 2020 doi: 10.1016/j.jinf.2020.03.035. [DOI] [Google Scholar]
- 26.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. MedRxiv. 2020;2020(3) doi: 10.1101/2020.03.02.20029975. 02.20029975. [DOI] [Google Scholar]
- 27.Dholaria B.R., Bachmeier C., Locke F. Mechanisms and management of chimeric antigen receptor T-cell therapy related toxicities. BioDrugs Clin Immunother Biopharm Gene Ther. 2019;33:45–60. doi: 10.1007/s40259-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 29.Jensen S., Thomsen A.R. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86:2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehwinkel J., Gack M.U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 33.Martinon F., Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z. Rochester, NY; Social Science Research Network: 2020. Overly exuberant innate immune response to SARS-CoV-2 infection. [DOI] [Google Scholar]
- 35.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinarello C.A. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabay C., Fautrel B., Rech J., Spertini F., Feist E., Kötter I. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann Rheum Dis. 2018;77:840–847. doi: 10.1136/annrheumdis-2017-212608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Dis. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siu K.-L., Yuen K.-S., Castaño-Rodriguez C., Ye Z.-W., Yeung M.-L., Fung S.-Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus Viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 43.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49 doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 44.Crouse J., Kalinke U., Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 45.Makris S., Paulsen M., Johansson C. Type I interferons as regulators of lung inflammation. Front Immunol. 2017;8:259. doi: 10.3389/fimmu.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster B., Assil S., Dreux M. Cell-cell sensing of viral infection by plasmacytoid dendritic cells. J Virol. 2016;90:10050–10053. doi: 10.1128/JVI.01692-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cervantes-Barragan L., Lewis K.L., Firner S., Thiel V., Hugues S., Reith W. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assil S., Coléon S., Dong C., Décembre E., Sherry L., Allatif O. Plasmacytoid dendritic cells and infected cells form an interferogenic synapse required for antiviral responses. Cell Host Microbe. 2019;25 doi: 10.1016/j.chom.2019.03.005. 730-745.e6. [DOI] [PubMed] [Google Scholar]
- 50.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Law H.K.W., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau S.K.P., Lau C.C.Y., Chan K.-H., Li C.P.Y., Chen H., Jin D.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 55.Högner K., Wolff T., Pleschka S., Plog S., Gruber A.D., Kalinke U. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 is sensitive to type I interferon pretreatment. BioRxiv. 2020;2020(3):07.982264. doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- 57.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.19.20068015. 19.20068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. 2020. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). infectious diseases (except HIV/AIDS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yue Y., Nabar N.R., Shi C.-S., Kamenyeva O., Xiao X., Hwang I.-Y. SARS-coronavirus open reading frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018;9:904. doi: 10.1038/s41419-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Y.-X., Tan T.H.P., Lee M.J.-R., Tham P.-Y., Gunalan V., Druce J. Induction of apoptosis by the severe acute respiratory syndrome coronavirus 7a protein is dependent on its interaction with the Bcl-XL protein. J Virol. 2007;81:6346–6355. doi: 10.1128/JVI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brooks D.G., Trifilo M.J., Edelmann K.H., Teyton L., McGavern D.B., Oldstone M.B.A. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamphuis E., Junt T., Waibler Z., Forster R., Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 69.Croker B.A., Krebs D.L., Zhang J.-G., Wormald S., Willson T.A., Stanley E.G. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 70.Yu C.-R., Mahdi R.M., Ebong S., Vistica B.P., Gery I., Egwuagu C.E. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–29759. doi: 10.1074/jbc.M300489200. [DOI] [PubMed] [Google Scholar]
- 71.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;0 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 73.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;102537 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia Borrega J., Gödel P., Rüger M.A., Onur Ö.A., Shimabukuro-Vornhagen A., Kochanek M. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. HemaSphere. 2019;3 doi: 10.1097/HS9.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet Lond Engl. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 78.Seguin A., Galicier L., Boutboul D., Lemiale V., Azoulay E. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest. 2016;149:1294–1301. doi: 10.1016/j.chest.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gerfaud-Valentin M., Maucort-Boulch D., Hot A., Iwaz J., Ninet J., Durieu I. Adult-onset still disease. Medicine (Baltimore) 2014;93:91–99. doi: 10.1097/MD.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jamilloux Y., Gerfaud-Valentin M., Martinon F., Belot A., Henry T., Sève P. Pathogenesis of adult-onset Still’s disease: new insights from the juvenile counterpart. Immunol Res. 2015;61:53–62. doi: 10.1007/s12026-014-8561-9. [DOI] [PubMed] [Google Scholar]
- 82.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filipovich A.H. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol. 2011;11:512–516. doi: 10.1097/ACI.0b013e32834c22f5. [DOI] [PubMed] [Google Scholar]
- 84.Stepp S.E., Mathew P.A., Bennett M., de Saint Basile G., Kumar V. Perforin: more than just an effector molecule. Immunol Today. 2000;21:254–256. doi: 10.1016/s0167-5699(00)01622-4. [DOI] [PubMed] [Google Scholar]
- 85.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter S.J., Tattersall R.S., Ramanan A.V. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology (Oxford) 2019;58:5–17. doi: 10.1093/rheumatology/key006. [DOI] [PubMed] [Google Scholar]
- 87.Bracaglia C., Prencipe G., De Benedetti F. Macrophage activation syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15:5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brisse E., Wouters C.H., Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016;174:203–217. doi: 10.1111/bjh.14147. [DOI] [PubMed] [Google Scholar]
- 89.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosário C., Zandman-Goddard G., Meyron-Holtz E.G., D’Cruz D.P., Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;102538 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruscitti P., Cipriani P., Di Benedetto P., Ciccia F., Liakouli V., Carubbi F. Increased level of H-ferritin and its imbalance with L-ferritin, in bone marrow and liver of patients with adult onset Still’s disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev. 2015;14:429–437. doi: 10.1016/j.autrev.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Nicholls J.M., Poon L.L.M., Lee K.C., Ng W.F., Lai S.T., Leung C.Y. Lung pathology of fatal severe acute respiratory syndrome. Lancet Lond Engl. 2003;361:1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng Y.-M., Xu X.-L., He X.-Q., Tang S.-Q., Li Y., Huang Y.-Q. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E. Ribavirin and interferon therapy for critically ill patients with Middle East Respiratory Syndrome: a multicenter observational study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hensley L.E., Fritz E.A., Jahrling P.B., Karp C., Huggins J.W., Geisbert T.W. Interferon-β 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scagnolari C., Vicenzi E., Bellomi F., Stillitano M.G., Pinna D., Poli G. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9:1003–1011. [PubMed] [Google Scholar]
- 106.Gonçalves A., Bertrand J., Ke R., Comets E., de Lamballerie X., Malvy D. Timing of antiviral treatment initiation is critical to reduce SARS-Cov-2 viral load. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.04.20047886. 04.20047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chasset F., Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev. 2018;17:44–52. doi: 10.1016/j.autrev.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 108.Shen K.-L., Yang Y.-H. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr WJP. 2020 doi: 10.1007/s12519-020-00344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mager D.E., Jusko W.J. Receptor-mediated pharmacokinetic/pharmacodynamic model of interferon-beta 1a in humans. Pharm Res. 2002;19:1537–1543. doi: 10.1023/a:1020468902694. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Q., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Chen V. Interferon-a2b treatment for COVID-19. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.06.20042580. 06.20042580. [DOI] [Google Scholar]
- 111.Meng Z., Wang T., Li C., Chen X., Li L., Qin X. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.11.20061473. 11.20061473. [DOI] [PubMed] [Google Scholar]
- 112.Nanjappa S.G., Kim E.H., Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117:5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perales M.-A., Goldberg J.D., Yuan J., Koehne G., Lechner L., Papadopoulos E.B. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sportès C., Hakim F.T., Memon S.A., Zhang H., Chua K.S., Brown M.R. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Unsinger J., McGlynn M., Kasten K.R., Hoekzema A.S., Watanabe E., Muenzer J.T. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol Baltim Md. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Francois B., Jeannet R., Daix T., Walton A.H., Shotwell M.S., Unsinger J. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiébaut R., Jarne A., Routy J.-P., Sereti I., Fischl M., Ive P. Repeated cycles of recombinant human interleukin 7 in HIV-infected patients with low CD4 T-cell reconstitution on antiretroviral therapy: results of 2 phase II multicenter studies. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62:1178–1185. doi: 10.1093/cid/ciw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. MedRxiv. 2020;2020(2) doi: 10.1101/2020.02.29.20029520. 29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. MedRxiv. 2020;2020(3) doi: 10.1101/2020.03.30.20048058. 30.20048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watanabe E., Sugawara H., Yamashita T., Ishii A., Oda A., Terai C. Successful tocilizumab therapy for macrophage activation syndrome associated with adult-onset still’s disease: a case-based review. Case Rep Med. 2016;2016:5656320. doi: 10.1155/2016/5656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;102452 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;202003:V1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.01.20048561. 01.20048561. [DOI] [Google Scholar]
- 126.Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol Off J Eur Soc Med Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roumier M., Paule R., Groh M., Vallee A., Ackermann F. Interleukin-6 blockade for severe COVID-19. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.20.20061861. 20.20061861. [DOI] [Google Scholar]
- 128.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. 2019. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 16 April 2020) [DOI] [PMC free article] [PubMed]
- 130.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang Y., Li J., Teng Y., Sun H., Tian G., He L. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 2019;11 doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pugin J., Ricou B., Steinberg K.P., Suter P.M., Martin T.R. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 133.Schulert G.S., Grom A.A. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol. 2014;28:277–292. doi: 10.1016/j.berh.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gerfaud-Valentin M., Jamilloux Y., Iwaz J., Sève P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13:708–722. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 135.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020 doi: 10.1002/acr2.11135. [published online ahead of print, 2020 Apr 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wampler Muskardin T.L. IV anakinra for macrophage activation syndrome may hold lessons for treatment of cytokine storm in the setting of COVID19. ACR Open Rheumatol. 2020 doi: 10.1002/acr2.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jamilloux Y., Belot A., Magnotti F., Benezech S., Gerfaud-Valentin M., Bourdonnay E. Geoepidemiology and immunologic features of autoinflammatory diseases: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:454–479. doi: 10.1007/s12016-017-8613-8. [DOI] [PubMed] [Google Scholar]
- 138.Ben-Zvi I., Kukuy O., Giat E., Pras E., Feld O., Kivity S. Anakinra for colchicine-resistant familial mediterranean fever: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol Hoboken NJ. 2017;69:854–862. doi: 10.1002/art.39995. [DOI] [PubMed] [Google Scholar]
- 139.Soylu A., Yıldız G., Torun Bayram M., Kavukçu S. IL-1β blockade in periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: case-based review. Rheumatol Int. 2019 doi: 10.1007/s00296-019-04389-3. [DOI] [PubMed] [Google Scholar]
- 140.Vastert S.J., Jamilloux Y., Quartier P., Ohlman S., Osterling Koskinen L., Kullenberg T. Anakinra in children and adults with Still’s disease. Rheumatology (Oxford) 2019;58 doi: 10.1093/rheumatology/kez350. vi9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martinon F., Mayor A., Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 143.Weiss E.S., Girard-Guyonvarc’h C., Holzinger D., de Jesus A.A., Tariq Z., Picarsic J. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131:1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020;2020(2) doi: 10.1101/2020.02.10.20021832. 10.20021832. [DOI] [Google Scholar]
- 145.Colafrancesco S., Priori R., Alessandri C., Perricone C., Pendolino M., Picarelli G. IL-18 serum level in adult onset still’s disease: a marker of disease activity. Int J Inflamm. 2012;2012:156890. doi: 10.1155/2012/156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Jesus A.A., Hou Y., Brooks S., Malle L., Biancotto A., Huang Y. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest. 2020;130:1669–1682. doi: 10.1172/JCI129301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Canna S.W., Girard C., Malle L., de Jesus A., Romberg N., Kelsen J. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–1701. doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang K.-J., Su I.-J., Theron M., Wu Y.-C., Lai S.-K., Liu C.-C. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020;2020(2) doi: 10.1101/2020.02.10.20021832. 10.20021832. [DOI] [Google Scholar]
- 150.Song C.-Y., Xu J., He J.-Q., Lu Y.-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. MedRxiv. 2020;2020(3) doi: 10.1101/2020.03.05.20031906. 05.20031906. [DOI] [Google Scholar]
- 151.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Al-Salama Z.T. Emapalumab: first global approval. Drugs. 2019;79:99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- 153.Vallurupalli M., Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019;134:1783–1786. doi: 10.1182/blood.2019002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Albeituni S., Verbist K.C., Tedrick P.E., Tillman H., Picarsic J., Bassett R. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134:147–159. doi: 10.1182/blood.2019000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Das R., Guan P., Sprague L., Verbist K., Tedrick P., An Q.A. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127:1666–1675. doi: 10.1182/blood-2015-12-684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gong J., Dong H., Xia S.Q., Huang Y.Z., Wang D., Zhao Y. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. 2020;2020(2) doi: 10.1101/2020.02.25.20025643. 25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet Lond Engl. 2020 doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prahalad S., Bove K.E., Dickens D., Lovell D.J., Grom A.A. Etanercept in the treatment of macrophage activation syndrome. J Rheumatol. 2001;28:2120–2124. [PubMed] [Google Scholar]
- 159.Flammiger A., Fiedler W., Bacher U., Bokemeyer C., Schneider M., Binder M. Critical imbalance of TNF-α and soluble TNF receptor 1 in a patient with macrophage activation syndrome: potential implications for diagnostics and treatment. Acta Haematol. 2012;128:69–72. doi: 10.1159/000338179. [DOI] [PubMed] [Google Scholar]
- 160.Makay B., Yilmaz S., Türkyilmaz Z., Unal N., Oren H., Unsal E. Etanercept for therapy-resistant macrophage activation syndrome. Pediatr Blood Cancer. 2008;50:419–421. doi: 10.1002/pbc.21019. [DOI] [PubMed] [Google Scholar]
- 161.Sterba G., Sterba Y., Stempel C., Blank J., Azor E., Gomez L. Macrophage activation syndrome induced by etanercept in a patient with systemic sclerosis. Isr Med Assoc J IMAJ. 2010;12:443–445. [PubMed] [Google Scholar]
- 162.Ramanan A.V., Schneider R. Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2003;30:401–403. [PubMed] [Google Scholar]
- 163.Stern A., Riley R., Buckley L. Worsening of macrophage activation syndrome in a patient with adult onset Still’s disease after initiation of etanercept therapy. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2001;7:252–256. doi: 10.1097/00124743-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 164.McDermott J.E., Mitchell H.D., Gralinski L.E., Eisfeld A.J., Josset L., Bankhead A. The effect of inhibition of PP1 and TNFα signaling on pathogenesis of SARS coronavirus. BMC Syst Biol. 2016;10:93. doi: 10.1186/s12918-016-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Charles P., Elliott M.J., Davis D., Potter A., Kalden J.R., Antoni C. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol Baltim Md. 1999;163:1521–1528. 1950. [PubMed] [Google Scholar]
- 166.Paleolog E.M., Young S., Stark A.C., McCloskey R.V., Feldmann M., Maini R.N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41:1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 167.Majewska E., Paleolog E., Baj Z., Kralisz U., Feldmann M., Tchórzewski H. Role of tyrosine kinase enzymes in TNF-alpha and IL-1 induced expression of ICAM-1 and VCAM-1 on human umbilical vein endothelial cells. Scand J Immunol. 1997;45:385–392. doi: 10.1046/j.1365-3083.1997.d01-412.x. [DOI] [PubMed] [Google Scholar]
- 168.Taylor P.C., Peters A.M., Paleolog E., Chapman P.T., Elliott M.J., McCloskey R. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 169.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet Lond Engl. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet Lond Engl. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated cochrane systematic review and meta-analysis. Crit Care Med. 2020;48:e98–106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 173.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 174.Lamontagne F., Rochwerg B., Lytvyn L., Guyatt G.H., Møller M.H., Annane D. Corticosteroid therapy for sepsis: a clinical practice guideline. The BMJ. 2018;362 doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clinical management of severe acute respiratory infection when COVID-19 is suspected. 2019. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 20 April 2020)
- 176.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumar A., Liang B., Aarthy M., Singh S.K., Garg N., Mysorekar I.U. Hydroxychloroquine Inhibits Zika Virus NS2B-NS3 Protease. ACS Omega. 2018;3:18132–18141. doi: 10.1021/acsomega.8b01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Akpovwa H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem Funct. 2016;34:191–196. doi: 10.1002/cbf.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fantini J., Scala C.D., Chahinian H., Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;105960 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kalia S., Dutz J.P. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20:160–174. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ziegler H.K., Unanue E.R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 185.Wozniacka A., Lesiak A., Narbutt J., McCauliffe D.P., Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus. 2006;15:268–275. doi: 10.1191/0961203306lu2299oa. [DOI] [PubMed] [Google Scholar]
- 186.Lafyatis R., York M., Marshak-Rothstein A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum. 2006;54:3068–3070. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 187.Löffler B.M., Bohn E., Hesse B., Kunze H. Effects of antimalarial drugs on phospholipase A and lysophospholipase activities in plasma membrane, mitochondrial, microsomal and cytosolic subcellular fractions of rat liver. Biochim Biophys Acta. 1985;835:448–455. doi: 10.1016/0005-2760(85)90114-6. [DOI] [PubMed] [Google Scholar]
- 188.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol Baltim Md. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. 1950. [DOI] [PubMed] [Google Scholar]
- 189.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.10.20060558. 10.20060558. [DOI] [Google Scholar]
- 190.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 191.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020;2020(3) doi: 10.1101/2020.03.22.20040758. 22.20040758. [DOI] [Google Scholar]
- 192.Gautret P., Lagier J.C., Parola P., Hoang V.T., Medded L., Mailhe M. Hydroxychloroquine and Azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. MedRxiv. 2020;2020(3) doi: 10.1101/2020.03.16.20037135. 16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 193.Chen Jun L.D., Chen Jun L.D. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ Med Sci. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.03.03. 0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Borba M.G.S., de Val F A., Sampaio V.S., Ara M.A., Alexandre U., Melo G.C. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.07.20056424. 07.20056424. [DOI] [Google Scholar]
- 196.Mahevas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.10.20060699. 10.20060699. [DOI] [Google Scholar]
- 197.Shamshirian A., Hessami A., Heydari K., Alizadeh-Navaei R., Ebrahimzadeh M.A., Ghasemian R. Hydroxychloroquine versus COVID-19: a rapid systematic review and meta-analysis. MedRxiv. 2020;2020(4) doi: 10.1101/2020.04.14.20065276. 14.20065276. [DOI] [Google Scholar]
- 198.Jamilloux Y., El Jammal T., Vuitton L., Gerfaud-Valentin M., Kerever S., Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18:102390. doi: 10.1016/j.autrev.2019.102390. [DOI] [PubMed] [Google Scholar]
- 199.FDA issues tofacitinib safety alert. RheumatologyMedicinemattersCom. 2019. https://rheumatology.medicinematters.com/rheumatoid-arthritis-/tofacitinib/fda-issues-tofacitinib-safety-alert/16527134 (accessed April 20, 2020)
- 200.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.El Jammal T., Gerfaud-Valentin M., Sève P., Jamilloux Y. JAK inhibitors: perspectives in internal medicine. Rev Med Interne. 2019;40:816–825. doi: 10.1016/j.revmed.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 202.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 203.Trouillet-Assant S., Viel S., Gaymard A. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.029. [published online ahead of print, 2020 Apr 29], S0091-6749(20)30578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]