Abstract
Magnetism is one of the physical methods affecting water properties. It is considered as an environmental factor that plays a role in the physiological and biochemical reactions. A hydroponic experiment was conducted using four types of treated water (distilled water, magnetically treated distilled water, magnetically treated tap water, and tap water). Tobacco plants (Nicotiana tabacum var. Turkish) were placed in a growth chamber for three weeks. Plants irrigated with magnetically treated distilled water had a significant increase in the physiological parameters including shoot height and root length (P < 0.0001). The same pattern was seen in the photosynthetic rate and protein content, but no significant differences in the stomatal conductance and transpiration rate (P < 0.5601). In contrast, a significant increase of total carbohydrate content was exhibited in plant irrigated with tap water (P < 0.0064). Electron micrographs showed deformed chloroplasts with damaged thylakoid membranes associated with plastoglobules in plants irrigated with tap water and magnetically treated tap water. Lastly, this study suggests that magnetically treated water is an excellent option to improve irrigation methods and thus obtains agricultural production with high efficiency.
Keywords: Nicotiana tabacum, Magnetized water, Photosynthetic rate, Thylakoids, Chloroplast
Introduction
In the last few decades, scientists intent their thinking to magnetically treated water to change its properties, so it could be used in different applications and many fields including agriculture. Water properties change after being magnetized. The changes occur in the structure of water (Chang and Weng 2006; Hozayn and Ahmed 2019). The magnetic effect causes changes in ion distribution in the diffuse layers and leads to organizing those ions (Szkatula et al. 2002; Abedinpour and Rohani 2017). The movement of positive charges is by the right-hand rule, while the movement of negative charges in the opposite direction causing more collisions force between molecules. Thus, the velocity will increase leading to metals precipitation and presence of insoluble compounds (Gholizadeh and Arabshahi 2008). Magnetically treated water (MTW) has a significant effect on one of the most important environment problem-water pollution. It has been shown that magnetically treated water treatment would decrease the bad odor of water and decrease the bacterial content when using static and shaked magnetized water treatment (Alkhazan and Saddiq 2010). It has been noticed that water exposed to the magnetic field directly affects living cells; reducing microbial water polluting (Smirnov 2003). Magnetized treatment of natural water improved the growth and yield of both monocot and dicot plants (Ijaz et al. 2014; Abedinpour and Rohani 2017). Several researches conducted over the years included different varieties of plants such as tomatoes, wheat, tobacco, chickpeas, lettuce and others showed positive increase in the crop yield, maturation of crops, reduction of plant diseases, improvement of crop quality, increase in fertilizers’ efficiency, and reduction the cost of farm operations, and optimal nutrient availability (Aladjadjiyan 2002; Aladjadjiyan and Ylieva 2003; Podleśny et al. 2004; Maheshwari and Grewal 2009; Hozayn et al. 2010; Moussa 2011; Chern 2012; Zúñiga et al. 2016; Massah et al. 2019). Asghar et al. (2017) reported that using both laser and magnetic field in pre-sowing Glycine max seed treatments had positive effect on the germination, seedling growth, and yield characteristics. Moreover, previous studies showed that magnetically treated water increased the chemical content of plants, improved chlorophyll composition, minerals, nucleic acids and protein content in plants (El-Sayed and El-Sayed 2014; Iqbal et al. 2016). Additionally, the exposure of wheat seeds before planting to magnetic field increased productivity particularly when the seeds were preliminarily soaked with water (Ijaz et al. 2014). Irrigattion with magnetically treated water significantly improved the growth characteristics, Gibberellic acid (GA3), and kinetin hormone in bean plant (Chern 2012). Also, it has been shown that magnetically treated water increases synthesis of photosynthetic pigments, photosynthetic rate and translocation of photoassimilates (Chern 2012); significant increase in plant height, spike length/weight, straw yield, and grain yield (Moussa 2011). It has been shown that magnetically treated water has accelerated seedling emergence of wheat when compared to wheat irrigated with tap water (Hilal et al. 2002).
Therefore, the aim of this study was to evaluate tobacco physio-biochemical and anatomical characteristics in response to magnetically treated water. Tobacco plant was used in this experiment as a model plant in the field of plant research.
Materials and methods
Plant materials and growth conditions
Tobacco seeds (Nicotiana Tabacum var. Turkish) were planted in nutrient-rich soil. Seedlings with same heights and number of leaves were selected for this study. Three independent experiments were conducted, each included 40 pots. Plants were divided into four groups [plants supplied with distilled water (D), plants supplied with magnetically treated distilled water (MD), plants supplied with tap water (T), and plants supplied with magnetically treated tap water (MT)], and all placed in Hoagland’s nutrient solution (Caisson Laboratories Inc., UT, USA). Plants were grown hydroponically for 21 days in a growth chamber (Bionex, model VS-3DM, Korea) as described by Alkhatib et al. (2016). The intensity of magnetic fields was about 0.07 T. Randomized complete block design was used in this experiment.
Morphological characteristics
At the end of each the experiment, shoots and roots were detached from each other. Then, the shoot height and root length were measured for each plant using a metric ruler. Post measuring, shoots and roots were put in paper bags and placed in the oven (Thermo lab Industries, HI 950D, Italy) at 60 °C for 2 days and their dry weight was recorded using analytical balance (Alkhatib et al. 2016).
Physiological characteristics
Net photosynthetic rate (PN), stomatal conductance (gs), transpiration rate (E), were measured using an infrared gas analyzer-based photosynthesis system (LI-6400, Li-Cor., Lincoln, NE, USA).
Total carbohydrate determination
Anthrone chemical (Yemm and Willis 1954; Parthiban et al. 2012) was used to determine the total carbohydrate content. Briefly, 100 mg of tobacco leaf was ground in liquid nitrogen. Then, a freshly prepared 0.2% (m/v) anthrone solution [0.2 g of anthrone dissolved in 100 cm3 of chilled 75% (m/m) H2SO4 with 98% purity] was added and stirred well. A spectrophotometer (UVM51 UV–VIS, BEL, BEL Engineering, Monza, Italy) was used to measure the absorbance of the solution at 630 nm.
Protein content determination
Bradford protocol (Bradford 1976) was followed to estimate the protein content. Protein concentration in all tobacco-treated plants was determined against a standard curve using bovine serum albumin (BSA). Absorbance of all solutions was measured using a spectrophotometer (UV-M51 UV–VIS, BEL, BEL Engineering) at 595 nm.
Electron microscopy
Leaf samples (D, MD, T, and MT) were prepared for electron transmission microscopy as described by Alkhatib et al. (2019). Briefly, leaf sections (70 nm) were cut using a Leica ultramicrotome (Leica, Switzerland), fixed, dehydrated, stained with uranyl acetate (Epstein and Holt 1963) then by lead citrate (Reynolds 1963), and checked using a transmission electron microscope (TEM) [FEI Morgagni 268(D), Netherland].
Statistical analysis
All obtained data were analyzed using the analysis of variance (ANOVA) and Dunnett’s tests at α = 0.05. PC SAS software was used for all statistical analysis (v. 9.2; SAS Institute, Cary, NC, USA). To assess the significant differences between different treatments, Dunnett’s test was used.
Results
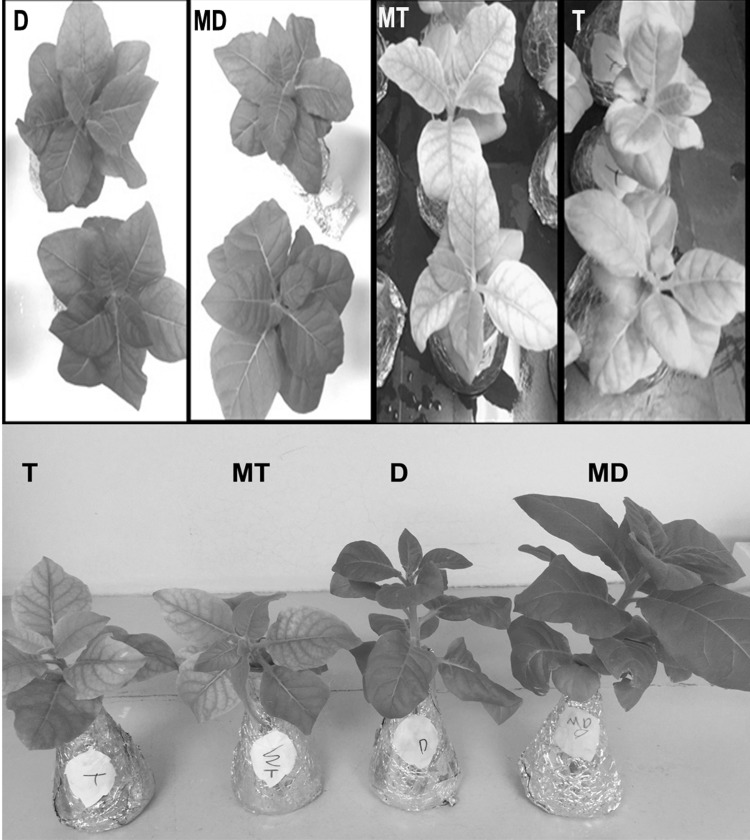
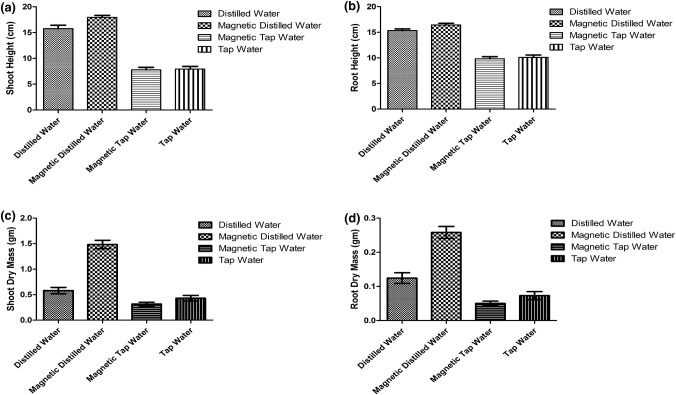
The morphology of plants grown in tap water (T) and magnetically treated tap water (MT) showed a significant reduction in their shoot height and root length as compared to plants grown in distilled water (D) and magnetically treated distilled water (MD) (Fig. 1). Tap water (T) and MT-treated plants showed growth retardation with obvious reduction in leaf area. In addition, severe chlorosis was observed on the surface of the leaves (Fig. 1). In contrast, plants grown in distilled water (D) and magnetically treated water (MD) showed significant increase in their shoot heights and root lengths as compared to other treated plants (P < 0.0001) (Fig. 2a, b). The dry weight of shoots and roots of plants grown in magnetically treated distilled water was significantly increased compared to plants irrigated with distilled, magnetized tap water and tap water (P < 0.0001). Moreover, no significant differences was observed between plants grown in magnetically treated tap water and tap water (Fig. 2c, d).
Fig. 1.
The physical appearance of Nicotiana tabacum plants treated by four types of water: Distilled water (D), magnetically treated distilled water (MD), magnetically treated tap water (MT), and tap water (T)
Fig. 2.
Effects of different types of water (D, MD, MT, T) on Nicotiana tabacum panel a shoot height (cm), b root length (cm), c shoot dry mass (g), d root dry mass (g). The average mean and standard error were plotted
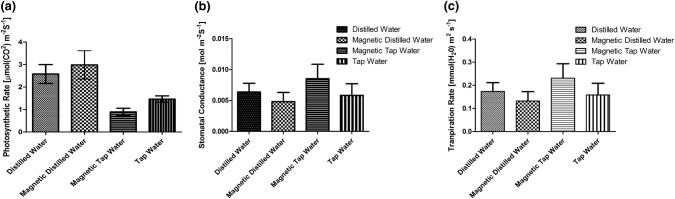
For photosynthetic parameters, there was a significant effect of magnetically treated water on the photosynthetic rate of the tobacco plants (P < 0.0023). However, there was no significant difference between plants grown in distilled water and plants grown in magnetically treated distilled water (Fig. 3a). Interestingly, there were no significant differences between plants grown in the four types of water in essence of stomatal conductance and transpiration rate (P < 0.5601) (Fig. 3b, c, respectively).
Fig. 3.
Photosynthetic parameters in Nicotiana tabacum under different types of water (D, MD, MT, T). a Photosynthetic rate [μmol(CO2) m−2 s−1]. b Stomatal conductance [mol m−2 s−1]. c Transpiration rate [nmol m−2 s−1]. The average mean and standard error were plotted
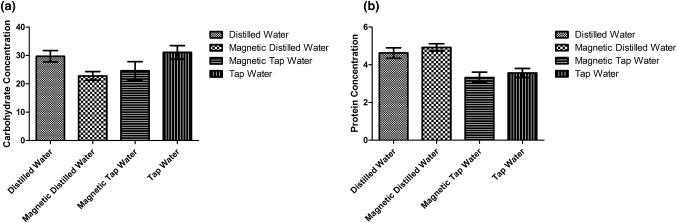
For total carbohydrate content, a significant increase (P < 0.0064) was observed in plants grown in tap water compared to other treated plants (Fig. 4a). For protein content, our data showed that there was a significant difference (P < 0.0001) in plants grown in distilled water and magnetically treated distilled water compared to magnetically treated tap and tap water (Fig. 4b).
Fig. 4.
Effects of different treated types of water (D, MD, MT, T) on Nicotiana tabacum. a Total carbohydrate content [% of (f.m)]; b protein content [% of (f.m)]. The average mean and standard error were plotted
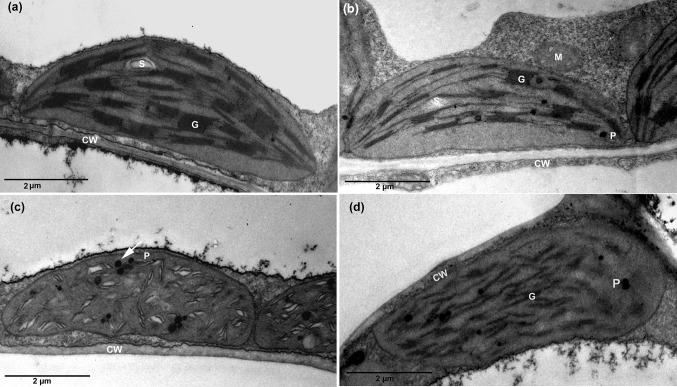
The electron micrographs of the mesophyll chloroplasts from the plants grown in tap water and magnetically treated tap water were characterized by deformed grana and numerous plastoglobules, especially in plants grown in tap water (Fig. 5c, d, respectively) as compared in plants grown in distilled and magnetically treated distilled water (Fig. 5a, b) which exhibit normal chloroplast with regular thylakoid membranes.
Fig. 5.
Electron micrographs of leaf chloroplast. a Distilled water treated tobacco leaf showing normal distribution of the grana (G) formed from several thylakoid membranes. b Magnetically treated distilled water tobacco leaf showing intact arrangement of the grana (G) and normal starch grain (S). c, d Tap water and magnetically treated tapwater treated tobacco leaf, respectively, showed damaged thylakoid membranes and several plastoglobules (P). Scale bars: 2 µm
Discussion
Several studies indicated that magnetic field increases the internal energy of the seed causing a high germination rate (Vashisth and Nagarajan 2008; Sudsiri et al. 2016; Asghar et al. 2017). In our study, plants treated with magnetically treated distilled water showed a significant increase in shoot height and root length compared to plants grown in tap and distilled water. This increase could be due to the interference in some metabolic processes such as photosynthesis, transpiration, respiration (Greger et al. 1992; Yamamoto et al. 2008; Iqbal et al. 2016). While the analysis of our data shows an increase in shoot/root ratio in plants grown in magnetically treated distilled water compared with magnetically treated tap water, this may result from the high concentration of minerals and metals fused inside the tap water, which could be an interruption of magnetic field interaction.
It has been revealed that the increase in growth parameters (shoots/roots) under the effect of magnetically treated water resulted from the capacity improvement for nutrient and water uptake (Sadeghipour and Aghaei 2013; Massah et al. 2019). Moreover, a significant increment in the rate of water absorption accompanied by an increase in total mass of lettuce was observed with the increase of magnetic force (Reina et al. 2001). Our data showed that magnetically treated water had the greatest effect on root weight. This suggests that growth enhancement was related to the increase of root growth which improved water and ions absorption. Thus, magnetic field increases cell division and protein synthesis subsequently causing an increase in plant growth (Çelik et al. 2008; El-Sayed and El-Sayed 2014).
For photosynthetic rate, plants treated with magnetically treated distilled water showed significant increase compared to other plants. This might be related to the increase in the leaf area in plants grown in MD water compared to other treatments, which led to enhancement in the photosynthetic rate due to the greater amount of absorption and capturing of light and the increase of assimilates available for vegetative growth (Sadeghipour and Aghaei 2013). Podleśna et al. (2015) reported that laser irradiation of pea seeds increased leaf area; increasing chlorophyll content in leaves which is a crucial factor in photosynthetic activity. Also, it may be due to the increase of photosynthetic pigments such as chlorophyll a, b and carotenoids (Racuciu et al. 2007, Selim and El-Nady 2011; Iqbal et al. 2016). Moreover, Asghar et al. (2016) reported that under the effect of both laser and magnetic pre-sowing treatments, the biochemical and chlorophyll contents were significantly enhanced in soybean seeds and seedlings. On the other hand, no significant effect for both stomatal conductance and transpiration rate were observed. This suggests that the increment of the photosynthetic rate on magnetically treated distilled water is not a consequence of stomatal opening which is consistent with previous work on rice and tobacco (Lidon et al. 1993; Alkhatib et al. 2016). Also, this indicates that the drop and/or increase in the photosynthetic reactions are not influenced by the state of stomatal closure and/or opening, and this influence is rather indirect. Shine et al. (2011) reported that in magnetically treated soybean plants, the SDS–polyacrylamide gel electrophoresis exhibit increased intensities of the bands of the larger and smaller subunits of Rubisco, which is a key factor in increasing the photosynthetic rate.
For protein content, a significant increase in total protein contents in plants treated with magnetically treated distilled water was observed which is consistent with the results obtained from Fomicheva et al. (1992a, b) which reported that protein content in broad bean yield had significant increase under magnetically treated water irrigation. Moreover, it has been reported that magnetically treated water had a significant effect in inducing cell metabolism, enzymatic activities, and mitosis in a pea, lentil, turnip, and flax (Belyavskaya 2001, Ul Haq et al. 2016). In addition, magnetic treatment affects the Ca2+ channel proteins, which is considered the second messengers cytokines, inducing cytokinins synthesis which then stimulate protein synthesis (Brault and Maldiney 1999; D’Agostino and Kieber 1999; Carimi et al. 2003).
For total carbohydrate content, plants grown in tap water had significant increase. This suggests that carbohydrate content of plants under abiotic stress increased (Gilbert et al. 1997; Balibrea et al. 2000; Pattanagul and Thitisaksakul 2008). This accumulation in plant leaves may result due to the reduction of both phloem loading and assimilate transport capacity, or due to the decrease in the rate of utilization of the assimilates in the sink organs (Alaoui-Sossé et al. 2004; Alkhatib et al. 2016).
In plants grown in magnetically treated distilled and distilled water, the ultrastructural organization of the chloroplasts and the integrity of the thylakoids were intact (Fig. 5a, b). In contrast, chloroplasts of plants grown in magnetically treated tap water and tap water exhibited severe reduction in grana and the integrity of their thylakoids were lost completely (Fig. 5c, d). This suggests the severe drop in the photosynthetic rate in these plants as compared to plants grown in magnetically treated distilled and distilled water is correlated with thylakoid membranes integrity (Alkhatib et al. 2016, 2019). In addition, Tai et al. (2008) reported that magnetized water blocks the uptake of roots to harmful metals, such as lead and nickel preventing chloroplast damage. Moreover, plastoglobules in chloroplasts were more pronounced in plants exposed to different stresses (Gillet et al. 1998; Yang et al. 2006; Alkhatib et al. 2016, 2019). For example, Arabidopsis and tobacco chloroplasts exposed to light stress exhibit high levels of plastoglobulin proteins (Rey et al. 2000; Yang et al. 2006). It was suggested that high amounts of plastoglobulin proteins and antioxidants such as tocopherols are found in plastoglobules (Claire et al. 2007).
Conclusion
Our data showed that the total impact of magnetically treated distilled water on plants leads to increase in the physiological and biochemical parameters including: shoot height, root length, shoot/root dry mass, photosynthetic rate and protein content. Therefore, using magnetically treated water in irrigation might be a very practical way to improve agricultural and horticultural production under greenhouse or field conditions. Also, it is considered one of the safe and eco-friendly non-traditional technologies to improve soil and water properties. However, further studies are needed to elucidate our knowledge about magnetized water applications in agriculture.
Acknowledgements
This project was financially funded by the Deanship of Scientific Research at Jordan University of Science and Technology (Grant No. 20160109). Also, the authors would like to thank Ms. Batool Alkhatib for helping in sample preparation for TEM, Ms. Haifa Hattab and Ms. Duaa Qattan for their technical assistance in the TEM facility at the University of Jordan, Faculty of Medicine, Amman, Jordan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abedinpour M, Rohani E. Effects of magnetized water application on soil and maize growth indices under different amounts of salt in the water. J Water Reuse Desalt. 2017;7:319–325. [Google Scholar]
- Aladjadjiyan A. Study of the influence of magnetized field on some biological characteristics of Zea maize. J Cent Eur Agric. 2002;3:90–94. [Google Scholar]
- Aladjadjiyan A, Ylieva T. Influence of stationary magnetized field on the early stages of the development of tobacco seeds (Nicotiana tabacum L.) J Cent Eur Agric. 2003;4:131–138. [Google Scholar]
- Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004;166:1213–1218. [Google Scholar]
- Alkhatib R, Alkhatib B, Al-Quraan N, Al-Eitan L, Abdo N, Muhaidat R. Impact of exogenous caffeine on morphological, biochemical, and ultrastructural characteristics of Nicotiana tabacum. Biol Plant. 2016;60:706–714. [Google Scholar]
- Alkhatib R, Alkhatib B, Abdo N, Al-Eitan L, Creamer R. Physio-biochemical and ultrastructural impact of (Fe3O4) nanoparticles on tobacco. BMC Plant Biol. 2019;19:253–265. doi: 10.1186/s12870-019-1864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhazan MM, Saddiq AA. The effect of magnetized field on the physical, chemical and microbiological properties of the lake water in Saudi Arabia. J Evol Biol Res. 2010;2:7–14. [Google Scholar]
- Asghar T, Jamil Y, Iqbal M, Zia-ul-Haq AM. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J Photochem Photobiol B. 2016;165:283–290. doi: 10.1016/j.jphotobiol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Asghar T, Iqbal M, Jamil Y, Zia-ul-Haq NJ, Shahid M. Comparison of He-Ne laser and sinusoidal non-uniform magnetic field seed pre-sowing treatment effect on Glycine max (Var 90-I) germination, growth and yield. J Photochem Photobiol B. 2017;166:212–2019. doi: 10.1016/j.jphotobiol.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Balibrea ME, Amico JD, Bolarin MC, Perez- Alfocca F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol Plant. 2000;110:503–511. [Google Scholar]
- Belyavskaya NA. Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetized fields. Adv Space Res. 2001;28:645–650. doi: 10.1016/s0273-1177(01)00373-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brault M, Maldiney R. Mechanisms of cytokinin action. Plant Physiol Biochem. 1999;37:403–412. [Google Scholar]
- Carimi F, Zottini M, Formentin E, Terzi M, Schiavo FL. Cytokinins: new apoptotic inducers in plants. Planta. 2003;216:413–421. doi: 10.1007/s00425-002-0862-x. [DOI] [PubMed] [Google Scholar]
- Çelik Ö, Atak Ç, Rzakulieva A. Stimulation of rapid regeneration by a magnetized field in Paulownia node cultures. J Cent Eur Agric. 2008;9:297–304. [Google Scholar]
- Chang KT, Weng CI. The effect of an external magnetized field on the structure of liquid water using molecular dynamics simulation. J Appl Phys. 2006;100:043917. [Google Scholar]
- Chern CC (2012) Application of magnetic water to stimulate the lady’s finger (Abelmoschus esculentus L.) moench plant growth. Dissertation, Universiti Teknologi, Malaysia
- Claire B, Felix K, Klaas J, Van W. Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci. 2007;12:260–266. doi: 10.1016/j.tplants.2007.04.003. [DOI] [PubMed] [Google Scholar]
- D’Agostino IB, Kieber JJ. Molecular mechanisms of cytokinin action. Curr Opin Plant Biol. 1999;2:359–364. doi: 10.1016/s1369-5266(99)00005-9. [DOI] [PubMed] [Google Scholar]
- El-Sayed H, El-Sayed A. Impact of magnetized water irrigation for improve the growth, chemical composition and yield production of broad bean (Vicia faba L.) plant. Am J Exp Agric. 2014;4:476–496. [Google Scholar]
- Epstein MA, Holt SJ. The localization by electron microscopy of Hela cell surface enzymes splitting adenosine triphosphate. J Cell Biol. 1963;19:325–336. doi: 10.1083/jcb.19.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomicheva VM, Govorun RD, Danilov VT. Proliferative activity and cell reproduction in the root meristem of pea lentil and flax in the conditioning of screening the geomagnetized field. Biophysics. 1992;37:645–648. [Google Scholar]
- Fomicheva VM, Zaslavskii VA, Govorun RD, Danilov VI. Dynamics of RNA and protein synthesis in the cells of the root meristem of the pea, lentil and flax. Biophysics. 1992;37:649–656. [Google Scholar]
- Gholizadeh M, Arabshahi H. The effect of magnetized water on growth and quality improvement of poultry. Middle-East J Sci Res. 2008;3:140–144. [Google Scholar]
- Gilbert GA, Wilson C, Madore MA. Root-zone salinity alters raffinose family oligosaccharide metabolism and transport in Coleus. Plant Physiol. 1997;115:1267–1276. doi: 10.1104/pp.115.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet B, Beyly A, Peltier G, Rey P. Molecular characterization of CDSP 34, a chloroplastic protein induced by water deficit in Solanum tuberosum L. plants, and regulation of CDSP 34 expression by ABA and high illumination. Plant J. 1998;16:257–262. doi: 10.1046/j.1365-313x.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Greger M, Tillberg JE, Johansson M. Aluminum effects on Scenedesmus obtusiusculus with different phosphorus status. II. Growth, photosynthesis and pH. Physiol Plant. 1992;84:202–208. [Google Scholar]
- Hilal MH, Shata SM, Abdel-Dayem AA, Hilal MM. Application of magnetized technologies in desert agriculture: III. Effect of magnetized water on yield and uptake of certain elements by citrus in relation to nutrients mobilization in soil. Egypt J Soil Sci. 2002;42:43–56. [Google Scholar]
- Hozayn M, Ahmed AA. Effect of Magneto-priming by tryptophan and ascorbic acid on germination attributes of barley (Hordeum vulgare, L.) under salinity stress. EurAsian J BioSci. 2019;13:245–251. [Google Scholar]
- Hozayn M, Qados A, Saeed AM. Magnetized water application for improving wheat (Triticum aestivum L.) crop production. Agric Biol J North Am. 2010;1:677–682. [Google Scholar]
- Ijaz B, Jatoi SA, Ahmad D, Masood MS, Siddiqui SU. Changes in germination behavior of wheat seeds exposed to magnetized field and magnetizedally structured water. Afr J Biotechnol. 2014;11:3575–3585. [Google Scholar]
- Iqbal M, Ul Haq Z, Jamil Y, Nisar J. Pre-sowing seed magnetic field treatment influence on germination, seedling growth and enzymatic activities of melon (Cucumis melo L.) Biocatal Agric Biotechnol. 2016;6:176–183. [Google Scholar]
- Lidon FC, Ramalho JC, Henriques FS. Copper inhibition of rice photosynthesis. J of Plant Physiol. 1993;142:12–17. [Google Scholar]
- Maheshwari BL, Grewal HS. Magnetized treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agr Water Manage. 2009;96:1229–1236. [Google Scholar]
- Massah J, Dousti A, Khazaei J, Vaezzadeh M. Effects of water magnetic treatment on seed germination and seedling growth of wheat. J Plant Nutr. 2019;42:1283–1289. [Google Scholar]
- Moussa HR. The impact of magnetized water application for improving common bean (Phaseolus vulgaris L.) production. New York Sci J. 2011;4:15–20. [Google Scholar]
- Parthiban VK, Prakasam K, Prabakar K. Changes in the biochemical constituents of carrot roots due to bacterial soft rot. Int J Appl Biol Pharm Technol. 2012;3:231–238. [Google Scholar]
- Pattanagul W, Thitisaksakul M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Biol. 2008;46:736–742. [PubMed] [Google Scholar]
- Podleśny J, Pietruszewski S, Podlesna A. Efficiency of the magnetized treatment of broad bean seeds cultivated under experimental plot conditions. Int Agrophys. 2004;18:6571. [Google Scholar]
- Podleśna A, Gładyszewska B, Podleśny J, Zgrajka W. Changes in the germination process and growth of pea in effect of laser seed irradiation. Int Agrophys. 2015;29:485–492. [Google Scholar]
- Racuciu M, Creanga D, Amoraritei C. Biochemical changes induced by low frequency magnetized field exposure of vegetal organisms. Rom J Phys. 2007;52:645–651. [Google Scholar]
- Reina FG, Pascual LA, Fundora IA. Influence of a stationary magnetized field on water relations in lettuce seeds. Part II: experimental results. Bioelectromagnetizeds. 2001;22:596–602. doi: 10.1002/bem.89. [DOI] [PubMed] [Google Scholar]
- Rey P, Gillet B, Römer S, Eymery F, Massimino J, Peltier G, Kuntz M. Over-expression of a pepper plastid lipid-associated protein in tobacco leads to changes in plastid ultrastructure and plant development upon stress. Plant J. 2000;21:483–494. doi: 10.1046/j.1365-313x.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghipour O, Aghaei P. Improving the growth of cowpea (Vigna unguiculata L. Walp.) by magnetized water. J Biodivers Environ Sci. 2013;3:37–43. [Google Scholar]
- Selim AH, El-Nady MF. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011;69:387–396. [Google Scholar]
- Shine MB, Guruprasad KN, Anand A. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics. 2011;32:474–484. doi: 10.1002/bem.20656. [DOI] [PubMed] [Google Scholar]
- Smirnov JV (2003) BioMagnetized hydrology. In: The effect of a specially modified electromagnetized field on the molecular structure of liquid water. Global Quantec. Inc., Lexington, pp 122–125
- Sudsiri CJ, Nattawat J, Kongchana P, Ritchie RJ. Effect of magnetically treated water on germination and seedling growth of oil palm (Elaeis guineensis) Seed Sci Technol. 2016;44:267–280. [Google Scholar]
- Szkatula A, Balanda M, Kopeć M. Magnetized treatment of industrial water. Silica activation. Eur Phys J Appl Phys. 2002;18:41–49. [Google Scholar]
- Tai CY, Wu CK, Chang MC. Effects of magnetic field on the crystallization of CaCO3 using permanent magnets. J Chem Eng Sci. 2008;63:5606–5612. [Google Scholar]
- Ul Haq Z, Iqbal M, Jamil Y, Anwar H, Younis A, Arif M, Fareed MZ, Hussain F. Magnetically treated water irrigation effect on turnip seed germination, seedling growth and enzymatic activities. Inf Process Agric. 2016;3:99–106. [Google Scholar]
- Vashisth A, Nagarajan S. Exposure of seeds to static magnetized field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.) Bioelectromagnetizeds. 2008;29:571–578. doi: 10.1002/bem.20426. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Rama Devi R, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cell. Plant Physiol. 2008;128:63–72. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sulpice R, Himmelbach A, Meinhard M, Christmann A, Grill E. Fibrillin expression is regulated by abscisic acid response regulators and is involved in abscisic acid-mediated photoprotection. Proc Natl Acad Sci USA. 2006;103:6061–6066. doi: 10.1073/pnas.0501720103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga O, Benavides JA, Ospina-Salazar DI, Jiménez CO, Gutiérrez MA. Magnetic treatment of irrigation water and seeds in agriculture. Ingeniería y Competitividad. 2016;18:217–232. [Google Scholar]