Abstract
Reactivation of dormant meristem in banjhi (dormant) shoots is important to enhance the quality and quantity of tea production. The field grown tea bushes were subjected to treatment with dormancy breaking agents such as potassium nitrate (KNO3), thiourea, sodium nitro prusside (SNP), the phytohormones kinetin (Kn) and gibberellins (GA). The efficacy of Kn and GA were comparatively lesser than KNO3 while the combination of Kn and GA (50 and100 ppm respectively) resulted in better dormancy reduction in tea buds. This observation was supported by our results from gene expression study where accumulation patterns of mRNAs corresponding to histones (H2A, H2B, H3 and H4), cyclins (B2, D1 and D3), cyclin-dependent kinase (CDKA), ubiquitination enzymes (FUS, EXT CE2), cyclophilin, E2F, and tubulin were analyzed during growth-dormancy cycles in tea apical buds under the influence of Kn, GA and their combinations. The level of these mRNAs was low in dormant buds, which was significantly increased by foliar application of GA and Kn combination. The present study indicated that the foliar application of GA in combination with Kn will help to improve quality and quantity of tea production by breaking dormancy and stimulating the bud growth.
Keywords: Camellia sinensis, Dormancy, Gene expression, Gibberellic acid, Kinetin
Introduction
Tea, a non-alcoholic beverage widely consumed in most of the countries, is manufactured from two leaves and a bud of tea plant (Camellia sinensis). The quality and quantity of tea production are affected by the presence or formation of dormant bud. In case of south Indian tea plantation, presence of banjhi (temporary dormancy) severely affects the production of tea.
Dormancy in tea is defined as the temporary cessation of growth in terminal bud. Tea shoot does not have a true phase of dormancy due to the development of leaf initials inside the dormant bud (Barua and Das 1979). In physiological point of view, dormancy is classified as paradormancy, endodormancy, and ecodormancy (Tanton 1981). Under paradormancy condition, the endogenous signals from apical bud are perceived by the dormant auxiliary buds, growth was resumed on removal of the apical bud. In case of endodormancy, the dormancy factor exists endogenously inside the dormant meristem. Whereas in ecodormancy, unfavorable external factors, such as nutrients, water and low temperature are responsible for dormancy in a growth-competent meristem and under absence of such unfavorable factors, growth-competent meristem resumes the growth (Lang 1987; Lang et al. 1987; Hao et al. 2017). Tea buds faces two types of dormancy that includes winter dormancy and banjhi. Tea buds undergo winter dormancy under low temperature (< 13 °C for at least 6 weeks) and short day length (photoperiod < 11 h 15 min) (Barua 1969; Hao et al. 2017). Tea plant grows well throughout the year under favorable environmental conditions, however, banjhi is observed several times in a year in unpruned and even in regularly harvested tea bush. Subsequently, banjhi is defined as a state in which the apical buds do not flush, even under optimal growth conditions (Krishnaraj et al. 2011). The mechanism responsible for the induction of dormancy in tea is not clearly understood, however banjhi is reported to be the result of either hormonal interaction (Krishnaraj et al. 2011) or lack of nutrient supply to actively growing apices (Bond 1942). In addition to the pruning cycle, temperature, depletion of food reserves, types and levels of fertilizers (Kulasegarum 1969), photoperiod (Stephens and Carr 1990) and clonal characteristics have a significant influence on onset and release of dormancy. Shoots which originate from the deep canopy (secondary shoot) are mostly found to be in the dormant state when only a few leaves have unfolded. Research on dormancy in plants are focused on the control mechanisms of dormancy in buds (Erez et al. 2000) and regulation of dormancy certainly involves interference with growth hormones (Rosin et al. 2003).
Among the different growth regulators, the gibberellins (GA) have a primordial function. Exogenous application of gibberellins counters balance the inhibition imposed by the abscisic acid and also causes an endogenous increase in GA (Koller et al. 1962). Combination of kinetin (Kn) with GA was proved better for the release of dormancy in Pinus brutia (Kabar 1998). The development and senescence of the whole plant are controlled by cytokinins (CKs). Plant responses to CKs are often judged from their responses to exogenously applied CKs (Brault and Maldiney 1999). CKs regulates the process such as seed germination and dormancy, the release of buds from apical dominance, formation of de novo bud, expansion of leaf, reproductive development and delay of senescence (Mok 1994). An increased amount of G1 Cyclin D3 (CycD3) required for the G1-S phase transition in the cell cycle (Soni et al. 1995) in the presence of cytokinin suggests its involvement in the G1-S phase transition and thus keep cells dividing. ABA imposed dormancy in seeds of lettuce was reversed and the dormancy was released by the application of kinetin (Dunlap and Morgan 1977).
Our earlier transcriptomic studies revealed that interaction of plant growth hormones and ubiquitination plays a major role in onset and release of banjhi (Krishnaraj et al. 2011; Thirugnanasambantham et al. 2013). Thus, in the present work, we used different dormancy breaking agents for releasing the dormant bud of tea from dormancy and the best dormancy breaking agent was selected, which was further confirmed with the expression level of different cell cycle regulatory genes with a time course experiment. Thus, the present investigation and the results obtained will help to select a better dormancy breaking agent for tea.
Materials and methods
Plant material
UPASI-3, an Assam type of tea cultivar that was planted in 1963 and growing well in the farm the UPASI- tea research institute, Valparai, Tamil Nadu, India was selected in the present study (Balasaravanan et al. 2003). All experiments were carried out at field condition.
Application of dormancy breaking agents
Tea bushes with average growth condition were selected for the experiment. Fifteen individual plots each with twenty numbers of bushes were selected for the spray experiment. Each of the plots was labeled for the appropriate agents and the lateral buds were sheared with mechanical shear to balance bush size and to reduce competition with the elongating terminal bud. Each of the respective plots was sprayed with the appropriate dormancy breaking agent(s) with the concentration as mentioned in Table 1. Foliar application of dormancy breaking agents was performed only after plucking in order to expose the maintenance leaf to perceive the foliar-applied fluids. After 16 days, the samples were collected with mechanical shear and the plots were sprayed with the respective dormancy breaking agent(s). The same method of foliar application of dormancy breaking agent was followed for four more times. During each shear harvest, the banjhi percentage of each plot was calculated, compared with the control and the results were expressed as the reduction in banjhi percentage. In brief, about 100 g of tea shoots were randomly collected from each group of freshly harvested plots. The collected tea shoots were grouped as active (actively growing flush shoot) and banjhi (shoots with dormant terminal bud) and the reduction in banjhi percent was calculated as follows:
| 1 |
| 2 |
Table 1.
List of different dormancy breaking agents
| S. No | Dormancy breaking agent | Concentration |
|---|---|---|
| 1 | Gibberellic acid | 10 ppm |
| 2 | Gibberellic acid | 50 ppm |
| 3 | Gibberellic acid | 100 ppm |
| 4 | Kinetin | 50 ppm |
| 5 | Kinetin | 100 ppm |
| 6 | Thiourea | 0.5% |
| 7 | Thiourea | 1% |
| 8 | Sodium nitro prusside | 100 µM |
| 9 | Sodium nitro prusside | 250 µM |
| 10 | Potassium nitrate | 1% |
| 11 | Potassium nitrate | 2% |
| 12 | Gibberellic acid + Kinetin | 50 + 100 ppm |
| 13 | Gibberellic acid + Kinetin | 100 + 50 ppm |
Application of dormancy breaking agent(s) for analysis of gene expression
Based on the results of the effect of different dormancy breaking agents, GA, Kn and their combination were found to be comparative to the existing dormancy breaking agent (KNO3). For the analysis of the influence of the above hormones in dormancy release, these hormones of required concentration were sprayed on seven plots which were prepared as above. Bud samples (both dormant and active) were collected in liquid nitrogen at 0th, 12th, 24th, 36th, 48th and 72th h, transferred to the laboratory for further analysis.
Semi-quantitative RT-PCR
Single strand cDNAs were synthesized from total RNA (1 µg) isolated from the bud tissues using cDNA synthesis kit (Clontech) as described by (Thirugnanasambantham et al. 2013). Gene-specific primers were used to amplify cDNA samples (50 ng) in PCR (Table 2). All primers were designed using the Primer-BLAST (Ye et al. 2012) tool based on the National Center for Biotechnology Information published sequences (www.ncbi.nlm.nih.gov) and the genes names considered as per the latest tea genome annotation (Wei et al. 2018). PCR conditions for each primer set were optimized and amplification was carried for a specific number of cycles. The semi-quantitative PCR assay was performed and analyzed at least two times independently. The band intensity was normalized against Cs5.8S rRNA and quantified using ImageJ software (http://rsb.info.nih.gov/ij/). The expression ratio was visualized as heatmap using the heatmap.2 function in the gplots package of R (Warnes et al. 2015).
Table 2.
Primer sequences and PCR assay parameter of genes encoding cell cycle regulatory proteins of tea used in semi-quantitative RT-PCR assay
| S. No | Gene name | NCBI Acc. No | TIPA locus name | Primer sequence (5′-3′) | Product size (bp) | No. of cycles | Annealing temperature (°C) |
|---|---|---|---|---|---|---|---|
| 1 | Histone H2A | HM003232 | TEA025242.1 | F: GGAGCTCCGGTCTACCTCGC | 150 | 26 | 65 |
| R: TGAATGTTAGGCATCACTCCGCCA | |||||||
| 2 | Histone H2B.3 | GW696866 | TEA025244.1 | F: GAGAAAGCTCCGGCGGAGAAGAA | 300 | 24 | 63 |
| R: TCAAGAGCTAGTGAACTTAGTAACCGCC | |||||||
| 3 | Histone H3 | HM003305 | TEA032511.1 | F: ATGGCTCGTACAAAGCAGACAGCTCG | 350 | 24 | 63 |
| R: TCAAGCTCTCTCGCCTCGAATCCTCC | |||||||
| 4 | Histone H4 | HM003230 | NA | F: ATGTCGGGAAGAGGAAAGGGAGGAA | 250 | 24 | 63 |
| R: CTAACCCCCAAACCCATAGAGAGTTCTC | |||||||
| 5 | Cyclin B | JF795471 | TEA021407.1 | F: TGATTGACTGGCTCATTGAGGTGC | 450 | 28 | 60 |
| R: ACTTGGTATGCCTCTCGCTTGTCT | |||||||
| 6 | Cyclophilin | HM003242 | TEA002681.1 | F: ATGTTGAGATTGCCGGAAAACCTGCTG | 325 | 23 | 66 |
| R: TCACAAAGGAAGTTCGCCGCTGTCA | |||||||
| 7 | Cyclin-D1 | X83369 | TEA017808.1 | F: ATCCTCTGTTGTCAATCCCCAC | 200 | 27 | 56 |
| R: AAGAGGATTCATCACTTGGCCT | |||||||
| 8 | Cyclin D3 | AB247282 | TEA014197.1 | F: CAGCTCTCACTGCAATTCTTGCCA | 300 | 27 | 65 |
| R: TGGGTTTTCAGTCCAAGCCTCCT | |||||||
| 9 | Cyclin dependent kinase A | AB247281 | TEA024500.1 | F: TGTGGATGTGTGGTCAGTGG | 200 | 22 | 65 |
| R: ACAGTGGCCAGGTCCTTAGA | |||||||
| 10 | Elongation factor 2 | JK341471 | TEA022285.1 | F: TCTGAGGGCAGCAACTTCAG | 350 | 34 | 55 |
| R: CGGGGCATTATACTCCAGGG | |||||||
| 11 | Tubulin alpha-3 | DQ444294 | TEA008342.1 | F: TTGCTTTTGGAGCGCTTGTC | 350 | 26 | 55 |
| R: CGAGGGTATGGCACAAGGTT | |||||||
| 12 | Ubiquitin-conjugating enzyme E2 variant 1D | HM003308 | TEA006425.1 | F: GAGCACACTTCAGCCCATGGCA | 500 | 28 | 65 |
| R: TGGGGGCCACATGACAGTCCTT | |||||||
| 13 | Ubiquitin-40S ribosomal protein S27 | HM003234 | TEA024638.1 | F: ACCCTCGCCGATTACAACATCCA | 300 | 27 | 65 |
| R: CCCCAGAACACTCAGAAACCACCA | |||||||
| 14 | Ubiquitin-40S ribosomal protein S27 protein | HM003307 | TEA024638.1 | F: TATGCCGGGGGAGAGAGACAGA | 400 | 22 | 63 |
| R: AGTCAACAGGGTCTTCTCAAGTGCG | |||||||
| 15 | Cs5.8S rRNA | FJ004886 | NA | F: GCCCTCAACCTAATGGCTTC | 125 | 22 | 63 |
| R: GATATCTCGGCTCTCGCATC |
Statistical analysis
The experiments were conducted in triplicates. Data are shown as mean ± SD of the obtained results and statically analysed using SPSS software v.11.0. The mean pairwise comparison was performed with Tukey’s multiple range post hoc test and P < 0.05 value was considered significant.
Results
Application of dormancy breaking agents
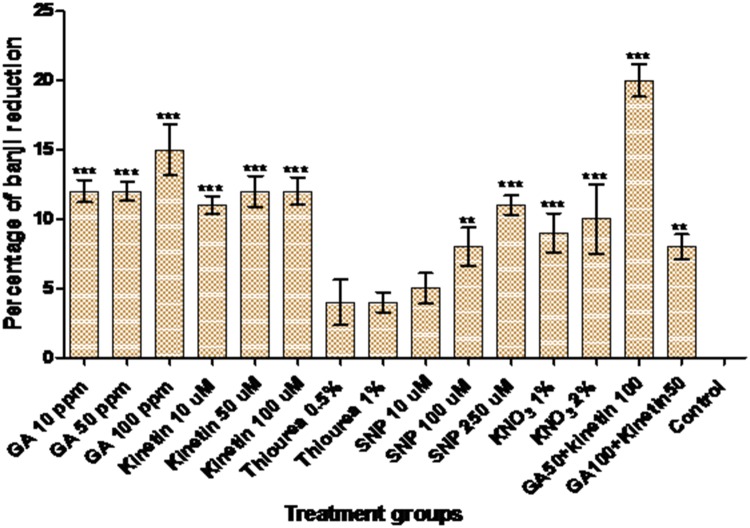
To find out the best dormancy breaking agent(s), five dormancy breaking agents in different concentrations were tested (Fig. 1). Foliar application of all these agents resulted in the reduction in banjhi percentage. We have screened the effect of GA in banjhi percentage with 3 different concentrations (10, 50 and 100 ppm), where the banjhi percentage was inversely proportional to the concentration of GA. GA at 100 ppm resulted in the better reduction of banjhi content when compared with other concentrations. The efficiency of kinetin in the reduction of banjhi percentage was less compared with GA. Foliar application of kinetin revealed a dose-dependent reduction in banjhi content (10 ppm < 50 ppm < 100 ppm). In addition to the plant hormones, the effect of antioxidative enzymes inhibitor thiourea and sodium nitro prusside (SNP) was also tested in banjhi percentage. Among the two inhibitors of antioxidative systems, SNP resulted in a better reduction of dormancy. Comparison of selected agents for banjhi percentage with the existing dormancy breaking agent, KNO3 was better than other agents. Though the reduction in banjhi was noticed with the application of single hormone, it was not up to the level of KNO3 application. Further to study the effect of the combination of GA and kinetin, we sprayed these hormones in two combinations (GA 50 ppm + Kn 100 ppm and GA 100 ppm + Kn 50 ppm). The first combination resulted in a better reduction of banjhi shoots and was also slightly higher than the existing KNO3 (Fig. 1).
Fig. 1.
Effect of different dormancy breaking agents on bud dormancy in tea. Data is shown as mean ± SD of representative experiments (n = 6, ns = no significant, P ≤ 0.05, **P ≤ 0.01, ***P < 0.001)
Analysis of cell cycle regulatory gene expression
Result of the above field experiment indicated the involvement of hormones for the process of dormancy release in tea. Further, to study the effect of the foliar application of GA and Kn in the regulation of transcripts essential for cell cycle regulatory protein (Table 2), we analyzed their expression in tea bud under the influence of these hormones by performing RT-PCR experiments.
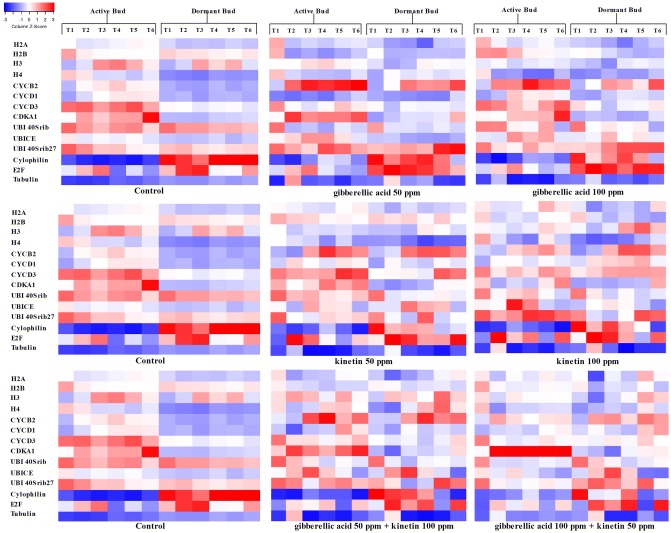
Analysis of expression pattern of histone H2A revealed their importance in active shoot growth. In the active buds of control plot, the expression of H2A increased gradually from 24 h. In case of dormant bud, the expression level of H2A was low and it was not increased till 72 h (Fig. 2). In case of foliar application of GA at a concentration of 50 ppm, expression of H2A mRNA in active bud started increasing from 12th h, while in dormant bud, increase in H2A expression took 48 h to reach the level of active bud (Fig. 2). Expression of H2A in the active bud of plots under foliar application of GA at concentration 100 ppm was constant till 24th h and a slight increase in expression level were noticed. In dormant bud, it started to increase from 12th h (Fig. 2). Foliar application of Kn both at a concentration of 50 and 100 ppm resulted in an increase in expression of H2A mRNA with time in both the buds but the level in a dormant bud was low at all stages. In dormant bud, H2A expression reached the level of active bud in control plots at 48th h with foliar application of Kn at 50 and 100 ppm respectively (Fig. 2). Foliar application of a combination of GA and Kn (50 + 100 ppm) resulted in an increase in H2A expression from 12th h in both active and dormant bud. The expression level of H2A in dormant bud reached the level of active bud after 36th h (Fig. 2). In case of a combination of GA and Kn (100 + 50 ppm), H2A expression in dormant bud starts to increase at 24th h and reached the expression level of active bud (Fig. 2).
Fig. 2.
Heat map representation of RT-qPCR validation of genes involved in cell cycle and ubiquitination process under the influence of exogenous treatments. The heat map was reported as the Z score, which is the scaled as gene expression measurement (blue = − 3 to red = 3).The time interval adopted for sample collection were listed as T1-0th h, T2-12th h, T3-24th h, T4-36th h, T5-48th h and T6-72nd h. Where the list of genes considered for analysis includes H2A-histone H2A (NCBI Acc. No. HM003232), H2B-histone H2B (NCBI Acc. No. GW696866), H3-histone H3 (NCBI Acc. No. HM003305), H4-histone H4 (NCBI Acc. No. HM003230), CYCB2-cyclin B (NCBI Acc. No. JF795471), CYCD1- cyclin-D1 (NCBI Acc. No. X83369), CYCD3- cyclin D3 (NCBI Acc. No. AB247282), CDKA1- cyclin dependent kinase A (NCBI Acc. No. AB247281), UBI40Srib-ubiquitin-40S ribosomal protein S27 (NCBI Acc. No. HM003234), UBICE-ubiquitin-conjugating enzyme E2 variant 1D (NCBI Acc. No. HM003308), UBI40Srib27-ubiquitin-40S ribosomal protein S27 protein (NCBI Acc. No. HM003307), Cyclophilin (NCBI Acc. No. HM003242), E2F-elongation factor 2 (NCBI Acc. No. JK341471) and Tubulin-tubulin alpha-3 (NCBI Acc. No. DQ444294) (color figure online)
Expression of histone H2B in active bud increased with time, while it was constant in the dormant bud of control plot (Fig. 2). Due to foliar application of 50 ppm GA, expression of H2B in dormant bud started to increase from 24th h, reaches the expression level of active bud after 36th h and increased gradually like the active bud. In case of foliar application of GA at a concentration of 100 ppm, its expression in both dormant and active bud increased till 12 h after which it remained static till the end of the experiment (Fig. 2). Here the dormant bud took 72 h to attain the level of active bud. Application of 50 ppm Kn resulted in increased expression of H2B after 12 h in the active bud, then it was uniform till completion of the experiment. In dormant bud, the expression of H2B increased after12 h and reached the initial level of active bud at 36 h (Fig. 2). In both active and dormant bud, application of 100 ppm Kn increased the expression of H2B gradually from 12 h. Dormant bud took 48 h to reach the level of active bud in the expression of H2B. In both active and dormant buds, a combination of GA and Kn at concentrations of 50 and 100 ppm respectively lead to increased expression of H2B from 12 h. Application of hormones with the above combination made the dormant bud to take 12 h to reach the level of active bud (Fig. 2). The same trend was noticed in case of a combination of GA and Kn at concentrations of 100 and 50 ppm respectively and the dormant bud took 36 h to reach the level of active bud (Fig. 2).
Histone H3 expression in the active bud of control plot was increased till 36 h and then maintained at uniform level till completion of the experiment while in the dormant bud, it was static till the end of the experiment. After 50 ppm GA application, H3 expression in active bud increased till 36 h and then it was maintained in uniform level and a similar trend was noticed in dormant bud also but it took 24 h to reach the level of active bud (Fig. 2). In 100 ppm GA treatment, the trend was similar to that of 50 ppm GA treatment, but the dormant bud took 36 h to reach the level of active bud (Fig. 2). A similar trend as above was observed with 50 ppm Kn treatment but the expression ratio was lower than the GA application. Foliar application of Kn at a concentration of 100 ppm resulted in gradual increase in expression of H3 in both active and dormant bud and the dormant bud just took 24 h to reach the level of active bud (Fig. 2). Foliar application with a combination of GA and Kn leads to a gradual increase in expression of H3 as above and in case of GA 50 ppm + Kn 100 ppm treatment, the dormant bud took just 12 h to reach the expression ratio of active bud. Foliar application of a combination of GA and Kn (100 and 50 ppm respectively) induced a gradual increase in expression of H3 in both active and dormant bud, dormant bud required 24 h to reach the level of active bud (Fig. 2).
In control plot, expression of Histone H4 in an active bud was static till 36 h and increased from 48 h, while in dormant bud, its expression was low and static throughout the experimental period (Fig. 2). Foliar application of GA at 50 ppm lead to gradual increase in expression of H4 in both active and dormant bud, the dormant bud took 12 h to reach the level of active bud. In the active bud, no change in the expression pattern of H4 with the application of GA at 100 ppm, but in dormant bud, increase in expression was noticed and required 36 h to reach the level of active bud (Fig. 2). In both active and dormant buds, application of Kn at 50 ppm lead to increase in H4 expression till 48 h and the expression ratio of dormant bud did not reach the level of active bud even after 72 h. Foliar application of Kn at 100 ppm lead to gradual increase in expression of H4 in both active and dormant buds and dormant bud needed 72 h to reach the level of active bud (Fig. 2). In a combination of GA + Kn (50 + 100 ppm respectively), expression of H4 increased till 36 h and dormant bud took 24 h to reach the level of active bud. Expression pattern of H4 under the influence of a combination of GA + Kn (100 + 50 ppm) increased gradually with time and the dormant bud required 24 h to reach the level of active bud (Fig. 2).
In general, expression of Cyclin B2 (CYC B2) was high in the active bud. In control plot, the expression of CYC B2 in active bud increased gradually and in the dormant bud, it was static till completion of the experiment. In all the plots sprayed with respective hormones, increased expression of CYC B2 in both active and dormant bud was noticed. In all treatments, dormant bud took just 12 h to reach the expression level of CYC B2 of active bud (Fig. 2).
Expression pattern of CYC D1 was similar to CYC B2 in both control and treated plots (Fig. 2). Under the influence of both the concentrations of GA, expression of CYC D1 in dormant bud required 24 h to reach the level of active bud. Whereas at both the concentrations of Kn (50 and 100 ppm), dormant bud required just 12 h to reach the expression ratio of active bud (Fig. 2). Combination of GA and Kn (50 + 100 ppm) also took 12 h to reach the level of active bud, then from 24 h, it was found to be static till the completion of the experiment. But the combination of GA and Kn with other concentration (100 + 50 ppm), CYC D1 expression in dormant bud reached the level of active bud within 12 h and then increased with time (Fig. 2).
The expression pattern of CYC D3 in active bud increased gradually with time. Expression pattern of CYC D3 in dormant buds of control plots were static throughout the experimental period (Fig. 2). Foliar application of GA at 50 ppm resulted in activation of CYC D3 expression in the dormant bud and it took 24 h to reach the level of active bud. A similar trend was noticed in plots sprayed with GA at 100 ppm but the dormant bud required 36 h to reach the CYC D3 expression level of active bud (Fig. 2). Foliar application of Kn at both the concentrations resulted in gradual increase in CYC D3 expression till the completion of the experiment. But the dormant buds of plots sprayed with 50 and 100 ppm Kn required more than 36 and 24 h respectively to reach the expression ratio of active bud (Fig. 2). Combination of GA and Kn resulted in an increase in expression of CYC D3 in the active and dormant buds. Combination of GA and Kn (50 + 100 ppm) required greater than 48 h for the expression level of CYC D3 in dormant bud to reach the level of active bud. In dormant bud of other combination of GA and Kn (100 + 50 ppm respectively) took 24 h to reach the level of active bud (Fig. 2).
In control plot, CDK A1 expression was higher in active bud compared to a dormant bud. In active bud, it increased gradually with time, whereas in the dormant bud, it was static (Fig. 2). Treatments with both the hormones lead to increase in expression of CDK A1 in both active and dormant buds. But the duration of time required by the dormant bud to reach the level of active bud varied with the concentration of hormone applied. In case of foliar application of GA at concentrations of 50 and 100 ppm, dormant bud required 12 h and 36 h respectively to reach the level of active bud (Fig. 2). Dormant buds of plots under the influence of foliar application of Kn at both concentrations (50 and 100 ppm) required 48 h to reach the level of active bud. Combination of GA and Kn at concentrations of 100 + 50 ppm respectively in dormant bud took 12 h (Fig. 2) and the combination of GA and Kn at concentrations of 50 + 100 ppm respectively took 24 h to reach the CDK A1 expression level of active bud.
Expression of UBI CE2 was high in the active bud. In control plot, its expression increased gradually, but in the dormant bud, its expression was lower than the active bud and it was throughout the experiment (Fig. 2). In case of foliar application of GA at 50 ppm, UBI CE2 expression in active bud increased till 24 h and then it decreased gradually. In case of dormant bud, expression of UBI CE2 increased gradually till completion of the experiment and the dormant bud took more than 36 h to reach the level of active bud. In the active bud, expression of UBI CE2 increased with application of GA at 100 ppm and it was decreased after 48 h of treatment. In dormant bud, application of GA at 100 ppm leads to a gradual increase in expression of UBI CE2 till 24 h and maintained at a uniform level. Dormant bud from the treatment of GA at 100 ppm required just 12 h to reach the level of active bud (Fig. 2). Foliar application of Kn at 50 ppm increased the expression of UBI CE2 till 24 h in both active and dormant bud, then it was maintained at uniform level till 48 h and decreased at the completion of the experiment. For the dormant bud under the treatment of Kn at 50 ppm, it required more than 12 h to reach the level of active bud, after which the expression ratio was same as an active bud (Fig. 2). Expression of UBI CE2 under the influence of Kn at 100 ppm in an active bud was similar to the application of Kn at 50 ppm, but it declined immediately after 24 h. Data from Fig. 2 revealed that expression of UBI CE2 in dormant bud under the influence of Kn at 100 ppm gradually increased till 24 h and then it was uniform till completion of the experiment. In case of combinations of GA and Kn, a similar trend was noticed where expression of UBI CE2 increased till 24 h and then decreased gradually till completion of the experiment (Fig. 2).
In control plot, expression of UBI Ext was high in the active bud that increased slowly and in the dormant bud, it was lower than the active bud and maintained the same level all over the experiment (Fig. 2). In plots treated with GA at 50 ppm, expression of UBI Ext increased from 12 h in both the buds. Under the influence of GA at 50 ppm, dormant bud required 12 h to reach the level of active bud, after which the expression was high in the dormant bud. Expression pattern of UBI Ext under the influence of GA at 100 ppm was similar to the GA at 50 ppm, but the expression ratio was lesser than the plots treatment with 50 ppm of GA (Fig. 2). Under the influence of Kn at 50 ppm, expression of UBI Ext in both active and dormant bud was static till 24 h and then increased with time. In general, the expression level was higher in the active bud. Foliar application of Kn at 100 ppm leads to a gradual increase in the expression of UBI Ext in both dormant and active bud; dormant bud took 48 h to reach the level of active bud (Fig. 2). Combination of GA and Kn at concentrations of 50 and 100 ppm respectively resulted in gradual increase in expression of UBI Ext in both dormant and active bud, but the dormant bud took 48 h to reach the level of the active bud. While in case of foliar application of GA and Kn at concentrations of 100 + 50 ppm respectively, though the expression pattern was similar to the earlier combination, the dormant bud required 12 h to reach the level of active bud (Fig. 2).
Expression of UBI FUS in the active bud of control plot increased gradually, while in the dormant bud, it was lower than the active bud and remained at the same level throughout the experiment (Fig. 2). Under the influence of GA at 50 ppm, expression of UBI FUS in both active and dormant bud increased gradually till 48 h, then it was decreased in the active bud, but the level was maintained in the dormant bud. Dormant bud required more than 24 h to reach the level of active bud. In plots treated with Kn at 50 ppm, its expression in an active bud was static till 48 h and then it increased, but in the dormant bud, it increased gradually till the completion of the experiment. In active bud, UBI FUS expression increased gradually till 36 h and decreased gradually thereafter in the plots treated with GA (100 ppm), while in case of dormant bud, it increased till completion of the experiment. At both the concentrations of Kn, dormant bud required 72 h to reach the level of active bud (Fig. 2). In plots treated with the combination of GA and Kn (50 and 100 ppm respectively), expression of UBI FUS was initially static in the active bud and increased from 24 h, while in the dormant bud, it was static till 24 h and then increased gradually from 36 h. In plots treated with a combination of GA and Kn (100 and 50 ppm respectively), expression of UBI FUS increased gradually in both active and dormant buds (Fig. 2). In the above treatment, the dormant bud required just 12 h to reach the expression level of active bud.
Expression of Cyclophilin in the active bud of control plot was lower than the dormant bud and alteration in expression pattern was not noticed in the active bud (Fig. 2). Foliar applications of GA at 50 ppm lead to increase in expression of Cyclophilin in both active and dormant bud till 36 h and then decreased. Similar expression pattern of Cyclophilin under the influence of foliar application of GA at 100 ppm was noticed, but with much lower expression level (Fig. 2). In dormant bud under the influence of Kn at 50 ppm, expression of Cyclophilin was unaltered. Expression pattern of Cyclophilin in the active bud of plots treated with Kn at 100 ppm and the combination of GA and Kn (50 and 100 ppm respectively) were similar to the plots treated with GA at 50 ppm (Fig. 2). In both active and dormant bud, foliar application of a combination of GA and Kn (100 and 50 ppm respectively) increased the expression of Cyclophilin till 36 h and then it decreased.
In control plot, expression of E2F in both active and dormant bud increased till 24 h, and then gradually decreased (Fig. 2). The pattern of expression in both the buds under the influence of GA at 50 ppm was similar to the control plot; the expression ratio was higher in the dormant bud. In the active bud of plots sprayed with 100 ppm of GA, expression of E2F increased till 24 h and then a gradual decline in expression was noticed. While in the dormant bud of plots sprayed with GA at 100 ppm, E2F expression increased continuously till the end of the experiment (Fig. 2). In plots treated with both Kn at 50 and 100 ppm, expression of E2F in active and dormant buds reached its maximal expression at 12 h, gradually decreased thereafter, and increased at the final stage of the experiment. While under the treatment of GA + Kn (50 + 100 ppm and 100 + 50 ppm respectively), expression of E2F increased gradually till 36 h, decreased at 48 h and again increased at the completion of the experiment (Fig. 2).
Expression of Tubulin in the active buds of control plot increased till 48 h and finally decreased. But in dormant, its expression was static (Fig. 2). In plots treated with GA at 50 ppm, its expression in both active and dormant bud reached a maximal level at 12 h and then decreased gradually. Expression of Tubulin was high in the active bud. Under the influence of GA at 100 ppm, till 24 h, the trend was similar to the foliar application of GA (50 ppm) and no change in expression was observed from 36 h (Fig. 2). In the active bud of plots treated with Kn at 50 and 100 ppm, expression of Tubulin increased and reached the maximal level at 12 h and decreased till 24 h. In case of dormant bud in plots treated with Kn at 100 ppm, expression of Tubulin decreased from 12 h till 36 h and increased again thereafter (Fig. 2). In case of combined treatment of GA and Kn, the expression of Tubulin in both active and dormant bud increased till 12 h (Fig. 2). The expression of Tubulin in dormant bud decreased till 36 h and then again increased gradually till the completion of the experiment.
Discussion
In the present work, GA at a concentration of 100 ppm resulted in a better reduction of banjhi (dormant bud). A similar effect of dormancy release with GA at higher concentration was observed in potato (Hartmann et al. 2011). Endogenous plant hormones play a critical role in the regulation of dormancy and bud break (Suttle 2004). Increase in GA level was also evident during the release of bud dormancy (Khattab et al. 2000). At a concentration of 50 ppm, we noticed a similar effect on reduction in banjhi content with foliar application of both GA and kinetin. Application of GA (50 ppm) to the dormant buds induced breaking of dormancy and onset of bud outgrowth. Rebers et al. (1994) confirmed that exogenously applied gibberellins partially substitute the cold treatment of dormant tulip bulbs, and stimulate shoot growth and flowering, concluded that gibberellins can act as a dormancy-breaking agent. GA3 might activate cell division in the meristematic tissue, and on the orientation of the cell microtubules in the direction of growth (Cleland 1999). Even though it is clear that GA can reduce dormancy, in the present study, we have made an attempt to determine the effective concentration of GA and observed the gene expression pattern of proteins which are important in the cell cycle. Our earlier studies on identification of dormancy specific genes revealed hormonal regulation of cell cycle in relevance to dormancy (Krishnaraj et al. 2011), we have made an attempt to reveal the hypothesis with field experiments. Our observation revealed that the combination of Kn and GA resulted in a better reduction of banjhi content when compared to the other treatments. Foliar application of KNO3 in banjhi control is a well-known phenomenon; in our study, we have reported for the first time that combination of GA and Kn is better than the existing KNO3 treatment. KNO3 application alone and in combination with Waiken® stimulated vegetative bud break, floral bud break, and promoted early leaf growth and development in peach and nectarine (Noppakoonwong et al. 2005). Cytokinin is needed to initiate cell cycle, but gibberellins are also needed to initiate and maintain cell growth (Rossouw 2008). Thus, the combination of the hormones produced better results on dormancy release in tea buds.
The relationship between ROS metabolism and dormancy breakage in both plant seeds (Fontaine et al. 1994) and vegetative buds (Pérez and Lira 2005) has been reported. Application of catalase inhibitor such as thiourea in dormancy break is a well-known phenomenon (Nir et al. 1986; Bajji et al. 2007). But in our study, it was observed that foliar application of catalase inhibitor resulted in a trace level of banjhi reduction. On the other hand, our study revealed that the combination of GA and Kn (50 and 100 ppm respectively) resulted in better dormancy break (Fig. 1). The combination at a concentration of 100 and 50 ppm of GA and Kn respectively was not satisfactory. High concentrations of CKs are required to override the effects of ABA; thus reduction in CK supply might amplify shoot responses to an increasing concentration of ABA (Davies and Zhang 1991). Observation from our study revealed that the effects of the combination of hormones are at par with existing KNO3.
Further, in addition to the study on the effect of foliar application of dormancy breaking agents, we have monitored the expression level of different cell cycle regulatory genes. Among the treatments, a combination of hormones (GA and Kn) resulted in rapid increase in expression of histone mRNA in the dormant bud and helped to reach the level of active bud (Fig. 2). Changes in cell-cycle-specific gene expression during dormancy release of auxiliary bud was observed in pea (Pisum sativum) (Devitt and Stafstrom 1995), potato (Campbell et al. 1996), adventitious buds of leafy spurge (Horvath et al. 2002) and Jerusalem artichoke (Freeman et al. 2003). During dormancy release, a gradual increase in mRNA levels of histone H2 and histone H3 (Mazzitelli et al. 2007). There is a close correlation between accumulation of histone mRNA and the S-phase which is an excellent marker for proliferating cells (Fobert et al. 1994). Breaking of dormancy up-regulates genes for G1-S phase transition like histones (Horvath et al. 2002). In pea, the expression patterns of cell cycle regulators such as histone H4 (S phase marker), cyclin B (G2/M phase marker) and cyclinD (G1 phase marker) were characterized to understand the molecular mechanisms of cell cycle control during the dormancy-to-growth transition (Shimizu and Mori 1998).
During the seasonal growth cycle, there is a significant transcriptional regulation in the cell cycle machinery where cyclins are primarily affected. Cyclin expression in a dormant bud was rapidly increased under the influence of kinetin and the combination with GA (Fig. 2). In plants, cytokinin can induce the expression of cyclin D3 (Hu et al. 2000). In the absence of kinetin, the suspension cultured cells of Nicotiana plumbaginifolia arrest in G2 phase with an inactive CDKA kinase (Zhang et al. 1996). Different plant hormones largely control the cell cycle. Activation of cell division by cytokinin is by exerting control over the activity of D-type cyclins (Riou-Khamlichi et al. 1999). GA enhanced expression of the cyclin genes and the time course of the induction was compatible in regulating the G2/M phase transition. CK induced the expression of mitotic B-type cyclins (Cyc1 and Cyc4) in lupine, that are also regulated by auxin (Jeleńska et al. 2000). It was observed that the application of external growth hormones induced the expression of gene encoding enzymes involved in Ubiquitin-dependent protein degradation (Chen et al. 1995). In the late G2 phase of the cell cycle, tubilin is up-regulated which served as a marker for this phase of cell division (Vantard et al. 2000). Expression of tubulin was enhanced by application of GA3 (Horvath et al. 2002). Our earlier study revealed that the cyclophilin expression in banjhi bud was higher than the actively growing bud. Further the expression of cyclophilin expression in dormant bud decreased during the conversion of dormancy to active growth phase (Thirugnanasambantham et al. 2014). This was in support with the results of the present study, where the expression of cyclophilin was down regulated by both GA and Kn (Fig. 2).
In conclusion, the effect of Kn and GA are comparable to KNO3. Combination of Kn and GA (50 + 100 ppm) resulted in better dormancy reduction in tea buds (Fig. 1), while the other hormonal combinations were not much satisfactory. This observation is corroborated by analysis of cell cycle regulatory gene expression data. The expression level of cell cycle regulatory genes was low in the dormant bud and increased with foliar application of hormones, which results in dormancy release. Combination of hormones induced the expression of cell cycle regulatory genes more rapidly than the other treatments. Thus, the combination of Kn and GA can act as a good dormancy breaking agent with rapid induction of proteins essential for active cell cycle.
Acknowledgements
The authors are thankful to the Director, UPASI Tea Research Foundation for his encouragement and support during the course of study.
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bajji M, MʼHamdi M, Gastiny F, et al. Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) tubers. Biotechnol Agron Soc Environ. 2007;11:121–131. [Google Scholar]
- Balasaravanan T, Pius PK, Raj Kumar R, et al. Genetic diversity among south Indian tea germplasm (Camellia sinensis, C. assamica and C. assamica spp. lasiocalyx) using AFLP markers. Plant Sci. 2003;165:365–372. doi: 10.1016/S0168-9452(03)00196-1. [DOI] [Google Scholar]
- Barua DN. Seasonal Dormancy in Tea (Camellia sinensis L.) Nature. 1969;224:514. doi: 10.1038/224514a0. [DOI] [Google Scholar]
- Barua DN, Das SC. Mechanism of growth periodicity in tea [India] Two A Bud. 1979;26:36–40. [Google Scholar]
- Bond TET. Studies in the vegetative growth and anatomy of the tea plant (Camellia thea Link.) with special reference to the phloem. Ann Bot. 1942;6:607–630. doi: 10.1093/oxfordjournals.aob.a088424. [DOI] [Google Scholar]
- Brault M, Maldiney R. Mechanisms of cytokinin action. Plant Physiol Biochem. 1999;37:403–412. doi: 10.1016/S0981-9428(99)80046-1. [DOI] [Google Scholar]
- Campbell MA, Suttle JC, Sell TW. Changes in cell cycle status and expression of p34cdc2 kinase during potato tuber meristem dormancy. Physiol Plant. 1996;98:743–752. doi: 10.1111/j.1399-3054.1996.tb06680.x. [DOI] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Cleland RE. Nature, occurrence, and functioning of plant hormones. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and molecularbiology of plant hormones. 1. Amsterdam: Elsevier Science; 1999. pp. 3–22. [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:55–76. doi: 10.1146/annurev.pp.42.060191.000415. [DOI] [Google Scholar]
- Devitt ML, Stafstrom JP. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol. 1995;29:255–265. doi: 10.1007/BF00043650. [DOI] [PubMed] [Google Scholar]
- Dunlap JR, Morgan PW. Reversal of induced dormancy in lettuce by ethylene, kinetin, and gibberellic acid. Plant Physiol. 1977;60:222–224. doi: 10.1104/pp.60.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A, Viémont JD, Crabbé J. Bud dormancy: a suggestion for the control mechanism and its evolution, dormancy in plants: from whole plant behaviour to cellular control. Wallingford: UKCAB International; 2000. [Google Scholar]
- Fobert PR, Coen ES, Murphy GJ, Doonan JH. Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J. 1994;13:616–624. doi: 10.1002/j.1460-2075.1994.tb06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine O, Huault C, Pavis N, Billard JP. Dormancy breakage of Hordeum vulgare seeds: effects of hydrogen peroxide and scarification on glutathione level and glutathione reductase activity. Plant Physiol Biochem. 1994;32:677–683. [Google Scholar]
- Freeman D, Riou-Khamlichi C, Oakenfull EA, Murray JAH. Isolation, characterization and expression of cyclin and cyclin-dependent kinase genes in Jerusalem artichoke (Helianthus tuberosus L.) J Exp Bot. 2003;54:303–308. doi: 10.1093/jxb/erg047. [DOI] [PubMed] [Google Scholar]
- Hao X, Yang Y, Yue C, et al. Comprehensive transcriptome analyses reveal differential gene expression profiles of Camellia sinensis axillary buds at para-, endo-, ecodormancy, and bud flush stages. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Senning M, Hedden P, et al. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011;155:776–796. doi: 10.1104/pp.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Chao WS, Anderson JV. Molecular analysis of signals controlling dormancy and growth in underground adventitious buds of leafy spurge. Plant Physiol. 2002;128:1439–1446. doi: 10.1104/pp.010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Bao F, Li J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000;24:693–701. doi: 10.1046/j.1365-313x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Jeleńska J, Deckert J, Kondorosi E, Legocki AB. Mitotic B-type cyclins are differentially regulated by phytohormones and during yellow lupine nodule development. Plant Sci. 2000;150:29–39. doi: 10.1016/S0168-9452(99)00158-2. [DOI] [Google Scholar]
- Kabar K. Comparative effects of kinetin, benzyladenine, and gibberellic acid on abscisic acid inhibited seed germination and seedling growth of red pine and arbor vitae. Turk J Bot. 1998;22:1–6. [Google Scholar]
- Khattab HI, Emam MM, Shehata MM. The correlative changes associated with bud dormancy and rooting of cane cuttings in grapevine. Egypt J Biotechnol. 2000;7:255–274. [Google Scholar]
- Koller D, Mayer AM, Poljakoff-Mayber A, Klein S. Seed germination. Annu Rev Plant Physiol. 1962;13:437–464. doi: 10.1146/annurev.pp.13.060162.002253. [DOI] [Google Scholar]
- Krishnaraj T, Gajjeraman P, Palanisamy S, et al. Identification of differentially expressed genes in dormant (banjhi) bud of tea (Camellia sinensis (L.) O. Kuntze) using subtractive hybridization approach. Plant Physiol Biochem. 2011;49:565–571. doi: 10.1016/j.plaphy.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Kulasegarum S. Studies on the dormancy of tea shoots. I. Hormonal stimulation of the growth of dormant buds. Tea Q. 1969;40:31–46. [Google Scholar]
- Lang GA. Dormancy: a new universal terminology. HortScience. 1987;22:817–820. [Google Scholar]
- Lang G, Early J, Martin G, Darnell R. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience. 1987;22:371–377. [Google Scholar]
- Mazzitelli L, Hancock RD, Haupt S, et al. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J Exp Bot. 2007;58:1035–1045. doi: 10.1093/jxb/erl266. [DOI] [PubMed] [Google Scholar]
- Mok MC. Cytokinins and plant development—an overview. In: Mok DWS, Mok MC, editors. Cytokinins chemistry, activity and function. Boca Raton: CRC Press; 1994. pp. 155–166. [Google Scholar]
- Nir G, Shulman Y, Fanberstein L, Lavee S. changes in the activity of catalase (EC 1.11.1.6) in relation to the dormancy of grapevine (Vitis vinifera L.) buds. Plant Physiol. 1986;81:1140–1142. doi: 10.1104/pp.81.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppakoonwong U, Sripinta P, Pasopa P et al (2005) A trial of rest-breaking chemicals on low-chill peach and nectarine. ACIAR Tech Reports Ser 73–80
- Pérez FJ, Lira W. Possible role of catalase in post-dormancy bud break in grapevines. J Plant Physiol. 2005;162:301–308. doi: 10.1016/j.jplph.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Rebers M, Romeijn G, Knegt E, Plas LHW. Effects of exogenous gibberellins and paclobutrazol on floral stalk growth of tulip sprouts isolated from cooled and non-cooled tulip bulbs. Physiol Plant. 1994;92:661–667. doi: 10.1111/j.1399-3054.1994.tb03037.x. [DOI] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Rosin FM, Hart JK, Van Onckelen H, Hannapel DJ. Suppression of a vegetative MADS box gene of potato activates axillary meristem development. Plant Physiol. 2003;131:1613–1622. doi: 10.1104/pp.102.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JA. Effect of cytokinin and gibberellin on potato tuber dormancy. Pretoria: University of Pretoria; 2008. [Google Scholar]
- Shimizu S, Mori H. Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant Cell Physiol. 1998;39:255–262. doi: 10.1093/oxfordjournals.pcp.a029365. [DOI] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Shah ZH, Murray JA. A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell. 1995;7:85–103. doi: 10.1105/tpc.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens W, Carr MKV. Seasonal and clonal differences in shoot extension rates and numbers in tea (Camellia sinensis) Exp Agric. 1990;26:83. doi: 10.1017/S001447970001543X. [DOI] [Google Scholar]
- Suttle JC. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. J Plant Physiol. 2004;161:157–164. doi: 10.1078/0176-1617-01222. [DOI] [PubMed] [Google Scholar]
- Tanton TW. The Banjhi (Dormancy) Cycle in Tea (Camellia sinensis) Exp Agric. 1981;17:149–156. doi: 10.1017/S001447970001139X. [DOI] [Google Scholar]
- Thirugnanasambantham K, Prabu G, Palanisamy S, et al. Analysis of dormant bud (Banjhi) specific transcriptome of tea (Camellia sinensis (L.) O. Kuntze) from cDNA library revealed dormancy-related genes. Appl Biochem Biotechnol. 2013;169:1405–1417. doi: 10.1007/s12010-012-0070-5. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambantham K, Prabu GR, Mandal AKA. Isolation and characterization of cDNA encoding cyclophilin gene from dormant bud of Camellia sinensis (L.) O. Kuntze. 2014;42:256–261. [Google Scholar]
- Vantard M, Cowling R, Delichère C. Cell cycle regulation of the microtubular cytoskeleton. Plant Mol Biol. 2000;43:691–703. doi: 10.1023/A:1006346107807. [DOI] [PubMed] [Google Scholar]
- Warnes GR, Bolker B, Bonebakker L, Gentleman R, Andy Liaw WH, Lumley T, Maechler M, Magnusson A, Schwartz MV (2015) gplots: Various R programming tools for plotting data. R Package Version 3.0.1. http://cran.r-project.org/package=gplots. Accessed 21 Nov 2019
- Wei C, Yang H, Wang S, et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci. 2018;115:E4151–E4158. doi: 10.1073/pnas.1719622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Letham DS, John PC. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta. 1996;200:2–12. doi: 10.1007/BF00196642. [DOI] [PubMed] [Google Scholar]