Abstract
Hippophae rhamnoides L. provides an enormous range of medicinal and nutritional benefits. The significant abilities of this plant to survive in Himalayan high altitudes enticed our study to investigate its rhizosphere. Seventeen rhizobacterial strains were isolated from the rhizospheric soil and plant root nodules, belonging to genus Frankia, Azorhizobium, Bacillus, Paenibacillus, Brevibacillus and Pseudomonas, as identified by 16SrRNA sequencing. This varying bacterial population was further examined for the presence of root degrading enzymes pectinase and cellulase, which enable them to intrude the plant roots. Based on the growth and substrate utilization by these rhizobacteria on pectinase screening agar medium and Mandels and Reese agar medium, all the seventeen strains were identified as pectinase and cellulase producing rhizobacteria. The quantitative analysis by DNS method demonstrated varying enzyme activities, spot-lighting the physiological variation in the microbiome. The divergence in the enzyme activities shown by all the strains was analysed statistically, using the software ASSISTAT.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00778-2) contains supplementary material, which is available to authorized users.
Keywords: Hippophae rhamnoides L., Pectinase, Cellulase, Enzyme activity
Introduction
Microorganisms play vital roles in maintaining soil fertility and plant health. Plant growth promoting rhizobacteria (PGPR) benefit the host plants in different ways like indole acetic acid (IAA) production, phosphate solubilisation, HCN production, siderophore formation etc. (Santoyo et al. 2016). Several enzyme-producing bacteria can destroy oospores of phytopathogenic fungi and affect the spore germination and germ-tube elongation of phytopathogenic fungi. Therefore, they can also act as biofertilizers and increase the resistance to biotic and abiotic stress (Sharma et al. 2007). These rhizobacteria aid in plant growth and are often classified as extracellular-(ePGPR) and intracellular-(iPGPR). ePGPR reside in the rhizosphere, rhizoplane or in the spaces between the cells of root cortex, e.g. Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcus, Pseudomonas and Serratia. iPGPR are localized inside the nodular structure of plant root cells, e.g. Allorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium and Frankia (Viveros et al. 2010; Bhattacharyya and Jha 2012).
Complex enzyme “cellulase” breaks down cellulose, which is made up of D-glucose molecules linked together by β-1,4-glycosidic bonds. Major applications of the enzyme include removal of crude enzymes and fibres; production of soluble sugars, production of lignin, decomposition of wastes and various applications in food industries (Sadhu and Maiti 2013). Pectin degrading enzyme “pectinase” is produced by bacteria and fungi, which play various roles in cell physiology, growth, ripening, as well as in intracellular adhesion and separation. With the gradual increase in their demand and marketing, pectinases are one of the most promising enzymes of the biotechnology sector (Garg et al. 2016). The soil surrounding plant roots is enriched with nutritious plant exudates and hence it’s a magnificent spot for the growth of microorganisms which are benefitted in terms of nutrition and habitat. Some of these rhizosphere dwelling bacteria are also capable of producing lytic enzymes, and hence can be parasitizing disease-causing fungi. Such bacteria can also destroy oospores of phytopathogenic fungi, thus affecting spore germination and germ-tube elongation of phytopathogenic fungi. Therefore, they not only serve as biofertilizers, increase the host-plants resistance to biotic and abiotic stress, but can also cause breaks in plant cell walls, by the degrading action of lytic enzymes (Slack et al. 2017). Being capable of degrading cellulose and pectin, these lytic-enzyme-producing-rhizospheric-bacteria can not only easily enter the host-plant roots, but also decompose cellulose and/or pectin-based organic waste- and harmful-material. Such plant-beneficial rhizospheric bacteria are also utilized in various stream of biotechnology to produce significant biomolecules like enzymes, proteins, antibiotics etc. For example, immobilized enzymes linked to a solid matrix or entrapped in a gel are widely used to catalyse biotransformations; Sharma et al. (2007) developed a combination of three enzymes (pectinase, cellulase and xylanase) called PECTINEX, which is an excellent alternative to carry-out three different catalytic activities as compared to free individual enzymes.
Leh, Himalayas has harsh environmental conditions, with temperature varying from − 30 to 30 °C, very low annual precipitation, and limited atmospheric oxygen. This drew our attention to the mystery of the related microorganisms which help this wonder plant to survive in these adverse climatic conditions. These microorganisms are also capable of surviving in such harsh conditions, which also is a topic of concern for industrial applications. In the present study, we have isolated different bacteria from the rhizospheric soil, and plant roots of Hippophae rhamnoides L. (H. rhamnoides L.) from Leh, India. So, to reveal the mechanism behind the ability of these bacteria to enter the plant, we conducted this study to examine the production of plant root degrading enzymes pectinase and cellulose. These can be of great industrial significance and therefore, are mass-cultivated commercially.
Materials and methods
Sample sites and sample collection
The area under this study was the cold desert of Ladakh, situated in Himalayas, India. The rhizospheric soil and root samples of the plant H. rhamnoides L. were collected from different sites of Leh, differing in altitudes, viz. Nubra (3048 m above sea level), Indus (3600 m above sea level) and Zanskar (4000 m above sea level). Ladakh is located at an elevation height of 3000 m above sea level with coordinates 34°10′12″N 77°34′48″E. Root samples from five plants from each site were collected. Rhizospheric soil samples from each plant were carefully collected from the close periphery of the root zone. All the soil and root samples were stored in sterilized zip-lock bags and were stored at -20 °C for further analysis.
Soil physicochemical properties
All the rhizospheric soil samples were analysed for the physicochemical properties pH, electric conductivity, organic carbon, phosphorus content and potash content (Hseu 2004).
Root histology
H. rhamnoides L. plant’s root and rhizospheric soil samples were collected from three different sites at Leh, Kashmir, India. The collected root samples were examined for root morphology to have better insight into the structural details. Roots, as well as the attached nodules (both brown and white), were subjected to histology for studying tissue architecture and variations therein if any. Root dimensions were measured by micrometre (make-99MAA001M2). Thin sections of the roots were prepared using a microtome (Senior Rotary Microtome, RMT-19). The sections were processed for dehydration and staining as per standard protocols. Series of slides were prepared from different root portions, viz., those containing white nodules, brown nodules as well as root without any nodules. To detect the microbial infection in meristematic zones, the sections were stained with lactophenol blue (Raja et al. 2016) and methylene blue (Gentili et al. 2006). The stained sections were further observed under image projection microscope (Olympus, Magnus).
Isolation of microorganisms from rhizospheric soil
Selective media like nutrient agar (HiMedia) for bacterial species (Gowsalya et al. 2014), potato dextrose agar (HiMedia) for fungal species, actinomycetes agar medium (HiMedia) for actinomycetes strains (Kumar et al. 2015), YEMA medium (HiMedia) for Rhizobium (Agah et al. 2016), and DPM agar media for Frankia were used. Composition of DPM (gm/L): sodium propionate 0.5, sodium succinate 0.5, K2HPO4 1.0, MgSO4 0.1, CaCl2 0.001, FeSO4 0.0055, Na2.EDTA 0.0074, H3BO3 0.005, MnCl2 0.0032, ZnSO4 0.0004, CuSO4 0.00014, NaMo.O4 0.00045, CoCl2 0.00045, agar 18 and made final volume of 1000 mL with distilled water (Gtari et al. 2004). 10 µL of 1:10 soil dilutions (1 mg soil in 10 mL sterile distilled water) of all the rhizospheric soil samples collected from all three sites were spread-inoculated on enriched media plates and the plates were incubated at 30 °C for 3–4 days.
Isolation of microorganisms from the root and root nodules
For isolation of microbial species present in the nodules, each root sample and root nodule was individually surface sterilized by using 30% H2O2. The sterilized nodules were then crushed in a sterile plate with a sterile rod. The ooze from each nodule was inoculated on plates of nutrient agar, potato dextrose agar, actinomycetes agar, YEMA and DPM agar medium plates, and these plates were incubated at 30 °C for 24–48 h (Baeucehmin et al. 2012). The term ooze here refers to exopolysaccharides and bacterial cells extracted/collected as droplets from the host plants’ roots and root nodules (Slack et al. 2017).
Identification of the isolated bacterial strains
Seventeen different strains of bacteria were obtained from the soil and root nodule samples which were analysed by 16SrRNA. Genomic DNA from the procured cultures was isolated (Sambrook and Russel 2001), and gradient PCR (40, 46.9, 51, 54.2, 56.5 and 58 °C) was run to identify and optimize the annealing temperature for each strain. Primers used were: 16S (F) 5′-AGAGTTTGATCCTGGCTCAG-3′ 16S I 5′-ACGGCTACCTTGTTACGACTT-3′; ITS-1 (F) 5′-TCCGTAGGTGAACCTGCGG-3′ ITS-4 I5′-TCCTCCGCTTATTGATATGC-3′. Using the 16S sequences, a cladogram was created with CLUSTAL-OMEGA, to detect the relatedness of the isolates (Gregory 2008). The gDNA from gel cuttings was eluted using BioBasic Gel Elution Kit. The eluted DNA with the concentration of 40 ng/µL was submitted for sequencing. For sequencing, reactions with the gDNA were run with forward and reverse primers and the reaction product sequences were aligned using the software “Codoncode Aligner” and hence the final aligned sequence for each strain were collected. These sequences were analysed on BLAST to obtain information regarding genus and species and later submitted in NCBI GenBank to receive accession number for each strain (Supplementary Fig. 1).
Qualitative analysis of pectinase and cellulase producing strains
Qualitative analysis for enzyme production was done by plate-assay, using pectinase screening agar medium (PSAM) and Mandels and Reese agar medium (MRAM). Pectinase and cellulase producing bacteria can utilize the pectin and cellulose present in the medium and form a zone around the colonies, respectively. PSAM plates were flooded with 50 mM I2 solution and incubated at 30 °C for 15 min. MRAM plates were stained with congo red solution for 15 min and de-stain with 1 M NaCl for 15 min. The plates were observed for clear zones around the colonies. For quantitative analysis of enzymes, active broth cultures of the isolates were centrifuged in 4 °C cooling centrifuge, at 12,000 rpm for 10 min and the supernatant was collected as the crude enzyme, to be used in the reaction mixture (Kashyap et al. 2000; Dantur et al. 2015).
Quantitative analysis of pectinase and cellulase
All the isolated bacterial strains were found capable of producing the enzymes pectinase and cellulase. To analyse the quantitative difference, active broth cultures of the isolated strains were centrifuged in 4 °C cooling centrifuge, at 12,000 rpm for 10 min. The supernatants were collected as a crude enzyme to measure the quantities (Dantur et al. 2015; Kashyap et al. 2000).
Enzyme assays for pectinase and cellulase
The enzyme assay was done by DNS (di-nitrosalicylic acid) method, to determine the amount of reduce sugar (glucose equivalent) released. A standard graph was plotted with various dilutions of 0.2 mg/mL glucose solution, measuring OD at 540 nm. For analysis of sugar reduction, reaction mixtures were prepared by adding 0.5 mL of the test sample to 0.5 mL 1% pectin solution and 0.5 mL of 1% CMC solution. The tubes were incubated in a 50 °C water bath for 30 min. 2 mL DNS reagent was added in each tube and again incubated in 100 °C boiling water bath for 10 min. OD was measured at 540 nm (Wanmolee et al. 2016). The concentration of substrate released was measured using the glucose standard curve.
Protein estimation and enzyme activity
Protein estimation was done by Lowry’s method. A standard graph was plotted using different dilutions of BSA stock solution and OD was measured at 660 nm. For estimation of proteins in the test samples, reaction mixtures were prepared by adding 0.4 mL of the crude enzyme to 4 mL alkaline CuSO4 solution. These tubes were incubated in dark for 10 min. 0.4 mL Follin’s reagent was added in each tube and incubated in dark for 30 min. The OD was measured at 660 nm, and the graph was plotted to estimate protein concentration in each test sample of crude enzyme. One unit of enzyme activity is defined as the amount of enzyme that releases 1 µM of reducing sugars equivalent to glucose, per minute during the reaction. Enzyme activity calculated for each sample was expressed as “U/mLmin”.
Statistical analysis
All the experiments were performed thrice, and the mean values were further analysed for comparing the divergence. Analysis of variation in the amount of extracellular folic acid production by the isolated rhizobacteria was done using the software “ASSISTAT” version 7.7 (Silva and Azevedo 2016). The averages followed by the same letter do not differ statistically between themselves.
Results
Soil physicochemical properties
The physicochemical properties of rhizospheric soil samples collected from the three sites are depicted in Table 1. As presented in the table, all the samples were alkaline in nature, with pH not deviating to a greater extent from the standard range of pH 8.8. Soil samples from the site no. 1 had pH of 8.5, soil from the site no. 2 had pH of 9 and soil from the site no. 3 had a pH of 8.7. Soil samples from all the three sites showed higher salinity values, especially soil samples from the site no. 1 and 2 had salinity values of 1 mS/cm and 1.06 mS/cm, respectively, which is many folds higher than the normal range of 0.16 mS/cm. Soil from the site no. 3 had moderate salinity value, 0.21 mS/cm. The organic carbon content in soil samples from all the three sites was almost the same as the normal range of 0.51%, with 0.54%, 0.6% and 0.54% from site 1, 2 and 3, respectively. The phosphorus content in soil samples from the site no 1, 2 and 3 were estimated equivalent to 31.1%, 19% and 5.6%, respectively, with that from the site no 3 varying to a greater extent from the normal range of 22.53%. Soil samples from the site no 1 and 2 had higher potash content of 273% and 274%, respectively, but that from site no.3 had a very low potash content of only 42%, deviating a lot from the normal range of 174%.
Table 1.
Physicochemical analysis of rhizospheric soil
| Property | Site 1 | Site 2 | Site 3 | Normal Range |
|---|---|---|---|---|
| pH | 8.5 | 9.0 | 8.7 | 8.8 |
| Salinity | 1.00 | 1.06 | 0.21 | 0.16 |
| Organic carbon (%) | 0.54 | 0.60 | 0.54 | 0.51 |
| Phosphorous (%) | 31.1 | 19.0 | 5.6 | 22.53 |
| Potash (%) | 273 | 274 | 42 | 174 |
Site 1—Nubra, Site 2—Indus and Site 3—Zanskar
Root histology
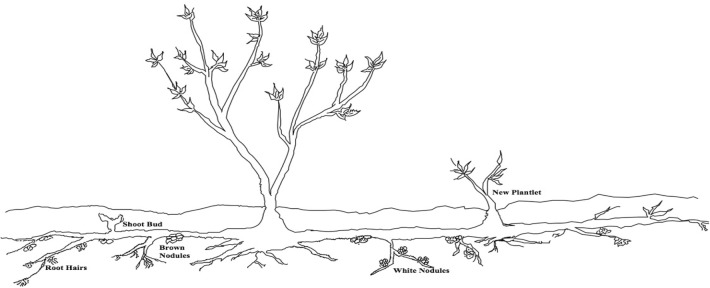
As depicted through a root sketch in Fig. 1, the horizontal roots had broad networking, which ranged from 2.0 to 5.0 m, whereas, the vertical roots ranged from 10 to 50 cm in length. The diameter of the main root varied from 1.957 to 2.485 cm at the junction and 0.129–0.033 cm at the tapered end. The feeding root system of H. rhamnoides reached up to 30–40 cm depth and made extensive branching to form complex networking. Newly spurred plantlets were found to be originating directly from certain zones of the main horizontal root, which were submerged in the soil at 40–50 cm depth. The main plant and the plantlets were interconnected to form the network. Two distinct types of nodules were observed on the roots of H. rhamnoides, which were either brown or whitish in colour. The brown nodules were distributed randomly on certain root branches while white nodules were arranged sequentially on certain other branches of roots. The branches with white nodules did not have brown nodules and vice versa. The other characteristics of this extensive and prominently horizontal root system of H. rhamnoides roots, like shoot buds, root hair, white-coloured nodules and brown-coloured nodules engrafted on the main root, are presented pictorially in Fig. 2. Various structures like different coloured root nodules and root hairs drew our attention and to unveil the inner content of these attached structures, microtomic thin sections of the main root, the brown nodule and the white nodule were stained and as expected, we got differing sectioning for the three. The normal root, brown nodules and white nodules, had diverged arrangement of epidermal, endodermal and stele cells. The cortex cells of root with brown nodules retained the dye lactophenol blue, and the epidermal cells of roots with white nodules retained methylene blue dye, proficiently convincing the presence of microorganisms in the roots with brown and white nodules. This suggested that the invading microbial communities must also produce root cell wall degrading enzymes that must allow them to degrade the host plant’s roots and enter to accommodate there.
Fig. 1.
Sketch showing root morphology of Hippophae rhamnoides
Fig. 2.
Various root modification observed in Hippophae rhamnoidesa brown nodules; b white nodules and c root hair
Identification of rhizobacteria
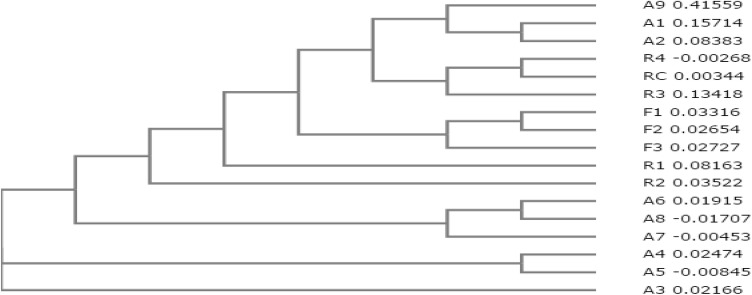
A total of seventeen bacterial strains were isolated from the rhizospheric soil and root samples of H. rhamnoides, as presented in Table 2. Two strains of Frankia, viz., Frankia strain Cea 1.3 and Frankia strain Agb 1–9 were isolated from the root nodules, and strains of Bacillus, Paenibacillus, Brevibacillus, Pseudomonas, Azorhizobium and one more strain of Frankia, were isolated from the rhizospheric soil. Figure 3 is the cladogram highlighting close relatedness of the associated rhizobacteria from plant roots and rhizosphere soil.
Table 2.
List of isolated bacterial strains from the rhizospheric soil and root samples
| Sr. no. | Name | Accession no. | Source | Site |
|---|---|---|---|---|
| 1 | Frankia strain Cea 1.3 | KY559044 | Root brown nodule | Zanskar |
| 2 | Frankia strain Ccl 3 | KY559045 | Rhizospheric soil | Zanskar |
| 3 | Frankia strain Agb 1–9 | KY559046 | Root white nodule | Zanskar |
| 4 | Azorhizobium strain ORS 571 | KY556720 | Rhizospheric soil | Zanskar |
| 5 | Azorhizobium strain BR5401 | KY559051 | Rhizospheric soil | Indus |
| 6 | Paenibacillus strain WP-1 | KY559047 | Rhizospheric soil | Indus |
| 7 | Brevibacillus strain T7 | KY559048 | Rhizospheric soil | Indus |
| 8 | Pseudomonas strain LBUM647 | KY559049 | Rhizospheric soil | Indus |
| 9 | Azorhizobium strain F1 | KY559050 | Rhizospheric soil | Indus |
| 10 | Uncultured bacterium De316 | KY549927 | Rhizospheric soil | Nubra |
| 11 | Uncultured bacterium De317 | KY549928 | Rhizospheric soil | Nubra |
| 12 | Bacillus strain A-SRETCR | KY556714 | Rhizospheric soil | Nubra |
| 13 | Bacillus strain LZ046 | KY556715 | Rhizospheric soil | Indus |
| 14 | Bacillus strain GY779 | KY556716 | Rhizospheric soil | Indus |
| 15 | Bacillus strain PS1 | KY556717 | Rhizospheric soil | Indus |
| 16 | Bacillus cereus st.66 | KY556718 | Rhizospheric soil | Indus |
| 17 | Bacillus cereus st.67 | KY556719 | Rhizospheric soil | Zanskar |
Fig. 3.
Cladogram of the isolated strains representing their relatedness (Uncultured Clostridium A1; Uncultured Clostridium A2; Bacillus subtilis A3; Bacillus A4; Bacillus A5; Bacillus A6; Bacillus cereus A7; Bacillus cereus A8; Azorhizobium caulinodans A9; Frankia F1; Frankia casuarinae F2; Frankia F3; Paenibacillus thermophilus R1; Brevibacillus R2; Pseudomonas R3; Azorhizobium R4 and Azorhizobium doebereinerae RC, respectively)
Qualitative analysis of pectinase and cellulase producing rhizobacteria
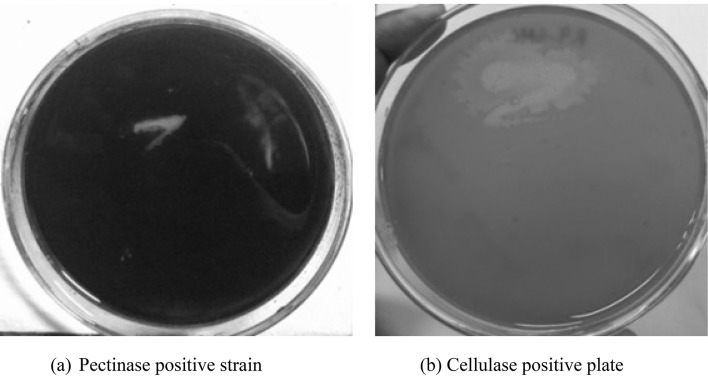
To unveil how these microbes must infest the host plant, we made an attempt to investigate whether these rhizobacteria were able to produce cell-wall degrading enzymes and our data recorded that all the seventeen isolated rhizobacteria were able to produce cellulase and pectinase enzymes. The clear zone formed on PSAM and MRAM plates, by pectinase-positive and cellulase-positive strains, respectively (Fig. 4). The clear zone proves the consumption of the substrates pectin and CMC by the isolates.
Fig. 4.
Pectinase positive strain on PSAM plate (a) and cellulase positive strain on MRAM plate (b) by bacterial strains isolated from the root nodules and rhizospheric soil of Hippophae rhamnoides
Enzyme assays: pectinase and cellulase
Interestingly, all the isolates exhibit both cellulolytic and pectinolytic activities, in differing levels, spotlighting the divergence of the degree of carbohydrate (cell-wall) degradation. The variations in proteins concentration of pectinase and cellulase present in the crude enzyme extract from each sample are presented in Table 3.
Table 3.
NCBI links for isolated bacterial strains
Enzyme activity: pectinase and cellulase
Surprisingly, the amount of protein present in each sample varied to a great extent, irrespective of the genus and source of isolation. Table 4 presents the pectinase and cellulase enzyme activities, which also exhibits variations, irrespective of the bacterial genus.
Table 4.
Protein concentration of crude enzyme extract (BSA standard graph) from the isolated bacterial
| Sample | Pectinase (mg/mL) | Cellulase (mg/mL) |
|---|---|---|
| Frankia strain Cea 1.3 | 31.75 | 3.225 |
| Frankia strain Ccl 3 | 28.825 | 2.7 |
| Frankia strain Agb 1–9 | 34.475 | 3.925 |
| Azorhizobium strain ORS 571 | 27.975 | 4.025 |
| Azorhizobium strain BR5401 | 54.15 | 4.425 |
| Paenibacillus strain WP-1 | 3.175 | 3.1 |
| Brevibacillus strain T7 | 41.575 | 2.425 |
| Pseudomonas strain LBUM647 | 23.775 | 3.825 |
| Azorhizobium strain F1 | 22.85 | 3.2 |
| Uncultured bacterium De316 | 27.325 | 5.75 |
| Uncultured bacterium De317 | 26.825 | 7.95 |
| Bacillus strain A-SRETCR | 26.775 | 8.8 |
| Bacillus strain LZ046 | 21.175 | 5.525 |
| Bacillus strain GY779 | 26.55 | 5.425 |
| Bacillus strain PS1 | 30.625 | 4.625 |
| Bacillus cereus st.66 | 33.475 | 5.475 |
| Bacillus cereus st.67 | 36.1 | 6.7 |
Statistical analysis
As presented in Table 5, the enzyme activities of pectinase and cellulase isolates from these seventeen strains differ statistically from each other based on Tuckey test at a level of 5% of probability, as analyzed using ASSISTAT. This analysis showcases noticeable divergence in cellulase activities by the rhizobacteria, but not a major difference in pectinase activities.
Table 5.
Enzyme activities (U/mL/min) per sample, along with the statistical analysis of the same (denoted by the alphabets), from the isolated bacterial strains
| Sample | Pectinase (U/mL/min) | Cellulase (U/mL/min) |
|---|---|---|
| Frankia strain Cea 1.3 | 0.00189c | 0.0322d |
| Frankia strain Ccl 3 | 0.00233c | 0.0314d |
| Frankia strain Agb 1–9 | 0.00233c | 0.0251f |
| Azorhizobium strain ORS 571 | 0.0021c | 0.0274e |
| Azorhizobium strain BR5401 | 0.00227c | 0.0172i |
| Paenibacillus strain WP-1 | 0.03172a | 0.0232g |
| Brevibacillus strain T7 | 0.00187c | 0.0430b |
| Pseudomonas strain LBUM647 | 0.00327c | 0.0278e |
| Azorhizobium strain F1 | 0.005b | 0.033c |
| Uncultured bacterium De316 | 0.00243c | 0.0129j |
| Uncultured bacterium De317 | 0.00275c | 0.0112l |
| Bacillus strain A-SRETCR | 0.00211c | 0.0070a |
| Bacillus strain LZ046 | 0.00311c | 0.0191h |
| Bacillus strain GY779 | 0.00269c | 0.0190h |
| Bacillus strain PS1 | 0.00197c | 0.0235g |
| Bacillus cereus st. 66 | 0.00179c | 0.0131j |
| Bacillus cereus st.67 | 0.00312c | 0.0125j |
The averages followed by the same letter do not differ statistically between themselves
Discussion
The plant H. rhamnoides L. exhibits an amazing root structure for infesting symbiotic bacteria in anatomically variant nodules and maximum collection of water and nutrients through root branches and root hairs. Presence of diverging microbial groups in the root and different root nodules directed us to the possibility for the production of plant root degrading enzymes by these associated rhizobacteria. Seventeen rhizobacteria were isolated from the rhizospheric soil and root nodules of the plant H. rhamnoides L., belonging to genus Azorhizobium, Bacillus, Brevibacillus Frankia, Paenibacillus, and Pseudomonas, all reported as PGPR, which help the plant with activities like nitrogen fixation, phosphate solubilisation, HCN production, siderophore formation, IAA, supply of valuable molecules like folic acid etc., enhancing the soil fertility and plant health (Bhattacharyya and Jha 2012; Kumar et al. 2014; Choudhary and Johri 2009).
Soil samples from the three site were found to be alkaline and saline. The soil of alkaline nature is well known for harbouring bacterial communities (Rousk et al. 2010), and same has been observed in our study where we were able to isolate fifteen bacterial strains from the rhizosphere soil samples. Soil samples from all three sites have favourable organic content (Azlan et al. 2012), but the phosphorous content varied noticeably from the normal range (Spohn and Kuzyakov 2013). Higher potash content was observed in the soil samples from site 1 and 2, though it was low in sample from site 3, showing variations.
The plant’s root represents incredible histology with the presence of two different coloured root nodules, extensive branching and root hairs. Preservation of the very essential ‘water’ for young root tissues and root perforation has been credited to root hairs which also prevent the plant roots from drying in extreme conditions. Presence of root branches gives strength to the plant, helps the roots in absorbing more water by increasing the surface area and provides soil reclamation (Haling et al. 2013; Tanaka et al. 2014).
In the current study, fifteen rhizobacteria belonging to Bacillus, Frankia, Paenibacillus, Brevibacillus, Azorhizobium and Pseudomonas were isolated from the rhizosphere soil and two different strains of Frankia were isolated from the two different root nodules. Gram-positive rhizobacteria Bacillus has been reported for plant growth enhancement by the production of IAA, HCN, siderophores, gibberellic acid, phosphate solubilization, exopolysaccharides and biocontrol of pathogenic fungi (Huang et al. 2015; Agrawal and Agrawal 2013; Apastambh et al. 2016; Rayavarapu and Padmavathi 2016). Nitrogen-fixing rhizobacteria Frankia is well known for helping the host plant survive in conditions of biotic and abiotic stress like high pH, naphthenic acid, axenic condition and limited nitrogen (Bissonnette et al. 2014; Echbab et al. 2007; Karthikeyan et al. 2013). Nitrogen-fixing rhizobacteria Azorhizobium has been gifted with various essential plant growth promoting activities (Akhtar et al. 2012; Joe and Sivakumaar 2009). Rhizobacteria Paenibacillus enhances plant growth by production of IAA, siderophores, ammonium, gibberellic acid, HCN ACC deaminase, volatile compounds, exopolysaccharides and enhances seed germination (Grady et al. 2016; Yadav et al. 2016; Goswami et al. 2016; Kefela et al. 2015; Rybakova et al. 2015; Weselowski et al. 2016). Ubiquitous Pseudomonas has also been very well known for improving plant growth by IAA, siderophore, HCN, protease, solubilization of phosphates, promoting seed germination and antifungal properties (Sivasakthi et al. 2014; Deshwal and Kumar 2013; Iqbal and Hasnain 2013; Przemieniecki et al. 2015; Dominguez-Nunez et al. 2013).
Rhizospheric Bacillus and Paenibacillus strains have been reported to produce cellulase and pectinase enzymes with valuable enzyme activities (Liang et al. 2016; Kaur et al. 2016; Khianngam et al. 2014; Woo et al. 2014; Torimiro and Okonji 2013). Frankia bacteria have been known for the production of cellulases, pectinases and proteases which provide the bacteria and host plants with additional benefits (Mastronunzio et al. 2008). Pseudomonas has been documented for cellulolytic and pectinolytic activities and provides the host plant with anti-fungal properties against pathogenic fungi (Menendez et al. 2015; Khatiwada et al. 2016; Franzetti and Scarpellini 2007). In our study, though all the isolates have shown production of root degrading enzymes pectinase and cellulose, interestingly, they vary not only at the genus level but also in the extent of their physiological activity, well-delineated by the deviations shown by the enzyme activities. Bacillus cereus st. 66 produces very efficient pectinase, whereas cellulose with the highest affinity is produced by the Azorhizobium st. BR 5401. Not only are these enzymes used by the rhizobacteria to enter the plant roots, they may also allow them to hinder the invasion of any harmful, pathogenic fungal species. Therefore, these enzymes can be used for farming purposes also, to support the growth of pants whose growth and propagation get endangered by pathogenic fungal encroachers. Being of great commercial values, these microbial strains can be mass-cultivated at optimal conditions to produce enzymes in higher amounts. Ulrich et al. (2008) isolated strains culturable cellulolytic bacteria, viz. Bacillus, Oerskovia, Streptomyces, Burkholderia, Kitasatospora, Fulvimonas, Arthrobacter, Paenibacter, Cellulomonas, Methylobacterium, Variovorax, Flavobacterium and Burkholderia. They worked on 537 different strains of bacteria in fields, to study their cellulolytic activities, on a phylogenetic basis. But, they ended-up concluding that their cellulolytic capabilities were highly variable and did not map phylogenetic affiliation. Similarly in our study, when analysed statistically, a great range of divergence was noted in the enzyme activities of these seventeen strains, spotlighting variation among the degree of root degrading capabilities of these strains.
Conclusion
Through the current study, we conclude that the rhizobacteria isolated from the root nodules and rhizospheric soil of H. rhamnoides L. are capable of producing root degrading enzymes pectinase and cellulase, in varying concentrations, with diversified levels of enzyme activities. Bacterial isolates which produced these enzymes with higher efficiencies can be very beneficial for agricultural and industrial applications. Future perspectives reside in the analysis of antimicrobial properties of these enzymes against pathogenic microorganisms. They can also be worked upon to analyse the optimal conditions to be cultivated for increased production for industrial uses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- PSAM
Pectinase screening agar medium
- MRAM
Mandels and Reese agar medium
- PGPR
Plant growth promoting rhizobacteria
- IAA
Indole acetic acid
- CLEA
Cross-linked enzyme aggregates
- DNS
Di-nitrosalicylic acid
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joginder Singh, Email: joginder.15005@lpu.co.in.
Manoj Kumar, Email: manoj@cuj.ac.in, Email: head.cls@cuj.ac.in.
References
- Agah MV, Orji JO, Nnachi AU, Chukwu OS, Udu-Ibiam OE, Nwachi AC, Olaosebikan OO. Isolation and identification of Rhizobium species from root nodules of Arachis hypogaea L. and Telfairia occidentalis in south-east Nigeria. Int J Sci Res. 2016;5(6):227–230. [Google Scholar]
- Agrawal DPK, Agrawal S. Characterization of Bacillus sp. strains isolated from rhizosphere of tomato plants (Lycopersicon esculentum) for their use as potential plant growth promoting rhizobacteria. Int J Curr Microbiol Appl Sci. 2013;2(10):406–417. [Google Scholar]
- Akhtar A, Hisamuddin RHI, Abbasi SR. Plant growth promoting rhizobacteria: an overview. J Nat Prod Plant Resour. 2012;2(1):19–31. [Google Scholar]
- Apastambh AR, Tanveer K, Baig MMV. Isolation and characterization of plant growth promoting rhizobacteria from banana rhizosphere. Int J Curr Microbiol Appl Sci. 2016;5(2):59–65. [Google Scholar]
- Azlan A, Aweng ER, Ibrahim CO, Noorhaidah A. Correlation between soil organic matter, total organic matter and water content with climate and depths of soil at different land use in Kelantan, Malaysia. J Appl Sci Environ Manag. 2012;16(4):353–358. [Google Scholar]
- Baeucehmin NJ, Furnholm T, Lavenus J, Svistoonoff S, Doumas P, Bogusz D, Laplaze L, Tisa LS. Casuarina root exudates alter the physiology, surface properties, and plant infectivity of Frankia sp. strain CcI3. Appl Environ Microbiol. 2012;78(2):575–580. doi: 10.1128/AEM.06183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Bissonnette C, Fahlman B, Peru KM, Khasa DP, Greer CW, Headley JV, Roy S. Symbiosis with Frankia sp. benefits the establishment of Alnus viridis sp. crispa and Alnus incana sp. rugosa in tailings sand from the Canadian oil sands industry. Ecol Eng. 2014;68:167–175. [Google Scholar]
- Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance. Microbiol Res. 2009;164(5):493–513. doi: 10.1016/j.micres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Dantur KI, Enrique R, Welin B, Castagnaro AP. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express. 2015;5(1):15. doi: 10.1186/s13568-015-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshwal VK, Kumar P. Production of plant growth promoting substance by Pseudomonads. J Acad Ind Res. 2013;2(4):221–225. [Google Scholar]
- Dominguez-Nunez JA, Munoz D, de la Cruz A, de Omenaca JLS. Effects of Pseudomonas fluorescens on the water parameters of mycorrhizal and non-mycorrhizal seedlings of Pinus halepensis. Agronomy. 2013;3:571–582. [Google Scholar]
- Echbab H, Arahou M, Ducousso M, Nourissier-Mountou S, Duponnois R, Lahlou H, Prin Y. Successful nodulation of Casuarina by Frankia in axenic conditions. J Appl Microbiol. 2007;103:1728–1737. doi: 10.1111/j.1365-2672.2007.03425.x. [DOI] [PubMed] [Google Scholar]
- Franzetti L, Scarpellini M. Characterisation of Pseudomonas spp. isolated from foods. Ann Microbiol. 2007;57(1):39–47. [Google Scholar]
- Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R. Microbial pectinases: an eco-friendly tool of nature for industries. Biotech. 2016;6:47. doi: 10.1007/s13205-016-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili F, Wall LG, Huss-Danell K. Effects of phosphorus and nitrogen on nodulation are seen already at the stage of early cortical cell divisions in Alnus incana. Ann Bot. 2006;98:309–315. doi: 10.1093/aob/mcl109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D, Thakker JN, Dhandhukia PC, Moral MT. Portraying mechanics of plant growth promoting rhizobacteria. Cogent Food Agric. 2016;2:1. [Google Scholar]
- Gowsalya V, Ponnusami S, Sugumaran KR. Isolation of bacteria from soil sample for exopolysaccharide production. Int J Chem Tech Res. 2014;6(5):2925–2928. [Google Scholar]
- Grady EN, MacDonald J, Liu L, Richman A, Yuan Z. Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory TR. Understanding evolutionary trees. Evol Educ Outreach. 2008;1:121–137. [Google Scholar]
- Gtari M, Brusetti L, Skander G, Mora D, Boudabous A, Daffonchio D. Isolation of Elaeagnus-compatible Frankia from soils collected in Tunisia. FEMS Microbiol Lett. 2004;234:349–355. doi: 10.1016/j.femsle.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS. Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J Exp Bot. 2013;64:3711–3721. doi: 10.1093/jxb/ert200. [DOI] [PubMed] [Google Scholar]
- Hseu ZY. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol. 2004;95:53–59. doi: 10.1016/j.biortech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Huang XF, Zhou D, Guo J, Manter DK, Reardon KF, Vivanco JM. Bacillus sp. from rainforest soil promote plant growth under limited nitrogen conditions. J Appl Microbiol. 2015;118:672–684. doi: 10.1111/jam.12720. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Hasnain S. Auxin producing Pseudomonas Strains: biological candidates to modulate the growth of Triticum aestivum beneficially. Am J Plant Sci. 2013;4:1693–1700. [Google Scholar]
- Joe MM, Sivakumaar PK. Long term survivability of Azospirillum co-aggregates: bioinoculation effect on the growth and yield of sunflower. Agricultura. 2009;6:71–77. [Google Scholar]
- Karthikeyan A, Chandrasekaran K, Geetha M, Kalaiselvi R. Growth response of Casuarina equisetifolia Forst rooted stem cuttings to Frankia in nursery and field conditions. J Biosci. 2013;38(4):741–747. doi: 10.1007/s12038-013-9362-3. [DOI] [PubMed] [Google Scholar]
- Kashyap DR, Chandra S, Kaul A, Tewari R. Production, purification and characterization of pectinase from a Bacillus sp. DT7. World J Microbiol Biotechnol. 2000;16:277. [Google Scholar]
- Kaur S, Kaur HP, Prasad B, Bharti T. Production and optimization of pectinase by Bacillus sp. isolated from vegetable waste soil. Indo Am J Pharm Res. 2016;6(01):4185–4190. [Google Scholar]
- Kefela T, Gachomo EW, Kotchoni SO. Paenibacillus polymyxa, Bacillus licheniformis and Bradyrhizobium japonicum IRAT FA3 promote faster seed germination rate, growth and disease resistance under pathogenic pressure. J Plant Biochem Physiol. 2015;3:145. [Google Scholar]
- Khatiwada P, Ahmed J, Sohag MH, Islam K, Azad AK. Isolation, screening and characterization of cellulase producing bacterial isolates from municipal solid wastes and rice straw wastes. J Bioprocess Biotech. 2016;6:280. [Google Scholar]
- Khianngam S, Pootaengon Y, Techakriengkrai T, Tanasupawat S. Screening and identification of cellulase producing bacteria isolated from oil palm meal. J Appl Pharm Sci. 2014;4(04):90–96. [Google Scholar]
- Kumar A, Kumar A, Pratush A. Molecular diversity and functional variability of environmental isolates of Bacillus species. Nature. 2014;3(1):312. doi: 10.1186/2193-1801-3-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PKR, Hemanth G, Niharika PS, Kolli SK. Isolation and identification of soil mycoflora in agricultural fields at Tekkali Mandal in Srikakulam district. Int J Adv Pharm Biol Chem. 2015;4(2):484–490. [Google Scholar]
- Liang TW, Tseng SC, Wang SL. Production and characterization of antioxidant properties. Mar Drugs. 2016;14(2):40. doi: 10.3390/md14020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronunzio JE, Tisa LS, Normand P, Benson DR. Comparative secretome analysis suggests low plant cell wall degrading capacity in Frankia symbionts. BMC Genomics. 2008;9:47. doi: 10.1186/1471-2164-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez E, Bahena MHR, Fabryova A, Igual JM, Benada O, Mateos PF, Peix A, Kolarik M, Fraile PG. Pseudomonas coleopterorum sp. nov., a cellulase-producing bacterium isolated from the bark beetle Hylesinus fraxini. Int J Syst Evol Microbiol. 2015;65:2852–2858. doi: 10.1099/ijs.0.000344. [DOI] [PubMed] [Google Scholar]
- Przemieniecki SW, Kurowski TP, Karwowska A. Plant growth promoting potential of Pseudomonas sp sp0113 isolated from potable water from a closed water well. Arch Biol Sci. 2015;67(2):663–673. [Google Scholar]
- Raja S, Subhashini P, Thangaradjou T. Differential methods of localization of fungal endophytes in the seagrasses. Mycology. 2016;7(3):112–123. doi: 10.1080/21501203.2016.1218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayavarapu VGB, Padmavathi T. Bacillus sp. as potential plant growth promoting rhizobacteria. Int J Adv Life Sci. 2016;9(1):29–36. [Google Scholar]
- Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010;2010:1–12. doi: 10.1038/ismej.2010.58. [DOI] [PubMed] [Google Scholar]
- Rybakova D, Cernava T, Koberl M, Liebminger S, Etemadi M, Berg G. Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil. 2015;405:125–140. doi: 10.1007/s11104-015-2526-1. [DOI] [Google Scholar]
- Sadhu S, Maiti TK. Cellulase production by bacteria: a review. Br Microbiol Res J. 2013;3(3):235–258. [Google Scholar]
- Sambrook J, Russel DW. Rapid isolation of yeast DNA. New York: Cold Spring Harbor Laboratory; 2001. pp. 631–632. [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda MC, Glick BR. Plant growth promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dalal S, Gupta MN. A multipurpose immobilized biocatalyst with pectinase, xylanase and cellulase activities. Chem Cent J. 2007;1(1):16. doi: 10.1186/1752-153X-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FAS, Azevedo CAV. The Assistat Software Version 7.7 and its uses in the analysis of experimental data. Afr J Agric Res. 2016;11(39):3733–3740. [Google Scholar]
- Sivasakthi S, Usharani G, Saranraj P. Biocontrol potentiality of plant growth promoting bacteria Pseudomonas fluorescens and Bacillus subtilis: a review. Afr J Agric Res. 2014;9(16):1265–1277. [Google Scholar]
- Slack SM, Zeng Q, Outwater CA, Sundin GW. Microbiological examination of Erwinia amylovora exopolysaccharide ooze. Phytopathology. 2017;107:403–411. doi: 10.1094/PHYTO-09-16-0352-R. [DOI] [PubMed] [Google Scholar]
- Spohn M, Kuzyakov Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol Biochem. 2013;61:69–75. [Google Scholar]
- Tanaka N, Kato M, Tomioka R, Kurata R, Fukao Y, Aoyama T, Maeshima M. Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. J Exp Bot. 2014;65:1497–1512. doi: 10.1093/jxb/eru014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torimiro N, Okonji RE. A comparative study of pectinolytic enzyme production by Bacillus species. Afr J Biotechnol. 2013;12(46):6498–6503. [Google Scholar]
- Ulrich A, Klimke G, Wirth S. Diversity and activity of cellulose-decomposing bacteria, isolated from a sandy and a loamy soil after long-term manure application. Microb Ecol. 2008;55:512–522. doi: 10.1007/s00248-007-9296-0. [DOI] [PubMed] [Google Scholar]
- Viveros OM, Jorquera MA, Crowley DE, Gajardo G, Mora ML. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr. 2010;10:293–319. [Google Scholar]
- Wanmolee W, Sornlake W, Rattanaphan N, Suwarnnarangsee S, Laosiripojana N, Champreda V. Biochemical characterization and synergism of cellulolytic enzyme system from Chaetomium globosumon rice straw saccharification. BMV Biotechnol. 2016;16:82. doi: 10.1186/s12896-016-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselowski B, Nathoo N, Eastman AW, MacDonald J, Yuan Z. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol. 2016;16:244. doi: 10.1186/s12866-016-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HL, Hazen TC, Simmons BA, DeAngelis KA. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst Appl Microbiol. 2014;37:60–67. doi: 10.1016/j.syapm.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Yadav AN, Sachan SG, Verma P, Saxena AK. Bioprospecting of plant growth promoting psychrotrophic Bacilli from the cold desert of north western Indian Himalayas. Indian J Exp Biol. 2016;54:142–150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.